Abstract
Eosinophilic esophagitis (EoE) is a chronic allergic disease characterized by esophageal intraepithelial eosinophils, extracellular eosinophil granule deposition, induced mast cell accumulation, and epithelial cell hyperplasia. However, the processes involved in the development of a number of these characteristics are largely unknown. Herein, we tested the hypothesis whether induced mast cell accumulation in the esophagus has a role in promoting EoE pathogenesis. Accordingly, we induced experimental EoE in wild-type mice, mast cell-deficient WWv mice, and mast cell-reconstituted WWv mice. We report that esophageal mast cell numbers increase in parallel with eosinophils in a dose- and time-dependent manner following the induction of allergen-induced EoE. The induced mast cells are localized in the esophageal lamina propria and muscular mucosa but have no influence on promoting esophageal eosinophilia. The 5′-bromodeoxyuridine (BrdU) incorporation analysis indicated that mast cells have a significant role in muscle cell hyperplasia and hypertrophy. In addition, the wild-type and mast cell-reconstituted WWv mice showed a comparable number of BrdU+ cells in the esophageal muscular mucosa following allergen-induced EoE. In conclusion, we provide for the first time direct evidence that mast cell promotes muscle cell hyperplasia and hypertrophy and may have a significant role in promoting esophageal functional abnormalities in EoE.
Keywords: allergen, 5′-bromodeoxyuridine, hyperplasia, hypertrophy, mast cells, muscular mucosa, eosinophilic esophagitis
eosinophilic esophagitis (EoE) is an increasingly recognized chronic inflammatory disease of the esophagus that clinically mimics gastroesophageal reflux disease. EoE is induced by food or aeroallergen exposure, characterized by intraepithelial eosinophil accumulation, eosinophil granule deposition in the extracellular tissue, and epithelial cell hyperplasia and is resistant to acid suppression therapy (10, 11, 14, 20, 27, 31). Eosinophils and mast cells are increased in the esophageal mucosa of experimental and human EoE (2, 9, 17, 19, 25, 30), but their role in disease pathogenesis is unclear. Mast cells express the high-affinity receptor for IgE on their surface (3, 4, 15, 16) that initiates a complex process of signal transduction that releases proinflammatory mediators, cytokines, and chemokines (3, 4, 12, 16). Mast cells produce the eosinophil chemoattractant eotaxin-1 that influences allergen-induced eosinophil accumulation (29) in select tissues, raising the possibility that they may mediate eosinophil accumulation in the esophagus.
Additionally, it has been shown that mast cells have a clear-cut pathological role in a number of allergic diseases (6, 7, 13, 18), and increased esophageal mast cells may have the potential to influence multiple aspects of the EoE pathogenesis. We recently reported that esophageal peristaltic dysfunction in experimental EoE is independent of eosinophils in experimental EoE (19). Therefore, we hypothesized that allergen-induced mast cell accumulation in the esophagus may have an important role in some of the esophageal abnormal function in EoE. Accordingly, we monitored the influence of mast cells on allergen-induced inflammation in the esophagus. We demonstrate that the esophageal mast cells increase in parallel with eosinophils; however, the esophageal eosinophilia is independent of the esophageal mast cells in EoE. Importantly, mast cell-deficient WWv mice are protected from the induction of muscle cell hyperplasia in allergen-induced EoE. Although using WWv mice has some limitations, as they have some reduced neutrophils and basophils as reported earlier (28), our experimentation, however, with reconstituted mast cells ruled out any effect on our study conclusions. Taken together, we provide direct evidence that mast cells may have a significant role in esophageal functional abnormalities, particularly the development of esophageal peristaltic dysfunction.
MATERIALS AND METHODS
Mice.
Specific pathogen-free wild-type, mast cell-deficient WWv mice with their respective controls were obtained from the Jackson Laboratory (Bar Harbor, ME). All mice were maintained in a barrier facility, and animals were handled according to institutional guidelines; all animal protocols were approved by the Case Western Reserve University Animal Care and Use Committee. All of the experiments were performed on age- and sex-matched mice that were 6–8 wk old.
Induction of experimental EoE in mice.
Experimental EoE was induced in mice following an established protocol described previously (20). In brief, mice were lightly anesthetized with isofluorane inhalation (methoxy-fluorane; Pittman-Moore, Mundelein, IL), and 100 μg (50 μl) of Aspergillus fumigatus (Greer Laboratories, Lenoir, NC) or 50 μl of normal saline alone was applied to the nares using a micropipette with the mouse held in the supine position. After instillation, mice were held upright until alert. After three treatments per week for 3 wk, mice were killed between 18 and 20 h after the last intranasal challenge.
Mast cell analysis.
The 5-μm esophageal paraffin tissue sections were deparaffinized and stained with hexazonized new fuchsin (Sigma-Aldrich, St. Louis, MO) with 4% sodium nitrate in naphthol-AS-D chloroacetate (Sigma-Aldrich) and phosphate-buffered saline solution for 30 min and counterstained with hematoxylin. In addition, Giemsa and Toluidine blue staining was also performed to identify degranulated and activated mast cells in the tissue sections. The tissue sections were stained for 15 min with either Giemsa or Toluidine reagent (Fisher Scientific, Pittsburgh, PA) followed by rinsing with running water of the tissue slides for 7–10 min. The histological analysis was performed using light microscopy.
Eosinophil analysis in the esophagus.
The 5-μm esophageal paraffin tissue sections were immunostained with antiserum against mouse eosinophil major basic protein (anti-MBP) as described earlier (20–22). In brief, endogenous peroxidase in the tissues was quenched with 0.3% hydrogen peroxide in methanol followed by nonspecific protein blocking with normal goat serum. Tissue sections were then incubated with rat anti-MBP (1:2,000) overnight at 4°C (kindly provided by Drs. James and Nancy Lee), followed by a 1:200 dilution of biotinylated goat anti-rat IgG secondary antibody and avidin-peroxidase complex (Vector Laboratories, Burlingame, CA) for 30 min each. These slides were further developed with nickel diaminobenzidine-cobalt chloride solution to form a black precipitate and counterstained with nuclear fast red. Negative controls included replacing the primary antibody with normal rabbit serum to check endogenous biotin and peroxidase activity.
Quantification of eosinophils and mast cells.
Eosinophils and mast cells were quantified by counting the positively stained cells on each tissue section with the assistance of digital morphometry and expressed as cells/mm2 tissue area as described earlier (21, 23).
Mast cell protease-1 analysis.
Mast cell protease (MMCP)-1 plasma and tissue levels were measured by using commercially available ELISA (Moredun Scientific, Midlothian, UK) following manufacturer's instructions and protocol as reported previously (6). Esophageal 10% homogenates were prepared by homogenizing each esophagus of saline and Aspergillus-challenged mice in phosphate buffer, pH 7.2; the tissue homogenates were centrifuged at 100 g for 2 min, and supernatants were collected for ELISA assay. The ELISA plate (DYNEX Technologies, Chantilly, VA) was coated with capture antibody diluted to 2 μg/ml in 0.1 M carbonate buffer pH 9.6 overnight at 4°C. Mouse serum, tissue samples, and purified mouse standards were applied to the plate. The plate was incubated 2 h at room temperature, washed, and incubated with optimally diluted conjugate. Finally, tetramethylbenzidine substrate solution (BD Biosciences, San Diego, CA) was added to each well, and the color was developed in the dark at room temperature. The reaction was stopped using 0.25 M H2SO4, and optical density was determined at 450 nM. The MMCP-1 concentration of each sample was calculated based on a standard curve.
Mast cell culture and reconstitution in WWv mice.
Mouse bone marrow cells were harvested in RPMI media from both the femur and tibia. Cells were centrifuged at 250 g for 5 min; cell pellets were resuspended and counted. The total cells were cultured with 30 ng/ml of IL-3 (eBioscience, San Diego, CA) for 4 wk. The cultured mast cells (96% pure at 107 cells/mouse) were reconstituted into the mast cell-deficient mice by tail vain injection. Three months after mast cell injection in mast cell-deficient mice along with sex- and age-matched wild-type mice, the mice were challenged with saline or Aspergillus following the experimental EoE protocol described above. The mast cell reconstitution in each mouse was confirmed by performing chloroacetate staining of the paraffin-embedded ear sections. The numbers of mast cells in the ear sections were detected in mast cell-reconstituted WWv mice.
Analysis of muscle cell hyperplasia in the esophagus.
To determine the degree of cellular hyperplasia, 5′-bromodeoxyuridine (BrdU) (Zymed Laboratories, San Francisco, CA) incorporation analysis was performed as previously reported (20, 23). In brief, saline- or Aspergillus-challenged mice were injected intraperitoneally with 0.25 ml of 5′-BrdU (0.75 μg), 24 h before death. The esophagus was fixed with 10% neutral buffered formalin (Sigma-Aldrich) for 24 h. After fixation, the tissue was embedded in paraffin, and a 5-μm section was processed using standard histological approaches. Tissues were digested with trypsin (0.125%) for 3 min at 37°C followed by incubation for 45 min at room temperature. Sections were washed with PBS three times for 2 min and further incubated with monoclonal biotinylated anti-BrdU antibody for 60 min at room temperature. Negative controls included replacing the primary antibody with PBS and manufacturer-provided positive control. BrdU nuclear incorporated-positive cells were detected with streptavidin-peroxidase and DAB substrate (Zymed Laboratories) followed by counterstaining with hematoxylin. The muscle cell hyperplasia was analyzed by counting the percentage of BrdU+-positive cells in the muscle cell mucosa of the esophagus.
Statistical analysis.
Data are expressed as means ± SD. Statistical significance comparing different sets of mice was determined by unpaired InStat GraphPad t-test software (InSat Software, San Diego, CA). A P value <0.05 was considered statistically significant.
RESULTS
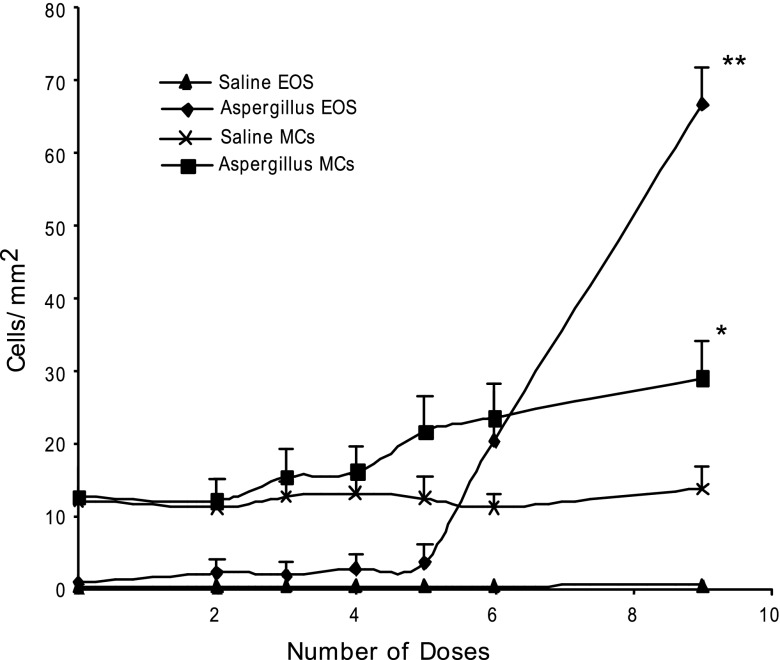
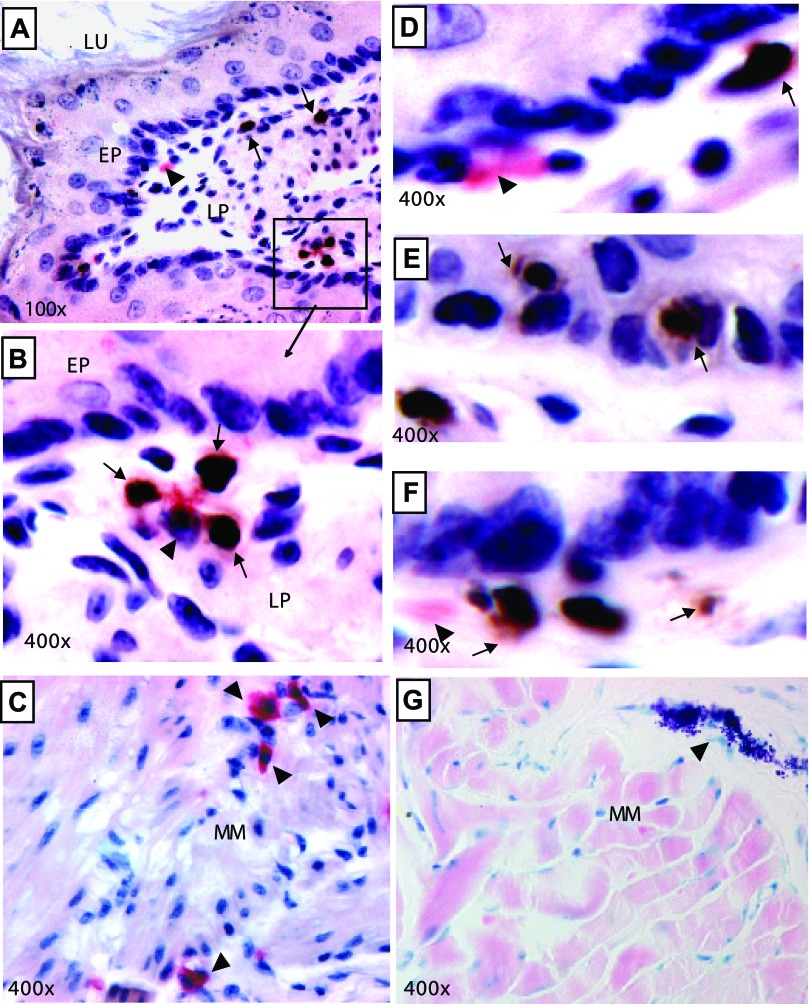
Mast cell numbers are increased in experimental EoE. It is well reported that mast cells and eosinophils are induced in human and experimental EoE; however, it is not yet clear whether mast cell recruitment in EoE is an eosinophil response or an allergen-induced response. Therefore, we were interested in determining whether mast cell levels increase in parallel with eosinophilia following aeroallergen exposure. Accordingly, a kinetic analysis of eosinophils and mast cells in the esophagus was performed following intranasal allergen challenge to the mice. Mice received intranasal saline or Aspergillus fumigatus extract separated by 24 h and were killed 18–20 h after zero, two, three, four, five, six, and nine doses. Eosinophil and mast cell numbers in the esophageal tissue sections were determined following anti-MBP and chloroacetate staining, respectively. Both eosinophil and mast cell levels increased following four doses of allergen and continued to rise following subsequent allergen exposure (Fig. 1). Mast cell numbers were increased from 12.5 ± 4/mm2 in saline to 28.8 ± 5/mm2 (means ± SD, n = 10 mouse/data point, P < 0.001) following nine allergen challenges. Mice given intranasal saline did not show any increase in the levels of mast cells at any time point. The histopathological analysis of esophageal tissue sections identified mast cells in the lamina propria of allergen-challenged mice along with the eosinophils. Both eosinophils and mast cells were detected gathering in the same region of esophageal lamina propria of allergen-challenged mice (Fig. 2, A and B). Interestingly, a number of mast cells was detected in the muscle cell mucosa of allergen-challenged mice compared with the saline-challenged mice (Fig. 2C). Additionally, intraepithelial mast cells and eosinophils including extracellular granules of eosinophils and mast cells were noted (Fig. 2, D–F). Activated degranulating mast cells were detected in the muscular mucosa of allergen-challenged wild-type mice (Fig. 2G). Interestingly, no significant change in the number of mast cells was observed in the other segment of the gastrointestinal tract (i.e., stomach or jejunum) in the mouse model of allergen-induced EoE (data not shown).
Fig. 1.
Kinetic analysis of mast cells (MCs) and eosinophils (EOS) in allergen-induced eosinophilic esophagitis (EoE). Mice were exposed to 9 intranasal Aspergillus fumigatus challenges, 3 times a week for 3 wk. The level of MCs and EOS in the esophagus 10–20 h after 2, 3, 4, 5, 6, 8, and 9 challenges was quantified by performing morphometric analysis following chloroacetate tissue staining and anti-major basic protein tissue immunostaining. The data are expressed as means ± SD of 3 experiments, n = 9 mice each time point, *P < 0.001, **P < 0.0001.
Fig. 2.
Esophageal eosinophil and mast cell localization in experimental EoE. A: histopathological representative photomicrograph of the localization of eosinophils and mast cells in the lamina propria of allergen-challenged wild-type mice (original magnification is ×100, eosinophils are represented by the arrow and mast cells by the arrow head). B: mast cell and eosinophil tissue colocalization in a high magnification (×400) of inserted area. C: allergen-induced chloroacetate-stained mast cell accumulation in the muscular mucosa. The intraepithelial mast cells (D), eosinophils (E), eosinophil degranulation (granules pointed by arrow) (F), and degranulating muscular mucosal mast cell are shown following Toluidine blue staining, ×400 (G). LU, lumen; EP, epithelium, LP, lamina propria; MM, muscular mucosa.
Mast cell recruitment in the esophagus is independent of IL-5.
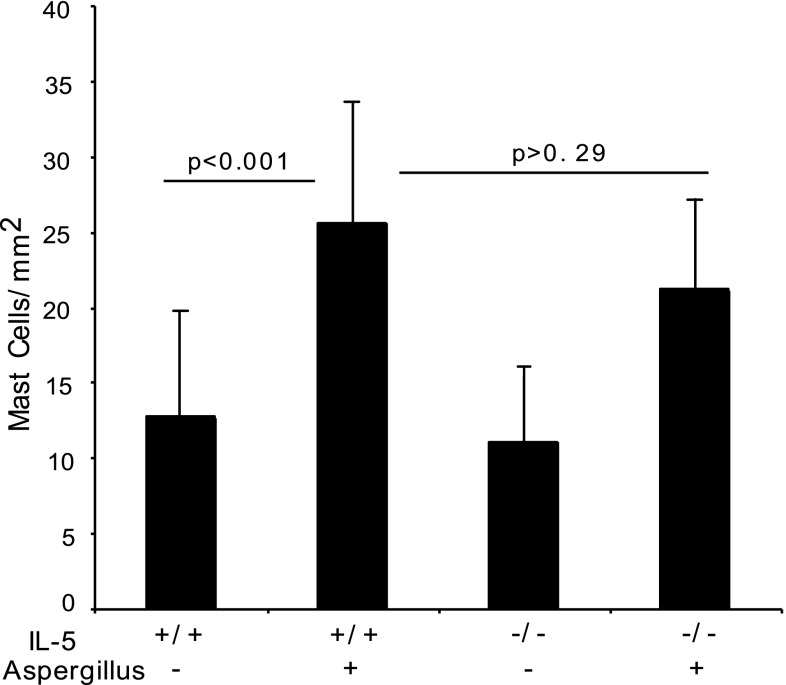
Allergen-challenge induces IL-5 in the esophagus (24), and eosinophil accumulation in the esophagus is dependent on IL-5 (20); therefore, we were interested in testing the hypothesis whether IL-5 also influences mast cell accumulation in the esophagus following allergen-induced experimental EoE. We examined mast cell numbers in wild-type and IL-5 gene-deficient mice following allergen challenge. A comparable level of mast cells in allergen-challenged IL-5 gene-deficient and wild-type mice were observed (Fig. 3). The number of mast cells in the esophagus of allergen-challenged wild-type and IL-5 gene-deficient mice was 25.6 ± 8 and 21.2 ± 6/mm2 (mean ± SD, n = 10) compared with their saline-challenged controls, 12.6 ± 7 and 11 ± 5/mm2, respectively (mean ± SD, n = 10). In contrast, the number of eosinophils in the esophagus of allergen-challenged wild-type and IL-5 gene-deficient mice was 30.5 ± 10.8 and 0.2 ± 0.4/mm2 (mean ± SD, n = 8) compared with their saline-challenged controls, 0.3 ± 0.3 and 0.2 ± 0.2/mm2, respectively (mean ± SD, n = 8). These data indicate that IL-5 and eosinophils are not required for allergen-induced esophageal mastocytosis.
Fig. 3.
Quantification of esophageal mast cells in wild-type and IL-5-deficient mice. Mast cell numbers in the esophagus of saline- and allergen-challenged wild-type mice and IL-5-deficient mice were examined by performing morphometric analysis. Data are expressed as means ± SD, n = 10–11 mice (P < 0.001).
Esophageal eosinophil recruitment is independent of mast cells.
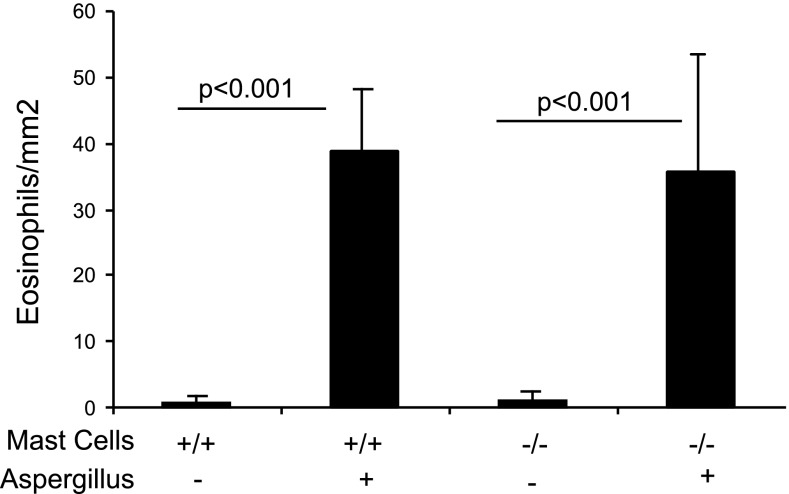
Earlier, it has been shown that mast cells influence eosinophil recruitment (29); therefore, we next tested the role of mast cells in the recruitment of eosinophils in allergen-induced experimental EoE. We induced experimental EoE in wild-type and mast cell-deficient WWv mice, and esophageal eosinophilia was examined. Eosinophil localization was observed in each segment of the esophagus, including the muscular mucosa of allergen-challenged wild-type and mast cell-deficient mice (data not shown). The quantification of eosinophils in wild-type and WWv mice showed similar levels of esophageal eosinophilic inflammation (Fig. 4). The number of eosinophils in the esophagus was 44 ± 11 and 1.33 ± 1.8/mm2 (mean ± SD, n = 10–12, P < 0.001) in allergen- and saline-challenged wild-type mice, respectively. Similarly, the eosinophil numbers in WWV allergen- or saline-challenged mice were 40.6 ± 13.7/mm2 and 1.3 ± 2.1/mm2 (mean ± SD, n = 12/group, P < 0.001), respectively. No mast cells were detected by performing chloroacetate staining in saline- or allergen-challenged WWV mice.
Fig. 4.
Allergen-induced eosinophil accumulation in mast cell-deficient mice. WWv mice along with wild-type controls were challenged with saline and Aspergillus antigen. Eosinophil numbers in the esophagus were analyzed and shown. Data are expressed as means ± SD (n = 12 mice). Wild-type (+/+), WWv (−/−).
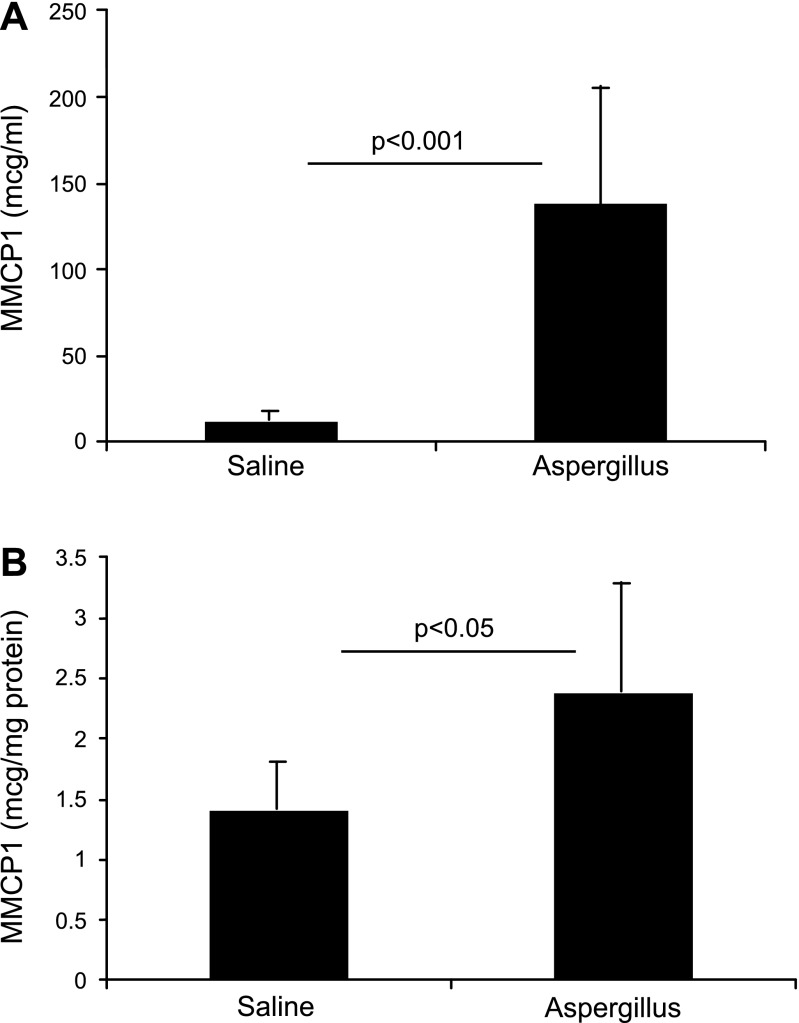
MMCP-1 level is increased in the blood and esophagus following allergen-induced experimental EoE.
Because degranulated mast cells were detected in the esophagus in experimental EoE, we next examined the mast cell mediators in the mice following the induction of EoE. The MMCP-1 levels in the blood and esophageal homogenate of saline- and allergen-challenged mice were measured. We detected ∼12-fold increase of MMCP-1 levels in the blood (Fig. 5A) and only ∼2-fold increase in the esophageal homogenates (Fig. 5B) of allergen-challenged mice compared with saline-challenged mice. The levels of MMCP-1 in the blood were 11.9 ± 1.6 and 138.1 ± 59.3 μg/ml (mean ± SD, n = 10, P < 0.001), whereas MMCP-1 in the esophagus was 1.4 ± 0.3 and 2.4 ± 0.6 μg/ml (mean ± SD, n = 10/group, P < 0.05) following saline and allergen challenge, respectively. The approximately twofold increase in MMCP-1 in the esophagus is consistent with the greater than twofold increase of mast cells in the esophagus.
Fig. 5.
Mast cell protease (MMCP)-1 levels in the blood and esophagus following induction of experimental EoE. MMCP-1 was measured in the blood and esophageal homogenates of saline- and allergen-challenged mice. The levels of MMCP-1 are shown in the blood (A) and the esophagus (B). The data are expressed as means ± SD, n = 10 mice.
Esophageal muscular cell hyperplasia is mediated by mast cells in experimental EoE.
We observed an increased number of mast cells in the esophageal lamina propria and mast cell infiltration into the muscular mucosa. Therefore, we next tested the hypothesis whether mast cells have a role in the induction of muscle cell hyperplasia, resulting in hypertrophy in EoE. Accordingly, we induced experimental EoE in wild-type and WWv mice. A thickened muscular mucosa and a number of BrdU+ cells were detected in muscular mucosa of allergen-challenged wild-type mice. The BrdU+ cells were commonly detected in the epithelial mucosa and rarely found in muscular mucosa of saline-challenged mice (Fig. 6, A and B). The allergen-challenged wild-type mice showed a significant increase in BrdU+ muscle cells and the muscular mucosa thickness compared with the saline-challenged wild-type and WWV mice (Fig. 6, C and D). The histological photomicrograph clearly demonstrates muscular hypertrophy following allergen challenge as a bundle of smooth muscles separated by fibrous septa and smooth muscle fibers with enlarged and irregular nuclei in the mucosa (Fig. 6B). The morphometric analysis revealed that BrdU+ cells in the muscular mucosa of allergen-challenged wild-type and WWv mice were 17.5 ± 12.0/mm2 and 2.1 ± 4.4/mm2 compared with a nondetectable value in saline-challenged wild-type and WWv mice (mean ± SD, n = 12).
Fig. 6.
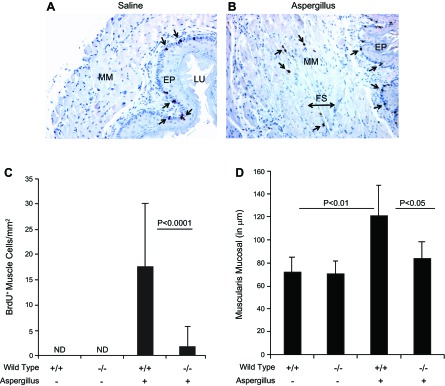
Morphometric quantification of muscular cell hyperplasia and hypertrophy. The 5′-bromodeoxyuridine (BrdU)+ cell quantification and muscular mucosa thickening was measured in saline- and allergen-challenged wild-type and mast cell-deficient mice. Representative photomicrographs (×125 original magnification) show BrdU+ cells in the epithelial mucosa of saline-challenged mice (A) and in the epithelium and muscular mucosa of allergen-challenged mice (B). The arrows in both the figures indicate the BrdU+ cells. The quantification of BrdU+ cells was performed in saline- and allergen-challenged mice by performing morphometric analysis and is shown in C, and the quantification of BrdU+ cells in saline-and allergen-challenged wild-type mice are shown in D. The data are expressed as means ± SD, n = 12 mice. FS, fibrous septa. Wild-type (+/+), WWv (−/−), Aspergillus (+), saline (-).
Reconstitution of mast cells in the WWv shows comparable muscle cell hyperplasia and hypertrophy.
Furthermore, to establish whether mast cells have a critical role in esophageal muscle cell hyperplasia and hypertrophy in EoE, we generated bone marrow-derived mast cells and adoptively transferred in mast cell-deficient WWv mice. Both wild-type mice and mast cell-reconstituted WWV mice were challenged with Aspergillus extract to induce experimental EoE. Each mast cell-reconstituted WWv mouse was examined for mast cells before being included in the experiment as stated in materials and methods, and, following the allergen challenge, both wild-type and mast cell-reconstituted WWv mice indicated mast cells in the esophagus (Fig. 7, A and B). Our analysis showed comparable BrdU+ esophageal muscle cells and muscular mucosa thickness in allergen-challenged wild-type mice and mast cell-reconstituted WWV mice (Fig. 7, C and D). The morphometric analysis revealed that BrdU+ cells in the muscular mucosa of wild-type and mast cell-reconstituted WWv mice were 20.8 ± 7.4/mm2 and 17.6 ± 8.3/mm2 compared with 1.1 ± 2.3/mm2 and 3.3 ± 4.7/mm2 in saline-challenged wild-type and mast cell-reconstituted WWv mice (mean ± SD, n = 6–8). Furthermore, the muscular mucosa thickness in wild-type and mast cell-reconstituted WWv mice was 119.3 ± 22.8/μm and 98.6 ± 20.7/μm compared with 78.4 ± 18.2/μm and 61.7 ± 5.9/μm in saline-challenged wild-type and mast cell-reconstituted WWv mice (mean ± SD, n = 6–8).
Fig. 7.
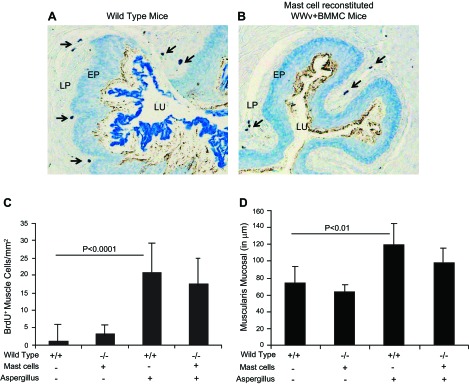
Comparable muscular cell hyperplasia and thickened muscular mucosa in wild-type and mast cell-reconstituted WWV mice. The allergen-challenged wild-type and mast cell-reconstituted WWv mice esophagus were examined for the mast cells by Toluidine blue staining, and a representative photomicrograph (×125 original magnification) is shown (A and B). Mast cells in wild-type mice and reconstituted in WWv mice are shown by the arrow in both photomicrographs (A and B). The BrdU+ cell quantification and muscular mucosa thickening was measured in saline- and allergen-challenged wild-type and mast cell-reconstituted WWv mice. The morphometric quantification of BrdU+ cells (C) and the muscular mucosa thickness in saline- and allergen-challenged wild-type and mast cell-reconstituted mice are shown (D). The data are expressed as means ± SD, n = 10 mice. [Mice type: wild-type (+/+), WWv (−/−)]. Mast cell reconstitution indicated by (+) and no mast cell reconstitution (-). Aspergillus challenge (+), saline challenge (-).
DISCUSSION
The pathogenesis of EoE is largely focused on the role of allergens, allergen-induced Th2 cytokines, chemokines including esophageal eosinophils, and antigen-induced antibodies (20, 21, 23). Herein, we report that esophageal mast cells increase in parallel with eosinophils in a dose- and time-dependent manner in experimental EoE, which is in accordance with the a number of earlier reported studies of human EoE (2, 9, 17, 19, 25, 30). We earlier showed that the esophagus is devoid of eosinophils (22), but mast cells are resident cells in the esophagus. Our present study demonstrated that two different pathways are operational in mast cells and eosinophil recruitment in EoE. Earlier, few reports indicated that mast cells produce eosinophil chemotactic factor, eotaxin-1 (8, 32); therefore, our study tested whether esophageal mast cells are responsible for the esophageal eosinophilia in allergen-induced EoE. We demonstrated that the recruitment of allergen-induced esophageal eosinophilia is independent to the mast cell function in EoE. Additionally, we also demonstrated that mast cell induction in allergen-induced EoE is independent to IL-5, in contrast to esophageal eosinophilia that is largely dependent on IL-5 (20). However, the tissue distribution of eosinophils and mast cells in the EoE indicated a possible crosstalk between these two types of inflammatory cells. Our data provide the evidence and demonstrate that mast cells localize near the eosinophils in the lamina propria, and we show extracellular mast cell granules and MBP immunoreactivity along with the increase of mast cell product MMCP-1 in the esophagus in allergen-induce EoE. The level of MMCP-1 is increased more in the blood compared with the esophagus and is not suggestive of mast cell systemic response. MMCP-1 also is released in the blood from other organs like lung and skin. This evidence indicates that mast cells may have a critical role in EoE pathogenesis.
These observations further reinforced our interest in exploring the role of mast cells in the EoE pathogenesis, particularly the muscle cell hyperplasia and hypertrophy. Because mast cells are infiltrated into the muscular mucosa and a number of degranulating mast cells were detected in the esophageal muscular region following the induction of allergen-induced EoE, we therefore largely focused our studies on the mast cell role in promoting muscular cell hyperplasia and hypertrophy in experimental EoE. Induced BrdU+ cells were detected in the muscular mucosa of allergen-challenged wild-type mice, whereas no increase was observed in the allergen-challenged mast cell-deficient WWv mice. It is interesting to note that muscle cell hyperplasia is observed in muscularis mucosa; however, we also observed few mast cells infiltrating the thin smooth muscle layer of allergen-challenged wild-type mice, but not the WWv mice. The thickness of smooth muscle cells is also changed in wild-type mice compared with WWv mice but not quantitated separately (data not shown). This observation explains our earlier findings why esophageal peristaltic dysfunction is independent of esophageal eosinophilia in a chronic mouse model of EoE (19) and explains the significance of induced mast cell function in EoE. Furthermore, to establish that mast cells were responsible to the muscle cell hyperplasia and hypertrophy, we reintroduced bone marrow-derived mast cells by adoptive transferring into the in WWv mice, which reinstated the wild-type phenotype. The adoptively transferred bone marrow-derived mast cells in WWv mice and wild-type mice showed comparable levels of BrdU+ cells in the muscular mucosa following the induction of allergen-induced EoE. These studies provide the significance to the findings of increased mast cells and extracellular MBP immunoreactivity in the esophageal biopsies of patients with EoE and draw attention to the pathogenic role of increased mast cells in human EoE (26). Notably, mast cells have long been considered to play a significant role in the pathophysiology of allergic diseases through their ability to release a host of pleiotropic autacoid mediators, proteases, and cytokines in response to activation by both IgE-dependent and diverse nonimmunological stimuli. They are the source of several neutral proteases, such as tryptase and chymase, which interact with many cells and potentially contribute to the tissue-remodeling process (5). Earlier, it has been shown that tryptase and chymase are induced in human EoE (1); however, the significance of these mast cell mediators is not well understood. We provide for the first time evidence that mast cells are infiltrated and degranulated in the muscular mucosa of the esophagus in experimental EoE. The tryptase and chymase are the degranulating proteins of the mast cells. Therefore, the possibility is that these mast cell-degranulating proteins may influence the remodeling process in the esophageal muscular mucosa that promotes muscle cell hyperplasia and hypertrophy. Furthermore, the evidence that mast cell-deficient mice do not promote muscle cell hyperplasia in experimental EoE and reconstitutions of mast cells in mast cell-deficient mice again restores the wild-type mice phenotype in experimental EoE, further supporting the significant role of mast cells in promoting remodeling of muscular mucosa in allergen-induced EoE. In addition, these studies further support our most recent report that mast cells may be responsible for the induction of esophageal functional abnormalities such as esophageal peristaltic dysfunction in experimental EoE (19). Notably, mast cell mediators in mouse are different than those in humans and guinea pig. In mouse, activated mast cells release more serotonin than histamine, whereas, in human and guinea pig, mast cells release more histamine. It has been earlier shown that histamine plays an important role in attracting eosinophils in the airway; however, this observation is not yet proven in esophagus. Therefore, it may be possible that, in human EoE, mast cells may have a role in esophageal eosinophil accumulation. Of note, using WWv mice may have some limitations, including their sterility low numbers of neutrophils and basophils (28). However, the roles of these cells are not examined, but we ruled out the role of these cells in promoting muscle cell hyperplasia and hypertrophy in EoE because our mast cell-reconstituted WWv mice show a wild-type phenotype following the induction of allergen-induced experimental EoE.
In conclusion, this study suggests an important role for mast cells in EoE pathogenesis in a number of ways. First, in experimental EoE, esophageal mast cell numbers increase and their accumulation is independent of IL-5. Second, an increase of mast cell mediators in the blood and esophagus can be seen, at least in the setting of allergen-induced experimental EoE. Third, mast cells infiltrate and degranulate into the esophageal muscular mucosa. Lastly, mast cells have a critical role in muscular cell hyperplasia and possibly esophageal functional impairment in EoE. Taken together, the present study directly implicates mast cells in the pathogenesis of EoE.
GRANTS
This work was supported by in part by NIH R01 DK067255 (A. Mishra) and NIH R01 AI080581 (A. Mishra).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.N., P.M., and M.R. performed experiments; R.N. and S.D. prepared figures; S.D. analyzed data; A.M. conception and design of research; A.M. drafted manuscript.
ACKNOWLEDGMENTS
We thank Drs. James and Nancy Lee (Mayo Clinic, Scottsdale, AZ) for the generous supply of anti-MBP. We also acknowledge Dr. Marc Rothenberg at CCHMC, Cincinnati, OH for providing IL-5 gene-deficient mice and Dr. Fabio Cominelli, Division of Gastroenterology and Liver Disease, for providing the facility at Case Western Reserve University, Cleveland to continue our EoE research program.
REFERENCES
- 1. Abonia JP, Blanchard C, Butz BB, Rainey HF, Collins MH, Stringer K, Putnam PE, Rothenberg ME. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol 126: 140–149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa'ad AH, Putnam PE, Aronow BJ, Rothenberg ME. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 116: 536–547, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blank U, Jouvin MH, Guerin-Marchand C, Kinet JP. [The high-affinity IgE receptor: lessons from structural analysis]. Med Sci 19: 63–69, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol 25: 266–273, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol 117: 1277–1284, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest 112: 1666–1677, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Broide DH. Molecular and cellular mechanisms of allergic disease. J Allergy Clin Immunol 108: S65–S71, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Dahinden CA, Geiser T, Brunner T, Vontscharner V, Caput D, Ferrara P, Minty A, Baggiolini M. Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J Exp Med 179: 751–756, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dellon ES, Chen X, Miller CR, Fritchie KJ, Rubinas TC, Woosley JT, Shaheen NJ. Tryptase staining of mast cells may differentiate eosinophilic esophagitis from gastroesophageal reflux disease. Am J Gastroenterol 106: 264–271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fox VL, Nurko S, Furuta GT. Eosinophilic esophagitis: It's not just kid's stuff. Gastrointest Endosc 56: 260–270, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Furuta GT. Eosinophils in the esophagus: acid is not the only cause. J Pediatr Gastroenterol Nutr 26: 468–471, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol 23: 749–786, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol 6: 135–142, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Justinich CJ, Ricci A, Jr, Kalafus DA, Treem WR, Hyams JS, Kreutzer DL. Activated eosinophils in esophagitis in children: a transmission electron microscopic study. J Pediatr Gastroenterol Nutr 25: 194–198, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol 2: 773–786, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Kawakami T, Kitaura J, Xiao W, Kawakami Y. IgE regulation of mast cell survival and function. Novartis Found Symp 271: 100–107; discussion 108–114, 145–151, 2005 [PubMed] [Google Scholar]
- 17. Lucendo AJ, Lucendo B. An update on the immunopathogenesis of eosinophilic esophagitis. Expert Rev Gastroenterol Hepatol 4: 141–148, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol 114: 21–27, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Mavi P, Rajavelu P, Rayapudi M, Paul RJ, Mishra A. Esophageal functional impairments in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 302: G1347–G1355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 107: 83–90, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol 168: 2464–2469, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest 103: 1719–1727, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 125: 1419–1427, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Mishra A, Wang M, Pemmaraju VR, Collins MH, Fulkerson PC, Abonia JP, Blanchard C, Putnam PE, Rothenberg ME. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology 134: 204–214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mulder DJ, Mak N, Hurlbut DJ, Justinich CJ. Atopic and non-atopic eosinophilic oesophagitis are distinguished by immunoglobulin E-bearing intraepithelial mast cells. Histopathology 61: 810–822, 2012 [DOI] [PubMed] [Google Scholar]
- 26. Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med 351: 940–941, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Rajavelu P, Rayapudi M, Moffitt M, Mishra A, Mishra A. Significance of para-esophageal lymph nodes in food or aeroallergen-induced iNKT cell-mediated experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 302: G645–G654, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends Immunol 33: 613–625, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Romagnani P, De Paulis A, Beltrame C, Annunziato F, Dente V, Maggi E, Romagnani S, Marone G. Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. Am J Pathol 155: 1195–1204, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sridhara S, Ravi K, Smyrk TC, Kita H, Kephart GM, Weiler CR, Katzka DA. Increased numbers of eosinophils, rather than only etiology, predict histologic changes in patients with esophageal eosinophilia. Clin Gastroenterol Hepatol 10: 735–741, 2012 [DOI] [PubMed] [Google Scholar]
- 31. Teitelbaum JE, Fox VL, Twarog FJ, Nurko S, Antonioli D, Gleich G, Badizadegan K, Furuta GT. Eosinophilic esophagitis in children: Immunopathological analysis and response to fluticasone propionate. Gastroenterology 122: 1216–1225, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest 116: 1633–1641, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]