Abstract
The aim of this study was to determine the extent to which gastric layering and retention of a meal could be used to reduce appetite using the same caloric load. Liquid (control) and semi-solid (active) meals were produced with the same protein, fat, carbohydrate, and mass. These were fed to 10 volunteers on separate days in a crossover study, and subjective appetite ratings, gastric contents, and plasma cholecystokinin (CCK) were assessed over a period of 3 h. The active meal showed food boluses in the stomach persisting for ∼45 min, slower emptying rates, and lower plasma CCK levels over the first hour. After the first hour, both gastric emptying rates and plasma CCK levels were similar for both systems and slightly increased compared with the unfed situation. Despite the lower plasma CCK levels for the active meal over the first hour, this meal reduced appetite more than the control meal over the 3 h of the study. For a moderately increased plasma CCK level in the fed state, appetite was correlated with the volume of gastric contents rather than gastric emptying rates or plasma CCK. This suggests that enhanced gastric retention was the key factor in decreasing appetite and was probably mediated by a combination of intestinal nutrient sensing and increased viscosity in the stomach.
Keywords: appetite, dairy food structure, gastric retention, cholecystokinin
over the last 30 years, there has been a steady rise in obesity and related health issues, such as diabetes. Type 2 diabetes and obesity are now major public health problems, with over 50% of the adult population being overweight and 1 in 20 having diabetes in the UK (2). Type 2 diabetes is associated with weight gain, and both diabetes and weight gain are associated with aging. A range of approaches have been used to address the increase in obesity based around dieting, but the continued increase in the prevalence of obesity suggests that a greater understanding of the mechanisms involved in controlling appetite and satiety is needed (33). Nutrition studies have started to look in detail at how specific macroscopic food structures are broken down in the gastrointestinal (GI) tract and how they release their nutrients (12, 23, 30). Such studies have shown that one of the major factors influencing nutrient release is the control of GI flow (21). In this regard, nutrient sensing in the small intestine has also been shown to be important and acts through the release of GI hormones such as cholecystokinin (CCK), GLP-1, and PYY. Studies looking at the sensing of specific nutrients such as protein and lipids have shown that intra-duodenal protein (29) or fat (18–20) can contribute to the suppression of energy intake. Thus understanding the rules that link food structure to GI flow, nutrient sensing, and satiety responses will be of primary importance in designing foods that are acceptable to the consumer and that provide the required physiological responses for maintaining health.
A number of studies have investigated the effect of food structure on gastric emptying and appetite regulation. At the most basic level, simple protein solutions have been assessed for their ability to affect gastric retention. In a study using dairy proteins, whey was compared with casein and cross-linked casein (10, 11). Both casein and whey were more potent in lowering postprandial glucose than the cross-linked protein, which had a less pronounced peak in insulin and significantly lower CCK release. Clemente et al. (4) studied the effects of structure on nutrient release for a range of dairy-based foods and showed that Mozzarella cheese was able to delay the peak in plasma triglycerides compared with milk. They also demonstrated a significantly faster reduction in antral volume as assessed by ultrasound, which may, however, not be directly related to a reduced gastric emptying time as suggested by the authors. Lipids form another group of macro nutrients that affect GI flow, and of particular interest for this are systems used to control the release of lipids. In a study comparing stable and unstable emulsions (21), gastric emptying was slower for an acid-stable emulsion, although the rate of energy delivery to the duodenum was not different up to 2 h. The acid-stable emulsion also induced increased fullness and decreased hunger and appetite. The use of emulsion systems with tailored release properties is increasingly being considered to control hyperlipidemia (3, 24, 33).
These studies demonstrate the importance of food structure in the modulation of postprandial satiety-related physiology. Indeed, foods such as fresh whole fruits and vegetables, whole grain bread, and meat are digested more slowly and as a consequence are more satiating than foods that have a softer, more highly processed structure (25). However, a review of a cross section of these types of studies in which food structures have been developed to enhance satiety (35) shows that we are still a long way from fully understanding the complex process of satiety, which involves physiological processes of the entire metabolism as well as psychological and social processes, although progress is being made in all these areas.
In this study, we have attempted to confirm that food structure alone can impact satiety and to determine the role of gastric retention and nutrient sensing as indicated by CCK secretion. The design of the meals in this study was based on the concept that gastric emptying is regulated by the caloric density of the chime delivered to the small intestine and the in vitro observation (34) that liquid food emulsions can be designed to either form a creaming or sedimenting energy-rich phase in the stomach. This feature can be used to control the timing of energy release from the stomach, which in case of a sedimenting energy-rich layer would induce increased satiety compared with meals that remain homogeneous under gastric conditions. In the present study, we wanted to test this concept by comparing two isocaloric (same fat, protein, and carbohydrate content) meals to assess the impact of food structuring on gastric retention and short-term appetite regulation. Because we did not want to be dependent on the process of gastric acidification since the mechanism that would produce a sedimenting protein and fat-rich phase in the stomach, we constructed a sedimenting system in the form of a slurry of small, dense cheese particles. These cheese particles were suspended in yogurt to form a thick semi-fluid. Detailed information about the dissolution of structures in the stomach and the volume of gastric contents were obtained by MRI, allowing the persistence of structure to be correlated with gastric flow rates, appetite, and CCK secretion.
MATERIALS AND METHODS
The meals.
The two meals used in the study were prepared under food grade conditions. The homogeneous meal referred to in this article as the control meal was made as follows. An emulsion was made comprising 27.5 g of sunflower oil and 242.5 g of 1.24% sodium caseinate solution in a blender (BL450 series, Kenwood). The shear cycle comprised 30 s at the low shear setting, 30 s of rest, 30 s at the high shear setting, 30 s of rest, and 30 s at high shear setting. The emulsion was then mixed with 199.5 g of a solution containing 1.24% sodium caseinate and 10% whey protein isolate (Bipro, Davisco). Sugar (6.1 g) was then added to the emulsion along with a few drops of vanilla flavoring. The emulsion was stored at 4°C until use (<24 h). The structured meal referred to as the active meal was prepared by mixing 88 g of finely grated Gouda cheese (Waitrose Essential Dutch Gouda) and 73 g of low-fat yogurt (Waitrose Essential low-fat yogurt), both of which were purchased from the local supermarket. The meal was consumed with 339 ml of bottled water, which was stored with the cheese and yogurt mixture at 4°C until use (<24 h). The sodium content of the active meal was 64 mM based on a concentration in the cheese of 2.1%, whereas the sodium content in the control meal was 20 mM based on the protein content. Figures for the nutrient content of the meals are given in Table 1. As far as possible, the two meals were isocaloric, the only differences being the higher salt content and the large degree of proteolysis of the proteins introduced into the active meal with the cheese.
Table 1.
The nutritional composition of the two meals used in the study
| Control Meal | Active Meal | |
|---|---|---|
| Fat, g | 27.5 | 27.5 |
| Carbohydrate, g | 6.1 | 6.1 |
| Protein, g | 25.3 | 25.3 |
| Total, g | 58.9 | 58.9 |
| Sodium, mM | 20 | 64 |
| Energy, kCal | 373.1 | 373.4 |
| Weight of meal, g | 500 | 500 |
Imaging of gastric contents.
The gastric contents of the volunteers were determined using a conventional 1.5-T magnetic resonance imaging (MRI) scanner (Siemens Avanto 1.5T). Imaging used a TRUFISP (fast imaging with steady-state precession) protocol developed to scan the stomach in a breath-hold of the order of 15–25 s, depending on the fullness of the stomach [reptitition time (TR)/echo delay time (TE) 3.5/1.5 ms; field of view 24 × 32 cm; matrix 154 × 256; slice thickness 0.5 cm]. This yields contiguous 5-mm axial slices through the stomach, enabling calculation of total stomach volume. Both transverse and coronal images were acquired to ensure that the gastric volume could be accurately defined. Total volumes of gastric contents (excluding gas) and the nature of layers formed as a result of sedimentation were determined at each time point using free-hand tracings of the region of interest around the stomach contents for each 5-mm-thick slice, and from this the total stomach volume was calculated using cardiac ventricular volume measurement software (Siemens Argus workstation). This involved assessment of the position of the pylorus. Each scan took ∼5 min, and between scans the volunteers underwent minimal physical movement and remained seated upright close to the scanner. From the variation of the gastric volume with time, we deduced an apparent emptying rate, which gives and impression of, but is not precisely the same as, the rate at which the food emptied from the stomach, because of the inhomogeneous distribution of the food material inside the stomach and because of the simultaneous addition of gastric secretion.
Visual analog scales.
We assessed volunteer satiety with a self-reported visual analog scale (VAS) technique (32). Before the meal and at specific time intervals post-meal, as given in Table 2, the volunteers completed a five-question satiety questionnaire with a VAS for each of the following questions: 1) “How hungry are you?” 2) “How full do you feel?” 3) “How satisfied do you feel?” 4) “How big is your desire to eat?” 5) “How thirsty are you?” The analog scores for each question were then converted to numeric scores based on the following: 1) 1 = not at all hungry, 10 = very hungry; 2) 1 = not full at all, 10 = very full; 3) 1 = not satisfied at all, 10 = very satisfied; 4) 1 = no desire to eat at all, 10 = very big desire to eat; 5) 1 = not thirsty at all, 10 = very thirsty.
Table 2.
Timing of clinical activities relative to consumption of the meal (min)
| Activity | MRI Scan | Blood Drawing | VAS Questionnaire |
|---|---|---|---|
| 1 | −15* | −10* | −5* |
| 2 | 5 | 10 | 15 |
| 3 | 25 | 20 | 30 |
| 4 | 45 | 35 | 40 |
| 5 | 65 | 60 | 55 |
| 6 | 85 | 90 | 95 |
| 7 | 105 | 120 | 115 |
| 8 | 125 | 150 | 155 |
| 9 | 145 | 180 | 185 |
| 10 | 165 |
Premeal was not considered to be critical, so these values are largely indicative.
Determination of CCK.
At the start of each study session, volunteers were fitted with a cannula so that blood could be drawn periodically. At each required time point, 4 ml of blood was drawn and stored on ice for <2 h before being centrifuged. Blood was collected into tubes (Vacutainer K2 EDTA, Becton Dickenson) containing 170.9 μl of aprotinin (Sigma-Aldrich), and, after centrifugation for 10 min at 1,500 g and 4°C, the plasma was removed and stored in prelabeled tubes at −80°C. The plasma was subsequently analyzed for CCK content using a radio-immunoassay (RIA) (27, 28) performed by the TNO organization in The Netherlands.
The study method.
The crossover study was designed to assess differences in gastric emptying, satiety indicators, and levels of the GI hormone CCK. The study included only male volunteers aged between 20 and 50 yr and with a body mass index (BMI) between 19 and 30. The mean age of the cohort was 35 yr, and the mean BMI was 24.7. All 10 volunteers recruited to the study were apparently healthy and provided written, informed consent before taking part in the study, which was approved by the local research ethics committee (Approval 11/EE/0192). Each volunteer attended the study center on two occasions at least 7 days apart and consumed a different meal on each occasion. The order in which the meals were consumed was randomly allocated. All volunteers were able consume all of the test meals within 5 min.
On each study day, volunteers were asked to eat their breakfast at home (before 9:00 AM). They were allowed to drink as much water as they need but only until 10:00 AM. After this time, no further consumption was allowed. The experimental protocol was started at between 1:30 PM and 2:00 PM, which corresponds to the first time point in Table 2. After initial formalities, each volunteer had a cannula inserted into an arm ready for blood drawing. They then underwent the first MRI scan, a 4-ml sample of blood was drawn, and they were asked to complete a VAS questionnaire (baseline measurements). The volunteer consumed the meal, allocated at random. Immediately after the meal was consumed, the second MRI scan was performed, with subsequent scans being undertaken as laid out in Table 2. The volunteers were asked to repeatedly complete a VAS satiety questionnaire and have a 4-ml sample of blood drawn, and the timing for these are given in Table 2.
Data analysis.
SPSS for Windows software (SPSS for Windows, version 19.0) was used to analyze the data. The results are expressed as means and SE, with a P value of ≤0.05 (two-sided) as a criterion for the statistical significance. The statistical significance of the data was determined from differences in the areas under the curves using a paired two-sided t-test (1).
RESULTS
Satiety.
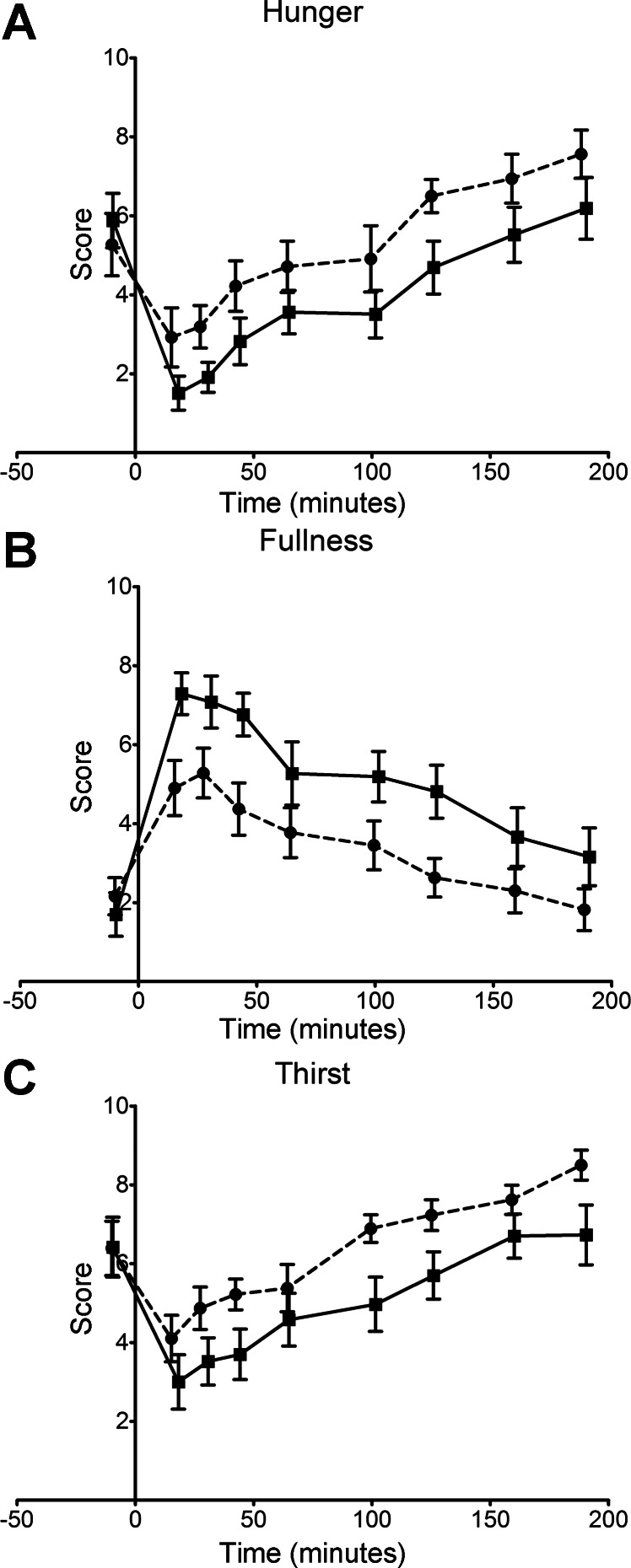
The impact of the two meals on the scores for hunger and fullness are shown in Fig. 1, and the areas under the curves (AUC) summarized in Table 3. There is a significant difference in the hunger scores for the two meals, with the active meal reducing hunger more than the control meal at virtually every time point, although the slopes of the two curves are very similar. Statistical analysis of the AUC for the two data sets yielded a P value of 0.002 (Table 3). A similar pattern is seen for the fullness data, with the active meal eliciting a higher fullness score at every time point, although the difference was more pronounced over the first 45 min after ingestion. Interestingly, the liquid control meal gave the highest fullness score at the first point collected after the meal was consumed, whereas after the active meal the second time point was highest. Although this difference was not statistically significant, it may indicate a slightly slower onset of the sensation of fullness with the active meal. The VAS determination of satisfaction and desire to eat very much reflect the data in Fig. 1, A and B, as can be seen from the AUC data in Table 3. Given the composition of the active meal and the relatively high salt content, it might be expected that the VAS thirst data would show a high value for the active meal. However, the data in Fig. 1C shows consistently lower thirst scores for the active meal at every post-meal time point.
Fig. 1.
Visual-analog scale (VAS) score data for hunger (A), fullness (B), and thirst (C) for the control (broken line) and active (solid line) meals. Mean and SE values are shown.
Table 3.
Mean areas under the curve ± SE of the visual-analog scale data collected for the control and active meals
| Control Meal | Active Meal | P Value | |
|---|---|---|---|
| Hunger | 6,187 ± 697 | 4,477 ± 592 | 0.002053 |
| Fullness | 4,345 ± 627 | 6,538 ± 635 | 0.004911 |
| Satisfaction | 4,616 ± 711 | 6,759 ± 647 | 0.00881 |
| Desire to eat | 6,561 ± 685 | 4,787 ± 701 | 0.007301 |
| Thirst | 7,518 ± 358 | 5,598 ± 659 | 0.001126 |
P values shown are based on a paired, two tailed t-test.
CCK modulation.
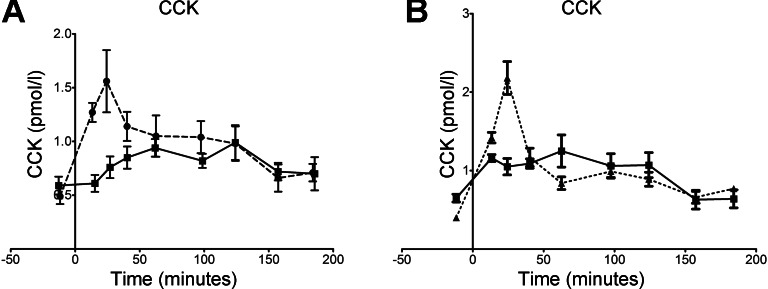
The mean levels of CCK measured in the blood of the volunteers after consuming the two meals is shown in Fig. 2A. The data for both meals is an average for nine of the volunteers, since the data from the active meal for one was clearly an outlier (data not shown). It is clear that differences in the release of the hormone were only seen in the first hour after consumption of the meal. After the first hour, serum CCK levels were increased from 0.6 pmol/l in the unfed state to ∼1 pmol/l during gastric emptying for both meals. In the case of the control meal, there was a steep rise to a maximum of 1.6 pmol/l at 25 min post-ingestion. In fact, the data for the control meal fell into two different groups, as shown in Fig. 2B. One group of five volunteers with a maximum in CCK concentration of 2.2 pmol/l and another with four volunteers where all the values were close to 1 pmol/l over the first 2 h. The data from both control groups was significantly different from that for the active meal over the first 25 min (P = 0.029, paired, two-sided t-test). The CCK levels generated by the active meal were significantly lower than the control meal for at least the first 40 min post-ingestion.
Fig. 2.
A: blood plasma concentrations of CCK measured before and after consumption of the control (broken line) and active (solid line) meals. B: two groups of controls: those showing a significant increase in CCK at 30 min (n = 5; broken line) and those that did not (n = 4; solid line). Mean and SE values are shown in both graphs.
Gastric emptying and layering.
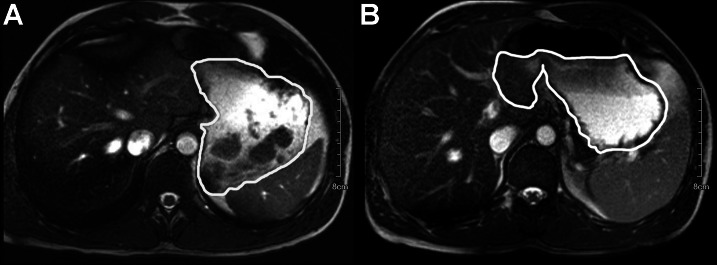
The data shown above has been governed to a large extent by the behavior of the two meals in the gastric compartment. To assess this, we have used MRI as illustrated in Fig. 3. Figure 3A shows the active meal in the stomach 5 min after ingestion, and individual boluses of cheese are clearly visible as dark regions in a fairly homogenous surrounding medium. The other image in the figure shows the control meal in the same volunteer some 25 min after consumption. In this image, layering of the gastric contents is clearly visible as the fat in the control meal starts to cream, leaving a darker, higher fat content region as an upper layer. We have measured the time over which these structures formed and persisted.
Fig. 3.
MRI images of the active meal in the stomach (outlined) 5 min after consumption (A) and the control meal in the stomach (outlined) 25 min after consumption (B).
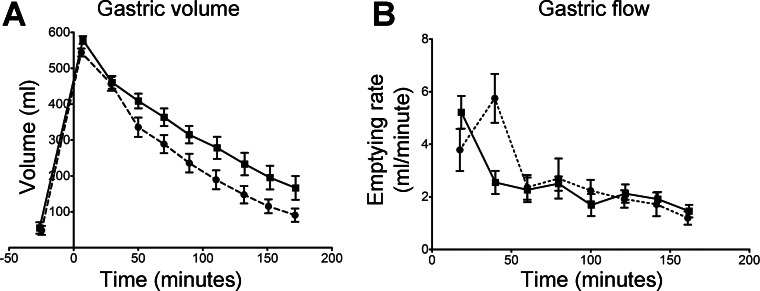
The structures in Fig. 3 reflect the heterogeneous and changing nature of the gastric contents and highlight the challenges in interpreting gastric retention times. The structures seen in Fig. 3A were persistent over the first 45 min after ingestion, and this time scale is typical of what was seen in the volunteers more generally. The layering seen in Fig. 3B tended to be persistent for rather longer and was typically still visible up to 105 min after ingestion. In addition to assessing intra-gastric layering, we measured mean gastric volume over the time course of the study, and the data are given in Fig. 4. After ingestion, there was little difference in the volume of gastric contents between the two meals for the first 25 min. The active meal showed a slightly higher volume at 5 min, but this was not statistically significant. The active meal also emptied slightly faster over the first 25 min, but again this was not statistically significant. Between 25 and 45 min, the two meals presented markedly different emptying rates, with the control meal emptying more than twice as fast as the active meal (P = 0.0058, paired, two-tailed t-test). From 60 min onward, the emptying rates of the two meals were very similar at ∼2 ml/min, showing that the cause of the 30-min difference in gastric half-times of 69 min and 100 min for the control meal and active meal, respectively, was behavior in the first 45 min after ingestion.
Fig. 4.
A: mean volumes of gastric contents as a function of time before and after consumption of the control (broken line) and active (solid line) meals. B: the gastric emptying rates (derivatives) calculated from the individual volume data. The data are the mean and SE values.
DISCUSSION
In the study described in this article, we have used two iso-caloric meals with different structures, semi-solid vs. liquid, to assess the impact of food structure on gastric emptying rates and short-term perception of appetite. We have used MRI to assess not just gastric volume but also the persistence of macroscopic structure in the gastric compartment, any layering formed, and its subsequent persistence. The active meal had a gastric retention half-time 30 min longer than the control meal. Although this seems to be in disagreement with a study showing that a mixed solid/liquid food empties faster and is less satiating than the same meal after homogenization to a “soup” (22), in that case the authors suggested that the mixed solid/liquid system initially emptied the liquid portion, which had a low energy density and in this way more quickly reduced the gastric volume and hence the sensation of fullness. Another study demonstrated faster gastric emptying when the viscosity of a meal was increased by adding pectin (30). However, in a study comparing liquid and semi-solid meals, the latter was more satiating, even though there was no difference in CCK-8 or GLP-1 secretion (36). It has also been shown that the detection of fat in the duodenum can significantly reduce hunger, increase fullness, and delay gastric emptying (20).
There are a number of feedback mechanisms that control gastric emptying, but one of the important ones involves the peptide hormone CCK. Detection of nutrients in the proximal small intestine by I-cells leads to the release of CCK, which plays a key role in regulating a range of intestinal responses that integrate and optimize the digestion of fat and protein (15). CCK is released in response to nutrients in the duodenum, with fat and protein producing a greater postprandial release than carbohydrates (13). This regulation includes three physiological effects of threshold levels of plasma CCK, which are a stimulation of pancreatic secretion through relaxation of the sphincter of Oddi; gall bladder emptying through contraction of the gall bladder and a modulating effect on gastric emptying (7–9, 15, 17, 31). Normally, following ingestion of a meal, CCK levels rise rapidly from a resting value of ∼1 pmol/l to a peak of 6–8 pmol/l during the first 15 min and then decline to a submaximal level, which is maintained for up to 2 h after eating (16, 17, 26). This peak in plasma CCK is responsible for gall bladder contraction (7). In the present study, the observed effect of the meals on CCK blood levels was smaller than typically observed for liquid emulsions (e.g., peaking to 8 pmol/l). This might be due to the relatively high viscosity of both meals in this study, which impeded the initial fast emptying found for thin liquids. The role of plasma CCK concentrations on inhibition of gastric motility and emptying seems more complex. CCK probably stimulates the mechanoreceptors in the gastric wall that signal gastric distension, which through the enteric nervous systems stimulates neurons that ultimately lead to a relaxation of the gastric fundus, a reduced motor activity of the antrum, and a reduced gastric emptying rate (14). The same type of effect is also induced by osmotic pressures in the duodenum (typically caused by sugars) and acidity (from the chime), independent of the CCK regulation (5–7).
Most significantly, in the present study, the main difference in empting rates occurred up to ∼50 min after ingestion, when the apparent emptying rate of the control meal was more than twice that for the active meal. This coincides with the time over which semi-solid parts of the active meal were seen to persist. Over this same time period, the rate of CCK secretion was significantly supressed relative to the control meal, apparently related to a reduced release of energy in the form of protein and fat during this time period. In fact, the observed steady-state emptying rate of ∼2 ml/min for both meals after 60 min translates to an emptying rate of ∼1.5 kcal/min, which is typical for energy-controlled gastric emptying. Thus it seems that the body was better able to control the initial emptying rate of the active meal at an appropriate energy release rate than the control meal. This might be because the less viscous control meal had already emptied by a substantial amount before CCK-regulated control of the emptying rate became fully effective. This would also explain why the active meal also generated a significantly higher feeling of fullness over the same period: since plasma CCK levels were similarly elevated in both cases, the remaining volume in the stomach would have been sensed by the distension mechanoreceptors, signaling more fullness for the active meal compared with the control meal, extending over the whole time of gastric emptying. Although the greater feeling of fullness persisted for the duration of the study, the differences seen after 45 min were much reduced and only just statistically significant. As discussed above, CCK works through activation of vagal afferent mechanosensors in the stomach and in the duodenum. Consequently, the satiating effect of gastric distension increases the anorectic effects of CCK in humans (14).
In summary, in the study reported here, the more structured active meal supressed the initial secretion of CCK compared with the liquid control meal, presumably because, for the active meal, initially a more viscous layer containing food boluses was present in the antrum, preventing significant early emptying. Thus a larger volume was retained longer in the stomach, leading to an increased sense of fullness. Altogether, this suggests that a nutrient-induced increase in serum CCK levels did not have a direct role in the control of appetite sensation in the active meal. In contrast, the liquid meal showed a peak in both emptying rate and plasma CCK at 30 min, related to the initial quick emptying of this meal. This is the first time that macroscopic structure persistence and formation have been linked to satiety via gastric retention and CCK secretion. The results suggest that, for the studied situation in which the plasma CCK level is moderately increased during nutrient-controlled gastric emptying, gastric retention was the key factor in decreasing appetite rather than the detection of nutrients in the duodenum and that plasma CCK was not directly linked to suppression of either gastric emptying or appetite. This study paves the way for further work to assess the role of other GI hormones and the impact of even more persistent structures.
GRANTS
The work in this article was funded by Top Institute for Food and Nutrition through project B1007 and BBSRC through their core strategic grant to the Institute of Food Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.R.M., P.M., and G.v.A. conception and design of research; A.R.M. and L.S. performed experiments; A.R.M., H.R., and P.M. analyzed data; A.R.M., H.R., P.M., and G.v.A. interpreted results of experiments; A.R.M. prepared figures; A.R.M. drafted manuscript; A.R.M., P.M., and G.v.A. edited and revised manuscript; A.R.M., P.M., and G.v.A. approved final version of manuscript.
REFERENCES
- 1. Bland JM, Altman DG. Statistical-methods for assessing agreement between 2 methods of clinical measurement. Lancet 1: 307–310, 1986 [PubMed] [Google Scholar]
- 2. Boniface S, Bridges S, Craig R, Darton R, Fuller E, Hancock R, Henderson C, Knott C, Mandalia D, Mindell J, Moody A, Morciano M, Ng Fat L, Oyebode O, Robinson C, Sadler K, Sutton R, Wittenberg R. Health Survey for England, edited by Craig R, Mindell J. London: National Centre for Social Research, Department of Epidemiology and Public Health, University College London, 2011 [Google Scholar]
- 3. Chu BS, Rich GT, Ridout MJ, Faulks RM, Wickham MSJ, Wilde PJ. Modulating pancreatic lipase activity with galactolipids: effects of emulsion interfacial composition. Langmuir 25: 9352–9360, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Clemente G, Mancini M, Nazzaro F, Lasorella G, Rivieccio A, Palumbo AM, Rivellese AA, Ferrara L, Giacco R. Effects of different dairy products on postprandial lipemia. Nutr Metab Cardiovasc Dis 13: 377–383, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Dockray GJ. Neuronal actions of cholecystokinin. In: Gastrointestinal Endocrinology: Receptors and Post-Receptor Mechanisms, edited by Thompson J. New York: Academic, 1990, p. 321–332 [Google Scholar]
- 6. Forster ER, Green T, Elliot M, Bremner A, Dockray GJ. Gastric-emptying in rats: role of afferent neurons and cholecystokinin. Am J Physiol Gastrointest Liver Physiol 258: G552–G556, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Grider JR. Role of cholecystokinin in the regulation of gastrointestinal motility. J Nutr 124: 1334S–1339S, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Higham A, Vaillant C, Yegen B, Thompson DG, Dockray GJ. Relation between cholecystokinin and antral innervation in the control of gastric emptying in the rat. Gut 41: 24–32, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hopman WPM, Kerstens P, Jansen J, Rosenbusch G, Lamers C. Effect of graded physiologic doses of cholecystokinin on gallbladder contraction measured by ultrasonography - determination of threshold, dose-response relationships and comparison with intraduodenal bilirubin output. Gastroenterology 89: 1242–1247, 1985 [DOI] [PubMed] [Google Scholar]
- 10. Juvonen KR, Karhunen LJ, Vuori E, Lille ME, Karhu T, Jurado-Acosta A, Laaksonen DE, Mykkanen HM, Niskanen LK, Poutanen KS, Herzig KH. Structure modification of a milk protein-based model food affects postprandial intestinal peptide release and fullness in healthy young men. Br J Nutr 106: 1890–1898, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Juvonen KR, Lille ME, Laaksonen DE, Mykkanen HM, Niskanen LK, Herzig KH, Poutanen KS, Karhunen LJ. Crosslinking with transglutaminase does not change metabolic effects of sodium caseinate in model beverage in healthy young individuals. Nutr J 11: 35, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Juvonen KR, Purhonen AK, Salmenkallio-Marttila M, Lahteenmaki L, Laaksonen DE, Herzig KH, Uusitupa MIJ, Poutanen KS, Karhunen LJ. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J Nutr 139: 461–466, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Karhunen LJ, Juvonen KR, Huotari A, Purhonen AK, Herzig KH. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul Pept 149: 70–78, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Kissileff HR, Carretta JC, Geliebter A, Pi-Sunyer F. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol 285: R992–R998, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Liddle RA. Cholecystokinin cells. Ann Rev Physiol 59: 221–242, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human-plasma - molecular-forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest 75: 1144–1152, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liddle RA, Morita ET, Conrad CK, Williams JA. Regulation of gastric-emptying in humans by cholecystokinin. J Clin Invest 77: 992–996, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maljaars J, Romeyn EA, Haddeman E, Peters HPF, Masclee AAM. Effect of fat saturation on satiety, hormone release, and food intake. Am J Clin Nutr 89: 1019–1024, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Maljaars PWJ, Peters HPF, Kodde A, Geraedts M, Troost FJ, Haddeman E, Masclee AAM. Length and site of the small intestine exposed to fat influences hunger and food intake. Br J Nutr 106: 1609–1615, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Maljaars PWJ, van der Wal RJP, Wiersma T, Peters HPF, Haddeman E, Masclee AAM. The effect of lipid droplet size on satiety and peptide secretion is intestinal site-specific. Clin Nutr 31: 535–542, 2012 [DOI] [PubMed] [Google Scholar]
- 21. Marciani L, Faulks R, Wickham MSJ, Bush D, Pick B, Wright J, Cox EF, Fillery-Travis A, Gowland PA, Spiller RC. Effect of intragastric acid stability of fat emulsions on gastric emptying, plasma lipid profile and postprandial satiety. Br J Nutr 101: 919–928, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Marciani L, Hall N, Pritchard SE, Cox EF, Totman JJ, Lad M, Hoad CL, Foster TJ, Gowland PA, Spiller RC. Preventing gastric sieving by blending a solid/water meal enhances satiation in healthy humans. J Nutr 142: 1253–1258, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Marciani L, Wickham M, Singh G, Bush D, Pick B, Cox E, Fillery-Travis A, Faulks R, Marsden C, Gowland PA, Spiller RC. Delaying gastric emptying and enhancing cholecystokinin release and satiety by using acid stable fat emulsions. Gastroenterology 130: A227–A227, 2006 [Google Scholar]
- 24. McClements DJ, Li Y. Structured emulsion-based delivery systems: controlling the digestion and release of lipophilic food components. Adv Colloid Interface Sci 159: 213–228, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Porrini M, Crovetti R, Riso P, Santangelo A, Testolin G. Effects of physical and chemical characteristics of food on specific and general satiety. Physiol Behavior 57: 461–468, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Rehfeld J. Cholecystokinin. In: Handbook of Physiology. The Gastrointestinal System. Neural and Endocrine Biology. Bethesda, MD: Am. Physiol. Soc., 1989, sect. 6, vol. II, chapt. 16, p. 337–358 [Google Scholar]
- 27. Rehfeld JF. Accurate measurement of cholecystokinin in plasma. Clin Chem 44: 991–1001, 1998 [PubMed] [Google Scholar]
- 28. Rehfeld JF. How to measure cholecystokinin in tissue, plasma and cerebrospinal fluid. Regul Pept 78: 31–39, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Ryan AT, Feinle-Bisset C, Kallas A, Wishart JM, Clifton PM, Horowitz M, Luscombe-Marsh ND. Intraduodenal protein modulates antropyloroduodenal motility, hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr 96: 474–482, 2012 [DOI] [PubMed] [Google Scholar]
- 30. Shimoyama Y, Kusano M, Kawamura O, Zai H, Kuribayashi S, Higuchi T, Nagoshi A, Maeda M, Mori M. High-viscosity liquid meal accelerates gastric emptying. Neurogastroenterol Motil 19: 879–886, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Smith GP, Gibbs J. Satiating effect of cholecystokinin. In: Cholecystokinin, edited by Reeve JR, Eysselein V, Solomon TE, Go VLW. New York: NY Acad. Sciences, 1994, p. 236–241 [DOI] [PubMed] [Google Scholar]
- 32. Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M, Stratton R, Delargy H, King N, Blundell JE. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr 84: 405–415, 2000 [DOI] [PubMed] [Google Scholar]
- 33. van Aken GA. Relating food emulsion structure and composition to the way it is processed in the gastrointestinal tract and physiological responses: What are the opportunities? Food Biophys 5: 258–283, 2010 [Google Scholar]
- 34. van Aken GA, Bomhof E, Zoet FD, Verbeek M, Oosterveld A. Differences in in vitro gastric behaviour between homogenized milk and emulsions stabilised by Tween 80, whey protein, or whey protein and caseinate. Food Hydrocolloids 25: 781–788, 2011 [Google Scholar]
- 35. Van Kleef E, Van Trijp JCM, Van den Borne J, Zondervan C. Successful development of satiety enhancing food products: towards a multidisciplinary agenda of research challenges. Crit Rev Food Sci Nutr 52: 611–628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zijlstra N, Mars M, de Wijk RA, Westerterp-Plantenga MS, Holst JJ, de Graaf C. Effect of viscosity on appetite and gastro-intestinal hormones. Physiol Behavior 97: 68–75, 2009 [DOI] [PubMed] [Google Scholar]