Abstract
The enteric nervous system (ENS), referred to as the “second brain,” comprises a vast number of neurons that form an elegant network throughout the gastrointestinal tract. Neuropeptides produced by the ENS play a crucial role in the regulation of inflammatory processes via cross talk with the enteric immune system. In addition, neuropeptides have paracrine effects on epithelial secretion, thus regulating epithelial barrier functions and thereby susceptibility to inflammation. Ultimately the inflammatory response damages the enteric neurons themselves, resulting in deregulations in circuitry and gut motility. In this review, we have emphasized the concept of neurogenic inflammation and the interaction between the enteric immune system and enteric nervous system, focusing on neuropeptide Y (NPY) and vasoactive intestinal peptide (VIP). The alterations in the expression of NPY and VIP in inflammation and their significant roles in immunomodulation are discussed. We highlight the mechanism of action of these neuropeptides on immune cells, focusing on the key receptors as well as the intracellular signaling pathways that are activated to regulate the release of cytokines. In addition, we also examine the direct and indirect mechanisms of neuropeptide regulation of epithelial tight junctions and permeability, which are a crucial determinant of susceptibility to inflammation. Finally, we also discuss the potential of emerging neuropeptide-based therapies that utilize peptide agonists, antagonists, siRNA, oligonucleotides, and lentiviral vectors.
Keywords: ENS, inflammation, neuropeptides, neurogenic inflammation
the enteric nervous system (ENS) is organized into two main plexi that innervate the submucosa (submucosal plexus/Meissner's plexus) and the muscularis propria (myenteric plexus). The neural networks of the plexi regulate the secretory and motor functions of the gastrointestinal tract via multiple neurotransmitters. In inflammatory diseases of the gut such as inflammatory bowel disease (IBD), the neural morphology, circuitry, and physiology are adversely affected (36). Inflammation induces abnormalities like neuronal hyperplasia, ganglion and axonal degeneration, and necrosis, alterations in the synthesis and release of neurotransmitters and/or their receptor systems, leading to impairment of secretory and motor gastrointestinal functions (82). These adaptive changes in the enteric neurons lead to significant neuronal remodeling that underlies the plasticity of the ENS.
In addition to being a target of inflammation, the neurotransmitters produced by the ENS also play a pivotal role in orchestrating the inflammatory processes in the gastrointestinal tract via effects on gut-associated lymphoid tissue (GALT), the largest immune repertoire in the body (77). GALT recognizes the “self/commensal” bacterial flora and maintains tolerance in a normal state. Considering the proximity to immune cells, it is not surprising that neuropeptides from the ENS can modulate immune cell functions like neutrophil chemotaxis (42), histamine release from mast cells (22), phagocytosis, chemokine expression (12, 79), and immunoglobulin production (51). Extensive research in the past decade has demonstrated the significance of enteric neuroenteric immune interactions in potentiating or dampening the inflammatory responses.
Neurogenic Inflammation
In late 1980, peripheral nerves were first recognized to play an active role in modulating immune responses and disease pathology of inflammatory diseases (17). Neuropeptides released from small-diameter sensory nerves were observed to regulate mast cell activation and vascular responses (14, 22), chemotaxis of neutrophils (42), and differentiation of T helper cells (63). Collectively, the responses evoked by neuropeptides, which were analogous to the inflammatory responses, were characterized as “neurogenic inflammation.” However, the effects of neuropeptides last longer because of the feedback regulation between neuropeptide expression and cytokine release from immune cells, compared with vasodilation and plasma extravasation, which are acute and rapid responses. Recently it was demonstrated that enteric hyperinnervation can actively drive intestinal inflammation, thus emphasizing the relevance of neurogenic inflammation in the gut (57). Since dysregulated immune signaling is central to IBD (18) and changes in neuropeptides have been associated with IBD (33, 59), understanding the role of enteric neuropeptides and neurogenic inflammation would help gain better insights into the pathophysiology of inflammatory diseases like IBD.
Mechanisms Mediating Neurotransmitter Release
Various mechanisms that mediate neurotransmitter release from sensory neurons include voltage-gated calcium channels (65) (capsaicin, heat, and protons), protein kinase C (PKC) (bradykinin) (83), and tryptase (via proteinase activated receptors, PAR-2) (84). It has been demonstrated recently that the activation of Toll-like receptor (TLR)-4 on enteric and sensory neurons can induce neuronal excitability, calcium signaling, and neuropeptide release (56).
Neuropeptide Effects on Inflammation
Neuropeptides may have anti-inflammatory [vasoactive intestinal peptide (VIP) and galanin] or proinflammatory effects [neuropeptide Y (NPY), substance P], serotonin, and neurotensin. These differences are due to activation of specific signaling pathways in immune cells that further propagate the inflammatory signals. Key signaling pathways in macrophages, T cells, or mast cells that are activated by neuropeptides include nuclear factor-κB (NF-κB), cyclooxygenase-2 (COX-2), or mitogen-activated protein kinase (MAPK). In addition, neuropeptides like VIP can also regulate TLR signals whereby it can modulate innate or adaptive immune responses (7).
Of the neuropeptides, NPY and VIP have been studied extensively, and the therapeutic potential of their receptors in the design of anti-inflammatory drugs is gaining significance. Hence in this review we have summarized the current understanding of the roles of these two important neuropeptides in modulating inflammatory signaling in the gut.
NPY Is an Immunomodulator
NPY is a 36-amino acid peptide produced abundantly in the central and peripheral nervous system. NPY modulates anxiety (91), appetite (32), blood pressure, and nociception (11). In the gut, NPY is abundantly produced in the sympathetic nerves and is coreleased with norepinephrine (45), where it induces colonic relaxation. Of the five receptors, NPYY1 receptor is critical to inflammatory signaling as Y1 activation has been shown to exert negative effects on T cell responses, but activates antigen presenting cells (87). Thus NPY producing neurons in the gut are potent modulators of immune responses during inflammation.
Alterations in NPY Levels During Inflammation
Cytokines from immune cells can modulate neuropeptide expression via their specific receptors, thus establishing a feedback loop between the enteric immune and the enteric nervous system (ENS). In several inflammatory diseases, neuropeptide levels have been reported to change in the ENS. As the epithelia and smooth muscles are richly innervated, this neuronal remodeling can have downstream effects on epithelial permeability (60), gut motility (16) and vascular tone (13). We (16) and others (23) have shown that NPY is upregulated in the ENS during experimental colitis. We found that NPY upregulation induces nitrosative stress resulting in persistent colonic motility impairments in mice (16). Our study also demonstrated that NPY knockout mice exhibited attenuated inflammation compared with wild-type mice (16). However, in human IBD, plasma levels of NPY have been reported to be unchanged (33), and downregulation of NPY has been demonstrated in the mesenteric ganglia of guinea pigs during inflammation (89). These findings suggest that inflammation-induced neuronal remodeling may have tissue and species-specific variations.
NPY Potentiates Inflammation by Modulating Key Immune Cell Functions via Y1/Y2 Receptors
Several studies have been instrumental in understanding NPY actions on immune cells. Interestingly, NPY is produced from T cells, macrophages, and dendritic cells during inflammation (88). NPY effects on immune and inflammatory responses are regulated by tissue-specific expression of different receptor subtypes that belong to the family of 7-transmembrane G protein-coupled receptors (GPCRs) (72). It is documented that NPY modulates inflammation by regulating key immune cell functions like neutrophil chemotaxis (42), granulocyte oxidative burst and nitric oxide production (29), T helper cell differentiation (12), natural killer cell activity (74), suppression of lymphocyte proliferation (58), and activation of antigen-presenting cells (APC) (88). The NPYY1 receptor plays a crucial role in immunomodulation as attenuation of inflammation has been noted in Y1-receptor knockout mice (43). In addition, elegant studies have delineated the bimodal role of Y1 receptor in the immune system, where it has been demonstrated that NPY exerts negative regulation on T cells but activates APC functions (88). NPYY1 receptors have been also found to mediate μ-opioid receptor (MOR)-induced reductions of natural killer cell activity (74). MORs are widely expressed in the central and peripheral neurons (68). Cytokines enhance MOR expression in mucosal immune cells and myenteric neurons during experimental inflammation (68). Hence NPY-MOR interactions in immune cells may have profound effects in modulating inflammatory signaling. The Y1 receptor is rapidly internalized by clathrin-dependent events (suggesting interactions with β-arrestin) and is also recycled rapidly (85).
NPY Y2 receptor is localized on immune cells (30) and plays a significant role in migration and adhesion of leucocytes. Contrary to the Y1 receptor, Y2 binds N-truncated fragments of the NPY peptide generated by activity of the enzyme dipeptidyl peptidase 4 (DPP4 or CD26) (30). NPY is a substrate for DPP4, a serine-ectoprotease that is highly expressed on T cells and other immune cells. DPP4 is crucial because it terminates the action of the NPY (1-36) on Y1 receptor by cleaving it into NPY (3-36) peptide. Studies using DPP4 inhibitors, synergistic with NPY, are suggestive of potentiation of inflammation by NPY-Y1 axis. Studies on knockout murine models of DPP4 have revealed attenuation of disease activity in CD26-/- mice with significant upregulation of macrophages (28).
A schematic representation of interaction between enteric immune cells and NPY is summarized in Fig. 1. As depicted in Fig. 1, NPY can activate macrophages leading to production of cytokines like IL-12, TNF-α, nitric oxide, and IL-4. Interestingly NPY inhibits T cell cytokines like IFN-γ. It is therefore suspected that Y1 receptor expression on T cells is induced only after T cell activation, thus maintaining a negative feedback loop that prevents sustained or hyperactivation of T cell responses (87).
Fig. 1.
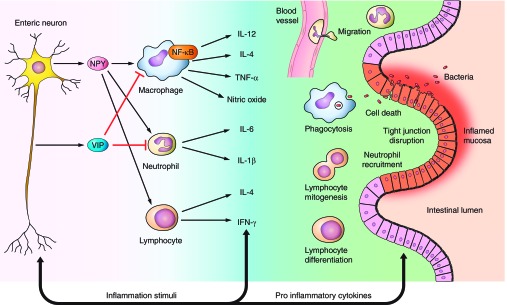
Interactions between enteric nervous system (ENS) and the enteric immune system regulate inflammatory signaling in the gut. Enteric neuropeptides like neuropeptide Y (NPY), and vasoactive intestinal peptide (VIP) can activate immune cells like macrophages, neutrophils, or lymphocytes and induce the release of various proinflammatory cytokines like TNF-α, IL-6, and IFN-γ. The cytokines in turn activate receptors on the ENS, thus establishing a bidirectional communication. This cross talk between the ENS and enteric immune system regulates the magnitude of inflammatory signaling via modulation of lymphocyte mitogenesis and differentiation, phagocytosis, neutrophil recruitment, production of cytokines, disruption of epithelial tight junctions, and finally cell death.
NPY Activation of Intracellular Signaling Pathways in Immune Cells Mediates Cytokine Secretion in the Gut
NPY binding to the Y1/Y2 receptors activates various signaling pathways that mediate immunity and inflammation. These include adenylate cyclase-cAMP, NF-κB, COX-2, protein kinases (MAPK), protein kinase A (PKA), phospholipase C, PKC, and phosphatidyl inositol-3-kinase.
The transcription factor NF-κB is a key regulator of cellular response to various stressors and mediates secretion of proinflammatory cytokines (8). NPY has been shown to inhibit nuclear translocation of NF-κB in microglia challenged with IL-1β, thus inhibiting IL-1β-induced nitric oxide release (35). In addition, the presence of potential binding sites for NF-κB has been identified in the human and murine NPY-Y1 receptor gene (80). Interestingly, inflamed mucosa from IBD patients display increased NF-κB activity in macrophages and epithelial cells, which correlates significantly with the severity of inflammation (61). Accordingly, downregulation of inflammation has been shown by administration of antisense oligonucleotides to the p65 subunit of NF-κB (49). Recent studies using NPY antisense oligonucleotides in mouse model of DSS colitis also revealed a reduction in TNF-α and phosphorylation of NF-κB (67). Thus NF-κB is a potent regulator of NPY-induced inflammatory signaling that could be targeted for therapy. NPY via PKC modulates intracellular Ca2+ mobilization and thus plays a significant role in controlling the excitability of submucosal neurons and hyperexcitability after inflammation (69). In addition, NPY modulates macrophage functions like oxidative burst via PKC (30).
All NPY receptor subtypes activate MAPK pathways, and as a consequence influence the control of gene expression and cell fate. NPY via activation of c-Jun NH2-terminal kinase (JNK) and p38 kinase play a significant role in the innate and adaptive immune systems as activation of TLRs can activate MAPK. MAPK activation in sensory neurons can also regulate inflammatory pain (64).
Stress and NPY
Recent studies have demonstrated that perceived stress, negative mood and major life events can significantly trigger the flare of inflammatory diseases (5). Stress response involves mainly the glucocorticoids and catecholamines (norepinephrine and epinephrine) and is mediated by hypothalamic-pituitary axis and sympathoadrenomedullary system. NPY may or may not be coreleased with catecholamines during stress. Stress-induced NPY release through Y2 receptors can inhibit production of catecholamines or cortisol presynaptically and thus modulate the overall magnitude of stress response (62). Recently it has been demonstrated that stress exaggerates diet-induced obesity via NPY and its receptor (NPY2R) upregulation in the white adipose tissue. NPY upregulation in turn induced a metabolic syndrome-like condition involving angiogenesis, macrophage infiltration, adipocyte proliferation, and differentiation, resulting in abdominal obesity. Elegant studies demonstrated that these effects were reversed by pharmacological inhibition or adipose-specific knockdown of NPY2R, thus opening new treatment options for treatment of metabolic syndrome focused on inhibition of NPY receptors (53).
Effects of NPY on Epithelial Permeability
The maintenance of the integrity of the intestinal epithelium is crucial in preventing inflammatory processes. Barrier integrity within intestinal epithelia is mediated by desmosomes, adherens junctions, and tight junctions. Tight junctions are composed of at least 20 transmembrane proteins embedded across the plasma membrane, with extracellular domains joining one another directly and intracellular domains linked to the actin cytoskeleton of the cell (4). A compromised epithelial barrier adversely affects the phagocytosis of pathogens, secretion, and nutrient uptake, thus disturbing epithelial homeostasis and increasing the susceptibility to diseases like IBD (86).
As submucosal neurons innervate the epithelium, various coculture models of neuronal and epithelial cell lines have been utilized to better understand the neuronal modulation of epithelial barrier functions. It has been recently shown that dysfunction of tight junctions can also induce activation of the immune system in experimental colitis (78). Stress has been postulated to play a significant role in causing active flares of diseases like IBD and irritable bowel syndrome leading to disorders in epithelial secretion and permeability (3).
NPY, abundantly produced by submucosal neurons, is released within the lamina propria of both crypts and villi. The effects of NPY on epithelial cells are mainly mediated by G protein-coupled receptors Y1 and Y2 (72, 81). These receptors regulate epithelial secretory function and hence contribute to changes in permeability. Recently it has been demonstrated that NPY via stimulation of Erk/MAPK-dependent pathways can stimulate Cl-/HCO3-(OH-) exchange activity, and alterations in the association of Cl-/HCO3-(OH-) exchanger with lipid rafts might contribute to proabsorptive effects of NPY (73). In addition to its direct effect on epithelial permeability via its receptors, NPY can also have indirect effects on epithelia via regulation of cytokine production.
Direct Effects of NPY on Intestinal Epithelium via Y1 Receptors
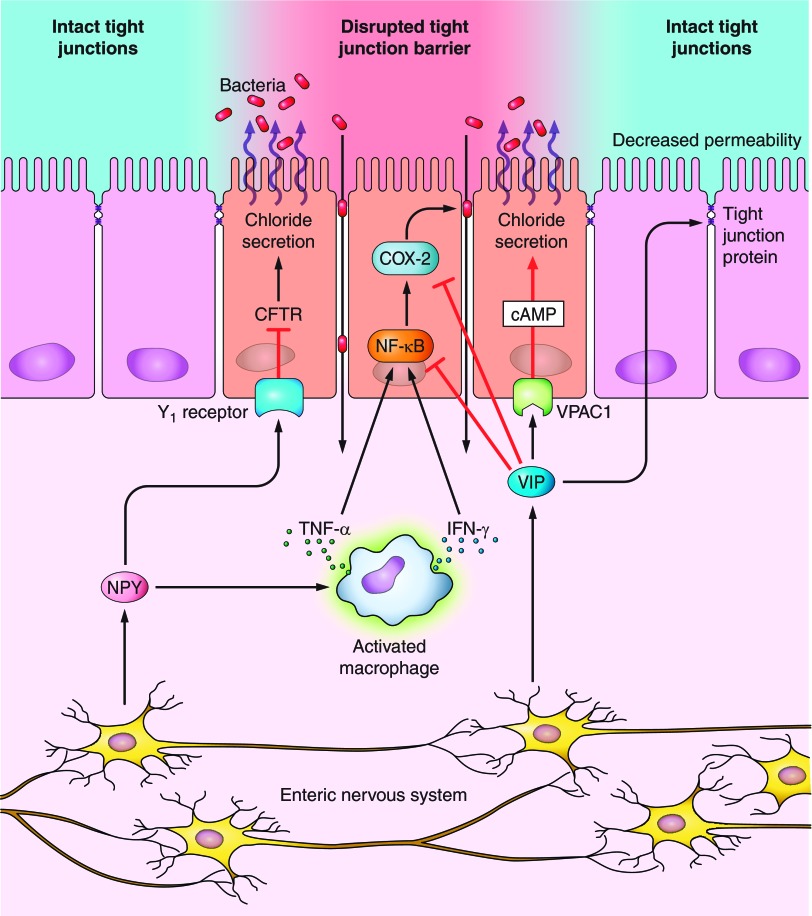
A schematic representation of mechanism of NPY regulation of epithelial permeability is given in Fig. 2. NPY peptide via Y1 receptors exerts an antisecretory role via attenuation of cytoplasmic cAMP (81), thereby reducing the activity of the PKA-sensitive chloride channels (CFTR) and basolateral K+ conductance (21). As CFTR-mediated chloride secretion modulates secretory diarrhea phenotype, it is probable that stable Y1 agonists could be therapeutically beneficial as novel antidiarrheals. However, in experimental colitis, antisecretory effects are observed to be diminished as reported recently because of reduction in Y1 receptor expression (52).
Fig. 2.
Direct and indirect effects of neuropeptides on epithelial secretion and barrier functions. The enteric neuropeptides can directly induce or inhibit chloride secretion via their respective receptors on epithelia, or indirectly modulate chloride secretion via cytokines. For example, NPY via Y1 receptors directly exerts an antisecretory role via attenuation of cytoplasmic cAMP, thereby reducing the activity of the PKA-sensitive chloride channels (CFTR). In addition, NPY via regulating TNF-α release from macrophages can also induce chloride secretion. VIP normally promotes secretory functions via its receptor VPAC1 through cAMP. However, VIP can also have antisecretory effects by inhibiting cyclooxygenase-2 (COX-2) and NF-κB. In addition VIP reduces paracellular permeability and decreases epithelial permeability via regulation of tight junction proteins.
Indirect Effects of NPY on Intestinal Epithelium via Modulation of Immune Cell Cytokine Release
NPY can also modulate epithelial permeability by regulating TNF-α release from immune cells or histamine from mast cells as depicted in Fig. 2, resulting in chloride secretion (66). Binding of histamine to H2 receptors on enteric neuronal cell bodies can elevate excitability of submucosal secretomotor neurons, increasing the volume of mucosal secretions of electrolytes and H2O, thus causing neurogenic secretory diarrhea commonly observed in IBD (20). The diarrhea may be helpful in expulsion of pathogenic bacteria and thus beneficial to the organism.
Vasoactive Intestinal Peptide
VIP is a 28-amino acid, inhibitory neuropeptide that induces colonic relaxation like NPY. Unlike NPY, VIP promotes secretory effects on the colonic epithelia and is proven to have anti-inflammatory role. VIP downregulates proinflammatory cytokines and mediators like IL-6, TNF-α, IL-12, nitric oxide, and chemokines (26, 54, 75). Recent research has demonstrated that VIP is also produced by type 2 T lymphocytes (Th2), raising the possibility of viewing it as a Th2 cytokine (24, 70).
VIP Is an Immunomodulator
VIP has potent anti-inflammatory effects due to varied effects on immune cell function particularly by favoring T helper (Th) cell differentiation toward a “Th2” phenotype (25). In addition, VIP also stimulates regulatory T cell production and inhibits macrophage proinflammatory actions. All these effects contribute to downregulation of inflammation. Since VIP administration has demonstrated downregulation of chemokine production and expression of chemokine receptors (34), VIP has been successfully administered in several models of neurodegenerative disorders (39). VIP also exerts its protective effect via stimulation of T cells to produce neurotrophic factors such as brain-derived neurotrophic factor to compensate for inflammation-induced neuronal loss (71).
Alterations in VIP Levels During Inflammation
Despite the well documented anti-inflammatory effects of VIP, there has not been consistency in linking VIP expression to pathophysiology of intestinal diseases. Differential changes in VIP expression has been noted in the submucosal and myenteric plexi of pediatric Crohn's patients (15). A positive association between plasma levels of VIP and active flares of IBD have been demonstrated (31). On the contrary, recent studies also demonstrate decreased binding and epithelial downregulation of VIP and its receptor (VPAC1) in patients with ulcerative colitis (48, 90). These observations suggest that VIP production from neuronal and nonneuronal cell types (inflammatory cells and epithelia) differ during normal and inflammatory conditions.
VIP Attenuates Inflammation by Modulating Key Immune Cell Functions via VPAC1/VPAC2
VIP maintains immunological tolerance and homeostasis in the gut mainly by regulation of T cell responses and TLR-mediated innate immune responses. The effects of VIP are mediated by the receptors VPAC1 and VPAC2, the GPCRs expressed on T cells. VPAC1 are constitutively expressed on T cells whereas VPAC2 expression is induced by inflammation (25). VIP promotes Th2-like responses and inhibits Th1 immune responses via VPAC2 as VPAC2 gene depletion leads to increased Th1-type responses (46). In this study short-deletion splice variant of VPAC2 was utilized, which, despite having similar affinity to VIP, failed to induce chemotaxis, T cell adenylate cyclase signaling, and inhibition of IL-2 production (41). Intraperitoneal administration of VIP has been shown to downregulate the Th1-driven responses, including the systemic levels of proinflammatory cytokines like TNF-α, and IL-6. Another mechanism of VIP-mediated protection is by promoting regulatory T cell function via increased expression of Foxp3 and TGF-β (6). In addition, VIP regulates innate and adaptive immunity by downregulation of TLR-2 and TLR-4 expression, and chemokine CXCL1 production (7, 44). On the contrary TLR ligands (TLR-2, 4) can effectively downregulate the VPAC2 receptor in macrophages via MAPK kinase signaling pathway (44). Thus VIP is a potent regulator of T cell- and TLR-mediated responses and plays a significantly role in gut homeostasis.
VIP Activation of Intracellular Signaling Pathways via VPAC1/VPAC2 Mediates Anti-Inflammatory Effects
Several studies have examined the signaling pathways activated by VIP on immune cells. Mainly, anti-inflammatory effect of VIP is ascribed to anti-inflammatory cytokine IL-10 synthesis via PKA-mediated phosphorylation of cAMP response element binding (54). Also VIP downregulates TNF-α expression by inhibiting downstream phosphorylation of MEK4, JNK, and c-Jun mediated through the specific VPAC1 receptor and the cAMP/PKA pathway (50). VIP also reprograms macrophages to produce Th2-type cytokines by favoring the development of bone marrow-derived tolerogenic dendritic cells via VPAC1 receptor and PKA, which involves NF-κB inhibition (37, 55). VIP also exerts its anti-inflammatory effects via downregulation of NF-κB-dependent gene activation of the COX-2 promoter (38).
A schematic representation of interaction between enteric immune cells and enteric neuropeptide VIP is summarized in Fig. 1.
VIP Exerts Direct and Indirect Effects on Epithelial Permeability
VIP also regulates epithelial barrier function, thereby determining the susceptibility to inflammation. VIP-ergic neurons in the submucosa directly innervate intestinal epithelial crypt cells and promote intestinal ion and fluid secretion via VPAC1-cAMP (10, 20). In addition, VIP indirectly modulates epithelial permeability via regulation of epithelial tight junction proteins. VIP-ergic pathways have been recognized to mediate increased expression of the tight junction protein zona occludens in the human submucosal neuronal layer and reduce paracellular epithelial permeability in Caco2 and HT29-Cl.16E monolayers (60). VIP has also been demonstrated to attenuate barrier dysfunction associated with Citrobacter rodentium-induced colitis in mice via reduction of paracellular permeability by prevention of translocation of tight junction proteins (19).
Therapeutic Potential
Neuropeptides and their receptor agonists or antagonists can be exploited as powerful therapeutic tools to attenuate inflammation. This is because neuropeptides modulate various aspects of inflammatory signaling as well as epithelial permeability via their receptors localized on immune and epithelial cells. A change in the content of neuropeptides and their receptor expression during pathological states induces a state of neuronal remodeling that can tip the balance of neuroimmune and neuroepithelial interactions. Hence administration of neuropeptide receptor agonists, antagonists, antisense oligonucleotides, or silencing the neuropeptide expression (using siRNAs) offers promise in attenuating or improving some of the pathological manifestations in inflammatory diseases.
NPYY1 Receptor Antagonists
NPYY1 antagonists may be exploited for treating inflammatory signaling as Y1 receptor is widely expressed on immune cells and promotes inflammatory signaling. Several peptide and nonpeptide agonists and antagonists have been synthesized for the Y1 receptor, and studies in rodent models have explored the effects of these compounds on orexigenic and vasoconstrictor responses. Peptidic antagonists of NPY are mainly the N-truncated NPY analogs. The first nonpeptide antagonist developed was BIBP-3226, i.e., (R)-N2-(diphenylacetyl)-N-[(4-hydroxyphenyl) methyl]-argininamide, which is very potent and highly selective for Y1 receptor (2).
Another significant role of NPY is in bone homeostasis. As many as 30 to 60% of people with IBD report osteoporosis or bone abnormalities due to steroid therapy, inflammation, or vitamin D deficiency (47). These conditions occur more frequently in people with Crohn's disease than in those with ulcerative colitis and are more common in women than in men (www.ccfa.org). NPYY1 knockout mice have greater bone mass and formation, and Y1 receptors have been observed on osteoblasts (76). Current pharmacological treatments for osteoporosis that utilize bisphosphonates that incorporate into bone and prevent bone reabsorption by osteoclasts are not successful in case of advanced osteoporosis. NPYY2 antagonists can inhibit osteoclast activity via a hypothalamic mechanism and also lead to reduced NPYY1 function that in turn would lead to increased osteoblast function and increased bone density (9).
Under normal conditions, NPY regulates water absorption in the colonic epithelia via Y1 (antisecretory) and Y2 receptors (promotes secretion). NPY also induces colonic relaxation and modulates colonic motility. Hence NPY receptors may be targeted to treat motility disorders like constipation or diarrhea. For example, a Y1 agonist would promote water absorption, which could be used to curb diarrhea, and a Y1 antagonist may also be utilized to treat constipation. On the other hand, enteric inflammation can skew the normal functions of NPY, resulting in loss of antisecretory effects of NPY, which is attributed to the downregulation of NPYY1 receptor mRNA and protein in the colonic epithelia (52). Inflammation also induces extensive damage to neurons in the gut, causing impaired gut motility. Moreover, the limited availability of receptor ligands coupled with the lack of studies to validate their efficacy and side effects on gut health are also decisive factors that restrict their therapeutic potential. Hence, considering the relevance and multifaceted roles of NPY in various human diseases like inflammation, pain, cancer, obesity, epilepsy, alcoholism, cardiovascular diseases, anxiety, bone metabolism, etc., more research is needed to exploit the full potential of these receptor agonists and antagonists in novel treatment strategies.
VIP Vectors
Despite the well-documented anti-inflammatory potential, VIP-based drug design has not been successful because of the peptide instability and limited bioavailability. Recent advances in the field include synthesis of lipophilic analogs of VIP, peptide derivatives that mimic the activity of VIP (40), and lentiviral vectors in a model of autoimmune arthritis (27). Administration of the peptide itself in mouse models of colitis have been shown to improve the clinical score and gastrointestinal inflammation (1).
Challenges in Therapy
Despite the well-documented recognition of the role of neuroimmune interactions in the pathology of several inflammatory diseases, there has not been a corresponding success in the application of neuropeptides in therapy. A key factor that limits the usage of peptide agonists or antagonists in therapy is their multiple cellular targets. Hence the targeted delivery of the neuropeptide or its receptor agonists or antagonists via nanoparticles is a desirable option. Another approach would be to utilize gene therapy to maintain a desired level of expression of the neuropeptide or its receptor via a tissue-specific promoter. Here again, the adverse effects of commonly used viral vectors like toxicity, immune activation, and complications due to interference with other genes are undesirable side effects. Hence combining a nonviral approach in a nanoparticle-based delivery system may represent an attractive option to exploit the therapeutic potential of neuropeptides.
Summary
In summary, neuropeptides NPY and VIP, via their receptors on immune cells, can activate or dampen inflammatory signaling. The expression of a specific neuropeptide and its receptor can vary during active inflammatory and latent phases of the disease. This can significantly alter immune cell responses and production of cytokines. On the other hand, inflammation can alter the neuropeptide milieu in the gut (inflammation-associated neuronal remodeling), which in turn can have drastic paracrine effects on immune and epithelial functions.
Conclusions
Enteric neuropeptides NPY and VIP, and their receptors, play a significant role in modulating immune cell functions and epithelial barrier functions. Thus they are important to the predisposition, propagation, and sustainability of inflammation. Hence further understanding of their role and receptors in inflammatory signaling and epithelial barrier functions can help develop new therapeutic targets to treat inflammatory diseases.
GRANTS
We acknowledge the career development grant support from Crohn's and Colitis Foundation of America (B. Chandrasekharan), NIH-RO1-DK80684 (S. Srinivasan), and VA-MERIT award (S. Srinivasan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.C. and S.S. conception and design of research; B.C., B.G.N., and S.S. prepared figures; B.C. drafted manuscript; B.C. and S.S. edited and revised manuscript; B.C. and S.S. approved final version of manuscript.
REFERENCES
- 1. Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, Gomariz RP. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology 124: 961–971, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Aiglstorfer I, Uffrecht A, Gessele K, Moser C, Schuster A, Merz S, Malawska B, Bernhardt G, Dove S, Buschauer A. NPY Y1 antagonists: structure-activity relationships of arginine derivatives and hybrid compounds with arpromidine-like partial structures. Regul Pept 75–76: 9–21, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Alonso C, Guilarte M, Vicario M, Ramos L, Rezzi S, Martinez C, Lobo B, Martin FP, Pigrau M, Gonzalez-Castro AM, Gallart M, Malagelada JR, Azpiroz F, Kochhar S, Santos J. Acute experimental stress evokes a differential gender-determined increase in human intestinal macromolecular permeability. Neurogastroenterol Motil 24: 740–746, e348–e749, 2012 [DOI] [PubMed] [Google Scholar]
- 4. Anderson JM, Van Itallie CM. Tight junctions. Curr Biol 18: R941–R943, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Andrews JM, Holtmann G. IBD: stress causes flares of IBD—how much evidence is enough? Nat Rev Gastroenterol Hepatol 8: 13–14, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Arranz A, Abad C, Juarranz Y, Torroba M, Rosignoli F, Leceta J, Gomariz RP, Martinez C. Effect of VIP on TLR2 and TLR4 expression in lymph node immune cells during TNBS-induced colitis. Ann NY Acad Sci 1070: 129–134, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Arranz A, Juarranz Y, Leceta J, Gomariz RP, Martinez C. VIP balances innate and adaptive immune responses induced by specific stimulation of TLR2 and TLR4. Peptides 29: 948–956, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol 12: 141–179, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest 109: 915–921, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Banks MR, Farthing MJ, Robberecht P, Burleigh DE. Antisecretory actions of a novel vasoactive intestinal polypeptide (VIP) antagonist in human and rat small intestine. Br J Pharmacol 144: 994–1001, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B, McCaleb ML. Behavioral characterization of neuropeptide Y knockout mice. Brain Res 868: 79–87, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Bedoui S, Kawamura N, Straub RH, Pabst R, Yamamura T, von Horsten S. Relevance of neuropeptide Y for the neuroimmune crosstalk. J Neuroimmunol 134: 1–11, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Birch D, Knight GE, Boulos PB, Burnstock G. Analysis of innervation of human mesenteric vessels in non-inflamed and inflamed bowel—a confocal and functional study. Neurogastroenterol Motil 20: 660–670, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Bot I, de Jager SC, Bot M, van Heiningen SH, de Groot P, Veldhuizen RW, van Berkel TJ, von der Thusen JH, Biessen EA. The neuropeptide substance P mediates adventitial mast cell activation and induces intraplaque hemorrhage in advanced atherosclerosis. Circ Res 106: 89–92, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Boyer L, Sidpra D, Jevon G, Buchan AM, Jacobson K. Differential responses of VIPergic and nitrergic neurons in paediatric patients with Crohn's disease. Auton Neurosci 134: 106–114, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Chandrasekharan B, Bala V, Kolachala VL, Vijay-Kumar M, Jones D, Gewirtz AT, Sitaraman SV, Srinivasan S. Targeted deletion of neuropeptide Y (NPY) modulates experimental colitis. PLoS One 3: e3304, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 15: 1063–1067, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christophi GP, Rong R, Holtzapple PG, Massa PT, Landas SK. Immune markers and differential signaling networks in ulcerative colitis and Crohn's disease. Inflamm Bowel Dis 18: 2342–2356, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Conlin VS, Wu X, Nguyen C, Dai C, Vallance BA, Buchan AM, Boyer L, Jacobson K. Vasoactive intestinal peptide ameliorates intestinal barrier disruption associated with Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol 297: (4) G735–G750, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann NY Acad Sci 915: 77–80, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Cox HM. Neuropeptide Y receptors; antisecretory control of intestinal epithelial function. Auton Neurosci 133: 76–85, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Cross LJ, Heaney LG, Ennis M. Further characterisation of substance P induced histamine release from human bronchoalveolar lavage mast cells. Inflamm Res 45, Suppl 1: S11–S12, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Czaja K, Kaleczyc J, Sienkiewicz W, Lakomy M. The influence of experimental ileitis on the neuropeptide coding of enteric neurons in the pig. Pol J Vet Sci 8: 155–163, 2005 [PubMed] [Google Scholar]
- 24. Delgado M, Ganea D. Cutting edge: is vasoactive intestinal peptide a type 2 cytokine? J Immunol 166: 2907–2912, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Delgado M, Gonzalez-Rey E, Ganea D. VIP/PACAP preferentially attract Th2 effectors through differential regulation of chemokine production by dendritic cells. FASEB J 18: 1453–1455, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Delgado M, Leceta J, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit the production of inflammatory mediators by activated microglia. J Leukoc Biol 73: 155–164, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Delgado M, Toscano MG, Benabdellah K, Cobo M, O'Valle F, Gonzalez-Rey E, Martin F. In vivo delivery of lentiviral vectors expressing vasoactive intestinal peptide complementary DNA as gene therapy for collagen-induced arthritis. Arthritis Rheum 58: 1026–1037, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Detel D, Pugel EP, Pucar LB, Buljevic S, Varljen J. Development and resolution of colitis in mice with target deletion of dipeptidyl peptidase IV. Exp Physiol 97: 486–496, 2012 [DOI] [PubMed] [Google Scholar]
- 29. Dimitrijevic M, Stanojevic S, Micic S, Vujic V, Kovacevic-Jovanovic V, Mitic K, von Horsten S, Kosec D. Neuropeptide Y (NPY) modulates oxidative burst and nitric oxide production in carrageenan-elicited granulocytes from rat air pouch. Peptides 27: 3208–3215, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Dimitrijevic M, Stanojevic S, Vujic V, Beck-Sickinger A, von Horsten S. Neuropeptide Y and its receptor subtypes specifically modulate rat peritoneal macrophage functions in vitro: counter regulation through Y1 and Y2/5 receptors. Regul Pept 124: 163–172, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Duffy LC, Zielezny MA, Riepenhoff-Talty M, Byers TE, Marshall J, Weiser MM, Graham S, Ogra PL. Vasoactive intestinal peptide as a laboratory supplement to clinical activity index in inflammatory bowel disease. Dig Dis Sci 34: 1528–1535, 1989 [DOI] [PubMed] [Google Scholar]
- 32. Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature 381: 415–421, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Fagerstam JP, Whiss PA, Strom M, Andersson RG. Expression of platelet P-selectin and detection of soluble P-selectin, NPY and RANTES in patients with inflammatory bowel disease. Inflamm Res 49: 466–472, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Fernandez-Martin A, Gonzalez-Rey E, Chorny A, Martin J, Pozo D, Ganea D, Delgado M. VIP prevents experimental multiple sclerosis by downregulating both inflammatory and autoimmune components of the disease. Ann NY Acad Sci 1070: 276–281, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Ferreira R, Xapelli S, Santos T, Silva AP, Cristovao A, Cortes L, Malva JO. Neuropeptide Y modulation of interleukin-1β (IL-1β)-induced nitric oxide production in microglia. J Biol Chem 285: 41921–41934, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Furness JB. The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol Motil 20, Suppl 1: 32–38, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Gonzalez-Rey E, Chorny A, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood 107: 3632–3638, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gonzalez-Rey E, Delgado M. Vasoactive intestinal peptide inhibits cyclooxygenase-2 expression in activated macrophages, microglia, and dendritic cells. Brain Behav Immun 22: 35–41, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Gonzalez-Rey E, Varela N, Chorny A, Delgado M. Therapeutical approaches of vasoactive intestinal peptide as a pleiotropic immunomodulator. Curr Pharm Des 13: 1113–1139, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Gozes I, Divinsky I, Pilzer I, Fridkin M, Brenneman DE, Spier AD. From vasoactive intestinal peptide (VIP) through activity-dependent neuroprotective protein (ADNP) to NAP: a view of neuroprotection and cell division. J Mol Neurosci 20: 315–322, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Grinninger C, Wang W, Oskoui KB, Voice JK, Goetzl EJ. A natural variant type II G protein-coupled receptor for vasoactive intestinal peptide with altered function. J Biol Chem 279: 40259–40262, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Hafstrom I, Ringertz B, Lundeberg T, Palmblad J. The effect of endothelin, neuropeptide Y, calcitonin gene-related peptide and substance P on neutrophil functions. Acta Physiol Scand 148: 341–346, 1993 [DOI] [PubMed] [Google Scholar]
- 43. Hassani H, Lucas G, Rozell B, Ernfors P. Attenuation of acute experimental colitis by preventing NPY Y1 receptor signaling. Am J Physiol Gastrointest Liver Physiol 288: G550–G556, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Herrera JL, Gonzalez-Rey E, Fernandez-Montesinos R, Quintana FJ, Najmanovich R, Pozo D. Toll-like receptor stimulation differentially regulates vasoactive intestinal peptide type 2 receptor in macrophages. J Cell Mol Med 13: 3209–3217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 46: 261–274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang MC, Miller AL, Wang W, Kong Y, Paul S, Goetzl EJ. Differential signaling of T cell generation of IL-4 by wild-type and short-deletion variant of type 2 G protein-coupled receptor for vasoactive intestinal peptide (VPAC2). J Immunol 176: 6640–6646, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iijima H, Shinzaki S, Takehara T. The importance of vitamins D and K for the bone health and immune function in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care 15: 635–640, 2012 [DOI] [PubMed] [Google Scholar]
- 48. Jonsson M, Norrgard O, Forsgren S. Epithelial expression of vasoactive intestinal peptide in ulcerative colitis: down-regulation in markedly inflamed colon. Dig Dis Sci 57: 303–310, 2012 [DOI] [PubMed] [Google Scholar]
- 49. Kim SG, Kim JS, Kim JM, Jung HC, Song IS. Inhibition of proinflammatory cytokine expression by NF-κB (p65) antisense oligonucleotide in Helicobacter pylori-infected mice. Helicobacter 10: 559–566, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Kim WK, Kan Y, Ganea D, Hart RP, Gozes I, Jonakait GM. Vasoactive intestinal peptide and pituitary adenylyl cyclase-activating polypeptide inhibit tumor necrosis factor-alpha production in injured spinal cord and in activated microglia via a cAMP-dependent pathway. J Neurosci 20: 3622–3630, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kimata H, Yoshida A, Ishioka C, Fujimoto M, Furusho K. Vasoactive intestinal peptide enhances immunoglobulin production and growth in human plasma cells via mechanisms that may involve protein kinase C. J Clin Endocrinol Metab 81: 3024–3032, 1996 [DOI] [PubMed] [Google Scholar]
- 52. Klompus M, Ho W, Sharkey KA, McKay DM. Antisecretory effects of neuropeptide Y in the mouse colon are region-specific and are lost in DSS-induced colitis. Regul Pept 165: 138–145, 2010 [DOI] [PubMed] [Google Scholar]
- 53. Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 13: 803–811, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Leceta J, Gomariz RP, Martinez C, Abad C, Ganea D, Delgado M. Receptors and transcriptional factors involved in the anti-inflammatory activity of VIP and PACAP. Ann NY Acad Sci 921: 92–102, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Lu J, Zheng MH, Yan J, Chen YP, Pan JP. Effects of vasoactive intestinal peptide on phenotypic and functional maturation of dendritic cells. Int Immunopharmacol 8: 1449–1454, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Lukewich MK, Lomax AE. Toll-like receptor 4 activation reduces adrenal chromaffin cell excitability through a nuclear factor-κB-dependent pathway. Endocrinology 154: 351–362, 2013 [DOI] [PubMed] [Google Scholar]
- 57. Margolis KG, Stevanovic K, Karamooz N, Li ZS, Ahuja A, D'Autreaux F, Saurman V, Chalazonitis A, Gershon MD. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology 141: 588–598, 598.e1–e2, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Medina S, Del Rio M, Hernanz A, De la Fuente M. Age-related changes in the neuropeptide Y effects on murine lymphoproliferation and interleukin-2 production. Peptides 21: 1403–1409, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Michalski CW, Autschbach F, Selvaggi F, Shi X, Di Mola FF, Roggo A, Muller MW, Di Sebastiano P, Buchler MW, Giese T, Friess H. Increase in substance P precursor mRNA in noninflamed small-bowel sections in patients with Crohn's disease. Am J Surg 193: 476–481, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol 285: G1028–G1036, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Neurath MF, Becker C, Barbulescu K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut 43: 856–860, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nguyen NK, Sartori SB, Herzog H, Tasan R, Sperk G, Singewald N. Effect of neuropeptide Y Y2 receptor deletion on emotional stress-induced neuronal activation in mice. Synapse 63: 236–246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nijhuis LE, Olivier BJ, de Jonge WJ. Neurogenic regulation of dendritic cells in the intestine. Biochem Pharmacol 80: 2002–2008, 2010 [DOI] [PubMed] [Google Scholar]
- 64. Obata K, Yamanaka H, Dai Y, Tachibana T, Fukuoka T, Tokunaga A, Yoshikawa H, Noguchi K. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J Neurosci 23: 4117–4126, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Olah Z, Szabo T, Karai L, Hough C, Fields RD, Caudle RM, Blumberg PM, Iadarola MJ. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J Biol Chem 276: 11021–11030, 2001 [DOI] [PubMed] [Google Scholar]
- 66. Oprins JC, van der Burg C, Meijer HP, Munnik T, Groot JA. Tumour necrosis factor alpha potentiates ion secretion induced by histamine in a human intestinal epithelial cell line and in mouse colon: involvement of the phospholipase D pathway. Gut 50: 314–321, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pang XH, Li TK, Xie Q, He FQ, Cui de J, Chen YQ, Huang XL, Gan HT. Amelioration of dextran sulfate sodium-induced colitis by neuropeptide Y antisense oligodeoxynucleotide. Int J Colorect Dis 25: 1047–1053, 2010 [DOI] [PubMed] [Google Scholar]
- 68. Philippe D, Dubuquoy L, Groux H, Brun V, Chuoi-Mariot MT, Gaveriaux-Ruff C, Colombel JF, Kieffer BL, Desreumaux P. Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest 111: 1329–1338, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Poole DP, Matsuyama H, Nguyen TV, Eriksson EM, Fowler CJ, Furness JB. Inflammation and inflammatory agents activate protein kinase C epsilon translocation and excite guinea-pig submucosal neurons. Gastroenterology 133: 1229–1239, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Pozo D, Delgado M. The many faces of VIP in neuroimmunology: a cytokine rather a neuropeptide? FASEB J 18: 1325–1334, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Quintana FJ, Zaltzman R, Fernandez-Montesinos R, Herrera JL, Gozes I, Cohen IR, Pozo D. NAP, a peptide derived from the activity-dependent neuroprotective protein, modulates macrophage function. Ann NY Acad Sci 1070: 500–506, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Rozengurt E. Signal transduction pathways in the mitogenic response to G protein-coupled neuropeptide receptor agonists. J Cell Physiol 177: 507–517, 1998 [DOI] [PubMed] [Google Scholar]
- 73. Saksena S, Tyagi S, Goyal S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Stimulation of apical Cl-/HCO3-(OH-) exchanger, SLC26A3 by neuropeptide Y is lipid raft dependent. Am J Physiol Gastrointest Liver Physiol 299: G1334–G1343, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Saurer TB, Ijames SG, Lysle DT. Neuropeptide Y Y1 receptors mediate morphine-induced reductions of natural killer cell activity. J Neuroimmunol 177: 18–26, 2006 [DOI] [PubMed] [Google Scholar]
- 75. Sigalet DL, Wallace LE, Holst JJ, Martin GR, Kaji T, Tanaka H, Sharkey KA. Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol 293: G211–G221, 2007 [DOI] [PubMed] [Google Scholar]
- 76. Sousa DM, Baldock PA, Enriquez RF, Zhang L, Sainsbury A, Lamghari M, Herzog H. Neuropeptide Y Y1 receptor antagonism increases bone mass in mice. Bone 51: 8–16, 2012 [DOI] [PubMed] [Google Scholar]
- 77. Straub RH, Wiest R, Strauch UG, Harle P, Scholmerich J. The role of the sympathetic nervous system in intestinal inflammation. Gut 55: 1640–1649, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136: 551–563, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sun J, Ramnath RD, Bhatia M. Neuropeptide substance P upregulates chemokine and chemokine receptor expression in primary mouse neutrophils. Am J Physiol Cell Physiol 293: C696–C704, 2007 [DOI] [PubMed] [Google Scholar]
- 80. Teixeira L, Sousa DM, Nunes AF, Sousa MM, Herzog H, Lamghari M. NPY revealed as a critical modulator of osteoblast function in vitro: new insights into the role of Y1 and Y2 receptors. J Cell Biochem 107: 908–916, 2009 [DOI] [PubMed] [Google Scholar]
- 81. Tough IR, Forbes S, Tolhurst R, Ellis M, Herzog H, Bornstein JC, Cox HM. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y1 and Y2 receptors. Br J Pharmacol 164: 471–484, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vasina V, Barbara G, Talamonti L, Stanghellini V, Corinaldesi R, Tonini M, De Ponti F, De Giorgio R. Enteric neuroplasticity evoked by inflammation. Auton Neurosci 126–127: 264–272, 2006 [DOI] [PubMed] [Google Scholar]
- 83. Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol 534: 813–825, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci 22: 146–152, 2001 [DOI] [PubMed] [Google Scholar]
- 85. Walther C, Morl K, Beck-Sickinger AG. Neuropeptide Y receptors: ligand binding and trafficking suggest novel approaches in drug development. J Pept Sci 17: 233–246, 2011 [DOI] [PubMed] [Google Scholar]
- 86. Welcker K, Martin A, Kolle P, Siebeck M, Gross M. Increased intestinal permeability in patients with inflammatory bowel disease. Eur J Med Res 9: 456–460, 2004 [PubMed] [Google Scholar]
- 87. Wheway J, Herzog H, Mackay F. The Y1 receptor for NPY: a key modulator of the adaptive immune system. Peptides 28: 453–458, 2007 [DOI] [PubMed] [Google Scholar]
- 88. Wheway J, Mackay CR, Newton RA, Sainsbury A, Boey D, Herzog H, Mackay F. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med 202: 1527–1538, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wojtkiewicz J, Rowniak M, Crayton R, Barczewska M, Bladowski M, Robak A, Pidsudko Z, Majewski M. Inflammation-induced changes in the chemical coding pattern of colon-projecting neurons in the inferior mesenteric ganglia of the pig. J Mol Neurosci 46: 450–458, 2012 [DOI] [PubMed] [Google Scholar]
- 90. Yukawa T, Oshitani N, Yamagami H, Watanabe K, Higuchi K, Arakawa T. Differential expression of vasoactive intestinal peptide receptor 1 expression in inflammatory bowel disease. Int J Mol Med 20: 161–167, 2007 [PubMed] [Google Scholar]
- 91. Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature 452: 997–1001, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]