Abstract
TGR5, the G protein-coupled bile acid receptor that transmits bile acid signaling into a cell functional response via the intracellular cAMP signaling pathway, is expressed in human and rodent cholangiocytes. However, detailed information on the localization and function of cholangiocyte TGR5 is limited. We demonstrated that in human (H69 cells) and rat cholangiocytes, TGR5 is localized to multiple, diverse subcellular compartments, with its strongest expression on the apical plasma, ciliary, and nuclear membranes. To evaluate the relationship between ciliary TGR5 and the cholangiocyte functional response to bile acid signaling, we used a model of ciliated and nonciliated H69 cells and demonstrated that TGR5 agonists induce opposite changes in cAMP and ERK levels in cells with and without primary cilia. The cAMP level was increased in nonciliated cholangiocytes but decreased in ciliated cells. In contrast, ERK signaling was induced in ciliated cholangiocytes but suppressed in cells without cilia. TGR5 agonists inhibited proliferation of ciliated cholangiocytes but activated proliferation of nonciliated cells. The observed differential effects of TGR5 agonists were associated with the coupling of TGR5 to Gαi protein in ciliated cells and Gαs protein in nonciliated cholangiocytes. The functional responses of nonciliated and ciliated cholangiocytes to TGR5-mediated bile acid signaling may have important pathophysiological significance in cilia-related liver disorders (i.e., cholangiociliopathies), such as polycystic liver disease. In summary, TGR5 is expressed on diverse cholangiocyte compartments, including a primary cilium, and its ciliary localization determines the cholangiocyte functional response to bile acid signaling.
Keywords: cholangiocytes, primary cilia, TGR5, G protein-coupled bile acid receptor, bile acid signaling
bile acids act as signaling molecules primarily via activation of nuclear farnesoid X receptor (FXR) and pregnane X receptor (PXRs) and a plasma membrane-bound G protein-coupled receptor, TGR5 (13, 32). In the liver, FXR and PXR are highly expressed in hepatocytes and function as ligand-modulated transcriptional factors that regulate target genes implicated mainly in hepatic bile acid transport and metabolism (13, 32). Cholangiocytes show weak or no expression of FXR and PXR (7, 41). In contrast, TGR5, which mediates the rapid, transcription-independent actions of bile acids, is not expressed in hepatocytes (22) but is expressed in cholangiocytes (16–18, 20) and is, presumably, a major receptor for bile acid signaling in biliary epithelia. In the liver, TGR5 is also expressed in Kupffer cells (16) and sinusoidal endothelial cells (19). Functionally, TGR5 is linked to the cAMP signaling pathway, and its activation in different cell types by free, taurine-conjugated, and glycine-conjugated bile acids induces an increase in intracellular cAMP levels followed by a cell-specific response (14, 17, 18, 22).
In cholangiocytes, TGR5 has been reported to be localized to the apical plasma membrane, subapical compartment, and primary cilia (15, 17, 18, 20). However, the functional significance of this diverse localization of TGR5 remains unknown. The expression of TGR5 in cholangiocyte cilia, which we have demonstrated are mechano-, chemo-, and osmosensory organelles (9, 25, 27, 28), suggests the potential existence of a novel ciliary-associated TGR5-mediated bile acid signaling pathway in biliary epithelia. This pathway may have physiological importance given the fact that cholangiocyte cilia are constantly exposed to varying amount and types of bile acids moving through the intrahepatic bile ducts (IBDs). The localization of TGR5 to the cholangiocyte apical plasma membrane, primary cilia, and subapical compartment also suggested to us that the cell functional response to bile acid signaling might depend on the subcellular localization of TGR5.
Based on these observations, the aims of the present study were to further characterize the localization of TGR5 in human and rodent cholangiocytes and to evaluate the relationship between its subcellular expression and the cholangiocyte functional response to TGR5-mediated bile acid signaling. We demonstrated that TGR5 is localized to multiple, diverse cholangiocyte subcellular compartments, with its strongest expression on the apical plasma, ciliary and nuclear membranes. To assess the functional role of ciliary-associated TGR5, we used experimental models of ciliated and nonciliated cholangiocytes, which allowed us to observe the differential effects of TGR5 agonists on cAMP and ERK levels in cells with and without cilia as well as their proliferation. The differential effects of TGR5 agonists were associated with the coupling of TGR5 to Gαi protein in ciliated cells and Gαs protein in nonciliated cells. Our results indicate that the subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling.
MATERIALS AND METHODS
Chemicals.
All chemicals were of the highest purity commercially available and were purchased from Sigma Chemical (St. Louis, MO) unless otherwise indicated.
Models.
IBDs dissected from male Sprague-Dawley rat livers (Harlan Sprague Dawley, Indianapolis, IN) (24) and H69 cells, which are simian virus 40-transformed cholangiocytes originally derived from the normal human liver (10), were used in this study. H69 cells were grown in 96-well plates (Corning, Corning, NY) for measuring cAMP levels and the rate of cell proliferation and in 24-well plates (Becton Dickinson, Franklin Lakes, NJ) for ERK-related experiments. TGR5 agonists were added to a monolayer of H69 cells, i.e., to the apical side of the cells. Dissected IBDs were placed in a microperfusion apparatus, as we have previously described in detail (24), and the TGR5 agonist 6α-ethyl-23(S)-methyl-cholic acid (INT-777) was delivered to the lumen of IBDs using a system of micropipettes; this technique allows stimulation of the apical side of naturally ciliated cholangiocytes lining the IBDs. All experimental procedures using animals were approved by the Institutional Animal Care and Use Committee of Mayo Clinic.
TGR5 ligands.
Two strongly potent natural TGR5 agonists, taurolithocholic acid (TLCA) and lithocholic acid (LCA) (14, 22, 32), and two well-characterized selective agonists, INT-777 (Intercept Pharmaceuticals, New York, NY) (38), which is a semisynthetic derivative of cholic acid, and oleanolic acid (OA), which is a triterpene naturally present in leaves of the olive tree (33), were used in this study. Ursodeoxycholic acid (UDCA), which does not have ligand capacity for TGR5, was used as a negative control. Forskolin (FSK; 10 μM), which increases cAMP levels directly through adenylyl cyclases, was used as a positive control. EGF (20 ng/ml) was used as a positive control for ERK signaling and cholangiocyte proliferation. H69 cells and isolated rat IBDs were treated for 30 min with 25 μM TGR5 agonists and UDCA, a concentration that is commonly used in TGR5 functional studies (15, 16, 19).
TGR5-green fluorescent protein construct.
Total RNA was prepared from H69 cells using TRIzol (Invitrogen) and reverse transcribed to cDNA using a Superscript III First Strand Synthesis kit (Invitrogen). TGR5 was amplified using the forward primer 5′-CCGGAATTCCTCCAGGACTCCCCTGTCCC-3′ and the reverse primer: 5′-CGCGGATCCGCGTTCAAGTCCAGGTCGACACTG-3′. The forward primer was designed to contain an EcoRI restriction site and a BamH1 site that was included in the reverse primer (underlined). The TGR5 stop codon was eliminated, and two nucleotides (GC, bold) were inserted to put the TGR5 coding sequence in frame with the green fluorescent protein (GFP) coding sequence. The PCR product was cloned to the pEGFP-N1 vector using the EcoRI and BamH1 restriction sites. The TGR5-GFP recombinant clone was confirmed by sequencing.
Apical sodium-dependent bile acid transporter small interfering RNA transfection.
H69 cells at 70–80% confluence were transfected with apical sodium-dependent bile acid transporter (ASBT) small interfering (si)RNA (sc-141294, Santa Cruz Biotechnology, Santa Cruz, CA) or the corresponding scrambled siRNA control (sc-36869, Santa Cruz Biotechnology) using siPORT Lipid Transfection Agent (Ambion, Austin, TX) according to the manufacturer's instruction. The transfection required the use of Opti-MEM1 medium, which stimulates rapid (i.e., in 48 h) growth of primary cilia in cultured cells (31). In our experiments, H69 cells were treated with 5 nmol ASBT siRNA or the corresponding scrambled siRNA control for 72 h. Thus, using this protocol, we generated ciliated ASBT siRNA-transfected H69 cells. The transfection efficacy was determined by fluorescent microscopy using control siRNA (FITC conjugate)-A (sc-36869, Santa Cruz Bioltechnology) and approached 80% at 72 h. The expression of ASBT protein in ASBT siRNA-transfected H69 cells was detected by Western blot analysis with actin as a loading control.
Immunofluorescence confocal microscopy.
Livers from normal rats (Harlan Sprague Dawley) and mice (C57BL/6, our own colony) were fixed and embedded in paraffin. Normal human liver tissues were obtained from the Mayo Clinical Core and National Disease Research Interchange. H69 cells, H69 cells transfected with the TGR5-GFP construct, and paraffin-embedded sections of rat, mouse, and human livers were incubated with the following antibodies: acetylated α-tubulin, a ciliary marker (T6793, Sigma Chemical); lamin B2, a nuclear membrane marker (ab84366, Abcam, Cambridge, MA); TGR5 (sc-48687, Santa Cruz Biotechnology); Gαs (ab83735, Abcam); and Gαi (5290S, Cell Signaling Technology, Danvers, MA) and then incubated for 1 h at room temperature with fluorescent secondary antibodies (Molecular Probes, Eugene, OR). Nuclei were stained blue with Prolong Gold Antifade Reagent with 4′,6-diamidino-2-phenylindole (P36935, Invitrogen). The corresponding negative controls (i.e., no primary antibodies) were performed for all experiments. A Zeiss LSM-510 microscope (Carl Zeiss, Thornwood, NY) was used in this study.
Immunogold transmission electron microscopy.
Isolated rat IBDs and H69 cells grown to 7 days postconfluence on collagen-coated coverslips (BD Biosciences, Sparks, MD) were fixed in 4% paraformaldehyde and 0.2% glutaraldehyde for 1 h at room temperature. After fixation, samples were washed in 0.1 M phosphate buffer (PB; pH 7.2–7.4) and in PB containing 0.1% sodium borohydride (10 min, 4 times) to inactivate residual aldehyde groups. Samples were then washed four times in PB and treated for 5 min with PB containing 0.1% Triton X-100. After being washed four times in PB, samples were incubated for 60 min at 4°C in blocking solution, which was PBS containing 1% FCS. Samples were then incubated overnight at 4°C with TGR5 (ab72608, Abcam) and Gαi (5290S, Cell Signaling Technology) primary antibodies diluted 1:20 with PBS containing 2% FCS. After six washes with PBS containing 2% FCS, samples were incubated for 1 h at room temperature with a secondary goat anti-mouse antibody conjugated with ultrasmall gold (Electron Microscopy Sciences, diluted 1:100 with blocking solution). Samples were washed in PBS, postfixed with 2.5% glutaraldehyde in PB for 2 h, enhanced with silver enhancement mixture (R-Gent SE-EM) for 30 min, and the osmicated with 1% osmium tetroxide for 30 min. In the corresponding controls, the incubation of samples with primary antibodies was omitted. For transmission electron microscopy (TEM), samples were dehydrated, embedded in Spurrs resin, sectioned at 90 nm, and observed using a Joel 12 electron microscope (Joel USA, Peabody, MA).
Negative staining immunogold TEM of biliary exosomes.
Exosomes were isolated from rat bile and processed for negative staining immunogold TEM, as we have previously described in detail (26). The expression of the exosomal marker CD63 in biliary exosomes was detected with a corresponding primary antibody (sc-51662, Santa Cruz Biotechnology).
Immunogold scanning electron microscopy.
Isolated rat IBDs were immersed in 4% phosphate-buffered glutaraldehyde (pH 7.4) for 1 h, treated with 0.1% Triton X-100 for 5 min, and rinsed with PB three times. Samples were incubated overnight at 4°C with antibodies to TGR5 (sc-48687, Santa Cruz Biotechnology, dilution: 1:100) and then incubated for 2 h at room temperature with anti-rabbit IgG conjugated with 1.4-nm nanogold. Samples were fixed in 1% glutaraldehyde for 10 min, and gold enhancement was performed with a gold enhancement kit (Nanoprobes). Samples were dehydrated, critical point dried, and carbon coated. Images were generated by a Hitachi S-4700 microscope (Hitachi, Pleasanton, CA).
Western blot analysis.
Proteins were separated by 4–15% SDS-PAGE, transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA), and the probed with antibodies against phosphorylated (p-)ERK (612359, BD Biosciences, San Jose, CA), total ERK (ab36991, Abcam), TGR5 (sc-48687, Santa Cruz Biotechnology), CD63 (sc-51662, Santa Cruz Biotechnology), ASBT (sc-27493, Santa Cruz Biotechnology), Gαs (ab83735, Abcam), and Gαi (5290S, Cell Signaling Technology) overnight at 4°C. Actin staining was used for the normalization of protein loading. Membranes were washed and incubated for 1 h at room temperature with the corresponding horseradish peroxidase-conjugated (Invitrogen) or IRdye 680 or 800 (Odyssey) secondary antibodies. Bands were visualized with the ECL Plus Western Blotting Detection kit (BD Biosciences) or Odyssey Li-Cor Scanner (Li-Cor, Lincoln, NE).
Measurement of cAMP.
cAMP levels in cholangiocytes were measured by the Bridge-It cAMP designer cAMP assay (Mediomics, St. Louis, MO), as we have previously described (25), and expressed in picomoles of cAMP per microgram of protein (H69 cells) or in picomoles of cAMP per microgram of DNA (microdissected rat IBDs).
Cell proliferation assay.
H69 cells were grown at 37°C, 5% CO2, and 100% humidity. Twenty-four hours before the assay, cells were treated with TGR5 agonists (25 μM). The rate of cell proliferation was determined using a CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega). The relative rates of cholangiocyte proliferation are expressed as percentages of the proliferation of nontreated cholangiocytes (100%).
Statistical analysis.
GraphPadPrism software (version 4.0, San Diego, CA) was used for statistical analysis. The statistical significance between two groups was tested by a two-tailed Student's t-test. All values are expressed as means ± SE, and differences of P < 0.05 were considered significant.
RESULTS
TGR5 is localized to cholangiocyte cilia.
The localization of TGR5 to cholangiocyte cilia was demonstrated by several complementary experimental approaches. By immunofluorescence confocal microscopy of paraffin-embedded sections of rat, mouse, and human livers (Fig. 1A), cilia and ciliary-associated TGR5 were identified with antibodies to a ciliary marker, acetylated α-tubulin, and TGR5, respectively. The images shown in Fig. 1A show acetylated α-tubulin- and TGR5-positive cilia. The overlay images show colocalization of these two proteins, i.e., the localization of TGR5 to cholangiocyte cilia in rat, mouse, and human livers.
Fig. 1.
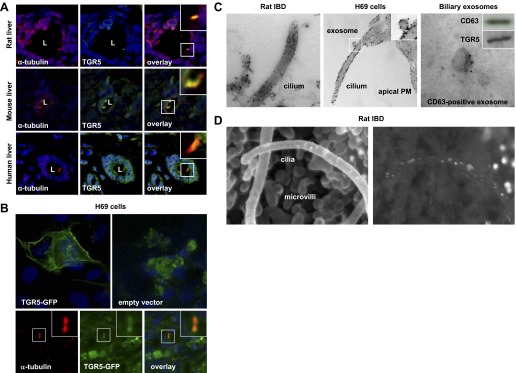
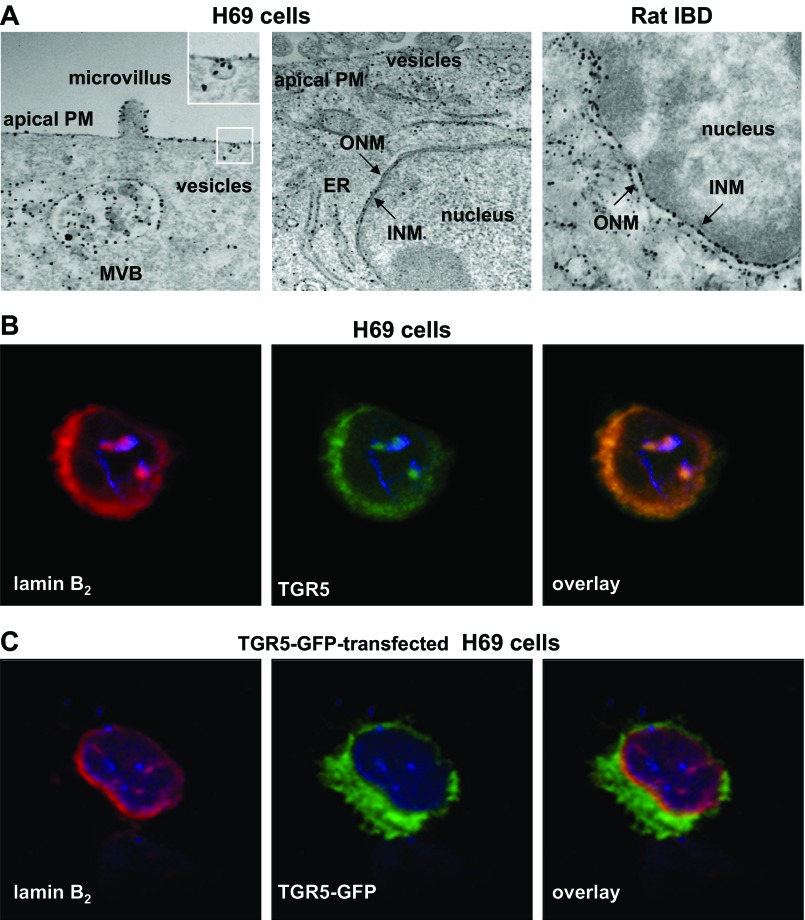
TGR5 is localized to cholangiocyte cilia. A: paraffin-embedded sections of rat, mouse, and human livers were stained with antibodies to the ciliary marker acetylated α-tubulin (red) and TGR5 (green). Cholangiocyte nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI; blue). The overlay images show colocalization of acetylated α-tubulin and TGR5 in yellow. L, lumen of the intrahepatic bile duct (IBD). B: H69 cells transiently transfected with a recombinant human TGR5-green fluorescent protein (GFP) construct showed localization of TGR5-GFP to the plasma membrane (PM) and primary cilium. In contrast, the GFP-empty vector (control) was distributed homogeneously in the cytoplasm of transfected H69 cells. Primary cilia were visualized with antibodies to acetylated α-tubulin (red) and colocalization with TGR5-GFP shown in yellow. Insets show higher-magnification images. C: immunogold tranmission electron microscopy showed localization of TGR5 on the ciliary membrane in rat IBD and H69 cells. A TGR5-positive exosome (inset) was attached to the ciliary membrane. Exosomes isolated from rat bile were positive for the exosomal marker CD63 and TGR5. D: conventional scanning electron microscopy image of an isolated split-open rat IBD showing primary cilia and microvilli extending from the cholangiocyte apical PM. The image generated by backscattered electrons showed numerous white dots, revealing the presence of TGR5 on cholangiocyte cilia and microvilli.
To generate additional evidence on the localization of TGR5 to cholangiocyte cilia, H69 cells were transiently transfected with a TGR5-GFP construct, and its presence in cilia was analyzed by confocal immunofluorescence microscopy (Fig. 1B). The ciliary expression of TGR5-GFP was determined by its colocalization with a ciliary marker, acetylated α-tubulin. A strong accumulation of TGR5-GFP was found on the cholangiocyte plasma membrane and cilia. In contrast, no specific subcellular distribution of GFP was found in H69 cells transfected with the empty vector.
The localization of TGR5 to cholangiocyte cilia was further confirmed by immunogold TEM (Fig. 1C) and scanning electron microscopy (SEM; Fig. 1D). By immunogold TEM, TGR5 was found on the ciliary membrane of freshly microdissected rat IBDs and cultured human cholangiocytes (H69 cells). The TGR5-positive vesicle shown in Fig. 1C (and also in the inset) attaching to the cholangiocyte cilium is likely an exosome. This interpretation is further supported by immunogold TEM and Western blot analysis results (Fig. 1C) demonstrating that exosomes isolated from rat bile contain both the exosomal marker CD63 and TGR5 and by our previous observation that exosomes interact with cholangiocyte cilia (26).
Finally, cilia extending from the cholangiocyte apical plasma membrane could be clearly seen by conventional SEM of the lumen of a microdissected split-open rat IBD (Fig. 1D). The image of the same field generated by backscattered electrons shows the gold-labeled secondary antibodies, which are shown on the ciliary membrane as bright white dots. The bright white dots can also be seen on the cholangiocyte microvilli, supporting our and others (18) observations that TGR5 is distributed on cilia and on the nonciliary portion of the cholangiocyte apical plasma membrane.
TGR5 is localized to diverse cholangiocyte compartments.
The localization of TGR5 was not limited to the apical surface of cholangiocytes. By immunogold TEM of both microdissected rat IBDs and ciliated H69 cells, TGR5 immunoreactivity was observed in the endoplasmic reticulum (ER), nucleus, multivesicular bodies (MVBs), and intracellular vesicles (Fig. 2A). The pattern of TGR5 labeling in rat and human (H69 cells) cholangiocytes was similar. Notably, TGR5 was present at high densities on the cholangiocyte nuclear membrane. As shown in the high-power photomicrographs of cholangiocyte nuclei (Fig. 2A), TGR5 immunoreactivity was associated with both outer and inner nuclear membranes (ONM and INM, respectively). Because the ONM is continuous with the ER, the observed TGR5 immunoreactivity may reflect both the ER and nuclear localization of TGR5. However, the TGR5 immunoreactivity on the cholangiocyte INM strongly implies the nuclear localization of this protein.
Fig. 2.
TGR5 is localized to diverse cholangiocyte compartments. A: TGR5 was localized on the apical PM, microvilli, multivesicular body (MVB), endoplasmic reticulum (ER), vesicles, and outer and inner nuclear membranes (ONM and INM, respectively) of H69 cells and microdissected rat IBDs. B: strong expression of the nuclear membrane marker lamin B2 (red) and TGR5 (green) was observed on the nuclear membrane of H69 cells, and their colocalization is shown in yellow. C: lamin B2 (red) was colocalized with TGR5-GFP in H69 cells transiently transfected with a TGR5-GFP construct (shown in yellow). The nuclei of H69 cells were stained with DAPI (blue).
The localization of TGR5 to the cholangiocyte INM was further confirmed by confocal immunofluorescence microscopy of H69 cells and H69 cells transiently transfected with a TGR5-GFP construct. To identify the expression of TGR5 on the nuclear membrane of H69 cells, cells were stained with antibodies to a marker of the INM, lamin B2, and TGR5. Figure 2B shows the strong expression of lamin B2 (red) and TGR5 (green) on the cholangiocyte nuclear membrane. The colocalization of these two proteins is shown in yellow. In H69 cells transiently transfected with a TGR5-GFP construct (Fig. 2C), TGR5-GFP was colocalized with lamin B2 on the cholangiocyte INM.
Taken together, these data demonstrate that TGR5 is localized to diverse cholangiocyte compartments, with its strongest expression on the apical, ciliary, and nuclear membranes.
TGR5 agonists decrease cAMP levels in ciliated cholangiocytes.
To address the role of ciliary TGR5 in bile acid signaling in cholangiocytes, we used H69 cells and compared the effects of TGR5 agonists on cAMP levels in cells with and without cilia (Fig. 3). H69 cells, when grown on the appropriate matrixes, form polarized monolayers and retain the phenotypic and functional characteristics of cholangiocytes in vivo (10). The formation of primary cilia in cholangiocytes depends on cell confluence and the duration of cell growth (12). H69 cells at day 0 after confluence are naturally occurring nonciliated cholangiocytes (Fig. 3A). H69 cells on day 7 after confluence possess well-developed primary cilia, i.e., are naturally occurring ciliated cholangiocytes (Fig. 3A).
Fig. 3.
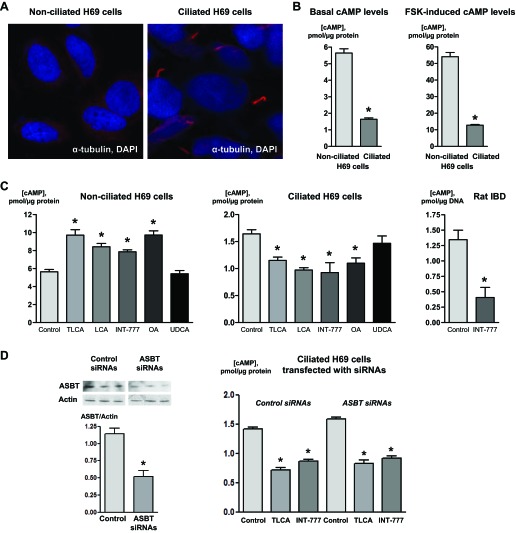
TGR5 agonists differentially affect cAMP levels in nonciliated and ciliated cholangiocytes. A: immunofluorescence confocal microscopy images showing nonciliated and ciliated H69 cells. Cells were stained with the ciliary marker acetylated α-tubulin (red). DAPI (blue) indicates nuclei. Multiple cilia were found in H69 cells cultured for 7 days after confluence (ciliated) but not in confluent H69 cells (day 0, nonciliated). B: the cAMP level was higher in nonciliated H69 cells compared with ciliated cells under basal and forskolin (FSK; 10 μmol)-induced conditions. C: in response to taurolithocholic acid (TLCA), lithocholic acid (LCA), INT-777, and oleanolic acid (OA) (all 25 μmol), cAMP levels were increased in nonciliated cells but decreased in ciliated H69 cells. Ursodeoxycholic acid (UDCA; 25 μmol), which does not have affinity for TGR5 (negative control), did not affect cAMP levels in both nonciliated and ciliated cholangiocytes. Also, INT-777 (25 μmol) inhibited cAMP levels in ciliated cholangiocytes of rat IBDs microperfused through their lumen. n = 12 wells from 3 separate experiments; n = 7 bile ducts isolated from 3 rats. *P < 0.05. D: representative Western blots (selected portions of the original blots are shown) and quantitative data demonstrating suppression of apical sodium-dependent bile acid transporter (ASBT) expression in H69 cells transfected with ASBT small interfering (si)RNAs. Suppression of ASBT expression in H69 cells did not abolish the inhibitory effects of the TGR5 agonists TLCA and INT-777 (both 25 μmol) on cAMP levels. n = 6 wells from 3 separate experiments. *P < 0.05.
Nonciliated and ciliated H69 cells are different with regard to their cAMP levels. In particular, basal and FSK-induced cAMP levels in ciliated H69 cells were 3.4 and 4.2 times lower compared with nonciliated cells (i.e., 1.64 ± 0.08 vs. 5.65 ± 0.25 pmol/μg protein and 12.76 ± 0.44 vs. 54.08 ± 2.54 pmol/μg protein, respectively; Fig. 3B). In response to the two most potent natural TGR5 agonists, TLCA and LCA, and two selective TGR5 agonists, INT-777 and OA, cAMP levels decreased in ciliated H69 cells by 29.9 ± 6.0%, 40.8 ± 8.7%, 43.6 ± 10.2%, and 33.0 ± 8.2%, respectively (Fig. 3C), whereas treatment of nonciliated H69 cells with TGR5 agonists under similar experimental conditions resulted in an increase of cAMP levels by 63.8 ± 10.7%, 49.2 ± 7.9%, 39.3 ± 5.9%, and 72.2 ± 8.7%, respectively (Fig. 3C). UDCA (a negative control), as expected, did not affect cAMP levels in nonciliated and ciliated cholangiocytes (Fig. 3C). In all experiments, the effects of FSK, which activates selective adenylyl cyclases via a TGR5-independent mechanism (a positive control), did not depend on the ciliary status of H69 cells and resulted in similar (i.e., by 9.6- and 7.8-fold) increases in cAMP levels in nonciliated and ciliated cholangiocytes, respectively (Fig. 3B). Taken together, these data demonstrate that in ciliated cholangiocytes, TGR5 agonists inhibit cAMP signaling.
Consistent with our results in cultured human cholangiocytes, an inhibitory effect of the TGR5 selective agonist INT-777 was also observed in isolated rat IBDs, which are lined by ciliated cholangiocytes. The cAMP level in rat IBDs in response to INT-777 was decreased by 69.7 ± 31.1% (Fig. 3C).
Taking into account the multiple intracellular locations of TGR5 that could be activated by TGR5 agonists delivered into the cell via the bile acid transporter ASBT, rather than at the ciliary membrane, we tested the effects of two TGR5 agonists, TLCA and INT-777, on cAMP levels in ciliated H69 cells in which ASBT expression was suppressed by specific siRNAs. ASBT siRNAs inhibited the expression of ASBT protein in ciliated H69 cells by 54.5 ± 7.9% (Fig. 3D), but this decrease in ASBT expression did not affect the inhibitory action of TGR5 agonists on cAMP levels. Similar to previously observed effects, TLCA and INT-777 inhibited cAMP levels in ciliated H69 cells transfected with ASBT siRNAs by 41.4 ± 4.2% and 41.9 ± 2.5%, respectively, which were not significantly different (i.e., inhibition by 49.1 ± 2.8% and 38.9 ± 1.7%, respectively) from H69 cells transfected with ASBT scrambled control siRNAs.
In conclusion, the effects of TGR5 agonists on cAMP levels in cholangiocytes occur at the apical membrane and depend on the presence of cilia. In ciliated cholangiocytes, TGR5 agonists induce a decrease in cAMP levels, whereas they provoke an opposite effect, i.e., stimulate cAMP production, in nonciliated cholangiocytes.
TGR5 agonists differentially affect ERK1/2 in nonciliated and ciliated cholangiocytes.
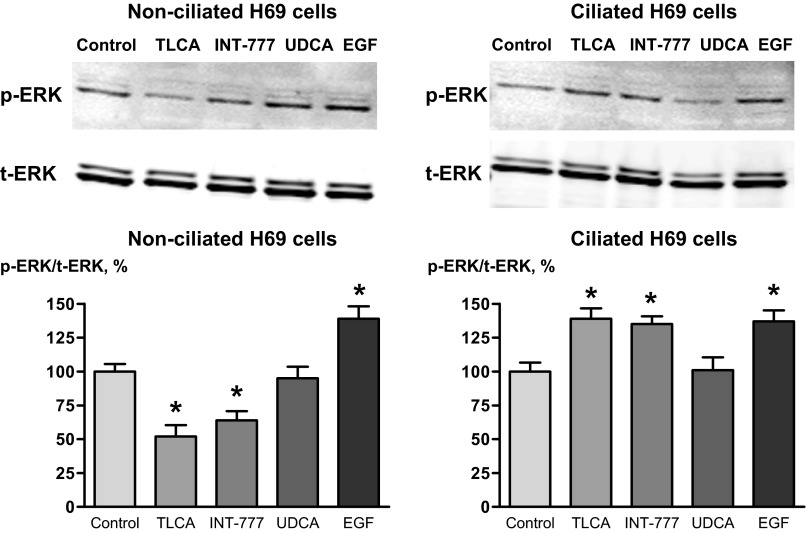
Because signal transduction by G protein-coupled receptors involves the activation of ERK, we studied whether TGR5 agonists affect ERK signaling in nonciliated and ciliated cholangiocytes and how. We found that the effects of TGR5 agonists on ERK1/2 in nonciliated and ciliated cholangiocytes were different and opposite to their effects on cAMP levels. A natural TGR5 agonist, TLCA, and a selective TGR5 agonist, INT-777, decreased the p-ERK-to-total ERK ratio in nonciliated H69 cells by 48.2 ± 8.4% and 36.6 ± 6.1%, respectively, but increased this ratio in ciliated H69 cells by 39.2 ± 7.1% and 35.7 ± 6.3% (Fig. 4). The effects of UDCA and EGF, which were used as negative and positive controls, respectively, did not depend on the ciliation status of cholangiocytes. UDCA did not affect ERK in both nonciliated and ciliated cholangiocytes, whereas EGF increased the p-ERK-to-total ERK ratio in both nonciliated and ciliated cells by 39.5 ± 9.0% and 37.7 ± 8.1%, respectively (Fig. 4). Thus, these data demonstrate that the cholangiocyte ERK signaling response to TGR5 agonists also depends on cilia.
Fig. 4.
TGR5 agonists differentially affect ERK signaling in nonciliated and ciliated cholangiocytes. The TGR5 agonists TLCA and INT-777 (both 25 μmol) decreased the ratio of phosphorylated ERK to total ERK (p-ERK/t-ERK ratio) in nonciliated H69 cells but increased this ratio in ciliated cholangiocytes. UDCA (25 μmol, negative control) did not affect ERK signaling in both nonciliated and ciliated cholangiocytes, whereas EGF (20 ng/ml, positive control) increased the p-ERK/t-ERK ratio in both cell types. Representative Western blots and quantitative data from 3 separate experiments are shown. *P < 0.05.
The effects of TGR5 agonists on cholangiocyte proliferation depend on the presence of cilia.
To determine whether ciliary-associated TGR5 contributes to a bile acid-regulated functional cellular response, we tested the effects of TGR5 agonists on the proliferation of nonciliated and ciliated H69 cells. UDCA and EGF were used as negative and positive controls, respectively.
The data shown in Fig. 5 demonstrate that all three TGR5 agonists, TLCA, INT-777, and OA, increased the proliferation of nonciliated H69 cells by 38.0 ± 7.3%, 31.4 ± 4.5%, and 25.8 ± 5.2%, respectively. The proliferative effects of TGR5 agonists were comparable with the effect of EGF (positive control), which increased the proliferation of nonciliated H69 cells by 45.3 ± 4.7%. UDCA did not affect cholangiocyte proliferation.
Fig. 5.
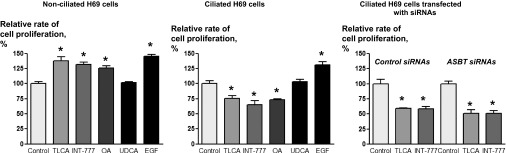
TGR5 agonists differentially affect the proliferation of nonciliated and ciliated H69 cells. The TGR5 agonists TLCA, INT-777, and OA (all 25 μmol) increased the proliferation of nonciliated H69 cells but decreased the proliferation of ciliated cells. UDCA (25 μmol, negative control) did not affect the proliferation of nonciliated or ciliated cholangiocytes. In contrast, EGF (20 ng/ml, positive control) increased the proliferation of both cell types. Suppression of ASBT expression in H69 cells did not abolish the inhibitory effects of the TGR5 agonists TLCA and INT-777 (both 25 μmol) on cell proliferation. n = 16 wells from 3 separate experiments. *P < 0.05.
The proliferative response of ciliated H69 cells to TGR5 agonists was opposite: all three TGR5 agonists inhibited cholangiocyte proliferation by 24.6.0 ± 6.5%, 35.0 ± 7.7%, and 27.0 ± 6.7%, respectively. UDCA did not affect cell proliferation, whereas EGF (positive control) increased the proliferation of ciliated H69 cells by 31.3.3 ± 6.8% (Fig. 5).
Given the importance of the bile acid transporter ASBT in cholangiocyte proliferation (40), we tested the effects of two TGR5 agonists, TLCA and INT-777, on the proliferation of ciliated H69 cells in which ASBT expression was suppressed by specific siRNAs. Our data (Fig. 5) show that ASBT suppression did not affect the inhibitory effects of TGR5 agonists on cholangiocyte proliferation. TLCA and INT-777 inhibited the proliferation of ciliated H69 cells transfected with ASBT siRNAs by 48.5 ± 5.7% and 49.2 ± 4.8%, respectively. Similar effects of TGR5 agonists (i.e., inhibition by 40.9 ± 1.1% and 41.6 ± 3.6%, respectively) were observed in H69 cells transfected with ASBT scrambled control siRNAs.
These data show that the proliferative response of H69 cells to TGR5 agonists depends on the presence of cilia.
Differential effects of TGR5 agonists in ciliated and nonciliated cholangiocytes are associated with the coupling of TGR5 to different Gα proteins.
One of the potential mechanisms through which TGR5 agonists may induce differential functional responses of ciliated and nonciliated cholangiocytes is associated with the coupling of TGR5 to different Gα proteins. To test if the inhibitory effects of TGR5 agonists on cAMP levels in ciliated cholangiocytes are associated with Gαi, whereas their stimulatory effects in nonciliated cells are associated with Gαs, we studied the expression of Gαi and Gαs proteins on cholangiocyte cilia and their colocalization with TGR5 in nonciliated and ciliated H69 cells under basal conditions and in response to a specific TGR5 agonist, INT-777.
The images shown in Fig. 6A demonstrate that Gαi but not Gαs protein is localized to primary cilia in H69 cells. In these images, Gαi localization appeared to be restricted to the base of cilia but not over the entire length of the ciliary shaft. A characteristic localization of Gαi protein to the base of cholangiocyte cilia was further confirmed on freshly isolated rat IBDs by immunogold TEM (Fig. 6B).
Fig. 6.
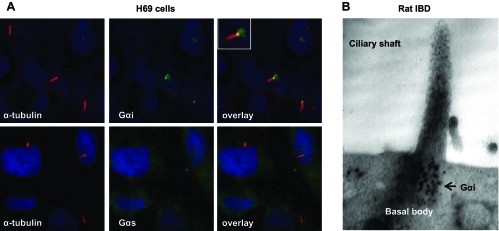
Gαi protein is expressed on cholangiocyte cilia. A: expression of Gαi protein and its localization at the base of cilia in H69 cells detected by immunofluorescence confocal microscopy. B: expression and localization of Gαi protein at the base of cholangiocyte cilia detected by immunogold tranmission electron microscopy in isolated rat IBDs. Numerous black dots reveal the expression of Gαi in the basal body.
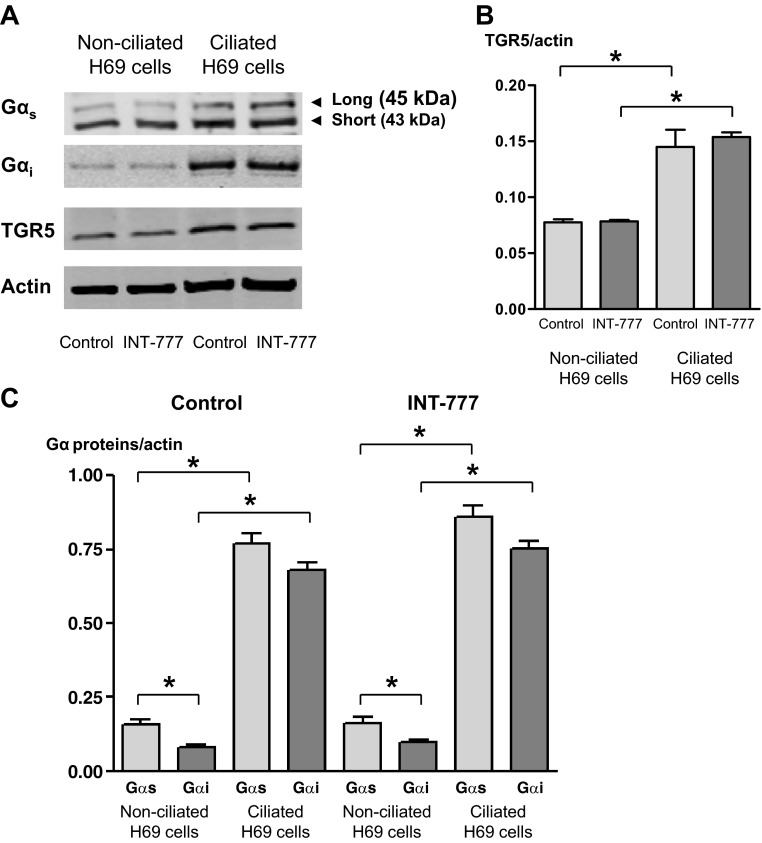
As demonstrated by Western blot analysis (Fig. 7) and confocal immunofluorescence microscopy (Fig. 8), both Gαs and Gαi proteins were expressed in nonciliated and ciliated cholangiocytes; however, in nonciliated cells, the amount of Gαs protein normalized to actin (i.e., 0.16 ± 0.02) was two times higher than the amount of Gαi protein (i.e., 0.08 ± 0.01). Compared with nonciliated H69 cells, ciliated cholangiocytes expressed larger amount of both Gαs and Gαi proteins (the amount of Gαs and Gαi proteins was increased by 4.8- and 8.5-fold, respectively; Fig. 7, A and C). The amount of TGR5 was also increased in ciliated cholangiocytes compared with nonciliated cells by twofold, i.e., less dramatically than the amount of Gαs or Gαi proteins (Fig. 7B). Stimulation of nonciliated and ciliated H69 cells with INT-777 did not affect the amount of both Gαs and Gαi proteins as well as TGR5 (Fig. 7) but induced changes in their colocalization (Fig. 8). In nonciliated cholangiocytes, INT-777 increased colocalization of TGR5 with Gαs protein but not with Gαi protein, whereas in ciliated cells the effect of INT-777 was opposite: TGR5 was primarily colocalized with Gαi protein but not with Gαs protein. These data support our hypothesis that differential effects of TGR5 agonists in ciliated and nonciliated cholangiocytes are associated with the coupling of TGR5 to Gαi and Gαs proteins, respectively.
Fig. 7.
Gαs and Gαi proteins are differentially expressed in nonciliated and ciliated H69 cells. Representative Western blots of Gα proteins and TGR5 (A) and quantitative data (B and C) are shown. Actin was used as a loading control. Gαs was present in H69 cells in two isoforms: long (45 kDa) and short (43 kDa) (A). The amount of TGR5 (B) and Gα proteins (C) increased in ciliated cells compared with nonciliated cells. The amount of Gαs in nonciliated cholangiocytes was larger than the amount of Gαi (C). The TGR5-specific agonist INT-777 (25 μmol) did not affect the amount of TGR5 (B) and Gα proteins (C) in nonciliated and ciliated cholangiocytes.
Fig. 8.
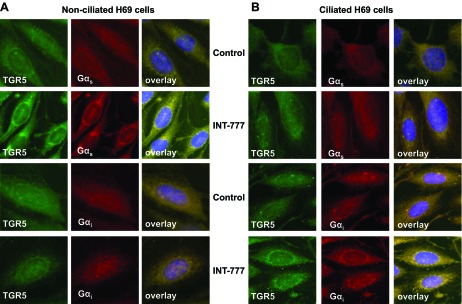
Gαs and Gαi proteins are differentially involved in the effects of the TGR5 agonist INT-777 in nonciliated and ciliated H69 cells. A: in nonciliated H69 cells, INT-777 increased the colocalization of TGR5 with Gαs protein but not Gαi protein. B: in ciliated cholangiocytes, the effect of INT-777 was opposite: colocalization of TGR5 increased with Gαi protein but not with Gαs protein.
DISCUSSION
The key findings of this study relate to the localization and function of the G protein-coupled bile acid receptor TGR5 in cholangiocytes. We demonstrated that 1) TGR5 is localized to diverse cholangiocyte compartments, with its strongest expression on the cholangiocyte apical plasma, ciliary, and nuclear membranes; 2) activation of TGR5 in ciliated and nonciliated cholangiocytes resulted in opposite changes in cAMP levels (i.e., in a decrease and an increase, respectively), demonstrating that ciliary TGR5 is associated with inhibition of cAMP signaling; 3) TGR5 is linked to ERK signaling, which is activated in ciliated cholangiocytes but inhibited in nonciliated cholangiocytes; 4) activation of TGR5 promotes the proliferation of nonciliated cholangiocytes but inhibits the proliferation of ciliated cholangiocytes; and 5) the differential effects of TGR5 agonists in ciliated and nonciliated cholangiocytes are associated with the differential coupling of TGR5 to Gαi and Gαs proteins, respectively.
TGR5 is recognized as a novel bile acid receptor that is expressed in different cells exposed to bile acids. The highest levels of TGR5 expression have been detected in the gallbladder (39) and in the intestine, primarily in the ileum and colon (22, 23, 39). TGR5 is also expressed in human and rodent cholangiocytes (16, 18, 20). The initial observations on TGR5 suggested that in different cell types, this bile acid receptor is predominantly localized in a subapical compartment and, to a smaller extent, on the plasma membrane (14, 16). The intracellular localization of TGR5 was proposed to be a result of its internalization into the cytoplasm in response to bile acids, whereas its expression on the plasma membrane links the signaling properties of bile acids to the intracellular cAMP signaling pathway (14).
Our study demonstrates that the cellular localization of TGR5 in human and rat cholangiocytes is complex. We found that in addition to its localization to the plasma and ciliary membranes, TGR5 is also expressed on the cholangiocyte ER, in MVBs and exosomes, and on the nuclear membrane. The presence of TGR5 on the cholangiocyte ER may simply reflect its synthesis in this organelle. However, based on the observations that bile acids can affect the ER via organelle-associated signaling mechanisms (3, 8), we cannot exclude that TGR5 in the cholangiocyte ER is functional. The expression of TGR5 in MVBs and intracellular vesicles suggests that TGR5 may undergo internalization, recycling, and release from the cell as a component of exosomes, which are small extracellular vesicles containing numerous biologically active molecules (26). However, the functional significance of exosomal TGR5 remains unclear. The expression of TGR5 on the cholangiocyte nuclear membrane may provide a novel mechanism for intranuclear bile acid signaling. The expression and function of a number of the G protein-coupled receptors on the nuclear membrane in different cell types are known physiologic phenomena (21, 37), and these phenomena could be extended to TGR5 localized to the cholangiocyte nuclear membrane.
We hypothesized in this study that the localization of TGR5 in different cellular compartments may provide mechanisms for subcellular, site-specific bile acid signaling. As an initial attempt to test this hypothesis, we addressed the functional significance of TGR5 localized to the cholangiocyte ciliary membrane. We found that cAMP levels in ciliated cholangiocytes differ from cAMP levels in nonciliated cholangiocytes, being higher in nonciliated H69 cells compared with ciliated cells. These data are in agreement with recently published observations showing that nonciliated and ciliated renal epithelial cells differ with regard to cAMP levels and that primary cilia in renal epithelial cells negatively control intracellular cAMP signaling (5). Our present and previous work on sensory functions of cholangiocyte cilia (25, 27, 28) also suggest that these organelles are negatively linked to cAMP signaling. Activation of ciliary TGR5 by bile acids and selective agonists inhibits cAMP signaling in cholangiocytes similarly to other extracellular stimuli, such as bile flow and biliary nucleotides, which act via ciliary polycystin-1/polycystin-2 and P2Y12/adenylate cyclase 6 complexes (25, 28). Thus, there appears to be a consistent inhibitory ciliary-associated signaling pathway, at least as it relates to the cAMP response.
Our observations showing that in nonciliated H69 cells TGR5 agonists increase cAMP levels are in agreement with previously observed TGR5-mediated cAMP increases in different cell types in culture since in all of these reports, cells were cultured no longer than 24 h (i.e., not grown long enough to develop cilia). Thus, an increase in cAMP levels in response to TGR5 agonists in such cells should be considered as a functional response of naturally occurring nonciliated cells to TGR5 agonists.
TGR5 agonists also affect the ERK signaling pathway in cholangiocytes. In general, the linkage of TGR5 to ERK signaling is not yet well established. It has been mentioned that activation of TGR5 in TGR5-transfected Chinese hamster ovary (CHO) cells resulted in changes in ERK1/2 (14) and that in gastric carcinoma cells TGR5 is involved in the activation of ERK1/2 by deoxycholic acid (42). The precise mechanisms of TGR5-mediated regulation of ERK signaling remain unknown. It has been shown that cAMP is involved in both activation and inhibition of ERK signaling depending on the functional state of the cell (6, 36). For example, cAMP-induced inactivation of ERK was observed in NIH-3T3-A14 cells, whereas in CHO and OVCAR3 cells, ERK was activated by cAMP (6). Also, inhibition of cAMP production and activation of ERK in response to progestin hormones were observed in MDA-MB-231 cells (43). In our experiments, an increase in cAMP levels in response to TGR5 agonists resulted in inhibition of ERK signaling and vice versa, suggesting a potential TGR5-mediated cross talk between cAMP and ERK signaling pathways. The changes in the p-ERK-to-total ERK ratio in response to TGR5 agonists are presumably secondary to changes in cAMP levels.
Cholangiocytes are epithelial cells with a great capacity to proliferate under different experimental conditions and in the course of chronic liver diseases, including cholangiociliopathies, a group of diseases that are caused by abnormalities in the structure and function of cholangiocyte cilia (29). We (29) have previously reported that cholangiocyte proliferation increases when cilia are absent or structurally or functionally abnormal. This observation is in agreement with the existing concept that primary cilia are involved in the inhibition of cell proliferation in a healthy adult organism (2). Our data demonstrating that activation of ciliary-associated TGR5 triggers inhibition of cholangiocyte proliferation further support this concept. In contrast, nonciliated cholangiocytes respond to TGR5 agonists by an increase in proliferation. The involvement of TGR5 in bile acid-induced proliferation of nonciliated cells has been recently observed in human Barrett's adenocarcinoma cell line FLO (11) and cholangiocytes isolated from wild-type and TGR5 knockout mice (18). It is likely that nonciliary TGR5 transduces bile acid signaling via mechanisms that are distinct from the ciliary-associated TGR5-mediated mechanisms controlling cholangiocyte proliferation.
As for any G protein-coupled receptor, coupling to Gα proteins is a major mechanism in TGR5-mediated bile acid signaling. TGR5 is primarily coupled to Gαs protein; however, its coupling to Gαi protein has also been reported (4, 11). By studying the relevance of Gαs and Gαi proteins in TGR5-mediated bile acid signaling in nonciliated and ciliated cholangiocytes, we found that Gαs protein is likely responsible for the TGR5-mediated effects of bile acids in nonciliated cholangiocytes, whereas Gαi protein is involved in mechanisms of TGR5-mediated bile acid signaling in ciliated cells. The expression of Gαs and Gαi proteins was not changed in both nonciliated and ciliated cholangiocytes in response to a TGR5 agonist, but the colocalization and redistribution of Gα proteins and TGR5 were specifically and differentially affected in cells with and without cilia. However, the cellular and molecular mechanisms that determine the coupling of TGR5 to different Gα proteins in nonciliated and ciliated cells remain to be elucidated.
In the present study, we showed that in cholangiocytes, Gαi is localized at the base of cilia. Several published observations support our findings. For example, Gαi2, a member of the Gαi family, is localized to motile cilia in the rat ependyma, oviduct, and trachea (35) and in the human endometrium and fallopian tubes (30). Gαi1, Gαi2, and Gαi3 are distributed between the base and shaft of the primary cilium when expressed in cultured cerebellar granular neuronal precursors (1).
The primary cilium is currently recognized as a dynamic organelle consisting of several morphological and functional compartments. The proximal segment of the primary cilium and the ciliary base are considered as a continuous functional compartment where different signaling molecules cycle and interact with each other in a dynamic manner (1, 34). Thus, the localization of TGR5 and Gαi protein to cholangiocyte cilia suggests that these organelles function as a main integration center that determines cholangiocyte functional responses to bile acid signaling.
Taking into account the increasing evidence for a role of TGR5 in liver diseases (17), the observed differential functional responses of nonciliated and ciliated cholangiocytes to TGR5-mediated bile acid signaling may have important pathophysiological significance. Our preliminary data (unpublished observations) suggest that in cilia-related liver disorders such as autosomal dominant and autosomal recessive polycystic kidney diseases, TGR5 is overexpressed in cholangiocytes but not localized on cilia. On the other hand, autosomal dominant and autosomal recessive polycystic kidney disease-affected cholangiocytes are characterized by malformed cilia and increased levels of cAMP (27), suggesting that they have lost ciliary-associated mechanisms negatively controlling cAMP levels. This may contribute to a cAMP increase in cystic cholangiocytes and, thus, reflect the potential involvement of TGR5 in cholangiociliopathies.
In a working model (Fig. 9), we summarized the results of this study, indicating that the subcellular localization of TGR5 determines cholangiocyte functional responses to bile acid signaling.
Fig. 9.
Working model of TGR-mediated bile acid signaling in nonciliated and ciliated cholangiocytes. The localization of TGR5 on the PM or ciliary membrane determines the cholangiocyte functional response to bile acid (BA) signaling. In nonciliated cholangiocytes, TGR5 agonists increase cAMP levels and cell proliferation but inhibit ERK signaling. In ciliated cholangiocytes, the effects of TGR5 agonists are opposite, i.e., cAMP levels and cell proliferation decreased, but ERK signaling is activated.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK-24031 (to N. F. LaRusso), by the Optical Microscopy Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (through NIDDK Grant P30-DK-084567), and by the Mayo Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.I.M. and N.F.L. conception and design of research; A.I.M., B.Q.H., B.N.R., G.B.G., P.L.S., T.V.M., and S.A.G. performed experiments; A.I.M., B.N.R., T.V.M., and S.A.G. analyzed data; A.I.M., B.Q.H., and T.V.M. interpreted results of experiments; A.I.M. and B.Q.H. prepared figures; A.I.M. drafted manuscript; A.I.M. and N.F.L. edited and revised manuscript; A.I.M. and N.F.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Mark Pruzanski and Dr. Luciano Adorini (Intercept Pharmaceuticals, New York, NY, and Perugia, Italy) for providing the selective TGR5 agonist INT-777.
REFERENCES
- 1. Barzi M, Kostrz D, Menendez A, Pons S. Sonic hedgehog-induced proliferation requires specific Gα inhibitory proteins. J Biol Chem 286: 8067–8074, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell PD, Fitzgibbon W, Sas K, Stenbit AE, Amria M, Houston A, Reichert R, Gilley S, Siegal GP, Bissler J, Bilgen M, Chou PC, Guay-Woodford L, Yoder B, Haycraft CJ, Siroky B. Loss of primary cilia upregulates renal hypertrophic signaling and promotes cystogenesis. J Am Soc Nephrol 22: 839–848, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Byrne AM, Foran E, Sharma R, Davies A, Mahon C, O'Sullivan J, O'Donoghue D, Kelleher D, Long A. Bile acids modulate the Golgi membrane fission process via a protein kinase Cη and protein kinase D-dependent pathway in colonic epithelial cells. Carcinogenesis 31: 737–744, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Cao W, Tian W, Hong J, Li D, Tavares R, Noble L, Moss SF, Resnick MB. Expression of bile acid receptor TGR5 in gastric adenocarcinoma. Am J Physiol Gastrointest Liver Physiol 304: G322–G327, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi YH, Suzuki A, Hajarnis S, Ma Z, Chapin HC, Caplan MJ, Pontoglio M, Somlo S, Igarashi P. Polycystin-2 and phosphodiesterase 4C are components of a ciliary A-kinase anchoring protein complex that is disrupted in cystic kidney diseases. Proc Natl Acad Sci USA 108: 10679–10684, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4: 901–906, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Fickert P, Fuchsbichler A, Moustafa T, Wagner M, Zollner G, Halilbasic E, Stoger U, Arrese M, Pizarro M, Solis N, Carrasco G, Caligiuri A, Sombetzki M, Reisinger E, Tsybrovskyy O, Zatloukal K, Denk H, Jaeschke H, Pinzani M, Trauner M. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am J Pathol 175: 2392–2405, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerasimenko JV, Flowerdew SE, Voronina SG, Sukhomlin TK, Tepikin AV, Petersen OH, Gerasimenko OV. Bile acids induce Ca2+ release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors. J Biol Chem 281: 40154–40163, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, Larusso NF. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci USA 104: 19138–19143, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, Mulberg AE, Cherington V, Jefferson DM. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol Gastrointest Liver Physiol 266: G1060–G1070, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Hong J, Behar J, Wands J, Resnick M, Wang LJ, DeLellis RA, Lambeth D, Souza RF, Spechler SJ, Cao W. Role of a novel bile acid receptor TGR5 in the development of oesophageal adenocarcinoma. Gut 59: 170–180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang BQ, Masyuk TV, Muff MA, Tietz PS, Masyuk AI, Larusso NF. Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol 291: G500–G509, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res 50: 1509–1520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem 278: 9435–9440, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Keitel V, Cupisti K, Ullmer C, Knoefel WT, Kubitz R, Haussinger D. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology 50: 861–870, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun 372: 78–84, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Keitel V, Haussinger D. Perspective: TGR5 (Gpbar-1) in liver physiology and disease. Clin Res Hepatol Gastroenterol 36: 412–419, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Keitel V, Haussinger D. TGR5 in the biliary tree. Dig Dis 29: 45–47, 2011 [DOI] [PubMed] [Google Scholar]
- 19. Keitel V, Reinehr R, Gatsios P, Rupprecht C, Gorg B, Selbach O, Haussinger D, Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology 45: 695–704, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Keitel V, Ullmer C, Haussinger D. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem 391: 785–789, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Kumar V, Fahey PG, Jong YJ, Ramanan N, O'Malley KL. Activation of intracellular metabotropic glutamate receptor 5 in striatal neurons leads to up-regulation of genes associated with sustained synaptic transmission including Arc/Arg3.1 protein. J Biol Chem 287: 5412–5425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 298: 714–719, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Maruyama T, Tanaka K, Suzuki J, Miyoshi H, Harada N, Nakamura T, Miyamoto Y, Kanatani A, Tamai Y. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol 191: 197–205, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Masyuk AI, Gong AY, Kip S, Burke MJ, LaRusso NF. Perfused rat intrahepatic bile ducts secrete and absorb water, solute, and ions. Gastroenterology 119: 1672–1680, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, Stroope AJ, Larusso NF. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol 295: G725–G734, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV, Radtke B, Splinter PL, LaRusso NF. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol 299: G990–G999, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masyuk AI, Masyuk TV, LaRusso NF. Cholangiocyte primary cilia in liver health and disease. Dev Dyn 237: 2007–2012, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131: 911–920, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Masyuk T, Masyuk A, LaRusso N. Cholangiociliopathies: genetics, molecular mechanisms and potential therapies. Curr Opin Gastroenterol 25: 265–271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Monkkonen KS, Aflatoonian R, Lee KF, Yeung WS, Tsao SW, Laitinen JT, Tuckerman EM, Li TC, Fazeli A. Localization and variable expression of Gαi2 in human endometrium and fallopian tubes. Hum Reprod 22: 1224–1230, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Plotnikova OV, Pugacheva EN, Golemis EA. Primary cilia and the cell cycle. Methods Cell Biol 94: 137–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol 54: 1263–1272, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, Mioskowski C, Auwerx J, Saladin R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun 362: 793–798, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Shiba D, Yamaoka Y, Hagiwara H, Takamatsu T, Hamada H, Yokoyama T. Localization of Inv in a distinctive intraciliary compartment requires the C-terminal ninein-homolog-containing region. J Cell Sci 122: 44–54, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Shinohara H, Asano T, Kato K, Kameshima T, Semba R. Localization of a G protein Gi2 in the cilia of rat ependyma, oviduct and trachea. Eur J Neurosci 10: 699–707, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Soltoff SP, Hedden L. Isoproterenol and cAMP block ERK phosphorylation and enhance [Ca2+]i increases and oxygen consumption by muscarinic receptor stimulation in rat parotid and submandibular acinar cells. J Biol Chem 285: 13337–13348, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tadevosyan A, Vaniotis G, Allen BG, Hebert TE, Nattel S. G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. J Physiol 590: 1313–1330, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vassileva G, Golovko A, Markowitz L, Abbondanzo SJ, Zeng M, Yang S, Hoos L, Tetzloff G, Levitan D, Murgolo NJ, Keane K, Davis HR, Jr, Hedrick J, Gustafson EL. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J 398: 423–430, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xia X, Francis H, Glaser S, Alpini G, LeSage G. Bile acid interactions with cholangiocytes. World J Gastroenterol 12: 3553–3563, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xia X, Jung D, Webb P, Zhang A, Zhang B, Li L, Ayers SD, Gabbi C, Ueno Y, Gustafsson JA, Alpini G, Moore DD, Lesage GD. Liver X receptor β and peroxisome proliferator-activated receptor delta regulate cholesterol transport in murine cholangiocytes. Hepatology 56: 2288–2296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yasuda H, Hirata S, Inoue K, Mashima H, Ohnishi H, Yoshiba M. Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem Biophys Res Commun 354: 154–159, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100: 2231–2236, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]