Abstract
Necrotizing enterocolitis (NEC) is typified by mucosal destruction, which subsequently can lead to intestinal necrosis. Prematurity, enteral feeding, and bacterial colonization are the main risk factors and, combined with other stressors, can cause increased intestinal permeability, injury, and an exaggerated inflammatory response. Heme oxygenase-1 (HO-1) mediates intestinal protection due to anti-inflammatory, antioxidative, and antiapoptotic effects of its products carbon monoxide, biliverdin, and bilirubin. This study investigates a possible role of HO-1 in the pathogenesis of NEC using a newborn mouse model. We induced NEC-like intestinal injury in 7-day-old HO-1 heterozygous (HO-1 Het, Hmox1+/-) and wild-type (Wt, Hmox1+/+) mice by gavage feeding and hypoxic exposures. Control (Con) pups of both genotypes were dam-fed. Intestines of HO-1 Het Con pups appeared predisposed to injury, with higher histological damage scores, more TUNEL-positive cells, and a significant reduction in muscularis externa thickness compared with Wt Con pups. The increase in HO activity after HO-1 induction by the substrate heme or by hypoxic stress was significantly impaired in HO-1 Het pups. After induction of intestinal injury, HO-1 Het pups displayed significantly higher NEC incidence (78 vs. 43%), mortality (83 vs. 54%), and median scores (2.5 vs. 1.5) than Wt NEC pups. PCR array analyses revealed increased expressions of IL-1β, P-selectin, matrix metallopeptidase 2, collagen type XVIII-α1, serpine 1, and others in NEC-induced HO-1 Het ileal and jejunal tissues. We conclude that a partial HO-1 deficiency promotes experimental NEC-like intestinal injury, possibly mediated by exaggerated inflammation and disruption in tissue repair.
Keywords: apoptosis, bilirubin/biliverdin, carbon monoxide, intestinal inflammation, necrotizing enterocolitis
necrotizing enterocolitis (NEC) is a leading cause of death in the neonatal intensive care unit (6). With an overall incidence of ∼1 per 1,000 live births, NEC occurs in up to 7% of infants with birth weights between 500 and 1,500 g and has an estimated mortality rate between 20 and 30% (45). Up to 50% of the survivors have long-term sequelae, such as short bowel syndrome, poor growth, and neurodevelopmental dysfunction, often manifested as speech and motor impairments, intellectual delays, and problems with social and interpersonal skills (48, 54, 61). Despite advancing knowledge due to intense research in recent years, the pathogenesis of NEC is still incompletely understood, and therapeutic treatments are limited in number and success with no preventive treatment strategy proven adequate to date (34). The cause of NEC is multifactorial, with the most consistent and greatest risk factor being prematurity and thus intestinal immaturity, low birth weight, enteral feeding, bacterial colonization, and possibly a genetic predisposition (18, 33, 45). Immaturities in intestinal motility and digestion, barrier function, circulatory regulation, and immunity increase the vulnerability of the intestinal mucosa and the likelihood of injury in the premature infant (18, 32, 45). An inflammatory cascade follows the initial injury, signaling neutrophil activation, increasing permeability of the vasculature, releasing reactive oxygen species, and thus leading to vasoconstriction and ischemia-reperfusion (I/R) injury (18) and finally to mucosal barrier breakdown.
Heme oxygenase-1 (HO-1) is the inducible of three isoforms of HO, the enzyme that catalyzes heme breakdown, generating equimolar amounts of bilirubin, free iron, and carbon monoxide (CO) (64). Bilirubin is a potent endogenous free radical scavenger (63). CO has long been recognized as having protective properties in hemorrhagic shock and being a modulator of vascular tone (26, 41). Moreover, CO possesses antiapoptotic, anti-inflammatory, and immunomodulatory properties (47). Owing to the combined effects of its products, HO-1 has been shown to be a mediator of gastroprotective pathways.
Although it is being debated whether I/R injury is the initiating event in the development of NEC or a consequence of an inflammatory process (46), the contribution of I/R to the disease pathogenesis is critical and serves as the basis for several animal models. A number of studies have shown that an increased HO-1 expression protects against I/R injury in the intestine (1–3, 36, 37, 43, 58) and liver (55). HO-1-mediated modulation of intestinal leukocyte adhesion (58) and decrease in myeloperoxidase activity (3, 67) are possible underlying mechanisms that may reduce mucosal injury and improve intestinal motility following I/R injury in the gut. In experimental colitis models, HO-1 induction has been shown to decrease inflammation and hasten tissue repair (5, 24, 49, 60, 74). The protective role of HO-1 appears to be mediated by the anti-inflammatory and immunomodulatory effects of its products, CO (24, 60), biliverdin, and bilirubin (5). Chung and coworkers (10) demonstrated that there is an exaggerated lethality in HO-1 homozygous knockout mice (KO, Hmox1-/-) following sepsis due to gross tissue destruction and loss of bowel integrity in the ileum and colon. Overexpression of HO-1 in smooth muscle cells and myofibroblasts of blood vessels was shown to increase bacterial clearance by enhancing phagocytosis and the endogenous antimicrobial response, thus improving survival.
Taken together, there is strong evidence that HO-1 could potentially positively affect three important pathogenic factors of NEC: circulatory dysfunction (I/R injury), exaggerated inflammatory response, and impaired intestinal mucosa integrity. Zuckerbraun et al. (76) reported that HO-1 protein is upregulated in human and rat neonatal NEC tissues and that exogenous CO delivery can protect against the development of intestinal inflammation and decrease mortality in an experimental NEC rat model.
The objective of this present study is to investigate whether a partial deficiency in HO-1 increases the risk of NEC development in an experimental mouse model of NEC-like intestinal injury. We then further elucidate the mechanisms underlying the enhanced tissue injury associated with a deficiency in HO-1 expression.
MATERIALS AND METHODS
Animals.
FVB wild-type (Wt, Hmox1+/+) breeders (6–8 wk old) were obtained from Charles River Laboratories (Wilmington, MA). The original Hmox1-/- (HO-1 KO) mouse strain, carrying a targeted deletion of a large portion of Hmox1 gene, was established on a C57BL/6 background and obtained from Poss and Tonegawa (50). The FVB/Hmox1+/- (HO-1 Het) strain was generously provided by Dr. Phyllis A. Dennery (Philadelphia, PA). To establish this strain on a predominant FVB background, C57BL/6 Hmox1-/- mice were backcrossed with FVB Wt mice for at least six generations (71). Mice were maintained under strict adherence to Stanford University institutional guidelines and under conventional housing. The Institutional Animal Care Committee of Stanford University approved all animal procedures.
Genotyping.
Genomic DNA from tail clippings was isolated by use of the Tissue DNeasy kit (Qiagen, Frederick, MD) and typed by PCR. For Hmox1+/+, Hmox1+/-, and Hmox1-/- screening, two sets of primers designed for the Wt, Het, and KO were used. Conditions for PCR were as follows: 95°C for 10 min for denaturing the genomic DNA, 94°C for 20 s, 68°C for 30 s, and 72°C for 40 s, repeating 40 cycles. Wt (510 bp), HO-1 Het, and HO-1 KO (390 bp) bands were analyzed by electrophoresis.
NEC induction model.
To induce NEC-like intestinal injury, a modification of the NEC mouse model described by Leaphart et al. (30) was employed. Seven-day-old HO-1 Het and Wt pups were removed from dams and fed 200-μl formula (1 g Similac 60/40 in 10 ml Esbilac)/5 g body wt by oral gavage (OG) every 4 h for 48 h. On days 1.5 and 7, pups were exposed to hypoxia (5% O2, balanced nitrogen, Praxair, San Ramon, CA) for 1 min. Control (Wt Con, HO-1 Het Con) pups were dam fed and kept in room air. After 48 h, intestines were harvested and sectioned for staining with hematoxylin and eosin (H&E). Tissues from pups (both genotypes) who developed signs of distress or imminent death were included in the study as long as they survived at least 24 h of NEC induction. Presence of NEC after 24 h of NEC induction has been shown previously in an experimental rat model (25).
NEC scoring.
Histological changes in the intestine were scored blindly and independently by two pathologists on a scale of 0 to 4, with a score of 2 or higher being classified as experimental NEC, according to a NEC scoring system previously published by Clark et al. (11). Briefly, scores were assigned as follows: 0 = no damage (normal); 1 = slight submucosal and/or lamina propria separation (mild); 2 = moderate separation of the submucosa and/or lamina propria and/or edema in the submucosa and muscular layers (moderate); 3 = severe separation of the submucosa and/or lamina propria and/or severe edema in the submucosa and muscular layers with regional villous sloughing (severe); or 4 = loss of villi and necrosis (necrosis). Intermediate scores of X.0 or X.5 were also used to more precisely assign the degree of intestinal damage.
Histological staining.
After NEC induction, intestinal sections were isolated and placed in 10% (vol/vol) neutral buffered formalin (Fisher Scientific, Waltham, MA) for 24 h. Fixed tissues were then embedded in paraffin according to standard protocols. Tissues 6 μm thick were cut from paraffin-embedded blocks by use of a microtome. Following deparaffinization, sections were stained with H&E (American Master*Tech Scientific, Lodi, CA) and histological changes were visualized by light microscopy (Nikon Eclipse E 800, Tokyo, Japan).
TUNEL staining.
Six-micrometer sectioned paraffin-embedded tissues were deparaffinized in xylene. After rehydration via a series of graded ethanol dilutions, tissue sections were incubated in 0.6 U/ml of proteinase K solution (Roche, Mannheim, Germany) for 15 min at room temperature. TUNEL staining was performed with the In Situ Cell Death Detection Kit, TMR red (Roche). Slides incubated with only labeling solution served as negative controls. Samples treated with recombinant DNaseI (RNase-free, Roche) followed by the standard protocol served as positive controls. All slides were then counterstained with DAPI nucleic acid stain (Invitrogen, Grand Island, NY) and mounted with ProLong Gold Antifade reagent (Invitrogen). Total cell number and apoptotic cells were counted under ×100 and ×200 magnification in six “hot spots” (jejunum and ileum) per slide and the extent of apoptosis was expressed as the apoptotic index.
Isolation of intestinal cells.
To yield single-cell suspensions from small intestines, the lamina propria isolation kit from Miltenyi Biotec (Auburn, CA) was used according to manufacturer's instructions. Briefly, 9-day-old Wt and HO-1 Het Con pups were euthanized. Small intestines (excluding the cecum) were removed in toto and flushed with PBS. Small intestines were first cut longitudinally, then laterally into 0.5-cm pieces, and placed into tubes containing HBSS (without Ca2+ and Mg2+), 5 mM EDTA, 5% FBS, and 1 mM DTT (predigestion solution). Cells were incubated at 37°C with fresh predigestion solution three times for 20 min. Between incubations, epithelial cells and intraepithelial lymphocytes (IEL) were removed mechanically using a vortex mixer and separated from the lamina propria by filtration through a 100-μm cell strainer. After the predigestion steps, lamina propria cells were incubated with digestion solution containing HBSS (with Ca2+ and Mg2+), 5% FBS, and a proprietary enzyme mix (Miltenyi Biotec) for 30 min at 37°C. To obtain single-cell suspensions, IEL, epithelial, and lamina propria cells were filtered through a 100-μm cell strainer and dissociated in MACS buffer (Miltenyi Biotec) by use of the gentleMACS Dissociator (Miltenyi Biotec). After dissociation, cells were filtered through a 70-μm cell strainer, washed with MACS buffer, and stained with the appropriate antibodies.
Flow cytometry.
The Annexin V Apoptosis Detection Kit APC (eBioscience, San Diego, CA) in combination with the viability dye eFluor780 (eBioscience) was used to detect apoptosis and nonviable cells. Following staining with eFluor780 for 30 min at 2 to 8°C, 1 × 104 to 5 × 105 cells were incubated with annexin V APC for 15 min at room temperature. Incubation of an intestinal single-cell suspension with 5 μM camptothecin (eBioscience) for 1 h at 37°C served as the positive apoptosis control. Flow cytometry was performed with LSR-II.2 (BD Bioscience) and analyzed with FlowJo (Tree Star, Ashland, OR). Debris and doublets were excluded by sequential gating on forward scatter height vs. forward scatter area.
HO activity.
To induce HO-1, Wt and HO-1 Het pups were exposed to hypoxia (5% O2 with balanced nitrogen) for 1 min at days 1.5, 3, and 6 of life or given 30-μmol heme/kg body wt or vehicle by OG on day 6 of life. Twenty-four hours after heme/vehicle treatment or after the last hypoxic exposure, HO activity was measured as CO production by gas chromatography with a reduction gas analyzer as previously described (66). Briefly, 20 μl of tissue sonicate (representing 2 mg fresh weight) was incubated for 15 min at 37°C in CO-free septum-sealed glass vials containing 20 μl of 150 μM/15 mM methemalbumin and 20 μl of 4.5 mM β-nicotinamide adenine dinucleotide phosphate, reduced (β-NADPH). Blank reaction vials contained buffer (0.1 M KPO4) in place of NADPH. Reactions were terminated by adding 5-μl sulfosalicylic acid (15% wt/vol) and placing the vials in wet ice. CO released into the headspace was quantitated and calculated as picomoles CO per hour per milligram fresh weight.
PCR array (84 genes, mouse endothelial cell biology).
After euthanasia, intestines were immediately harvested and samples from equal regions of the jejunum and distal ileum were placed in RNAlater (Qiagen) and stored at -80°C until analysis. Total RNA was extracted using the RNeasy Mini Kit (Qiagen). cDNA was synthesized by use of a RT2 First Strand Kit (Qiagen). Real-time PCR was performed with RT2 Real-Time SYBR Green/ROX PCR Master Mix (Qiagen) on a Stratagene Mx3005P QPCR system (Agilent, Santa Clara, CA). Optimal selections of internal control/housekeeping/normalization genes for analyses were automatically chosen by using the web-based PCR Array analysis program (Qiagen). Fold changes in gene expressions in HO-1 Het NEC jejunums and ileums over Wt NEC jejunum and ileum levels were then calculated by the ΔΔCt method (www.sabiosciences.com/pcrarraydataanalysis.php).
Quantitative RT-PCR analysis.
After euthanasia, samples from equal regions of the jejunum and distal ileum were immediately placed in RNAlater (Qiagen). Total RNA was extracted by use of the RNAeasy Mini Kit (Qiagen). Hmox1, Hmox2, and β-actin mRNA levels were quantified by using the iScript SYBR Green RT-PCR kit (Bio-Rad, Hercules, CA) as described previously (73). Amplification was performed with a CFX384 Touch Real-Time PCR Detection System (Bio-Rad).
Statistics.
Statistical comparisons of median NEC scores were performed by the Mann-Whitney test. Fisher's exact test was used to assess differences in NEC incidence. Kaplan-Meier plots were used to determine survival over time and compared by the log-rank test. All other group comparisons were analyzed by Student's unpaired t-test, one-way ANOVA followed by Bonferroni's multiple comparison test, or two-way ANOVA. Data are expressed as medians and means ± SD, and differences were considered significant when P ≤ 0.05.
RESULTS
Ontogeny of intestinal HO activity.
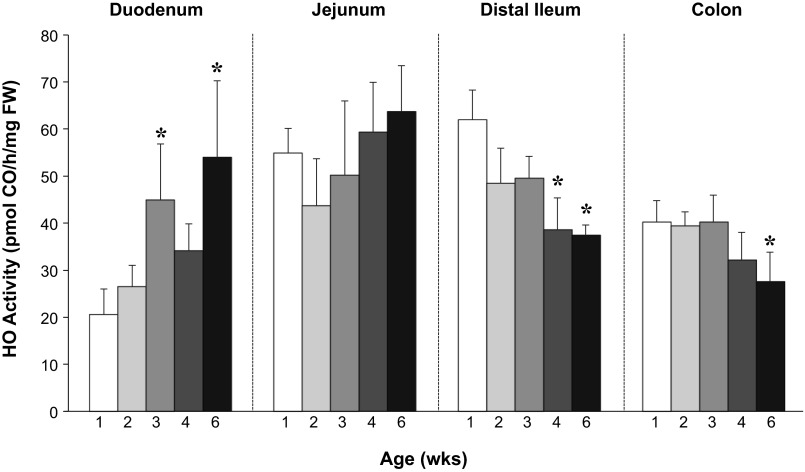
Because the gut matures with age (51), we first investigated whether intestinal HO activity is developmentally regulated. To this end, we determined HO activity in the duodenums, jejunums, distal ileums, and colons of Wt mice from 1 to 6 wk of age. HO activity not only was found to vary with age but also was region specific (Fig. 1). In the duodenum and jejunum, HO activity increased in an age-dependent manner, whereas, in the distal ileum and colon, activity was highest at 1 wk and then significantly decreased.
Fig. 1.
Ontogeny of intestinal heme oxygenase (HO) activity. Intestinal HO activity throughout all regions of the intestine was determined by measurements of carbon monoxide (CO) production by gas chromatography in wild-type (Wt) mice aged 1 (white bars) to 6 wk (black bars) with n ≥ 6 animals per group. *P ≤ 0.05 vs. 1-wk-old mice (1-way ANOVA).
HO-1 deficiency is associated with abnormal intestinal histology and increased apoptosis.
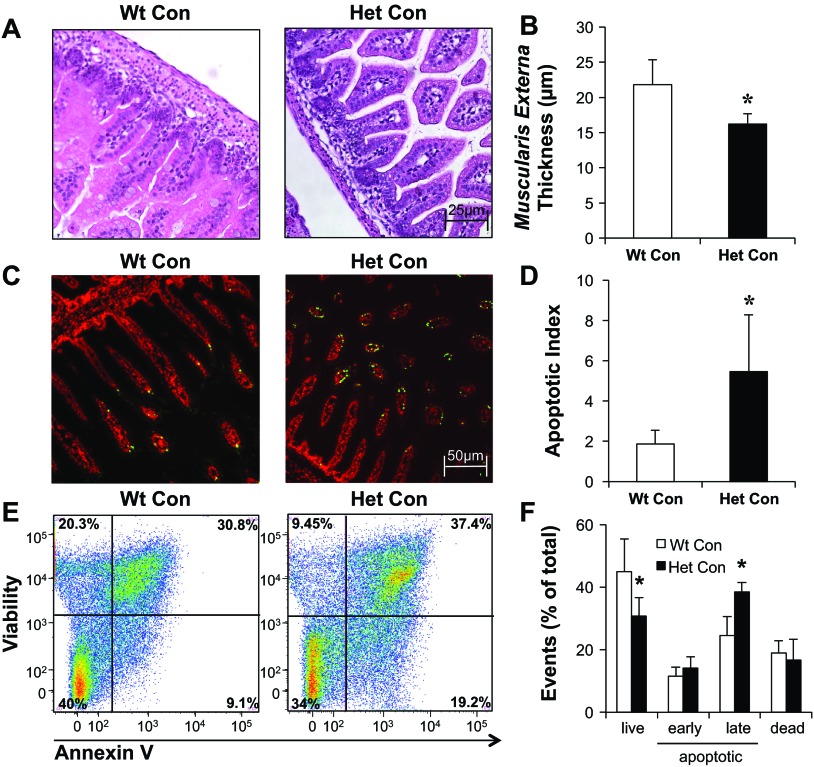
We compared H&E-stained slides of intestinal regions from dam-fed control mice from both genotypes (Wt Con, HO-1 Het Con). Figure 2A shows representative histological damage scores of 1.0, 2.0, 3.0, and 4.0 in NEC-induced ileums. We found slight submucosal separation in the jejunum, distal ileum, and colon of HO-1 Het pups (Fig. 2B). Moreover, the intestinal villi in the small intestine of HO-1 Het pups appeared slightly atrophic and less dense compared with those of Wt pups (Fig. 2B). Using the NEC scoring criteria described above, we found a median histological damage score of 1.0 for HO-1 Het pups, which was significantly higher than the median of 0.5 for Wt pups (Fig. 2C). Moreover, under light microscopy, the muscularis externa appeared to be thinner in HO-1 Het compared with Wt ileums (Fig. 3A). To further confirm this observation, we randomly selected nine regions per tissue slide of the inner circular muscle fibers of the muscularis externa in the ileums [cut longitudinally, magnification: ×200, regions of interests (ROIs)] of Wt and HO-1 Het Con pups. With ImageJ software (NIH, Bethesda, MD), we then measured the thickness in three random spots per ROI (a total of 27 measurements per slide) and calculated the mean thickness. We found a significant 25% reduction in muscularis externa thickness in HO-1 Het (n = 5) compared with Wt (n = 4) ileums (Fig. 3B).
Fig. 2.
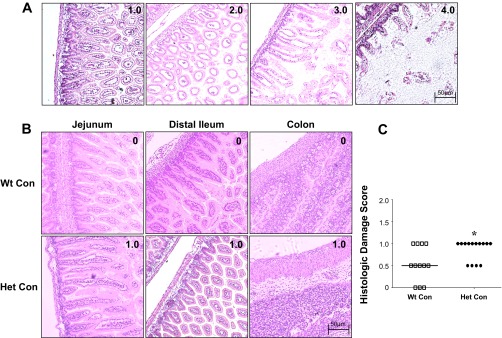
Representative hematoxylin and eosin (H&E)-stained tissue sections of the jejunum, distal ileum, and colon of control (Con) pups with corresponding histological damage scores. A: H&E-stained ileal tissue showing representative sections for each intestinal damage score from 1.0 to 4.0 graded as follows: 0 = no damage (normal, B); 1 = slight submucosal and/or lamina propria separation (mild); 2 = moderate separation of the submucosa and/or lamina propria and/or edema in the submucosa and muscular layers (moderate); 3 = severe separation of the submucosa and/or lamina propria and/or severe edema in the submucosa and muscular layers with regional villous sloughing (severe); or 4 = loss of villi and necrosis (necrosis). B: representative H&E-stained tissue sections and corresponding scores from wild-type (Wt) Con pups at top and HO-1 heterozygous (Het) Con pups at bottom. Magnification: ×100. C: cumulative maximum score of intestinal abnormality (histological damage score), independent of the intestinal region, for Wt and Het Con pups. Bars indicate median. *P = 0.028, by Mann-Whitney test.
Fig. 3.
Muscularis externa thicknesses and assessments of apoptosis of Con, Wt, and Het pups. A: representative H&E-stained ileums from Wt Con and Het Con pups. To assess the thickness of the muscularis externa, 9 regions of interest (ROIs) per tissue section were randomly selected (at ×200 magnification). The thickness in 3 random areas per ROI for a total of 27 measurements per tissue slide was quantitated with ImageJ and then averaged. B: quantification of muscularis externa thickness in the ileum of Wt (n = 4) and Het Con pups (n = 5). C: representative TUNEL staining of Wt and Het Con small intestines (jejunum/ileum) show apoptotic cells in green and nuclear counterstained cells with DAPI in red. Magnification: ×100. D: quantification of apoptosis in the small intestine of Wt and Het Con pups determined as the apoptotic index. E: representative flow cytometry analyses of single-cell suspensions of small intestinal tissue from Wt Con and Het Con pups. The values in the corner of each quadrant represent the percentage of viable (bottom left, annexin V APC-/viability eFluor780-), early apoptotic (bottom right, annexin V APC+/viability eFluor780-), late apoptotic/necrotic (top right, annexin V APC+/viability eFluor780+), and nonviable (top left, annexin V APC-/viability eFluor780+) cells. F: summarized flow cytometry data of annexin V APC/viability eFluor780 staining of Wt (white bars) and Het (black bars) Con pups (n = 5 per group). *P ≤ 0.05, by Student's t-test.
HO-1 deficiency is associated with a proapoptotic phenotype (72), and intestinal apoptosis has been shown to be a key player in initiating intestinal necrosis in experimental rat NEC models (12, 25) and in NEC patients (16). We therefore performed TUNEL staining to assess possible differences in apoptosis between the two genotypes (Fig. 3C). The apoptotic index, defined as the number of apoptotic cells per total cell number, was significantly increased in the small intestine of dam-fed HO-1 Het Con compared with Wt Con pups (Fig. 3D). Similar enhancement of apoptosis was also found in the colon of HO-1 Het pups (data not shown). Additionally, we performed annexin V staining to better distinguish between apoptotic and necrotic processes using single-cell suspensions of small intestines from Wt Con and HO-1 Het Con pups. We found a significantly reduced percentage of live cells (Fig. 3, E and F, bottom left quadrant) and a significantly increased percentage of late apoptotic/necrotic cells (Fig. 3, E and F, top right quadrant) compared with Wt Con pups. The percentage of early apoptotic cells also increased but did not reach statistical significance (Fig. 3, E and F).
HO activity is impaired in HO-1 deficiency under stress conditions.
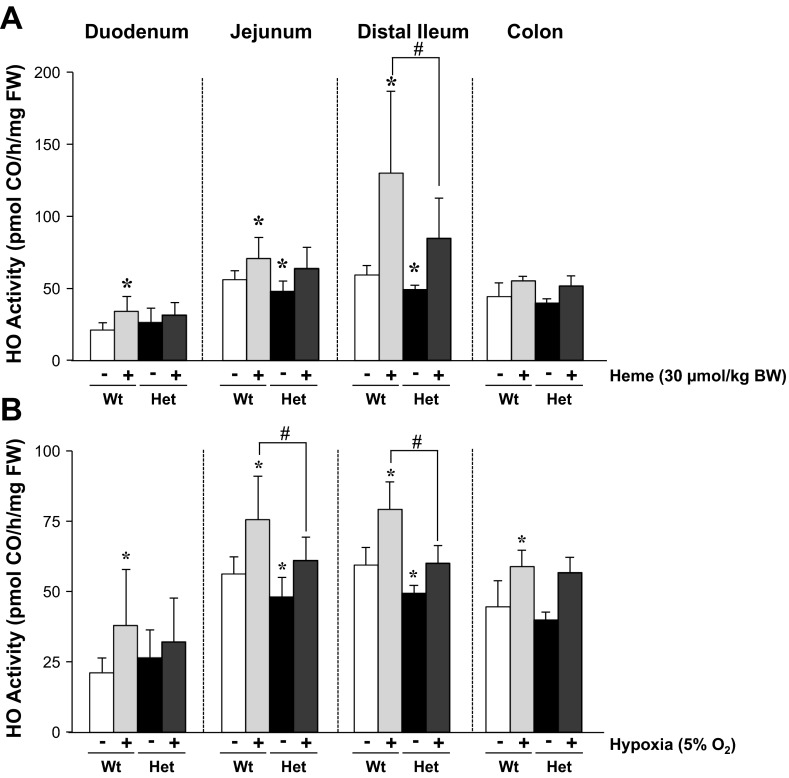
The intestine primarily expresses the constitutive HO-2 (19). Thus we hypothesized that our observed differences in histological damage were due to an impaired capacity of HO-1 Het pups to induce HO-1 in response to stressors. We therefore determined intestinal HO activity of 1-wk-old mice of both genotypes, nonstimulated and stimulated with either heme or hypoxia (5% O2). We found that HO activity of the jejunums and ileums of HO-1 Het mice was 20% lower than HO activity of Wt mice under nonstimulated conditions (Fig. 4). Twenty-four hours after OG of 30 μmol heme/kg body wt, HO activity significantly increased in all intestinal regions of Wt pups. In HO-1 Het pups, the induction of HO activity was less in all regions, and significantly lower in the ileum (Fig. 4A). To see whether this impaired response to heme by HO-1 Het pups also occurred with other stressors, we exposed pups to hypoxia for 1 min on days 1.5, 3, and 6 of life. On day 7, we found that hypoxia increased HO activity in all regions of the intestine in both genotypes, but to a significantly lower degree in HO-1 Het pups (Fig. 4B).
Fig. 4.
Induction of intestinal HO activity. A: HO activity throughout all regions of the intestine measured as CO production by gas chromatography 24 h after the administration of 30-μmol heme/kg body wt by oral gavage to 6-day-old Wt and HO-1 Het pups. B: HO activity throughout all regions of the intestine measured as CO production by using gas chromatography after hypoxia (5% O2) treatment of Wt and Het pups at days 1.5, 3, and 6 of life. *P ≤ 0.05 vs. Wt Con (white bars), using Student's t-test; #P ≤ 0.05 vs. Wt treated (light gray bars, Heme or Hypoxia), by 2-way ANOVA.
HO-1 deficiency results in higher NEC scores, incidence, and mortality.
Because we used a less aggressive model of NEC induction compared with other established mouse models (9, 22, 23, 31), intestinal injury may also be present in more proximal areas of the small intestine, possibly dependent on where the initial insult occurs. We therefore scored the histological damage in the jejunums/proximal ileums, distal ileums, and proximal colons of each genotype. Intestinal damage scores were higher in the small intestine than in the colon. We observed a higher frequency of damage in the jejunum/proximal ileum than in the distal ileum for both genotypes. In all intestinal regions, the damage was more severe in HO-1 Het NEC pups (Fig. 5). To compare NEC scores, the maximum score for each animal was plotted independent of the intestinal region. Significantly higher NEC scores were found in HO-1 Het NEC compared with Wt NEC pups. The median Wt NEC score was 1.5 compared with a median of 2.5 for HO-1 Het NEC mice (Fig. 6A) (P = 0.005). We observed a significantly higher NEC incidence in HO-1 Het pups (77.8%, 21/27) compared with Wt pups (42.9%, 15/35) (Fig. 6B) (P = 0.009). Furthermore, a significantly lower survival rate was observed in HO-1 Het NEC (17%) pups compared with Wt NEC pups (46%) (Fig. 6C), with mortality occurring earlier (36 h) for HO-1 Het NEC pups compared with 44 h for Wt NEC pups (P = 0.005).
Fig. 5.
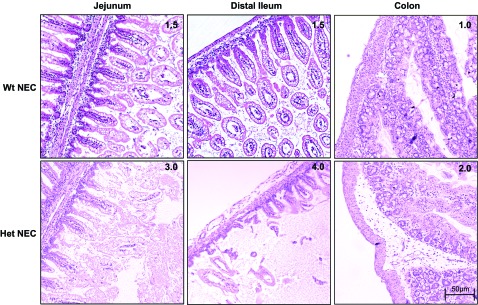
Histological damage scores in nectrotizing enterocolitis (NEC)-induced pups. Representative images of H&E-stained jejunum, distal ileum, and colon with corresponding NEC scores from Wt NEC-induced pups are shown at top and Het NEC-induced pups at bottom. Magnification: ×100.
Fig. 6.
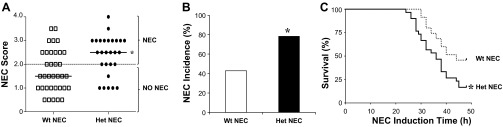
Cumulative maximum NEC scores, incidence, and survival plots for Wt and Het NEC-induced pups. A: to compare NEC scores, the maximum score of each pup, independent of the intestinal region, was plotted. A score ≥2 was defined as NEC. Bars indicate median. *P = 0.005, by Mann-Whitney test. B: incidence of NEC reflecting the percentage of pups having NEC scores ≥2 for each genotype. *P = 0.009, by Fisher's exact test. C: Kaplan-Meier plots were used to compare survival over time for Wt and Het NEC-induced pups. *P = 0.005, log-rank (Mantel-Cox) test.
Genes involved in inflammation, leukocyte adhesion, and coagulation are increased in HO-1-deficient NEC-induced mice.
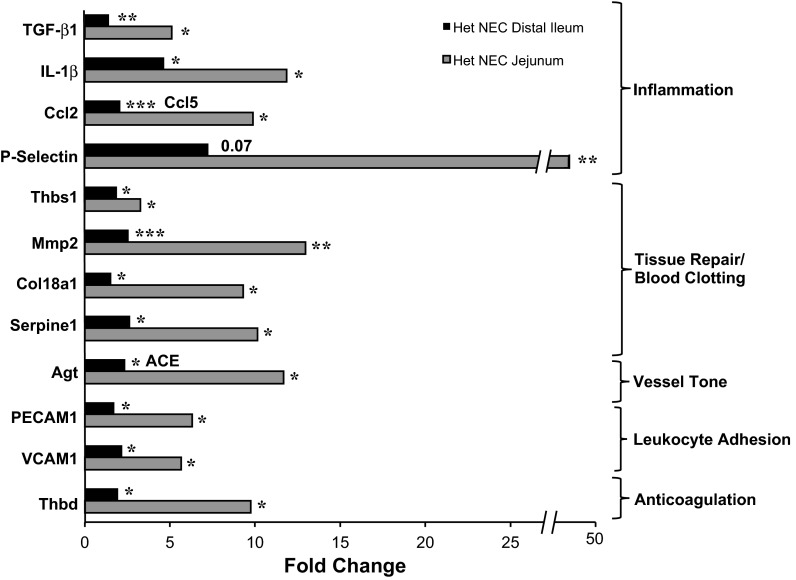
To try to further elucidate the molecular mechanism underlying the higher NEC incidence of HO-1 Het pups, we screened for genes involved in cell activation, injury, and vessel tone using the Mouse RT2 Profiler PCR Array (SA BioSciences, Valencia, CA). The web-based ΔΔCt method was used to calculate fold changes in gene expression in the jejunums and distal ileums of HO-1 Het NEC over that of Wt NEC tissues. The expression of genes involved in inflammation (transforming growth factor-β1, IL-1β, chemokine ligand 2 and 5, P-selectin, angiotensinogen), tissue repair and coagulation (thrombospondin 1, matrix metallopeptidase 2, collagen type XVIII-α1), vessel tone (angiotensinogen, angiotensin I converting enzyme), leukocyte adhesion [platelet/endothelial cell adhesion molecule 1 (PECAM1), vascular cell adhesion molecule 1 (VCAM1)], and anticoagulation (thrombumodulin) increased significantly in the distal ileums and jejunums of HO-1 Het NEC compared with Wt NEC pups (Fig. 7). When the ileal tissue of Wt NEC and HO-1 Het NEC is compared with Wt Con, genes in response to stress [nitric oxide synthase 2 (iNOS), SOD 1], and TNF-α-induced protein 3 were significantly elevated in HO-1 Het NEC, but not in Wt NEC tissues (data not shown). Interestingly, chemokine ligand 2, iNOS, endothelial nitric oxide synthase, collagen XVIII-α1, natriuretic peptide receptor 1, and von Willebrand factor (P = 0.07 for Wt NEC) were significantly increased in both NEC genotypes, but with a higher magnitude in HO-1 Het NEC tissue (data not shown).
Fig. 7.
Cell injury PCR array. Eighty-four genes involved in cell injury were screened and fold changes in the expression levels from [Het NEC (NEC score ≥ 2)] distal ileums (black bar) and jejunums (light gray bar) overexpression levels from Wt NEC (NEC score ≥ 2) distal ileums and jejunums were calculated by the web-based ΔΔCt method. TGF-β1, transforming growth factor-β1; Ccl2, chemokine ligand 2 (only in jejunum); Ccl5 chemokine ligand 5 (only in ileum); Thbs1, thrombospondin 1; Mmp2, matrix metallopeptidase 2; Col18a1, collagen type XVIII-α1; Agt, angiotensinogen (only in jejunum); ACE, angiotensin I converting enzyme (only in ileum); PECAM1, platelet/endothelial cell adhesion molecule 1; VCAM1, vascular cell adhesion molecule 1; Thbd, thrombomodulin. *P ≤ 0.05; **P ≤ 0.005; ***P ≤ 0.0005 vs. Wt NEC, by Student's t-test.
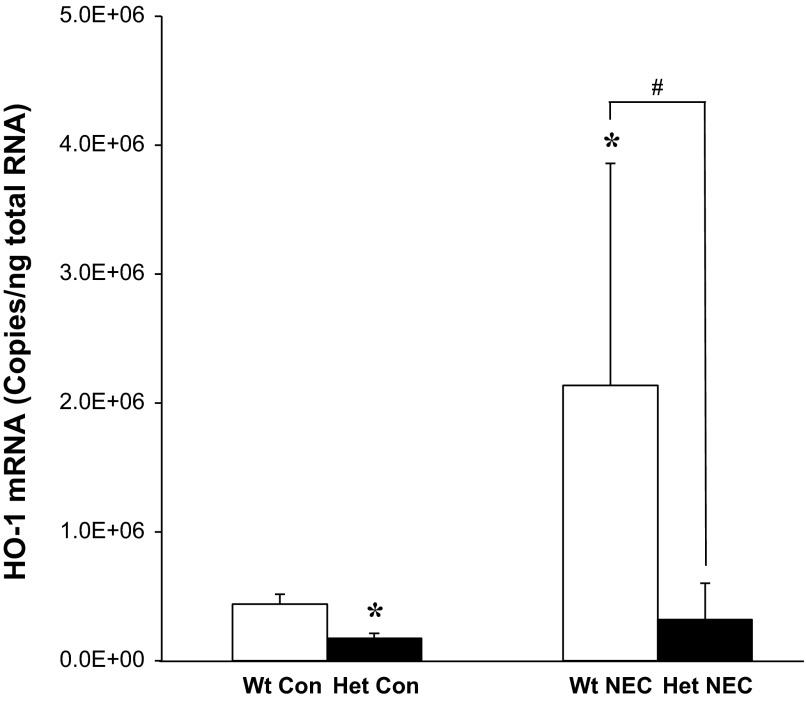
HO-1 mRNA is upregulated in NEC-induced Wt pups.
At baseline, as expected HO-1 mRNA in the jejunum and distal ileum in Wt Con pups was ∼50–60% higher than that in HO-1 Het Con pups. HO-2 mRNA levels were not different between genotypes (data not shown). After NEC induction, HO-1 mRNA increased significantly in the distal ileums of Wt NEC pups compared with Wt Con and Het NEC pups (Fig. 8). The HO-1 expression in the jejunum did not change significantly compared with Con of both genotypes. In addition, HO-2 mRNA did not change in the jejunums and distal ileums for both genotypes (data not shown).
Fig. 8.
HO-1 mRNA expression in the distal ileum of Con and NEC-induced Wt and Het pups determined by quantitative RT-PCR. Values are normalized to β-actin. *P ≤ 0.05 vs. Wt Con, by Student's t-test; #P ≤ 0.05 vs. Wt NEC, by 2-way ANOVA.
DISCUSSION
Three major risk factors for NEC development are nearly always present: prematurity, enteral feeding, and bacterial colonization (18, 33). The majority of very low birth weight infants have those risk factors and also show intermittent gastrointestinal (GI) symptoms, such as abdominal distention, heme-positive stools, and feeding intolerance, but only a minority of infants develop NEC (45). This relative infrequency of NEC development suggests robust systems, which generally prevent the occurrence of NEC, even if identified risk factors are present (29). Kim and coworkers (29) propose that one initiating condition in the pathogenesis of NEC is an impaired ability of the neonatal gut to cope with oxidative stress, and, if neonates are then exposed to enteral feeding, this additional metabolic oxidative stress leads to apoptosis, inflammation, bacterial activation, and eventually necrosis.
HO-1 expression has been shown to increase in the GI tract in response to oxidative stress, preconditioning, and acute inflammation (42). A number of different GI injury models suggests that HO-1 activation could act as an endogenous defensive mechanism to maintain the integrity of the intestinal barrier and reduce inflammation and tissue injury (2, 5, 42, 49, 60). The present study demonstrates that a deficiency of the HO-1 enzyme promotes the development of NEC-like intestinal injury. We found that HO activity in the intestine is not only developmentally regulated but also region specific. Intestines of HO-1 Het mice appear to be predisposed to injury, showing abnormal morphology and enhanced apoptosis under basal conditions. In addition, the phenotype is characterized by an impaired ability to respond to stressors. Moreover, NEC scores, incidence, mortality, and expression of inflammatory and cell injury mediators were significantly higher in HO-1-deficient mice.
The intestine predominantly expresses the constitutive HO-2 (15, 19). HO-2 immunoreactivity is found in enteric ganglionic neurons of the myenteric plexus, in nerve fibers innervating the circular smooth muscle fibers, and in smooth muscle cells in mouse (20, 39), dog (15), and human intestine (40). Also interstitial cells of Cajal, essential for GI motility, express HO-2 in mouse colon and small intestine (19). Exogenous CO has been shown to modulate intestinal smooth muscle electrical activity (15, 39, 40, 69), which underlies organized motility patterns. We found a significant reduction in the thickness of the inner circular muscle fibers of the muscularis externa in HO-1 Het mice, possibly reflecting a decrease in muscle activity. Studies have shown that not only may HO-2-derived CO be involved in the regulation of motility, but also functional HO-1 contributes to intestinal smooth muscle activity (19). Although most studies failed to show HO-1 immunoreactivity in the small intestine (15, 40), the authors conclude that levels of the inducible HO-1 are below detection limits under unstimulated conditions but may increase significantly under stimulation with HO-1 inducers and stress conditions (15). In fact, increased HO-1 protein mainly in the mucosal epithelial cells and apical villi after LPS stimulation and in the macrophages of inflamed mucosa have been shown in other studies (17, 42). We have also shown a significant upregulation of HO activity in the intestine of Wt pups, due to HO-1 induction by heme or hypoxia. We speculate that the decreased thickness of the muscularis externa in HO-1 Het mice is due to limited CO availability, and thus reduced smooth muscle contractility. Delayed intestinal transit time has been shown in HO-2 KO mice (69). In a mouse model of postoperative ileus, pretreatment with CO-releasing molecules markedly restored intestinal contractility and transit time (13). Similarly, inhaled CO improved posttransplant motility by reducing the autocrine effects of COX-2 and iNOS produced prostanoids and NO in a rat model of small intestinal transplantation (44). Impaired intestinal motility may lead to a prolonged deposition of irritating nutrients and promote bacterial overgrowth. Both factors can then trigger the development of NEC.
Studies from animal models and human samples highlight the relevance of augmented apoptosis in NEC development, possibly leading to impaired intestinal barrier function and thus increased permeability, all which may precede necrotic processes (16, 25, 56). It has been shown that the protection from NEC development by epidermal growth factor (EGF) and Bifidobacterium bifidum is associated with their ability to alter apoptotic gene expression, shifting the balance of pro- and antiapoptotic genes toward cell survival (12, 27). Moreover, heparin-binding EGF-like growth factor (HB-EGF) KO mice displayed increased epithelial apoptosis and mucosal permeability in a model of hemorrhagic shock and resuscitation and are also more susceptible to NEC (52). It is proposed that the downregulation of intestinal apoptosis could be a molecular mechanism that decreases mucosal injury and maintains intestinal integrity (27). An antiapoptotic role of HO-1, mediated by CO, has been demonstrated in vitro and in vivo in other tissues (7, 75). CO exposure (250 ppm) inhibited TNF-α plus actinomycin D-induced apoptosis in intestinal epithelial cells (76). However, whether reduced tissue availability of CO and/or decreased antioxidant capacity (low levels of bilirubin/biliverdin) in HO-1-deficient mice causes increased early and late apoptosis in the intestine as observed in our study needs to be further elucidated.
Our findings highlight the importance of having the ability to increase intestinal HO activity in response to substrate (heme) or stress (hypoxia) stimulation. Although we found significant differences at baseline levels between jejunums and ileums of Wt and HO-1 Het mice, the HO-1-deficient phenotype was much more apparent under HO-1 stimulation (heme or hypoxia treatment). We speculate that this impaired ability to increase HO-1 gene expression underlies the increased susceptibility of HO-1 Het mice to our NEC-like induction protocol. The fact that HO-1 mRNA was significantly increased in Wt NEC pups supports this hypothesis. Because HO-1 Het pups were not able to upregulate HO-1, they developed NEC earlier and much more frequently. It would be interesting to elucidate whether the HO-1-deficient genotype can be rescued through the administration of either (or both) HO products, CO and/or bilirubin.
Interestingly, HO activity differs along the intestinal regions and is also age dependent. In 1-wk-old mice, HO activity was highest in the ileum, the region primarily affected by NEC. HO-1 mRNA expression followed the same pattern along the intestine, whereas HO-2 mRNA was relatively equally expressed in all intestinal sections (data not shown), confirming that the HO activity differences along the intestine reflect changes in HO-1 expression. Low HO-1 expressors in humans due to HO-1 polymorphisms [microsatellite polymorphisms of (GT)n repeats (longer repeats = lower expression; shorter repeats = higher expression), a single-nucleotide polymorphism, T(-143)/A, HO-1 mutant alleles] (57) may be particularly susceptible to ileal injury, if HO-1 expression in human newborns follows the same intestinal expression pattern. Our intestinal ontogeny data are consistent with other reports that also show highest HO activity in the duodenum, progressively decreasing toward distal regions in adult rats (17, 53). Interestingly, Kim and coworkers (28) also found an inverse relationship of HO activity along the intestine between suckling (HO activity lowest in duodenum) and adult rats (HO activity highest in duodenum). Raffin et al. (53) postulated that the high HO activity in the duodenum (for adults) correlates with the most efficient hemoglobin iron absorption in the proximal intestinal part. The age-dependent and regional difference of HO activity, whether it is a matter of heme-iron absorption or of changing HO-1-to-HO-2 ratios, should be further explored.
Although “classic” NEC refers to intestinal damage primarily in the distal ileum and proximal colon, we also scored the jejunum and found frequent NEC-like damage with mucosal and submucosal edema, hemorrhage, and loss of villous integrity. Barlow and coworkers (4) described in their initial NEC rat model, using hypoxia and enteral feeding, that gaseous distention was not limited to the ileum but also involving the entire intestine during prolonged NEC induction (≥36 h). Interestingly, HO activity is highest in the ileum of 1-wk-old mice and decreases age dependently; the ileum is also the site of the most significant HO activity increase due to substrate and hypoxia stimulation, and the ileal HO-1 mRNA is highly upregulated in Wt NEC pups. Taken together, these data may indicate that the ileum is highly protected by HO-1 against disruption of intestinal homeostasis.
Elevated levels of IL-1β, leukocyte-recruiting chemokines, and cell adhesion molecules (chemokines 2 and 5, P-selectin, VCAM1, and PECAM1) in ileal and jejunal tissues of HO-1 Het NEC pups indicate an exaggerated inflammatory response under HO-1 deficiency, which may lead to a faster progression of NEC. Increased levels of IL-1β have been described consistently in human NEC cases (59, 65, 68) and have also been found in experimental NEC models (70). IL-6 expression did not change; it was elevated in the majority of the previously mentioned studies but was also found unchanged between human control and acute NEC samples by Viscardi et al. (65). TGF-β2 has been shown to protect mouse pups against NEC-like intestinal injury, with similar effects found for TGF-β1 (35). As discussed by other authors, the simultaneous elevation of pro- and anti-inflammatory mediators might be related to a disruption of balance between these two systems (70). We found similar contradictory elevations of genes involved in blood clotting (von Willebrand factor, collagen XVIII-α1), anticoagulation (thrombomodulin), and fibrinolysis (serpine 1) for blood coagulation and tissue repair.
Interestingly, high P-selectin staining has been found on endothelium of large and medium-sized vessels and microvasculature located in all layers of the intestine in human NEC samples compared with control tissue. P-selectin was specifically high in specimens with severe histological damage (62). The authors speculate that P-selectin promotes neutrophil rolling, adhesion, and the degree of infiltration, mediated by intercellular adhesion molecule-1 (ICAM1) and VCAM1. Expression of both genes (ICAM1 only in the jejunum, data not shown) was significantly upregulated in HO-1 Het NEC pups.
In the present study, we have demonstrated that HO-1-deficient pups were predisposed to intestinal injury and showed an increased risk for NEC-like intestinal injury development. The underlying mechanism for this observation may be an inability to react to stress conditions via an upregulation of intestinal HO-1, leading to an exaggerated inflammatory process and a disruption in tissue repair. Whether a preventive or therapeutic strategy that upregulates HO-1 can protect against NEC-like intestinal injury and whether the HO-1-deficient phenotype can be rescued with either or both of the HO-1 products, CO and bilirubin, should be further studied.
GRANTS
This work was supported by the Lucile Packard Foundation for Children's Health, and the Stanford CTSA (UL1 RR025744), the Mary L. Johnson Research Fund, the Christopher Hess Research Fund, and the H. M. Lui Research Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.S., R.J.W., H.Z., and D.K.S. conception and design of research; S.S., R.J.W., and F.K. performed experiments; S.S., R.J.W., K.Y.J., and K.M.C. analyzed data; S.S., R.J.W., and K.Y.J. interpreted results of experiments; S.S. and R.J.W. prepared figures; S.S., R.J.W., H.J.V., K.G.S., and D.K.S. drafted manuscript; S.S., R.J.W., H.Z., H.J.V., K.G.S., and D.K.S. edited and revised manuscript; S.S., R.J.W., K.Y.J., F.K., K.M.C., H.Z., H.J.V., K.G.S., and D.K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Stephanie Torreilles, Department of Comparative Medicine, for advice on the care and feeding of the pups. We thank Pauline Chu, from the Department of Comparative Medicine, Stanford University, for technical expertise. We also thank Dr. Yang Yang from the Department of Genetics, Stanford University, for valuable discussions.
REFERENCES
- 1. Attuwaybi B, Kozar RA, Gates KS, Moore-Olufemi S, Sato N, Weisbrodt NW, Moore FA. Hypertonic saline prevents inflammation, injury, and impaired intestinal transit after gut ischemia/reperfusion by inducing heme oxygenase 1 enzyme. J Trauma 56: 749–758, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Attuwaybi BO, Hassoun HT, Zou L, Kozar RA, Kone BC, Weisbrodt NW, Moore FA. Hypothermia protects against gut ischemia/reperfusion-induced impaired intestinal transit by inducing heme oxygenase-1. J Surg Res 115: 48–55, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Attuwaybi BO, Kozar RA, Moore-Olufemi SD, Sato N, Hassoun HT, Weisbrodt NW, Moore FA. Heme oxygenase-1 induction by hemin protects against gut ischemia/reperfusion injury. J Surg Res 118: 53–57, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Barlow B, Santulli TV, Heird WC, Pitt J, Blanc WA, Schullinger JN. An experimental study of acute neonatal enterocolitis—the importance of breast milk. J Pediatr Surg 9: 587–595, 1974 [DOI] [PubMed] [Google Scholar]
- 5. Berberat PO, A-Rahim YI, Yamashita K, Warny MM, Csizmadia E, Robson SC, Bach FH. Heme oxygenase-1-generated biliverdin ameliorates experimental murine colitis. Inflamm Bowel Dis 11: 350–359, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Berman L, Moss RL. Necrotizing enterocolitis: an update. Semin Fetal Neonatal Med 16: 145–150, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 192: 1015–1026, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caplan MS, Hedlund E, Adler L, Hsueh W. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol 14: 1017–1028, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Cetin S, Leaphart CL, Li J, Ischenko I, Hayman M, Upperman J, Zamora R, Watkins S, Ford HR, Wang J, Hackam DJ. Nitric oxide inhibits enterocyte migration through activation of RhoA-GTPase in a SHP-2-dependent manner. Am J Physiol Gastrointest Liver Physiol 292: G1347–G1358, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest 118: 239–247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol 291: G938–G949, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Clark JA, Lane RH, Maclennan NK, Holubec H, Dvorakova K, Halpern MD, Williams CS, Payne CM, Dvorak B. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 288: G755–G762, 2005 [DOI] [PubMed] [Google Scholar]
- 13. De Backer O, Elinck E, Blanckaert B, Leybaert L, Motterlini R, Lefebvre RA. Water-soluble CO-releasing molecules reduce the development of postoperative ileus via modulation of MAPK/HO-1 signalling and reduction of oxidative stress. Gut 58: 347–356, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 282: G156–G164, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Farrugia G, Miller SM, Rich A, Liu X, Maines MD, Rae JL, Szurszewski JH. Distribution of heme oxygenase and effects of exogenous carbon monoxide in canine jejunum. Am J Physiol Gastrointest Liver Physiol 274: G350–G358, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg 32: 275–282, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Fujii H, Takahashi T, Nakahira K, Uehara K, Shimizu H, Matsumi M, Morita K, Hirakawa M, Akagi R, Sassa S. Protective role of heme oxygenase-1 in the intestinal tissue injury in an experimental model of sepsis. Crit Care Med 31: 893–902, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Gephart SM, McGrath JM, Effken JA, Halpern MD. Necrotizing enterocolitis risk: state of the science. Adv Neonatal Care 12: 77–87; quiz 88–79, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gibbons SJ, Farrugia G. The role of carbon monoxide in the gastrointestinal tract. J Physiol 556: 325–336, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grozdanovic Z, Gossrau R. Expression of heme oxygenase-2 (HO-2)-like immunoreactivity in rat tissues. Acta Histochem 98: 203–214, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Halpern MD, Dominguez JA, Dvorakova K, Holubec H, Williams CS, Meza YG, Ruth MC, Dvorak B. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr 36: 126–133, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Halpern MD, Khailova L, Molla-Hosseini D, Arganbright K, Reynolds C, Yajima M, Hoshiba J, Dvorak B. Decreased development of necrotizing enterocolitis in IL-18-deficient mice. Am J Physiol Gastrointest Liver Physiol 294: G20–G26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Halpern MD, Weitkamp JH, Mount Patrick SK, Dobrenen HJ, Khailova L, Correa H, Dvorak B. Apical sodium-dependent bile acid transporter upregulation is associated with necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299: G623–G631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med 202: 1703–1713, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res 55: 622–629, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Kanagawa F, Takahashi T, Inoue K, Shimizu H, Omori E, Morimatsu H, Maeda S, Katayama H, Nakao A, Morita K. Protective effect of carbon monoxide inhalation on lung injury after hemorrhagic shock/resuscitation in rats. J Trauma 69: 185–194, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Khailova L, Mount Patrick SK, Arganbright KM, Halpern MD, Kinouchi T, Dvorak B. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299: G1118–G1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim CB, Hintz SR, Vreman HJ, Stevenson DK. In vitro carbon monoxide production by the small intestine of suckling and adult Wistar rats: effect of parenteral tin-protoporphyrin. Dev Pharmacol Ther 11: 166–172, 1988 [DOI] [PubMed] [Google Scholar]
- 29. Kim M, Christley S, Alverdy JC, Liu D, An G. Immature oxidative stress management as a unifying principle in the pathogenesis of necrotizing enterocolitis: insights from an agent-based model. Surg Infect (Larchmt) 13: 18–32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 179: 4808–4820, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Leaphart CL, Qureshi F, Cetin S, Li J, Dubowski T, Baty C, Beer-Stolz D, Guo F, Murray SA, Hackam DJ. Interferon-gamma inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology 132: 2395–2411, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, Tsao LY, Chen CH, Su BH. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 122: 693–700, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol 32: 70–82, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 368: 1271–1283, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Maheshwari A, Kelly DR, Nicola T, Ambalavanan N, Jain SK, Murphy-Ullrich J, Athar M, Shimamura M, Bhandari V, Aprahamian C, Dimmitt RA, Serra R, Ohls RK. TGF-beta2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 140: 242–253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mallick IH, Winslet MC, Seifalian AM. Ischemic preconditioning of small bowel mitigates the late phase of reperfusion injury: heme oxygenase mediates cytoprotection. Am J Surg 199: 223–231, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Mallick IH, Winslet MC, Seifalian AM. Pyrrolidine dithiocarbamate protects the small bowel from warm ischaemia/reperfusion injury of the intestine: the role of haem oxygenase. Clin Sci (Lond) 111: 373–380, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Martin NA, Mount Patrick SK, Estrada TE, Frisk HA, Rogan DT, Dvorak B, Halpern MD. Active transport of bile acids decreases mucin 2 in neonatal ileum: implications for development of necrotizing enterocolitis. PLoS One 6: e27191, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller SM, Farrugia G, Schmalz PF, Ermilov LG, Maines MD, Szurszewski JH. Heme oxygenase 2 is present in interstitial cell networks of the mouse small intestine. Gastroenterology 114: 239–244, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Miller SM, Reed D, Sarr MG, Farrugia G, Szurszewski JH. Haem oxygenase in enteric nervous system of human stomach and jejunum and co-localization with nitric oxide synthase. Neurogastroenterol Motil 13: 121–131, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest 96: 2676–2682, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Naito Y, Takagi T, Uchiyama K, Yoshikawa T. Heme oxygenase-1: a novel therapeutic target for gastrointestinal diseases. J Clin Biochem Nutr 48: 126–133, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakao A, Kaczorowski DJ, Sugimoto R, Billiar TR, McCurry KR. Application of heme oxygenase-1, carbon monoxide and biliverdin for the prevention of intestinal ischemia/reperfusion injury. J Clin Biochem Nutr 42: 78–88, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nakao A, Moore BA, Murase N, Liu F, Zuckerbraun BS, Bach FH, Choi AM, Nalesnik MA, Otterbein LE, Bauer AJ. Immunomodulatory effects of inhaled carbon monoxide on rat syngeneic small bowel graft motility. Gut 52: 1278–1285, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 364: 255–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nowicki PT, Nankervis CA. The role of the circulation in the pathogenesis of necrotizing enterocolitis. Clin Perinatol 21: 219–234, 1994 [PubMed] [Google Scholar]
- 47. Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol 24: 449–455, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Patel JC, Tepas JJ, 3rd, Huffman SD, Evans JS. Neonatal necrotizing enterocolitis: the long-term perspective. Am Surg 64: 575–579, 1998 [PubMed] [Google Scholar]
- 49. Paul G, Bataille F, Obermeier F, Bock J, Klebl F, Strauch U, Lochbaum D, Rummele P, Farkas S, Scholmerich J, Fleck M, Rogler G, Herfarth H. Analysis of intestinal haem-oxygenase-1 (HO-1) in clinical and experimental colitis. Clin Exp Immunol 140: 547–555, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA 94: 10919–10924, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Puiman P, Stoll B. Animal models to study neonatal nutrition in humans. Curr Opin Clin Nutr Metab Care 11: 601–606, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Radulescu A, Yu X, Orvets ND, Chen Y, Zhang HY, Besner GE. Deletion of the heparin-binding epidermal growth factor-like growth factor gene increases susceptibility to necrotizing enterocolitis. J Pediatr Surg 45: 729–734, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Raffin SB, Woo CH, Roost KT, Price DC, Schmid R. Intestinal absorption of hemoglobin iron-heme cleavage by mucosal heme oxygenase. J Clin Invest 54: 1344–1352, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed 92: F193–F198, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Richards JA, Wigmore SJ, Devey LR. Heme oxygenase system in hepatic ischemia-reperfusion injury. World J Gastroenterol 16: 6068–6078, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Santaolalla R, Abreu MT. Innate immunity in the small intestine. Curr Opin Gastroenterol 28: 124–129, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schulz S, Zhao H, Wong RJ, Stevenson DK. Heme oxygenase biology during the perinatal period: Part 1: Prenatal considerations. NeoReviews 13: e151–e157, 2012 [Google Scholar]
- 58. Scott JR, Gray DK, Bihari A, Badhwar A, Zhang X, Shan P, Lee PJ, Chakrabarti S, Harris KA, Potter RF. Heme oxygenase modulates small intestine leukocyte adhesion following hindlimb ischemia/reperfusion by regulating the expression of intercellular adhesion molecule-1. Crit Care Med 33: 2563–2570, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Sharma R, Tepas JJ, 3rd, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, Premachandra BR. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg 42: 454–461, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Sheikh SZ, Hegazi RA, Kobayashi T, Onyiah JC, Russo SM, Matsuoka K, Sepulveda AR, Li F, Otterbein LE, Plevy SE. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol 186: 5506–5513, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sonntag J, Grimmer I, Scholz T, Metze B, Wit J, Obladen M. Growth and neurodevelopmental outcome of very low birthweight infants with necrotizing enterocolitis. Acta Paediatr 89: 528–532, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Stefanutti G, Lister P, Smith VV, Peters MJ, Klein NJ, Pierro A, Eaton S. P-selectin expression, neutrophil infiltration, and histologic injury in neonates with necrotizing enterocolitis. J Pediatr Surg 40: 942–947, 2005 [DOI] [PubMed] [Google Scholar]
- 63. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 235: 1043–1046, 1987 [DOI] [PubMed] [Google Scholar]
- 64. Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 61: 748–755, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Viscardi RM, Lyon NH, Sun CC, Hebel JR, Hasday JD. Inflammatory cytokine mRNAs in surgical specimens of necrotizing enterocolitis and normal newborn intestine. Pediatr Pathol Lab Med 17: 547–559, 1997 [PubMed] [Google Scholar]
- 66. Vreman HJ, Stevenson DK. Detection of heme oxygenase activity by measurement of CO. In: Current Protocols in Toxicology, edited by Maines MD, Costa G, Reed DJ, Sassa S, Sipes IG. New York: Wiley, 1999, p. 9.2.1–9.2.10 [DOI] [PubMed] [Google Scholar]
- 67. Wasserberg N, Pileggi A, Salgar SK, Ruiz P, Ricordi C, Inverardi L, Tzakis AG. Heme oxygenase-1 upregulation protects against intestinal ischemia/reperfusion injury: a laboratory based study. Int J Surg 5: 216–224, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Weitkamp JH, Koyama T, Rock MT, Correa H, Goettel JA, Matta P, Oswald-Richter K, Rosen MJ, Engelhardt BG, Moore DJ, Polk DB. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 62: 73–82, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci USA 94: 14848–14853, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang C, Sherman MP, Prince LS, Bader D, Weitkamp JH, Slaughter JC, McElroy SJ. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis Model Mech 5: 522–532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhao H, Azuma J, Kalish F, Wong RJ, Stevenson DK. Maternal heme oxygenase 1 regulates placental vasculature development via angiogenic factors in mice. Biol Reprod 85: 1005–1012, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhao H, Wong RJ, Kalish FS, Nayak NR, Stevenson DK. Effect of heme oxygenase-1 deficiency on placental development. Placenta 30: 861–868, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhao H, Wong RJ, Nguyen X, Kalish F, Mizobuchi M, Vreman HJ, Stevenson DK, Contag CH. Expression and regulation of heme oxygenase isozymes in the developing mouse cortex. Pediatr Res 60: 518–523, 2006 [DOI] [PubMed] [Google Scholar]
- 74. Zhong W, Xia Z, Hinrichs D, Rosenbaum JT, Wegmann KW, Meyrowitz J, Zhang Z. Hemin exerts multiple protective mechanisms and attenuates dextran sulfate sodium-induced colitis. J Pediatr Gastroenterol Nutr 50: 132–139, 2010 [DOI] [PubMed] [Google Scholar]
- 75. Zuckerbraun BS, Billiar TR, Otterbein SL, Kim PK, Liu F, Choi AM, Bach FH, Otterbein LE. Carbon monoxide protects against liver failure through nitric oxide-induced heme oxygenase 1. J Exp Med 198: 1707–1716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zuckerbraun BS, Otterbein LE, Boyle P, Jaffe R, Upperman J, Zamora R, Ford HR. Carbon monoxide protects against the development of experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 289: G607–G613, 2005 [DOI] [PubMed] [Google Scholar]