Abstract
In enterocytes, glutamine serves as the major source of energy; another metabolic fate of glutamine is conversion to citrulline. Because sepsis can affect gut function and integrity, alterations in glutamine metabolism may exist and lead to decreased citrulline production. This study aimed to investigate how sepsis affects glutamine metabolism, including its conversion to citrulline, by measuring glutamine and citrulline flux, fractional splanchnic extraction of glutamine and leucine, and the contribution of glutamine nitrogen to citrulline in septic patients and healthy controls. Eight patients with severe sepsis and 10 healthy controls were given primed, constant intravenous infusion of [2H2]citrulline and sequential administration of intravenous and enteral [α-15N]glutamine and [13C]leucine in the postabsorptive state. The results showed that, compared with healthy controls, septic patients had a significantly lower whole body citrulline flux and plasma concentration, higher endogenous leucine flux, and higher glutamine clearance. Fractional splanchnic extraction of leucine was higher in septic patients than in controls, but fractional extraction of glutamine was not different. The majority of the 15N label transferred from glutamine to citrulline was found at the α-position. These results demonstrate that lower glutamine plasma concentrations in sepsis were a result of increased glutamine clearance. Despite adequate splanchnic uptake of glutamine, there is decreased production of citrulline, suggesting a defect in the metabolic conversion of glutamine to citrulline, decreased uptake of glutamine by the enterocyte but increased uptake by the liver, and/or shunting of glutamine to other metabolic pathways.
Keywords: severe sepsis, stable isotopes, enterocyte
the gut is believed to play an important role in sepsis. In fact, it has been postulated that the gut can initiate, perpetuate, or exacerbate the systemic inflammatory response syndrome (SIRS) and lead to the development of multiorgan dysfunction syndrome (MODS) (29). Sepsis can affect gut function and mucosal integrity via a variety of mechanisms, including ischemia-reperfusion injury, mitochondrial dysfunction, and increased intestinal permeability (9). Because maintenance of gut mucosal integrity depends almost exclusively on an adequate enteral supply of amino acids both as primary source of fuel and as substrate for protein synthesis, more information is needed about the metabolic fates of enterally administered amino acids in sepsis.
In health, the gastrointestinal tract is the major organ of glutamine utilization (32). Glutamine is the major source of energy for proliferating enterocytes, providing energy for ATP-dependent processes, including rapid intracellular protein turnover and nutrient transport (39). Adult rat enterocytes extract 25–33% of arterial glutamine and 66% of luminal glutamine (39). Normally the most abundant amino acid in human plasma and muscle (3), glutamine becomes conditionally essential during sepsis, because the need for glutamine exceeds its endogenous rate of synthesis (18). Low plasma glutamine concentrations are found in patients with sepsis (16), and in critically ill patients, low glutamine concentrations are correlated with mortality (4, 28). At present there is a paucity of data on the mechanism(s) responsible for this reduction of glutamine in sepsis.
Along with its use by the gut for energy production and protein synthesis, another metabolic fate of glutamine in the gut is its conversion to citrulline in enterocytes (6). In addition, citrulline is a modulator of protein anabolism (7) and is the only precursor for de novo synthesis of arginine in the body. Low plasma citrulline concentrations in sepsis are the result of decreased citrulline production (16, 22). This decrease in citrulline synthesis may be caused by reduced availability of glutamine or impaired metabolic conversion of glutamine to citrulline within the enterocyte. At present, there are no data on the mechanism responsible for this reduction of citrulline in sepsis.
Recently, there has been some controversy about the exact role of glutamine as a precursor for citrulline synthesis. Windmueller and Spaeth first demonstrated the importance of glutamine as a precursor for citrulline synthesis (37), and subsequent stable isotope tracer studies using an [α-15N]glutamine tracer reported that ∼80% of citrulline is derived from glutamine in fasted humans (20, 35). However, the role of glutamine as a carbon skeleton donor to citrulline has recently been questioned. Marini et al. (16) examined the relationship between glutamine and citrulline in fed mice by use of different isotopomer tracers of glutamine. They reported that glutamine is a poor carbon skeleton precursor for the synthesis of citrulline but rather contributes nonspecific nitrogen and carbon to citrulline synthesis (24). On the other hand, in a study of fed humans, Tomlinson et al. (17) found significant synthesis of arginine from the carbon skeleton of dietary glutamine, as well as transfer of the amino N of glutamine to arginine largely via transamination, providing indirect evidence that glutamine contributes both carbon and nitrogen to citrulline synthesis.
The primary aim of this study was to investigate how glutamine metabolism is affected by sepsis; the secondary aim was to characterize the metabolic conversion of glutamine to citrulline. Using stable isotope tracers, we measured the rate of glutamine entry into the plasma, whole body citrulline flux, glutamine clearance, splanchnic extraction of glutamine, and the fraction of administered glutamine used for citrulline synthesis in patients with sepsis and healthy controls. Splanchnic metabolism of glutamine was compared with splanchnic metabolism of leucine, an essential amino acid. We hypothesized that the lower plasma glutamine seen in patients with sepsis is due to decreased rate of entry into the plasma plus increased utilization (clearance), that glutamine is the main carbon precursor for the synthesis of citrulline, and that the rate of de novo citrulline production is directly related to the rate of splanchnic extraction of dietary glutamine.
MATERIALS AND METHODS
Study subjects.
The study was reviewed and approved by the Institutional Review Board of Baylor College of Medicine in Houston, Texas. Eight adult patients admitted with severe sepsis to the Medical Intensive Care Unit (MICU) at Ben Taub General Hospital in Houston were enrolled in the study. Severe sepsis was defined according to the International Sepsis Definition Conference (19), and all patients were receiving mechanical ventilation. Septic patients were studied within 48 h of admission to the MICU. None of the patients were receiving chronic systemic corticosteroids equivalent to 10 mg/day prednisone or higher or had a diagnosis of end-stage renal disease or solid organ malignancy.
Ten healthy adult volunteers participated in the study as control subjects. All control subjects were in good health as established by medical history, physical examination, and blood chemistry measurements. They were selected to be similar in age, sex, and body mass index (BMI) to the patients. All patients and controls were enrolled after written, informed consent was obtained.
Isotope tracer infusion.
Tracer infusions were performed in healthy subjects at either the adult General Clinical Research Center (GCRC) of Baylor College of Medicine or the Metabolic Research Unit (MRU) of the Children's Nutrition Research Center. Tracer infusions were performed in the septic patients in the MICU at Ben Taub General Hospital. Sterile solutions of [1-13C]leucine, [α-15N]glutamine, and [5,5-2H2]citrulline (99%; Cambridge Isotope Laboratories, Woburn, MA) were prepared in normal saline using strict aseptic techniques and were tested for sterility and lack of pyrogens prior to infusion.
After a 10-h overnight fast, healthy participants were admitted to the GCRC or MRU, and an intravenous catheter was placed in an antecubital vein for isotope infusions and in a hand vein of the contralateral arm for blood sampling. The hand was heated to arterialize blood samples. The use of heated dorsal hand vein sampling as a surrogate for direct arterial sampling has been previously validated (1, 5). After a baseline blood sample was obtained, a primed, continuous, intravenous infusion of [5,5-2H2]citrulline (prime = 1 μmol/kg, infusion = 1 μmol·kg−1·h−1) was started and maintained for 7 h. In addition, primed, continuous intravenous infusions of [1-13C]leucine (prime = 6 μmol/kg, infusion = 6 μmol·kg−1·h−1) and [α-15N]glutamine (prime = 24 μmol/kg, infusion = 16 μmol·kg−1·h−1) were also started and maintained for 3.5 h. After 3.5 h, the intravenous infusions of [13C]leucine and [α-15N]glutamine were stopped. Starting at 3.5 h, the participants received 10-ml boluses of a solution of [1-13C]leucine and [α-15N]glutamine enterally every 30 min for six total doses (equivalent to a continuous infusion of [1-13C]leucine at 6 μmol·kg−1·h−1 and [α-15N]glutamine at 16 μmol·kg−1·h−1 for 3.5 h). Blood samples were obtained every 15 min between 2 h 45 min and 3 h 30 min of the intravenous infusion and 2 h 45 min and 3 h 30 min of the enteral isotope administration (6 h 15 min to 7 h of the complete protocol).
The same tracer infusions were performed in septic patients who had been fasting for at least 10 h. However, the septic patients received continuous enteral infusions rather than bolus doses of [13C]leucine (infusion = 6 μmol·kg−1·h−1) and [15N]glutamine (infusion = 16 μmol·kg−1·h−1) starting at 3.5 h through preexisting feeding tubes placed past the pylorus, and enteral infusions were maintained for 3.5 h. In addition, the intravenous tracers were given through a preexisting central venous catheter, and blood samples were obtained from preexisting arterial catheters.
Sample analysis.
The blood samples were drawn into prechilled tubes containing sodium heparin. The tubes were centrifuged immediately at 4°C, and the plasma was separated and stored immediately at −70°C for later analysis. C-reactive protein was measured in all baseline samples using a commercially available ELISA kit (EMD Millipore, Billerica, MA).
The plasma isotopic enrichment of α-ketoisocaproic acid (KICA), a surrogate of intracellular leucine, was measured by negative chemical ionization gas chromatography-mass spectrometry (GC-MS) of its pentaflurobenzyl derivative and monitoring of ions at m/z 129 and 130. The plasma isotope enrichments of leucine and citrulline were measured by tandem LC-MS. Plasma leucine and citrulline were converted into their 5-dimethlamino-1-napthalene sulfonamide (DANS) derivatives, and ions were analyzed by selected reaction monitoring (SRM) on a triple quadrapole mass spectrometer. The transitions observed were precursor ion m/z 409 to product ion m/z 392 at 14 eV for citrulline and precursor ion m/z 365 and product ion m/z 170 for leucine. Plasma isotope enrichments of glutamine were also measured by tandem LC-MS as well as the location of the 15N positional isomers of glutamine and citrulline, as previously described (23). Briefly, high and low collision energies were applied to the protonated DANS-citrulline derivative, with multiple reactions monitoring of transitions m/z 410 to 392 to identify labeling at the ureido-N position of citrulline, 410 to 71 for labeling at the α-N position, and 410 to 393 for labeling at the α-N or δ-N position of citrulline. Similarly, multiple reactions monitoring of transition m/z 381 to 85 was performed to determine enrichment of [α-15N]glutamine. Plasma concentrations of glutamine, citrulline, arginine, and leucine were measured by ultraperformance liquid chromatography (Waters, Milford, MA) using precolumn derivitization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate.
Calculations.
The rate of appearance or total flux (Q) of leucine, glutamine, and citrulline were calculated from the steady-state equation
where Einf is the isotopic enrichment of leucine, glutamine, or citrulline in the infusate; Eplat is the isotopic enrichment of α-KICA, glutamine, or citrulline in plasma at the isotopic steady state; and i is the infusion rate of the tracer (μmol·kg−1·h−1). Endogenous leucine and citrulline fluxes were determined by subtracting their infusion rates, i, from Q.
Under steady-state conditions, the rate of appearance of glutamine equals the rate of disappearance. Therefore, glutamine clearance (ml·kg−1·min−1) = QGln/plasma glutamine concentration.
The percentage of the enteral tracer of leucine or glutamine extracted by the splanchnic tissues was calculated from
where EpEN and EpIV are the plateau isotopic enrichments in the plasma of the enteral and intravenous tracers (leucine and glutamine), and iIV and iEN are the infusion rates of the intravenous and enteral tracers. Absolute splanchnic extraction of the leucine or glutamine tracer was calculated as the product of the fraction of tracer extracted by the splanchnic tissues and the enteral tracer infusion rate.
The fraction of administered glutamine tracer used for citrulline production was calculated from
where ECIT(GLN) is the plateau isotopic enrichment in plasma of [α-15N]citrulline derived from [α-15N]glutamine, QCIT is the citrulline flux, Einf is the isotopic enrichment of the glutamine tracer, and i is the infusion rate of glutamine.
Statistics.
Continuous variables were summarized by group as means ± SE unless otherwise indicated. Differences between groups of subjects were assessed by the unpaired Student's t-test. Differences in the isotopomer distribution of categorical variables were compared using χ2 test. Tests were considered statistically significant if P < 0.05. Correlations were performed using Pearson's correlation. Data analysis was performed with STATA software (version 11).
RESULTS
Subject characteristics.
The characteristics of the patients and controls are presented in Table 1. There were no significant differences in age, sex, or BMI between the two groups. Of the patients with sepsis, all had respiratory failure and were intubated and required mechanical ventilation, and five of the eight patients had septic shock. The source of sepsis was pneumonia in five patients, meningitis in one patient, endocarditis in one patient, and septic emboli in one patient. Blood cultures were positive in 50% of the patients (pneumococcus in 2, methicillin-resistant staphylococcus aureus in 1, and citrobacter freundii in 1). Patients had an APACHE II score of 23.0 ± 2.0 (mean ± SE), and three died during their hospitalization. Septic patients had significantly higher plasma concentrations of C-reactive protein than controls.
Table 1.
Demographic characteristics, vital signs, and blood chemistry indexes in controls (n = 10) vs. septic patients (n = 8)
| Controls | Septic Patients | |
|---|---|---|
| Age | 43 ± 5 | 51 ± 5 |
| Sex | 8M:2F | 7M:1F |
| Weight, kg | 84.2 ± 4.4 | 78.4 ± 10.8 |
| BMI, kg/m2 | 24.8 ± 0.7 | 25.7 ± 2.9 |
| Mean arterial pressure, mmHg | 99.3 ± 4.6 | 75.7 ± 5.9* |
| Heart rate, beats/min | 76 ± 5 | 101 ± 7* |
| White blood cell count, k/μl | 6.0 ± 0.4 | 16.0 ± 4.6† |
| Creatinine, mg/dl | 0.9 ± 0.1 | 1.4 ± 0.2† |
| Albumin, g/dl | 4.6 ± 0.1 | 1.5 ± 0.2* |
| AST, U/l | 29.8 ± 2.3 | 69.3 ± 15.8* |
| ALT, U/l | 19.6 ± 1.7 | 49.9 ± 11.4* |
| C-reactive protein, μg/ml | 1.1 ± 1.1 | 119.3 ± 57.7* |
Values are expressed as means ± SD. AST, aspartate aminotransferase. ALT, alanine aminotransferase.
P < 0.01 vs. controls by unpaired t-test;
P < 0.05 vs. controls by unpaired t-test.
Leucine, glutamine, and citrulline kinetics and plasma concentrations.
The amino acid kinetics and plasma concentrations are presented in Tables 2 and 3. Because leucine is an essential amino acid, in the fasted state its flux is derived only from whole body protein breakdown. Therefore, the endogenous flux of leucine is an index of the rate of whole body protein breakdown. Endogenous leucine flux was significantly higher in patients with sepsis than in controls (P = 0.01; Table 2). Glutamine flux reflects the interorgan transport rates of glutamine through plasma (8). There was no difference in glutamine flux between patients and controls (P = 0.51), but the plasma concentration of glutamine was significantly lower in the patients (P < 0.001; Table 3) due to an increase in its clearance from the plasma (P = 0.003). Septic patients had a significantly lower citrulline flux (P < 0.001) as well as plasma citrulline concentration (P < 0.001) compared with controls.
Table 2.
Amino acid fluxes in controls (n = 10) vs. septic patients (n = 8)
| Controls | Septic Patients | |
|---|---|---|
| Endogenous leucine flux, μmol·kg−1·h−1 | 97.8 ± 4.6 | 177.7 ± 31.4† |
| Glutamine flux, μmol·kg−1·h−1 | 308.1 ± 11.9 | 326.6 ± 27.3 |
| Glutamine clearance, ml·kg−1·min−1 | 10.5 ± 0.9 | 26.8 ± 5.2* |
| Citrulline flux, μmol·kg−1·h−1 | 8.9 ± 0.5 | 4.4 ± 0.2* |
Values are expressed as means ± SE.
P < 0.05 vs. controls by unpaired t-test;
P < 0.01 vs. controls by unpaired t-test.
Table 3.
Plasma amino acid concentrations in controls (n = 10) vs. septic patients (n = 8)
| Controls | Septic Patients | |
|---|---|---|
| Glutamine, μM | 477.8 ± 25.8 | 237.5 ± 45.1* |
| Citrulline, μM | 28.8 ± 2.4 | 9.9 ± 1.7* |
| Arginine, μM | 76.1 ± 2.9 | 68.7 ± 11.1 |
| Leucine, μM | 105.0 ± 7.0 | 101.4 ± 18.0 |
Values are expressed as means ± SE.
P < 0.001 vs. controls by unpaired t-test.
There was a significant correlation between plasma glutamine and citrulline concentrations (r = 0.77, P < 0.001). There were also significant correlations between citrulline flux and citrulline plasma concentration (r = 0.84, P < 0.001) and citrulline flux and plasma glutamine concentration (r = 0.78, P < 0.001). There was a significant correlation between glutamine flux and endogenous leucine flux when one outlying value was removed (r = 0.68, P = 0.003).
Splanchnic extraction of leucine and glutamine.
Percent splanchnic extraction of the leucine tracer was significantly higher in septic patients than in controls (P = 0.045), and there was a trend toward a higher absolute extraction of the tracer in sepsis (P = 0.051). There was no difference in percent splanchnic extraction or absolute splanchnic extraction of the glutamine tracer in the two groups (P = 0.17). These results are summarized in Table 4. The ratio of [13C]KICA to [13C]leucine in the plasma was 0.63 ± 0.05 in septic patients and 0.71 ± 0.03 in controls when the tracer was given intravenously, and 0.84 ± 0.06 in septic patients and 0.77 ± 0.07 in controls when the tracer was given enterally. This ratio was significantly higher when given enterally compared with intravenously in the septic patients (P = 0.01).
Table 4.
Percent and absolute splanchnic extraction of leucine and glutamine tracers in controls (n = 10) vs. septic patients (n = 8)
| Controls | Septic Patients | |
|---|---|---|
| Percent extraction of leucine | 18.0 ± 3.2 | 30.8 ± 5.0* |
| Absolute splanchnic extraction of leucine, μmol tracer·kg−1·h−1 | 0.9 ± 0.2 | 1.5 ± 0.2 |
| Percent extraction of glutamine | 39.9 ± 4.5 | 49.0 ± 5.6 |
| Absolute splanchnic extraction of glutamine, μmol tracer·kg−1·h−1 | 6.4 ± 0.7 | 7.8 ± 0.9 |
Values are expressed as means ± SE.
P < 0.05 vs. controls by unpaired t-test.
Relationship between glutamine and citrulline.
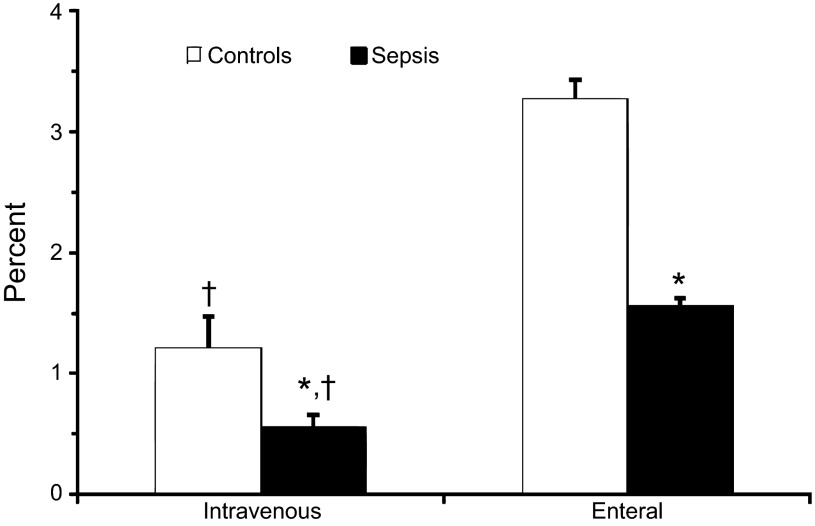
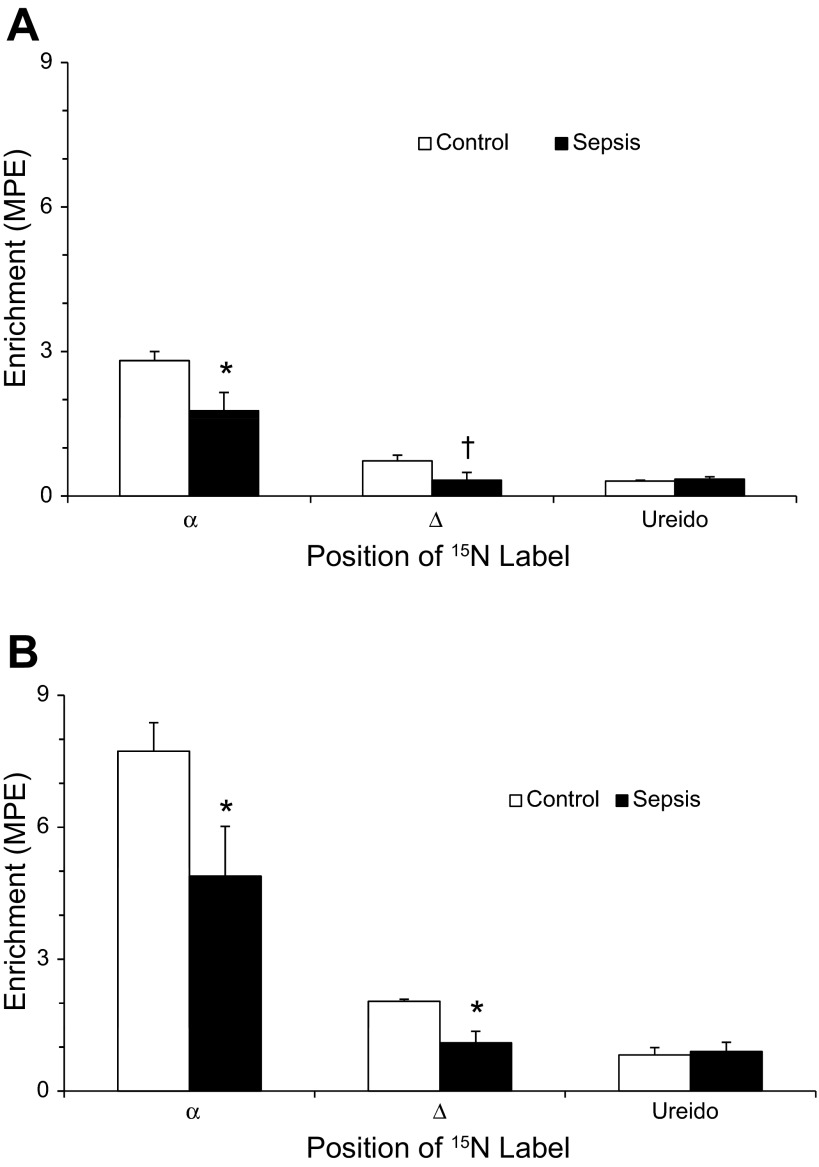
As shown in Fig. 1, the fraction of administered glutamine used for citrulline synthesis was lower in septic patients than in controls regardless of the route of administration. A greater amount of glutamine was used for citrulline production when it was given enterally compared with parenterally. The majority of the 15N label transferred from glutamine to citrulline was found at the α-position whether glutamine was administered intravenously or enterally (Fig. 2, A and B). At both the α- and δ-positions, but not the ureido position, there was significantly lower enrichment of [15N]citrulline in septic patients regardless of the route of administration of glutamine.
Fig. 1.
Percentage of intravenously and enterally administered [α-15N]glutamine tracer used to synthesize [α-15N]citrulline in patients with sepsis (n = 8) and controls (n = 10). *P < 0.05 vs. controls; †P < 0.05 vs. enteral tracer using two-factor ANOVA.
Fig. 2.
A: isotopic enrichment of 15N isotopomers of citrulline derived from intravenous [α-15N]glutamine in patients with sepsis (n = 8) and controls (n = 10). *P < 0.05 vs. controls; †P = 0.06 vs. controls by unpaired t-test. B: isotopic enrichment of 15N isotopomers of citrulline derived from enteral [α-15N]glutamine in patients with sepsis (n = 8) and controls (n = 10). *P < 0.05 vs. controls.
DISCUSSION
The present study aimed to investigate how sepsis affects glutamine metabolism, with emphasis on its conversion to citrulline, in patients with severe sepsis or septic shock and in healthy controls. To our knowledge, this is the first study to examine the relationship between splanchnic glutamine extraction and citrulline production as well as the contribution of glutamine nitrogen to citrulline in septic humans. Contrary to our hypothesis, the fractional splanchnic extraction of glutamine was not different in sepsis vs. controls, although the fraction of administered glutamine used for citrulline production was lower in sepsis, suggesting that factors other than diminished supply of glutamine lead to decreased citrulline production. In support of this, we also found that in sepsis there is a greater proportion of nitrogen label transferred from [α-15N]glutamine to citrulline at the α- and ureido positions relative to the δ-position, indicating a possible defect in the nitrogen transfer via transamination to glutamate semialdehyde by the enzyme ornithine transaminase.
Splanchnic extraction of glutamine and leucine.
Our finding that in healthy controls the fractional extraction of glutamine was 39.9 ± 4.5%. and the fractional splanchnic extraction of leucine was 18.0 ± 3.2% is consistent with prior studies in healthy humans showing that ∼40–75% of enterally administered glutamine (13, 25, 33) and 18–23% of enterally administered leucine (14, 26) undergo first-pass extraction within the splanchnic bed. Septic patients in this study had a 67% greater splanchnic uptake of leucine compared with controls but did not have a significant change in glutamine splanchnic uptake. In a study of healthy volunteers given endotoxin, net splanchnic uptake of leucine similarly increased 76%, from 21 ± 3% to 37 ± 7% (10). The ultimate fate of leucine taken up by the splanchnic bed in this study cannot be determined, as leucine oxidation was not measured. However, Matthews et al. (26) showed that the majority was either converted to KICA or taken up for incorporation to newly synthesized protein. We found that, in both septic patients and controls, the ratio of KICA to leucine isotopic enrichments was increased when leucine was administered enterally compared with intravenously, although this difference is greater in the septic patients. This suggests that enteral leucine is undergoing transamination to KICA.
During health the small intestine is the principal organ of glutamine uptake, but in sepsis the liver is the major site of glutamine consumption, and there is a decrease in intestinal glutamine uptake (2, 31). The tracer methodologies used in this study cannot distinguish between retention of amino acids by the gut and first-pass clearance of amino acids by the liver. However, because it is likely that glutamine uptake by the liver is increased, this suggests that uptake of glutamine by the gut may be decreased. The different responses in splanchnic metabolism of leucine and glutamine in sepsis may be due to changes in utilization of these amino acids rather than a consequence of gut dysfunction. Of note, these studies were performed in the postabsorptive state, and intestinal response to a tracer in the fed state may be different.
Glutamine metabolism.
Endogenous leucine flux, an index of whole body protein breakdown, was greater in patients with sepsis than in controls, a finding that is consistent with studies in both animals and humans (17, 38, 40). Because glutamine is present in high concentrations in all organs and tissues, glutamine can account for as much as 50% of amino acids released from whole body protein breakdown (38). Accordingly, we found a significant correlation between endogenous leucine flux and glutamine rate of entry into the plasma, indicating that glutamine entry into the plasma is closely related to its release from protein breakdown. However, despite the faster rate of protein breakdown in the septic patients, glutamine entry into plasma was not also increased. This finding is consistent with a previous study of glutamine and leucine kinetics in critical illness (15). The plasma concentration of glutamine in septic patients was significantly lower compared with controls despite a similar rate of entry into the plasma compartment, confirming that rate of glutamine removal exceeded its rate of entry into the plasma. This was supported by the increased rate of clearance of glutamine by the septic patients.
Glutamine as a precursor of citrulline.
Our data suggest indirectly that glutamine serves as a carbon precursor for citrulline. In both septic patients and healthy controls, the majority of the 15N label (more than 70%) from glutamine was found on the α-nitrogen of citrulline. This is in contrast to the results of Marini et al. (24), who found that after infusion of [α-15N]glutamine, [15N]citrulline had the greatest enrichment at the ureido position. On the other hand, Tomlinson et al. (34) found the highest 15N enrichment at the δ-position of citrulline after administration of [α-15N]glutamine. Differences in study design and isotopomer analysis may explain these differences, as the first study was performed in mice, and although the other study was in humans, it was in the fed state. Although the carbon skeleton of glutamine was not labeled in our study, the α-nitrogen of glutamine is transferred along with the carbon skeleton in the pathway to form [α-15N]citrulline, indicating that glutamine also serves as a carbon donor for glutamine. Additionally, no labeled ornithine was administered; thus, ornithine could be a major contributor of carbon to citrulline. Finally, healthy controls, but not septic patients, had significant labeling at the δ-N position of citrulline. This suggests a defect in the transfer of nitrogen by transamination via ornithine aminotransferase. Together, the findings that less administered [α-15N]glutamine is used to form [α-15N]citrulline in sepsis combined with a decrease in nitrogen transfer to citrulline at the δ-position suggests defect(s) in the metabolic pathway between glutamine and citrulline, not just an inadequate supply of precursor.
Glutamine supplementation in critical illness.
Because of the finding of low plasma glutamine in sepsis, exogenous glutamine supplementation has been studied in large clinical trials. However, the metabolic effects of exogenous glutamine supplementation remain poorly understood. Current guidelines recommend intravenous glutamine supplementation in parenterally fed critically ill patients (30) on the basis of evidence that parental, but not enteral, glutamine supplementation may affect mortality and infectious complications (11, 12, 27). It has been hypothesized that the benefit of exogenous glutamine may be from its conversion to citrulline and then to arginine (36). However, the metabolic data here do not support this hypothesis for multiple reasons. First, we found that a very small percentage of glutamine is used for the synthesis of citrulline, a finding consistent with a prior study of humans during surgery (21). Second, we found that about twice as much glutamine tracer was used for citrulline synthesis when it was administered enterally compared with intravenously. Thus, if its mechanism of action were through citrulline, glutamine's clinical benefit should be greater when given enterally. In fact, because enteral administration of amino acids leads to uptake and utilization in the intestines, there is less delivery to the peripheral tissues with dietary supplementation. Therefore, the benefit of parenteral over enteral glutamine during critical illness suggests that its effect on peripheral tissues is of greater importance. On the other hand, if one potential benefit of glutamine supplementation is to improve gut barrier function, its effect may be greater if administered enterally and prior to the onset of MODS, even in fasting patients.
Study limitations.
This study had several limitations. Sepsis is a heterogeneous syndrome; therefore, the findings in this small sample may not be generalizable to all patients with sepsis. The subjects selected for the study had severe sepsis or septic shock with a mean APACHE II score of 23 ± 2. Nevertheless, significant differences were seen in splanchnic leucine extraction, citrulline flux, and the conversion of glutamine to citrulline. Also, the methods used in this study could not distinguish between intestinal and liver uptake during first-pass metabolism of tracers. However, this could not be done without invasive procedures and sampling of the portal circulation. Finally, the study was conducted only in the fasted state, and utilization and metabolism of glutamine may change in the fed state.
Conclusions.
Despite adequate splanchnic uptake of glutamine, there is decreased production of citrulline, suggesting there is 1) a defect in one or more steps in the metabolic conversion of glutamine to citrulline, 2) decreased uptake of glutamine by the enterocyte but increased uptake by the liver, and/or 3) shunting of glutamine to other metabolic pathways. The findings of this study suggest that the beneficial effect of glutamine supplementation is probably not via enhanced citrulline and arginine production but rather from the delivery of glutamine to the peripheral tissues.
GRANTS
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Disease (1K23DK08115). Work at the General Clinical Research Center was supported by the National Institutes of Health (M01-RR-00188). This research was also supported with federal funds from the US Department of Agriculture, Agricultural Research Service under Cooperative Agreement number 58-6250-6001.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.C.K., V.B., and F.J. conception and design of research; C.C.K., J.W.H., and V.B. performed experiments; C.C.K. and J.W.H. analyzed data; C.C.K., J.W.H., V.B., and F.J. interpreted results of experiments; C.C.K. prepared figures; C.C.K. drafted manuscript; C.C.K., J.W.H., V.B., and F.J. edited and revised manuscript; C.C.K., J.W.H., V.B., and F.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the nursing staff of the General Clinical Research Center and the Metabolic Research Unit for care of the participants and Grace Tang for assistance in laboratory analyses.
REFERENCES
- 1. Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism 30: 936–940, 1981 [DOI] [PubMed] [Google Scholar]
- 2. Austgen TR, Chen MK, Flynn TC, Souba WW. The effects of endotoxin on the splanchnic metabolism of glutamine and related substrates. J Trauma 31: 742–751; discussion 751–742, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Bergstrom J, Furst P, Noree LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol 36: 693–697, 1974 [DOI] [PubMed] [Google Scholar]
- 4. Castillo Rodas P, Rooyackers O, Hebert C, Norberg A, Wernerman J. Glutamine and glutathione at ICU admission in relation to outcome. Clin Sci (Lond) 122: 591–597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Copeland KC, Kenney FA, Nair KS. Heated dorsal hand vein sampling for metabolic studies: a reappraisal. Am J Physiol Endocrinol Metab 263: E1010–E1014, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, Cynober L. Almost all about citrulline in mammals. Amino Acids 29: 177–205, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Cynober L, Moinard C, De Bandt JP. The 2009 ESPEN Sir David Cuthbertson. Citrulline: a new major signaling molecule or just another player in the pharmaconutrition game? Clin Nutr 29: 545–551, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Darmaun D, Matthews DE, Bier DM. Glutamine and glutamate kinetics in humans. Am J Physiol Endocrinol Metab 251: E117–E126, 1986 [DOI] [PubMed] [Google Scholar]
- 9. Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg 216: 117–134, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fong Y, Matthews DE, He W, Marano MA, Moldawer LL, Lowry SF. Whole body and splanchnic leucine, phenylalanine, and glucose kinetics during endotoxemia in humans. Am J Physiol Regul Integr Comp Physiol 266: R419–R425, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Goeters C, Wenn A, Mertes N, Wempe C, Van Aken H, Stehle P, Bone HG. Parenteral l-alanyl-l-glutamine improves 6-month outcome in critically ill patients. Crit Care Med 30: 2032–2037, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Griffiths RD, Jones C, Palmer TE. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition 13: 295–302, 1997 [PubMed] [Google Scholar]
- 13. Hankard RG, Darmaun D, Sager BK, D'Amore D, Parsons WR, Haymond M. Response of glutamine metabolism to exogenous glutamine in humans. Am J Physiol Endocrinol Metab 269: E663–E670, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Hoerr RA, Matthews DE, Bier DM, Young VR. Leucine kinetics from [2H3]- and [13C]leucine infused simultaneously by gut and vein. Am J Physiol Endocrinol Metab 260: E111–E117, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Jackson NC, Carroll PV, Russell-Jones DL, Sonksen PH, Treacher DF, Umpleby AM. The metabolic consequences of critical illness: acute effects on glutamine and protein metabolism. Am J Physiol Endocrinol Metab 276: E163–E170, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Kao CC, Bandi V, Guntupalli KK, Wu M, Castillo L, Jahoor F. Arginine, citrulline and nitric oxide metabolism in sepsis. Clin Sci (Lond) 117: 23–30, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Kao CC, Guntupalli KK, Bandi V, Jahoor F. Whole-body CO2 production as an index of the metabolic response to sepsis. Shock 32: 23–28, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nutr Rev 48: 297–309, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31: 1250–1256, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Ligthart-Melis GC, van de Poll MC, Boelens PG, Dejong CH, Deutz NE, van Leeuwen PA. Glutamine is an important precursor for de novo synthesis of arginine in humans. Am J Clin Nutr 87: 1282–1289, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Ligthart-Melis GC, van de Poll MC, Vermeulen MA, Boelens PG, van den Tol MP, van Schaik C, De Bandt JP, Deutz NE, Dejong CH, van Leeuwen PA. Enteral administration of alanyl-[2-(15)N]glutamine contributes more to the de novo synthesis of arginine than does intravenous infusion of the dipeptide in humans. Am J Clin Nutr 90: 95–105, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Luiking YC, Poeze M, Ramsay G, Deutz NE. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr 89: 142–152, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Marini JC. Quantitative analysis of 15N-labeled positional isomers of glutamine and citrulline via electrospray ionization tandem mass spectrometry of their dansyl derivatives. Rapid Commun Mass Spectrom 25: 1291–1296, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Marini JC, Didelija IC, Castillo L, Lee B. Glutamine: precursor or nitrogen donor for citrulline synthesis? Am J Physiol Endocrinol Metab 299: E69–E79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of glutamine and glutamic acid in humans. Am J Physiol Endocrinol Metab 264: E848–E854, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol Endocrinol Metab 264: E109–E118, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med 30: 2022–2029, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Oudemans-van Straaten HM, Bosman RJ, Treskes M, van der Spoel HJ, Zandstra DF. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med 27: 84–90, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Rotstein OD. Pathogenesis of multiple organ dysfunction syndrome: gut origin, protection, and decontamination. Surg Infect (Larchmt) 1: 217–223; discussion 223–215, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, Griffiths R, Kreyman G, Leverve X, Pichard C, Espen ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr 28: 387–400, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Souba WW, Herskowitz K, Klimberg VS, Salloum RM, Plumley DA, Flynn TC, Copeland EM., 3rd The effects of sepsis and endotoxemia on gut glutamine metabolism. Ann Surg 211: 543–549; discussion 549–551, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Souba WW, Herskowitz K, Salloum RM, Chen MK, Austgen TR. Gut glutamine metabolism. J Parenter Enteral Nutr 14: 45S–50S, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Thibault R, Welch S, Mauras N, Sager B, Altomare A, Haymond M, Darmaun D. Corticosteroids increase glutamine utilization in human splanchnic bed. Am J Physiol Gastrointest Liver Physiol 294: G548–G553, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Tomlinson C, Rafii M, Ball RO, Pencharz P. Arginine synthesis from enteral glutamine in healthy adults in the fed state. Am J Physiol Endocrinol Metab 301: E267–E273, 2011 [DOI] [PubMed] [Google Scholar]
- 35. van de Poll MC, Ligthart-Melis GC, Boelens PG, Deutz NE, van Leeuwen PA, Dejong CH. Intestinal and hepatic metabolism of glutamine and citrulline in humans. J Physiol 581: 819–827, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vermeulen MA, van de Poll MC, Ligthart-Melis GC, Dejong CH, van den Tol MP, Boelens PG, van Leeuwen PA. Specific amino acids in the critically ill patient–exogenous glutamine/arginine: a common denominator? Crit Care Med 35: S568–576, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol Endocrinol Metab 241: E473–E480, 1981 [DOI] [PubMed] [Google Scholar]
- 38. Wolfe RR, Jahoor F, Hartl WH. Protein and amino acid metabolism after injury. Diabetes Metab Rev 5: 149–164, 1989 [DOI] [PubMed] [Google Scholar]
- 39. Wu G. Intestinal mucosal amino acid catabolism. J Nutr 128: 1249–1252, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Yoshida S, Lanza-Jacoby S, Stein TP. Leucine and glutamine metabolism in septic rats. Biochem J 276: 405–409, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]