Abstract
We investigated the relationship among oxidative stress, hypertension, renal injury, and angiotensin-converting enzyme-2 (ACE2) expression in type 1 diabetic Akita mice. Blood glucose, blood pressure, and albuminuria were monitored for up to 5 mo in adult male Akita and Akita catalase (Cat) transgenic (Tg) mice specifically overexpressing Cat, a key antioxidant enzyme in their renal proximal tubular cells (RPTCs). Same-age non-Akita littermates and Cat-Tg mice served as controls. In separate studies, adult male Akita mice (14 wk) were treated with ANG 1–7 (500 μg·kg−1·day−1 sc) ± A-779, an antagonist of the Mas receptor (10 mg·kg−1·day−1 sc), and euthanized at the age of 18 wk. The left kidneys were processed for histology and apoptosis studies. Renal proximal tubules were isolated from the right kidneys to assess protein and gene expression. Urinary angiotensinogen (AGT), angiotensin II (ANG II), and ANG 1–7 were quantified by specific ELISAs. Overexpression of Cat attenuated renal oxidative stress; prevented hypertension; normalized RPTC ACE2 expression and urinary ANG 1–7 levels (both were low in Akita mice); ameliorated glomerular filtration rate, albuminuria, kidney hypertrophy, tubulointerstitial fibrosis, and tubular apoptosis; and suppressed profibrotic and proapoptotic gene expression in RPTCs of Akita Cat-Tg mice compared with Akita mice. Furthermore, daily administration of ANG 1–7 normalized systemic hypertension in Akita mice, which was reversed by A-779. These data demonstrate that Cat overexpression prevents hypertension and progression of nephropathy and highlight the importance of intrarenal oxidative stress and ACE2 expression contributing to hypertension and renal injury in diabetes.
Keywords: catalase, hypertension, angiotensin-converting enzyme-2, tubulointerstitial fibrosis
oxidative stress is implicated in the progression of diabetic complications. High glucose (25 mM d-glucose) induces the generation of reactive oxygen species (ROS) that contribute to apoptosis in podocytes and mesangial and tubular cells (1, 20, 37). Angiotensin II (ANG II) also stimulates ROS generation via heightened nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase activity in various kidney cell types, whereas antioxidants provide renal protection, in part by ameliorating oxidative stress (19, 36, 43). Taken together, these data strongly link oxidative stress, renin-angiotensin system (RAS) activation, and kidney injury to diabetes.
Human and murine renal proximal tubular cells (RPTCs) express all RAS components (23, 27, 38, 44). In the kidneys, angiotensinogen (AGT, the sole substrate of all angiotensins) is expressed predominantly in RPTCs and sequentially cleaved by renin and angiotensin-converting enzyme (ACE) to yield active ANG II. ANG II is then cleaved by angiotensin-converting enzyme-2 (ACE2) yielding angiotensin 1–7 (ANG 1–7), which has opposing actions to ANG II (see recent reviews, Refs.2, 17).
ACE2 shares 40–42% homology with ACE but possesses distinct and different biochemical activities (9, 39). ACE2 specifically cleaves ANG I and ANG II to generate ANG 1–9 and ANG 1–7, respectively. However, ACE2 is 400-fold more effective cleaving ANG II than ANG I, resulting in predominant ANG 1–7 formation (34, 41). The identification of Mas as a receptor for ANG 1–7 and genetic deletion of the Mas receptor lead to kidney injury (31), which established ANG 1–7 as a part of the RAS cascade. Recombinant human ACE2 administration attenuates ANG II-dependent and pressure-overload-induced hypertension, myocardial remodeling and renal injury in ACE2 knockout mice (30, 45, 47), supporting an important counterregulatory role for ACE2 in ANG II-mediated cardiac and renal pathology.
We previously examined the role of RAS components in renal injury, reporting for example, that transgenic (Tg) mice specifically overexpressing rat AGT in their RPTCs develop hypertension, albuminuria, and kidney injury (25, 35). Further, in diabetic mice, hyperglycemia and intrarenal AGT overexpression act together to increase hypertension and kidney injury (24). We also recently observed that renal ACE2 expression and urinary ANG 1–7 were lower in type 1 diabetic Akita mice and that treatment with RAS blockers normalized ACE2 expression and prevented hypertension development in these Akita mice (26). The molecular mechanism(s) by which RAS blockade normalizes renal ACE2 expression, however, remains undefined.
Our present study investigated whether oxidative stress is involved in the development of hypertension and nephropathy in Akita mice via downregulation of renal ACE2 expression. We used a cDNA encoding catalase (Cat), an antioxidant enzyme that by converting hydrogen peroxide (H2O2) to water and oxygen mitigates the toxic effects of H2O2. In the kidneys, Cat is localized to the cytoplasm of RPTCs and is not detectable in other parts of the nephron (48). For these reasons, we created Akita Cat-Tg mice that overexpress rat Cat selectively in their RPTCs by cross-breeding heterozygous Akita mice with our established homozygous Tg mice overexpressing rat Cat in RPTCs (4). Here we report that overexpression of Cat in RPTCs of Akita mice normalizes renal ACE2 expression and urinary ANG 1–7 levels; prevents hypertension; attenuates glomerular filtration rate (GFR), renal hypertrophy, tubulointerstitial fibrosis, and tubular apoptosis; and suppresses profibrotic and apoptotic gene expression. Furthermore, daily administration of ANG 1–7 normalized systemic hypertension in Akita mice, which was reversed by the Mas receptor antagonist A-779.
RESEARCH DESIGN AND METHODS
Chemicals and constructs.
ANG 1–7 and the Mas receptor antagonist A-779 (D-Ala7-ANG I/II/1–7) were purchased from Bachem (Torrence, CA). The following antibodies were used: bovine Cat polyclonal antibody and β-actin monoclonal antibody (Sigma-Aldrich Canada, Oakville, ON, Canada); polyclonal anti-heme oxygenase-1 (HO-1; Assay Designs, Ann Arbor, MI); monoclonal anti-collagen type IV antibody (Chemicon International, Temecula, CA); polyclonal anti-TIM-1/KIM-1/HAVCR, anti-hLAP/TGF-β1, and anti-ACE2 antibody (R&D Systems, Minneapolis, MN); and polyclonal anti-transforming growth factor-β1 (TGF-β1), monoclonal anti-aquaporin-1, and anti-ACE antibody (Santa Cruz Biotechnology, Santa Cruz, CA). A rabbit polyclonal antibody against rAGT was generated in our laboratory [Chan and colleagues (42)]. This antibody is specific for intact rat and mouse AGT (55–62 kDa) and does not cross-react with pituitary hormone preparations or other rat or mouse plasma proteins. pKAP2 plasmid containing the kidney-specific androgen-regulated protein (KAP) promoter responsive to testosterone stimulation was a gift from Dr. Curt Sigmund (University of Iowa, Iowa, IA) (8). Oligonucleotides were synthesized by Invitrogen (Burlington, ON, Canada). Restriction and modifying enzymes were purchased from Invitrogen, Roche Biochemicals (Dorval, QC, Canada), or GE Healthcare Life Sciences (Baie d'Urfé, QC, Canada).
Generation of Akita Tg mice overexpressing rat Cat.
Tg mice (C57Bl/6 background) that overexpress rat Cat-HA [HA-tag, a sequence encoding amino acid residues 98–106 (YPYDVPDYA) of human influenza virus hemagglutinin] in their RPTCs (line no. 688) driven by the KAP gene promoter were created in our laboratory [Chan and colleagues (4)] and have been described elsewhere. Homozygous Cat-Tg mice were then crossed with heterozygous Akita mice (C57BL/6-Ins2Akita/J; Jackson Laboratories, Bar Harbor, ME; http://jaxmice.jax.org; N.B., homozygous Akita mice are infertile). Breeding was continued until Akita Cat-Tg mice were obtained. These mice are homozygous for the Cat transgene but heterozygous for the insulin2 gene mutation. The presence of the Cat-HA transgene in the Akita Cat-Tg mouse was confirmed by PCR of genomic DNA with specific primers against the Cat-HA transgene (4) and the mutated insulin2 gene (26, 46). The mutation in the insulin2 gene was identified by Fnu4H1 digestion (1 h at 37°C) of the PCR product revealing two fragments (280 and 140 bp) separated on 3% agarose gel electrophoresis.
Physiological studies.
Male adult non-Akita littermates, Akita, Cat-Tg, and Akita Cat-Tg mice (8 mice per group) were studied. All animals had ad libitum access to standard mouse chow and water. Animal care and procedures were approved by the Centre Hospitalier de l'Université de Montréal (CRCHUM) Animal Care Committee.
Systolic blood pressure (SBP) was monitored in the morning with a BP-2000 tail-cuff pressure monitor (Visitech Systems, Apex, NC) at least two to three times per week per animal, for 12 wk (24–26, 35). The mice were habituated to the procedure for at least 15–20 min per day for 5 days before the first SBP measurements. SBP values are expressed as the means ± SE. All animals were housed individually in metabolic cages for 24 h before euthanasia at the age of 20 wk. Body weight was recorded. Urine was collected and assayed for albumin and creatinine by ELISAs (Albuwell and Creatinine Companion; Exocell, Philadelphia, PA; Refs. 24–26, 35). Immediately following euthanasia, the kidneys were removed, decapsulated, and weighed. The left kidneys were processed for histology and immunostaining, and the right kidneys were harvested for isolation of renal proximal tubules (RPTs) by Percoll gradient (24–26, 35).
In separate studies, adult male Akita mice (age 14 wk) were treated subcutaneously with ANG 1–7 (500 μg·/kg−1·day−1) ± A-779 (10 mg·kg−1·day−1) and euthanized at age 18 wk (6 mice per group). Controls were untreated non-Akita wild type (WT). SBP was measured thrice weekly (24–26, 35).
The GFR was estimated as described by Qi et al. (32), as recommended by the Animal Models of Diabetic Complications (AMDCC; http://www.diacomp.org/) with slight modifications (5).
AGT, ANG 1–7, and ANG II measurement.
Mouse urinary AGT levels were assayed by ELISA (Immuno-Biological Laboratories; IBL America, Minneapolis, MN) and normalized by urinary creatinine levels. Serum and urinary ANG II and ANG 1–7 levels were assayed by specific ELISAs for ANG II and ANG 1–7, respectively, following extraction with a kit (Bachem Americas) (14, 26).
Histology.
Kidney sections (4–5 sections, 3- to 4-μm thick, per kidney) from eight animals per group were stained with PAS or Masson's trichrome and assessed by light microscopy by two independent blinded observers. The collected images were analyzed and quantified using the National Institutes of Health ImageJ software (http://rsb.info.nih.gov/ij/)(14, 24–26).
Mean glomerular volume on 30 random glomerular sections per mouse was assessed by the Weibel method (42a) with Motic Images Plus 2.0 image analysis software (Motic Instrument, Richmond, BC, Canada) (14, 24–26). Tubular luminal areas were measured on renal sections (6 animals/group; 4–5 sections per kidney, 4 random fields per section, 10 tubules around the glomerulus per field) with Motics Image Plus 2.0 image analysis software (14, 24–26). Outer cortical RPTs with similar cross-sectional views and clear nuclear structure were selected. Mean cell volume was estimated by the Nucleator method (15), as described previously (14, 24–26).
Immunohistochemical staining was performed according to the standard avidin-biotin-peroxidase complex method (ABC Staining System; Santa Cruz Biotechnologies) (14, 24–26, 35). Immunostaining with nonimmune normal rabbit serum in non-Akita mouse kidneys served as controls, and no immunostaining was observed (photographs not shown). Oxidative stress in RPTs in vivo was assessed by dihydroethidium (DHE; Sigma) staining in frozen kidney sections (6). In this assay, the nonfluorescent DHE is oxidized to fluorescent ethidium by superoxide anion (O2·−). The results were confirmed by standard immunohistochemical staining for HO-1 (an oxidative stress-inducible gene that confers cellular oxidative stress in vivo; Refs. 14, 18). The percentage of apoptotic RPTCs was estimated semiquantitatively (14, 24–26) by the terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) assay (Roche Diagnostics, Laval, QC, Canada).
Quantitative RT-PCR assays for gene expression.
AGT, ACE2, ACE, TGF-β1, collagen type IV, and β-actin mRNA expression in RPTs was quantified by quantitative RT-PCR with forward and reverse primers as described previously (14, 26).
Western blotting for estimation of protein expression.
Western blotting for ACE2, ACE, and AGT was performed with RPT lysates (14, 26). The membranes were first blotted with anti-ACE2, ACE, or AGT antibodies and then reblotted with anti-β-actin monoclonal antibodies and chemiluminescent developing reagent (Roche Biochemicals). The relative densities of ACE2, ACE, AGT, and β-actin bands were quantified by computerized laser densitometry (ImageQuant software, version 5.1; Molecular Dynamics, Sunnyvale, CA).
Statistical analysis.
Statistical significance between experimental groups was analyzed by Student's t-test or one-way ANOVA and the Bonferroni correction as appropriate. Data are expressed as means ± SE. P < 0.05 was considered to be statistically significant.
RESULTS
RPTC-specific expression of the Cat transgene in Akita and Tg mouse kidneys.
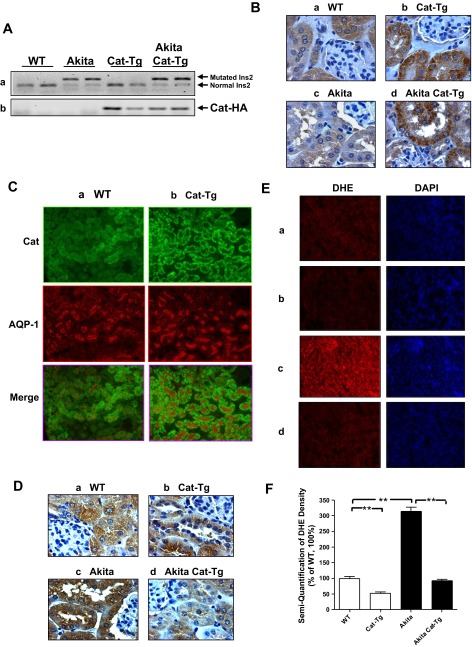
We confirmed the presence of the mutated insulin2 gene in the RPTs of Akita and Akita Cat-Tg mice but not in WT non-Akita or Cat-Tg mice (Fig. 1Aa). Likewise, the Cat-HA transgene was expressed only in RPTs of Cat-Tg and Akita Cat-Tg mice but not in RPTs of WT and Akita mice (Fig. 1Ab). Cat levels were significantly higher in RPTCs in Cat-Tg mice (Fig. 1Bb) and Akita Cat-Tg mice (Fig. 1Bd) than in non-Akita WT mice (Fig. 1Ba) or in Akita mice (Fig. 1Bc). The Cat expression was RPTC-specific and it colocalized to aquaporin-1-positive immunostained RPTCs (Fig. 1C). HO-1 immunostaining and DHE staining demonstrated lower levels of oxidative stress in RPTCs of Cat-Tg (Fig. 1, Db and Eb) than in non-Akita WT mice (Fig. 1, Da and Ea). Akita mice (Fig. 1, Dc and Ec) exhibited significantly higher oxidative stress and normalized in Akita Cat-Tg mice (Fig. 1, Dd and Ed). Quantitation of DHE staining confirmed these findings (Fig. 1F). These results confirm that the KAP gene promoter directs Cat transgene expression in the RPTCs of Cat-Tg and Akita Cat-Tg mice and that Cat expression effectively attenuates ROS production.
Fig. 1.
Generation of Akita catalase (Cat)-transgenic (Tg) mice. Aa: genotyping of the insulin2 mutation in non-Akita and Akita mice. Ab: Cat-HA transgene expression. PCR analysis of Cat-HA transgene in offspring of Cat-Tg line 688 cross-bred with heterozygous Akita mice. Akita Cat-Tg mice displaying Cat-HA transgene were used in subsequent experiments. B: immunohistochemical staining for Cat in male non-Akita wild-type (WT; a), Cat-Tg (b), Akita (c), and Akita Cat-Tg (d) mouse kidneys by employing rabbit anti-bovine Cat polyclonal antibody. Magnification is ×600. C: colocalization of immunostaining of Cat and aquaporin-1 (AQP-1) in male non-Akita WT (a) and Cat-Tg (b) mouse kidneys. Magnification is ×200. D: immunostaining of heme oxygenase-1 (HO-1) in male non-Akita WT (a), Cat-Tg (b), Akita (c), and Akita Cat-Tg (d) mouse kidney by employing HO-1 polyclonal antibody. Magnification is ×600. E: dihydroethidium (DHE; red) staining in mouse kidneys (a-d, same as in D). Magnification is ×200. F: semiquantification of DHE fluorescence in mouse kidneys. Values are expressed as means ± SE; n = 8 per group. **P < 0.01.
Physiological parameters in Akita and Tg mice.
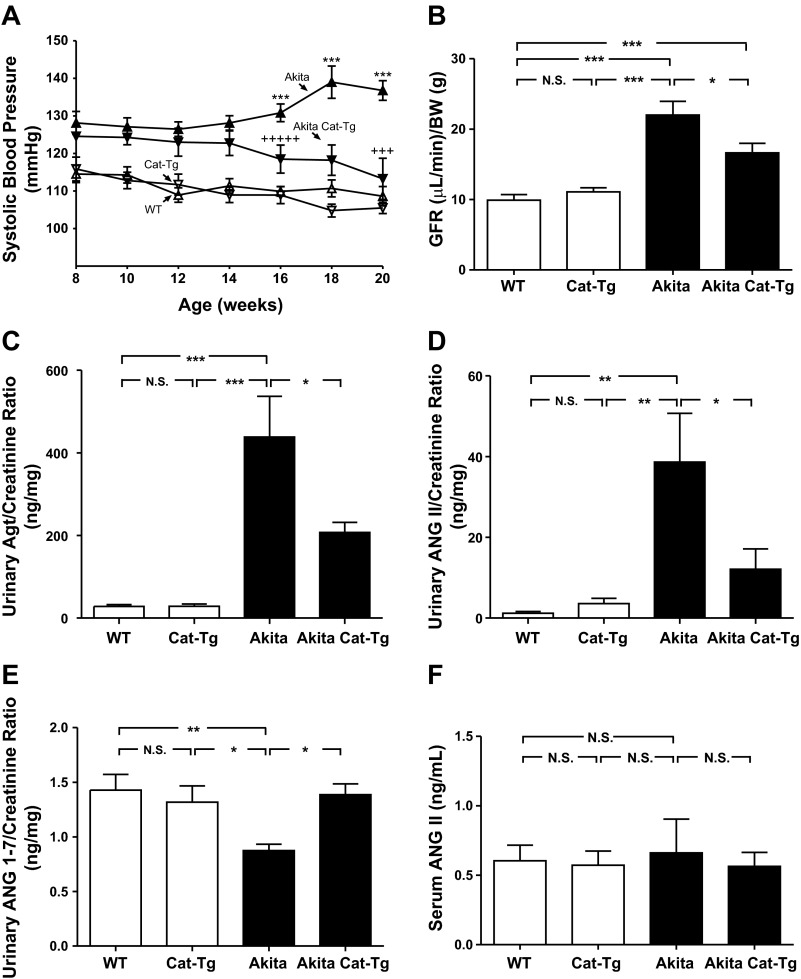
We detected significant differences in SBP between Akita and non-Akita WT mice as early as 8 wk of age. These differences increased with age (from week 14 until week 20; Fig. 2A). There were no significant differences in SBP between the Akita mouse and Akita Cat-Tg mouse until week 16 (Fig. 2A). Overexpression of the Cat transgene protected the Akita Cat-Tg mouse against increased SBP compared with Akita mice. SBP did not differ significantly in Cat-Tg mice and non-Akita WT mice (over time) and at 20 wk (Table 1).
Fig. 2.
Effect of overexpression of Cat in renal proximal tubular cells (RPTCs) on systolic blood pressure (SBP), glomerular filtration rate (GFR), urinary angiotensinogen (AGT), ANG II, and ANG 1–7 levels and serum ANG II level in Akita mice. A: longitudinal changes in mean SBP in male non-Akita WT (△), Cat-Tg (△), Akita (▲), and Akita Cat-Tg (▼). Baseline SBP was measured daily over a 5-day period before initiation of measurement. GFR (B), urinary levels of AGT (C), ANG II (D), and ANG 1–7 (E) and serum levels of ANG II (F) were measured at the age of week 20 in non-Akita WT, Cat-Tg, Akita, and Akita Cat-Tg mice. Values are expressed as means ± SE; n = 8 per group. *P < 0.05; **P < 0.01; ***P < 0.005; N.S., nonsignificant.
Table 1.
Physiological measurements
| WT | Cat-Tg | Akita | Akita Cat-Tg | |
|---|---|---|---|---|
| Blood glucose, mmol/l | 10.84 ± 0.65 | 11.16 ± 0.68 | 34.50 ± 0.72c | 35.10 ± 0.79c |
| Systolic blood pressure, mmHg | 108.6 ± 2.56 | 105.5 ± 1.47 | 136.7 ± 2.6c | 113.25 ± 5.48f |
| Body weight, g | 34.46 ± 0.80 | 34.80 ± 0.39 | 26.71 ± 0.63c | 26.99 ± 0.60c |
| Kidney weight, g | 0.40 ± 0.011 | 0.41 ± 0.011 | 0.63 ± 0.022c | 0.53 ± 0.019c,e |
| Heart weight, g | 0.151 ± 0.007 | 0.159 ± 0.006 | 0.173 ± 0.012 | 0.171 ± 0.011 |
| Kidney-to-body wt ratio, g/g | 0.0118 ± 0.000507 | 0.0119 ± 0.000361 | 0.0234 ± 0.000595c | 0.0201 ± 0.000543f |
| Heart-to-body wt ratio, g/g | 0.0044 ± 0.000117 | 0.0046 ± 0.000168 | 0.0071 ± 0.000441c | 0.0058 ± 0.00023b,d |
| Albumin-to-creatinine ratio, μg·ml−1·mg−1·dl−1 | 0.244 ± 0.027 | 0.174 ± 0.026 | 4.462 ± 1.361c | 2.15 ± 0.553e |
| Glomerular tuft volume, ×103 μm3 | 129.7 ± 3.99 | 137.0 ± 5.20 | 260.7 ± 16.52c | 180.3 ± 9.57c,f |
| RPTC volume, ×103 μm3 | 5.44 ± 0.11 | 4.82 ± 0.05b | 9.68 ± 0.26c | 5.86 ± 0.14f |
| Tubular luminal area, μm2 | 51.38 ± 5.37 | 48.76 ± 5.54 | 105.7 ± 14.55b | 66.27 ± 9.11a,e |
Values are means ± SE. WT, wild-type mice; Cat-Tg, catalase-transgenic mice; RPTC, renal proximal tubular cell.
P < 0.05,
P < 0.01, and
P < 0.005 vs. WT.
P < 0.05,
P < 0.01, and
P < 0.005 vs. Akita.
Interestingly, at 20 wk Akita mice had higher GFR (Fig. 2B), urinary AGT (Fig. 2C), and ANG II levels (Fig. 2D) and lower ANG 1–7 levels (Fig. 2E) than non-Akita mice or Cat-Tg mice. Overexpressing Cat attenuated elevated GFR, urinary AGT, and ANG II levels and normalized ANG 1–7 levels in Akita Cat-Tg mice (Fig. 2, B, C, D, and 2E, respectively). In contrast, serum ANG II levels did not differ significantly among the groups studied (Fig. 2F).
Blood glucose level was significantly higher in Akita and Akita Cat-Tg mice at 20 wk than in non-Akita WT or Cat-Tg mice, respectively (Table 1). Akita mice also exhibited elevated kidney-to-body weight ratio and heart-to-body weight ratio at the end of the experiment compared with non-Akita WT controls or Cat-Tg mice (Table 1). Cat overexpression markedly attenuated, though not completely normalized, these ratios in Akita Cat-Tg mice. Urinary albumin-to-creatinine ratio at 20 wk was significantly higher in Akita mice than in non-Akita WT controls or Cat-Tg mice (Table 1). Cat overexpression partially reduced the albumin-to-creatinine ratio in Akita Cat-Tg mice but did not completely normalize it to WT control levels. These findings indicate that Cat overexpression effectively attenuates kidney and heart hypertrophy and albuminuria without exerting an anti-hyperglycemic effect in the Akita Cat-Tg mice.
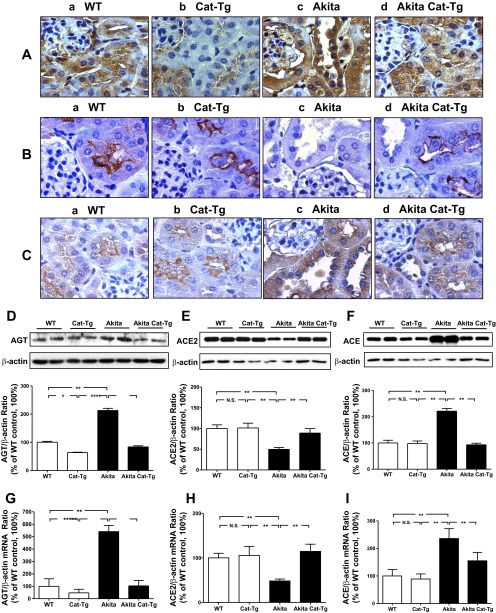
AGT, ACE2 and ACE expression in Akita and Tg kidneys.
We noted higher AGT immunostaining in RPTCs of WT controls (Fig. 3Aa) relative to Cat-Tg mice (Fig. 3Ab). AGT immunostaining was significantly increased in Akita mice (Fig. 3Ac) compared with WT controls and Cat-Tg mice and normalized in Akita Cat-Tg mice (Fig. 3Ad). Expression of ACE2 in the RPTCs of WT non-Akita controls (Fig. 3Ba) or Cat-Tg mice (Fig. 3Bb) was also significantly higher compared with Akita mice (Fig. 3Bc). Overexpression of Cat normalized ACE2 immunostaining in RPTCs of Akita Cat-Tg mice (Fig. 3Bd). In contrast, the RPTCs of WT controls (Fig. 3Ca) or Cat-Tg mice (Fig. 3Cb) exhibited decreased staining for ACE relative to Akita mice (Fig. 3Cc). Overexpressing Cat decreased the level of immunostaining for ACE in the RPTCs of Akita Cat-Tg mice (Fig. 3Cd) vs. Akita mice (Fig. 3Cc).
Fig. 3.
AGT, angiotensin-converting enzyme 2 (ACE2), and ACE expression in mouse kidneys at the age of week 20. AGT (A), ACE2 (B), and ACE (C) immunostaining: a: non-Akita WT control littermate; b: Cat-Tg mouse; c: Akita mouse; d: Akita Cat-Tg mouse. Magnification is ×600. Western blotting of AGT (D), ACE2 (E), and ACE (F) in mouse RPTs. Membranes were reblotted for β-actin. AGT, ACE2, and ACE levels were normalized by corresponding β-actin levels. Values are expressed as means ± SE (n = 8). *P < 0.05; **P < 0.01. RT-quantitative (q)PCR of AGT (G), ACE2 (H), and ACE (I) mRNAs in mouse RPTs. ACE2, ACE, and β-actin mRNAs were run simultaneously in the assays. AGT, ACE2, and ACE mRNA levels were normalized by corresponding β-actin mRNA levels. mRNA levels in non-Akita control littermates were considered as 100%. Values are expressed as means ± SE (n = 8) *P < 0.05; **P < 0.01.
We confirmed these findings by immunoblotting for AGT, ACE2 and ACE (Fig. 3, D–F) and by detecting AGT mRNA, ACE2 mRNA, and ACE mRNA expression (Fig. 3, G–I) in isolated RPTCs by quantitative RT-PCR.
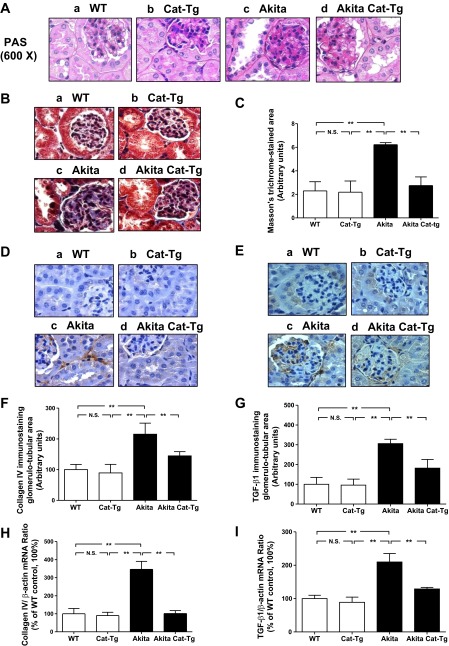
Histology at 20 wk.
Unlike WT non-Akita mice and Cat-Tg mice, Akita mice exhibited renal structural damage (Fig. 4A). Histologic findings included tubular luminal dilation and accumulation of cell debris in the tubular lumen. Some RPTCs were flattened. Remarkably, overexpression of Cat in the Akita Cat-Tg mice markedly suppressed but did not completely prevent these abnormalities. We observed a significantly enlarged tubular luminal area and increased glomerular tuft and RPTC volume in Akita mice compared with non-Akita WT or Cat-Tg mice (Table 1). Overexpression of the Cat transgene partially reduced tubular luminal area and glomerular tuft volume and completely normalized RPTC volume in Akita Cat-Tg mice.
Fig. 4.
Periodic acid-Schiff (PAS) staining, Masson's trichrome staining, collagen IV and TGF-β1 expression in mouse kidneys at the age of week 20. A: PAS staining. B: Masson's trichrome staining. C: quantification of extracellular matrix component accumulation (Masson's trichrome staining). D: immunostaining for collagen IV. E: immunostaining for TGF-β1. For A, B, D, and E: a: non-Akita WT control littermate; b: Cat-Tg mouse; c: Akita mouse; d: Akita Cat-Tg mouse. Magnification is ×600. F: quantitation of immunoreactive collagen IV deposition. G: quantitation of TGF-β1 immunostaining. RT-qPCR of collagen IV (H) and TGF-β1 (I) mRNA. Values are expressed as means ± SE (n = 8) **P < 0.01.
Tubulointerstitial fibrosis and profibrotic gene expression in Akita and Tg kidneys.
We assessed expression of collagenous components with Masson's trichrome staining and by immunostaining for collagen type IV or TGF-β1. Kidneys from non-Akita WT (Fig. 4, Ba, Da, and Ea) or Cat-Tg mice (Fig. 4, Bb, Db, and Eb) exhibited significantly lower expression of collagenous components, collagen type IV and TGF-β1 relative to Akita mice (Fig. 4, Bc, Dc, and Ec). Cat overexpression markedly reduced tubulointerstitial fibrosis (Fig. 4, Bd, Dd, and Ed) as demonstrated by quantitative analysis of Masson's trichrome staining (Fig. 4C) and immunostaining for collagen IV (Fig. 4F) and TGF-β1 (Fig. 4G). Quantitation of collagen IV (Fig. 4H) and TGF-β1 mRNA (Fig. 4I) expression further confirmed these findings. Collectively, these data indicate that Cat overexpression effectively prevents tubulointerstitial fibrosis in Akita Cat-Tg mice.
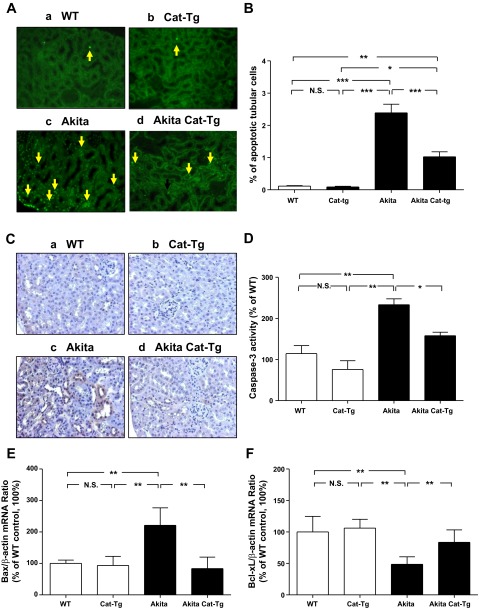
Tubular apoptosis in Akita and Tg kidneys.
Next, we investigated the impact of Cat overexpression on tubular apoptosis in Akita mice by the TUNEL assay. The number of TUNEL-positive nuclei in RPTCs of non-Akita WT mice (Fig. 5Aa) or Cat-Tg mice (Fig. 5Ab) were significantly lower than in Akita mice (Fig. 5Ac). Cat overexpression significantly reduced the number of TUNEL-positive cells in Akita Cat-Tg mice (Fig. 5Ad), and this was confirmed by semi-quantitation (Fig. 5B). Consistently, expression of active (cleaved) caspase-3 was also lower in non-Akita WT (Fig. 5Ca) and Cat-Tg mice (Fig. 5Cb) but was higher in RPTCs of Akita mice (Fig. 5Cc). Cat overexpression attenuated expression of active caspase-3 in Akita Cat-Tg mice (Fig. 5Cd). Caspase-3 activity assays in isolated RPTs confirmed these findings (Fig. 5D).
Fig. 5.
Apoptosis in mouse kidneys at the age of week 20. A: terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL; green) staining. Magnification is ×200. Arrows indicate apoptotic cells in proximal tubules. B: semiquantitation of apoptotic RPTCs in mouse kidneys. Immunostaining for cleaved (active) caspase-3 (C) and caspase-3 (D) activity in isolated RPTs. For A and C: a: non-Akita WT control littermate; b: Cat-Tg mouse; c: Akita mouse; d: Akita Cat-Tg mouse. Magnification is ×200. RT-qPCR for Bax (E) and Bcl-xL (F) mRNA. Bax, Bcl-xL, and β-actin mRNAs were run simultaneously in the assays. Bax and Bcl-xL mRNA levels were normalized by corresponding β-actin mRNA levels. mRNA levels in non-Akita control littermates were considered as 100%. Values are expressed as means ± SE (n = 8) *P < 0.05; **P < 0.01; ***P < 0.005.
Akita mice also exhibited increased expression of Bax and Bcl-2 mRNA (Fig. 5E) and decreased expression of Bcl-xL mRNA (Fig. 5F) compared with non-Akita WT mice or Cat-Tg mice. mRNA expression was normalized in Akita Cat-Tg mice (Fig. 5, E and F).
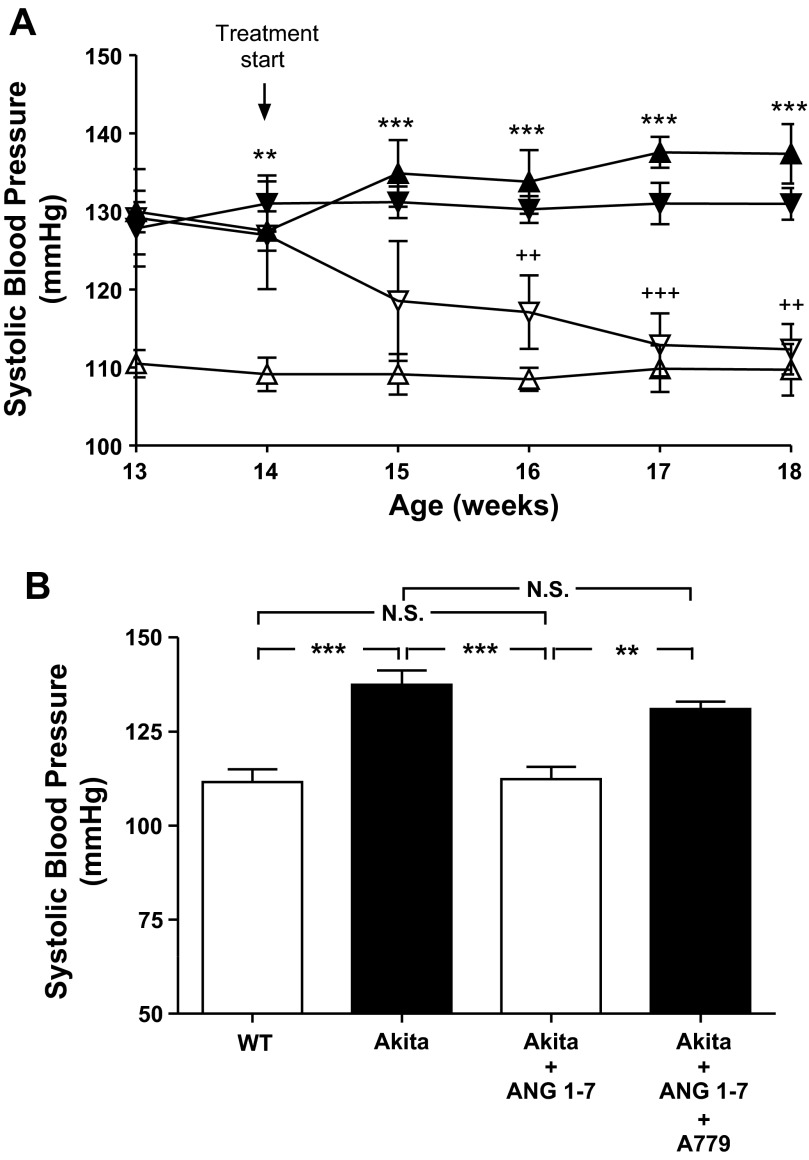
Effect of ANG 1–7 administration on SBP in Akita mice.
Mean SBP was significantly higher in Akita mice at 13 wk of age than in WT mice (Fig. 6A) and remained higher for the duration of the study. Daily administration of ANG 1–7 for 4 wk normalized SBP in Akita mice. Coadministration of ANG 1–7 with A-779 resulted in increases in SBP (Fig. 6, A and B).
Fig. 6.
Effect of ANG 1–7 with or without A-779 on SBP in Akita mice. A: longitudinal changes in mean SBP in male non-Akita WT mice (△), Akita mice (▲), Akita mice treated with ANG 1–7 (▽), and Akita mice treated with ANG 1–7 and A-779 (▼). Baseline SBP was measured daily over a 5-day period before initiation of treatment. B: cross-sectional analysis of SBP measured 2–3 times per animal per week in the morning without fasting, at the age of week 18 in non-Akita WT mice, Akita mice, Akita mice treated with ANG 1–7, and Akita mice treated with ANG 1–7 and A-779. Values are expressed as means ± SE (n = 6 per group). *P < 0.05; **P < 0.01; ***P < 0.005.
DISCUSSION
Our study demonstrates that Cat overexpression in RPTCs of Akita mice effectively attenuates oxidative stress, prevents systemic hypertension and renal injury, normalizes ACE2 expression in RPTCs, and suppresses AGT, profibrotic, and proapoptotic gene expression. Our observations indicate that ROS level in RPTC plays a critical role in regulating renal AGT gene expression and RAS activation and subsequently SBP and kidney injury in vivo.
Expanding our previous findings that overexpression of Cat in RPTCs prevents hypertension and attenuates tubulointerstitial fibrosis and RPTC apoptosis in nondiabetic AGT/Cat-Tg mice and type 2 diabetic db/db Cat-Tg mice (3, 14), here we demonstrate that overexpression of Cat also enhances renal ACE2 expression and ANG 1–7 formation. Administration of ANG 1–7 prevents systemic hypertension in Akita mice, indicating that intrarenal ANG 1–7 formation is critical in counteracting ANG II actions in Akita mice. These findings highlight an important role for intrarenal ACE2 expression and ANG 1–7 formation in preventing development of hypertension and nephropathy in diabetic mice.
The Akita mouse, an autosomal dominant model of spontaneous type 1 diabetes in which the insulin2 gene is mutated, have decreased numbers of β-cells of the pancreatic islets and develop hyperglycemia at age of 3–4 wk (46). By the age of 30 wk, male Akita mice manifest impaired renal function and increased oxidative stress markers in their RPTs (40) and elevated kidney-to-body weight and heart-to-body weight ratios, closely resembling those observed in patients with type 1 diabetes.
Our data indicate that mitigating oxidative stress via kidney-specific overexpression of Cat protects diabetes-prone Akita mice against hypertension. The mechanisms underlying elevated SBP in Akita mice are largely unknown. The possibility that downregulation of ACE2 gene expression and consequently high ANG II-to-ANG 1–7 ratio facilitates the development of hypertension has received considerable attention (2, 17). Indeed, our present findings demonstrate significantly lower ACE2 and urinary ANG 1–7 and higher ACE, urinary AGT, and ANG II levels in Akita mice than in non-Akita WT or Cat-Tg mice. Cat overexpression in RPTCs normalized these changes. These observations are consistent with our previous findings of markedly elevated ACE and depressed ACE2 expression in the kidneys of AGT-Tg mice (14) as well as with studies on normotensive Lewis rats, which showed that RAS blockade increases cortical ACE2 activity and urinary ANG 1–7 excretion (12). Normal human kidneys express low levels of ACE and high levels of ACE2, and this ratio is reversed in kidneys of hypertensive and diabetic patients (22, 28, 33). Furthermore, ANG II was found to upregulate ACE and downregulate ACE2 expression in HK2 cells in vitro (22). Taken together, these findings lend support to the concept that intrarenal RAS activation upregulates ACE activity and downregulates ACE2 expression via enhanced oxidative stress in RPTCs, ultimately contributing to development of hypertension.
As expected (26), we detected renal structural damage in Akita mice, including tubular luminal dilation, glomerular hypertrophy, and increased RPTC volume. Increases in tubular lumen in Akita mice would indicate enhanced susceptibility of RPTCs to atrophy (21). Furthermore, the occurrence of RPTC injury in Akita mouse kidneys was supported by augmented immunostaining for KIM-1, a molecular marker of RPTC injury (16 and unpublished results). Importantly, selective overexpression of Cat in RPTCs markedly attenuated these morphological changes. Thus hyperglycemia and RAS activation induce kidney damage in diabetes, which could be attenuated by Cat overexpression-mediated inhibition of RAS activation and action.
Since glomerular hyperfiltration and microalbuminuria are early clinical markers of hypertension- or diabetes-induced nephropathy, we monitored GFR and urinary albuminuria and detected enhanced GFR and microalbuminuria in Akita mice at the age of 20 wk. Cat overexpression significantly reduced, although not completely prevented, these changes. These observations imply a link among intrarenal oxidative stress, RAS activation, GFR, and albuminuria. However, the underlying molecular mechanism(s) remain largely unknown. One possible mechanism is that increased intrarenal ANG 1–7 formation and decreased ANG II formation in Akita Cat-Tg mice would prevent or attenuate the actions of ANG II on efferent arterioles, thereby reducing the glomerular pressure (hyperfiltration), glomerular volume, and eventually SBP. Increased urinary ANG 1–7 and reduced ANG II levels in Akita Cat-Tg mice lend support to this notion.
Treatment with ANG 1–7 prevented systemic hypertension in Akita mice, and this can be reversed by A-779. These findings are consistent with attenuation by recombinant human ACE2 of ANG II-dependent and pressure-overload-induced hypertension in ACE2 knockout mice (30, 45, 47), supporting an important counterregulatory role for ANG 1–7 in ANG II-mediated cardiac and renal abnormalities.
We observed modest thickening of the tubular basement membrane in Akita mice, which can be prevented by Cat overexpression. The mechanism by which oxidative stress leads to interstitial fibrosis in Akita mice remains unclear. One possibility is that augmented AGT and ANG II expression via ROS generation stimulates TGF-β1, subsequently enhancing the expression of extracellular matrix proteins, collagen type IV, profibrotic, and proapoptotic proteins in RPTCs, with resultant apoptosis and, ultimately interstitial fibrosis (7). Indeed, neutralizing TGF-β alleviates fibrosis and tubular cell apoptosis in animal models of diabetes (49). Our present study shows higher AGT, TGF-β1, and collagen IV expression and lower LAP/TGF-β1 expression (a marker of the inactive TGF-β1 complex; Ref. 10 and unpublished results) in RPTs of Akita mice than in non-Akita mice. These changes were mitigated in Akita Cat-Tg mice, linking intrarenal oxidative stress to interstitial fibrosis.
Confirming our previous findings in the kidneys of nondiabetic AGT-Tg, diabetic AGT-Tg, and Akita mice (14, 24–26), we detected more apoptotic RPTCs in Akita mice, shown by increased percentage of TUNEL-positive RPTCs parallel with increased active caspase-3 immunostaining and Bax mRNA expression and decreased Bcl-xL mRNA expression. An elevated Bax-to-Bcl-xL ratio is consistent with induction of tubular apoptosis in Akita mice. Once again, these changes were mitigated in Akita Cat-Tg mice.
Our results may be clinically relevant for type 1 diabetes. Tubulointerstitial fibrosis and tubular apoptosis occur in human type 1 diabetic kidneys (29), and tubular atrophy appears to be a better indicator of disease progression than glomerular pathology (13); we suggest that tubulointerstitial fibrosis and RPTC apoptosis may be initial events leading to tubular atrophy in diabetes. Oxidative stress-mediated decrease of ACE2 gene expression would further accelerate this process.
In summary, our data indicate a critical role for tubular oxidative stress in the development of hypertension, albuminuria, tubulointerstitial fibrosis, and RPTC apoptosis in Akita mice.
GRANTS
This work was supported in part by grants from the Kidney Foundation of Canada (to J. S. Chan); Canadian Institutes of Health Research Grants MOP-84363, MOP-93650, and MOP-106688 (to J. S. Chan), MOP-86450 (to S. L. Zhang), and MOP-12573 (to J. G. Filep); National Heart, Lung, and Blood Institute Grant HL-48455 (to J. R. Ingelfinger); and Foundation of the CHUM.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.S., C.-S.L., I.C., H.M., and S.-L.Z. performed experiments; Y.S., C.-S.L., I.C., H.M., S.-L.Z., and J.S.D.C. analyzed data; Y.S., C.-S.L., I.C., H.M., S.-L.Z., and J.S.D.C. interpreted results of experiments; Y.S., C.-S.L., I.C., H.M., S.-L.Z., and J.S.D.C. prepared figures; C.-S.L., S.-L.Z., and J.S.D.C. conception and design of research; J.G.F., J.R.I., S.-L.Z., and J.S.D.C. edited and revised manuscript; J.G.F., J.R.I., S.-L.Z., and J.S.D.C. approved final version of manuscript; S.-L.Z. and J.S.D.C. drafted manuscript.
ACKNOWLEDGMENTS
Editorial assistance was provided by the CRCHUM Research Support Office.
This manuscript or any significant part of it is not under consideration for publication elsewhere. The data, however, have been presented in part as free communication at the 42nd Annual Meeting of the American Society of Nephrology, San Diego, CA, Oct. 27 -Nov.1, 2009.
REFERENCES
- 1. Allen DA, Harwood S, Varagunam M, Raftery MJ, Yaqoob MM. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J 17: 908–910, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Batlle D, Wysocki J, Soler MJ, Ranganath K. Angiotensin-converting enzyme 2: enhancing the degradation of angiotensin II as a potential therapy for diabetic nephropathy. Kidney Int 81: 520–528, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Brezniceanu ML, Liu F, Wei CC, Chenier I, Godin N, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes 57: 451–459, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Brezniceanu ML, Liu F, Wei CC, Tran S, Sachetelli S, Zhang SL, Guo DF, Filep JG, Ingelfinger JR, Chan JS. Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int 71: 912–923, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Chang SY, Chen YW, Chenier I, Tran Sle M, Zhang SL. Angiotensin II type II receptor deficiency accelerates the development of nephropathy in type I diabetes via oxidative stress and ACE2. Exp Diab Res 2011: 521076, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen YW, Chenier I, Chang SY, Tran S, Ingelfinger JR, Zhang SL. High glucose promotes nascent nephron apoptosis via NF-κB and p53 pathways. Am J Physiol Renal Physiol 300: F147–F156, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Dai C, Yang J, Liu Y. Transforming growth factor-beta1 potentiates renal tubular epithelial cell death by a mechanism independent of Smad signaling. J Biol Chem 278: 12537–12545, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Ding Y, Sigmund CD. Androgen-dependent regulation of human angiotensinogen expression in KAP-hAGT transgenic mice. Am J Physiol Renal Physiol 280: F54–F60, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87: E1–9, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Ehrhart EJ, Segarini P, Tsang ML, Carroll AG, Barcellos-Hoff MH. Latent transforming growth factor beta1 activation in situ: quantitative and functional evidence after low-dose gamma-irradiation. FASEB J 11: 991–1002, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann Tallant E, Smith RD, Chappell MC. Effects of renin-angiotensin system blockade on renal angiotensin-(1–7) forming enzymes and receptors. Kidney Int 68: 2189–2196, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int 56: 1627–1637, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Godin N, Liu F, Lau GJ, Brezniceanu ML, Chenier I, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Catalase overexpression prevents hypertension and tubular apoptosis in angiotensinogen transgenic mice. Kidney Int 77: 1086–1097, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Gundersen HJ. The nucleator. J Microscopy 151: 3–21, 1988 [DOI] [PubMed] [Google Scholar]
- 16. Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is upregulated in renal cells after injury. J Biol Chem 273: 4135–4142, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Ingelfinger JR. Angiotensin-converting enzyme 2: implications for blood pressure and kidney disease. Curr Opin Nephrol Hypertens 18: 79–84, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Ishizaka N, Aizawa T, Ohno M, Usui Si S, Mori I, Tang SS, Ingelfinger JR, Kimura S, Nagai R. Regulation and localization of HSP70 and HSP25 in the kidney of rats undergoing long-term administration of angiotensin II. Hypertension 39: 122–128, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Jaimes EA, Galceran JM, Raij L. Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int 54: 775–784, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Kang BP, Frencher S, Reddy V, Kessler A, Malhotra A, Meggs LG. High glucose promotes mesangial cell apoptosis by oxidant-dependent mechanism. Am J Physiol Renal Physiol 284: F455–F466, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Kimura M, Asano M, Abe K, Miyazaki M, Suzuki T, Hishida A. Role of atrophic changes in proximal tubular cells in the peritubular deposition of type IV collagen in a rat renal ablation model. Nephrol Dial Transplant 20: 1559–1565, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Koka V, Huang XR, Chung AC, Wang W, Truong LD, Lan HY. Angiotensin II upregulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol 172: 1174–1183, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lai KN, Leung JC, Lai KB, To WY, Yeung VT, Lai FM. Gene expression of the renin-angiotensin system in human kidney. J Hypertens 16: 91–102, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Liu F, Brezniceanu ML, Wei CC, Chenier I, Sachetelli S, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Overexpression of angiotensinogen increases tubular apoptosis in diabetes. J Am Soc Nephrol 19: 269–280, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu F, Wei CC, Wu SJ, Chenier I, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Apocynin attenuates tubular apoptosis and tubulointerstitial fibrosis in transgenic mice independent of hypertension. Kidney Int 75: 156–166, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Lo CS, Liu F, Shi Y, Maachi H, Chenier I, Godin N, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Dual RAS blockade normalizes angiotensin-converting enzyme-2 expression and prevents hypertension and tubular apoptosis in Akita angiotensinogen-transgenic mice. Am J Physiol Renal Physiol 302: F840–F852, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loghman-Adham M, Rohrwasser A, Helin C, Zhang S, Terreros D, Inoue I, Lalouel JM. A conditionally immortalized cell line from murine proximal tubule. Kidney Int 52: 229–239, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Mizuiri S, Hemmi H, Arita M, Ohashi Y, Tanaka Y, Miyagi M, Sakai K, Ishikawa Y, Shibuya K, Hase H, Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis 51: 613–623, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Najafian B, Crosson JT, Kim Y, Mauer M. Glomerulotubular junction abnormalities are associated with proteinuria in type 1 diabetes. J Am Soc Nephrol 17: S53–60, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, Loibner H, Janzek E, Schuster M, Penninger JM, Herzenberg AM, Kassiri Z, Scholey JW. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes 59: 529–538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pinheiro SV, Ferreira AJ, Kitten GT, da Silveira KD, da Silva DA, Santos SH, Gava E, Castro CH, Magalhaes JA, da Mota RK, Botelho-Santos GA, Bader M, Alenina N, Santos RA, Simoes e Silva AC. Genetic deletion of the angiotensin-(1–7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int 75: 1184–1193, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int 74: 1610–1616, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 383: 45–51, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sachetelli S, Liu Q, Zhang SL, Liu F, Hsieh TJ, Brezniceanu ML, Guo DF, Filep JG, Ingelfinger JR, Sigmund CD, Hamet P, Chan JS. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int 69: 1016–1023, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 38. Tang SS, Jung F, Diamant D, Brown D, Bachinsky D, Hellman P, Ingelfinger JR. Temperature-sensitive SV40 immortalized rat proximal tubule cell line has functional renin-angiotensin system. Am J Physiol Renal Fluid Electrolyte Physiol 268: F435–F446, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Ueno Y, Horio F, Uchida K, Naito M, Nomura H, Kato Y, Tsuda T, Toyokuni S, Osawa T. Increase in oxidative stress in kidneys of diabetic Akita mice. Biosci Biotechnol Biochem 66: 869–872, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277: 14838–14843, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Wang L, Lei C, Zhang SL, Roberts KD, Tang SS, Ingelfinger JR, Chan JS. Synergistic effect of dexamethasone and isoproterenol on the expression of angiotensinogen in immortalized rat proximal tubular cells. Kidney Int 53: 287–295, 1998 [DOI] [PubMed] [Google Scholar]
- 42a. Weibel ER. Stereological Methods: Theoretical Foundations. London: Academc, vol 2, 1980, p. 149–152, 1980 [Google Scholar]
- 43. Wolf G. Free radical production and angiotensin. Curr Hypertens Rep 2: 167–173, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Wolf G, Neilson EG. Angiotensin II as a hypertrophogenic cytokine for proximal tubular cells. Kidney Int Suppl 39: S100–107, 1993 [PubMed] [Google Scholar]
- 45. Wysocki J, Ye M, Rodriguez E, Gonzalez-Pacheco FR, Barrios C, Evora K, Schuster M, Loibner H, Brosnihan KB, Ferrario CM, Penninger JM, Batlle D. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension 55: 90–98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 46: 887–894, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Zhong J, Guo D, Chen CB, Wang W, Schuster M, Loibner H, Penninger JM, Scholey JW, Kassiri Z, Oudit GY. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension 57: 314–322, 2011 [DOI] [PubMed] [Google Scholar]
- 48. Zhou Z, Kang YJ. Cellular and subcellular localization of catalase in the heart of transgenic mice. J Histochem Cytochem 48: 585–594, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA 97: 8015–8020, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]