Abstract
Prednisone is a mainstay of treatment for patients with focal segmental glomerulosclerosis (FSGS), a disease characterized by reduced podocyte number and glomerulosclerosis. Although the systemic immune-modulatory effects of prednisone are well-known, direct tissue effects on glomerular cells are poorly understood. Experimental FSGS was induced in mice with a cytotoxic anti-podocyte antibody, resulting in an abrupt decrease in podocyte number by day 3, proteinuria, and the development of glomerulosclerosis. Administering daily prednisone to mice with FSGS, beginning at day 3, significantly increased podocyte number at weeks 2 and 4. Podocyte number did not increase in control mice with FSGS given DMSO. The increase in podocyte number in prednisone-treated mice correlated significantly with reduced glomerulosclerosis. Prednisone reduced podocyte apoptosis measured by synaptopodin+/caspase-3+ double staining. Additionally, the number of podocyte progenitors, defined as cells expressing both a parietal epithelial cell protein and a podocyte protein, was significantly increased in prednisone-treated mice with FSGS at weeks 2 and 4. This was associated with increased phospho-ERK staining in both parietal epithelial cells (PAX2+/p-ERK+) and in podocyte progenitors (WT-1+/p-ERK+ lining Bowman's capsule). These data show that in this model of experimental FSGS, prednisone augments glomerular repair by increasing podocyte number through direct effects on both glomerular epithelial cells. Prednisone limits podocyte loss by reducing apoptosis, and it increases regeneration by augmenting the number of podocyte progenitors. The data support a direct glomerular cell action for prednisone in improving outcomes in FSGS.
Keywords: focal segmental glomerulosclerosis, apoptosis, proteinuria, glomerulosclerosis, prednisone, parietal epithelial cell, CD44, regeneration, repair
focal segmental glomerulosclerosis (FSGS) is the leading cause of nephrotic syndrome in adults (21). In this disease, the podocyte, a terminally differentiated epithelial cell, is the primary target of injury (36). The subsequent decrease in podocyte number is a critical determinant underlying the development of glomerulosclerosis (11, 36, 42). Although FSGS is typically considered a nonimmunological disease, immunosuppressive agents, such as glucocorticosteroids, are commonly used as first line therapy in clinical disease (16, 19). In addition to immunomodulatory effects, the therapeutic benefit of glucocorticoids may be in part due to direct cellular actions on podocytes including limiting apoptosis (40, 41), actin rearrangement (28), downregulating podocyte cytokines (43), and enhanced slit diaphragm complex protein synthesis (13).
Several recent lines of evidence suggest that glomerular parietal epithelial cells (PECs) may serve as progenitor cells for podocytes, and thus may have a potential role in glomerular repair by replacing, in part or completely, any decrease in podocyte number (1, 30, 32–34). Romagnani et al. (33, 34) reported a subset of parietal progenitors was present in normal human glomeruli. Moeller's group (1) showed that podocytes can derive from PECs in adolescent mice. We, and others, showed in certain experimental glomerular diseases such as FSGS, membranous nephropathy, and aging nephropathy that PECs begin to express proteins traditionally considered specific for podocytes, such as WT-1 and synaptopodin (25, 44, 45). Some have called glomerular cells expressing both PEC and podocyte proteins as glomerular epithelial transitional cells or progenitor cells. However, to date no published reports show that this subpopulation of PECs becomes fully functional podocytes.
To our knowledge, the effect of corticosteroids on podocyte number is not known, nor is it known whether corticosteroids enhance glomerular repair by affecting the number of glomerular epithelial progenitor cells. The purpose of this study was to determine whether the positive effects of prednisone in FSGS might be due to an increase in the number of glomerular epithelial progenitor cells (defined as glomerular cells expressing a PEC and podocyte protein) in experimental FSGS characterized by reduced podocyte number.
MATERIALS AND METHODS
Animal Model
Experimental FSGS mouse model.
We previously reported that giving mice sheep anti-glomerular antibody leads to features consistent with classic FSGS: reduced podocyte number, focal glomerulosclerosis, and proteinuria (21). This model differs from an antibody-induced mouse model characterized by glomerular epithelial cell proliferation (17, 23, 39). In this study, baseline data were collected on B6129SV/j mice before experimental FSGS was induced by administration of sheep anti-glomerular antibody (12.5 mg/20 g body wt, for 2 consecutive days) as we previously reported (25, 27, 38, 45). On day 3 of disease, mice were randomized into three groups: 1) seven mice were killed at day 3, 2) 12 age- and weight-matched mice were randomly assigned to receive prednisone (5 times weekly subcutaneous injection of prednisone 1 mg/kg body wt), and 3) 12 diseased mice were assigned to the untreated disease control group to receive DMSO, the vehicle for prednisone. After 2 wk of disease, six animals from the prednisone group, and six from the untreated group, were randomly selected for urine measurement, and then killed. The remaining animals in each group continued to receive prednisone or DMSO until 4 wk, when urine was collected, followed by death. A group of normal age- and weight-matched mice was killed at weeks 2 (n = 3) and 4 (n = 3) to ensure changes were not age related. Mice were housed in the animal care facility of the University of Washington under standardized pathogen-free conditions with food and water available ad libitum. These studies were reviewed and approved by the University of Washington Institutional Animal Care and Use Committee.
Urine Collection and Urine Protein Assay
Mice were placed into individual metabolic cages overnight and spontaneously voided urine was collected for 12 h before disease induction, and 12 h before death. Urine protein concentration was determined using the sulfosalicylic acid method, and urine creatinine was determined using a colorimetric micro-plate assay (Oxford Biomedical Research, Oxford, MI and Cayman Chemical, Ann Arbor, MI), as we previously reported (45).
Immunohistochemistry Staining
Single staining.
Indirect immunoperoxidase staining was performed on formalin-fixed biopsies as previously reported in detail (44, 45) for p57 (podocyte number), synaptopodin (podocyte density), and CD44 (PEC activation). A rabbit anti-Kip2 p57 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-synaptopodin (SYNA, podocyte cytoplasm protein) antibody (Fitzgerald, Concord, MA), and mouse anti-CD44 monoclonal antibody (Abcam) were used and visualized with diaminobenzidine (DAB), brown color (Fisher).
Double immunohistochemistry staining methods.
To identify and quantitate the number of glomerular epithelial progenitor cells that express both podocyte and PEC proteins, and to measure the number of proliferating cells, double staining was performed as we previously reported in detail (44, 45). To measure phosphorylated extracellular signal-regulated kinase 1 and 2 (p-ERK1/2), double staining for p-ERK with PAX2 and WT-1 was performed. The following primary antibodies were used: rabbit anti-rat paired box gene 2 (PAX2, PEC nuclear protein, Zymed Laboratories, South San Francisco, CA), rabbit anti-Wilms' Tumor-1 (WT-1; podocyte nuclear protein) antibody (Santa Cruz Biotechnology), mouse anti-synaptopodin (SYNA, podocyte cytoplasm protein) antibody (Fitzgerald), rabbit anti-Ki-67 (Ki-67, proliferating cell nuclear protein) antibody (Thermo, Fremont, CA), rabbit anti-Kip2 p57 (p57, podocyte nuclear protein) polyclonal antibody (Santa Cruz Biotechnology), and rabbit anti-phospho-p44/42 MAPK (ERK1/2; Thr202/Tyr204; Cell Signaling Technology, Boston, MA). Staining was visualized with the Vector SG substrate kit, blue/gray color and the Warp Red Chromogen Kit, red color (Biocare Medical) and DAB.
Double immunofluorescence staining for caspase-3 and synaptopodin was performed to measure podocyte apoptosis using a rabbit anti-cleaved caspase-3 (Asp175) antibody (Cell Signaling) (23, 24, 35). Cleaved caspase-3 was visualized with Alex 594, red color (Invitrogen) and synaptopodin was visualized with Alex 488, green color (Invitrogen).
Immunofluorescence Staining
Sheep IgG immunofluorescence staining.
To ensure that the effects of prednisone were not due to a decrease in the binding of the anti-glomerular antibody, sheep IgG was stained at 2 and 4 wk using a rabbit anti-sheep IgG H&L (FITC) polyclonal antibody (Abcam, Cambridge, MA) as reported previously (26).
Sirius Red Staining
To measure glomerular sclerosis, Sirius red staining was performed (20). Sections were treated with 0.2% phosphomolybdic acid (MP Biomedicals) for 5 min and then exposed to Picro-Sirius red (Polysciences & Sigma) for 90 min at room temperature, rinsed briefly with acidified water (0.01% HCl) before dehydration and mounting.
Quantitation and Statistical Analysis
Quantification of positively stained cells was performed on individual animals at each time point using a combination of bright field and fluorescent microscopy as we reported (44, 45). Double positive progenitor cells were identified as follows. The presence of blue/gray color in the nucleus by bright field microscopy indicated positive staining for PAX2. If the same nucleus also showed the presence of red color by fluorescent microscopy, this indicated positive staining for WT-1. Or, if the same blue/gray nucleus also showed the presence of brown color in the cytoplasm by bright field microscopy, this indicated positive staining for synaptopodin. This cell was then considered a double positive cell, and the number of these cells within the entire glomerulus [defined as the total number of positive cells lining Bowman's capsule (BC) and in the glomerular tuft] was quantitated. PAX2 and p-ERK, WT-1 and p-ERK double positive cells were defined as the presence of blue/gray color in the nucleus, and brown color in the cytoplasm by bright field microscopy.
Image J software was used to measure the intensity of synaptopodin according to The Image J User Guide (Version 1.44). Briefly, the pixel density represented by synaptopodin staining was measured in each individual glomerulus. This value was divided by the pixel density representing the glomerular tuft area in each individual glomerulus as we described previously (39, 45). The intensity in synaptopodin staining was shown as a percentage of the glomerular tuft area.
Glomerulosclerosis was determined using Sirius red staining, and graded quantitatively by the percentage of glomerular tuft area involvement as follows and as previously reported (30): score 0, no sclerosis; score 1, <25%; score 2, 25–50%; score 3, 50–75%; score 4, 75–100%. Global sclerosis means the glomeruli with over 75% tuft area scleroses, which is score 4.
The mean number of glomeruli analyzed was 45 (95% confidence interval: 18–68) per animal. For multiple comparisons, one-way ANOVA was used and post hoc analyses were performed by least significant difference test. Paired-samples t-test was performed when animal number was equal in each group. Pearson correlation coefficient (r value) analysis was performed on two correlated variables, podocyte number and sclerosis grade, synaptopodin staining intensity and sclerosis grade. P < 0.05 was considered significant. All values were expressed as means ± SE.
RESULTS
Prednisone Improves Renal Function in Experimental FSGS
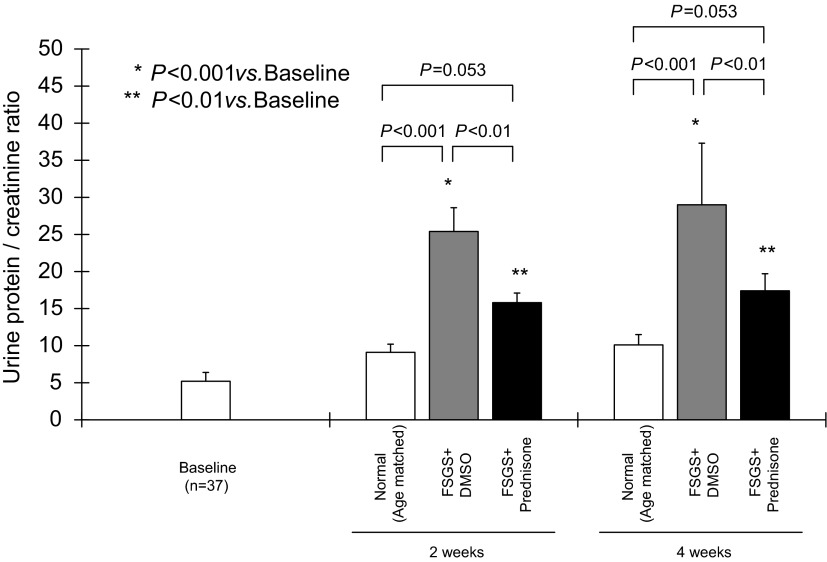
Proteinuria was measured to determine whether kidney function improved in prednisone-treated mice. As expected, the urinary protein:creatinine ratio increased in untreated control mice with FSGS given DMSO at 2 wk (25.4 ± 3.2 vs. 9.1 ± 1.1, normal age-matched mice, P < 0.001) and at 4 wk (29.0 ± 8.3 vs. 10.1 ± 1.4, normal age-matched mice, P < 0.001). Prednisone treatment in mice with FSGS significantly decreased the urinary protein:creatinine ratio both at 2 wk (15.8 ± 1.3 vs. 25.4 ± 3.2, untreated FSGS mice, P < 0.01) and 4 wk (17.4 ± 2.3 vs. 29 ± 8.3, untreated FSGS mice, P < 0.01; Fig. 1).
Fig. 1.
Renal function in experimental focal segmental glomerulosclerosis (FSGS). Renal function, as measured by urine protein:creatinine ratio, was not different in all normal age-matched animals compared with baseline before experiment. Compared with normal mice (open bars), there was an increase in the urine protein:creatinine ratio in mice with FSGS given DMSO at 2 and 4 wk (gray bars). Prednisone treatment decreased the urine protein:creatinine ratio in mice with FSGS at 2 and 4 wk (filled bars).
Prednisone Reduces Glomerulosclerosis in Experimental FSGS
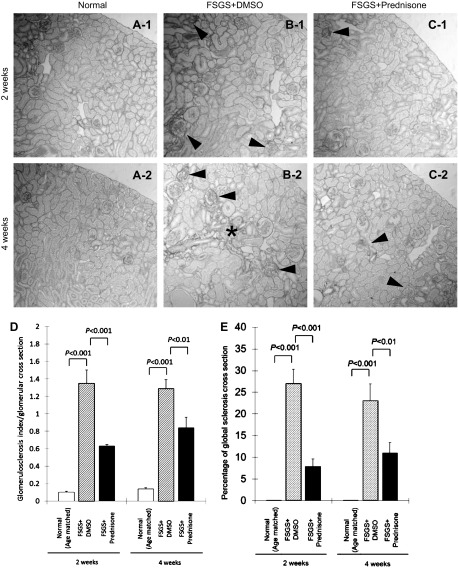
Compared with normal mice, control mice with FSGS given DMSO had a higher glomerulosclerosis index score per glomerular cross section at 2 wk (1.35 ± 0.15 vs. 0.10 ± 0.01, normal mice, P < 0.001) and 4 wk (1.29 ± 0.10 vs. 0.14 ± 0.01, normal mice, P < 0.001). Compared with untreated mice with FSGS, prednisone treatment significantly reduced sclerosis at 2 wk (0.63 ± 0.02 vs. 1.35 ± 0.15, untreated mice with FSGS, P < 0.001) and 4 wk (0.84 ± 0.12 vs. 1.29 ± 0.10, untreated mice with FSGS, P < 0.01; Fig. 2D).
Fig. 2.
Prednisone improves glomerulosclerosis in experimental FSGS. A–C: representative images of Sirius red staining at ×200 original magnification (arrowheads indicate examples of glomerulosclerosis, * shows an example of interstitial fibrosis in untreated mice with FSGS at 4 wk). D: glomerulosclerosis index increased in mice with FSGS given DMSO at 2 and 4 wk (hatched bars) compared with age-matched normal mice (open bars). Prednisone treatment (filled bars) was associated with a decrease in glomerulosclerosis index in mice with FSGS. E: percentage of glomeruli with global sclerosis, defined as over 75% tuft scarring (score 4), increased in mice with FSGS given DMSO (hatched bars). Prednisone treatment significantly decreased global sclerosis at 2 and 4 wk (filled bars).
Untreated mice with FSGS not only had a higher sclerosis index within glomeruli, but also had a significantly higher percentage of glomeruli with global sclerosis (glomeruli with score 4) at 2 wk (27 ± 3.3 vs. 0%, normal mice, P < 0.001) and 4 wk (23 ± 3.9 vs. 0%, normal mice, P < 0.001). Prednisone treatment of mice with FSGS decreased the percentage of glomeruli with global sclerosis both at 2 wk (7.9 ± 1.7 vs. 27 ± 3.3%, untreated mice with FSGS, P < 0.01) and at 4 wk (11 ± 2.4 vs. 23 ± 3.9%, untreated mice with FSGS, P < 0.01; Fig. 2E). These data show that prednisone augments repair by significantly reducing glomerular scarring in this model.
Prednisone Does Not Affect the Binding of the Anti-Glomerular Antibody
Although prednisone was administered for the first time at 3 days after disease induction and therefore antibody deposition, immunostaining for the disease-inducing sheep anti-glomerular antibody was performed. As expected, there was no difference in staining for sheep IgG within the glomerulus between untreated and prednisone-treated mice with FSGS at all time points (data not shown).
Prednisone Increases Podocyte Number in Experimental FSGS
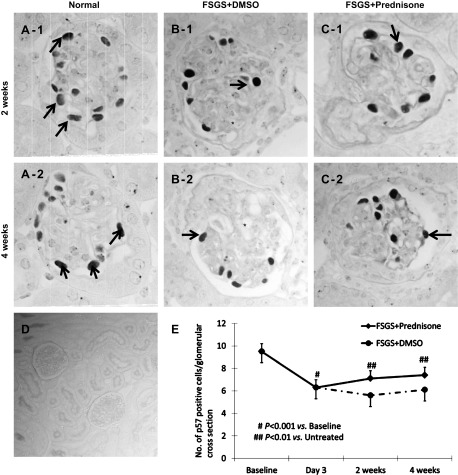
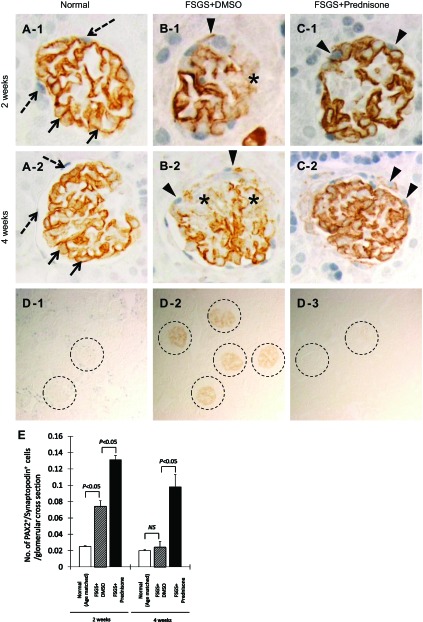
Podocyte number and density were quantitated by the number of glomerular cells staining for p57, and the intensity of synaptopodin staining, respectively. Similar to what we previously reported in this model (5, 6), Fig. 3 shows that in this study, podocyte number was significantly decreased by day 3 of FSGS (6.3 ± 0.4 vs. 9.5 ± 0.5 baseline, p57-positive cells/glomerular cross section in baseline normal mice, P < 0.001). To mimic the typical clinical scenario, prednisone in this study was first given at day 3, when podocyte number was already depleted. In untreated mice with FSGS (those given DMSO), reduced podocyte number persisted at 2 wk (5.6 ± 0.4 vs. 9.5 ± 0.5/glomerulus, baseline normal mice, P < 0.001) and at 4 wk (6.1 ± 0.4 vs. 9.5 ± 0.5/glomerulus, baseline normal mice, P < 0.001; Fig. 3E). In contrast, podocyte number was significantly higher in prednisone-treated mice with FSGS at 2 wk (7.1 ± 0.2 vs. 5.6 ± 0.4 p57-positive cells/glomerular cross section in untreated mice with FSGS, P < 0.05) and 4 wk (7.4 ± 0.3 vs. 6.1 ± 0.4/ glomerulus, untreated mice with FSGS, P < 0.01; Fig. 3E). These data showed that prednisone treatment, initiated following podocyte depletion and after the onset of proteinuria in mice with FSGS, was associated with a higher number of podocytes (measured by p57 staining) compared with untreated diseased mice.
Fig. 3.
Prednisone increases podocyte number in experimental FSGS. A–C: representative images of p57 staining (brown, nuclear) at 2 and 4 wk in normal mice (A-1, A-2), mice with FSGS given DMSO (B-1, B-2), and prednisone-treated mice with FSGS (C-1, C-2; ×630 original magnification). Arrows show examples of positive staining. D: staining is not detected in the negative control where the p57 primary antibody was omitted (×200 original magnification). E: podocyte number, measured as the number of p57-positive cells/glomerular cross section, was significantly depleted by day 3 of FSGS. Podocyte number remained low in mice with FSGS mice given DMSO at 2 and 4 wk (dotted line). Podocyte number increased significantly in mice with FSGS given prednisone (solid line) at 2 and 4 wk. These data show that when prednisone is administered following abrupt podocyte depletion, podocyte number increases compared with controls.
Synaptopodin stains podocytes specifically, and the intensity of synaptopodin staining measured by computer densitometry can be used as a measure of podocyte density (23, 39). The intensity in synaptopodin staining decreased in untreated mice with FSGS at 2 wk (34 ± 5.9 vs. 59 ± 6.7%, area synaptopodin staining/glomerular tuft cross section area in normal mice, P < 0.001) and 4 wk (39 ± 3.0 vs. 58 ± 7.3%, normal mice, P < 0.001). Prednisone treatment significantly increased synaptopodin staining intensity at 2 wk (46 ± 5.1 vs. 34 ± 5.9%, area synaptopodin staining/glomerular tuft cross section area in untreated mice with FSGS, P < 0.01). There was no significant difference between treated and untreated mice with FSGS at 4 wk. These data showed that prednisone treatment initiated after the initial decrease in podocyte number in mice with FSGS was associated with a higher intensity in synaptopodin staining compared with untreated diseased mice.
Finally, the degree of glomerulosclerosis described earlier was correlated with the changes in podocyte number in treated and untreated mice with FSGS. There was a positive correlation between p57-positive cells/glomerular cross section and glomerulosclerosis index, r = −0.701, P < 0.001. There was also a positive correlation between expression intensity of synaptopodin/glomerular cross section and glomerulosclerosis index, r = −0.737, P < 0.001. Taken together, the increase in podocyte number in the prednisone-treated group correlated significantly with decreased glomerulosclerosis.
Prednisone Decreases Podocyte Apoptosis in Experimental FSGS
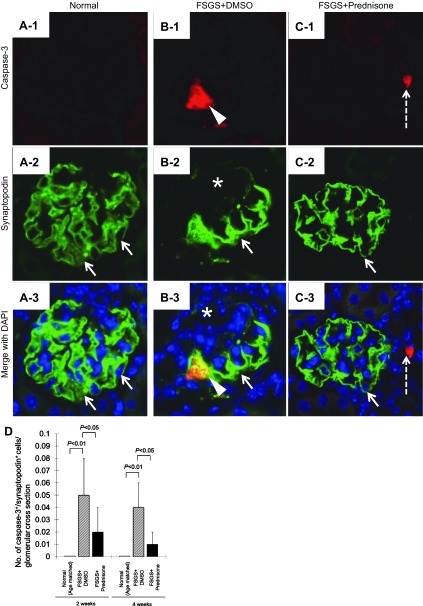
To determine whether prednisone-induced changes in podocyte number were due to effects on cell survival, as we have described in cultured cells (40, 41), double staining for caspase-3 and synaptopodin was performed to assess the magnitude of podocyte apoptosis. Caspase-3+/synaptopodin+-stained cells increased significantly in untreated FSGS mice at 2 wk (0.05 ± 0.03 vs. 0/glomerular cross section, normal mice, P < 0.01) and at 4 wk (0.04 ± 0.01 vs. 0/ glomerulus, normal mice, P < 0.01). Prednisone treatment significantly decreased the number of caspase-3+/synaptopodin+ double positive cells at 2 wk (0.02 ± 0.01 vs. 0.05 ± 0.03/glomerulus, untreated mice with FSGS, P < 0.05) and at 4 wk (0.01 ± 0.01 vs. 0.04 ± 0.02/glomerulus, untreated mice with FSGS, P < 0.05; Fig. 4D).
Fig. 4.
Prednisone decreases podocyte apoptosis in experimental FSGS. A–C: podocyte apoptosis was measured by double staining for caspase-3 (red color) and synaptopodin (green color), with DAPI staining nuclei (blue color; ×630 original magnification). A1–3: normal mice: synaptopodin staining is present in a typical podocyte distribution (arrows); caspase-3 staining is absent. B1–3: mice with FSGS given DMSO. B-1: caspase-3-stained cell (arrowhead) is shown. B-2: areas of reduced synaptopodin staining are noted (*). B-3: caspase-3 and synaptopodin double positive cell (yellow color, arrowhead) in the tuft. C1–3: mice with FSGS given prednisone. C-1: caspase-3-positive cells in the tubule (dashed arrow) adjacent to the glomerulus. C-2: intensity of synaptopodin staining is more marked in mice with FSGS given DMSO. C-3: no double staining is detected in the glomerulus. D: quantitation for caspase-3+/synaptopodin+ double staining: apoptosis is increased in mice with FSGS given DMSO at 2 and 4 wk (hatched bars) compared with normal mice (open bars). Prednisone treatment decreased apoptosis at 2 and 4 wk (filled bars).
These data suggest that prednisone significantly reduced podocyte apoptosis in experimental FSGS that occurred between 3 days and 2 wk. This may partially explain why podocyte number was higher in experimental FSGS mice treated with prednisone compared with untreated mice.
Prednisone Has No Effects on Podocyte Proliferation
Because the increase in podocyte number following prednisone treatment was greater than the decrease in apoptosis, we next sought to determine how prednisone might lead to higher podocyte number in mice with FSGS. To determine whether the increase was due to podocyte proliferation, double staining for Ki-67 and p57 was performed, and the results are shown in Fig. 5. Staining for Ki-67 was not detected in the glomerular tuft in treated or untreated mice with FSGS, and therefore no double staining for Ki-67+/p57+ was detected (Fig. 5).
Fig. 5.
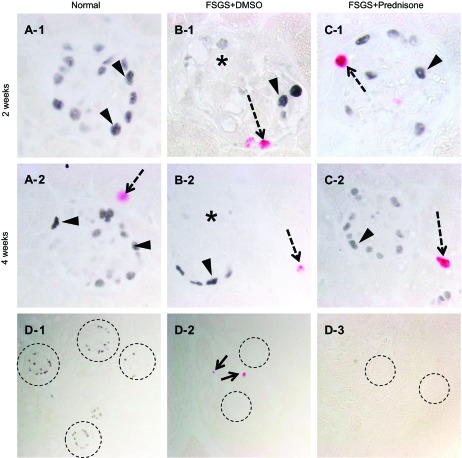
Podocyte proliferation measured by p57/Ki-67 double staining. A–C: representative images of p57/Ki-67 staining at 2 and 4 wk at ×630 original magnification. A-1, A-2: normal mice: p57-positive nuclei (blue/gray) are detected in a typical podocyte distribution (arrowheads). No Ki-67 staining is detected in normal glomeruli. A tubular cell stains positive (red, dashed arrow) at 4 wk (A-2). B-1, B-2: mice with FSGS given DMSO: overall fewer p57 staining cells are detected; p57 staining is not detected in areas of sclerosis (*). At 2 wk, Ki-67 staining is detected in a cell lining Bowman's capsule, and at 4 wk positive staining is detected in a tubular cell. C-1, C-2: mice with FSGS given prednisone: at 2 wk, Ki-67 staining is detected in a cell lining Bowman's capsule, and at 4 wk positive staining is detected in a tubular cell. D1–3: negative controls (×200 magnification). D-1: staining for Ki-67 was not detected when the primary antibody was omitted (dashed circles represent glomeruli with p57 staining). D-2: staining for p57 was not detected when the primary antibody was omitted (dashed circles represent glomeruli; arrow represents a positive Ki-67 cell). D-3: staining for p57 or Ki-67 was not detected when both primary antibodies were omitted (dashed circles represent glomeruli). These data show that podocytes do not proliferate in mice with FSGS given DMSO or prednisone. Ki-67 staining is detected in cells lining Bowman's capsule.
However, similar to what we previously reported (44, 45), staining for Ki-67 was detected in cells lining BC in mice with FSGS given DMSO and prednisone. To determine whether this translated to an increase in PEC number, the number of PAX2-positive cells lining BC was quantitated. PAX2+-stained cells increased significantly in untreated mice with FSGS at 2 wk (3.7 ± 0.2 vs. 2.5 ± 0.1/glomerular cross section, normal mice, P < 0.01) and at 4 wk (3.2 ± 0.2 vs. 2.5 ± 0.1/glomerular cross section, normal mice, P < 0.05; pictures not shown). However, there was no significant difference in the number of PAX2+ cells between FSGS mice that were treated vs. untreated (P > 0.05). These data show that podocytes do not proliferate in this model, but as reported (25, 44, 45), PECs do.
Glomerular Progenitors Increase in Prednisone-Treated Mice With Experimental FSGS
The data show that the increase in podocyte number with prednisone suggested a possible effect on regeneration. We therefore next sought to determine whether prednisone increased the number of glomerular epithelial progenitors (defined as cells in the glomerulus expressing both PEC- and podocyte-specific proteins), double-immunostaining was performed and quantitated for PAX2 and synaptopodin (Fig. 6), and for PAX2 and WT-1 (Fig. 7), and the results were as follows.
Fig. 6.
Glomerular progenitor cells, defined as cells double staining for PAX2 and synaptopodin, increase in experimental FSGS. A–C: representative images at ×630 original magnification for PAX2 (blue/gray nuclear, dashed arrow) and synaptopodin (brown, cytoplasmic, solid arrow) double staining. Arrowheads indicate double positive PAX2+/synaptopodin+ cells. A-1, A-2: normal mice: PAX2-stained cells are confined to Bowman's capsule, and synaptopodin stains in a typical podocyte distribution at 2 and 4 wk. B-1, B-2: mice with FSGS given DMSO: PAX2+/synaptopodin+ double-stained cells are detected at 2 and 4 wk (arrowheads). Segmental decreases in synaptopodin staining are represented by the *. C-1, C-2: mice with FSGS given prednisone: PAX2+/synaptopodin+ double-stained cells are detected at 2 and 4 wk. D1–3: negative controls (×200 magnification). D-1: staining for synaptopodin was not detected when the primary antibody was omitted (dashed circles represent glomeruli with PAX2 staining). D-2: staining for PAX2 was not detected when the primary antibody was omitted (dashed circles represent glomeruli). D-3: staining for PAX2 and synaptopodin was not detected when both primary antibodies were omitted (dashed circles represent glomeruli). E: progenitor cell number, measured as the number of PAX2+/synaptopodin+ double positive cells/glomerular cross section, was augmented by prednisone treatment (filled bar) at 2 and 4 wk compared with control mice with FSGS given DMSO.
Fig. 7.
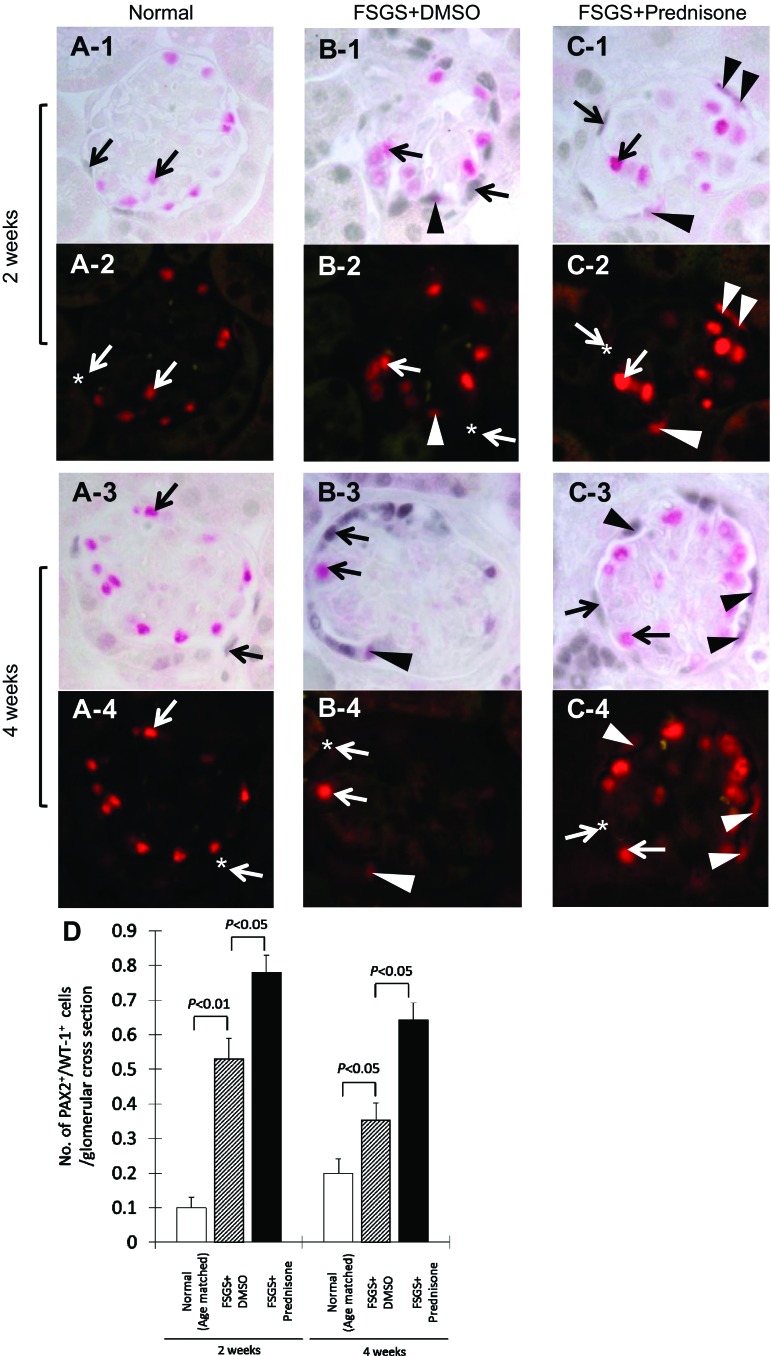
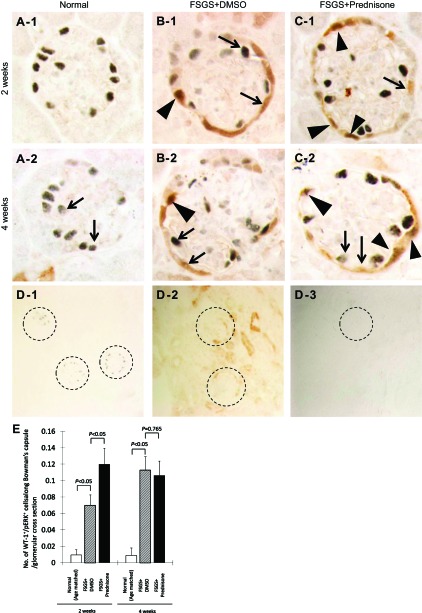
Glomerular progenitor cells, defined as cells double staining for PAX2 (blue/gray) and Wilms' Tumor-1 (WT-1; red), increase in experimental FSGS. A: representative images of PAX2+/WT-1+ double staining at ×630 original magnification in normal mice. A-1: arrows indicate PAX2 (blue/gray)/WT-1 (red) double staining at 2 wk. Arrows indicate examples of a PAX2-positive (blue/gray only, WT-1-negative) cell and a WT-1-positive (red only, PAX2-negative) cell. A-2: fluorescent microscopic view of the A-1 bright-field view, where only WT-1 staining is seen because only the warp-red substrate is visible by fluorescent microscopy. * Indicates the site where PAX2 stains but WT-1 (red fluorescence) is negative in the nucleus. A-3 and A-4: represent PAX2/+WT-1+ staining in normal mice at 4 wk. Arrows indicate PAX2 or WT-1 single positive cells, and * indicates the site where PAX2 stains but WT-1 is negative in the nucleus (A-4). B: representative images of PAX2+/WT-1+ double staining at ×630 original magnification in untreated mice with FSGS at 2 wk. B-1: arrows indicate PAX2 (blue/gray) or WT-1 (red) single positive cells. Arrowhead indicates a PAX2+/WT-1+ double positive cell lining along Bowman's capsule. B-2: fluorescent microscopic view of B-1 bright-field view, where only WT-1 staining is seen. Arrowhead indicates the same cell from B-1, termed glomerular progenitor cell, which is visible. B-3 and B-4: same staining in untreated mice with FSGS at 4 wk. Arrows indicate PAX2 or WT-1 single positive cells, arrowheads indicate a glomerular progenitor cell (PAX2+/WT-1+ double positive). C: representative images of PAX2+/WT-1+ double staining at ×630 original magnification in prednisone-treated mice with FSGS at 2 wk. C-1: arrows indicate PAX2 (blue/gray) or WT-1 (red) single positive cells. Arrowheads indicate PAX2+/WT-1+ double positive cells lining Bowman's capsule and in the glomerular tuft. C-2: fluorescent microscopic view of the C-1 bright-field view, only WT-1 staining is seen. Arrowheads indicate the same cells from C-1, which are visible by fluorescence. C-3 and C-4: same staining in prednisone-treated mice with FSGS at 4 wk. Arrows indicate PAX2 or WT-1 single positive cells; arrowheads indicate glomerular progenitor cells (PAX2+/WT-1+ double positive). D: number of cells staining positive for PAX2 and WT-1 per glomerular cross section was significantly higher at 2 and 4 wk in untreated mice with FSGS (hatched bar) compared with normal mice (open bar). Prednisone treatment increases the number of PAX2/WT-1-positive cells/glomerular cross section at 2 and 4 wk (filled bars), compared with untreated mice with FSGS.
PAX2 and synaptopodin staining.
Figure 6 shows that in untreated mice with FSGS, the number of cells within the glomerulus that double stained for both PAX2+ and synaptopodin+ increased at 2 wk (0.074 ± 0.007 vs. 0.025 ± 0.001 double positive cells/glomerular cross section, normal mice, P < 0.05). There was no significant difference in number of double positive cells between untreated FSGS mice and normal mice at 4 wk. Compared with untreated mice with FSGS, prednisone significantly augmented the increase in the number of progenitor cells at 2 wk (0.13 ± 0.05 vs. 0.074 ± 0.07/glomerulus untreated mice with FSGS, P < 0.05) and at 4 wk (0.098 ± 0.015 vs. 0.024 ± 0.07/glomerulus, untreated mice with FSGS, P < 0.05; Fig. 6E).
PAX2 and WT-1 staining.
Figure 7 shows that the number of cells staining double positive for both PAX2 and WT-1 increased in mice with FSGS at 2 wk (0.53 ± 0.06 vs. 0.1 ± 0.03 double positive PAX2+ and WT-1+ cells/glomerular cross section, normal mice, P < 0.01) and 4 wk (0.35 ± 0.05 vs. 0.2 ± 0.04/glomerulus, normal mice, P < 0.05). Compared with untreated FSGS mice, prednisone augmented the number of progenitor cells both at 2 wk (0.78 ± 0.05 vs. 0.53 ± 0.06/glomerulus, untreated mice with FSGS, P < 0.05) and 4 wk (0.64 ± 0.05 vs. 0.35 ± 0.05/glomerulus, untreated mice with FSGS, P < 0.05; Fig. 7D).
These data show that prednisone significantly increased the number of glomerular epithelial progenitor cells within glomeruli of mice with FSGS.
Prednisone Augments p-ERK1/2 in Experimental FSGS
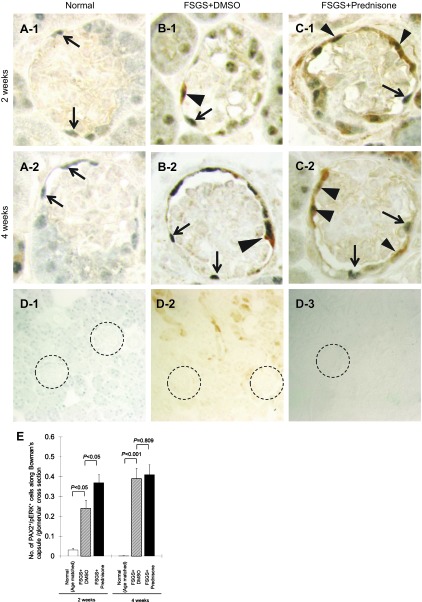
We next sought to determine whether prednisone-induced changes in progenitor cell number were associated with changes in the ERK pathway, given its role in survival, proliferation, and differentiation. Double staining was performed for the PEC marker PAX-2, and for phospho-ERK (p-ERK), the active form of ERK. The number of PAX2+/p-ERK+ double positive cells along BC was significantly increased in untreated mice with FSGS at 2 wk (0.24 ± 0.04 vs. 0.03 ± 0.01 PAX2+/p-ERK+ double positive cells along BC/glomerular cross section, normal mice, P < 0.05) and at 4 wk (0.39 ± 0.05 vs. 0 PAX2+/p-ERK+ double positive cells/BC cross section, normal mice, P < 0.001). Treatment with prednisone further increased the number of PAX2+/p-ERK+-positive cells at 2 wk (0.37 ± 0.04 vs. 0.24 ± 0.04 PAX2+/p-ERK+ double positive cells along BC/glomerular cross section, untreated FSGS mice, P < 0.05). There was no significant difference in the number of double positive cells at 4 wk (Fig. 8E).
Fig. 8.
Prednisone augments p-ERK1/2 in parietal epithelial cells (PECs) in experimental FSGS. A–C: representative images of PAX2/p-ERK double staining at ×630 original magnification. The arrowheads show double positive cells along Bowman's capsule, arrows show PAX2 single positive cells. D-1 to D-3: staining was not detected when the primary antibodies were omitted as negative controls (×200). PAX2 only (D-1), p-ERK only (D-2), no primary antibody (D-3). Glomeruli are circled for easier identification. E: number of PECs with phosphorylated ERK, measured as the number of PAX2/p-ERK double positive cells/glomerulus along Bowman's capsule, increased at 2 and 4 wk in mice with FSGS mice (hatched bar). Prednisone treatment was associated with an increase in this number at 2 wk (filled bar).
Next, to determine the relationship between p-ERK-positive cells and progenitor cells along BC, WT-1 and p-ERK double staining was performed. The number of WT-1+/p-ERK+ double positive cells along BC was significantly increased in mice with FSGS at 2 wk (0.07 ± 0.01 vs. 0.01 ± 0.01 WT-1+/p-ERK+ double positive cells along BC/glomerular cross section, normal mice, P < 0.05) and 4 wk (0.11 ± 0.02 vs. 0.01 ± 0.01 double positive cells along BC/glomerular cross section, normal mice, P < 0.05). Treatment with prednisone further increased the number of WT-1+/p-ERK+ double positive cells along BC at 2 wk (0.12 ± 0.02 vs. 0.07 ± 0.01 double positive cells along BC/glomerular cross section, untreated FSGS mice, P < 0.05). There was no significant difference in double positive cells between mice with FSGS treated with prednisone and untreated mice at 4 wk (Fig. 9E).
Fig. 9.
Prednisone augments glomerular progenitor cells' p-ERK1/2 along Bowman's capsule in experimental FSGS. A–C: representative images of WT-1/p-ERK double staining at ×630 original magnification. The arrowheads show double positive cells along Bowman's capsule; arrows show WT-1 or p-ERK single positive cells. D-1 to D-3: staining was not detected when the primary antibodies were omitted as negative controls (×200). WT-1 only (D-1), p-ERK only (D-2), and no primary antibody (D-3). Glomeruli are circled for easier identification. E: number of progenitor cells with phosphorylated ERK, measured as the number of WT-1/p-ERK double positive cells/glomerulus along Bowman's capsule, increased at 2 and 4 wk in mice with FSGS (hatched bar). Prednisone treatment was associated with an increase in this number at 2 wk (filled bar).
These data suggest that prednisone significantly increased activation of the ERK pathway in PECs, and in a subpopulation of p-ERK-positive PECs begin to express WT-1, which are likely progenitors.
Prednisone Decreases CD44 Expression in Experimental FSGS
Finally, we investigated CD44 expression in response to prednisone in mice with FSGS. CD44, a protein responsible for cell migration, interaction, and adhesion, is detected in activated PECs where it may play a key role in the formation of sclerosis (10, 37). CD44 staining was not detected in glomeruli in normal mice, but was detected in occasional tubular cells, indicating that the absence of glomerular staining was not a false negative (data not shown). The percentage of glomeruli staining positive for ≥1 CD44-positive cell was significantly increased in untreated mice with FSGS at 2 wk (6.3 ± 1.7 vs. 0%, normal mice, P < 0.01) and at 4 wk (10.4 ± 2.3 vs. 0%, normal mice, P < 0.001; staining not shown). Prednisone decreased the percentage of glomeruli with CD44-positive cells in mice with FSGS at 4 wk (5.6 ± 0.8 vs. 10.4 ± 2.3%, untreated mice with FSGS, P < 0.05), but not at 2 wk. At 4 wk, the decrease in the number of glomeruli expressing CD44 correlated with the decrease in glomerulosclerosis (r = 0.587, P < 0.01).
These data suggest that prednisone decreases the expression of CD44, a marker of PEC activation, in diseased glomeruli in this model.
DISCUSSION
The clinical benefits of corticosteroids in subsets of FSGS patients with nephrotic syndrome are well-described (8, 21). However, to date, there is little direct evidence supporting any systemic immune changes in most forms of FSGS. This raises the possibility that the benefits of corticosteroids may be direct cellular effects (36). In this study, we show that following an abrupt depletion in podocyte number in mice with experimental FSGS, prednisone administration increases podocyte number by augmenting regeneration of podocyte progenitors. This correlated with reduced proteinuria and glomerulosclerosis.
A decrease in podocyte number in FSGS portends a poor prognosis, as it is an independent determinant and predictor for the development of proteinuria and progressive glomerulosclerosis (11, 12, 14, 15, 36, 42). Because podocytes lack the cell cycle machinery necessary to adequately replace any decrease in their cell number, repair is limited. This begs the question how prednisone might lead to enhanced glomerular repair in FSGS? Accordingly, we used a mouse model of FSGS for these studies characterized by podocyte depletion that we previously reported (25, 38, 45). This model differs from an antibody-induced mouse model characterized by glomerular epithelial cell proliferation and pseudo-crescent formation (17, 23, 39). The model utility of the model used in this study was further validated by the data showing that prednisone augments glomerular repair measured by a reduction in proteinuria, and in glomerulosclerosis.
The first major finding in this study was that podocyte number and podocyte density, measured by p57 and synaptopodin staining, respectively, were higher in mice with FSGS given prednisone compared with control mice with FSGS given the vehicle DMSO (untreated group). Importantly, mice were first administered prednisone (or DMSO) 3 days after disease induction, when podocyte number had already declined significantly, and proteinuria was already present. The effect of prednisone was not due to changes in disease induction, because immunofluorescent staining for the cytotoxic disease-inducing anti-podocyte antibody was similar in the treated and untreated mice. The enhanced number of podocytes correlated directly with reduced glomerulosclerosis. To our knowledge, this is the first report in experimental glomerular disease that prednisone enhances glomerular repair by increasing podocyte number. Although several studies used WT-1 staining to quantitate podocyte number, WT-1 staining has also been detected in cells lining BC (2, 25, 33, 37, 44, 45). Accordingly, we prefer p57 staining which in glomeruli is restricted to podocytes (18).
To determine whether the higher podocyte number in the prednisone-treated group was due to proliferation, Ki-67 staining was performed. Similar to our previous reports in this model (44, 45), podocyte proliferation was not detected. However, as we reported, a subpopulation of PECs expresses Ki-67, and PEC number increases along BC.
Because we previously showed that corticosteroids reduce podocyte apoptosis in cultured cells (41), we next asked whether prednisone might affect podocyte number by directly enhancing podocyte survival in vivo. In the model used, the binding of the cytotoxic antibody to podocytes acutely depletes podocyte number within the first days of disease due to necrosis, apoptosis, and detachment (5, 6). A second finding in this study was that administering prednisone to mice with FSGS at day 3 of disease reduced the magnitude of podocyte apoptosis (measured by caspase-3 and synaptopodin double staining) later at 2 and 4 wk of disease.
Several direct cellular actions of prednisone have been shown on podocytes. These include limiting apoptosis (40, 41), actin rearrangement (28), downregulating podocyte cytokines (43), and enhanced slit diaphragm complex protein synthesis (13), etc. The data in this study are consistent with prednisone having direct biological effects on podocytes in vivo, by improving survival/reducing apoptosis.
Because the decrease in podocyte apoptosis in the absence of proliferation did not readily explain the increase in podocyte number in prednisone-treated mice, we next sought to determine whether prednisone increased podocyte regeneration by increasing podocyte progenitors. PEC progenitors are defined herein as cells that coexpress proteins considered specific for PECs and podocytes. The third major finding in this study was that prednisone increased the number of PEC progenitors at 2 and 4 wk of disease compared with untreated mice. Noteworthy was that PEC progenitors were detected along BC (normal location of PECs), and within the glomerular tuft (normal location of podocytes). These data suggest a possible direct biological effect of prednisone on PECs.
The rationale to study PECs as progenitors follows several paradigm-shifting studies that elegantly provided evidence that podocyte number can be increased under certain circumstances independent of proliferation (3, 29, 34). This is possibly because PEC progenitors normally reside along BC (2, 30–34). When human PEC progenitors are isolated and grown ex vivo, and are then injected into mice, they track to the glomerulus (34). Reporter mice showed that PECs are podocyte progenitors in adolescent mice (22). In experimental FSGS models (25), experimental membranous nephropathy (25), and aging (44), the number of PEC progenitors increases as podocyte number decreases. In this context, the data from the current study support the notion that prednisone increases the number of PEC progenitors in experimental FSGS. The data from the current study suggest that we can add prednisone to the list of other agents that improve podocyte regeneration by augmenting the number of PEC progenitors including ACE inhibitors (4), retinoids (45), notch inhibitors (22), and blockade of the chemokine stromal-derived factor-1 (9).
The signaling events underlying an increase in PEC progenitor number are not fully understood to date. In this study, we focused on phospho-ERK, because it has effects on survival, differentiation, and proliferation in several epithelial cells (7, 41). A fourth finding in this study was that staining for phospho-ERK increased significantly in PECs in disease. Moreover, the number of PEC progenitors lining BC identified as WT-1-positive that coexpressed phospho-ERK increased further when prednisone was administered. These results suggest, but do not prove, that ERK is important in increasing the number of PEC progenitors and that prednisone augments this pathway. We attempted to extend these studies to cultured PECs, but as we previously reported, inhibiting ERK led to substantial PEC apoptosis, which limited this mechanistic approach (7).
Finally, we studied CD44, a protein responsible for cell migration, interaction, and adhesion. Some have suggested that the presence of CD44 indicates “activated” PECs and that CD44 may play a key role in migration of these cells resulting in subsequent formation of sclerosis (1, 10, 37). The data in this study show that prednisone significantly reduced the percentage of glomeruli expressing CD44 in FSGS, which correlated with a decrease in glomerulosclerosis. Thus, one interpretation is that prednisone has a direct effect on PECs to reduce “activation.”
In summary, we provide evidence that when prednisone is given to mice with experimental FSGS at a time when podocyte number was already decreased, glomerular repair is augmented. Prednisone increases podocyte number, and this correlates with reduced proteinuria and decreased glomerulosclerosis. This may be due to direct biological effects on both glomerular epithelial cells, by reducing podocyte apoptosis and by enhancing podocyte regeneration by increasing the number of PEC progenitors. The latter is accompanied by increased phospho-ERK expression. Further studies are needed to resolve whether the decrease in PEC activation (CD44 expression) by prednisone is a direct cellular effect on PECs, and/or is due to an indirect effect by improving podocyte number.
GRANTS
J. Zhang was funded by an International Society of Nephrology fellowship. S. J. Shankland was supported by National Institutes of Health Grants R01DK056799 and R21DK081835.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.Z., J.W.P., Z.L., and S.J.S. conception and design of research; J.Z., J.W.P., R.D.K., and S.N. performed experiments; J.Z., J.W.P., R.D.K., S.N., and S.J.S. analyzed data; J.Z., J.W.P., R.D.K., S.N., Z.L., and S.J.S. interpreted results of experiments; J.Z., J.W.P., and S.J.S. prepared figures; J.Z., J.W.P., and S.J.S. drafted manuscript; J.Z., J.W.P., and S.J.S. edited and revised manuscript; J.Z., J.W.P., R.D.K., Z.L., and S.J.S. approved final version of manuscript.
REFERENCES
- 1. Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recuitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bariety J, Mandet C, Hill GS, Bruneval P. Parietal podocyte in normal human glomeruli. J Am Soc Nephrol 17: 2770–2780, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Benigni A, Morigi M, Remuzzi G. Kidney regeneration. Lancet 375: 1310–1317, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Benigni A, Morigi M, Rizzo P, Gagliardini E, Rota C, Abbate M, Ghezzi S, Remuzzi A, Remuzzi G. Inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am J Pathol 179: 628–638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brinkkoetter PT, Olivier P, Wu JS, Henderson S, Krofft RD, Pippin JW, Hockenbery D, Roberts JM, Shankland SJ. Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J Clin Invest 119: 3089–3101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brinkkoetter PT, Wu JS, Ohse T, Krofft RD, Schermer B, Benzing T, Pippin JW, Shankland SJ. p35, the noncyclin activator of Cdk5, protects podocytes against apoptosis in vitro and in vivo. Kidney Int 77: 690–699, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Chang AM, Ohse T, Krofft RD, Wu JS, Eddy AA, Pippin JW, Shankland SJ. Albumin-induced apoptosis of glomerular parietal epithelial cells is modulated by extracellular signal-regulated kinase 1/2. Nephrol Dial Transplant 27: 1330–1343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Darisipudi MN, Kulkarni OP, Sayyed SG, Ryu M, Migliorini A, Sagrinati C, Parente E, Vater A, Eulberg D, Klussmann S, Romagnani P, Anders HJ. Dual blockade of the homeostatic chemokine CXCL12 and the proinflammatory chemokine CCL2 has additive protective effects on diabetic kidney disease. Am J Pathol 179: 116–124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fatima H, Moeller MJ, Smeets B, Yang HC, D'Agati VD, Alpers CE, Fogo AB. Parietal epithelial cell activation marker in early recurrence of FSGS in the transplant. Clin J Am Soc Nephrol 7: 1852–1858, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fogo AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol 22: 2011–2022, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fogo AB. The target podocyte. J Clin Invest 121: 2142–2145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujii Y, Khoshnoodi J, Takenaka H, Hosoyamada M, Nakajo A, Bessho F, Kudo A, Takahashi S, Arimura Y, Yamada A, Nagasawa T, Ruotsalainen V, Tryggvason K, Lee AS, Yan K. The effect of dexamethasone on defective nephrin transport caused by ER stress: a potential mechanism for the therapeutic action of glucocorticoids in the acquired glomerular diseases. Kidney Int 69: 1350–1359, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC. Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol 23: 1351–1363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC. Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int 81: 40–55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Hogan SL, Middleton JP, Vehaskari VM, Flynn PA, Powell LM, Vento SM, McMahan JL, Siegel N, D'Agati VD, Friedman AL. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int 80: 868–878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griffin SV, Krofft RD, Pippin JW, Shankland SJ. Limitation of podocyte proliferation improves renal function in experimental crescentic glomerulonephritis. Kidney Int 67: 977–986, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hiromura K, Haseley LA, Zhang P, Monkawa T, Durvasula R, Petermann AT, Alpers CE, Mundel P, Shankland SJ. Podocyte expression of the CDK inhibitor p57 during development and disease. Kidney Int 60: 2235–2246, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Hogg RJ, Friedman A, Greene T, Radeva M, Budisavljevic MN, Gassman J, Gipson DS, Jefferson JA, John EG, Kaskel FJ, Moudgil A, Moxey-Mims M, Ortiz LA, Schelling JR, Schnaper W, Srivastava T, Trachtman H, Vehaskari VM, Wong C, Woronieki RP, Van Why SK, Zolotnitskaya A. Renal function and proteinuria after successful immunosuppressive therapies in patients with FSGS. Clin J Am Soc Nephrol 8: 211–218, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunter MG, Hurwitz S, Bellamy CO, Duffield JS. Quantitative morphometry of lupus nephritis: the significance of collagen, tubular space, and inflammatory infiltrate. Kidney Int 67: 94–102, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Korbet SM. Treatment of primary FSGS in adults. J Am Soc Nephrol 23: 1769–1776, 2012 [DOI] [PubMed] [Google Scholar]
- 22. Lasagni L, Ballerini L, Angelotti ML, Parente E, Sagrinati C, Mazzinghi B, Peired A, Ronconi E, Becherucci F, Bani D, Gacci M, Carini M, Lazzeri E, Romagnani P. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells 28: 1674–1685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Logar CM, Brinkkoetter PT, Krofft RD, Pippin JW, Shankland SJ. Darbepoetin alfa protects podocytes from apoptosis in vitro and in vivo. Kidney Int 72: 489–498, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Menini S, Iacobini C, Oddi G, Ricci C, Simonelli P, Fallucca S, Grattarola M, Pugliese F, Pesce C, Pugliese G. Increased glomerular cell (podocyte) apoptosis in rats with streptozotocin-induced diabetes mellitus: role in the development of diabetic glomerular disease. Diabetologia 50: 2591–2599, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, Hudkins KL, Alpers CE, Pippin JW, Shankland SJ. De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol 298: F702–F711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ophascharoensuk V, Pippin JW, Gordon KL, Shankland SJ, Couser WG, Johnson RJ. Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int 54: 416–425, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Ransom RF, Lam NG, Hallett MA, Atkinson SJ, Smoyer WE. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int 68: 2473–2483, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116: 288–296, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Romagnani P, Remuzzi G. Renal progenitors in nondiabetic and diabetic nephropathies. Trends Endocrinol Metab 24: 13–20, 2013 [DOI] [PubMed] [Google Scholar]
- 31. Romagnani P. Parietal epithelial cells: their role in health and disease. Contrib Nephrol 169: 23–36, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol 9: 137–146, 2013 [DOI] [PubMed] [Google Scholar]
- 33. Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bwoman's capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, Böttinger EP. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest 108: 807–816, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1262–1274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taniguchi Y, Pippin JW, Hagmann H, Krofft RD, Chang AM, Zhang J, Terada Y, Brinkkoetter P, Shankland SJ. Both cyclin I and p35 are required for maximal survival benefit of cyclin-dependent kinase 5 in kidney podocytes. Am J Physiol Renal Physiol 302: F1161–F1171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vaughan MR, Pippin JW, Griffin SV, Krofft R, Fleet M, Haseley L, Shankland SJ. ATRA induces podocyte differentiation and alters nephrin and podocin expression in vitro and in vivo. Kidney Int 68: 133–144, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Wada T, Pippin JW, Marshal CB, Griffin SV, Shankland SJ. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol 16: 2615–2625, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Wada T, Pippin JW, Nangaku M, Shankland SJ. Dexamethasone's prosurvival benefits in podocytes require extracellular signal-regulated kinase phosphorylation. Nephron Exp Nephrol 109: e8–e19, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW. Direct effects of dexamethasone on human podocytes. Kidney Int 70: 1038–1045, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Zhang J, Hansen KM, Pippin JW, Chang AM, Taniguchi Y, Krofft RD, Pickering SG, Liu ZH, Abrass CK, Shankland SJ. De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol 302: F571–F580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang J, Pippin JW, Vaughan MR, Krofft RD, Taniguchi Y, Romagnani P, Nelson PJ, Liu ZH, Shankland SJ. Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron Exp Nephrol 121: e23–e37, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]