Abstract
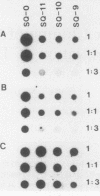
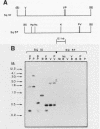
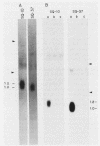
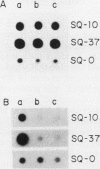
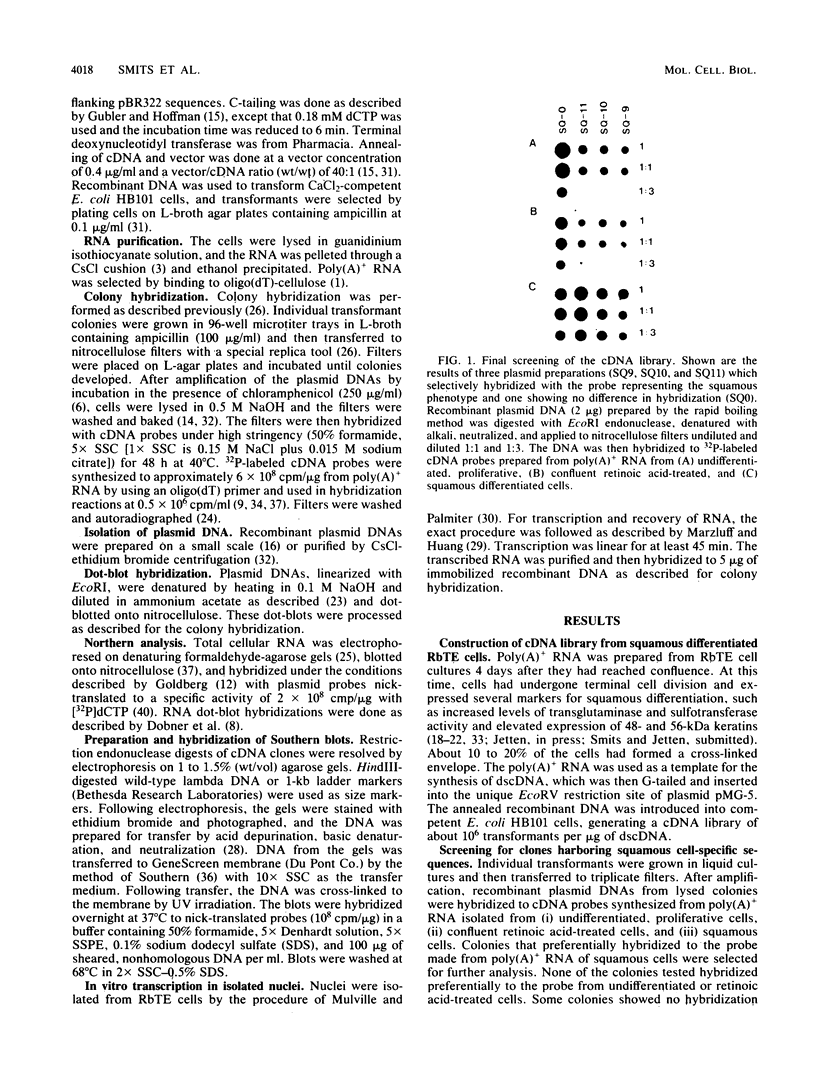
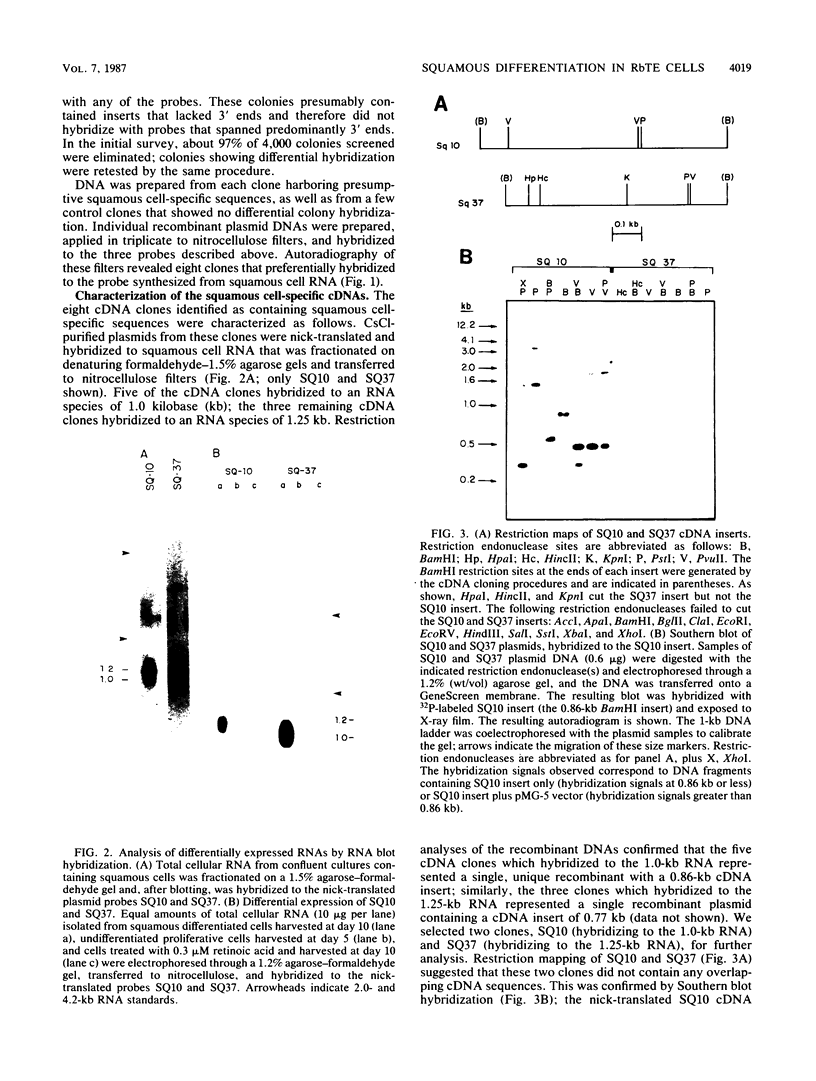
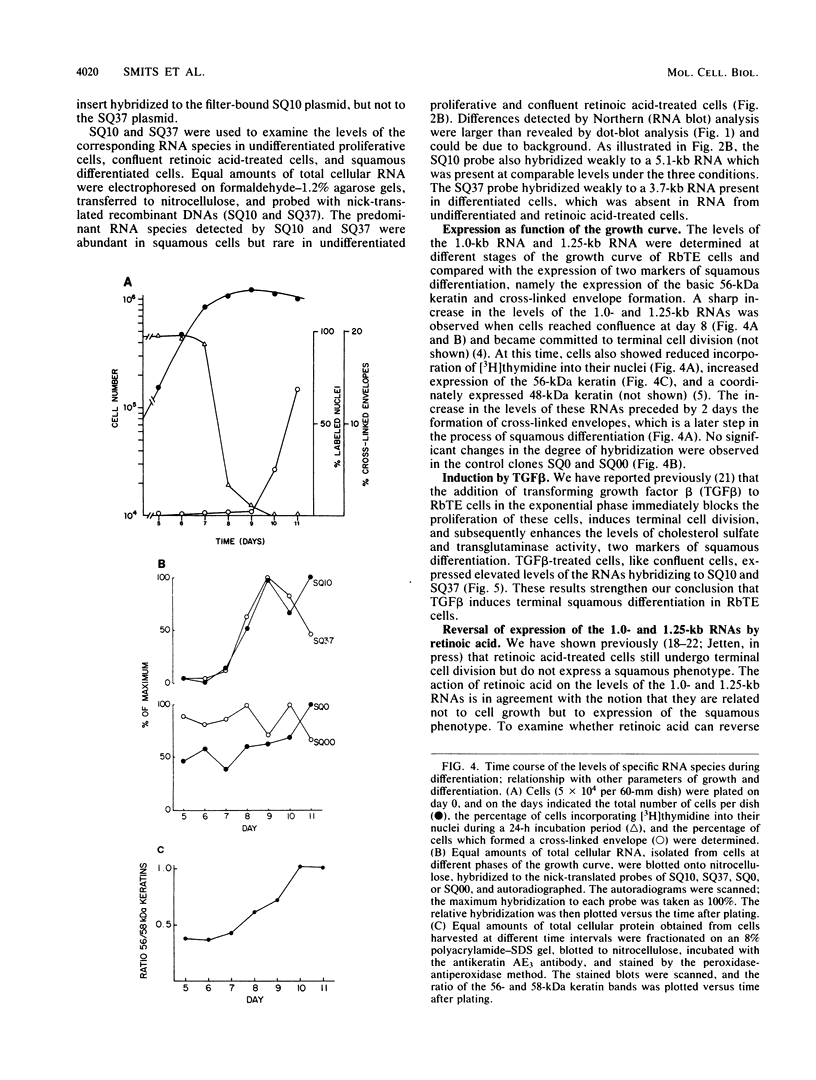
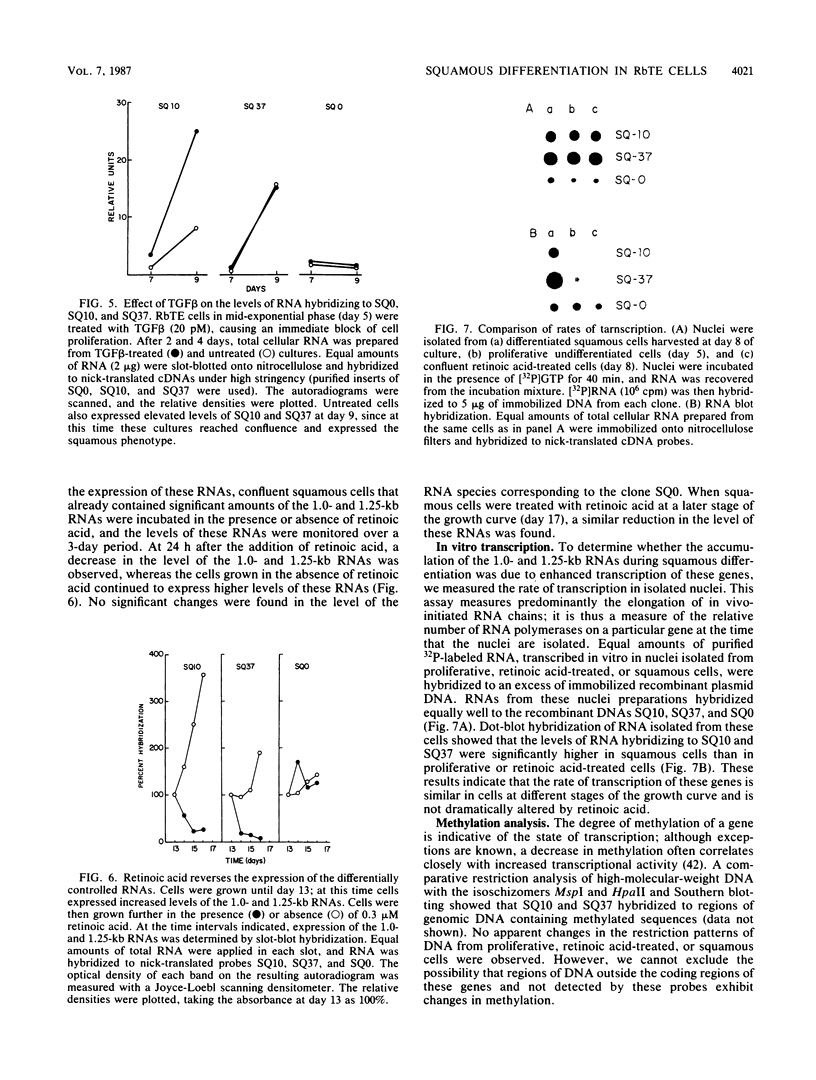
A cDNA library was constructed from polyadenylated RNA present in squamous differentiated rabbit tracheal epithelial cells. Screening of the cDNA library was aimed at identifying RNAs that were abundant in squamous cells and expressed at low levels in undifferentiated cells. Two different recombinants were obtained containing inserts, 0.86 and 0.77 kilobases (kb) in size, that hybridized to mRNAs 1.0 and 1.25 kb in length. These RNAs were present at approximately 50-fold higher levels in squamous cells than in proliferative or confluent retinoic acid-treated cells. The increase in the levels of the 1.0- and 1.25-kb RNAs correlated closely with the onset of squamous differentiation and was not related to induction of terminal cell division. Treatment of rabbit tracheal epithelial cells with transforming growth factor beta, which induces squamous differentiation in these cells, also resulted in elevated levels of the 1.0- and 1.25-kb RNAs. The increased levels of these RNAs in squamous cells appeared to a large extent to be regulated at a posttranscriptional level. Retinoic acid not only inhibited the increase in the levels of the 1.0- and 1.25-kb RNAs but also reversed the expression of these RNAs in squamous cells. These results suggest that retinoic acid affects, directly or indirectly, molecular events that induce alterations in the posttranscriptional processing of the transcripts corresponding to the 1.0- and 1.25-kb RNAs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen stabilizes vitellogenin mRNA against cytoplasmic degradation. Cell. 1983 Aug;34(1):207–214. doi: 10.1016/0092-8674(83)90151-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chytil F., Ong D. E. Cellular retinol- and retinoic acid-binding proteins in vitamin A action. Fed Proc. 1979 Oct;38(11):2510–2514. [PubMed] [Google Scholar]
- Chytil F., Ong D. E. Cellular retinol- and retinoic acid-binding proteins. Adv Nutr Res. 1983;5:13–29. doi: 10.1007/978-1-4613-9937-7_2. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond D. J., Goodman H. M. Regulation of growth hormone messenger RNA synthesis by dexamethasone and triiodothyronine. Transcriptional rate and mRNA stability changes in pituitary tumor cells. J Mol Biol. 1985 Jan 5;181(1):41–62. doi: 10.1016/0022-2836(85)90323-7. [DOI] [PubMed] [Google Scholar]
- Dobner P. R., Kawasaki E. S., Yu L. Y., Bancroft F. C. Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous M., Nir U., Wallach D., Merlin G., Rubinstein M., Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3082–3086. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton R., Birnie G. D., Knowler J. T. Post-transcriptional regulation of rat liver gene expression by glucocorticoids. Nucleic Acids Res. 1985 Sep 25;13(18):6467–6482. doi: 10.1093/nar/13.18.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfix B. M., Eckert R. L. Coordinate control by vitamin A of keratin gene expression in human keratinocytes. J Biol Chem. 1985 Nov 15;260(26):14026–14029. [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall G. J., Dominguez F., Horecker B. L. Molecular cloning of cDNA for human prothymosin alpha. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8926–8928. doi: 10.1073/pnas.83.23.8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Rearick J. I., Smits H. L. Regulation of differentiation of airway epithelial cells by retinoids. Biochem Soc Trans. 1986 Oct;14(5):930–933. doi: 10.1042/bst0140930. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Shirley J. E. Characterization of transglutaminase activity in rabbit tracheal epithelial cells. Regulation by retinoids. J Biol Chem. 1986 Nov 15;261(32):15097–15101. [PubMed] [Google Scholar]
- Jetten A. M., Shirley J. E., Stoner G. Regulation of proliferation and differentiation of respiratory tract epithelial cells by TGF beta. Exp Cell Res. 1986 Dec;167(2):539–549. doi: 10.1016/0014-4827(86)90193-x. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Smits H. Regulation of differentiation of tracheal epithelial cells by retinoids. Ciba Found Symp. 1985;113:61–76. doi: 10.1002/9780470720943.ch5. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Growth-related changes in specific mRNAs of cultured mouse cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4271–4275. doi: 10.1073/pnas.80.14.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Mulvihill E. R., Palmiter R. D. Relationship of nuclear estrogen receptor levels to induction of ovalbumin and conalbumin mRNA in chick oviduct. J Biol Chem. 1977 Mar 25;252(6):2060–2068. [PubMed] [Google Scholar]
- Peacock S. L., McIver C. M., Monahan J. J. Transformation of E. coli using homopolymer-linked plasmid chimeras. Biochim Biophys Acta. 1981 Sep 28;655(2):243–250. doi: 10.1016/0005-2787(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Peden K., Mounts P., Hayward G. S. Homology between mammalian cell DNA sequences and human herpesvirus genomes detected by a hybridization procedure with high-complexity probe. Cell. 1982 Nov;31(1):71–80. doi: 10.1016/0092-8674(82)90406-8. [DOI] [PubMed] [Google Scholar]
- Rearick J. I., Jetten A. M. Accumulation of cholesterol 3-sulfate during in vitro squamous differentiation of rabbit tracheal epithelial cells and its regulation by retinoids. J Biol Chem. 1986 Oct 25;261(30):13898–13904. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole J. J., Knopf J. L., Wozney J. M., Sultzman L. A., Buecker J. L., Pittman D. D., Kaufman R. J., Brown E., Shoemaker C., Orr E. C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984 Nov 22;312(5992):342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., LaRosa G. J., Gudas L. J. Molecular cloning of gene sequences transcriptionally regulated by retinoic acid and dibutyryl cyclic AMP in cultured mouse teratocarcinoma cells. Dev Biol. 1985 Jan;107(1):75–86. doi: 10.1016/0012-1606(85)90377-x. [DOI] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier M. L., Scott R. E. Regulation of the terminal event in cellular differentiation: biological mechanisms of the loss of proliferative potential. J Cell Biol. 1986 May;102(5):1955–1964. doi: 10.1083/jcb.102.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]