Abstract
Recent evidence suggests that the glucagon-like peptide-1 (GLP-1) neuronal projection to the nucleus accumbens core (NAcC) contributes to food intake control. To investigate the role of endogenous stimulation of GLP-1 receptors (GLP-1R) in NAcC, we examined the effects of the GLP-1R antagonist exendin-(9–39) (Ex9) on meal pattern and microstructure of ingestive behavior in rats. Intra-NAcC Ex9 treatment selectively increased meal size relative to vehicle in rats consuming 0.25 M sucrose solution or sweetened condensed milk. Microstructural analysis revealed effects of NAcC Ex9 on initial lick rate and the size and duration of licking bursts in rats consuming 0.1 or 0.25 M sucrose, suggesting that blockade of NAcC GLP-1R increases palatability. Because NAcC Ex9 did not affect licking for nonnutritive saccharin (0.1%), we suggest that the presence of nutrients in the gut may be required for endogenous stimulation of NAcC GLP-1R. Consistent with this, we also found that the meal size-suppressive effects of intragastric nutrient infusion were attenuated by NAcC delivery of Ex9 at a dose that had no effect when delivered alone. Analysis of licking patterns revealed that NAcC Ex9 did not reverse intragastric nutrient-induced suppression of burst number but rather blunted the effect of nutrient infusion on meal size primarily by increasing the size and duration of licking bursts. Together, our results suggest that NAcC Ex9 influences taste evaluation. We conclude that GLP-1 released in NAcC in response to gastrointestinal nutrients reduces the hedonic value of food.
Keywords: licking microstructure, palatability, satiation, glucagon-like peptide-1, nucleus accumbens
glucagon-like peptide-1 (glp-1) neurons in the hindbrain nucleus of the solitary tract (NTS) have been hypothesized to play a role in the control of food intake since it was first shown that intracerebroventricular injection of GLP-1 potently suppresses feeding (22). These neurons are activated by meal-related signals such as gastric distention (23) and receive direct vagal afferent input (13). A physiological role for brain GLP-1 is suggested by studies showing that either pharmacological blockade of GLP-1 receptors (GLP-1R) or siRNA-mediated knockdown of NTS GLP-1 expression increases food intake and body weight (16, 3). NTS GLP-1 cells project widely throughout the brain to nuclei traditionally considered important for energy homeostasis (e.g., the paraventricular nucleus of the hypothalamus) but also to nuclei associated with reward and motivation such as the ventral tegmental area and the nucleus accumbens (NAc) (1, 10, 18). Here, we focus on the role of GLP-1R in the NAc core (NAcC) subregion. We targeted the NAcC because our previous research suggests that GLP-1R in core, but not shell, affect feeding (10). Recent studies have shown that stimulation of GLP-1R in this nucleus suppresses food intake, whereas site-specific blockade of GLP-1R increases intake (1, 10). Importantly, NAcC GLP-1R stimulation does not condition a taste aversion (10), nor does it induce kaolin intake (1, 9), suggesting that its ability to suppress food intake is not due to gastrointestinal malaise. These data support the suggestion that GLP-1R in the NAcC play a physiological role in the control of feeding but do not elucidate the behavioral mechanisms through which these receptors affect total food intake.
The NAc is known to affect reward-motivated behavior, and Dickson et al. (9) recently reported that intra-NAc injection of the GLP-1R agonist exendin-4 reduces operant responding for sucrose on a progressive ratio schedule. The NAc can also affect food palatability. This has been evaluated using the taste reactivity method, based on unconditioned orofacial responses to tastants (4). For example, intra-NAc injection of the μ-opioid receptor agonist [d-Ala2,N-MePhe4,Gly-ol]-enkephalin (DAMGO) increases rats' ingestive responses to sucrose and reduces aversive responses to quinine, suggesting that DAMGO increases palatability (18). It is possible that stimulation of GLP-1R in NAcC has the opposite effect, suppressing intake by reducing palatability. The NAc has not been considered a mediator of postingestive negative feedback signals arising from the gastrointestinal tract, but the fact that GLP-1 neurons can be activated by such signals raises the possibility that NAcC GLP-1R contribute to satiation or satiety, as well. Simple measurement of food intake cannot distinguish among these possibilities, but we can obtain useful clues through detailed analysis of ingestive behavior (8).
In this series of experiments, we used the GLP-1R antagonist exendin-(9–39) (Ex9) to investigate the mechanisms through which endogenous activation of NAcC GLP-1R reduces food intake. First, we asked whether NAcC GLP-1R blockade increases intake of sweetened condensed milk or sucrose solution by increasing meal size, meal number, or both. We chose to examine sweetened condensed milk intake because this has been shown to induce c-Fos in GLP-1 neurons (11). However, the large body of literature on the microstructure of licking for sugar solutions (e.g., Refs. 7, 5, 20) makes sucrose a better choice for detailed analysis. Therefore, we asked whether NAcC Ex9 treatment affects the pattern of licking for sucrose solutions in a manner consistent with an effect on palatability or satiation. To determine whether NAcC Ex9 can affect nonnutritive palatable food intake, we examined its effect on licking for saccharin. Finally, because GLP-1 neurons are activated by nutrient-related gastrointestinal signals, we asked whether NAcC Ex9 injection could attenuate the intake-inhibitory effects of intragastric nutrient infusion.
MATERIALS AND METHODS
Subjects.
Naïve male Wistar rats (Harlan, Indianapolis, IN) were maintained individually in a temperature-controlled vivarium on a 12:12-h light-dark cycle in plastic cages. Rats had ad libitum access to distilled water and Purina 5001 pellet rat chow (St. Louis, MO) except where otherwise noted. They were handled daily and habituated to experimental procedures before start of experiments. Rats were each used in only one experiment except where otherwise noted. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee and conformed to the standard of the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Surgery.
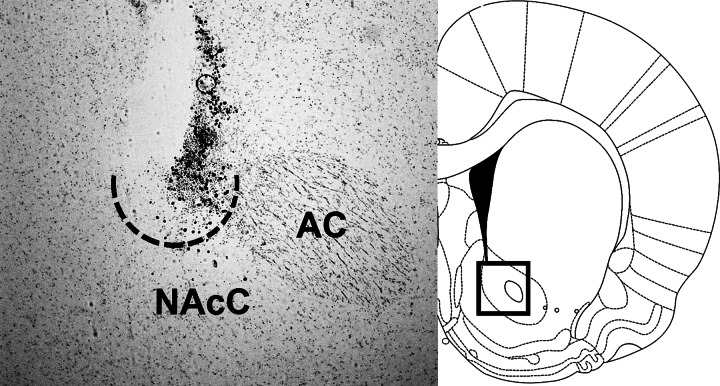
Under 2–4% isoflurane delivered at a rate of 1 l/min, rats were implanted with a unilateral 26-gauge guide cannula (Plastics One, Roanoke, VA) targeting the NAcC. The cannula was cemented to three jeweler's screws fastened to the skull surface and closed with a removable obturator. Coordinates for the guide cannula were 1.2 mm anterior to bregma, 1.5 mm lateral, and 4.9 mm ventral to skull surface (2.5 mm above the NAcC target). Rats in experiment 5 were implanted with a Silastic (DowCorning, Midland, MI) intragastric (IG) catheter according to a technique adapted from Davis and Campbell (6). IG catheters were flushed daily with 1 ml of saline to maintain patency. Rats were given 1 wk to recover from surgery prior to the start of experimentation. NAcC cannula placements were verified histologically after the end of behavioral experiments (Fig. 1). Rats were deeply anesthetized (180 mg/kg ketamine and 30 mg/kg xylazine ip) and transcardially perfused with 10 mM PBS followed by 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Brains were removed and sunk in 30% sucrose in PBS and then frozen in isopentane on dry ice. Coronal cryostat sections (20 μm) through the NAc were slide-mounted and stained with cresyl violet (Sigma-Aldrich, St. Louis, MO) for examination with an Olympus BX41 microscope. Monochromatic digital images were acquired with a Retiga EXI Aqua camera and Q-Capture software (Hunt Optics, Pittsburgh, PA). We identified injection sites by cannula track, injector-induced damage, and appearance of gliosis. Injection sites within the boundaries of the NAcC as drawn in the atlas of Paxinos and Watson (17) were considered correct.
Fig. 1.
Representative image of a nucleus accumbens core (NAcC) injection site (ventral aspect outlined with dotted line) in a cresyl violet-stained coronal section, and diagram based on Paxinos and Watson (17) with a box identifying the location at which the photograph was taken. AC, anterior commissure. This section is ∼1.4 mm anterior to bregma. All rats included in these studies had injection sites similar to this, in close proximity to the AC, at rostrocaudal levels ranging from 1 to 2 mm anterior to bregma.
Apparatus.
All behavioral testing took place in Habitest operant chambers enclosed in sound-attenuating cabinets (Coulbourn Instruments, Whitehall, PA). Each chamber was equipped with a recessed drinking spout located 2 cm above the grid floor. Licks were detected via photobeam positioned at the base of the spout. Licks were recorded by Graphic State 3.03 software (Coulbourn Instruments, Whitehall, PA) and stored on the computer for later analysis.
Experiment 1: effect of NAcC Ex9 on licking for sweetened condensed milk.
All testing took place during the mid-light phase. Rats (n = 6, mean body weight 548 g at the start of the experiment) were placed in the test chambers for daily training sessions, during which they had ad libitum access to sweetened condensed milk (Eagle Brand, Orrville, OH) diluted 50:50 with water (SCM, 1.57 kcal/ml) for 120 min. No other food or water was present in the test chamber. Daily training continued for 7 days, at which point SCM intake did not vary by more than 10% across consecutive days. At that point, rats were habituated to intra-NAcC injection procedures. Brain injections were made using a 10-μl Hamilton microsyringe (Reno, NV) connected to a 33-G injector (Plastics One, Roanoke, VA) via Tygon tubing (VWR, Radnor, PA). The injector extended 2.5 mm beyond the end of the guide cannula in order to reach the NAcC. Injections were delivered at a rate of 0.5 μl/min, and the injectors were then left in place for an additional minute before removal. For habituation to this procedure, rats received a 0.5 μl of sterile 0.9% saline injection 15 min before the start of the test session.
On experiment days, rats received an injection of either 0.5 μl of saline vehicle or 3 μg of Ex9 (California Peptide Research, Napa, CA) 15 min prior to the test session. This dose was chosen because we had previously observed that it effectively increases 2-h chow intake (10). All rats received both conditions in counterbalanced order, with treatments separated by at least 48 h. On noninjection days, rats still had daily 120-min SCM intake sessions. After the test session, rats were returned to their home cages. Body weight and overnight home cage chow intake were measured daily.
Experiment 2: effect of NAcC Ex9 on licking for 0.25M sucrose.
The procedures were identical to those described for experiment 1, except in this study, rats (n = 9, mean body weight 462 g) consumed 0.25 M sucrose (Sigma-Aldrich) during their daily test sessions.
Experiment 3: effect of NAcC Ex9 on licking for 0.1M sucrose.
We hypothesized that Ex9 might increase taste palatability and that this might be more clearly observed with lower concentrations of sucrose. Therefore, we performed this experiment with 0.1 M sucrose. The procedures were identical to those described above, except that in this study rats (n = 8, mean body weight 474 g) consumed 0.1 M sucrose during their daily test sessions.
Experiment 4: effect of NAcC Ex9 on licking for 0.1% saccharin.
The same rats used in experiment 3 were used in this study (n = 8). After the end of experiment 3, they received 2 wk of no test sessions or injections and then began training with 0.1% saccharin (Sigma-Aldrich) available in the test chambers. Intakes stabilized within 7 days and were similar to those seen previously in naïve rats (unpublished observations from our laboratory). At that point, the experiment proceeded as described above. We chose the 0.1% concentration of saccharin to approximately match microstructural variables, including initial lick rate, burst size, and burst duration to values observed in experiment 3. Although higher concentrations of saccharin are preferred by rats in two-bottle preference tests (21), we hypothesized that NAcC Ex9 would increase palatability. Higher, more palatable concentrations of saccharin could result in a ceiling effect that would reduce our ability to discern an effect of Ex9.
Experiment 5: effect of NAcC Ex9 on the response to intragastric nutrient infusion.
Rats (n = 8, mean body weight 426 g) in this experiment were trained to consume 0.25 M sucrose in daily test sessions as described above, but these rats were also connected to infusion lines in the test chambers. The infusion lines consisted of polyethylene tubing (PE; VWR, Radnor, PA) protected by a stainless steel spring. The PE tubing was connected to an infusion swivel (Instech Solomon, Plymouth Meeting, PA) mounted on a counterbalanced lever at the top of the chamber. This preserved the rats' ability to move freely around the chamber when the infusion line was connected to the back-mounted IG catheter port. When daily 0.25 M sucrose intakes were stable, rats received an additional habituation test session in which they received an IG infusion of 0.9% saline at a rate of 1 ml/min for the first 3 min of the test session.
This experiment had 4 conditions, which were administered to all rats in counterbalanced order and separated by at least 48 h. Rats received an intra-NAcC injection of either sterile 0.9% saline or 2 μg of Ex9 15 min before the start of the test intake session. This dose was chosen based on a pilot study in which we observed no effect of 2 μg of Ex9 delivered to the NAcC on 0.25 M sucrose intake or licking microstructure. Immediately upon the start of the test session, IG infusions began. Rats received 3 ml of either 0.9% saline or 40% sucrose (4.8 kcal) delivered at a rate of 1 ml/min (for total duration of 3 min). We chose this sucrose concentration and timing in order to strongly stimulate endogenous within-meal gastrointestinal satiation signaling. Thus, the conditions were: NAcC vehicle/IG saline; NAcC vehicle/IG sucrose; NAcC Ex9/IG saline; and NAcC Ex9/IG sucrose. After the 120-min test session had concluded, the IG catheters of rats that had received IG sucrose earlier were flushed with 1 ml of saline before the port was capped. Rats that received saline IG were simply disconnected and capped. All rats were then returned to their home cages, and body weight and overnight chow intake were monitored.
Data analysis.
Each lick emitted during test sessions was saved and analyzed with a customized program. A meal was defined as at least three licks, and the criterion for the end of a meal was a pause of 600 or more seconds (2, 20). Intermeal interval is defined as the time between the last lick of one meal and the first lick of the next. Satiety ratio is calculated as intermeal interval divided by preceding meal size multiplied by 100. Within each meal, a licking burst was defined as a series of at least two licks separated by an interlick interval of less than 1 s (20). Variables obtained from the program include within-meal burst number, mean number of licks per burst, mean licks per minute over the course of the meal, number of licks in the first minute of the meal, size and duration of the first three bursts of the meal, and average interburst interval. Within-burst interlick interval was calculated as an average of interlick intervals below 250 ms, because this captures more than 95% of interlick intervals (8). In addition, the first meals of the sessions were split into thirds based on burst number. This allowed assessment of drug effects on many of the above-mentioned variables early in the meal, when postingestive negative feedback was less prominent and licking was largely driven by taste vs. late in the meal when licking behavior was strongly influenced by postingestive satiation signals (20).
For all variables, data are reported as means ± SE. For experiments 1–4, most data were analyzed via paired-samples Student's t-test. Changes in licking variables across meals segmented into thirds were assessed via repeated-measures two-way ANOVA with meal segment and drug as factors. For experiment 5, data were analyzed via repeated-measures two-way ANOVA, with drug and IG infusion as factors, or 3-way ANOVA with meal segment, drug, and IG infusion as factors. Tukey's HSD was used to evaluate significant main effects or interactions obtained in two-way ANOVAs, and planned comparisons of least square means were performed after three-way ANOVAs for data from experiment 5. In all cases, P < 0.05 was taken to be significant.
RESULTS
Experiment 1: effect of NAcC Ex9 on licking for sweetened condensed milk.
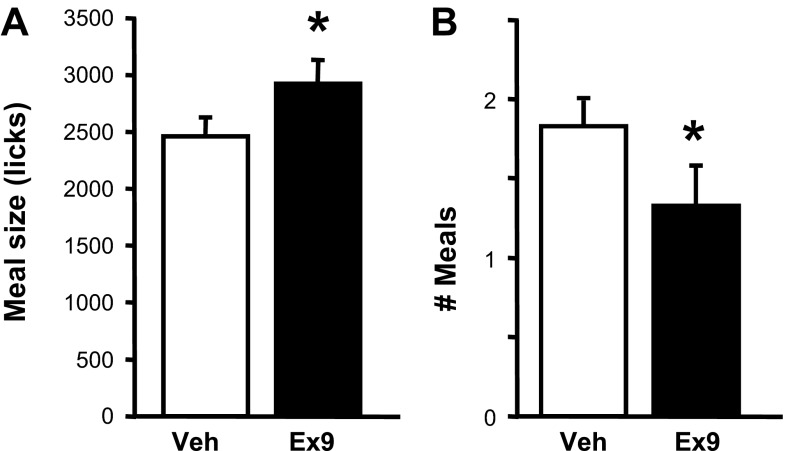
When rats were consuming SCM, intra-NAcC injection of 3 μg of Ex9 significantly increased the size of the first meal of the session relative to vehicle [t(5) = 4.31, P < 0.01; Fig. 2A]. Total number of licks during the 120-min session was unaffected by drug (2,910.8 ± 263.6 after vehicle vs. 3,151.0 ± 320.9 after Ex9). After intra-NAcC Ex9, rats took significantly fewer meals during the session [t(5) = 2.71, P < 0.05; Fig. 2B], with 5 of 6 of rats taking a second meal after vehicle but only one doing so after Ex9.
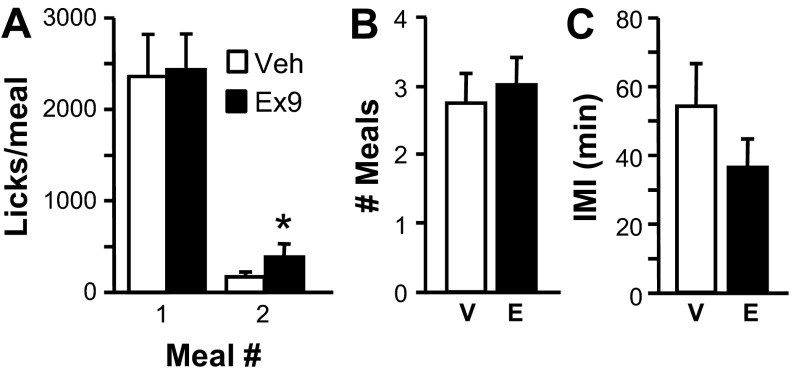
Fig. 2.
Effects of intra-NAcC injection of vehicle or 3 μg of exendin-(9–39) (Ex9) on licking for SCM (sweetened condensed milk diluted 50:50 with water). A: size (no. of licks) of the 1st meal of the test session. B: no. of meals taken during the 120-min session. Data are means ± SE. *P < 0.05, vehicle vs. Ex9.
Experiment 2: effect of NAcC Ex9 on licking for 0.25 M sucrose.
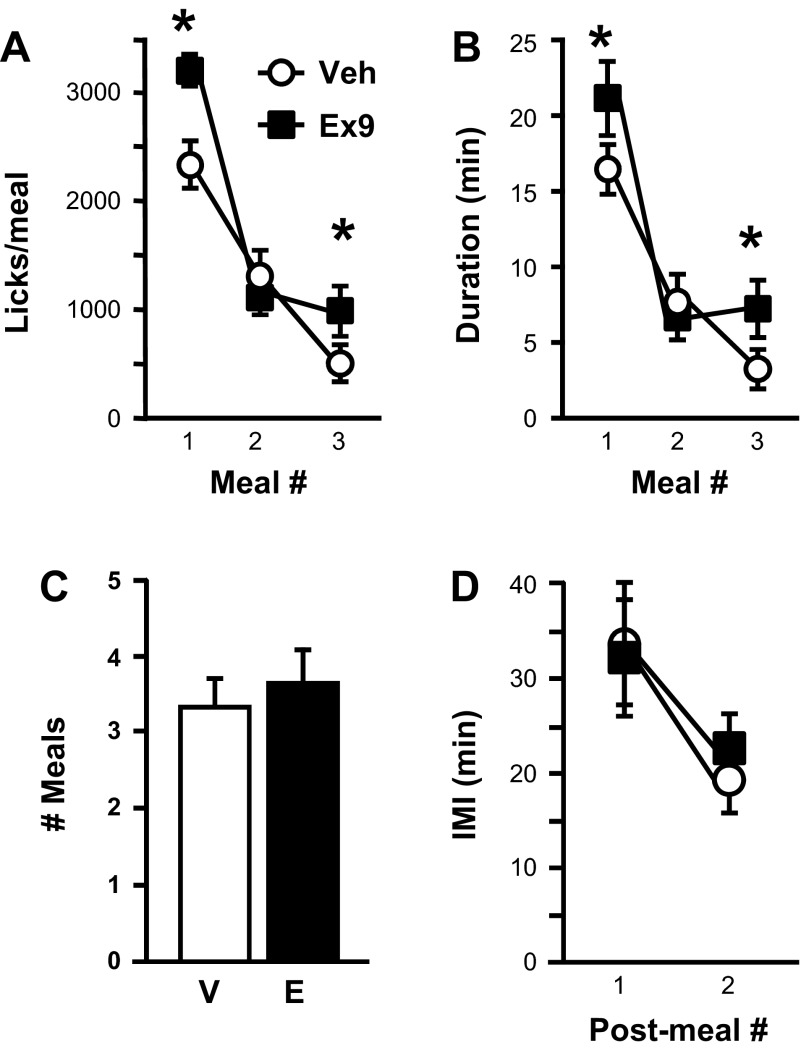
Rats licking for 0.25 M sucrose showed significant increases in meal size after intra-NAcC Ex9 compared with vehicle for the first [t(8) = 3.49, P < 0.01] and third [t(6) = 3.63, P < 0.05] meals of the test session (Fig. 3A). Total licks during the session were significantly increased by Ex9 as well [4,747.44 ± 384.89 after vehicle vs. 6,274.33 ± 378.50 after Ex9; t(8) = 3.41, P < 0.01]. All rats took at least two meals during the session under both drug treatment conditions, but only 7 of 9 took a third meal under both conditions. Additional meals were not taken by enough rats to allow statistical analysis. Average number of meals taken during the session was not affected by Ex9 (Fig. 3C), nor were the intervals between the first, second, and third meals (Fig. 3D). Satiety ratios were not affected by Ex9 (1st meal: vehicle 1.56 ± 0.36, Ex9 1.07 ± 0.23; 2nd meal vehicle 2.38 ± 0.93, Ex9 2.53 ± 0.42).
Fig. 3.
Effects of intra-NAcC injection of vehicle (V) or 3 μg Ex9 (E) on licking for 0.25 M sucrose. A: size (no. of licks) of the 1st 3 meals of the test session. B: duration (min) of each of the 1st 3 meals of the session. C: no. of meals taken during the session. D: Intermeal intervals (IMI) between 1st, 2nd, and 3rd meals of the session. Data are means ± SE. *P < 0.05, vehicle vs. Ex9.
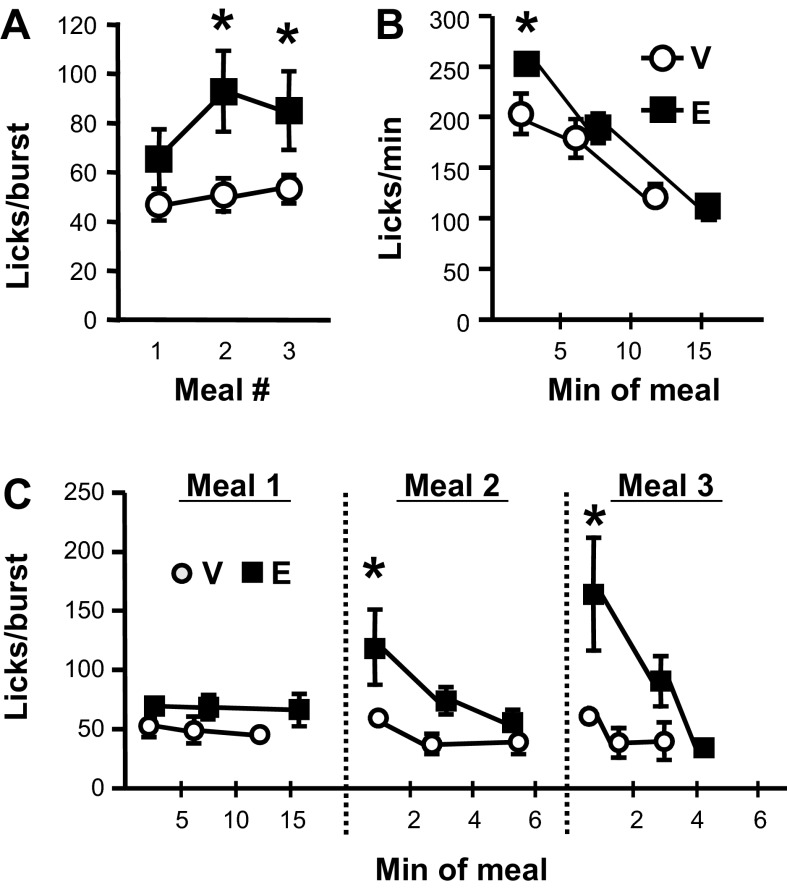
Intra-NAcC Ex9 significantly increased first meal duration [t(8) = 2.52, P < 0.05; Fig. 3B] but had no effect on number of bursts taken within that meal. There was also no effect on number of licks emitted during the first minute of the first meal (Table 1). Average burst size (Fig. 4A) and duration (Table 1) tended to be increased by Ex9, but this did not reach significance for the first meal. Effects on lick rate emerged when this meal was split into thirds. Intra-NAcC Ex9 significantly increased average licks per minute during the first segment of the meal, whereas there was no drug effect on lick rate during the second or third segments (Fig. 4B).
Table 1.
Licking microstructural variables measured in experiment 2
| 1st Meal |
2nd Meal |
3rd Meal |
||||
|---|---|---|---|---|---|---|
| Variable | Veh | Ex9 | Veh | Ex9 | Veh | Ex9 |
| Licks in the 1st min | 205.22 (20.80) | 215.00 (21.75) | 311.00 (32.42) | 289.11 (21.97) | 257.63 (43.16) | 279.50 (34.34) |
| Ingestion rate, licks/min | 147.11 (11.10) | 165.26 (16.03) | 213.13 (26.84) | 229.77 (30.16) | 190.97 (38.41) | 185.86 (39.96) |
| Burst duration, s | 6.72 (0.85) | 9.25 (1.63) | 8.08 (1.29) | 13.86* (2.85) | 7.70 (0.67) | 12.46* (2.49) |
| Burst number | 57.9 (8.74) | 56.8 (6.95) | 29.44 (6.21) | 15.4* (3.36) | 9.86 (3.39) | 13.43 (3.49) |
| Within-burst ILI, ms | 139.95 (4.06) | 140.16 (4.22) | 148.54 (4.95) | 143.39 (3.68) | 140.20 (8.33) | 142.20 (5.00) |
Data are means (±SE). In experiment 2, rats licking for 0.25 M sucrose were treated with vehicle (Veh) or 3 μg exendin 9-(9–39) (Ex9). ILI, interlick interval.
Significant difference between vehicle and Ex9 (P < 0.05).
Fig. 4.
Licking microstructure effects when rats were licking for 0.25 M sucrose after intra-NAcC injection of vehicle (V) or 3 μg Ex9 (E). A: meal average burst size (licks/burst) for the 1st 3 meals of the session. B: lick rate (licks/min) during the 1st meal segmented into 3rds, with data plotted at the mean temporal midpoint of each 3rd. C: burst size across 3rds of each of the 1st 3 meals of the session. Data are means ± SE. *P < 0.05, vehicle vs. Ex9.
During both the second and third meals of the test session, Ex9 significantly increased meal average burst size [Fig. 4A: 2nd meal t(8) = 3.28, P < 0.05; 3rd meal t(6) = 2.49, P < 0.05] and burst duration [Table 1: 2nd meal t(8) = 2.83, P < 0.05; 3rd meal t(6) = 2.44, P < 0.05]. These effects were driven by Ex9-induced increases early in the meals, with no effects on licking behavior toward the end of these meals (Fig. 4C). A significant reduction in burst number in meal 2 [t(8) = 2.47, P < 0.05] explains the lack of Ex9 effect on second meal size, but there was no effect on burst number in meal 3 (Table 1). Ex9 had no effect on within-burst interlick interval during any of these meals (Table 1).
Experiment 3: effect of NAcC Ex9 on licking for 0.1 M sucrose.
When rats were licking for 0.1 M sucrose, 3-μg Ex9 injection had no effect on first meal size but significantly increased the size of the second meal of the session [t(7) = 2.59, P < 0.05; Fig. 5A] and total number of licks taken during the 120-min session [vehicle 2,599.0 ± 492.58, Ex9 3,069.88 ± 474.71; t(7) = 2.3, P < 0.05]. All subjects took at least TWO meals under vehicle and drug conditions, but few took additional meals. Ex9 had did not affect number of meals in the session (Fig. 5B), interval between the first and second meal (Fig. 5C), or satiety ratio in this experiment (vehicle 3.22 ± 1.04, Ex9 1.78 ± 0.55).
Fig. 5.
Effects of intra-NAcC injection of vehicle (V) or 3 μg Ex9 (E) on licking for 0.1 M sucrose. A: size (no. of licks) of the 1st 2 meals of the test session. B: no. of meals taken during the session. C: IMI between 1st and s2nd meals of the session. Data are means ± SE. *P < 0.05, vehicle vs. Ex9.
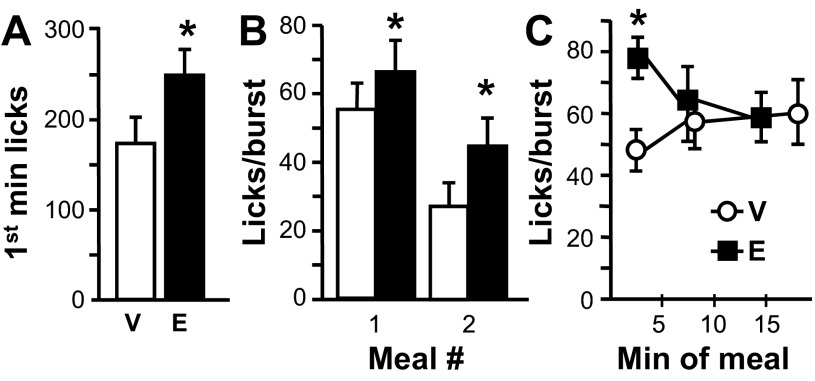
Although first meal size was not increased by Ex9, this treatment significantly increased the number of licks emitted during the first minute of the meal [t(7) = 2.27, P < 0.05; Fig. 6A]. First meal average burst size and burst duration were both significantly increased by intra-NAcC Ex9, as well [size t(7) = 3.3, P < 0.05; duration t(7) = 3.13, P < 0.05; Table 2 and Fig. 6B]. These effects were prominent during the first segment of the meal after Ex9 treatment, with no effect of Ex9 later in the meal [Fig. 6C: interaction between meal segment and Ex9, burst size, F(2,14) = 6.3, P < 0.05; burst duration, F(2,14) = 6.37, P < 0.05]. A similar pattern of Ex9 effects was observed for the second meal of the session. Ex9 increased burst size [Fig. 6B: t(7) = 3.85, P < 0.01] and burst duration [t(7) = 3.44, P < 0.05] with no effect on burst number (Table 2). Ex9 had no effects on meal duration, number of bursts, or average within-burst interlick interval in either meal of the session (Table 2).
Fig. 6.
Licking microstructure effects when rats were licking for 0.1 M sucrose after intra-NAcC injection of vehicle (V) or 3 μg Ex9 (E). A: no. of licks emitted during the 1st min of the 1st meal of the session. B: meal average licks/burst during the 1st and 2nd meals of the session. C: burst size across 3rds of 1st meal of the session, with data plotted at the mean temporal midpoint of each 3rd. Data are means ± SE. *P < 0.05, vehicle vs. Ex9.
Table 2.
Licking microstructural variables measured in experiment 3
| 1st Meal |
2nd Meal |
|||
|---|---|---|---|---|
| Variable | Veh | Ex9 | Veh | Ex9 |
| Meal duration, min | 25.21 (5.02) | 19.40 (2.38) | 2.53 (1.06) | 4.14 (1.43) |
| Ingestion rate, licks/min | 97.48 (9.56) | 123.73* (12.06) | 155.05 (57.11) | 126.92 (24.25) |
| Burst duration, s | 8.32 (1.06) | 10.20* (1.26) | 4.94 (1.47) | 8.11* (1.38) |
| Burst number | 45.38 (9.76) | 39.88 (7.95) | 5.88 (1.41) | 7.50 (1.28) |
| Within-burst ILI, ms | 146.00 (2.36) | 147.06 (2.65) | 147.31 (3.03) | 149.29 (2.64) |
Data are means (±SE). In experiment 3, rats licking for 0.1 M sucrose were treated with vehicle or 3 μg Ex9.
Significant difference between vehicle and Ex9 (P < 0.05).
Experiment 4: effect of NAcC Ex9 on licking for 0.1% saccharin.
Intra-NAcC Ex9 treatment had no significant effect on any variable examined when rats were licking for 0.1% saccharin (Table 3).
Table 3.
Variables measured in experiment 4
| Variable | Veh | Ex9 |
|---|---|---|
| First meal size, licks | 731.33 (153.71) | 767.00 (181.77) |
| Meal duration, min | 10.29 (2.41) | 9.88 (3.03) |
| Licks in 1st min | 156.33 (28.90) | 186.00 (19.42) |
| Ingestion rate, licks/min | 74.22 (8.26) | 105.72 (31.20) |
| Burst size, licks/burst | 54.40 (7.38) | 59.73 (11.14) |
| Burst duration, s | 8.13 (1.02) | 8.83 (1.57) |
| Burst number | 13.00 (2.07) | 14.00 (3.74) |
| Within-burst ILI, ms | 144.91 (3.76) | 145.51 (3.10) |
| Number of meals in session | 1.89 (0.28) | 2.33 (0.47) |
| Total licks/session | 934.56 (200.10) | 1,105.78 (200.25) |
Data are means (±SE). In experiment 4, rats licking for 0.1% saccharin were treated with vehicle or 3 μg Ex9.
Experiment 5: effect of NAcC Ex9 on the response to IG nutrient infusion.
Total number of licks during the session were reduced by IG sucrose infusion [main effect of infusion: F(1,7) = 15.93, P < 0.01; data not shown], but pairwise comparisons revealed no significant differences among conditions. Therefore, we focused further analysis on the first meal of the session.
IG sucrose infusion during the first 3 min of the first meal of the session significantly suppressed the size of that meal [main effect of sucrose infusion: F(1, 7) = 16.86, P < 0.01; Fig. 7A]. As expected, 2 μg of Ex9 injected into the NAcC had no effect when followed by a saline IG infusion. However, this dose of Ex9 significantly attenuated the suppressive effect of the sucrose load on the size of the first meal [interaction between infusion and Ex9: F(1,7) = 8.6, P < 0.05]. IG sucrose infusion significantly suppressed first meal size by 47% after intra-NAcC vehicle (P < 0.01), but this effect of sucrose infusion was eliminated when rats were treated with intra-NAcC Ex9 (Fig. 7A). The duration of the first meal was reduced by IG sucrose infusion [main effect: F(1, 7) = 15.31, P < 0.01], and Ex9 had no influence on this variable, but pairwise comparisons revealed no significant differences across conditions (Fig. 7B). Neither IG infusion nor Ex9 affected number of meals in the session, but among those rats that took at least two meals under all treatment conditions (n = 6), there was a significant main effect of IG sucrose infusion to prolong the interval between the first and second meals [F (1, 5) = 7.72, P < 0.05; Fig. 7C]. In those subjects, the size of the second meal was not affected by IG infusion or Ex9. Satiety ratio tended to be increased by IG sucrose infusion independent of Ex9 treatment, but this main effect of infusion did not reach significance (P = 0.06).
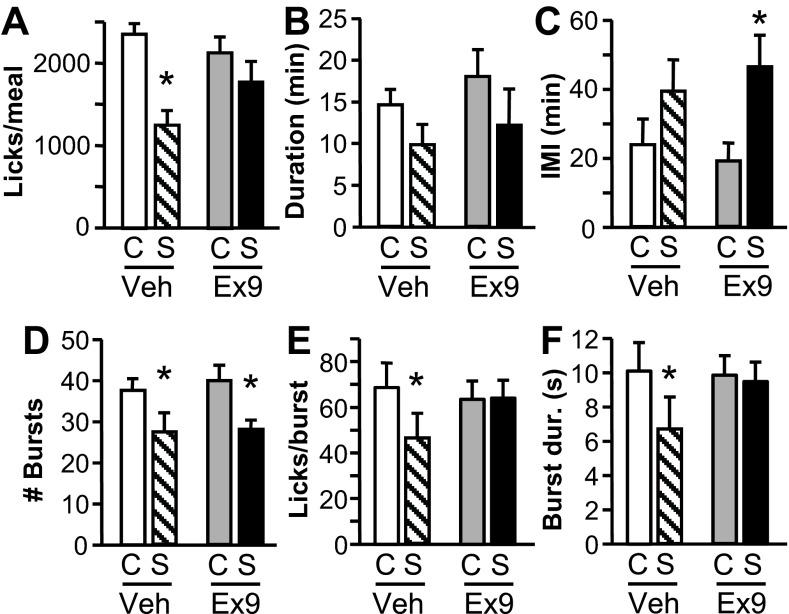
Fig. 7.
Effects of intra-NAcC injection of vehicle or 2 μg Ex9 on licking for 0.25 M sucrose when receiving intragastric (IG) control (C) or sucrose (S) infusions. A: size (no. of licks) of the 1st meal of the test session. B: duration (min) of that 1st meal. C: interval between the 1st and 2nd meals of the session. D: no. of licking bursts in the 1st meal. E: average burst size during the 1st 3rd of the 1st meal (approximately the first 3.3 min). F: average duration (s) of bursts during the 1st 3rd of the 1st meal. Data are means ± SE. *P < 0.05, control vs. sucrose infusion.
IG sucrose infusion reduced first meal size in large part by reducing the number of licking bursts in the meal [main effect of infusion: F(1, 7) = 20.79, P < 0.01], and this effect was similar whether rats were treated with vehicle or Ex9 (Fig. 7D). Ex9's ability to blunt the meal size effect of IG sucrose was instead related to an effect on the average burst size [interaction between infusion and Ex9: F(1,7) = 7.99, P < 0.05]. IG sucrose infusion significantly reduced burst size during the meal after rats were given intra-NAcC vehicle (P < 0.05), but there was a tendency for burst size to be increased by IG sucrose after intra-NAcC Ex9 (P = 0.09). A similar effect on burst duration was observed [interaction: F(1, 7) = 10.51, P < 0.05]. Examination of the meal divided into thirds revealed that effects on burst size and duration were seated in the first third of the meal, with significant interactions between meal segment and Ex9 [size, F(2,14) = 4.17, P < 0.05; duration, F(2,14) = 5.79, P < 0.05] and between Ex9 and IG infusion [size, F(1,7) = 7.91, P < 0.05; duration, F(1,7) = 9.6, P < 0.05]. The first third of the meal was an average of 3.3 ± 0.38 min in duration (no differences across conditions), which roughly corresponds to the period of the IG sucrose infusion (3 min). During this portion of the meal, burst size and duration were significantly suppressed by IG sucrose infusion after intra-NAcC vehicle injection (P's < 0.05), but there was no effect of IG sucrose after intra-NAcC Ex9 injection (Fig. 7, E and F). No effects on burst size or duration were observed for later segments of the meal. There were no effects of IG infusion or Ex9 on number of licks emitted in the first min of the meal or average within-burst interlick interval.
DISCUSSION
Our findings support the hypothesis that endogenous GLP-1 release in the NAcC plays a physiological role in the control of feeding and suggest that GLP-1R at that site are particularly influential in determining meal size but not meal frequency. Moreover, the data are consistent with an effect of these receptors on taste evaluation and support the idea that endogenous GLP-1 action in this nucleus reduces food intake, at least in part, by reducing palatability. On the other hand, we find less evidence that GLP-1R in NAcC play a role in mediating the effects of gastrointestinal satiation signals. This is consistent with the conventional view of the NAcC as being involved in the control of food intake primarily through effects on food reward. However, it is a surprising finding, considering that GLP-1 neurons are thought to be activated by meal-related gastrointestinal signals, and central GLP-1 action has been associated with satiation and satiety in previous studies (12, 22).
We observed increases in meal size when rats were licking for SCM, 0.25 M sucrose, and 0.1 M sucrose after blockade of NAcC GLP-1R with the antagonist Ex9. Meal frequency was not increased by Ex9 in any of our experiments but was instead either unchanged or reduced. The interval between meals was not affected by Ex9, nor was satiety ratio. These findings suggest that NAcC GLP-1R are involved in within-meal processes, as opposed to post-meal satiety. This is in contrast with the report of Hayes et al. (12) that hindbrain GLP-1R stimulation suppresses food intake through a reduction in meal number with no effect on meal size. We can therefore conclude that GLP-1R in different brain regions influence feeding through distinct mechanisms.
The results of our licking microstructural analyses suggest that NAcC GLP-1R influence rats' taste evaluation. Initial rate of licking, burst size, and burst duration are all associated with palatability of the test solution. For example, each increases with increasing sucrose concentration (8). In these experiments, we observed significant effects of intra-NAcC Ex9 to increase rate of licking during the early part of the first meal taken when rats were consuming 0.25 M sucrose and to increase licks taken in the first minute of the first meal when rats were consuming 0.1 M sucrose. We also saw increases in meal average burst size and burst duration after Ex9 treatment in the second and third meals of 0.25 M sucrose test sessions, as well as in the first and second meals of 0.1 M sucrose. These effects on average burst size and duration were driven by increases that occur in the early part of the meal, with no drug effect on burst size or duration late in the meal. These data suggest that blockade of NAcC GLP-1R enhances palatability of these sucrose solutions and support the hypothesis that endogenous GLP-1 action at that site reduces food palatability. Our observation that NAcC Ex9 treatment had significant effects on licking microstructure so early in the meal, within the first minute of the first meal when rats were licking for 0.1 M sucrose, raises the possibility that tonic GLP-1 release in this region acts to suppress palatability.
Because GLP-1 neurons are activated by gastrointestinal meal-related stimuli, we hypothesized that the GLP-1 neuronal projection to NAcC may play a role in mediating the satiating effects of gut nutrients. However, our studies provide mixed evidence for this. In most cases where intra-NAcC Ex9 increased meal size relative to vehicle, meal duration was also increased. This is often taken an indication that postingestive negative feedback or the brain's response to this feedback has been suppressed; however, the number of bursts in the meal was not increased by Ex9. In addition, when we segmented the first meals of the session into thirds, we saw that Ex9 had no effects late in the meal, when postingestive negative feedback is strongest. In the most direct test of this hypothesis in experiment 5, we asked whether a dose of intra-NAcC Ex9 that has no effect when delivered alone could attenuate the effects of intragastric nutrient infusion during a 0.25 M sucrose meal. The 40% sucrose IG infusion was designed to stimulate postingestive negative feedback signals that promote satiation and satiety. Within that meal, increased satiation after IG sucrose was reflected in the licking microstructure as a reduction in number of bursts. Although the nutrient-induced reduction in meal size was indeed blunted by NAcC GLP-1R blockade, Ex9 pretreatment did not prevent the suppressive effect of IG sucrose infusion on burst number. Surprisingly, the ability of Ex9 to reduce the intake-suppressive effect of IG nutrients seems to be have been mediated by an effect on burst size and duration. When the IG sucrose infusion was ongoing in the first few minutes of the meal, the IG nutrient load reduced burst size and duration. These effects were completely reversed by intra-NAcC Ex9. Taken together, these data suggest that NAcC GLP-1R influence feeding primarily through effects on hedonic aspects of eating.
Although NAcC GLP-1R clearly do not mediate all of the effects of IG sucrose on licking microstructural variables, we suggest that gastrointestinal nutrients are an important factor for stimulating endogenous GLP-1 action at this site. Because Ex9 primarily affected palatability-related variables when rats were licking for sucrose, we asked whether NAcC GLP-1R blockade would affect the pattern of licking for nonnutritive saccharin. We chose 0.1% saccharin because under vehicle conditions rats showed a similar number of licks in the first minute of the meal, burst size, and burst duration when consuming this concentration of saccharin compared with 0.1 M sucrose. The same dose of intra-NAcC Ex9 that significantly increased each of those measures when rats were ingesting 0.1 M sucrose had no effect on any variable when rats were consuming 0.1% saccharin. In addition to being nonnutritive, saccharin differs from sucrose in other respects (e.g., bitter taste), so it is possible that the lack of effect of NAcC Ex9 on licking for saccharin was not strictly due to lack of gastrointestinal nutrient stimulation. However, the ability of an otherwise subthreshold dose of NAcC Ex9 to increase meal size, burst size, and burst duration relative to vehicle when rats received IG infusion of sucrose in experiment 5 provides further support for the suggestion that gastrointestinal nutrients play a role in NAcC GLP-1R activity.
Overall, our results suggest a novel physiological role for central GLP-1 in taste evaluation and provide further confirmation that endogenous GLP-1 release in the NAcC plays a role in the control of food intake. This may have clinical relevance, because degradation-resistant GLP-1 agonists are now in clinical use for treatment of type 2 diabetes. For the studies presented here, we believe that the relevant source of endogenous GLP-1 in the NAcC is the GLP-1 neuronal projection to this site. GLP-1 released from the intestine is too rapidly degraded to its inactive form for a significant amount to reach the NAcC and act directly on GLP-1R in that nucleus (14). However, agonists such as exenatide and liraglutide can cross the blood-brain barrier, and at least some portion of their effects on food intake is mediated through stimulation of central GLP-1R (15). Therefore, it is possible that, in addition to promoting satiation and/or satiety by acting on GLP-1R elsewhere, peripherally administered GLP-1R agonists could suppress feeding by acting at NAcC GLP-R to reduce food palatability.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant R00 DK-078779 (to D. L. Williams) and in part by funding to A. M. Dossat from the National Institute of Mental Health Grant T32 MH-093311.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.M.D., R.D., L.G., A.P., and K.K. performed experiments; A.M.D. and D.L.W. analyzed data; A.M.D. and D.L.W. drafted manuscript; A.M.D., R.D., L.G., A.P., K.K., and D.L.W. approved final version of manuscript; D.L.W. conception and design of research; D.L.W. interpreted results of experiments; D.L.W. prepared figures; D.L.W. edited and revised manuscript.
REFERENCES
- 1. Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153: 647–658, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baird JP, Grill HJ, Kaplan JM. Effect of hepatic glucose infusion on glucose intake and licking microstructure in deprived and nondeprived rats. Am J Physiol Regul Integr Comp Physiol 277: R1136–R1143, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31: 3904–3913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev 24: 173–198, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Breslin PA, Davis JD, Rosenak R. Saccharin increases the effectiveness of glucose in stimulating ingestion in rats but has little effect on negative feedback. Physiol Behav 60: 411–416, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Davis JD, Campbell CS. Chronic intrajugular, intraportal, gastric, and duodenal cannulae for the rat. In: Physiological Techniques in Behavioral Research, Singh D, Avery DD. (eds.). Monterey, CA: Brooks Cole, 1975, p. 163–177 [Google Scholar]
- 7. Davis JD, Smith GP. Analysis of lick rate measures the positive and negative feedback effects of carbohydrates on eating. Appetite 11: 229–238, 1988 [DOI] [PubMed] [Google Scholar]
- 8. Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci 106: 217–228, 1992 [PubMed] [Google Scholar]
- 9. Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32: 4812–4820, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci 31: 14453–14457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaykema RP, Daniels TE, Shapiro NJ, Thacker GC, Park SM, Goehler LE. Immune challenge and satiety-related activation of both distinct and overlapping neuronal populations in the brainstem indicate parallel pathways for viscerosensory signaling. Brain Res 1294: 61–79, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150: 2654–2659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like Peptide 1 neurons. Diabetes 59: 1890–1898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia 48: 612–615, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103–3112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meeran K, O'Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, Taylor GM, Sunter D, Steere J, Choi SJ, Ghatei MA, Bloom SR. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology 140: 244–250, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic “liking” for food: map based on microinjection Fos plumes. Brain Res 863: 71–86, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res 1350: 18–34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci 112: 678–694, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Smith JC, Sclafani A. Saccharin as a sugar surrogate revisited. Appetite 38: 155–160, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 285: R470–R478, 2003 [DOI] [PubMed] [Google Scholar]