Abstract
Renal ischemia-reperfusion injury (IRI) is a common cause of acute kidney injury (AKI), occurring with hypotension and cardiovascular surgery and inevitably during kidney transplantation. Mortality from AKI is high due to incomplete knowledge of the pathogenesis of IRI and the lack of an effective therapy. Inflammation accompanies IRI and increases the blood level of C-reactive protein (CRP), a biomarker of worsened outcomes in AKI. To test if CRP is causal in AKI we subjected wild-type mice (WT) and human CRP transgenic mice (CRPtg) to bilateral renal IRI (both pedicles clamped for 30 min at 37°C then reperfused for 24 h). Serum human CRP level was increased approximately sixfold after IRI in CRPtg (10.62 ± 1.31 μg/ml at baseline vs. 72.01 ± 9.41 μg/ml at 24 h) but was not elevated by sham surgery wherein kidneys were manipulated but not clamped. Compared with WT, serum creatinine, urine albumin, and histological evidence of kidney damage were increased after IRI in CRPtg mice. RT-PCR analysis of mRNA isolated from whole kidneys of CRPtg and WT subjected to IRI revealed that in CRPtg kidneys 1) upregulation of markers of macrophage classical activation (M1 markers) was blunted, 2) downregulation of markers of macrophage alternative activation (M2 markers) was more robust, and 3) expression of the activating receptor FcγRI was increased. Our finding that CRP exacerbates IRI-induced AKI, perhaps by shifting the balance of macrophage activation and FcγR expression towards a detrimental portfolio, might make CRP a promising therapeutic target for the treatment of AKI.
Keywords: alternatively activated macrophages, acute phase proteins, AKI
acute kidney injury (AKI) can occur in any setting where renal ischemia reperfusion injury (IRI) is manifest, including during cardiovascular surgery (37) and kidney transplantation (50). In fact, AKI is a serious complication in ∼1% of all hospitalizations and has a mortality rate as high as 80% (22, 37). Despite this risk to patients and its burden on the health care system, there is still no effective therapy for AKI. The pathogenesis of renal IRI is not completely understood, but it is recognized that it is always accompanied by a systemic inflammatory response (35). Damage to the kidney is thought to evoke the release of inflammatory cytokines like TNF-α and IL-6 that in turn foster renal infiltration of leukocytes, neutrophils, dendritic cells, and macrophages (1, 20). Exactly how this inflammatory cascade culminates in kidney damage and how this process is regulated are unknown.
In accordance with the inflammatory nature of AKI, people with increased urinary albumin, a biomarker of AKI, also tend to have increased blood levels of the acute phase reactant C-reactive protein (CRP; Refs. 15, 39). Blood CRP generally increases as kidney function decreases (26, 36), and CRP levels are positively associated with worse outcomes and increased mortality in AKI (48, 49). In the context of renal transplantation, elevated CRP levels in graft recipients associate with graft failure, and local expression of CRP mRNA in donated kidneys correlates with both acute and chronic rejection (14, 31, 43). There is evidence from animal models of chronic kidney disease suggesting that CRP actively increases inflammation (21, 23). Despite all of this evidence of association, it is not known if CRP is causal in AKI.
CRP is an acute phase protein in humans, wherein its blood levels can rise dramatically from a typical baseline of <3 μg/ml to levels approaching 1 mg/ml within days after an inflammatory insult (8). This dynamic expression is regulated at the level of transcription, with IL-6 being the main inducer of the CRP acute phase response (40). Because of these properties, the CRP blood level is useful in the clinical setting as a marker of inflammatory status in patients (8). Furthermore, CRP also has several biological actions that are of potential direct relevance to disease. The best understood of these is the ability of CRP to bind the membrane lipid phosphatidylcholine (44), a molecule expressed on the cell surface of apoptotic and necrotic cells. Also, CRP exerts many biological actions by binding to activating Fcγ receptors (FcγRI, FcγRIIA, and FcγRIII) and inhibitory ones (FcγRIIB; Refs. 24, 25, 27, 38), which are all widely expressed by cells resident in the kidney (38) and infiltrating into it after injury (10, 34). Importantly, because CRP can bind to the different FcγRs with comparable affinities (24, 25, 38), the outcome of CRP signaling mediated via FcγR interactions in the kidney is predicted to be dictated by resident FcγR diversity and availability. Finally, although the liver is the major source of blood CRP (11), renal tubular epithelial cells also produce CRP (13, 41) and so CRP actions in the kidney may be manifest even during periods of restricted blood flow.
Of the many types of FcγR-expressing (and thus potentially CRP responsive) inflammatory cells that infiltrate the kidney during IRI, macrophages are thought to play a central role (16, 18, 33). Soon after the kidney is injured, monocytes are recruited into the organ where they undergo differentiation into macrophages. These become polarized and exhibit either a proinflammatory M1 (classically activated) phenotype or an anti-inflammatory M2 (alternatively activated) phenotype (2, 29). Once M1 macrophages are primed they are highly phagocytic and can produce high amounts of proinflammatory molecules such as TNF-α, IL-1, IL-6, and inducible nitric oxide synthase (iNOS) that together contribute to tissue injury (5, 28, 30). Once M2 macrophages are primed they exhibit increased efferocytosis and can produce beneficial molecules like IL-10, mannose receptor, and arginase that can foster a reparative phase of AKI (5, 28). In mice, M1 macrophages have been shown to predominate in the kidney in the hours immediately following renal IRI (19, 45) whereas days later there is a switch to M2 macrophages (19). Since CRP interaction with macrophage FcγRs has been shown to influence the outcome of both immune-complex-mediated nephritis in vivo (34) and macrophage polarization in vitro (7), it is possible that CRP (circulating through the kidney or expressed locally) could influence macrophage activity and thus the injury process during renal IRI (7).
We undertook the current study to ascertain the impact of CRP in a mouse model of AKI. We compared the outcomes of renal IRI in wild-type mice (WT; wherein CRP is present but is not a major acute phase reactant) and human CRP transgenic mice (CRPtg; wherein human CRP is expressed as an acute phase reactant; Ref. 6). The data obtained provide direct evidence that CRP exacerbates the injury process during renal IRI.
MATERIALS AND METHODS
Animals.
Human CRP transgenic mice (backcrossed to C57BL/6) have been fully described elsewhere (6, 40). These carry a 31-kb ClaI fragment of human genomic DNA comprised of the CRP gene, 17 kb of 5′-flanking sequence containing the human CRP promoter and all the known CRP regulatory elements, and 11.3 kb of 3′-flanking sequence that includes the CRP pseudogene (6). Human CRP is present in the blood of CRPtg at concentrations relevant to humans, i.e., low levels under steady-state conditions (<1 to 30 μg/ml) and much higher levels during the acute phase response (100–500 μg/ml; Refs. 8, 40). In WT and in CRPtg mice, mouse CRP is not a major acute phase protein (46). All mice were housed at constant humidity (60 ± 5%) and temperature (24 ± 1°C) with a 12-h light cycle (6 AM to 6 PM) and maintained ad libitum on sterile bottled water and regular chow (Harlan Teklad). Only male mice were used in experiments as males are more susceptible to renal IRI (32) and male CRPtg express human CRP more robustly than females (40). All animals were 8- to 12-wk-old when used, all animal protocols were approved by the Institutional Animal Care and Use Committees at the University of Alabama at Birmingham, and all mouse experiments were consistent with the Guide for the Care and Use of Laboratory Animals (NIH publication 96-01, revised 1996).
Ischemia-reperfusion injury.
To induce acute bilateral kidney IRI, mice were anesthetized with isoflurane 2.5% inhalation and, following flank incisions, the right and left pedicles were exposed, secured, and clamped with an atraumatic microserrefine vascular clamp (catalog no. 18055-05; Fine Science Tools) for 30 min. During this ischemia, the kidneys were kept moist and the mice were maintained at 37°C. The clamp was then removed to allow reperfusion, which was verified visually, and the kidneys were restored to their original position in the body cavity. The incision was closed, and the mice were allowed to recover. Blood and urine samples were taken 24 h before surgery and again 24 h after surgery when the mice were killed and their kidneys harvested. Each separate renal IRI experiment included at least six mice (3 mice per genotype), and each experiment was repeated at least three times. Sham-operated mice (abdominal surgery with kidney displacement but no ischemia) served as controls.
Bilateral nephrectomy and CRP injections.
Mice were anesthetized with isoflurane 2.5% inhalation, and a bilateral nephrectomy (BNx) was performed as described by Andres-Hernando et al. (3). Briefly, a midline incision was made and the right and left renal pedicles were exposed, tied off with sutures, and then cut distally. The ureters were pinched off with forceps, and each kidney was removed. Sham surgery involved the same procedure, but renal pedicles were not sutured and kidneys were not removed. The incisions were closed, and the mice were allowed to recover overnight before each received an intravenous injection of human CRP (1 mg/ml in phosphate-buffered saline; US Biological) estimated to achieve 8 mg CRP/kg body wt. Blood was collected via tail vein 2 and 4 h later for measurement of circulating CRP levels.
Measurement of biomarkers.
Mouse CRP was measured using the mouse C-Reactive Protein kit (Life Diagnostics) and the manufacturer's instructions. Human CRP was measured using an enzyme-linked immunosorbent assay (ELISA) developed in our laboratory (40). The latter does not detect mouse CRP and has a lower limit of detection of ∼20 ng of human CRP per ml of mouse serum. Urine albumin was measured by ELISA (Bethyl Laboratories) that has a lower limit of detection of 80 ng/ml. Serum creatinine was determined by tandem mass spectrometry (LC-MS/MS) as described previously (51).
Histology.
Kidneys were harvested, bisected transversely through the pelvis, formalin fixed, paraffin embedded, and cut into 5-μm-thick sections. Sections were stained with periodic acid-Schiff reagent and histological assessment of renal damage was performed in a blinded fashion (by M. Pegues and A. Zarjou). For representative thin sections prepared from each kidney, 10 high-powered (×400) nonoverlapping fields of both the cortex and outer stripe of the medulla were imaged and each image was examined for evidence of changes characteristic of AKI, i.e., the number of degenerating/necrotic tubules, the number of tubules with brush border loss, and the number of tubules containing casts was counted (51).
Protein and RNA extraction.
To examine hepatic and renal proteins we obtained organs from transcardially perfused mice. Twenty-four hours after renal IRI or sham surgery, mice were deeply anesthetized with isoflurane 2.5% inhalation. The thorax and abdomen were exposed via midline incision, and an 18-gauge needle was inserted into the left ventricle. The right atrium was snipped open, and immediately thereafter sterile 0.9% saline was injected into the left ventricle. Saline perfusion continued until the liver became noticeably pale in color, at which time the livers and kidneys were harvested. Samples of each organ were digested in homogenization buffer containing 0.5% Triton X-100 and a protease inhibitor cocktail (Sigma) in PBS, and digests of each organ were used for subsequent determination of total protein (DC protein assay kit from Bio-Rad) and CRP content (ELISA). Hepatic and renal RNA was obtained from organs isolated 24 h after renal IRI or sham surgery (without perfusion) and treated with TRIzol reagent according to the manufacturer's protocol (Invitrogen).
Quantitative RT-PCR.
DNase 1 treated RNA was converted to cDNA using the RETROscript kit (Ambion), and each quantitative (q) real-time PCR was performed with SYBR Green Mastermix (Bio-Rad) and specific primers for human CRP and mouse FcγRI, FcγRIIB, FcγRIII, TNF-α, mannose receptor (MR), iNOS, and arginase (ARG). Briefly, cDNA was amplified in an iCycler for 40 cycles, and for each mRNA species of interest the expression level was calculated with iCycler software (Bio-Rad). The specificity of each PCR reaction was monitored using melting curve analysis, and each reaction was performed in triplicate. The primers used for detection of specific mRNAs were as follows: for human CRP, sense TTTACAGTGGGTGGGTCTGAA and antisense CCACCGAAGGAATCCTG; for FcγRI, sense CTTCAGATTCGGAGGTCG and antisense AGCACTGGCGTGGTAAA; for FcγRIIB, sense TGTCGCAGCCATTGTTAT and antisense CGTATTCTCAGCCTCAGTT; for FcγRIII, sense ACAACCCTGGGAACTCTT and antisense CTCCATTTGACACCGATA; for arginase, sense CTCCAAGCCAAAGTCCTTAGAG and antisense AGGAGCTGTCATTAGGGACATC; for mannose receptor, sense CCTGTGCTCGAGAGGATATG and antisense GCAGTCTGCATACCACTTGT; for TNF-α, ACGGCATGGATCTCAAAGAC and antisense AGATAGCAAATCGGCTGACG; and for iNOS, sense CCAAGCCCTCACCTACTTCC and antisense CTCTGAGGGCTGACACAAGG. Expression of mRNA for each gene was normalized against mRNA for the housekeeping gene GAPDH (sense ATTCTTCCACCTTTGATGC and antisense TGGTCCAGGGTTTCTTACT). Genotype and treatment effects on mRNA expression were estimated using the ΔΔCt method relative to sham treated WT mice.
Statistical analysis.
All grouped data are presented as means with associated SE. Statistical analyses were performed using Graphpad Prism 3.02 and Statview 5.0.1. One-way ANOVA, post hoc protected least-squared difference tests, and Student's t-tests were used for comparisons among and between genotypes. Linear regression analysis was used to test for correlations between biomarkers, in which case Fisher's r to z test was used to calculate P values. In all analyses a P value of ≤0.05 was considered significant.
RESULTS AND DISCUSSION
Robust elevation of circulating CRP induced by renal IRI in CRPtg mice.
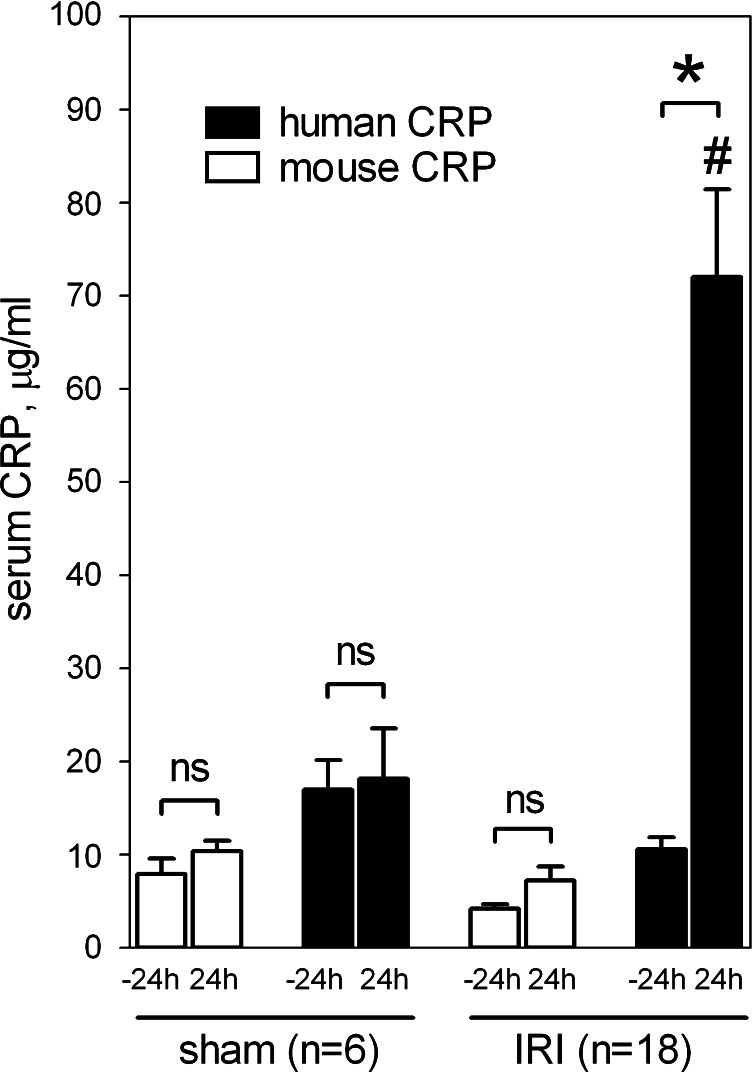
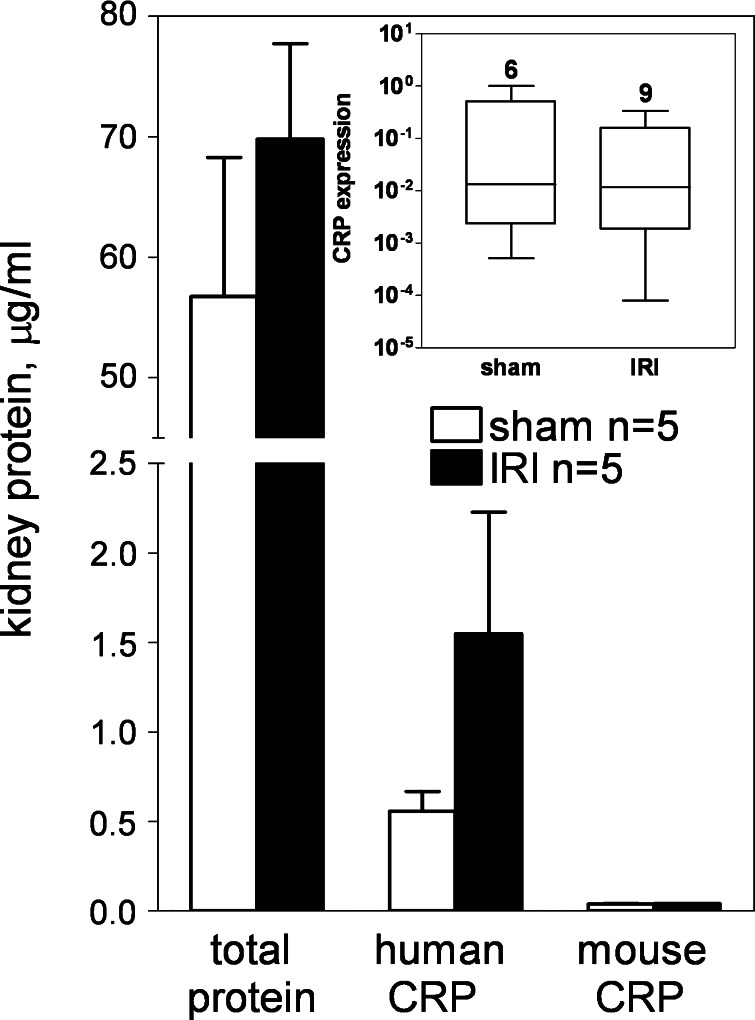
In CRPtg mice subjected to renal IRI, serum human CRP was significantly increased from 10.62 ± 1.31 μg/ml at baseline to 72.01 ± 9.41 μg/ml at 24 h (P < 0.0001, Student's t-test; Fig. 1, closed bars), but there was no significant change in human CRP level following renal surgery without IRI. Mouse CRP levels were not significantly affected by either treatment (Fig. 1, open bars). Since both human and mouse CRP are synthesized primarily in the liver (6, 8, 11) and only human CRP is a major acute phase reactant (6, 8, 11, 46), these blood CRP responses are consistent with the earlier proposition (9) that renal IRI-mediated signals communicating with the liver can evoke a systemic acute phase response. Human and mouse CRP were recovered from perfused kidneys of CRPtg mice subjected to both sham and IRI treatments; although the difference was not statistically significant, an IRI-associated increase in renal CRP of approximately threefold was evident for the human protein (Fig. 2). In a separate experiment (Fig. 2, inset) we found no evidence that the approximately threefold increased recovery of human CRP from injured kidneys was attributable to increased local expression of the human CRP transgene. In their sum, the data shown in Figs. 1 and 2 are consistent with accumulation of hepatically expressed/blood borne CRP in the kidney after IRI.
Fig. 1.
Serum C-reactive protein (CRP) response to renal ischemia-reperfusion injury (IRI). Human CRP (black bars) and mouse CRP (white bars) were measured by ELISA (see materials and methods) in sera collected from CRP transgenic mice (CRPtg) 24 h before and 24 h after sham surgery (sham) or renal ischemia-reperfusion injury (IRI). Each bar and whisker indicates a group means ± SE. *P < 0.0001 and nsP > 0.05, for unpaired t-tests comparing −24-h vs. 24-h values for the bracketed groups. #P < 0.005, for unpaired t-test comparing 24-h values of human CRP (sham treated vs. IRI treated). Sample sizes are indicated.
Fig. 2.
Renal CRP response to renal IRI. Total protein and human and mouse CRP recovered from CRPtg kidneys 24 h after sham or IRI surgeries was measured by ELISA. Each bar and whisker indicate the group means ± SE. Inset: box-and-whisker plot showing expression of human CRP mRNA (relative to GAPDH) in kidneys of CRPtg subjected to IRI vs. sham surgery. Sample sizes are given.
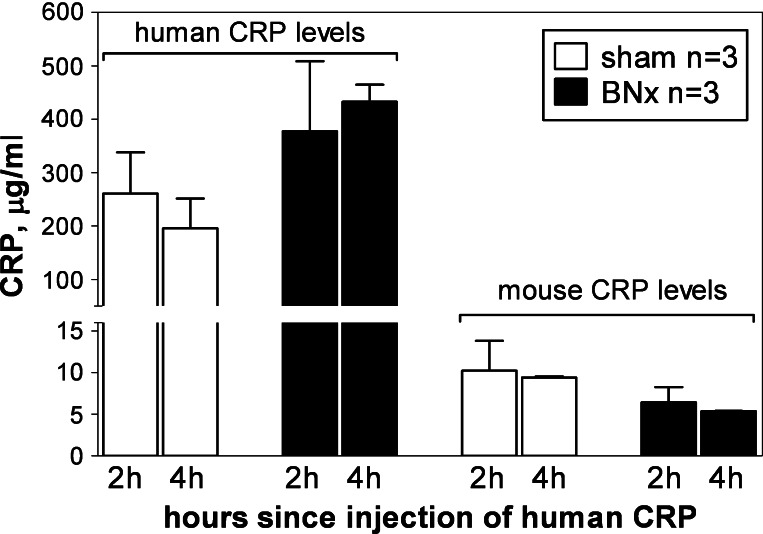
Despite the much higher level of human CRP than mouse CRP in the blood and in the kidneys after renal IRI (Figs. 1 and 2, respectively), the amount of human CRP detected in the voided urine (0.339 ± 0.061 μg/ml) was not increased compared with mouse CRP (0.405 ± 0.229 μg/ml). This finding suggested that reduced renal clearance was not likely a major reason for the observed increase in human CRP in the blood and kidney following AKI. To test directly if IRI-induced elevation of blood human CRP was due to reduced renal clearance, we compared the blood clearance of human CRP injected intravenously into WT mice that had undergone BNx. As shown in Fig. 3, neither the amount of human CRP in the circulation 2–4 h after its intravenous administration, nor the amount of endogenously expressed mouse CRP, was significantly different between sham-operated and BNx animals. The combined data thus indicate that elevation of blood-borne human CRP after renal IRI (Fig. 1), and likely its appearance in increased amounts in the injured kidney (Fig. 2), are the consequence of increased hepatic expression of the protein and not because of reduced renal clearance after AKI.
Fig. 3.
Renal clearance of human and mouse CRP. WT mice were given intravenous injections of human CRP after either sham or bilateral nephrectomy (BNx; see materials and methods). Blood was taken 2 and 4 h later and human CRP and mouse CRP levels assessed by ELISA. Note the break in the y-axis.
Biomarkers of renal damage correlate to increased human CRP.
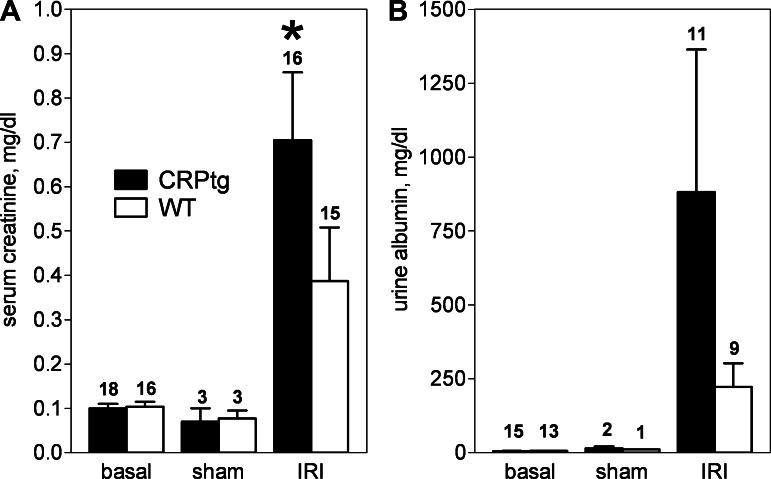
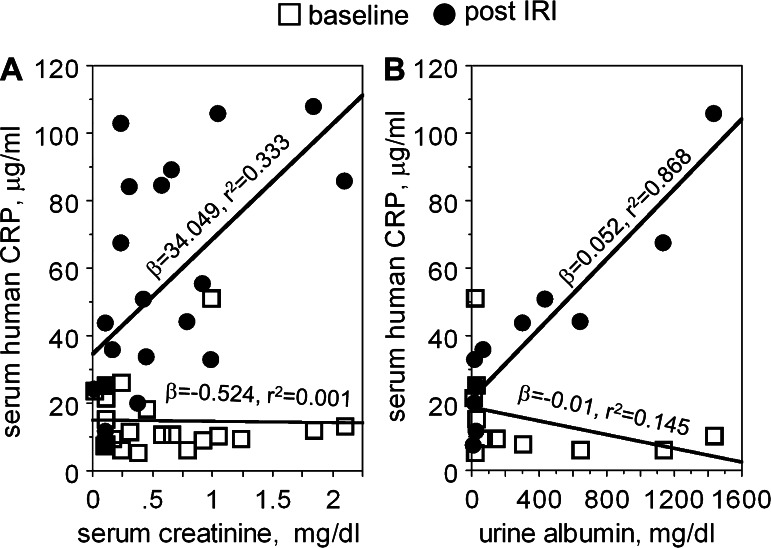
Elevation of both serum creatinine and urine albumin following renal IRI was more pronounced in CRPtg than WT mice (Fig. 4, A and B, respectively), with the difference in serum creatinine levels achieving statistical significance (P < 0.001, one-tailed t-test). Importantly, there was a strong positive association of human CRP serum levels measured 24 h after renal IRI with concurrently measured serum creatinine (Fig. 5A; β = 34.049; r2 = 0.33; P = 0.0067) and urine albumin (Fig. 5B; β = 0.052; r2 = 0.868; P = 0.001). In stark contrast the levels of these biomarkers 24 h after IRI was not associated to baseline levels of human CRP (Fig. 5). Thus, as in humans with AKI, in CRPtg with AKI serum human CRP level associates positively with biomarkers of renal injury. The fact that in CRPtg baseline human CRP does not correlate with biomarkers of kidney damage suggests that CRP might be a modifier of ongoing AKI rather than an initiator of AKI.
Fig. 4.
Biomarkers of acute kidney injury (AKI) after renal ischemia-reperfusion injury. A: serum creatinine 24 h before (basal) and 24 h after surgery (sham or IRI) as measured by tandem mass spectrometry (LC-MS/MS) for CRPtg and wild-type (WT) mice. B: urine albumin as measured by ELISA. *P < 0.001, one-tailed t-test, serum creatinine level was significantly greater in the CRPtg/IRI group than in WT/IRI. Sample sizes are indicated.
Fig. 5.
Association of human CRP with biomarkers of AKI. Blood and urine was collected from CRPtg mice 24 h before (□: baseline) and 24 h after undergoing renal IRI (●: post-IRI) and human CRP and creatinine and albumin levels were determined (see materials and methods). Serum creatinine (A) and urine albumin (B) measured after IRI were positively associated with serum human CRP measured after IRI but not with human CRP baseline levels. The β coefficient and r2 value for each regression line are shown.
Histological evidence of kidney damage is more evident in CRPtg mice subjected to renal IRI.
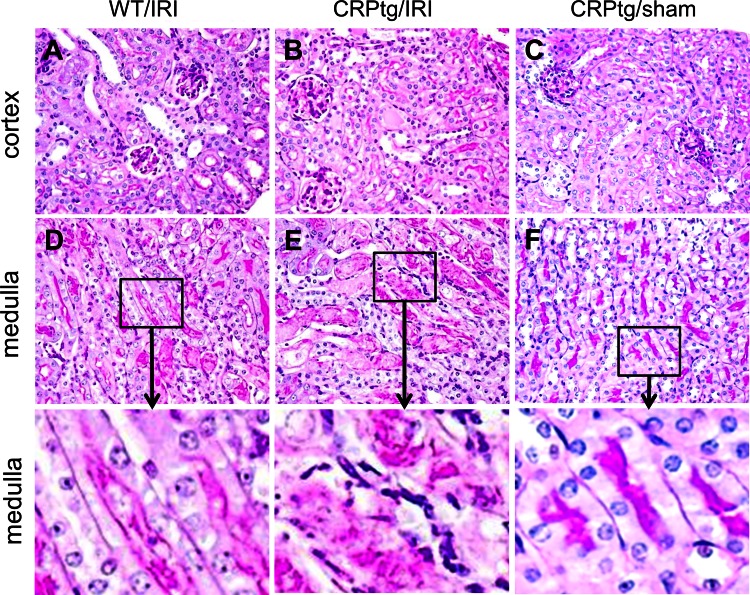
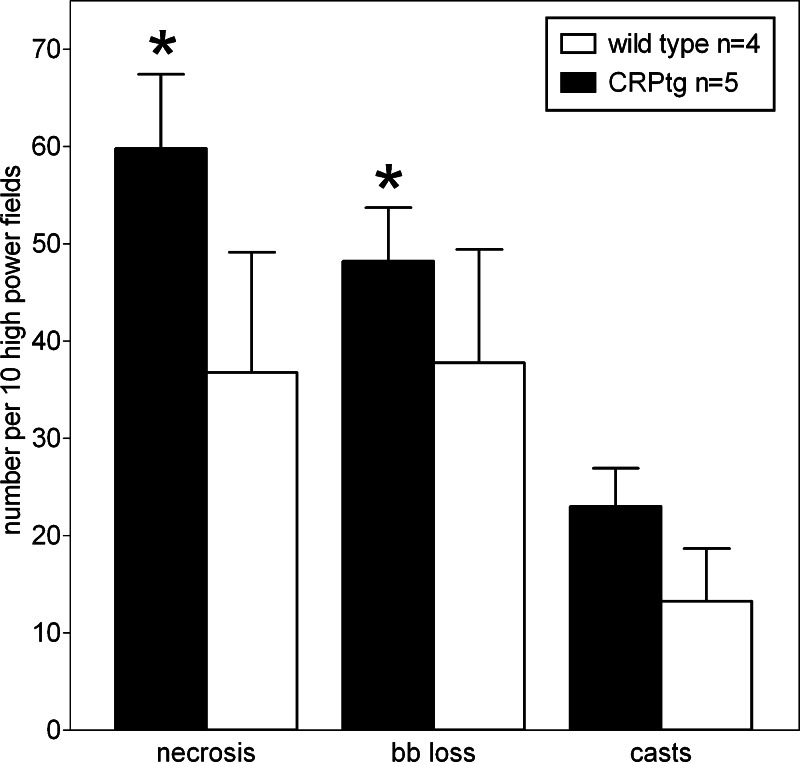
Periodic acid-Schiff-stained sections of kidneys were examined for histological evidence of renal damage. For all animals subjected to renal IRI, kidney damage was more pronounced in the outer medulla than in the cortex (Fig. 6). In alignment with the biomarker data, deleterious tissue changes in the medulla (tubular necrosis, casts, and brush border loss) were more evident in kidneys from CRPtg that had experienced renal IRI (Fig. 6E) than in kidneys from WT mice that had experienced renal IRI (Fig. 6D). Quantitation of the medullary changes verified that tissue necrosis, brush border loss, and the number of renal casts was increased for CRPtg compared with WT following renal IRI (Fig. 7), although the difference in numbers of casts did not achieve statistical significance. Like the human CRP acute phase response (Fig. 1), the worsening of pathology was associated with IRI per se, as the renal medulla in kidneys from CRPtg subjected to sham surgery without renal IRI was largely unaffected (Fig. 6F). These results show that CRP expression affects the tissue injury response to renal IRI.
Fig. 6.
Histological changes after renal IRI. Histology [periodic acid-Schiff (PAS) staining] of the renal cortex (A–C) and outer medulla (D–E) of representative kidneys collected from WT and CRPtg mice 24 h after renal ischemia reperfusion injury (IRI) or sham-surgery (sham). Original magnification of A–F is ×40. Bottom: 4-fold magnifications of the areas indicated in D–F. Note the PAS-positive brush border is intact in F and either damaged or lost in D and E. Also note the extensive tubular casts and necrotic cells in E.
Fig. 7.
Quantitation of histological changes after renal IRI. Tubular necrosis, brush border (bb) loss, and tubular casts were counted in the renal medulla using PAS-stained kidney sections (see materials and methods) collected 24 h after renal IRI surgery. Each bar represents the average (±SE) of 30–50 microscopic fields. *Significant difference compared with the same measure of damage for WT mice (one-tailed t-tests).
Human CRP changes expression of Fcγ receptors and markers of macrophage activation.
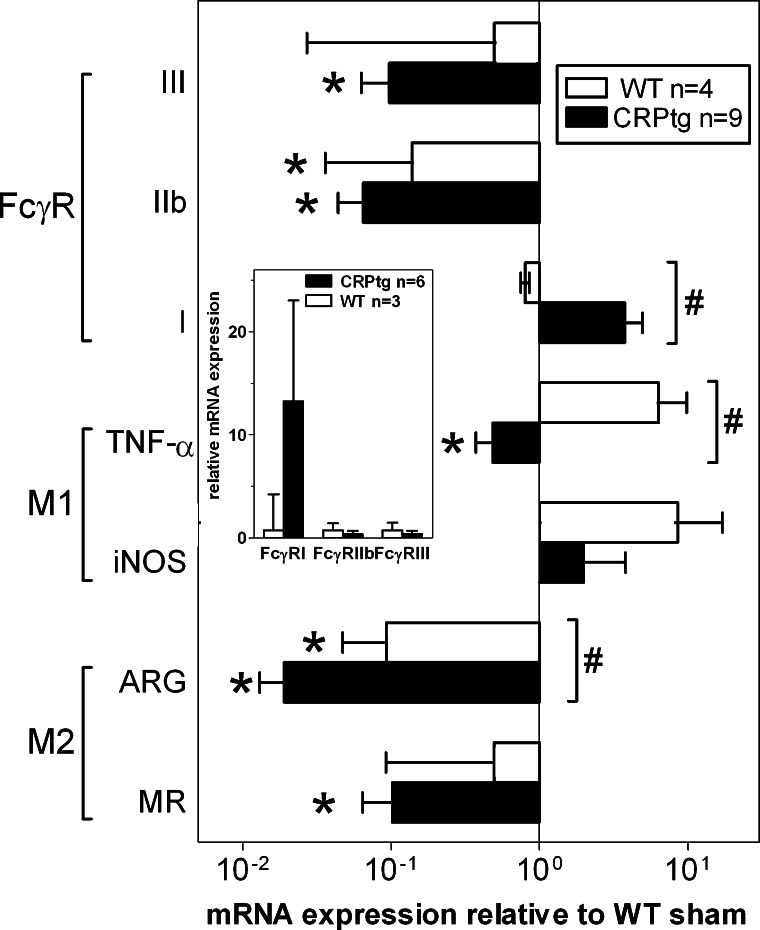
As a first step towards understanding how CRP overexpression might achieve its deleterious effect on renal IRI, we investigated the pattern of mRNA expression in whole kidneys of WT vs. CRPtg mice. Compared with the mRNA levels in kidneys from sham-treated WT mice, in kidneys from IRI-treated WT (Fig. 8, white bars) expression of the M2 macrophage markers mannose receptor and arginase was reduced ∼2-fold and 10-fold, respectively, whereas expression of the M1 macrophage markers iNOS and TNF-α were increased ∼9-fold and ∼6-fold, respectively. In the same WT/IRI mice, expression of the inhibitory receptor FcγRIIB was reduced approximately sevenfold. This pattern is consistent with reports that the early response to renal IRI in mice is dominated by a proinflammatory M1 macrophage response (19, 45) and aligns with the notion that decreased expression of FcγRIIB should de-inhibit macrophage activation (4, 42, 47). In comparison, in kidneys of CRPtg subjected to IRI the upregulation of M1 markers was blunted or reversed and the downregulation of M2 markers more robust (Fig. 8, black bars), consistent with a weaker M2 response in CRPtg than WT. In the same CRPtg kidneys, downregulation of FcγRIIB and FcγRIII was more evident than in treatment-matched WT, but there was a approximately fourfold increase in expression of the activating receptor FcγRI that was not seen in WT kidneys. Notably, kidney expression of FcγRI was also much greater in sham-treated CRPtg than sham-treated WT (Fig. 8, inset). CRP is known to engage all three of the FcγRs whose expression we measured (24, 25, 27), but their expression in the context of AKI and their possible role in propagation of organ damage following AKI have never been investigated. We posit that if FcγRs expressed on macrophages or elsewhere are required for propagating the deleterious action of CRP in the injured kidney, then this could be a consequence of the enhanced expression of the activating FcγRI receptor in CRPtg. In this scenario, any damage propagated by CRP→FcγRI interaction would possibly be more potent because of the IRI-associated suppression of the counteracting FcγRIIB receptor (4, 12, 42, 47).
Fig. 8.
Expression of mRNA for markers of renal inflammation in WT and CRPtg mice subjected to renal IRI. Expression of mRNA for FcγRs, markers of proinflammatory M1 macrophages [inducible nitric oxide synthase (iNOS) and TNF-α], and markers of anti-inflammatory M2 macrophages [arginase (ARG) and mannose receptor (MR)] in whole kidneys from WT (white bars) and CRPtg mice (black bars) subjected to renal IRI. Each bar represents the average mRNA expression after renal IRI, normalized to mRNA levels in sham-treated WT, and the SE is for 9 amplifications of mRNA isolated from at least 3 kidneys each. *P < 0.05, for one sample t-tests of the indicated group vs. WT/sham. #P < 0.05, for two-tailed t-tests of the indicated marker in CRPtg vs. WT. Inset: mRNA expression in kidneys of sham-treated CRPtg compared with sham-treated WT.
To our knowledge, this is the first study that indicates CRP might play an active role in AKI, with CRP worsening the damage caused by renal IRI. Our preliminary analysis indicates acute phase expression of human CRP is associated with diversion of macrophage activation away from an otherwise beneficial M2 phenotype, a process that itself might depend on the observed CRP-associated changes in the balance of activating and inhibitory FcγRs (12). In this AKI setting, increased expression of FcγRI possibly allows human CRP to promote a generally activating effect, which might be enhanced by the concomitant decrease in expression of the inhibitory receptor FcγRIIB. This is consistent with reports that in vitro human CRP polarizes macrophages towards an M1 phenotype and inhibits their transformation to an M2 phenotype in an FcγR-dependant manner (7). A recognized limitation of our study is that because it was limited to investigation of the early injury response, we were unable to compare any effect CRP might have on the later kidney repair process (19). This remains to be determined. Also not yet known is whether the observed changes in renal expression of M1 markers, M2 markers, and FcγRs reflect changes on a single cell type (macrophages) or multiple ones. Analysis of the communities of inflammatory cells recoverable from injured CRPtg vs. WT kidneys is required to address this question, which is currently ongoing. Finally, not consistent with our “weakened M2” model of CRP action in AKI is our finding that TNF-α expression is significantly lower in kidneys from CRPtg mice (Fig. 8).
AKI is a prevalent and potentially lethal condition that arises in many health care settings. It is difficult to predict and diagnose AKI due to the use of biomarkers that lack sensitivity and specificity, and AKI is difficult to treat. Here we have provided evidence that CRP, a widely recognized blood marker of AKI, might exacerbate the injury response after renal IRI, perhaps by altering macrophage polarization and FcγR expression early during the course of injury. It is not yet known if our results will translate to humans, but if they do, targeting CRP (17) might be a potential therapeutic approach to limit tissue damage in AKI.
GRANTS
This research was funded by a Pilot and Feasibility Grant (to A. J. Szalai) from the National Institutes of Health funded UAB-UCSD O'Brien Core Center (National Institute of Diabetes and Digestive and Kidney Diseases Grant 1P30 DK-079337) and a Predoctoral Fellowship (to M. A. Pegues) from the American Heart Association (13PRE14490057).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A.P., A.Z., and A.J.S. conception and design of research; M.A.P. and M.A.M. performed experiments; M.A.P., A.Z., and A.J.S. analyzed data; M.A.P. and A.J.S. interpreted results of experiments; M.A.P. and A.J.S. prepared figures; M.A.P. and A.J.S. drafted manuscript; M.A.P. and A.J.S. edited and revised manuscript; M.A.P. and A.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. James George and Ling Ling Guo at the University of Alabama at Birmingham (UAB)-University of California at San Diego (UCSD) O'Brien Center Small Animal Microsurgery Core Laboratory for assistance with all surgical procedures and Dr. Anupam Agarwal for helpful discussions.
REFERENCES
- 1. Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm 2009: 1–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int 80: 915–925, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Andres-Hernando A, Dursun B, Altmann C, Ahuja N, He Z, Bhargava R, Edelstein CE, Jani A, Hoke TS, Klein C, Faubel S. Cytokine production increases and cytokine clearance decreases in mice with bilateral nephrectomy. Nephrol Dial Transplant 27: 4339–4347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brownlie RJ, Lawlor KE, Niederer HA, Cutler AJ, Xiang Z, Clatworthy MR, Floto RA, Greaves DR, Lyons PA, Smith KG. Distinct cell-specific control of autoimmunity and infection by FcgammaRIIb. J Exp Med 205: 883–895, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao Q, Zheng D, Wang YP, Harris DC. Macrophages and dendritic cells for treating kidney disease. Nephron Exp Nephrol 117: e47–52, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Ciliberto G, Arcone R, Wagner EF, Rüther U. Inducible and tissue-specific expression of human C-reactive protein in transgenic mice. EMBO J 6: 4017–4022, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Devaraj S, Jialal I. C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arterioscler Thromb Vasc Biol 31: 1397–1402, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gabay C, Kushner I. Acute phase proteins and other systemic responses to inflammation. N Engl J Med 340: 448–454, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Grams ME, Rabb H. The distant organ effects of acute kidney injury. Kidney Int 81: 942–948, 2012 [DOI] [PubMed] [Google Scholar]
- 10. Hotta O, Yusa N, Ooyama M, Unno K, Furuta T, Taguma Y. Detection of urinary macrophages expressing the CD16 (Fc gamma RIII) molecule: a novel marker of acute inflammatory glomerular injury. Kidney Int 55: 1927–1934, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Hurlimann J, Thorbecke GJ, Hochwald GM. The liver as the site of C-reactive protein formation. J Exp Med 123: 356–378, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ichii O, Konno A, Sasaki N, Endoh D, Hashimoto Y, Kon Y. Altered balance of inhibitory and active Fc gamma receptors in murine autoimmune glomerulonephritis. Kidney Int 74: 339–347, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Jabs WJ, Lögering BA, Gerke P, Kreft B, Wolber EM, Klinger MH, Fricke L, Steinhoff J. The kidney as a second site of human C-reactive protein formation in vivo. Eur J Immunol 33: 152–161, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Jabs WJ, Meier M, Lamprecht P, Steinhoff J, Nitschke M. Local expression of C-reactive protein is associated with deteriorating graft function in acute and chronic failure of kidney transplants. Nephron Clin Pract 117: c390–c397, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD. C-reactive protein and soluble vascular cell adhesion molecule-1 are associated with elevated urinary albumin excretion but do not explain its link with cardiovascular risk. Arterioscler Thromb Vasc Biol 22: 593–598, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol 130: 41–50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones NR, Pegues MA, McCrory MA, Singleton W, Bethune C, Baker BF, Norris DA, Crooke RM, Graham MJ, Szalai AJ. A selective inhibitor of C-reactive translation is efficacious in vitro and in C-reactive protein transgenic mice and humans. Mol Ther Nucleic Acids 1: e52, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant 23: 842–852, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee DW, Faubel S, Edelstein CL. Cytokines in acute kidney injury (AKI). Clin Nephrol 76: 165–173, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Li ZI, Chung AC, Zhou L, Huang XR, Liu F, Fu P, Fan JM, Szalai AJ, Lan HY. C-reactive protein promotes acute renal inflammation and fibrosis in unilateral ureteral obstructive nephropathy in mice. Lab Invest 91: 837–851, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Liaño F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 50: 811–818, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Liu F, Chen HY, Huang XR, Chung AC, Zhou L, Fu P, Szalai AJ, Lan HY. C-reactive protein promotes diabetic kidney disease in a mouse model of type 1 diabetes. Diabetologia 54: 2713–2723, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Lu J, Marjon KD, Marnell LL, Wang R, Mold C, Du Clos TW, Sun P. Recognition and functional activation of the human IgA receptor (FcalphaRI) by C-reactive protein. Proc Natl Acad Sci USA 108: 4974–4979, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manolov DE, Röcker C, Hombach V, Nienhaus GU, Torzewski J. Ultrasensitive confocal fluorescence microscopy of C-reactive protein interacting with FcgammaRIIa. Arterioscler Thromb Vasc Biol 24: 2372–2377, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Mittalhenkle A, Stehman-Breen CO, Shlipak MG, Fried LF, Katz R, Young BA, Seliger S, Gillen D, Newman AB, Psaty BM, Siscovick D. Cardiovascular risk factors and incident acute renal failure in older adults: the cardiovascular health study. Clin J Am Soc Nephrol 3: 450–456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mold C, Baca R, Du Clos TW. Serum amyloid P component and C-reactive protein opsonize apoptotic cells for phagocytosis through Fcgamma receptors. J Autoimmun 19: 147–154, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 104: 2224–2234, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med 109: 665–678, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Ozdemir NF, Elsurer R, Ibis A, Arat Z, Haberal M. Serum C-reactive protein surge in renal transplant recipients: link with allograft survival. Transplant Proc 39: 934–937, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem 279: 52282–52292, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodriguez W, Mold C, Kataranovski M, Hutt JA, Marnell LL, Verbeek JS, Du Clos TW. C-reactive protein mediated suppression of nephrotoxic nephritis: role of macrophages, complement, and Fcgamma receptors. J Immunol 178: 530–538, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 107: 87–92, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Star RA. Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Stein MP, Edberg JC, Kimberly RP, Mangan EK, Bharadwaj D, Mold C, Du Clos TW. C-reactive protein binding to FcgammaRIIa on human monocytes and neutrophils is allele-specific. J Clin Invest 105: 369–376, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stuveling EM, Hillege HL, Bakker SJ, Gans RO, De Jong PE, De Zeeuw D. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int 63: 654–661, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Szalai AJ, van Ginkel FW, Dalrymple SA, Murray R, McGhee JR, Volanakis JE. Testosterone and IL-6 requirements for human C-reactive protein gene expression in transgenic mice. J Immunol 160: 5294–5299, 1998 [PubMed] [Google Scholar]
- 41. Szalai AJ, Weaver CT, McCrory MA, van Ginkel FW, Reiman RM, Kearney JF, Marion TN, Volanakis JE. Delayed lupus onset in (NZB x NZW)F1 mice expressing a human C-reactive protein transgene. Arthritis Rheum 48: 1602–1611, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Tridandapani S, Siefker K, Teillaud JL, Carter JE, Wewers MD, Anderson CL. Regulated expression and inhibitory function of FcgammaRIIb in human monocytic cells. J Biol Chem 277: 5082–5089, 2002 [DOI] [PubMed] [Google Scholar]
- 43. van Ree RM, Oterdoom LH, de Vries AP, Gansevoort RT, van der Heide JJ, van Son WJ, Ploeg RJ, de Jong PE, Gans RO, Bakker SJ. Elevated levels of C-reactive protein independently predict accelerated deterioration of graft function in renal transplant recipients. Nephrol Dial Transplant 22: 246–253, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol 38: 189–197, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Wang Y, Cao Q, Zheng G, Lee VW, Zheng D, Li X, Tan TK, Harris DC. By homing to the kidney, activated macrophages potently exacerbate renal injury. Am J Pathol 172: 1491–1499, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Whitehead AS, Zahedi K, Rits M, Mortensen RF, Lelias JM. Mouse C-reactive protein. Generation of cDNA clones, structural analysis, and induction of mRNA during inflammation. Biochem J 266: 283–290, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wijngaarden S, van de Winkel JG, Jacobs KM, Bijlsma JW, Lafeber FP, van Roon JA. A shift in the balance of inhibitory and activating Fcgamma receptors on monocytes toward the inhibitory Fcgamma receptor IIb is associated with prevention of monocyte activation in rheumatoid arthritis. Arthritis Rheum 50: 3878–3887, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Xie Q, Zhou Y, Xu Z, Yang Y, Kuang D, You H, Ma S, Hao C, Gu Y, Lin S, Ding F. The ratio of CRP to prealbumin levels predict mortality in patients with hospital-acquired acute kidney injury. BMC Nephrol 12: 30, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 35: 469–476, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Zager RA, Johnson AC, Lund S, Hanson S. Acute renal failure: determinants and characteristics of the injury-induced hyperinflammatory response. Am J Physiol Renal Physiol 291: F546–F556, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Zarjou A, Kim J, Traylor AM, Sanders PW, Balla J, Agarwal A, Curtis LM. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Renal Physiol 300: F254–F262, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]