Abstract
Antenatal treatment with glucocorticoids (GC) poses long-lasting effects on endocrine and cardiovascular function. Given that leptin attenuates adrenal function and the reported sex differences in plasma leptin concentration, we hypothesized that antenatal GC will affect leptin levels and leptin modulation of adrenal function in a sex-specific manner. Pregnant sheep were randomly given betamethasone or vehicle at 80 days of gestational age, and offspring were allowed to deliver at term. Adrenocortical cells (ADC) were studied from male and female animals at 1.5 yr of age. Plasma leptin was increased 66% in male and 41% in female GC-treated animals (P < 0.05), but adrenal leptin mRNA was increased only in GC-treated males (P < 0.05). Whereas mRNA expression of adrenal leptin receptor isoforms showed sex (Ob-Ra and Ob-Rb) and treatment-dependent (Ob-Rb) differences, protein expression remained unchanged. GC-treated females showed greater plasma cortisol and greater ACTH-stimulated cortisol production (P < 0.05) in ADC. Leptin exerted a greater inhibitory effect on basal and stimulated cortisol by ADC from GC-treated males (P < 0.05), with no differences in females. Similarly, greater inhibitory effects on basal and ACTH-stimulated StAR and ACTH-R mRNA expression by leptin were observed in cells from GC males (P < 0.05), with no changes in females. Persistent effects of antenatal GC on leptin levels and leptin modulation of adrenal function are expressed in a sex-specific manner; males are more sensitive than females to the inhibitory influences of leptin on adrenal function, and this effect appears to be mediated by a greater inhibition of StAR and ACTH-R expression in adrenals of adult GC-treated males.
Keywords: leptin, betamethasone, adrenal responsiveness, adrenocorticotropic hormone receptor, steroidogenic acute regulatory protein
synthetic glucocorticoids (GCs) such as betamethasone are widely administered to pregnant women at risk for premature delivery (30) to improve lung development and survival among premature infants born before 34 wk of gestation. Major concerns have arisen about the impact of GC treatment on cardiovascular and endocrine development, and some long-lasting programming effects of antenatal GC treatment on the hypothalamic-pituitary-adrenal (HPA) and stress responses have been observed in humans (8, 10, 11, 40, 57). However, information regarding the mechanisms of long-term effects of antenatal GC exposure on the HPA axis (HPAA) is limited. Alterations in the regulation of the HPAA after prenatal GC have been observed in the adult guinea pig (14), whereas in sheep, repeated but not single prenatal betamethasone administration inhibits cortisol production in adults (44). Recent evidence obtained in sheep indicates that the effects of antenatal GC treatment on HPAA activity extend to the second generation offspring (25), but there is a dearth of studies examining whether there are sex-related differences in the effects of antenatal GC exposure on HPAA function.
Leptin is a 16-kDa hormone that acts as a satiety signal in the central nervous system, and it has been proposed to be the link between obesity, diabetes, and cardiovascular risk (37, 63). Although leptin was originally thought to act largely via the central nervous system, studies have demonstrated that leptin exerts a wide repertoire of peripheral effects through its direct actions on target tissues, including the adrenal (2, 17, 22, 34, 45, 64). Evidence of leptin-HPAA interactions includes the inhibition by leptin of corticotropin-releasing hormone release from hypothalamic explants and blunting of plasma ACTH and cortisol responses to restraint (20) as well as inhibition of cortisol production by adrenocortical cells (2), among others (5). Our recent demonstration that leptin administration decreases expression of fetal adrenal steroidogenic enzymes suggests one in vivo mechanism of this HPAA inhibition at the level of the adrenal gland (50).
Sex-based differences in leptin levels, with women having higher levels of leptin than men (27), are independent of variations in total body fat mass (9, 19, 35, 36, 62), suggesting that women may display leptin resistance. Moreover, leptin receptors are ubiquitously expressed, including the short Ob-Ra and long Ob-Rb isoforms in the adrenal (4, 24, 28, 50), and sex differences have been observed in expression of leptin receptors (19). However, whether or not there are any interactions between leptin and antenatal GC exposure influencing adrenal function in adulthood is not known.
We hypothesize that antenatal GC will affect leptin levels and leptin modulation of adrenal steroidogenesis differently in male and female adult sheep. Therefore, we measured plasma levels of leptin and cortisol along with leptin expression in adrenal tissue as well as mRNA and protein levels for adrenal leptin receptors in adult male and female sheep that were exposed before birth to betamethasone or vehicle. Considering that adrenal responsiveness to ACTH, a key factor in cortisol production, is dependent upon the upregulation of the steroidogenic acute regulatory protein (StAR) and the ACTH receptor (ACTH-R), we also determined the effects of leptin on cortisol production, StAR, and ACTH-R mRNA expression levels in basal and ACTH-stimulated conditions in adrenocortical cells (ADC) from these animals.
MATERIALS AND METHODS
Animal preparation.
Pregnant sheep were randomly treated with a single course of betamethasone [1:1 mixture of betamethasone acetate and betamethasone phosphate (Celestone Soluspan; Schering, Kenilworth, NJ)] in two maternal intramuscular doses (0.17 mg/kg with a maximum of 12 mg) or vehicle 24 h apart at 80 and 81 days of gestation (term ∼145 days). This regime of betamethasone administration was chosen because it is equivalent to that currently used in clinical practice. The time of administration (60% of gestation) is comparable with a gestational age of ∼24 wk in humans and was chosen considering that more than 50% of pregnant women receiving antenatal steroids are treated between 23 and 28 wk of gestation (60, 61). In addition, the administered dose of betamethasone was equivalent to the dose a 70-kg pregnant woman would receive. The pregnant sheep were allowed to deliver at term. Offspring were weaned at 3 mo of age after spontaneous delivery and transferred to the laboratory at 1.5 yr of age for study. Animals were instrumented with intravascular catheters as described by us (51). Animals were maintained on a normal diet, with free access to tap water in our Association for Assessment and Accreditation of Laboratory Animal Care International -approved facility with a 12:12-h light-dark cycle. At least 5 days after surgery, while animals were conscious and resting in stalls, blood samples were taken, blood was centrifuged immediately after collection, and plasma was separated into aliquots and stored at −80°C for later analysis. After blood collection, animals were euthanized with an overdose of Euthasol (Virbac). A total of 31 adult animals were used: 15 males (control, n = 6; GC-treated, n = 9) and 16 females (control, n = 6; GC-treated, n = 10), with equivalent numbers of animals coming from twin pregnancies in each sex (males, n = 2; females, n = 2). Because of some technical issues, not all of the determinations were performed in each animal. All of the procedures for animal housing, management, and euthanasia were approved by Wake Forest University's Institutional Animal Care and Use Committee.
Leptin ELISA.
Plasma leptin concentrations were measured with a commercial ELISA kit (Multispecies Leptin Assay; Linco Research, St. Charles, MO) using recombinant ovine leptin standards. Calibration curves made using recombinant ovine leptin standards and the recombinant human leptin standards (provided with the kit) were similar. Previously, this kit has been reported to detect ovine leptin (13), and no differences have been observed in biological activity between ovine and human leptin (12, 15). The sensitivity of measurement was 1 ng/ml of recombinant ovine standard. The intra- and interassay coefficients of variation were 8.7 and 8.1%, respectively.
Cell culture.
Adrenal glands from control and GC-treated male and female animals were isolated at necropsy, cleaned, and weighed, and the medulla was gently peeled out. Cortical cells were dispersed in 0.4% collagenase type I (Worthington Biochemical, Lakewood, NJ) for 2–3 h, washed, centrifuged through 60% Percoll (Sigma, St. Louis, MO), resuspended in DMEM-Ham's F-12 containing 10% fetal calf serum, counted, and plated in six-well-plates at a density of 4 × 105 cells/well. Treatments were identical for cells from control and GC-treated male and female animals, and at least two wells per animal were studied. On day 3 of culture, after 72 h at 37°C in 5% CO2, cells were rinsed two times with DMEM-Ham's F-12 medium containing 0.1% Polypep (Sigma) and then incubated with human leptin (10 or 100 ng/ml) or medium alone for 24 h. On day 4 of culture, cells from the three groups were treated with medium alone (basal conditions) or medium containing ACTH1–24 (1.5 × 10−10 M, stimulated conditions, Cortrosyn; Organon, West Orange, NJ) for 3 h. This concentration of ACTH has been demonstrated in our laboratory to produce half-maximal cortisol secretion from ovine cells (41). After incubation with ACTH1–24, culture medium was stored at −20°C until it was assayed for cortisol, and cells were harvested for RNA and protein extraction.
Cortisol measurements.
Cortisol concentrations in plasma and culture medium from basal or ACTH1–24-stimulated cells were measured using commercial RIA kits, following the manufacturer's instructions (MP Biomedical, Orangeburg, NY). The sensitivity of measurement was 0.7 ng/ml. The intra- and interassay coefficients of variation were 6.8 and 9.5%, respectively.
RNA isolation.
Leptin and leptin receptor isoform mRNA expression was determined in adrenal tissue. Total RNA from adrenal cortex tissue was extracted using Trizol reagent (GIBCO-BRL, Carlsbad, CA) according to the manufacturer's recommendations. StAR and ACTH-R mRNA expression was determined in isolated adrenocortical cells, and total RNA from cells was prepared using an RNA preparation kit, following manufacturer's instructions (Qiagen).
Quantitative real-time RT-PCR.
The quantitative real-time RT-PCR was performed using methods that we have described previously and validated (50). RNA from cells and adrenal tissues was quantified by measuring absorbance at 260 nm, and 1 μg of total RNA was reverse-transcribed in a 20-μl reaction mixture using an ABI High Capacity cDNA Archive Kit according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). Leptin, Ob-Ra, Ob-Rb, ACTH-R, and StAR mRNA were measured by quantitative real-time RT-PCR, using TaqMan PCR as described as before (50). Taqman PCR was performed on the cDNA samples using an ABI Prism 7500 Sequence Detection System (Applied Biosystems). For each gene tested (see Table 1), PCR primers were designed from the sheep sequences with Genbank accession numbers AY278244 (Ob-Ra gene for leptin receptor short form), U62124 (Ob-Rb gene for leptin receptor long form), AF290202 (STAR gene for StAR), AF116874 (MC2R gene for ACTH-R), and NM_000230 (human leptin gene). Glyceraldehyde 3-phosphate dehydrogenase (Genbank accession no. U94889) was used as control. The relative quantification of target gene expression was done using the comparative cycle threshold (CT) method, where the CT variable is defined as the cycle number at which the fluorescent signal generated by cleavage of the dual-labeled probe is first detectable. CT values for Lep, Ob-Ra, Ob-Rb, StAR, and ACTH-R were corrected for the CT of GAPDH in each sample based on the within-sample deviation for GAPDH (ΔCT) from the average for the housekeeping gene before quantification of mRNA for these genes of interest.
Table 1.
Primers and probes used in real-time PCR assays
| Gene | Forward Primer | Reverse Primer | Probe Sequence |
|---|---|---|---|
| MC2R | GTATGAAAACATCAACAGTACAGCAAGAA | AAAACTCCGACAATGGATACTGTGA | FAM-CTGCTGTGATTTTGCC-MGB |
| StAR | GCCCCACCTGCATGGT | GAGTTTGGTCCTTGAGGGACTTC | FAM-TTCCTGCCAAGACACTGT-MGB |
| Leptin | CACCAGGATCAATGACATCTCACA | CCAGTGACCCTCTGTTTGGA | FAM-CACGCAGTCCGTCTCC-MGB |
| Ob-Ra | CCCCGAGGAAAGTTCACCTATG | TGGTGGCACTCTTGCTCATT | FAM-ACGCAGTGTACTGCTGC-MGB |
| Ob-Rb | GGAGACAGCCCTCTGTTAAATATGC | TGAGCTGTTTATAAGCCCTTGCT | FAM-CCTCCTCGGCTTCACC-MGB |
| GAPDH | GCATCGTGGAGGGACTTATGAC | GGGCCATCCACAGTCTTCTG | FAM-ACGCCATCACTGCCACCT-MGB |
Nucleotide sequences are 5′-3′. MC2R, ACTH receptor; StAR, steroidogenic acute regulatory protein; leptin, human leptin gene; Ob-Ra, leptin receptor, short form; Ob-Rb, leptin receptor, long form.
Isolation of total protein extracts.
Cells were homogenized in cold 50 mM Tris·HCl (pH 7.5), 0.1 mM EDTA, 0.5 mM DTT, and protease inhibitor cocktail (1:200 diluted; Sigma) and the homogenates centrifuged for 8 min at 2,000 g at 4°C. Protein concentration in the resultant supernatant was measured using Bradford reagent, and the total protein extract was used for Western blot analysis.
Western blot analysis of leptin receptors.
Protein samples (50 μg of cellular membranes) were loaded and separated on a 12% SDS-polyacrylamide gel. Proteins were transferred onto a polyvinylidene fluoride membrane and blocked with 5% nonfat dry milk in 0.05% Tween-20 in Tris-buffered saline for 60 min at room temperature before incubation with the rabbit polyclonal antibodies against Ob-Ra and Ob-Rb isoforms (Santa Cruz Biotechnology, Santa Cruz, CA), and immunoreactive proteins were visualized using the enhanced chemiluminescence method as described by the manufacturer and exposed to Amersham Hyperfilm ECL. Autoradiograms were quantified by scanning densitometry, and the results are reported in arbitrary optical density units.
Statistical analysis.
Results are expressed as means ± SE. Two-way ANOVA was used for statistical evaluation. Data from leptin-treated cells are expressed as percent of inhibition relative to media-treated cells. Statistical significance was considered as a P value of <0.05.
RESULTS
Animal weights.
No differences in weights were observed in adult male [72.3 ± 2.7 (control) vs. 67.6 ± 2.7 kg (GC-treated), P > 0.05] or female animals [66.6 ± 5.3 (control) vs. 72.3 ± 3.9 kg (GC-treated), P > 0.05]. Similarly, no effects of sex difference on weight were observed in control [66.6 ± 5.3 (females) vs. 72.3 ± 2.7 kg (males), P > 0.05] or GC-treated animals [72.3 ± 3.9 (females) vs. 67.6 ± 2.7 kg (males), P > 0.05].
Plasma and adrenal leptin levels.
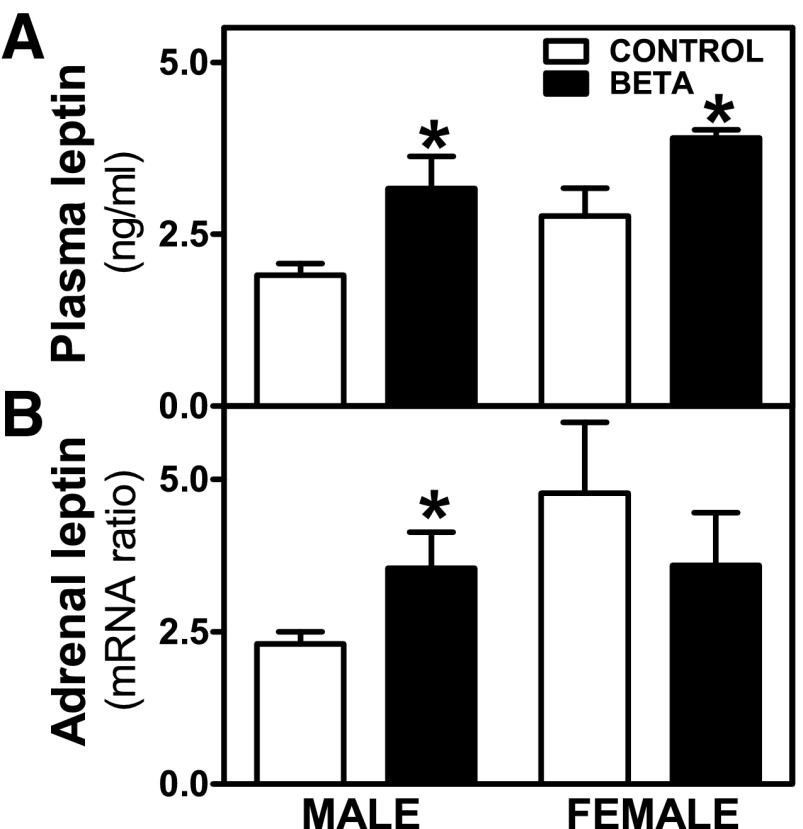
In control animals, higher plasma leptin values were observed in females compared with males, although these differences did not attain statistical significance (P = 0.08; Fig. 1A), whereas GC treatment elevated male and female plasma leptin significantly (P < 0.05; Fig. 1A). Higher levels of adrenal leptin mRNA expression were observed in GC-treated males, with no differences in females (Fig. 1B).
Fig. 1.
Leptin levels in plasma and adrenal tissue. Effects of antenatal betamethasone (Beta) on plasma leptin levels [glucocorticoid (GC)-treated (Beta; ■), male (n = 8) and female (n = 3); control (□), male (n = 6) and female (n = 5)] (A) and adrenal leptin mRNA [GC-treated (Beta), male (n = 8) and female (n = 10); control, male and (n = 6) and female (n = 6)] (B) on male and female adult sheep. Each number indicates number of animals analyzed. Data are expressed as means ± SE. *P < 0.05 vs. control.
Leptin receptor isoforms in adrenal cortical cells.
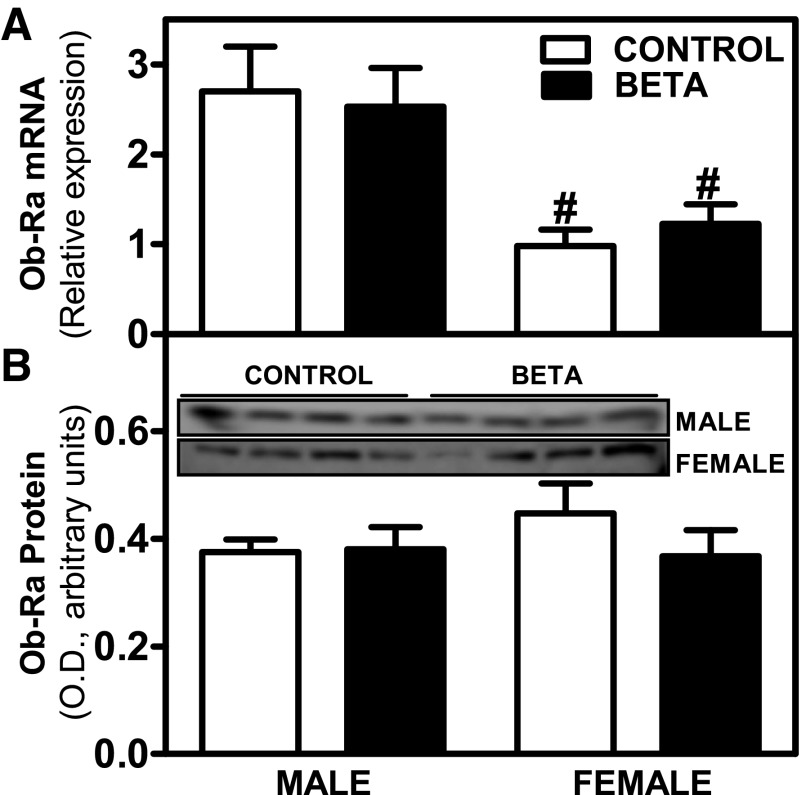
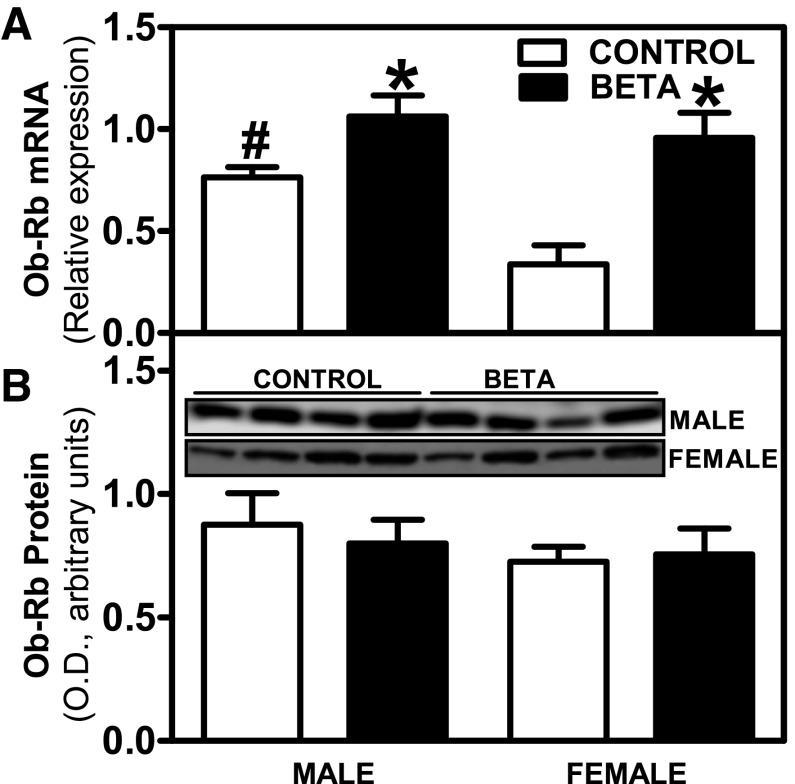
Ob-Ra mRNA isoform expression was significantly higher in males than in females (F = 18.2, P < 0.001), with no changes due to GC treatment (Fig. 2A). In terms of Ob-Ra protein expression, no differences were observed between sexes or treatments (Fig. 2B). Ob-Rb mRNA isoform expression was significantly higher in males than in females (males > females, F = 5.7, P < 0.03), and there was a treatment (GC-treated > control, F = 16.9, P < 0.001) effect (Fig. 3A). No differences were observed in Ob-Rb protein expression (Fig. 3B).
Fig. 2.
Expression levels of leptin receptor isoform Ob-Ra. Effects of antenatal Beta on mRNA [GC-treated (beta; ■), male and (n = 8) female = (n = 10); control (□), male (n = 6) and female (n = 6)] (A) and protein expression [GC-treated (Beta), male (n = 5) and female (n = 5); control, male (n = 5) and female (n = 5)] (B) of leptin receptor isoform Ob-Ra. Each no. indicates no. of animals analyzed. Data are expressed as means ± SE. #P < 0.05 vs. male. B, top, shows Western blot data. OD, optical density.
Fig. 3.
Expression levels of leptin receptor isoform Ob-Rb. Effects of antenatal Beta on mRNA [GC-treated (Beta; ■), male (n = 9) and female (n = 10); control (□) male (n = 5) and female (n = 5)] (A) and protein expression [GC-treated (Beta), male (n = 5) and female (n = 5); control, male (n = 5) and female (n =5)] (B) of leptin receptor isoform Ob-Rb. Each no. indicates no. of animals analyzed. Data are expressed as means ± SE. *P < 0.05 vs. control; #P < 0.05 vs. female.
Plasma cortisol and ADC basal and ACTH-stimulated cortisol secretion.
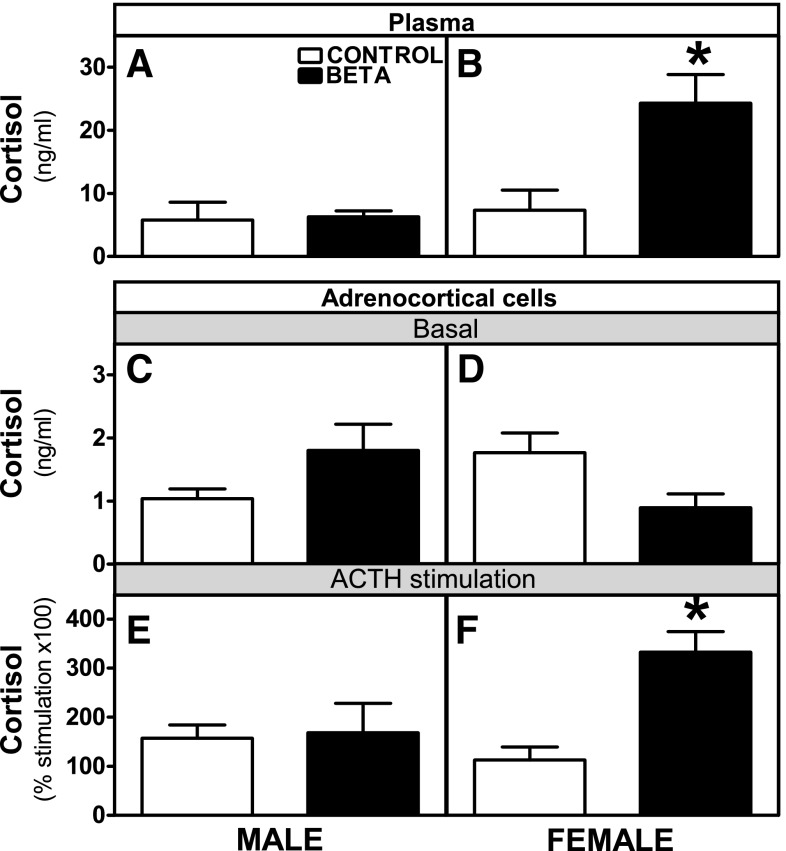
Basal plasma cortisol levels were higher in GC-treated compared with control females (P < 0.05; Fig. 4B), with no effects of GC treatment on males (Fig. 4A). Basal cortisol secretion by adrenocortical cells from male (Fig. 4C) and female (Fig. 4D) animals showed no differences. The rise in cortisol secretion following in vitro ACTH stimulation was significantly higher in cells from treated females compared with cells from the other groups (P < 0.05; Fig. 4, E and F).
Fig. 4.
Plasma and adrenocortical cell cortisol levels. Effects of antenatal Beta on plasma cortisol [GC-treated (Beta; ■), male (n = 5) and female (n = 5); control (□), male (n = 5) and female (n = 6)] (A and B), basal (C and D), and ACTH-stimulated [GC-treated (Beta; ■), male (n = 4) and female (n = 4); control (□) male (n =5) and female (n = 6)] cortisol secretion (E and F) in adrenocortical cells from male and female adult sheep. Each no. indicates no. of animals analyzed. Data are expressed as means ± SE. *P < 0.05 vs. control.
Effects of leptin on cortisol production by ovine adrenocortical cells.
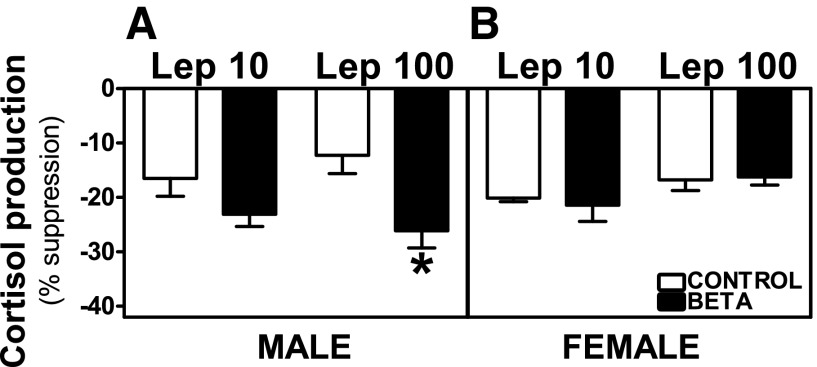
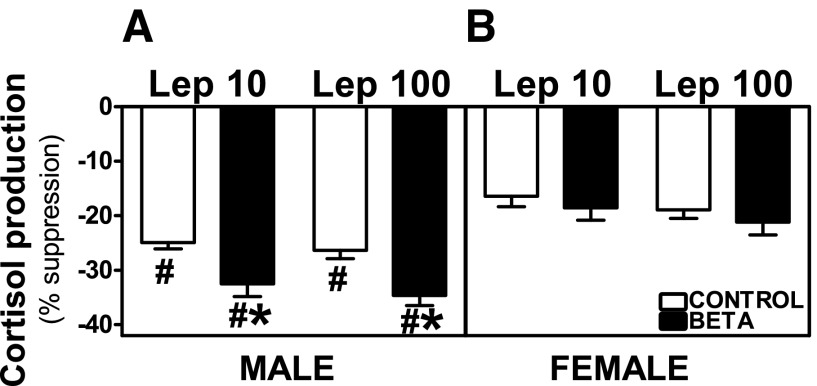
Preincubation with leptin [10 ng/ml (Lep 10) or 100 ng/ml (Lep 100)] reduced basal and ACTH-stimulated cortisol secretion from adrenocortical cells in control as well as GC-treated male and female animals. In cells from males, the percentage of suppression of basal cortisol production was higher in GC-treated animals incubated with Lep 100 (effects of treatment, F = 6.78, P < 0.01; Fig. 5A), whereas there was no effect of GC treatment on leptin-induced suppression of basal release in female cells (Fig. 5B). Both Lep 10 (F = 11.39, P < 0.005) and Lep 100 (F = 7.45, P < 0.01) incubations induced greater suppression of ACTH-stimulated cortisol production in ADC from GC-treated males (Fig. 6A) compared with cells from control animals. Following Lep 10 (F = 12.17, P < 0.005) or Lep 100 (F = 4.61, P < 0.05) incubations, ACTH-stimulated cortisol inhibition was greater in males compared with females (Fig. 6, A and B). In females, the inhibitory effects of leptin on ACTH-stimulated cortisol production were not different between control and treated animals (Fig. 6B).
Fig. 5.
Suppression of basal cortisol secretion by leptin. Effects of preincubation with 10 (Lep 10) or 100 ng/ml leptin (Lep 100) on basal cortisol secretion in adrenocortical cells from antenatal GC [Beta, male (n = 5) and female (n = 5); ■] and vehicle-treated [control, male (n = 5) and female (n = 6); □] male (A) and female (B) adult sheep. Each no. indicates no. of animals analyzed. Data are expressed as means ± SE. *P < 0.05 vs. control.
Fig. 6.
Suppression of ACTH-stimulated cortisol secretion by leptin. Effects of preincubation with Lep 10 or Lep 100 on ACTH-stimulated cortisol secretion in adrenocortical cells from antenatal GC [Beta, male (n = 4) and female (n = 4); ■] and vehicle-treated [control, male (n = 5) and female (n = 6); □] male (A) and female adult sheep (B). Each no. indicates no. of animals analyzed. Data are expressed as means ± SE. *P < 0.05 vs. control; #P < 0.05 vs. female.
StAR and ACTH-R mRNA basal expression in ovine adrenocortical cells.
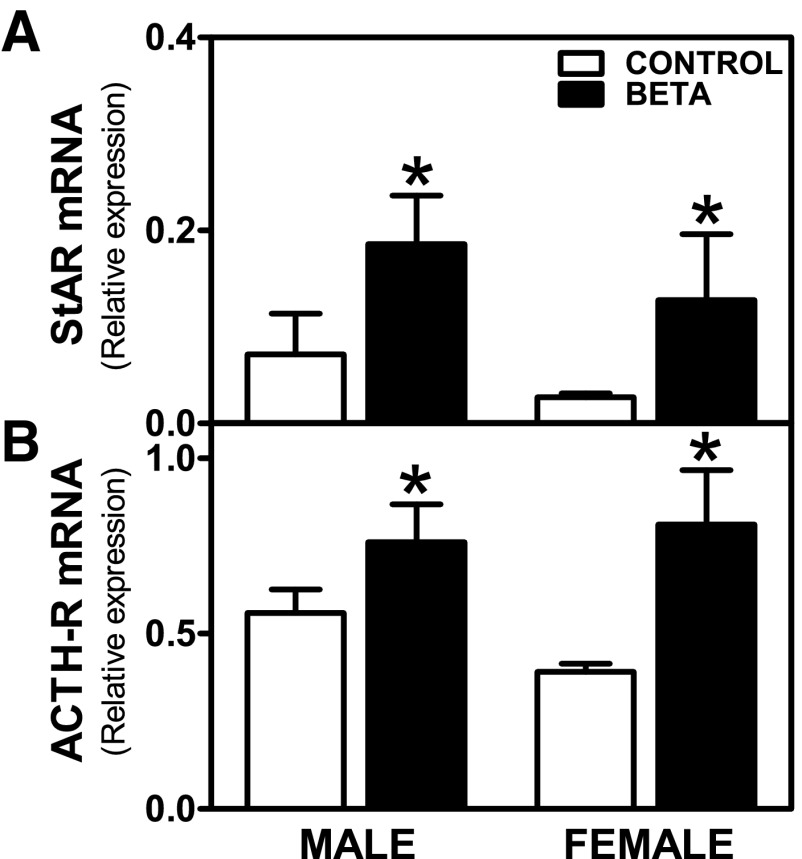
StAR (effects of treatment, F = 5.69, P < 0.05) and ACTH-R (effects of treatment, F = 10.02, P < 0.01) mRNA basal expression were higher in ADC from GC-treated animals, as shown in Fig. 7, A and B, respectively.
Fig. 7.
Expression of steroidogenic acute regulatory (StAR) and ACTH receptor (ACTH-R) mRNA in adrenocortical cells. Effects of antenatal Beta on basal mRNA expression of StAR [Beta, male (n = 5) and female (n = 5) (■); control, male (n = 5) and female (n = 6) (□)] (A) and ACTH-R [Beta, male (n = 4) and female (n = 4); control, male (n = 5) and female (n = 4)] (B) in adrenocortical cells. Each no. indicates number of animals analyzed. Data are expressed as means ± SE. *P < 0.05 vs. control.
Effects of leptin on StAR mRNA expression in ovine adrenocortical cells.
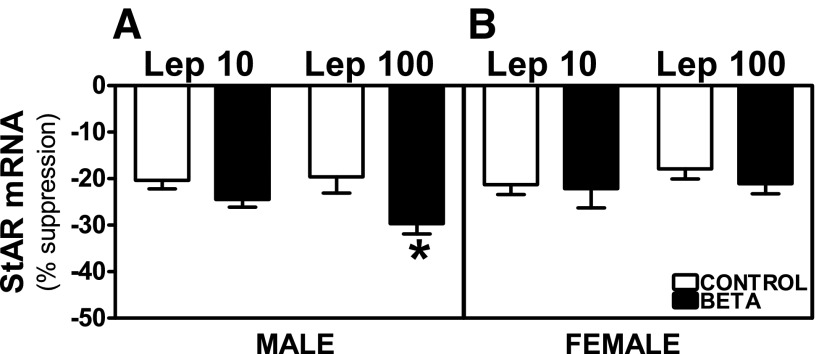
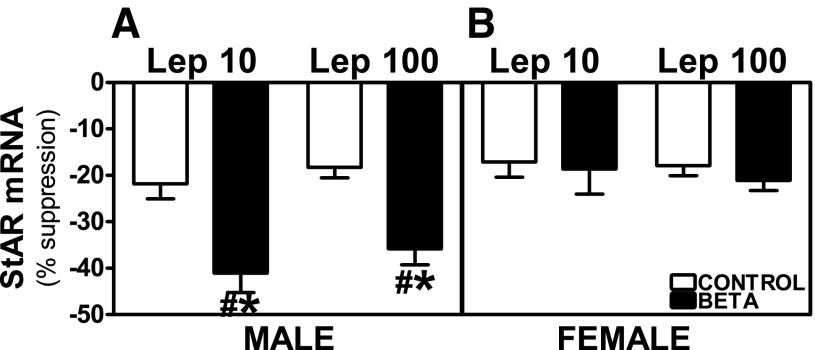
Preincubation with Lep 10 or Lep 100 reduced basal and ACTH-stimulated StAR mRNA expression in adrenocortical cells from control as well as GC-treated male and female animals. In males, the suppression of basal StAR mRNA expression was higher in cells from GC-treated animals incubated with Lep 100 (effects of treatment, F = 7.48, P < 0.01; Fig. 8A), whereas there was no effect of GC on basal expression in females (Fig. 8B). Both Lep 10 (F = 6.78, P < 0.05) and Lep 100 (F = 15.38, P < 0.001) incubations induced greater suppression of ACTH-stimulated StAR mRNA expression in GC-treated male cells (Fig. 9A). Following Lep 10 (F = 11.61, P < 0.004) or Lep 100 (F = 8.13, P < 0.01) incubation suppression of ACTH-stimulated StAR mRNA expression was greater in GC-treated males compared with females (Fig. 9, A and B). The inhibitory effects of leptin on ACTH-stimulated StAR mRNA expression in cells from female control and treated animals were similar (Fig. 9B).
Fig. 8.
Suppression of basal StAR mRNA expression by leptin. Effects of preincubation with Lep 10 or Lep 100 on basal StAR mRNA expression in adrenocortical cells from antenatal GC [Beta, male (n = 5) and female (n = 4); ■] or vehicle-treated [control, male and (n = 5) female (n = 5); □] male (A) and female (B) adult sheep. Each no. indicates no. of animals analyzed. Data are expressed as means ± SE. *P < 0.05 vs. control.
Fig. 9.
Suppression of ACTH-stimulated StAR mRNA expression by leptin. Effects of preincubation with Lep 10 or Lep 100 on ACTH-stimulated StAR mRNA expression in adrenocortical cells from antenatal GC [Beta, male (n = 5) and female (n = 4); ■] or vehicle-treated [control, male (n = 5) and female (n = 5); □] male (A) and female (B) adult sheep. Each no. indicates no. of animals analyzed. Data are expressed as means ± SE. *P < 0.05 vs. control; #P < 0.05 vs. female.
Effects of leptin on ACTH-R expression in ovine adrenocortical cells.
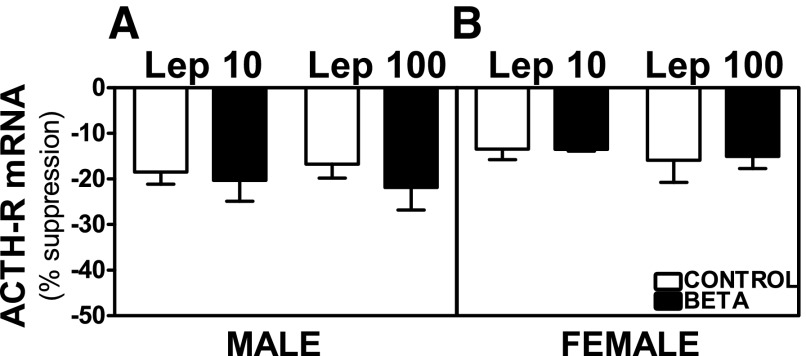
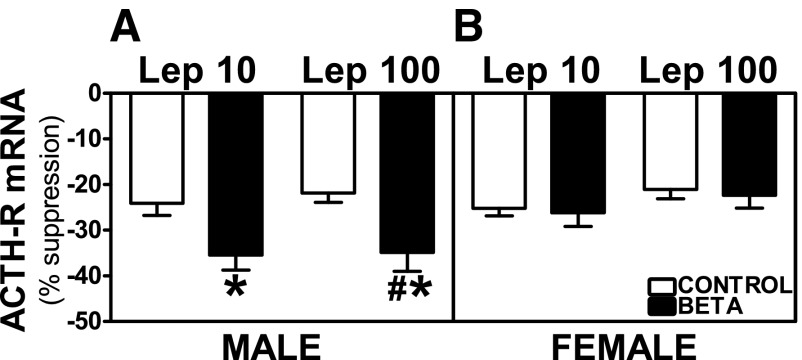
Preincubation with Lep 10 or Lep 100 reduced basal and ACTH-stimulated ACTH-R mRNA expression in adrenocortical cells from control as well as GC-treated male and female animals. No treatment-dependent differences were observed on basal ACTH-R mRNA expression in ADC from male or female animals (Fig. 10, A and B). Both Lep 10 (F = 5.04, P < 0.05) and Lep 100 incubations (F = 6.47, P < 0.05) induced greater suppression of ACTH-stimulated ACTH-R mRNA expression in GC-treated male cells compared with cells from vehicle-treated animals (Fig. 11A). Following Lep 100 (F = 5.58, P < 0.05) incubation, inhibition of ACTH-stimulated ACTH-R mRNA expression was greater in GC-treated males compared with females (Fig. 11, A and B). In females, the inhibitory effects of leptin remained unchanged between control and treated animals on ACTH-stimulated ACTH-R mRNA expression (Fig. 11B).
Fig. 10.
Suppression of basal ACTH-R mRNA expression by leptin. Effects of preincubation with Lep 10 or Lep 100 on basal ACTH-R mRNA expression in adrenocortical cells from antenatal GC [Beta, male (n = 5) and female (n = 5); ■] and vehicle-treated [control, male (n = 4) and female (n = 4); □] male (A) and female (B) adult sheep. Each no. indicates no. of animals analyzed. Data are expressed as means ± SE.
Fig. 11.
Suppression of ACTH-stimulated ACTH-R mRNA expression by leptin. Effects of preincubation with Lep 10 or Lep 100 on ACTH-stimulated ACTH-R mRNA expression in adrenocortical cells from antenatal GC [Beta, male (n = 5) and female (n = 4); ■] or vehicle-treated [control, male (n = 4) and female (n = 4); □] male (A) and female (B) adult sheep. Each no. indicates no. of animals analyzed. Data are expressed as means ± SE. *P < 0.05 vs. control; #P < 0.05 vs. female.
DISCUSSION
This study shows that prenatal exposure to GCs alters the impact of leptin on adult adrenal function in a sex-specific manner. This sex-related difference is evident in the suppression of cortisol production by leptin, which was greater in ADC from steroid-treated males and is associated with increased adrenal leptin mRNA levels in the betamethasone-exposed males. Our results also show that the greater inhibition of adrenal function by leptin may be at least partially explained by a greater suppression of StAR and ACTH-R expression in males.
In addition to the reported long-lasting effects of antenatal GCs on cardiovascular responses, our results provide evidence of a sex difference in the persistent effects of antenatal GCs on leptin modulation of adrenal function. Our findings of increased plasma levels of leptin in GC-treated animals are consistent with observations in humans, indicating that GCs stimulate leptin secretion by adipocytes and that dexamethasone increases leptin expression and secretion (43). Although differences in plasma leptin levels between sexes did not reach statistical significance, the trend we observed for greater plasma leptin in females is in agreement with several human studies that show greater plasma leptin in women, and given that plasma leptin is proportional to fat mass, this has been taken as evidence that women may be more resistant to the effects of leptin (35, 36, 46).
Considering that leptin signaling may be modulated by changes in the Ob-R leptin receptor, we sought to analyze leptin receptor levels in GC-treated male and female sheep. We demonstrated that leptin Ob-Ra receptor mRNA expression levels were lower in females, with no effects of sex difference or GC treatment, and no differences were observed in Ob-Ra protein expression. Significant treatment and sex difference effects were observed in Ob-Rb mRNA expression levels, and similarly to Ob-Ra, no differences were observed in Ob-Rb protein expression. Our results showed sex- and GC-specific effects on leptin receptor isoform mRNA, with no effects on protein expression. These results indicate that effects at the transcriptional level are not always transmitted directly to the protein translation levels.
Plasma cortisol was greater in GC-treated females, with no differences in males. We observed no differences in basal production of cortisol by ADC, whereas ACTH-stimulated production was again higher in GC-treated females. Leptin treatment of ADC in culture led to an inhibition of cortisol release (basal and ACTH stimulated) in all groups studied, confirming the predominantly inhibitory effects of leptin on steroidogenesis (2, 32, 52). In male ADC under basal conditions, Lep 100 induced a greater inhibition than Lep 10, whereas in ACTH-stimulated cells, both leptin doses are equally effective. Also, leptin inhibition was greater in cells from male GC-treated than control animals, with none of these effects observed in female ADC. The greater inhibition of ACTH-stimulated cortisol production by leptin observed in GC male cells suggests that GC-treated females (with less leptin-induced inhibition) are more responsive to ACTH in vivo, as reflected by increased plasma cortisol levels in this group.
The StAR protein regulates cholesterol delivery to the P450scc enzyme located in the inner mitochondrial membrane (7, 47), and its induction by ACTH is a key element in the rate-limiting step of steroid hormone biosynthesis (7, 47, 48). Since decreased expression of StAR has been associated with the inhibitory effect of leptin on adrenal responses (6), we intended to gain insight into whether in GC-treated animals the effects of leptin on cortisol production could be associated with an altered expression of StAR mRNA. We found that leptin attenuates basal and ACTH-stimulated steroidogenesis in ADC and prevents the hormone-induced increase in StAR mRNA steady-state levels. Preincubation with leptin also had a greater inhibitory effect on ACTH-stimulated ACTH-R mRNA expression. The greater effects on StAR and ACTH-R are limited exclusively to ADC from GC-treated males, with no effects of GC on the leptin-induced inhibition in cells from females. These data suggest that antenatal treatment with GCs programs the inhibition of steroidogenesis by leptin by affecting expression levels of adrenal steroidogenic enzymes in a sex-specific manner.
Steroidogenesis is highly dependent on intracellular cyclic AMP (cAMP) levels (49) that are controlled through synthesis by adenylyl cyclase and degradation by phosphodiesterase (PDE). Since we observed no differences in leptin protein receptor expression, the more pronounced leptin-dependent suppression of StAR and ACTH-R expression in cells from GC-treated males may result from GC-induced sex differences in the interaction between adrenal leptin and steroidogenic transduction pathways. In terms of the mechanisms involved in the actions of leptin, the modulation of cAMP levels by PDEs appears to be an important mediator of leptin intracellular signaling. Leptin-dependent activation of human platelets requires PDE3A (16), and leptin inhibits insulin secretion and gene expression in pancreatic β-cells in a process involving cAMP-dependent signaling (42) and phosphoinositide 3′-kinase (PI3K)-dependent activation of PDE3B (65). The role of the PI3K/PDE3B pathway in mediating leptin intracellular signaling in hypothalamic neurons has also been proposed (38, 39). Similarly, in human granulosa cells, leptin inhibits cAMP-stimulated StAR protein expression through a leptin-induced MAPK signal transduction pathway (23). All of these effects were demonstrated to be dependent on activation of leptin receptor transduction pathways. It has been shown that activation of PDEs also plays a role in the regulation of adrenal steroidogenesis (54, 56), and recent reports have suggested a relevant role of the cAMP-specific PDE8 in regulating adrenal steroidogenesis in mice (53). Moreover, knockout mice for PDE8B display adrenal ACTH hypersensitivity, which may be explained partially by an increase in mRNA levels of StAR and ACTH-R, with no variations in levels of other steroidogenic enzymes (55). In a human adrenocortical cell line, leptin activity has been shown to activate PDE3 and thus interfere with cAMP-mediated responses (21). Taken together, these data suggest that PDE activation by leptin is responsible for the inhibition on adrenal function that we observed in ADC. Moreover, the existence of PDEs with different affinities for cAMP (53, 56) makes it possible that different PDEs participate in leptin regulation of basal and ACTH-stimulated steroidogenesis, when different cAMP levels would be present.
In terms of the sex differences we observed in adrenal responses to leptin, a sex dependence on expression levels of PDE1A and PDE3B has been shown in endothelial cells derived from male and female rats in culture, with male cells having higher PDE1A and lower PDE3B compared with female cells (58). A greater expression of any of the PDE enzymes in the male adrenal and the consequent greater effect of their activation by leptin may explain the greater inhibitory effects of leptin in male ADC that we observed in this study.
PERSPECTIVES AND SIGNIFICANCE
The results of this study emphasize two general points related to the “fetal programming” or the “developmental origins of health and disease” hypothesis. The first is that administration of a single course of a clinically relevant dose of betamethasone at 60% of gestation in pregnant sheep produces sex-specific effects on pituitary adrenal function in the adult offspring. The treatment we used does not alter birth weight, suggesting that it does not change fetal growth rate (18, 51), nor does it impact weight in adulthood. However, multiple treatments with different glucocorticoids at a later gestational age have been shown to reduce birth weight in sheep (26). In humans the majority of studies have not found reduced birth weights with a single course of antenatal glucocorticoids (33, 59), but there are some conflicting data in the literature (3). The mechanisms by which GCs modulate fetal growth require further investigation. In the present work, administration of betamethasone to pregnant ewes at 80–81 days of gestation results some 400–450 days later in male/female differences in the ability of leptin to modulate adrenal function. That is, it produces what appears to be a relatively permanent endocrine change. The second finding is that this change retains its sex-specific nature in adult adrenal cells removed from the body and cultured in an artificial environment lacking the normal endocrine milieu found in vivo. For all practical purposes, this appears to be a phenotypic change that is different depending upon the sex of the animal exposed to antenatal glucocorticoids. These fundamental sex differences may contribute to the observed in vivo differences in normal and pathological endocrine responses between males and females and highlight the importance of considering the original sex of the cells used in in vitro cell culture research. How these changes are accomplished is fundamentally important, and much work needs to be done to understand the mechanisms involved.
Since preterm birth continues to be a major clinical obstetrical issue, the use of antenatal GCs as standard of care in cases of risk of preterm delivery is expected to continue. It is reported that antenatal GCs are given to more than 70% of women threatened by preterm delivery (29), and that number appears to be rising (31). The sex-specific changes induced by antenatal GCs on the adult endocrine responses we observed highlight the importance of considering sex difference in the study (1) and development of therapeutic strategies for antenatally programmed adult diseases.
GRANTS
This research was supported by National Institute of Child Health and Human Development Grants HD-11210 and HD-47584.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.S. and L.C.C. performed the experiments; Y.S., L.C.C., J.C.R., and V.M.P. analyzed the data; Y.S., L.C.C., J.C.R., and V.M.P. interpreted the results of the experiments; Y.S., J.C.R., and V.M.P. prepared the figures; Y.S., J.C.R., and V.M.P. drafted the manuscript; Y.S., J.C.R., and V.M.P. edited and revised the manuscript; Y.S., L.C.C., J.C.R., and V.M.P. approved the final version of the manuscript; J.C.R. and V.M.P. contributed to the conception and design of the research.
REFERENCES
- 1. Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction 145: R1–R13, 2013 [DOI] [PubMed] [Google Scholar]
- 2. Bornstein SR, Uhlmann K, Haidan A, Ehrhart-Bornstein M, Scherbaum WA. Evidence for a novel peripheral action of leptin as a metabolic signal to the adrenal gland: leptin inhibits cortisol release directly. Diabetes 46: 1235–1238, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Braun T, Husar A, Challis JR, Dudenhausen JW, Henrich W, Plagemann A, Sloboda DM. Growth restricting effects of a single course of antenatal betamethasone treatment and the role of human placental lactogen. Placenta 34: 407–415, 2013 [DOI] [PubMed] [Google Scholar]
- 4. Cao GY, Considine RV, Lynn RB. Leptin receptors in the adrenal medulla of the rat. Am J Physiol Endocrinol Metab 273: E448–E452, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Casanueva FF, Dieguez C. Neuroendocrine regulation and actions of leptin. Front Neuroendocrinol 20: 317–363, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Cherradi N, Capponi AM, Gaillard RC, Pralong FP. Decreased expression of steroidogenic acute regulatory protein: a novel mechanism participating in the leptin-induced inhibition of glucocorticoid biosynthesis. Endocrinology 142: 3302–3308, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Clark BJ, Stocco DM. StAR—A tissue specific acute mediator of steroidogenesis. Trends Endocrinol Metab 7: 227–233, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci 3: 19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Couillard C, Mauriège P, Prud'homme D, Nadeau A, Tremblay A, Bouchard C, Després JP. Plasma leptin concentrations: gender differences and associations with metabolic risk factors for cardiovascular disease. Diabetologia 40: 1178–1184, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Davis EP, Townsend EL, Gunnar MR, Guiang SF, Lussky RC, Cifuentes RF, Georgieff MK. Antenatal betamethasone treatment has a persisting influence on infant HPA axis regulation. J Perinatol 26: 147–153, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Davis EP, Waffarn F, Sandman CA. Prenatal treatment with glucocorticoids sensitizes the hpa axis response to stress among full-term infants. Dev Psychobiol 53: 175–183, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delavaud C, Bocquier F, Chilliard Y, Keisler DH, Gertler A, Kann G. Plasma leptin determination in ruminants: effect of nutritional status and body fatness on plasma leptin concentration assessed by a specific RIA in sheep. J Endocrinol 165: 519–526, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Ducsay CA, Hyatt K, Mlynarczyk M, Kaushal KM, Myers DA. Long-term hypoxia increases leptin receptors and plasma leptin concentrations in the late-gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol 291: R1406–R1413, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Dunn E, Kapoor A, Leen J, Matthews SG. Prenatal synthetic glucocorticoid exposure alters hypothalamic-pituitary-adrenal regulation and pregnancy outcomes in mature female guinea pigs. J Physiol 588: 887–899, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ehrhardt RA, Slepetis RM, Siegal-Willott J, Van Amburgh ME, Bell AW, Boisclair YR. Development of a specific radioimmunoassay to measure physiological changes of circulating leptin in cattle and sheep. J Endocrinol 166: 519–528, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Elbatarny HS, Maurice DH. Leptin-mediated activation of human platelets: involvement of a leptin receptor and phosphodiesterase 3A-containing cellular signaling complex. Am J Physiol Endocrinol Metab 289: E695–E702, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Emilsson V, Liu YL, Cawthorne MA, Morton NM, Davenport M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes 46: 313–316, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res 58: 510–515, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Guerra B, Fuentes T, Delgado-Guerra S, Guadalupe-Grau A, Olmedillas H, Santana A, Ponce-Gonzalez JG, Dorado C, Calbet JAL. Gender dimorphism in skeletal muscle leptin receptors, serum leptin and insulin sensitivity. PLoS One 3: e3466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology 138: 3859–3863, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Hsu HT, Chang YC, Chiu YN, Liu CL, Chang KJ, Guo IC. Leptin interferes with adrenocorticotropin/3′,5′-cyclic adenosine monophosphate (cAMP) signaling, possibly through a Janus kinase 2-phosphatidylinositol 3-kinase/Akt-phosphodiesterase 3-cAMP pathway, to down-regulate cholesterol side-chain cleavage cytochrome P450 enzyme in human adrenocortical NCI-H295 cell line. J Clin Endocrinol Metab 91: 2761–2769, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Kieffer TJ, Heller RS, Leech CA, Holz GG, Habener JF. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes 46: 1087–1093, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin Q, Poon S, Chen J, Cheng L, HoYuen B, Leung P. Leptin interferes with 3′,5′-cyclic adenosine monophosphate (cAMP) signaling to inhibit steroidogenesis in human granulosa cells. Reprod Biol Endocrinol 7: 115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu X, Dunn IC, Sharp PJ, Boswell T. Molecular cloning and tissue distribution of a short form chicken leptin receptor mRNA. Domest Anim Endocrinol 32: 155–166, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Long NM, Ford SP, Nathanielsz PW. Multigenerational effects of fetal dexamethasone exposure on the hypothalamic-pituitary-adrenal axis of first- and second-generation female offspring. Am J Obstet Gynecol 208: 217.e1–217.e8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long NM, Shasa DR, Ford SP, Nathanielsz PW. Growth and insulin dynamics in two generations of female offspring of mothers receiving a single course of synthetic glucocorticoids. Am J Obstet Gynecol 207: 203.e1–203.e8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lönnerdal B, Havel PJ. Serum leptin concentrations in infants: effects of diet, sex, and adiposity. Am J Clin Nutr 72: 484–489, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Malendowicz LK, Rucinski M, Belloni AS, Ziolkowska A, Nussdorfer GG. Leptin and the regulation of the hypothalamic-pituitary-adrenal axis. In: International Review of Cytology: A Survey of Cell Biology, edited by Kwang WJ. San Diego, CA: Academic, 2007, p. 63–102 [DOI] [PubMed] [Google Scholar]
- 29. McKinlay CJ, Crowther CA, Middleton P, Harding JE. Repeat antenatal glucocorticoids for women at risk of preterm birth: a Cochrane Systematic Review. Am J Obstet Gynecol 206: 187–194, 2012 [DOI] [PubMed] [Google Scholar]
- 30. National Institutes of Health Consensus Development Panel Antenatal corticosteroids revisited: repeat courses — National Institutes of Health Consensus Development Conference Statement, August 17–18, 2000. Obstet Gynecol 98: 144–150, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Polyakov A, Cohen S, Baum M, Trickey D, Jolley D, Wallace EM. Patterns of antenatal corticosteroid prescribing 1998–2004. Aust N Z J Obstet Gynaecol 47: 42–45, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Pralong FP, Roduit R, Waeber G, Castillo E, Mosimann F, Thorens B, Gaillard RC. Leptin inhibits directly glucocorticoid secretion by normal human and rat adrenal gland. Endocrinology 139: 4264–4268, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 19: CD004454, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Roduit R, Thorens B. Inhibition of glucose-induced insulin secretion by long-term preexposure of pancreatic islets to leptin. FEBS Lett 415: 179–182, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Rosenbaum M, Pietrobelli A, Vasselli JR, Heymsfield SB, Leibel RL. Sexual dimorphism in circulating leptin concentrations is not accounted for by differences in adipose tissue distribution. Int J Obes Relat Metab Disord 25: 1365–1371, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, El Tawil K, Rude RK, Kamdar V. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab 82: 579–584, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Sader S, Nian M, Liu P. Leptin: a novel link between obesity, diabetes, cardiovascular risk, and ventricular hypertrophy. Circulation 108: 644–646, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Sahu A. A role of phosphodiesterase-3B pathway in mediating leptin action on proopiomelanocortin and neurotensin neurons in the hypothalamus. Neurosci Lett 479: 18–21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sahu A. Intracellular leptin-signaling pathways in hypothalamic neurons: the emerging role of phosphatidylinositol-3 kinase-phosphodiesterase-3B-cAMP pathway. Neuroendocrinology 93: 201–210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schäffer L, Luzi F, Burkhardt T, Rauh M, Beinder E. Antenatal betamethasone administration alters stress physiology in healthy neonates. Obstet Gynecol 113: 1082–1088, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Schwartz J, Kleftogiannis F, Jacobs R, Thorburn GD, Crosby SR, White A. Biological activity of adrenocorticotropic hormone precursors on ovine adrenal cells. Am J Physiol Endocrinol Metab 268: E623–E629, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Seufert J, Kieffer TJ, Habener JF. Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proc Natl Acad Sci USA 96: 674–679, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Slieker LJ, Sloop KW, Surface PL, Kriauciunas A, LaQuier F, Manetta J, Bue-Valleskey J, Stephens TW. Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP. J Biol Chem 271: 5301–5304, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Sloboda DM, Moss TJ, Li S, Doherty D, Nitsos I, Challis JR, Newnham JP. Prenatal betamethasone exposure results in pituitary-adrenal hyporesponsiveness in adult sheep. Am J Physiol Endocrinol Metab 292: E61–E70, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Spicer LJ, Francisco CC. The adipose obese gene product, leptin: evidence of a direct inhibitory role in ovarian function. Endocrinology 138: 3374–3379, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Steinberg GR, McAinch AJ, Chen MB, O'Brien PE, Dixon JB, Cameron-Smith D, Kemp BE. The suppressor of cytokine signaling 3 inhibits leptin activation of AMP-kinase in cultured skeletal muscle of obese humans. J Clin Endocrinol Metab 91: 3592–3597, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 17: 221–244, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Stocco DM. Intramitochondrial cholesterol transfer. Biochim Biophys Acta 1486: 184–197, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol 19: 2647–2659, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Su Y, Carey LC, Rose JC, Pulgar VM. Leptin alters adrenal responsiveness by decreasing expression of ACTH-R, StAR, and P450c21 in hypoxemic fetal sheep. Reprod Sci 19: 1075–1084, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tang L, Carey LC, Bi J, Valego N, Sun X, Deibel P, Perrott J, Figueroa JP, Chappell MC, Rose JC. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am J Physiol Regul Integr Comp Physiol 296: R309–R317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tena-Sempere M, Pinilla L, Gonzalez LC, Casanueva FF, Dieguez C, Aguilar E. Homologous and heterologous down-regulation of leptin receptor messenger ribonucleic acid in rat adrenal gland. J Endocrinol 167: 479–486, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Tsai LC, Beavo JA. Regulation of adrenal steroidogenesis by the high-affinity phosphodiesterase 8 family. Horm Metab Res 44: 790–794, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tsai LC, Beavo JA. The roles of cyclic nucleotide phosphodiesterases (PDEs) in steroidogenesis. Curr Opin Pharmacol 11: 670–675, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsai LC, Shimizu-Albergine M, Beavo JA. The high-affinity cAMP-specific phosphodiesterase 8B controls steroidogenesis in the mouse adrenal gland. Mol Pharmacol 79: 639–648, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vezzosi D, Bertherat J. Phosphodiesterases in endocrine physiology and disease. Eur J Endocrinol 165: 177–188, 2011 [DOI] [PubMed] [Google Scholar]
- 57. Waffarn F, Davis EP. Effects of antenatal corticosteroids on the hypothalamic-pituitary-adrenocortical axis of the fetus and newborn: experimental findings and clinical considerations. Am J Obstet Gynecol 207: 446–454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang J, Bingaman S, Huxley VH. Intrinsic sex-specific differences in microvascular endothelial cell phosphodiesterases. Am J Physiol Heart Circ Physiol 298: H1146–H1154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wapner RJ, Sorokin Y, Thom EA, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Harper M, Caritis SN, Miodovnik M, Mercer B, Thorp JM, Moawad A, O'Sullivan MJ, Ramin S, Carpenter MW, Rouse DJ, Sibai B, Gabbe SG. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol 195: 633–642, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Wapner RJ, Sorokin Y, Mele L, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Malone F, Caritis SN, Mercer B, Harper M, Rouse DJ, Thorp JM, Ramin S, Carpenter MW, Gabbe SG. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med 357: 1190–1198, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Wirtschafter DD, Danielsen BH, Main EK, Korst LM, Gregory KD, Wertz A, Stevenson DK, Gould JB. Promoting antenatal steroid use for fetal maturation: results from the California Perinatal Quality Care Collaborative. J Pediatr 148: 606–612, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Wong SL, DePaoli AM, Lee JH, Mantzoros CS. Leptin hormonal kinetics in the fed state: effects of adiposity, age, and gender on endogenous leptin production and clearance rates. J Clin Endocrinol Metab 89: 2672–2677, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Yang R, Barouch LA. Leptin signaling and obesity. Circ Res 101: 545–559, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Zachow RJ, Magoffin DA. Direct intraovarian effects of leptin: impairment of the synergistic action of insulin-like growth factor-I on follicle-stimulating hormone-dependent estradiol-17 beta production by rat ovarian granulosa cells. Endocrinology 138: 847–850, 1997 [DOI] [PubMed] [Google Scholar]
- 65. Zhao AZ, Bornfeldt KE, Beavo JA. Leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J Clin Invest 102: 869–873, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]