Abstract
Since nitric oxide (NO) participates in the renal regulation of blood pressure, in part, by modulating transport of Na+ and Cl− in the kidney, we asked whether NO regulates net Cl− flux (JCl) in the cortical collecting duct (CCD) and determined the transporter(s) that mediate NO-sensitive Cl− absorption. Cl− absorption was measured in CCDs perfused in vitro that were taken from aldosterone-treated mice. Administration of an NO donor (10 μM MAHMA NONOate) reduced JCl and transepithelial voltage (VT) both in the presence or absence of angiotensin II. However, reducing endogenous NO production by inhibiting NO synthase (100 μM NG-nitro-l-arginine methyl ester) increased JCl only in the presence of angiotensin II, suggesting that angiotensin II stimulates NO synthase activity. To determine the transport process that mediates NO-sensitive changes in JCl, we examined the effect of NO on JCl following either genetic ablation or chemical inhibition of transporters in the CCD. Since the application of hydrochlorothiazide (100 μM) or bafilomycin (5 nM) to the perfusate or ablation of the gene encoding pendrin did not alter NO-sensitive JCl, NO modulates JCl independent of the Na+-dependent Cl−/HCO3− exchanger (NDCBE, Slc4a8), the A cell apical plasma membrane H+-ATPase and pendrin. In contrast, both total and NO-sensitive JCl and VT were abolished with application of an epithelial Na+ channel (ENaC) inhibitor (3 μM benzamil) to the perfusate. We conclude that NO reduces Cl− absorption in the CCD through a mechanism that is ENaC-dependent.
Keywords: nitric oxide, transepithelial voltage, cortical collecting duct
aldosterone and angiotensin II increase NaCl absorption in the cortical collecting duct (CCD) by stimulating transporters such as epithelial Na+ channel (ENaC) and pendrin (12, 21, 24, 28, 39, 40), which increases NaCl absorption, thereby contributing to the hypertension expected following treatment with these hormones (41, 44).
In vascular and in renal tissue, many of the effects of angiotensin II and aldosterone are mediated by changes in O2·−/H2O2 or nitric oxide (NO) (1, 11, 19, 23, 48, 49). In kidney cells, aldosterone stimulates ENaC activity, in part, by increasing O2·− production and by reducing NO bioavailability (52). With increased NO bioavailability, a natriuresis and diuresis are observed (20) due to inhibition of renal Na+ transporters, such as ENaC (33). However, NO may also reduce Cl− absorption by inhibiting pendrin since NO reduces HCO3− secretion in rabbit CCD (37) and since HCO3− secretion occurs largely through pendrin-dependent transport (31). Thus, the purpose of this study was to determine whether NO reduces Cl− absorption in the mouse CCD and to explore the transport mechanism(s) by which this occurs.
METHODS
Animals.
All experiments were performed on male and female Slc26a4 −/− (or Pds−/−) mice developed by Everett et al. (4) and in wild-type mice from the same strain (129S6/SvEv Tac; Taconic Farms), which were bred in parallel. Every third generation wild-type and pendrin-null mice were interbred to produce heterozygotes. These heterozygotes were interbred to generate new wild-type and homozygous pendrin-null breeders (25).
We generated mice that were both homozygous pendrin-null and homozygous for the Liddle's mutation (29) (Pds−/−& L/L), which constitutively upregulates the ENaC. To do so, mice homozygous for the Liddle's mutation (29) on a C57Bl6 background were bred with homozygous pendrin-null mice (4) on a 129S6SvEvTac background. The progeny were backcrossed onto a 129 S6SvEvTac background over at least 10 generations. Mouse genotype was determined from tail biopsies using real-time PCR with specific probes designed for each gene (Transnetyx, Cordova, TN).
For 5–7 days before death, mice ate a balanced diet (53881300; Zeigler Brothers) prepared as a gel (0.6% agar, 74.6% water, and 24.8% mouse chow) supplemented with NaCl (∼0.8 meq NaCl/day) (12) and were treated with aldosterone by minipump (250 μg·kg body wt−1·day−1). The Institutional Animal Care and Use Commmittee at Emory University approved all treatment protocols.
In vitro perfusion of isolated CCDs.
CCDs were dissected from medullary rays and perfused at flow rates of 2–3 nl/min in the presence of symmetric, physiological solutions containing (in mmol/l) 125 NaCl, 2.5 K2HPO4, 24 NaHCO3/5% CO2, 2 CaCl2, 1.2 MgSO4, and 5.5 glucose bubbled with 95% air-5% CO2 (31). Unless otherwise stated, 10 nM angiotensin II was present in the bath solution (24). Tubules were equilibrated at 37°C for 30 min before the collections were started. Stock solutions of benzamil hydrochloride (3 × 10−3 M) were prepared in deionized water. Hydrochlorothiazide stock solutions (10−1 M) were prepared in DMSO, whereas stock solutions of bafilomycin (10−5 M) were prepared in absolute ethanol. All chemicals were purchased from Sigma (St. Louis, MO). MAHMA NONOate was purchased from Axxora (San Diego, CA). Stock solutions of 20 mM MAHMA were prepared in 0.01 M NaOH and used within 24 h. Uric acid was prepared in 1 M NaOH, diluted 1:1,000, and then titrated to pH 7.4 with 1 M HCl. Stock solutions of tempol and NG-nitro-l-arginine methyl ester (l-NAME) were made in deionized water and diluted 1:1,000.
Measurement of net transepithelial Cl− flux.
Cl− concentration was measured in perfusate and collected samples using a continuous-flow fluorimeter and the Cl−-sensitive fluorophore 6 methoxy-N-(3-sulfopropyl) quinolinium (Molecular Probes, Eugene, OR), as described previously (5, 43, 44). Transepithelial Cl− flux (JCl) was calculated according to the equation: JCl = (Co − CL)Q/L, where Co and CL are perfusate and collected fluid Cl− concentrations, respectively. Q is flow rate in nanoliters per minute. L is tubule length. Net fluid transport was taken to be 0 since net fluid flux was observed in CCDs when perfused in vitro in the presence of symmetric solutions and in the absence of vasopressin (13, 14). JCl was expressed in picomoles per millimeter per minute.
Transepithelial voltage (VT) was measured in the perfusion pipette connected to a high-impedance electrometer through an agar bridge saturated with 0.16 M NaCl and a calomel cell as described previously (42). The reference was an agar bridge from the bath to a calomel cell.
Statistics.
All data are presented as means ± SE. Data displayed usually represented a single measurement. Occasionally, data from two collections were averaged to obtain a single value. Each “n” used in the statistical analysis represents data from separate mice. To test for statistical significance between two groups, a paired or an unpaired Student's t-test was used, as appropriate. The criterion for statistical significance was P < 0.05.
RESULTS
NO reduces Cl− absorption.
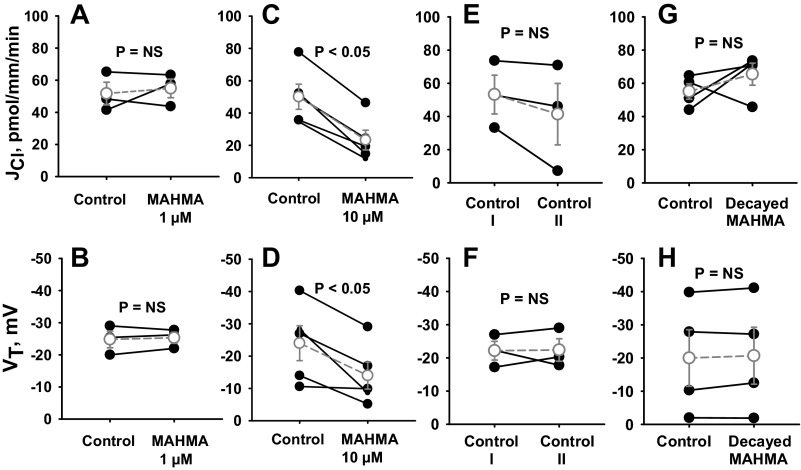
We examined the effect of NO donors on Cl− absorption in CCDs from aldosterone-treated mice that were perfused in vitro with angiotensin II (10 nM) in the bath solution. Aldosterone was applied in vivo and angiotensin II was applied in vitro to upregulate the ENaC (21, 28), the H+-ATPase (27, 50), and pendrin (24, 39). As shown, while 1 μM MAHMA NONOate did not alter JCl or VT (Fig. 1, A and B), application of 10 μM MAHMA NONOate reduced JCl and VT by ∼50% (Fig. 1, C and D). This fall in JCl and VT was not due to time-dependent changes (Fig. 1, E and F). To determine whether MAHMA reduces Cl− absorption through a breakdown product of MAHMA, Cl− absorption was measured before and after the addition of MAHMA that had been allowed to decay (Fig. 1, G and H). As shown, decayed MAHMA did not change JCl or VT. We conclude that MAHMA reduces Cl− absorption through a direct effect of NO.
Fig. 1.
Nitric oxide (NO) donors reduce Cl− absorption (JCl) and transepithelial voltage (VT). JCl and VT were measured in cortical collecting ducts (CCDs) from aldosterone-treated mice before and after the application of the NO donor {MAHMA NONOate, (Z)-1-N-methyl-N-[6-(N-methylammoniohexyl)amino]-diazen-1-ium-1,2-diolate} at concentrations of 1 μM (A and B) or 10 μM (C and D). Angiotensin II (10 nM) was present in the bath solution. E and F: display time control experiments in the absence of MAHMA NONOate. G and H: display JCl and VT when measured before and after application of 10 μM MAHMA NONOate that had been allowed to decay. To do so, MAHMA NONOate was incubated at 22°C for 60 min before use. Solid lines show individual tubules. Dashed lines show means ± SE. NS, not significant.
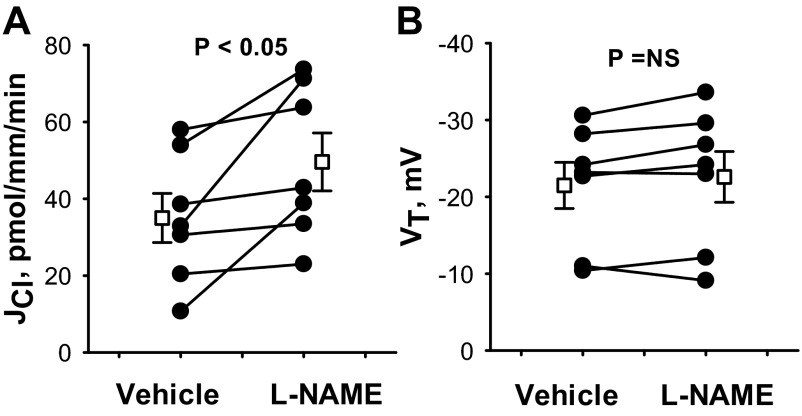
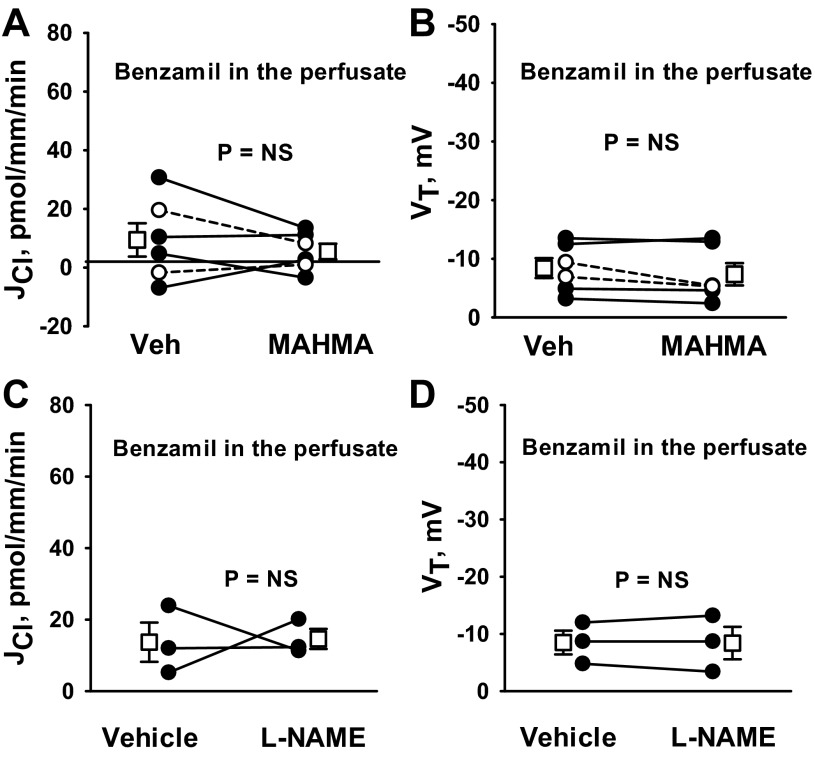
Further experiments asked whether reducing endogenous production of NO alters Cl− absorption. Thus, Cl− absorption was measured in the presence and absence of the NO synthase (NOS) inhibitor l-NAME (100 μM; Fig. 2) with angiotensin II in the bath solution. As shown, Cl− absorption increased ∼30% with l-NAME application, although VT did not change significantly.
Fig. 2.
Inhibiting endogenous NO synthases increases JCl. JCl (A) and VT (B) were measured in CCDs from aldosterone-treated mice before and after application of the NO synthase inhibitor l-NAME (100 μM) to the bath. Angiotensin II (10 nM) was present in the bath solution throughout the experiment. Solid lines show individual tubules. Open squares show means ± SE.
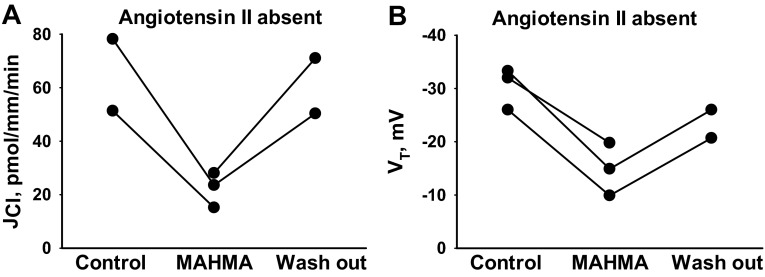
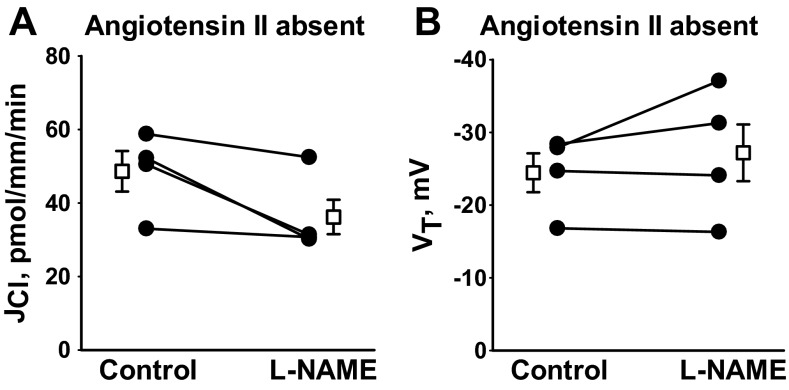
Angiotensin II increases NO production in vascular tissue by stimulating NOS activity (7, 10, 30, 32) and stimulates NADPH oxidase activity, thereby increasing the production of O2·− (17, 34). Since impaired O2·−/NO balance in the CCD may alter the response to NO donors or NOS inhibitors, we examined the effect of NO donors and NOS inhibitors on Cl− absorption in the absence of angiotensin II. As shown (Fig. 3), following NO donor (10 μM MAHMA) application, Cl− absorption fell 64% (n = 3, P < 0.05), while VT fell 47% (n = 3, P < 0.05) similar to the effects observed in the presence of angiotensin II. Therefore, NO donors modulate JCl both in the presence and in the absence of angiotensin II. In contrast, while NOS inhibition increased Cl− absorption in the presence of angiotensin II (Fig. 2), in the absence of angiotensin II, NOS inhibition (100 μM l-NAME; Fig. 4) did not change Cl− absorption. We conclude that Cl− flux is much more sensitive to NOS inhibition following angiotensin II application in vitro, likely because angiotensin II increases NO production by stimulating NOS activity.
Fig. 3.
Removing angiotensin II from the bath does not alter the effect of NO donors on JCl. JCl (A) and VT (B) were measured in CCDs from aldosterone-treated mice before and after the application of an NO donor (10 μM MAHMA NONOate) when angiotensin II was not present in the bath. In the absence of angiotensin II, application of MAHMA reduced JCl from 62.2 ± 5.8 to 22.2 ± 3.8 pmol·mm−1·min−1, and reduced VT from −28.4 ± 2.6 to −14.9 ± 2.9 mV, respectively.
Fig. 4.
NO synthase inhibition does not increase JCl when angiotensin II is removed from the bath. JCl (A) and VT (B) were measured in CCDs from aldosterone-treated mice before and after the application of the NO synthase inhibitor (100 μM l-NAME) without angiotensin II in the bath. In the absence of angiotensin II, application of l-NAME did not alter JCl or VT. Solid lines show individual tubules. Open squares show means ± SE.
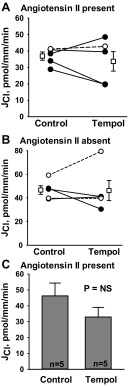
Since superoxide (O2·−) is involved in the breakdown of endothelium-derived vascular relaxing factor (NO) (6), we explored the effect of reducing O2·− through the application of a superoxide dismutase mimetic (50 μM tempol) to the bath. As shown (Fig. 5), tempol did not reduce Cl− absorption in CCDs from aldosterone-treated mice, when perfused either in the presence or in the absence of angiotensin II in the bath solution. To exclude the possibility that tempol failed to reduce Cl− absorption because of limited intracellular accumulation, we examined the effect of tempol in an unpaired experiment, in which CCDs were exposed to tempol for a longer period of time (i.e., at least 30 min) before each collection was started. Tubules were perfused in the presence of angiotensin II to stimulate O2·− production. Although Cl− absorption was numerically lower with tempol present in the bath, the difference did not reach statistical significance (Fig. 5). We conclude that O2·− plays a limited role in regulating Cl− absorption in CCDs from aldosterone-treated mice.1
Fig. 5.
Superoxide dismutase mimetics do not reduce JCl. CCDs from aldosterone-treated mice were perfused in vitro in the presence (A and C) or in the absence (B) of angiotensin II (10 nM) in the bath. A and B: JCl was measured in individual tubules before and then after the application of the superoxide mimetic tempol (4-hydroxy 2,2,6,6,-tetramethyl piperidinoxyl; 50 μM) to the bath. Solid lines show individual tubules. Dashed lines show experiments conducted in reverse order (i.e., tempol was applied in the first period and then removed in the second). Open squares show means ± SE. C: JCl was measured in separate tubules after at least 30-min incubation with tempol (50 μM) or vehicle present in both the bath and luminal solution. The bar graph shows means ± SE.
Since angiotensin II application in vitro increases the sensitivity of Cl− transport to NOS inhibition, further experiments were performed with angiotensin II present in the bath solution.
NO does not reduce Cl− absorption through the action of ONOO−.
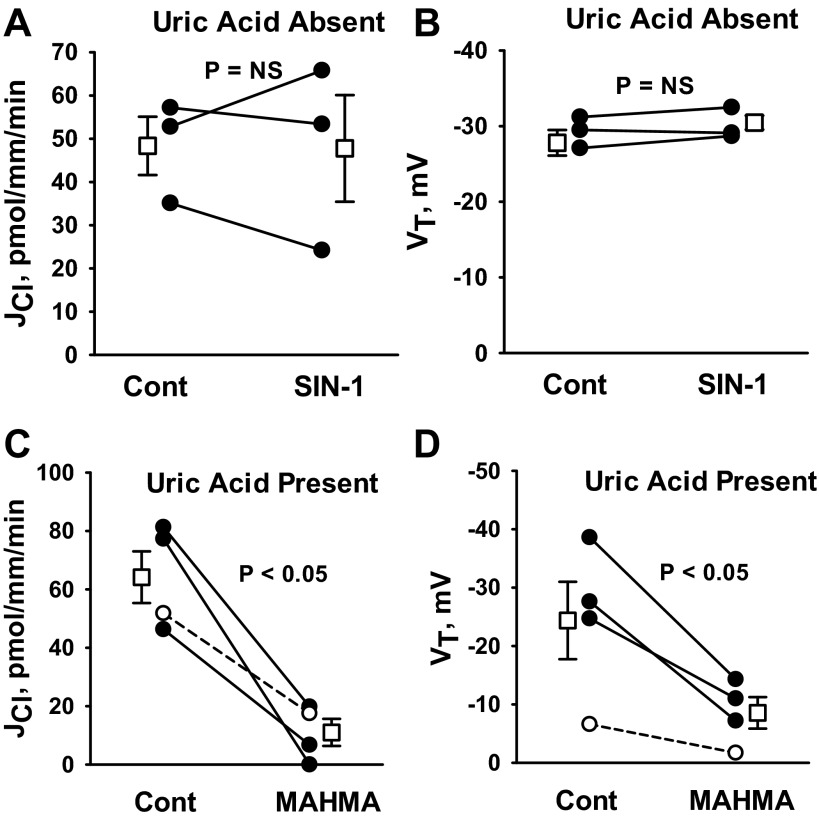
NO reacts with O2·− (superoxide) to form ONOO−, which is a highly oxidative and nitrosylating compound (49). We hypothesized that if NO reduces Cl− absorption through ONOO−, ONOO− donors should mimic the effect of NO on JCl. Therefore, we examined the effect of an ONOO− donor (SIN-1) (15) on JCl and VT. As shown (Fig. 6, A and B), Cl− absorption and VT were unchanged with SIN-1 application. These data suggest that the fall in Cl− absorption observed with the NO donor MAHMA does not occur through the formation of ONOO−.
Fig. 6.
NO does not reduce JCl in the CCD through the action of ONOO−. A and B: JCl and VT were measured before and after the application of the ONOO− donor SIN-1 [5-amino-3-(4-morpholinyl)-1,2,3,-oxadiazolium chloride; 1 mM] to the bath solution. C and D: CCDs were perfused in vitro with uric acid (100 μM) present in the perfusate and the bath solution. JCl and VT were measured before and after the application of MAHMA (10 μM). Angiotensin II (10 nM) was present in the bath in all experiments. Solid lines show individual tubules. Dashed lines show experiments conducted in reverse order (i.e., MAHMA was applied in the first period and then removed in the second). Open squares show means ± SE.
We further hypothesized that if NO reduces Cl− absorption through the action of ONOO−, then ONOO− scavengers should blunt the effect of NO on Cl− absorption. To test this hypothesis, we examined the effect of MAHMA on JCl in CCDs that were perfused in vitro in the presence of uric acid, which is an effective ONOO− scavenger (15). As shown, uric acid application did not prevent the fall in Cl− absorption or VT observed with NO donor (MAHMA) application (Fig. 6, C and D) (15). We conclude that NO does not reduce JCl through the action of ONOO−.
NO reduces Cl− absorption through a benzamil-sensitive mechanism that does not involve the H+-ATPase or the Na+-dependent Cl−/HCO3− exchanger (Slc4a8).
Further experiments explored the transport pathways mediating NO-sensitive Cl− absorption. Three transport inhibitors modulate Cl− absorption in the CCD. Cl− absorption falls in the CCD 1) when the basolateral plasma membrane H+-ATPase is inhibited with bafilomycin (24), 2) when the apical Na+-dependent Cl−/HCO3− exchanger encoded by Slc4a8 is inhibited with hydrochlorothiazide (18), and 3) when the apical plasma membrane ENaC is blocked with amiloride analogs (26).
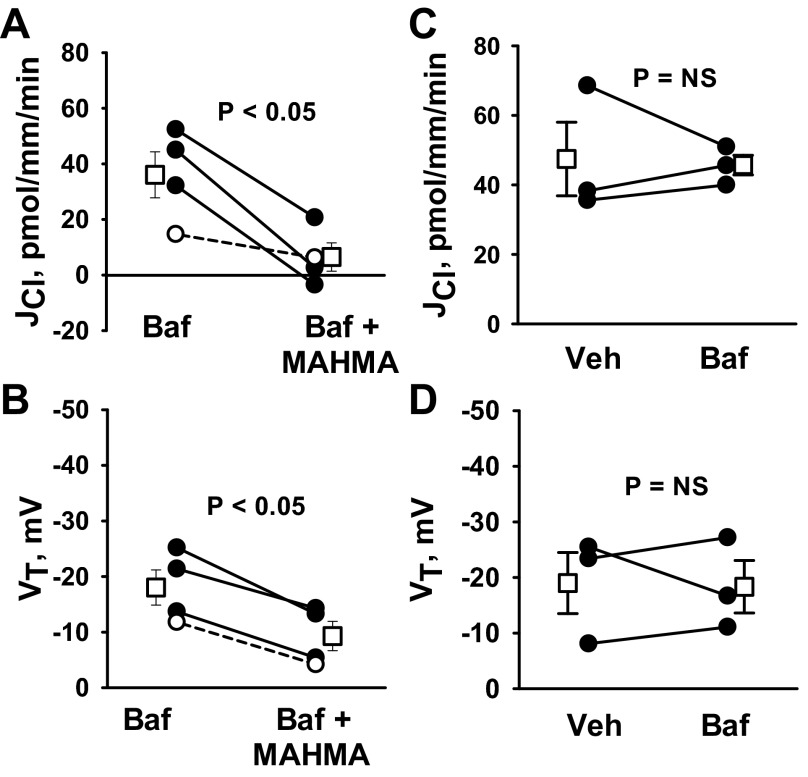
The apical plasma membrane of type A intercalated cells mediates H+ secretion by way of the H+-ATPase, which may increase the driving force for apical Cl−/HCO3− exchange by reducing luminal HCO3− concentration. Since NO reduces H+-ATPase activity (36), we hypothesized that NO reduces Cl− absorption by inhibiting the H+-ATPase. If so, the fall in Cl− absorption observed with NO donor application should be eliminated with H+-ATPase blockade. To test this hypothesis, we examined the effect of NO on JCl and VT with an H+-ATPase inhibitor (bafilomycin) present in the perfusate (Fig. 7, A and B). As shown, luminal bafilomycin did not eliminate MAHMA-induced changes in JCl and VT. We conclude that MAHMA does not reduce JCl by inhibiting the apical plasma membrane H+-ATPase.
Fig. 7.
NO donors do not reduce JCl through an effect on the H+-ATPase. JCl and VT were measured in CCDs from aldosterone-treated wild-type mice with angiotensin II (10 nM) in the bath solution. A and B: JCl and VT measured before and after MAHMA application to the bath (10 μM) when a H+-ATPase inhibitor [5 nM bafilomycin (Baf)] was present in the perfusate in both periods. C and D: effect of bafilomycin (5 nM) on JCl and VT when applied to the perfusate. Solid lines show individual tubules. Dashed lines show individual experiments conducted in reverse order. Open squares show means ± SE. Veh, vehicle.
Bafilomycin might not modify the effect of NO donors on JCl either because the H+-ATPase is not sensitive to this NO donor or because the H+-ATPase does not play a significant role in Cl− absorption in this treatment model. To resolve this question, we measured Cl− absorption in the CCD, in the presence and absence of the H+-ATPase inhibitor bafilomycin (5 nM) in the perfusate. As shown (Fig. 7, C and D), bafilomycin did not change JCl or VT. We therefore conclude that inhibiting the apical H+-ATPase does not significantly alter Cl− absorption in CCDs from aldosterone-treated mice.
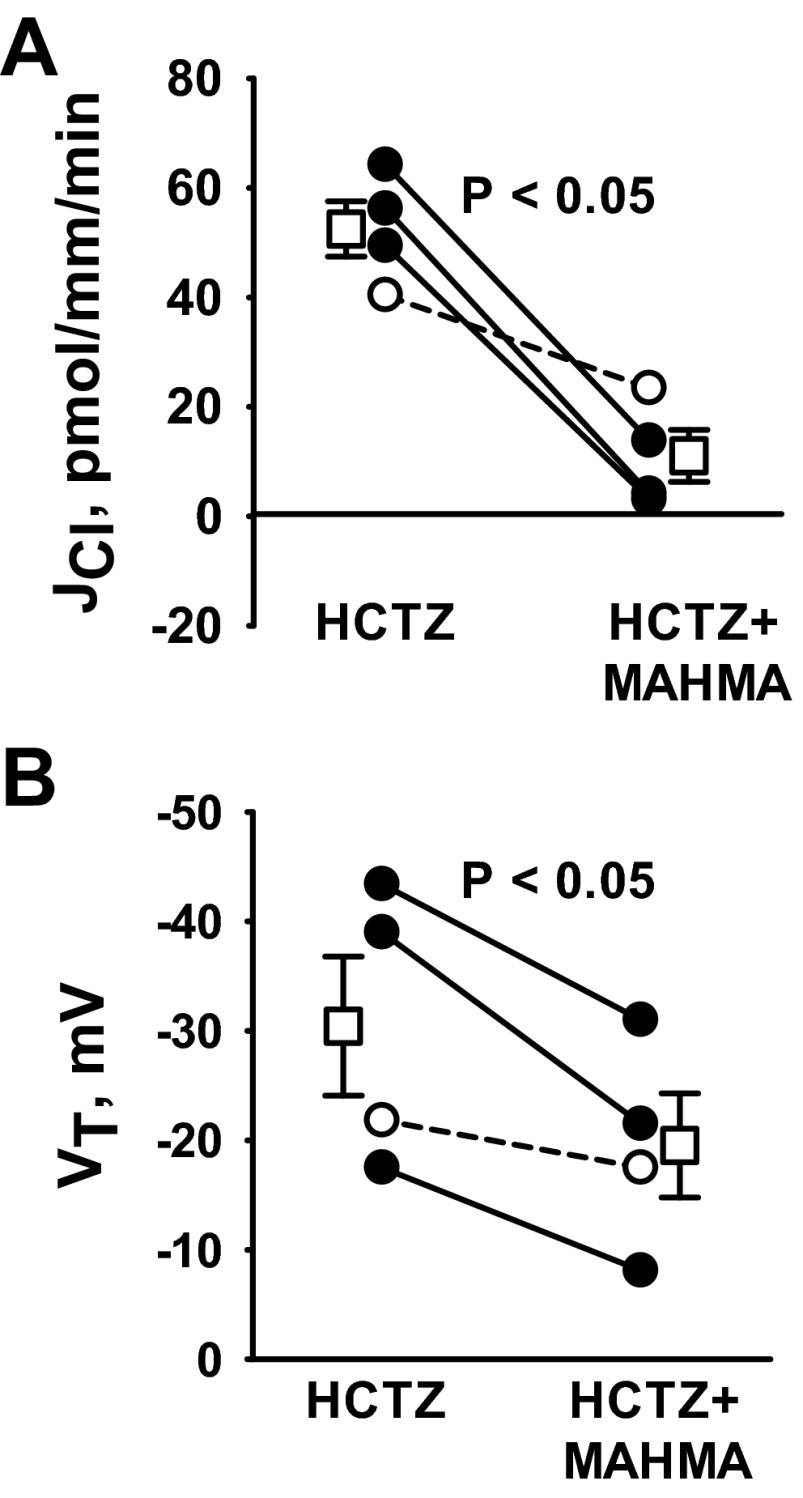
In the CCD of NaCl-restricted mice, Cl− absorption occurs through a thiazide-sensitive Na+-dependent Cl−/HCO3− exchanger encoded by Slc4a8 (18). Therefore, we asked whether thiazides attenuate the NO-sensitive component of Cl− absorption in CCDs from aldosterone-treated mice. To test this hypothesis, we examined the effect of MAHMA on Cl− absorption with hydrochlorothiazide (100 μM) in the perfusate. Figure 8 demonstrates that thiazides do not block the fall in Cl− absorption and VT observed with the application of NO donors. Because thiazides do not eliminate NO-sensitive changes in JCl, Slc4a8 is not likely a significant target of NO in CCDs from aldosterone-treated mice. These observations are in agreement with a recent study demonstrating that the thiazide-sensitive component of Cl− absorption is very low in CCDs taken from mice following aldosterone treatment (26).
Fig. 8.
NO donors reduce JCl through a thiazide-insensitive pathway. CCDs from aldosterone-treated wild-type mice were perfused in vitro. Throughout the experiments, hydrochlorothiazide (HCTZ; 100 μM) was present in the perfusate and angiotensin II (10 nM) was present in the bath. A and B: JCl and VT were measured before and after MAHMA application to the bath (10 μM). Solid lines show individual tubules. Dashed lines show individual experiments conducted in reverse order. Open squares show means ± SE.
To determine whether NO targets ENaC-dependent Cl− absorption, we tested whether NO donors modulate the benzamil-sensitive component of Cl− absorption. We observed that with an ENaC inhibitor (benzamil) in the perfusate, Cl− absorption and VT are low and unchanged with MAHMA application (Fig. 9, A and B). Moreover, in the presence of an ENaC inhibitor (benzamil), NOS inhibitors (l-NAME) did not stimulate Cl− absorption or VT (Fig. 9, C and D). We conclude that NO reduces Cl− absorption through a benzamil-sensitive pathway that depends on the epithelial Na+ channel.
Fig. 9.
NO targets the benzamil-sensitive component of JCl. CCDs from aldosterone-treated wild-type mice were perfused in vitro with benzamil (3 μM) in the perfusate. A and B: JCl and VT were measured in CCDs before and after MAHMA (10 μM) application to the bath. C and D: JCl and VT were measured before and after the application of l-NAME (100 μM) to the bath. Solid lines show individual tubules. Dashed lines show individual experiments conducted in the reverse order. Open squares show means ± SE. Angiotensin II (10 nM) was present in the bath solution in all experiments.
NO does not reduce Cl− absorption through a mechanism dependent on the Cl−/HCO3− exchanger pendrin.
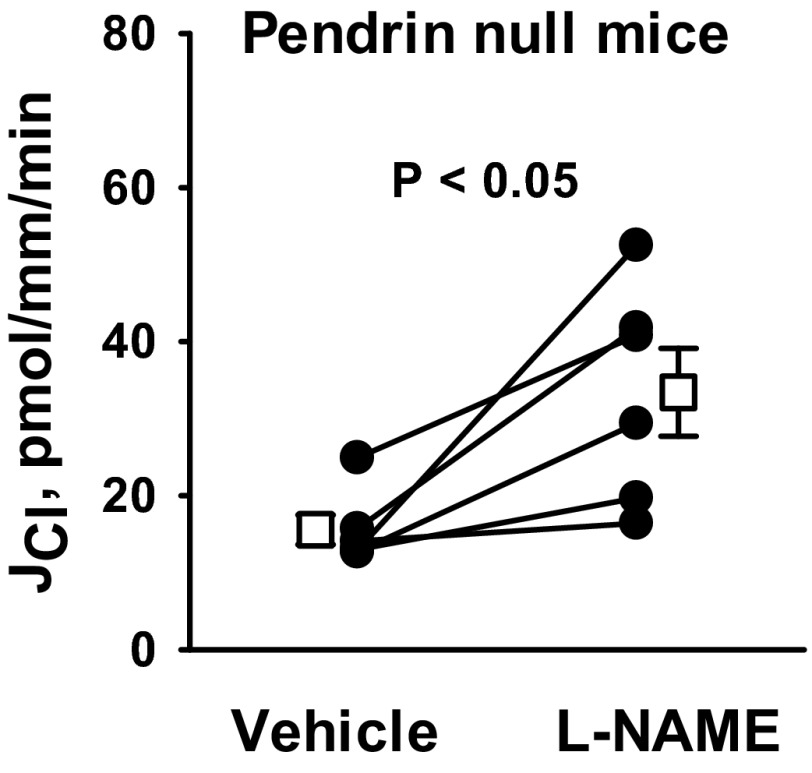
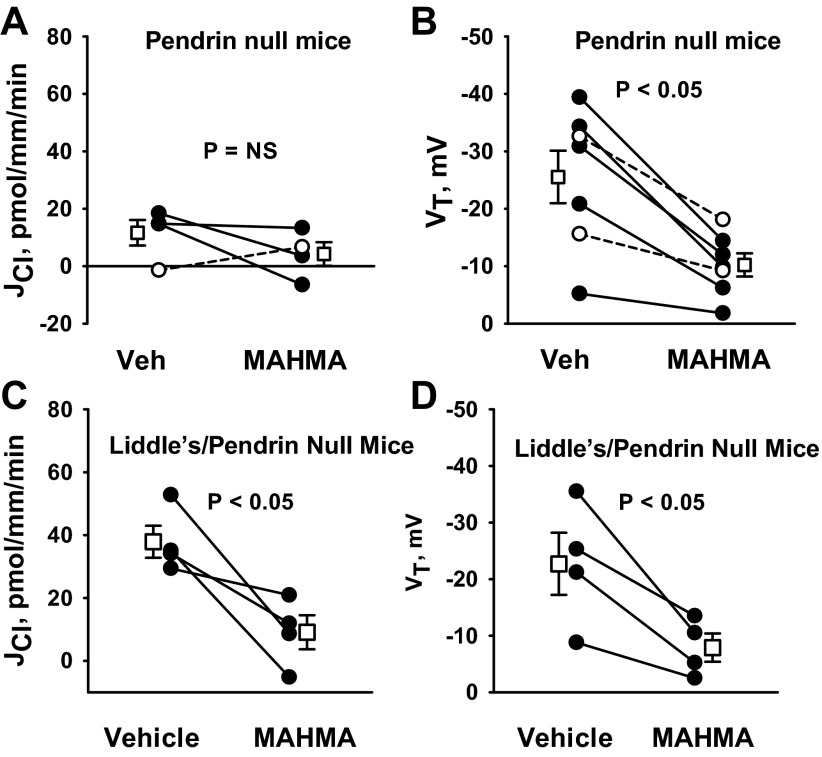
Since pendrin mediates HCO3− secretion (31) and since HCO3− secretion is reduced with NO (38), we asked whether pendrin gene ablation alters NO-sensitive Cl− absorption. To answer this question, we tested the effect of pendrin gene ablation (Slc26a4) on NO-sensitive Cl− absorption. As shown, pendrin gene ablation did not eliminate the increase in Cl− absorption observed with NOS inhibitor (100 μM l-NAME; Fig. 10) application. These data suggest that NO regulates JCl independent of pendrin. In contrast, pendrin gene ablation eliminated the fall in Cl− absorption observed with NO donor administration (Fig. 11, A and B). Why Cl− absorption changed with the application of a NOS inhibitor but not with an NO donor could be explained by two alternative hypotheses. If NO directly targets pendrin-mediated Cl− absorption, NO donors might not reduce Cl− absorption because the targeted transporter (pendrin) is absent in these mutant mice. However, if NO directly targets benzamil-sensitive Cl− absorption, rather than pendrin-mediated transport, NO donors would not reduce Cl− absorption because ENaC is already markedly downregulated in kidneys from pendrin-null mice (12), and therefore could not be downregulated much farther. To resolve this issue, we examined the effect of MAHMA on JCl in CCDs from mice that are homozygous for both the pendrin-null and the Liddle's mutation (Pds−/−& L/L; Fig. 11, C and D). With the introduction of the Liddle's mutation, ENaC is constitutively upregulated in the pendrin-null mice (3). As shown, in CCDs from mice that are homozygous for both the pendrin-null and the Liddle's mutations (Pds−/− & L/L), MAHMA reduced JCl and VT. Thus, pendrin gene ablation does not eliminate NO-sensitive JCl and VT. We conclude that NO does not inhibit JCl by directly targeting pendrin-mediated Cl− absorption.
Fig. 10.
Pendrin gene ablation does not eliminate the increase in JCl observed with NO synthase inhibition. JCl was measured in CCDs from aldosterone-treated pendrin-null mice before and after the application of a NO synthase inhibitor (100 μM l-NAME) to the bath. Angiotensin II (10 nM) was present in the bath solution in all experiments. Solid lines show individual tubules. Open squares show means ± SE.
Fig. 11.
Pendrin gene ablation does not eliminate the fall in JCl observed with NO donor application. A and B: JCl and VT were measured in CCDs from aldosterone-treated pendrin-null mice before and after the application of MAHMA (10 μM) to the bath. C and D: JCl and VT were measured in CCDs from aldosterone-treated pendrin-null/Liddle's mice (Pds−/−; L/L) before and after the application of MAHMA (10 μM) to the bath. Angiotensin II (10 nM) was present in the bath solution in all experiments. Solid lines show individual tubules. Dashed lines show individual experiments conducted in reverse order. Open squares show means ± SE.
DISCUSSION
NO is an important blood pressure regulator. With NO depletion, hypertension is observed in both rodents (16) and people (49). The present and previous studies showed that NO modulates blood pressure, in part, by targeting ENaC in the kidney. NO is produced through the action of NOS, which catalyze the oxidation of l-arginine to NO and l-citrulline (47, 51). Three NOS isoenzymes have been identified: neuronal NOS (nNOS, NOS 1), inducible NOS (iNOS, NOS II), and endothelial NOS (eNOS, NOS III) (47). eNOS (NOS III) expression has not been observed in the CCD. Within that segment, principal cells express nNOS (or NOS1) and ENaC (45), whereas type A and type B intercalated cells express iNOS, but do not express ENaC (2, 36). While the present study demonstrated that benzamil-sensitive Cl− absorption is reduced by endogenous NO production, which NOS are responsible for this NO production remains to be determined. The ENaC might be targeted by NO produced in principal cells. However, NO produced in other cells might act on principal cells through a paracrine effect.
NO is a hydrophobic compound that partitions from the aqueous phase into biological membranes (22). By virtue of its biophysical properties, NO freely diffuses across artificial bilayers at a rate inversely proportional to the cholesterol content of the membrane (22). Since NO can be transported by water channels, such as aquaporin-1 (AQP1) (8, 9) and AQP4 (46), we cannot exclude the possibility that NO traverses cell membranes in the mouse CCD through a mechanism other than diffusion.
While NO might reduce Cl− absorption in the CCD through NO·, NO+, ONOO−, or NO2−, it is unlikely that MAHMA reduces Cl− absorption through the action of NO2− since decayed MAHMA, which yields NO2− and NO3−, had no effect on Cl− absorption. Moreover, the present study shows that ONOO− donors do not mimic the effect of NO on Cl− absorption in the CCD. Instead, NO donors, such as MAHMA NONOate, most likely reduce Cl− absorption through the action of NO· and/or NO+.
Our laboratory observed previously that in aldosterone-treated mice, Cl− absorption and VT are reduced by more than 50% with chemical inhibitors of the ENaC (26). ENaC-dependent changes in JCl do not occur exclusively through changes in paracellular Cl− transport. Instead, ENaC blockade reduces Cl− absorption, at least in part, by stimulating a Cl− secretory pathway that is dependent on NKCC1 (26).
The present study demonstrates that in aldosterone-treated mice, benzamil-sensitive Cl− transport is a major target of NO. Therefore, NO reduces amiloride (benzamil)-sensitive Na+ (33) and Cl− absorption in the CCD. These data raise the possibility that the NO depletion that follows aldosterone administration contributes to the increase in benzamil-sensitive transport observed during this treatment model.
While NO does not modulate pendrin-dependent Cl− absorption in vitro, NO modulates pendrin total protein abundance in vivo and in vitro (35, 40). We observed that in NaCl-restricted mice, the angiotensin type 1 receptor modulates pendrin protein abundance through a mechanism that is dependent on NO (40). The present study shows that while NO reduces Cl− absorption in the CCD, it does so independent of pendrin. Thus, NO changes the number of pendrin transporters, and possibly pendrin-mediated Cl− transport, in a span of hours to days, whereas NO changes pendrin-independent, ENaC-dependent Cl− absorption within 10 min.
In summary, NO reduces benzamil-sensitive Cl− absorption in the mouse CCD. The downstream signaling cascade mediating NO-induced ENaC inhibition remains to be determined.
GRANTS
This work was supported by Grant DK 46493 (to S. M. Wall) and by the American Society of Nephrology Career Development Award no. 145596 (to V. Pech).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.P., S.I.D., D.G.H., and S.M.W. conception and design of research; V.P. and M.T. performed experiments; V.P. and S.M.W. analyzed data; V.P., S.I.D., D.G.H., and S.M.W. interpreted results of experiments; V.P. and S.M.W. prepared figures; V.P. and S.M.W. drafted manuscript; V.P. and S.M.W. edited and revised manuscript; V.P., M.T., S.I.D., E.H., B.C.R., D.G.H., and S.M.W. approved final version of manuscript.
Footnotes
Although we could not demonstrate a significant role for O2·− in Cl− absorption in the CCD from aldosterone-treated mice, O2·− may regulate Cl− absorption in other conditions. In the CCD from mice treated with a NaCl-replete diet and furosemide (24), application of tempol (50 μM) to the bath and lumen in the presence of angiotensin II reduced Cl− absorption from 15.6 ± 2.4 (n = 12) to 7.0 ± 2.0 pmol·mm−1·min−1 (n = 7), P < 0.05.
REFERENCES
- 1. Adler S, Huang H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Renal Physiol 287: F907–F913, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Ahn KY, Mohaupt MG, Madsen KM, Kone BC. In situ hybridization of mRNA encoding inducible nitric oxide synthase in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 267: F748–F757, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Dahlmann A, Pradervand S, Hummler E, Rossier BC, Frindt G, Palmer LG. Mineralocorticoid regulation of epithelial Na+ channels is maintained in a mouse model of Liddle's syndrome. Am J Physiol Renal Physiol 285: F310–F318, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Everett LA, Belyantseva IA, Noben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum Mol Genet 10: 153–161, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Garcia NH, Plato CF, Garvin JL. Fluorescent determination of chloride in nanoliter samples. Kidney Int 55: 321–325, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Gryglewski RJ, Palmer RMJ, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Hennington BS, Zhang H, Miller MT, Granger JP, Reckelhoff JF. Angiotensin II stimulates synthesis of endothelial nitric oxide synthase. Hypertension 31: 283–288, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Herrera M, Garvin JL. Novel role of AQP-1 in NO-dependent vasorelaxation. Am J Physiol Renal Physiol 292: F1443–F1451, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Herrera M, Hong NJ, Garvin JL. Aquaporin-1 transports NO across cell membranes. Hypertension 48: 157–164, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Jin CZ, Jang JH, Wang Y, Kim JG, Bae YM, Shi J, Che CR, Kim SJ, Zhang YH. Neuronal nitric oxide synthase is up-regulated by angiotensin II and attenuates NADPH oxidase activity and facilitates relaxation in murine left ventricular myocytes. J Mol Cell Cardiol 52: 1274–1281, 2012 [DOI] [PubMed] [Google Scholar]
- 11. Jin L, Beswick RA, Yamamoto T, Palmer T, Taylor TA, Pollock JS, Pollock DM, Webb RC. Increased reactive oxygen species contributes to kidney injury in mineralocorticoid hypertensive rats. J Physiol Pharmacol 57: 343–357, 2006 [PubMed] [Google Scholar]
- 12. Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin WK, Verlander JW, Sutliff RL, Wall SM. Reduced ENaC expression contributes to the lower blood pressure observed in pendrin null mice. Am J Physiol Renal Physiol 293: F1314–F1324, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Knepper MA, Good DW, Burg MB. Mechanism of ammonia secretion by cortical collecting ducts of rabbits. Am J Physiol Renal Fluid Electrolyte Physiol 247: F729–F738, 1984 [DOI] [PubMed] [Google Scholar]
- 14. Knepper MA, Good DW, Burg MB. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 249: F870–F877, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol 70: 343–354, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Lahera V, Salom MG, Miranda-Guardiola F, Moncada S, Romero JC. Effects of NG-nitro-l-arginine methyl ester on renal function and blood pressure. Am J Physiol Renal Fluid Electrolyte Physiol 261: F1033–F1037, 1991 [DOI] [PubMed] [Google Scholar]
- 17. Lai EY, Solis G, Luo Z, Carlstrom M, Sandberg K, Holland S, Wellstein A, Welch WJ, Wilcox CS. p47phox is required for afferent arteriolar contractile responses to angiotensin II and perfusion pressure in mice. Hypertension 59: 415–420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hatim H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. Identification of a novel electroneutral Na+ reabsorption process in the distal nephron mediated by the Na+-dependent chloride-bicarbonate exchanger Slc4A8. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lopez B, Garcia Salom M, Arregui B, Valero F, Fenoy FJ. Role of superoxide in modulating the renal effects of angiotensin II. Hypertension 42: 1150–1156, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Lopez B, Moreno C, Salom MG, Roman RJ, Fenoy FJ. Role of guanylyl cyclase and cytochrome P-450 on renal response to nitric oxide. Am J Physiol Renal Physiol 281: F420–F427, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α,β and γ subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miersch S, Espey MG, Chaube R, Akarca A, Tweten R, Ananvoranich S, Mutus B. Plasma membrane cholesterol content affects nitric oxide diffusion dynamics and signaling. J Biol Chem 283: 18513–18521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 24: 841–848, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292: F914–F920, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, Wall SM. Pendrin modulates ENaC function by changing luminal HCO3−. J Am Soc Nephrol 21: 1928–1941, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pech V, Thumova M, Kim YH, Agazatian D, Hummler E, Rossier BC, Weinstein AM, Nanami M, Wall SM. ENaC inhibition stimulates Cl− secretion in the mouse cortical collecting duct through an NKCC1-dependent mechanism. Am J Physiol Renal Physiol 303: F45–F55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells in mouse cortical collecting duct. J Am Soc Nephrol 19: 84–91, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT1 receptors. J Am Soc Nephrol 13: 1131–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Pradervand S, Wang Q, Burnier M, Beermann F, Horisberger JD, Hummler E, Rossier BC. A mouse model for Liddle's Syndrome. J Am Soc Nephrol 10: 2527–2533, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Pueyo ME, Arnal JF, Rami J, Michel JB. Angiotensin II stimulates the production of NO and perosynitrite in endothelial cells. Am J Physiol Cell Physiol 274: C214–C220, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saito S, Hirata Y, Emori T, Imai T, Marumo F. Angiotensin II activates endothelial constitutive nitric oxide synthase via AT1 receptors. Hypertens Res 19: 201–206, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol 6: 89–94, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Sun P, Yue P, Wang WH. Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. Am J Physiol Renal Physiol 302: F679–F687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thumova M, Pech V, Frohlich O, Agazatian D, Wang X, Verlander JW, Kim YH, Wall SM. Pendrin protein abundance in the kidney is regulated by nitric oxide and cAMP. Am J Physiol Renal Physiol 303: F812–F820, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tojo A, Guzman NJ, Garg LC, Tisher CC, Madsen KM. Nitric oxide inhibits bafilomycin-sensitive H+-ATPase activity in rat cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 267: F509–F515, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Tsuruoka S, Schwartz GJ. Adaptation of rabbit cortical collecting duct HCO3− transport to metabolic acidosis in vitro. J Clin Invest 97: 1076–1084, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsuruoka S, Watanabe S, Purkerson JM, Fujimura A, Schwartz GJ. Endothelin and nitric oxide mediate adaptation of the cortical collecting duct to metabolic acidosis. Am J Physiol Renal Physiol 291: F866–F873, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM. Deoxycorticosterone upregulates Pds (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Verlander JW, Hong S, Pech V, Bailey JL, Agazatian D, Matthews SW, Coffman TM, Le T, Inagami T, Whitehill FM, Weiner ID, Farley DB, Kim YH, Wall SM. Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am J Physiol Renal Physiol 301: F1314–F1325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Verlander JW, Kim YH, Shin WK, Pham TD, Hassell KA, Beierwaltes WH, Green ED, Everett LA, Matthews SW, Wall SM. Dietary Cl− restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol 291: F833–F839, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Wall SM. NH4+ augments net acid secretion by a ouabain-sensitive mechanism in isolated perfused inner medullary collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 270: F432–F439, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Wall SM, Fischer MP, Mehta P, Hassell KA, Park SJ. Contribution of the Na+-K+-2Cl− cotransporter (NKCC1) to Cl− secretion in rat outer medullary collecting duct. Am J Physiol Renal Physiol 280: F913–F921, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl− conservation. Hypertension 44: 1–6, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Wang X, Lu M, Gao Y, Papapetropoulos A, Sessa WC, Wang W. Neuronal nitric oxide synthase is expressed in principal cell of collecting duct. Am J Physiol Renal Physiol 275: F395–F399, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Tajkhorshid E. Nitric oxide conduction by the brain aquaporin AQP4. Proteins 78: 661–670, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wei CC, Wang ZQ, Durra D, Hemann C, Hille R, Garcin ED, Getzoff ED, Stuehr DJ. The three nitric oxide synthases differ in their kinetics of tetrahydrobiopterin radical formation, heme-dioxyreduction, and arginine hydroxylation. J Biol Chem 280: 8929–8935, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Welch WJ, Blau J, Xie H, Chabrashvilli T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol 288: H22–H28, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Winter C, Schulz N, Giebisch G, Geibel JP, Wagner CA. Nongenomic stimulation of vacuolar H+-ATPases in intercalated renal tubule cells by aldosterone. Proc Natl Acad Sci USA 101: 2636–2641, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yan C, Kim D, Aizawa T, Berk BC. Functional interplay between angiotensin II and nitric oxide. Arterioscler Thromb Vasc Biol 23: 26–36, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Yu L, Bao HF, Self JL, Eaton DC, Helms MN. Aldosterone-induced increases in superoxide production counters nitric oxide inhibition of epithelial Na channel activity in A6 distal nephron cells. Am J Physiol Renal Physiol 293: F1666–F1677, 2007 [DOI] [PubMed] [Google Scholar]