Abstract
The iron-diabetes hypothesis proposes an association between iron overload and glucose metabolism that is supported by a number of epidemiological studies. The prevalence of type 2 diabetes in patients with hereditary hemochromatosis and iron-loading thalassemia supports this hypothesis. The Belgrade rat carries a mutation in the iron transporter divalent metal transporter 1 (DMT1) resulting in iron-loading anemia. In this study, we characterized the glycometabolic status of the Belgrade rat. Belgrade rats displayed normal glycemic control. Insulin signaling and secretion were not impaired, and pancreatic tissue did not incur damage despite high levels of nonheme iron. These findings suggest that loss of DMT1 protects against oxidative damage to the pancreas and helps to maintain insulin sensitivity despite iron overload. Belgrade rats had lower body weight but increased food consumption compared with heterozygous littermates. The unexpected energy balance was associated with increased urinary glucose output. Increased urinary excretion of electrolytes, including iron, was also observed. Histopathological evidence suggests that altered renal function is secondary to changes in kidney morphology, including glomerulosclerosis. Thus, loss of DMT1 appears to protect the pancreas from injury but damages the integrity of kidney structure and function.
Keywords: iron overload, kidney
the iron-diabetes hypothesis is supported by a large body of epidemiological studies showing that body iron stores are positively associated with serum insulin and blood glucose levels (14, 33, 46). The high prevalence of type 2 diabetes observed in individuals with hereditary hemochromatosis (HH) further supports the idea that systemic iron loading is associated with impaired glucose metabolism (20, 32, 34, 37). However, the underlying pathological interactions between iron and glucose homeostasis are not well understood.
Iron homeostasis in vertebrates requires coordination between cells that export iron into plasma and those that take up iron for utilization or storage. Iron is an essential nutrient, but excessive iron accumulation can cause oxidative damage due to Fenton chemistry generating reactive oxygen species (ROS). Because regulated pathways for iron excretion are not known to exist, the major underlying concept in iron homeostasis is that dietary absorption is limited to meet needs and avoid toxicity (22).
Divalent metal transporter 1 (DMT1) plays a major role in regulating the iron absorptive capacity of villus enterocytes. The Belgrade rat (b/b) is a unique animal model that displays severe hypochromic microcytic anemia and hyperferrinemia (40). It harbors a point mutation (G185R) in the gene for DMT1, such that uptake of iron (and other divalent metals) across duodenal enterocytes is significantly reduced. Because DMT1 function is also required for uptake of iron into erythrocytes for hemoglobin production, impaired erythropoeisis results in excessive iron accumulation in serum (42) and liver (43). The unique iron status in b/b rats is reminiscent of the iron-loading condition observed in individuals with thalassemia. Iron loading is a critical problem for these patients, and the prevalence of impaired glucose tolerance and diabetes in this population ranges from 14.1 to 19.5% in different cross-sectional studies (7, 35).
The objective of this study was to characterize glucose metabolism in the Belgrade rat model with iron-loading anemia to better understand the relationships between glucose homeostasis and iron metabolism. Recent studies characterizing β-cell-specific DMT1 knockout mice suggest this transporter plays a role in ROS production in the pancreas in response to cytokines, a feature that contributes to metabolic syndrome and insulin resistance (19). Our investigation determined that b/b rats have normal glucose tolerance despite hyperferrinemia. During the course of this study, we found that Belgrade rats consume more food than heterozygous littermates despite their lower body weight. This unexpected phenotype was associated with increased urinary glucose excretion. Urinary loss of iron and other electrolytes was also observed. Histopathology reveals that effects on renal function are most likely secondary to glomerulosclerosis observed in Belgrade rat kidneys. This damage does not appear to result from tissue iron overload in kidneys; hence, these results suggest DMT1 may play an important role in renal physiology, an idea supported by the fact that this transporter is highly expressed in kidneys (12, 47).
MATERIALS AND METHODS
Animals and diets.
Animal protocols were approved by the Harvard Medical Area Animal Care and Use Committee. Belgrade rats were maintained on a 12-h light-dark cycle and consumed food and water ad libitum. Because of their phenotypic anemia, breeding animals were maintained on an iron-supplemented diet to improve husbandry (17). Mating pairs of female heterozygotes (+/b) and male Belgrade (b/b) rats were fed chow containing 480–600 mg iron/kg (TD 02385; Harlan Teklad). Female +/b rats were fed the iron-supplemented diet throughout pregnancy. At postnatal day 3–6, litters were cross-fostered to F344 Fischer dams (+/+; Charles River) fed with a standard diet containing 220 mg/kg iron (PicoLab 5053; PharmaServ). The b/b and +/b groups were established at the time of weaning and fed with the iron-supplemented diet. Belgrade rat genotype was verified by both visual discrimination and PCR genotyping (49). Body weight and daily food intake were measured two times a week from 3 to 18 wk old. Dietary iron overload was induced as previously described (45). Weanling Fischer F344 rats (Charles River) were fed an iron-loading diet containing 1% carbonyl iron (TD 09077; Harlan Teklad) or a diet with 50 mg iron/kg (TD 07800; Harlan Teklad) for 4 wk. Unlike the experimental Belgrade rats, which consumed more chow than controls, carbonyl iron-fed rats consumed less chow, and therefore control rats for this group were pair-fed. Daily food consumption by rats fed the carbonyl iron diet was measured two times a day, and the amount of food provided to the control group was adjusted accordingly to maintain similar body weights between both groups. Hematocrits and nonheme iron concentrations in liver, pancreas, and kidney were measured as previously described (42). Serum iron was measured as described (6) except that bathophenanthroline sulfonate was used. Total muscle iron content was determined from emission readings at the iron 238.204 emission line using Inductively-Coupled Plasma Optical Emission Spectroscopy (ICP-OES, Optima DV 2000; PerkinElmer).
Metabolic analyses.
At 12 wk of age, blood was collected after 6 h fasting for insulin and glucose determinations. Serum insulin was measured using a rat insulin assay kit according to the manufacturer's instructions (Meso Scale Discovery). Blood glucose was measured through the tail tip using the OneTouch Ultra Glucose-Monitoring System (Lifescan). Glucose tolerance tests (GTT) and insulin tolerance tests (ITT) were performed when rats were 12 wk old. The right jugular vein was catheterized under isoflurane anesthesia, using Micro-Renathane tubing (Braintree Scientific, Braintree, MA). Specifically, a small skin incision (1 cm) was made over the right jugular vein using a scalpel. The subcutaneous connective tissue was cleared by blunt dissection to expose the vessel. After a 7- to 8-mm section of the vessel was isolated, a loose ligature was placed caudally, and the cranial end of the vein was ligated. A small incision using a microscissor was made between the ligatures into which the catheter was inserted. The catheter (R-JVC-M37) was secured in place by tying the loose ligature around the catheterized vessel. A midscapular incision (1 cm) was made in the scapular region to serve as the exit site of the catheter. The free end of the catheter was exteriorized dorsally, which allowed GTT or ITT experiments to be carried out under unanesthetized and unrestrained conditions after 48 h recovery. On the day of experiment, animals were fasted for 6 h before intraperitoneal injection of d-glucose (1.5 g/kg) or insulin (1 IU/kg; Eli Lilly, Indianapolis, IN). Blood samples were obtained from the catheter at baseline and 10, 20, 30, 45, 60, and 90 min following injection. The levels of glucose or insulin were then determined for samples drawn at these time points.
Urinary glucose and electrolyte determination.
After 6 h fasting, b/b or +/b rats received intraperitoneal injection of d-glucose (1.5 g/kg), and urine samples were collected in metabolic cages for the next 6 h. Urinary glucose was measured using a glucose assay kit (Sigma, St. Louis, MO) following the manufacturer's instruction with the following modification. To control for the background of urine color, urine samples were mixed with equivalent amounts of working solution and stop solution (12 N H2SO4) to be used as negative controls during analysis. Absorption at 540 nm was determined with a BioTek plate reader (BioTek Instrument). Other urinary electolytes were determined after 24 h urine collection. The urinary concentrations of Cl, Na, K, and Ca were determined at the Kansas State Veterinary Diagnostic Laboratory.
Insulin signaling.
Rats were fasted for 6 h, anesthetized with isoflurane, followed by midline abdominal incision (1 cm) to expose the portal vein. Rats were then injected with insulin (1 IU/kg) or the same volume of saline under anesthesia. Fifteen minutes later, rats were killed with isoflurane overdose, and tissues were collected, snap-frozen, and stored in −80°C. Frozen skeletal muscle was homogenized in ice-cold lysis buffer containing 20 mM HEPES, pH 7.4, 100 mM KCl, 85 mM sucrose, 20 μM EGTA, 0.5% Nonidet P-40, and protease inhibitor (Complete Mini; Roche, South San Francisco, CA) and phosphatase inhibitor (Halt Protease Inhibitor Cocktails; Thermo Scientific, Rockford, IL). Protein samples were electrophoresed on 10–12% Bis-Tris gels (Bio-Rad, Hercules, CA) and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). Anti-phospho-Akt(Ser473) (1:1,000) or anti-Akt (1:1,000) antibodies (catalog nos. 4060 and 9272, respectively; Cell Signaling, Danvers, MA) were incubated overnight. Protein signals were visualized with secondary IR Dye 800 donkey anti-rabbit antibody (Li-Cor, Lincoln, NE) using a Li-Cor Odyssey infrared imaging system. The intensity of bands was quantified with the Odyssey Infrared Imaging System, and the ratio between pAkt/Akt was calculated to determine insulin-induced signaling.
Histology.
Fresh pancreas was fixed in Bouin's solution, and kidneys were fixed in 10% neutrally buffered formalin (pH 7.2) before embedding in paraffin. Each 5-μm-thick pancreas section was then stained with hematoxylin and eosin (H&E), and kidney sections were stained with periodic acid-Schiff (PAS). Briefly, to perform PAS staining, paraffin sections were deparaffinized, rinsed, and oxidized in 0.5% periodic acid solution. After incubation in Schiff reagent, sections were counterstained in hematoxylin. Images were acquired with a Zeiss Axiotome microscope equipped with Axiovision software.
Statistical analysis.
Reported values are means ± SE. Statistical significance was evaluated by two-tailed Student's t-test. Differences between +/b and b/b rats were considered significant at P < 0.05.
RESULTS
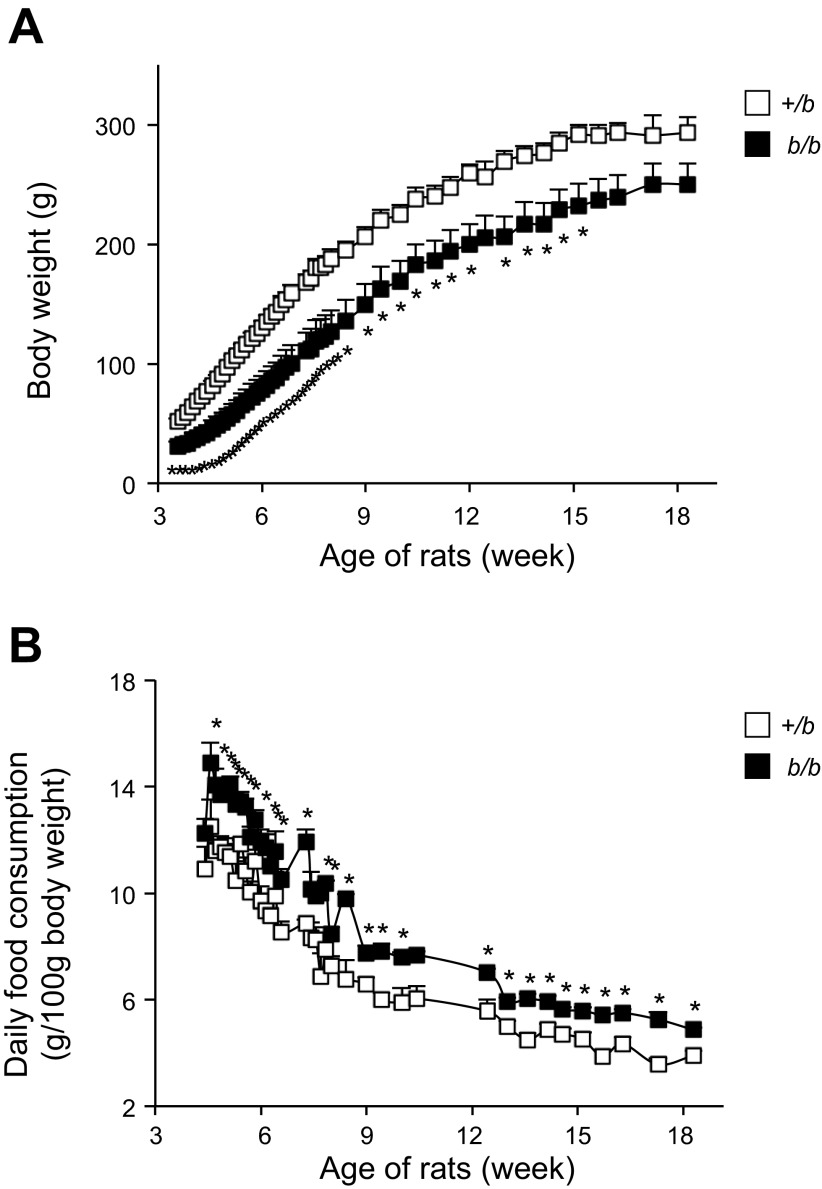
Characteristics of 12-wk-old Belgrade b/b rats and phenotypically null heterozygote littermate control (+/b) rats were compared (Table 1). Consistent with previous studies (43, 44), 12-wk-old b/b rats had reduced hematocrit values compared with +/b controls. Serum iron was increased threefold in the b/b group. These features arise from the mutation in DMT1, resulting in low iron uptake and ineffective erythropoiesis (42). Basal serum glucose and insulin levels in b/b rats were similar to +/b rats. In addition to lower birth weight (data not shown), Belgrade rats had significantly reduced body weight compared with +/b controls from 3 to 15 wk of age (n = 4/group; P < 0.05 for each time point up to 15 wk; Fig. 1A). Slopes of the growth curves were 3.7 ± 0.1 g/day and 3.1 ± 0.3 for +/b and b/b rats, respectively (means ± SE, P = 0.102). Daily food intake of b/b rats was greater than +/b rats (n = 4/group; P < 0.05 for each time point; Fig. 1B). These combined data indicate that Belgrade rats consume more food than control rats, and this difference continues over time.
Table 1.
Characteristics of 12-wk-old +/b and b/b rats
| +/b | n | b/b | n | P | |
|---|---|---|---|---|---|
| Serum iron, μg/ml | 1.6 ± 0.3 | 7 | 6.4 ± 0.5 | 7 | <0.001 |
| Hematocrit, % | 42.4 ± 1.4 | 8 | 30.6 ± 2.6 | 5 | 0.004 |
| Liver nonheme iron, μg/g | 32.0 ± 8.4 | 7 | 55.7 ± 17.0 | 7 | 0.140 |
| Blood glucose, mg/dl | 154 ± 10.9 | 6 | 138 ± 9.8 | 6 | 0.089 |
| Serum insulin, ng/ml | 1.13 ± 0.09 | 4 | 1.57 ± 0.31 | 6 | 0.177 |
Values are means ± SE; n, no. of rats. b/b, Belgrade rats; +/b, heterozygote rats.
Fig. 1.
Body weight (A) and daily food consumption per kg body wt (B) of Belgrade (b/b) and female heterozygote (+/b) rats. Data were recorded two times a week from 3 to 18 wk old. The food intake was adjusted by the body weight of each rat; n = 4 in both groups. *P < 0.05 vs. +/b in A at each time point. *P < 0.05 vs. +/b in B.
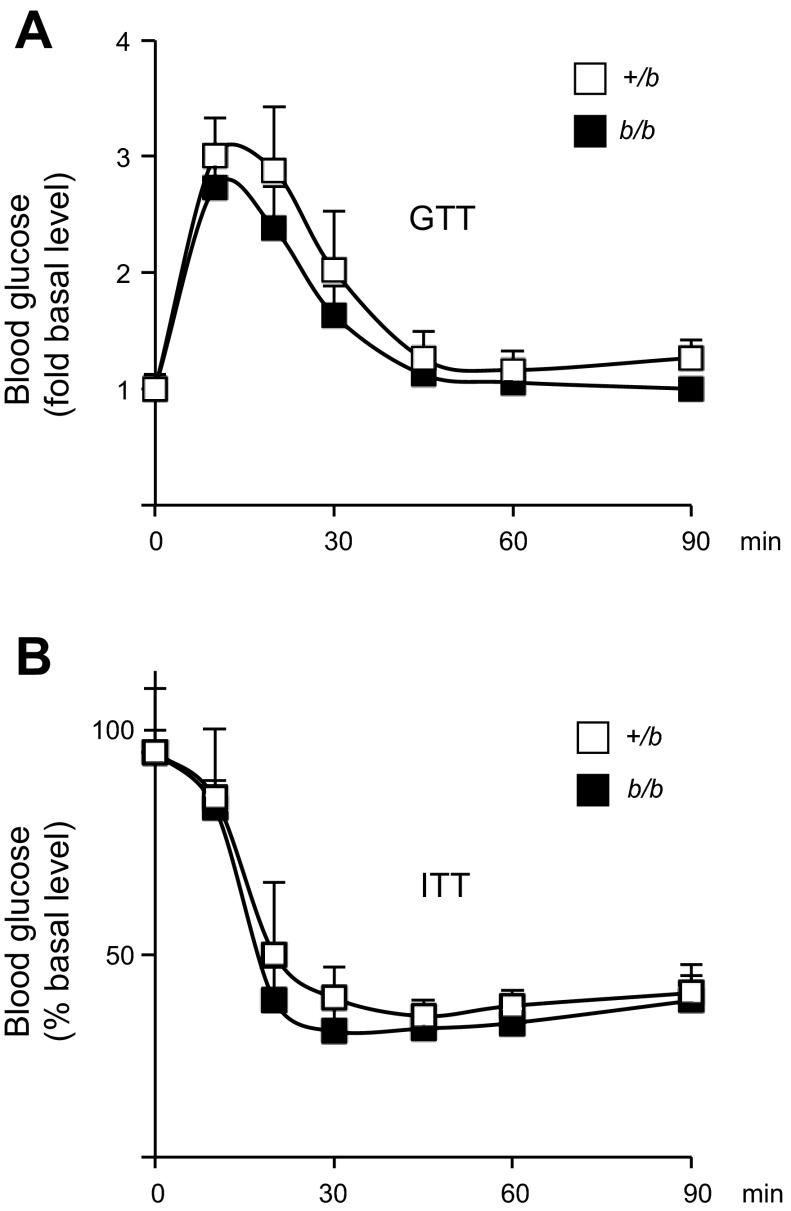
Typically, iron loading is associated with insulin resistance (5, 25). To further evaluate glucose homeostasis, GTT and ITT were performed. No significant difference was detected comparing +/b and b/b rats (Fig. 2, A and B). Insulin signaling was studied in skeletal muscle tissue collected from fasted rats after portal vein insulin injection. Phosphorylation of Akt, a downstream target of insulin action (27, 39), was measured by immunoblotting. The ratio between insulin-induced phosphorylation of pAkt(Ser473) and the total Akt level was calculated. Insulin-induced Akt phosphorylation in skeletal muscle was similar in b/b and +/b rats (Fig. 3A). Muscle tissue iron levels were also similar between the two groups (Fig. 3B). This result is consistent with studies that show muscle iron loading occurs only with advanced age in rats (48).
Fig. 2.
Glycometabolic status in +/b and b/b rats. Glucose tolerance test (GTT) (A) and insulin tolerance test (ITT) (B) were carried out after 6 h fasting. Twelve-week-old male b/b rats and age-matched +/b rats (n = 6 each group) were injected with d-glucose (1.5 g/kg body wt) or insulin (1 IU/kg body wt), and blood glucose was measured at 10, 20, 30, 45, 60, and 90 min after ip injection.
Fig. 3.
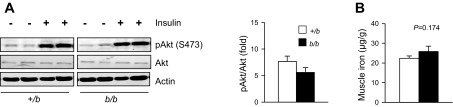
Insulin signaling in skeletal muscle of Belgrade rats. Phospho (p) Akt was determined by Western blot analysis (A), and the induced phosphorylation on Akt(Ser473) (S473) after insulin injection was compared with the total Akt level for b/b and +/b rats (n = 4 each group). Total Fe in skeletal muscle was measured by Inductively-Coupled Plasma Optical Emission Spectroscopy (B); n = 4 +/b and n = 3 b/b.
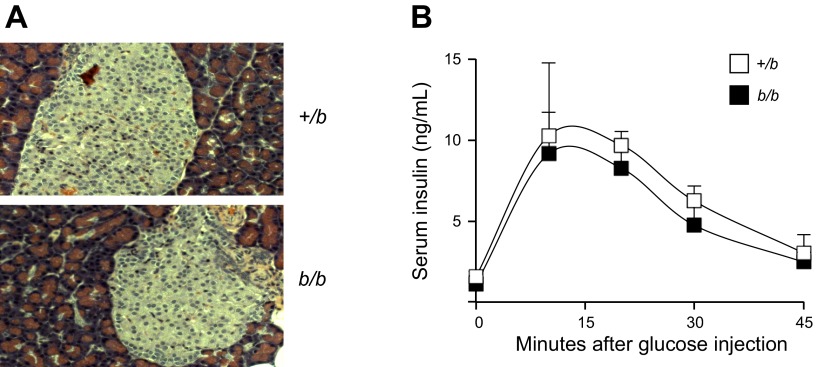
High serum iron is associated with tissue damage in the pancreas due to iron loading (24). However, no visible histological alterations were detected in H&E-stained pancreatic islets to indicate oxidative damage (Fig. 4A). To assess the glucose-induced insulin secretory capacity of the pancreas, insulin levels were determined in blood samples collected at 10-, 20-, 30-, and 45-min time points of the GTT. No significant difference was discerned in the average insulin level at each time point (Fig. 4B). These data, combined with the GTT and ITT results, indicate that pancreatic function and insulin sensitivity were not significantly affected by the excess iron deposition in Belgrade rat tissues.
Fig. 4.
Pancreatic function in Belgrade rats. Hematoxylin and eosin staining of pancreas in +/b and b/b rats is shown (A). Glucose-induced insulin secretion was assessed (B). Insulin levels were determined in blood samples collected at 0, 10, 20, 30, and 45 min after glucose injection during GTT assay; n = 6 for both groups in A and B.
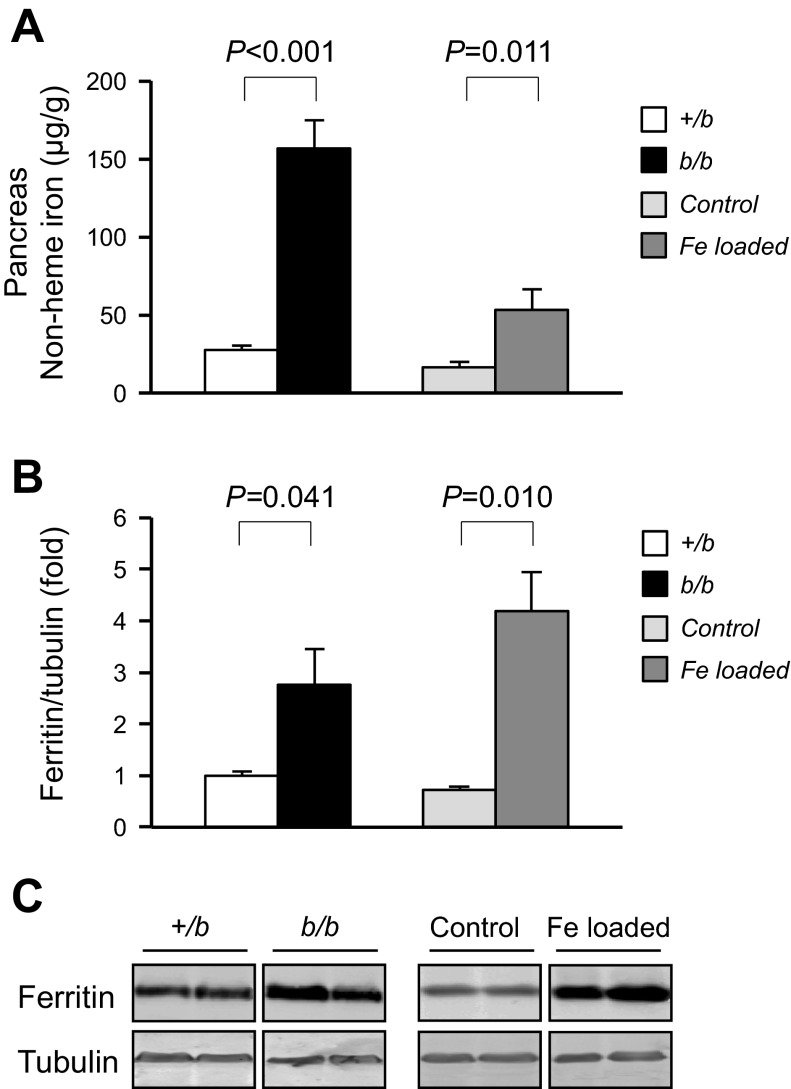
One protective mechanism that guards against oxidative tissue damage is the induction of ferritin synthesis. Ferritin levels are posttranslationally regulated through iron-responsive protein (IRP) control in response to increased cellular iron content (22). Ferritin mRNA contains a 5′-iron-responsive element that is bound by IRP under low iron conditions to prevent translation. Upon increased iron, IRP loses its RNA-binding activity, and synthesis of ferritin is promoted. Pancreatic nonheme iron levels were 5.6-fold higher in Belgrade rats compared with heterozygous littermate controls (Fig. 5A), and, correspondingly, tissue ferritin levels were increased 2.7-fold (Fig. 5, B and C).
Fig. 5.
Pancreatic nonheme iron levels and pancreatic ferritin expression. Pancreatic nonheme iron levels in +/b and b/b are shown (A), and they are compared with pancreatic levels of control rats and carbonyl iron-loaded rats; n = 6 +/b and b/b, n = 3 in control and iron-loaded rats. Ferritin was determined by Western blot analysis in +/b, b/b, control, and carbonyl iron-fed rats (B and C), n = 3 in each group.
These observations are consistent with previous studies of iron overload wherein increased pancreatic iron was associated with excess ferritin deposition (3, 31). However, in the latter studies, pancreatic tissue dysfunction was reported. To more directly compare similar conditions with the Belgrade rat model, the effects of dietary iron loading were studied in the strain background Fischer rat. To induce overload, a cohort of weanling rats was fed chow supplemented with 1% carbonyl iron for 4 wk. Age-matched control rats were pair-fed normal chow. Under these conditions, dietary iron loading resulted in liver nonheme iron levels that were substantially greater than in the 12-wk-old Belgrade rats (Table 2). Compared with age-matched rats pair-fed control chow, the carbonyl iron-loaded rat liver nonheme iron levels were 17.6-fold greater. Kidneys (Table 2) and pancreas (Fig. 5) also showed excess iron accumulation. Pancreatic dysfunction was noted in the marked decrease in serum insulin levels in carbonyl iron-loaded compared with control rats (0.13 ± 0.03 vs. 0.47 ± 0.04 ng/ml; n = 3, P = 0.010). Fasting glucose levels were not affected by loading as previously reported by May et al. (31). Like the Belgrade rat model, ferritin levels were induced in the pancreas due to iron loading (Fig. 5, B and C). Surprisingly, ferritin expression was comparable to that observed for Belgrade rats although the extent of pancreatic iron loading was much less in carbonyl iron-fed rats (Fig. 5, A and B).
Table 2.
Characteristics of 7-wk-old Fischer F344 rats fed 1% carbonyl iron or control diet
| Control | n | Fe Loaded | n | P | |
|---|---|---|---|---|---|
| Body wt, g | 155.0 ± 7.0 | 3 | 140.0 ± 5.2 | 3 | 0.160 |
| Hematocrit, % | 43.0 ± 3.6 | 3 | 36.6 ± 3.5 | 3 | 0.140 |
| Liver nonheme iron, μg/g | 41.2 ± 6.9 | 3 | 727.9 ± 63.5 | 3 | <0.001 |
| Kidney nonheme iron, μg/g | 42.35 ± 9.28 | 3 | 87.88 ± 12.29 | 3 | 0.041 |
| Blood glucose, mg/dl | 115.7 ± 9.8 | 3 | 119.7 ± 4.4 | 3 | 0.729 |
| Serum insulin, ng/ml | 0.47 ± 0.04 | 3 | 0.13 ± 0.04 | 3 | 0.003 |
Values are means ± SE; n, no. of rats.
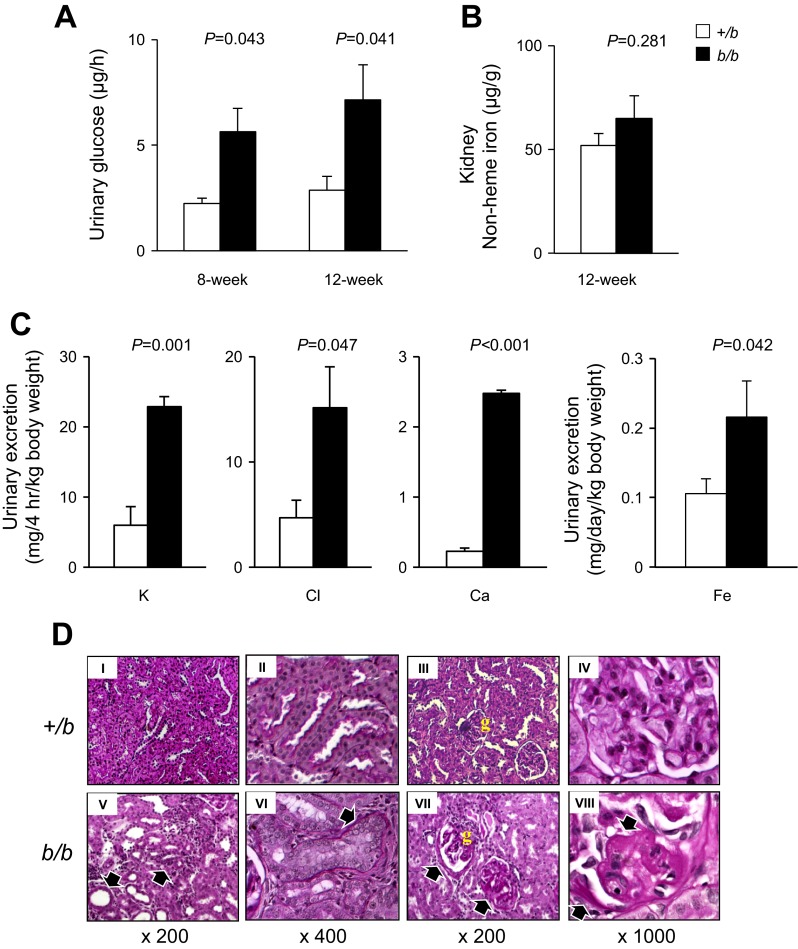
We next considered the observation that Belgrade rats consumed more food despite lower body weight and anemia. We postulated that perhaps the apparent imbalance in energy metabolism was due to excess glucose excretion. To test this possibility, we determined the amount of glucose excreted into both b/b and +/b rat urine within 6 h following intraperitoneal injection (1.5 g d-glucose/kg). The b/b rats demonstrated significant increase of urinary glucose excretion (Fig. 6A). Increased urinary glucose output was observed in both 8- and 12-wk-old rats. This effect was observed despite the fact that nonheme iron levels in kidneys were unchanged in b/b rats compared with +/b controls (Fig. 6B). In addition to glucose, Belgrade rats also showed significantly increased urinary excretion of K, Cl, Ca, and Fe compared with +/b controls (Fig. 6C). Na levels were below 10 mM in both b/b and +/b rats.
Fig. 6.
Kidney function and renal morphology in Belgrade rats. Urinary glucose excretion (A) was measured in 8- and 12-wk-old rats as described in materials and methods, n = 4 in both groups. Nonheme iron levels were determined in 12-wk-old rat kidneys (B), n = 4 in each group. Urinary K, Cl, Ca, and Fe were determined and adjusted to body weight (C). For K, Cl, and Ca, n = 3 each group. For Fe determinations, n = 6 +/b and n = 3 b/b. Periodic acid-Schiff (PAS) staining of paraformaldehyde-fixed sections demonstrated structural changes in the kidneys of b/b rats compared with +/b animals (D), n = 3 each group. The black arrows in DV show disorganization in renal cortex of b/b rats with an ill-defined corticomedullary junction, cellular infiltrations in renal tubules, and tubular dilatation. Arrows in DVI show occlusion of the luminal space in cortical tubules of b/b rats. The black arrows in D, VII and VIII, show thickening of glomerular basement membrane and an increased extracellular mesangial matrix. The yellow “g” in III and VII refers to the glomerulus.
Histochemical experiments revealed the changes in b/b rat renal function were accompanied by marked structural changes in PAS-stained kidneys (Fig. 6D). There was a clear disorganization in renal cortex of b/b rats with an ill-defined corticomedullary junction and cellular infiltrations (Fig. 6D, V). The histology study also showed tubular dilatation and flattened epithelium in some cortical tubules of b/b rats, whereas other tubules demonstrated an almost complete occlusion of the luminal space (Fig. 6D, V and VI). These changes occurred together with thickening of tubular basal membrane and interstitial sclerosis (Fig. 6D, V and VI). The b/b rats also showed glomerulosclerosis mainly characterized by thickening of glomerular basement membrane and increased extracellular mesangial matrix (Fig. 6D, VII and VIII). These features were not observed in age-matched +/b controls fed with the same diet (Fig. 6D, I–IV).
DISCUSSION
High iron status arising from genetic disorders such as HH and thalassemias is closely linked to diabetes and other metabolic problems (23, 36). To investigate how iron overload might influence glycometabolic status, we studied glucose metabolism in the Belgrade rat. This animal model harbors a mutation in DMT1 (14) that reduces intestinal iron absorption and leads to ineffective erythropoiesis (16, 43, 44). The resulting phenotype of iron-loading anemia resembles symptoms associated with thalassemia, including reduced hematocrit despite high serum iron.
Our results indicate that Belgrade rats have normal glycemic control despite hyperferrinemia and pancreatic iron loading. GTT and ITT outcomes were similar to phenotypically null heterozygous littermates. Moreover, insulin signaling in muscle is comparable to +/b controls. These features were unexpected based on previous studies reporting impaired glucose metabolism in iron-loaded patients and animals (1, 10, 18, 41). However, results from such studies have been inconsistent (2, 15, 23, 38). Some studies reported insulin resistance as a primary consequence of iron overload while others suggested that both insulin deficiency and insulin resistance contribute to glucose intolerance. The fact that glucose-induced insulin secretion was unaffected in Belgrade rats despite highly elevated pancreatic nonheme iron levels provides one explanation for their apparently normal glycometabolism. Previous studies have shown that high pancreatic iron stores reduce insulin secretion in thalassemic patients (35) and individuals with HH (32). In mouse models of hemochromatosis (9), lower levels of insulin were reported in response to glucose challenge. Our study shows that dietary loading with carbonyl iron also results in iron accumulation in the pancreas and reduced serum insulin. Despite the fact that pancreatic iron was elevated in the b/b rat, signs of tissue dysfunction or oxidative injury damage were not observed. Recently, it has been reported that β-cell-specific Dmt1 knockout mice are also resistant to high fat-diet-induced diabetes (19), and the sum of our data is consistent with this finding.
Although pancreatic β-cells are susceptible to damage by ROS (19), the lack of DMT1 transport function in the Belgrade rat appears to afford protection against tissue damage in this rat model of disease. It is not clear how the DMT1(G185R) mutation confers resistance in this animal model, but we note that, among the tissues studied, significantly greater nonheme iron levels were observed in pancreas but not kidneys or liver. This comparative sensitivity may suggest that, in the absence of functional DMT1, iron is taken up and stored in the Belgrade rat pancreas by mechanisms that avoid the generation of ROS. Functional DMT1 may mediate transport by mechanisms that produce free radicals to promote pancreatic dysfunction, which would be consistent with impaired glucose metabolism in iron-overload diseases (19). Protection from oxidative stress is most likely afforded by ferritin induction. Carbonyl iron-loaded rats also displayed upregulation of ferritin. Ferritin levels in these two iron-overload models were comparable, despite the fact that pancreatic nonheme iron was twofold greater in Belgrade rats. This difference may be explained by increased iron storage within the ferritin shell, but it is possible that iron accumulates in intracellular vesicles in the pancreas due to loss of DMT1 function. Further work is necessary to decipher this pathway, which may be unique to pancreatic function and could provide an attractive therapeutic target for protection in diabetes. It is interesting to note that the pancreas synthesizes the iron regulatory hormone hepcidin (28). Like the liver, the pancreas may have specific regulatory factors involved in iron homeostasis, as well as specialized iron-handling machinery.
One unexpected observation was that Belgrade rats consumed more food but weighed less than controls. Typically, anemia is associated with depressed activity and lower food intake. What appears to be negative energy balance in b/b rats could arise, at least in part, from enhanced glucosuria. The kidneys play an important role in glucose homeostasis through its reabsorption at the level of the proximal tubule. Under normal physiological conditions, 100% of glucose is reabsorbed by proximal tubes of kidney. We observed that both urinary glucose and electrolyte excretion in b/b rats was greater than +/b rats. The change in electrolytes agrees with previous studies of kidney function in the Belgrade rat (11). It is of interest that excretion of Fe into urine is enhanced in b/b rats, possibly suggesting a function for DMT1 in reabsorption. However, increased urinary Fe is more likely to be secondary to severe renal damage observed in b/b rats since it is associated with a generalized loss of electrolytes (Fig. 6). The kidney pathology also underlies the chronic loss of glucose to contribute to the lower body weight. However, one caveat is that homozygous Belgrade rats have reduced body weight at birth and therefore may simply display a lag in food consumption, although at later ages b/b and +/b body weights are similar while food consumption remains greater in Belgrade rats. Further work is necessary to investigate the molecular basis for the altered energy balance and deranged kidney morphology in Belgrade rats, but these findings should be appreciated in future studies of the DMT1(G185R) and other DMT1 knockout models. Patients carrying DMT1 mutations also display iron-loading anemia (4), and metabolic characterization of these individuals would be important to gain a better understanding of metabolic disease and iron homeostasis. Control of DMT1 function in the pancreas, and possibly the kidneys, could have important therapeutic value to these patients and others with iron-loading disease.
GRANTS
This work was supported by grants from the National Institutes of Health to M. Wessling-Resnick (R01-ES-14638, R01-DK-64750, and RC1-DK-086774) and to C.-H. Lee (R01-DK-J075046). J. Kim was supported by an NIH Roadmap Fellowship (R90-DK-71507) and the Yerby Postdoctoral Fellowship at the Harvard School of Public Health.
DISCLOSURES
The authors have no conflicting financial interests.
AUTHOR CONTRIBUTIONS
Author contributions: X.J., J.K., T.V., C.-H.L., and M.W.-R. conception and design of research; X.J., J.K., T.V., C.-H.L., and M.W.-R. performed experiments; X.J., J.K., T.V., C.-H.L., and M.W.-R. analyzed data; X.J., J.K., T.V., C.-H.L., and M.W.-R. interpreted results of experiments; X.J., J.K., T.V., C.-H.L., and M.W.-R. prepared figures; X.J., J.K., T.V., C.-H.L., and M.W.-R. drafted manuscript; X.J., J.K., T.V., C.-H.L., and M.W.-R. edited and revised manuscript; X.J., J.K., T.V., C.-H.L., and M.W.-R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Carlos Mendivil and Sihao Liu for helpful discussion.
REFERENCES
- 1. Angelopoulos NG, Zervas A, Livadas S, Adamopoulos I, Giannopoulos D, Goula A, Tolis G. Reduced insulin secretion in normoglycaemic patients with beta-thalassaemia major. Diab Med 23: 1327–1331, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Arredondo M, Fuentes M, Jorquera D, Candia V, Carrasco E, Leiva E, Mujica V, Hertrampf E, Perez F. Cross-talk between body iron stores and diabetes: iron stores are associated with activity and microsatellite polymorphism of the heme oxygenase and type 2 diabetes. Biol Trace Element Res 143: 625–636, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Awai M, Narasaki M, Yamanoi Y, Seno S. Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate. Am J Pathol 95: 663–674, 1979 [PMC free article] [PubMed] [Google Scholar]
- 4. Beaumont C, Delaunay J, Hetet G, Grandchamp B, de Montalembert M, Tchernia G. Two new human DMT1 gene mutations in a patient with microcytic anemia, low ferritinemia, and liver iron overload. Blood 107: 4168–4170, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Brudevold R, Hole T, Hammerstrom J. Hyperferritinemia is associated with insulin resistance and fatty liver in patients without iron overload. PloS one 3: e3547, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaudhury C, Kim J, Mehnaz S, Wani MA, Oberyszyn TM, Bronson CL, Mohanty S, Hayton WL, Robinson JM, Anderson CL. Accelerated transferrin degradation in HFE-deficient mice is associated with increased transferrin saturation. J Nutr 136: 2993–2998, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Chern JP, Lin KH, Lu MY, Lin DT, Lin KS, Chen JD, Fu CC. Abnormal glucose tolerance in transfusion-dependent beta-thalassemic patients. Diabetes Care 24: 850–854, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Cianciulli P. Pulmonary embolism and intravenous high-dose desferrioxamine. Haematologica 77: 368–369, 1992 [PubMed] [Google Scholar]
- 9. Cooksey RC, Jouihan HA, Ajioka RS, Hazel MW, Jones DL, Kushner JP, McClain DA. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 145: 5305–5312, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Dongiovanni P, Valenti L, Ludovica Fracanzani A, Gatti S, Cairo G, Fargion S. Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am J Pathol 172: 738–747, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferguson CJ, Wareing M, Delannoy M, Fenton R, McLarnon SJ, Ashton N, Cox AG, McMahon RF, Garrick LM, Green R, Smith CP, Riccardi D. Iron handling and gene expression of the divalent metal transporter, DMT1, in the kidney of the anemic Belgrade (b) rat. Kidney Int 64: 1755–1764, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Ferguson CJ, Wareing M, Ward DT, Green R, Smith CP, Riccardi D. Cellular localization of divalent metal transporter DMT-1 in rat kidney. Am J Physiol Renal Physiol 280: F803–F814, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Fernandez-Real JM, Ricart-Engel W, Arroyo E, Balanca R, Casamitjana-Abella R, Cabrero D, Fernandez-Castaner M, Soler J. Serum ferritin as a component of the insulin resistance syndrome. Diabetes Care 21: 62–68, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA 95: 1148–1153, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fung EB, Harmatz PR, Lee PD, Milet M, Bellevue R, Jeng MR, Kalinyak KA, Hudes M, Bhatia S, Vichinsky EP. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. Br J Haematol 135: 574–582, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Garrick LM, Dolan KG, Romano MA, Garrick MD. Non-transferrin-bound iron uptake in Belgrade and normal rat erythroid cells. J Cell Physiol 178: 349–358, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Garrick M, Scott D, Walpole S, Finkelstein E, Whitbred J, Chopra S, Trivikram L, Mayes D, Rhodes D, Cabbagestalk K, Oklu R, Sadiq A, Mascia B, Hoke J, Garrick L. Iron supplementation moderates but does not cure the Belgrade anemia. Biometals 10: 65–76, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Grunblatt E, Bartl J, Riederer P. The link between iron, metabolic syndrome, and Alzheimer's disease. J Neural Trans 118: 371–379, 2011 [DOI] [PubMed] [Google Scholar]
- 19. Hansen JB, Tonnesen MF, Madsen AN, Hagedorn PH, Friberg J, Grunnet LG, Heller RS, Nielsen AO, Storling J, Baeyens L, Anker-Kitai L, Qvortrup K, Bouwens L, Efrat S, Aalund M, Andrews NC, Billestrup N, Karlsen AE, Holst B, Pociot F, Mandrup-Poulsen T. Divalent metal transporter 1 regulates iron-mediated ROS and pancreatic beta cell fate in response to cytokines. Cell Metab 16: 449–461, 2012 [DOI] [PubMed] [Google Scholar]
- 20. Hatunic M, Finucane FM, Brennan AM, Norris S, Pacini G, Nolan JJ. Effect of iron overload on glucose metabolism in patients with hereditary hemochromatosis. Metabolism 59: 380–384 [DOI] [PubMed] [Google Scholar]
- 21. Hediger MA, Rhoads DB. Molecular physiology of sodium-glucose cotransporters. Physiol Rev 74: 993–1026, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell 117: 285–297, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Huang J, Jones D, Luo B, Sanderson M, Soto J, Abel ED, Cooksey RC, McClain DA. Iron overload and diabetes risk: a shift from glucose to Fatty Acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes 60: 80–87, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inoue Y, Nakanishi K, Hiraga T, Okubo M, Murase T, Kosaka K, Miyakoshi S, Mutoh Y, Kobayashi T. Recovery of pancreatic beta-cell function in hemochromatosis: combined treatment with recombinant human erythropoietin and phlebotomy. Am J Med Sci 314: 401–402, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Kim CH, Kim HK, Bae SJ, Park JY, Lee KU. Association of elevated serum ferritin concentration with insulin resistance and impaired glucose metabolism in Korean men and women. Metab Clin Exp 60: 414–420, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Koren G, Bentur Y, Strong D, Harvey E, Klein J, Baumal R, Spielberg SP, Freedman MH. Acute changes in renal function associated with deferoxamine therapy. Am J Dis Child 143: 1077–1080, 1989 [DOI] [PubMed] [Google Scholar]
- 27. Krook A, Kawano Y, Song XM, Efendic S, Roth RA, Wallberg-Henriksson H, Zierath JR. Improved glucose tolerance restores insulin-stimulated Akt kinase activity and glucose transport in skeletal muscle from diabetic Goto-Kakizaki rats. Diabetes 46: 2110–2114, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Kulaksiz H, Fein E, Redecker P, Stremmel W, Adler G, Cetin Y. Pancreatic beta-cells express hepcidin, an iron-uptake regulatory peptide. J Endocrinol 197: 241–249, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Ly JP, Onay T, Sison K, Sivaskandarajah G, Sabbisetti V, Li L, Bonventre JV, Flenniken A, Paragas N, Barasch JM, Adamson SL, Osborne L, Rossant J, Schnermann J, Quaggin SE. The Sweet Pee model for Sglt2 mutation. J Am Soc Nephrol 22: 113–123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mackenzie B, Loo DD, Panayotova-Heiermann M, Wright EM. Biophysical characteristics of the pig kidney Na+/glucose cotransporter SGLT2 reveal a common mechanism for SGLT1 and SGLT2. J Biol Chem 271: 32678–32683, 1996 [DOI] [PubMed] [Google Scholar]
- 31. May ME, Parmley RT, Spicer SS, Ravenel DP, May EE, Buse MG. Iron nitriloacetate-induced experimental diabetes in rats. J Lab Clin Med 95: 525–535, 1980 [PubMed] [Google Scholar]
- 32. McClain DA, Abraham D, Rogers J, Brady R, Gault P, Ajioka R, Kushner JP. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia 49: 1661–1669, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. New Engl J Med 313: 1256–1262, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Njajou OT, Vaessen N, Oostra B, Heutink P, Van Duijn CM. The hemochromatosis N144H mutation of SLC11A3 gene in patients with type 2 diabetes. Mol Gen Metabol 75: 290–291, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Noetzli LJ, Coates TD, Wood JC. Pancreatic iron loading in chronically transfused sickle cell disease is lower than in thalassaemia major. Br J Haematol 152: 229–233, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Noetzli LJ, Mittelman SD, Watanabe RM, Coates TD, Wood JC. Pancreatic iron and glucose dysregulation in thalassemia major. Am J Hematol 87: 155–160 2012 [DOI] [PubMed] [Google Scholar]
- 37. Papanikolaou G, Samuels ME, Ludwig EH, MacDonald ML, Franchini PL, Dube MP, Andres L, MacFarlane J, Sakellaropoulos N, Politou M, Nemeth E, Thompson J, Risler JK, Zaborowska C, Babakaiff R, Radomski CC, Pape TD, Davidas O, Christakis J, Brissot P, Lockitch G, Ganz T, Hayden MR, Goldberg YP. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Gen 36: 77–82, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Pappas S, Donohue SM, Denver AE, Mohamed-Ali V, Goubet S, Yudkin JS. Glucose intolerance in thalassemia major is related to insulin resistance and hepatic dysfunction. Metabolism 45: 652–657, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Paz K, Liu YF, Shorer H, Hemi R, LeRoith D, Quan M, Kanety H, Seger R, Zick Y. Phosphorylation of insulin receptor substrate-1 (IRS-1) by protein kinase B positively regulates IRS-1 function. J Biol Chem 274: 28816–28822, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Sladic-Simic D, Martinovitch PN, Zivkovic N, Pavic D, Martinovic J, Kahn M, Ranney HM. A thalassemia-like disorder in Belgrade laboratory rats. Ann NY Acad Sci 165: 93–99, 1969 [DOI] [PubMed] [Google Scholar]
- 41. Syrovatka P, Kraml P, Potockova J, Fialova L, Vejrazka M, Crkovska J, Andel M. Relationship between increased body iron stores, oxidative stress and insulin resistance in healthy men. Ann Nutr Metab 54: 268–274, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Thompson K, Molina RM, Brain JD, Wessling-Resnick M. Belgrade rats display liver iron loading. J Nutr 136: 3010–3014, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thompson K, Molina RM, Donaghey T, Brain JD, Wessling-Resnick M. Iron absorption by Belgrade rat pups during lactation Am J Physiol Gastrointest Liver Physiol 293: G640–G644, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J 21: 223–230, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thompson K, Moline RM, Donaghey T, Brain JD, Wessling-Resnick M. The influence of high iron diet on rat lung manganese absorption. Toxicol Appl Pharmacol 210: 17–23, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Tuomainen TP, Nyyssonen K, Salonen R, Tervahauta A, Korpela H, Lakka T, Kaplan GA, Salonen JT. Body iron stores are associated with serum insulin and blood glucose concentrations. Population study in 1,013 eastern Finnish men. Diabetes Care 20: 426–428, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Veuthey T, D'Anna MC, Roque ME. Role of the kidney in iron homeostasis: renal expression of Prohepcidin, Ferroportin, and DMT1 in anemic mice. Am J Physiol Renal Physiol 295: F1213–F1221, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Xu J, Hwang JCY, Lees HA, Wohlgemuth SE, Knutson MD, Judge AR, Dupont-Versteegden EE, Marzetti E, Leeuwenburgh C. Long-term perturbation of mucle iron homeostasis following hindlimb suspension in old rats is associated with high levels of oxidative stress and impaired recovery from atrophy. Exp Gerontol 47: 100–108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yeh KY, Yeh M, Polk P, Glass J. Hypoxia-inducible factor-2alpha and iron absorptive gene expression in Belgrade rat intestine. Am J Physiol Gastrointest Liver Physiol 301: G82–G90, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]