Abstract
We have recently identified the zinc finger and SCAN domain containing 4 (Zscan4), which is transiently expressed and regulates telomere elongation and genome stability in mouse embryonic stem (ES) cells. The aim of this study was to examine the expression of ZSCAN4 in the adult pancreas and elucidate the role of ZSCAN4 in tissue inflammation and subsequent regeneration. The expression of ZSCAN4 and other progenitor or differentiated cell markers in the human pancreas was immunohistochemically examined. Pancreas sections of alcoholic or autoimmune pancreatitis patients before and under maintenance corticosteroid treatment were used in this study. In the adult human pancreas a small number of ZSCAN4-positive (ZSCAN4+) cells are present among cells located in the islets of Langerhans, acini, ducts, and oval-shaped cells. These cells not only express differentiated cell markers for each compartment of the pancreas but also express other tissue stem/progenitor cell markers. Furthermore, the number of ZSCAN4+ cells dramatically increased in patients with chronic pancreatitis, especially in the pancreatic tissues of autoimmune pancreatitis actively regenerating under corticosteroid treatment. Interestingly, a number of ZSCAN4+ cells in the pancreas of autoimmune pancreatitis returned to the basal level after 1 yr of maintenance corticosteroid treatment. In conclusion, coexpression of progenitor cell markers and differentiated cell markers with ZSCAN4 in each compartment of the pancreas may indicate the presence of facultative progenitors for both exocrine and endocrine cells in the adult pancreas.
Keywords: autoimmune pancreatitis, facultative progenitor cells, regeneration
autoimmune pancreatitis is a form of chronic pancreatitis characterized by diffuse swelling of the affected gland and a high serum immunoglobulin G type 4 concentration (19). In patients with autoimmune pancreatitis, pancreatic exocrine (and also most of the time endocrine) function is severely damaged by inflammation. An anti-inflammatory regimen with corticosteroids is widely used as a treatment for autoimmune pancreatitis to ameliorate the swelling of the pancreas and obstructive jaundice (10). In a previous study we have shown that, in patients with autoimmune pancreatitis, corticosteroid restores severely damaged pancreatic tissues functionally and morphologically, which is accompanied by the disappearance of inflammatory cells and massive regeneration of pancreatic tissues (11). We have also shown that regeneration of exocrine cells paralleled the increase of immunoreactivity for CD133 (prominin-1), one of the markers for hematopoietic (27), intestinal (29), and pancreatic (20) progenitors. These findings lead us to the hypothesis that pancreatic progenitor cells reside even in severely damaged autoimmune pancreatitis tissues and give rise to mature differentiated cells in the adult human pancreas after corticosteroid treatment (11).
Recently we have identified zinc finger and SCAN domain containing 4 (Zscan4), a gene that is specific in mammals, expressed exclusively in late two-cell stage mouse embryos (4), and transiently expressed in mouse embryonic stem (ES) cells (28). Zscan4 can extend telomeres and plays an essential role in the maintenance of genome stability. Transient Zscan4 expression, or oscillation between the Zscan4-negative (Zscan4−) and -positive (Zscan4+) state, is indispensable for the maintenance of a normal karyotype, telomere length, and thus the immortality of ES cells (28). In addition, mouse Zscan4 gene was shown to be a potent reprogramming factor for the generation of induced pluripotent stem (iPS) cells (8). Considering the critical function of Zscan4 in ES cells, we hypothesized that ZSCAN4 may also be expressed transiently in stem/progenitor cells even in the adult human pancreas.
Here we have identified ZSCAN4+ cells in the adult human and mouse pancreas by immunohistochemistry. ZSCAN4+ cells are present not only in pancreatic ducts, which are the sites of the previously identified pancreatic stem/progenitor pool (5, 9, 20, 21, 24), but also in the islets of Langerhans (6), exocrine acinar cells (22), and oval-shaped cells [putative quiescent pancreatic stellate cells (14)]. ZSCAN4 was colocalized with known progenitor cell markers in the pancreas and other organs such as polycomb group protein BMI1 (22), carbonic anhydrate II (CAII) (6, 9), neurogenin 3 (NEUROG3) (6), leucine-rich repeat-containing, G protein-coupled receptor 5 (LGR5) (2), and Doublecortin and CaM kinase-like-1 (DCAMKL-1) (16) and with known differentiated cell markers for each compartment of the pancreas such as aquaporin 1 (12) for duct cells, amylase for acinar cells, pancreatic hormones for endocrine cells, and desmin and glial fibrillary acidic protein (GFAP) for pancreatic stellate cells (1). These findings indicate the presence of rare facultative progenitor cells in both the exocrine and endocrine compartments of the human and mouse pancreas.
MATERIALS AND METHODS
Subjects.
Surgically resected pancreatic tissues and pancreatic biopsy samples were used for immunohistochemical analyses. Pancreatic tissues that were resected for the treatment of biliary carcinoma (n = 3) were used as controls, and tissues resected for the treatment of chronic alcoholic pancreatitis (n = 3) were also used. Pancreas biopsy samples from patients with autoimmune pancreatitis were reported previously (18). All pancreatic biopsies were performed to exclude malignancy, and written informed consent was obtained from each patient before the procedure. Under the visual guidance of endoscopic ultrasonography (GF-UCT240; Olympus), pancreatic tissues were obtained from the body of the pancreas using a 19-gauge Trucut biopsy needle (Wilson-Cook). Patients met the following 2006 revised Japanese clinical diagnostic criteria for autoimmune pancreatitis: diffuse swelling of the pancreas, irregular narrowing of the main pancreatic duct, and a positive test for autoantibodies or a high immunoglobulin G (≥1,800 mg/dl)/immunoglobulin G4 concentration (≥135 mg/dl). Among patients with autoimmune pancreatitis, three patients were subjected to a pancreatic biopsy at the following three different times: at the time of diagnosis, 3 mo after the initiation of corticosteroid treatment, and after 1 yr of maintenance corticosteroid treatment (11). A standard protocol for oral corticosteroids was as follows: prednisolone at 30 mg/day for 1 wk as an initial dose, 20 mg/day for a second week, 10 mg/day for four additional weeks, and 5 mg/day as a maintenance dose all through the observation period (11). All of the procedures for obtaining human samples were approved by the Institutional Ethics Committee of the Nagoya University Hospital and the Aichi Cancer Center Hospital.
Animals.
C57BL/6 mice were purchased from Japan SLC (Hamamatsu, Japan). Male mice at 8–10 wk were used for acute pancreatitis experiments. Experimental procedures for animal samples were approved by the Institutional Animal Care and Use Committee of the Nagoya University Graduate School of Medicine.
Caerulein-induced acute pancreatitis.
Pancreatitis was induced by intraperitoneal injections of 50 μg/kg caerulein (Sigma, St. Louis, MO) in 0.9% NaCl. Controls received equal volumes of 0.9% NaCl injected intraperitoneally. The pancreas was removed from mice at 1 day and 4 days after the beginning of six caerulein injections at hourly intervals.
Immunohistochemistry and immunofluorescence.
Both human and mouse pancreases were fixed in 10% formalin and embedded in paraffin. Embedded tissues were thin-sliced with a Leica microtome (Leica Microsystems, Wetzlar, Germany) at 5 μm. Sections were deparaffinized, permeabilized, and used for immunohistochemical analyses. The numbers of immunopositive cells in both human and mouse pancreas were counted by two independent observers who were blinded to the conditions.
Antibodies.
Antibodies used in this study are summarized in Table 1. Antibodies were diluted according to the manufacturer's recommendation. For immunohistochemistry, immunoreactions were intensified using Histofine Simple Stain MAX-PO reagent (Nichirei Biosciences, Tokyo, Japan). Immunolabeling was visualized using 3,3′-diaminobenzidinetetrahydrochloride as substrate for horseradish peroxidase. Sections were counterstained with Mayer's hematoxylin. For immunofluorescence, Alexa Fluor 488 (green)-, Alexa Fluor 596 (red)-, or Alexa Fluor 350 (blue)-labeled secondary antibodies were used for double or triple staining as appropriate. Immunolabelings were microphotographed with Olympus fluorescence microscopy (AX80; Olympus, Tokyo, Japan). Cell nuclei were counterstained with Hoechst 33258.
Table 1.
List of antibodies used in this study
| Antibodies | Species | Manufacturer | Product ID | Working Dilution |
|
|---|---|---|---|---|---|
| Anti-ALDH | Rabbit | Abcam | ab52492 | IHC | 1:100 |
| IF | 1:100 | ||||
| Anti-AQP1 | Rabbit | Alpha Diagnostic | AQP11-A | IHC | 1:500 |
| IF | 1:500 | ||||
| Anti-α-amylase | Mouse | Abcam | ab54765 | IHC | 1:300 |
| IF | 1:100 | ||||
| Anti-αSMA | Mouse | Abcam | ab7817 | IHC | 1:100 |
| IF | 1:100 | ||||
| Anti-BMI1 | Mouse | Millipore | 05-637 | IHC | 1:200 |
| IF | 1:100 | ||||
| Anti-CAII | Rabbit | Abcam | ab6621 | IHC | 1:2,000 |
| IF | 1:1,000 | ||||
| Anti-CD163 | Mouse | Leica | NCL-CD163 | IHC | 1:300 |
| IF | 1:100 | ||||
| Anti-DCAMKL-1 | Rabbit | Abcam | ab37994 | IHC | 1:100 |
| IF | 1:100 | ||||
| Anti-desmin | Rabbit | Abcam | ab32362 | IHC | 1:500 |
| IF | 1:200 | ||||
| Anti-GFAP | Rabbit | Abcam | ab16997 | IHC | 1:100 |
| IF | 1:100 | ||||
| Anti-glucagon | Rabbit | Abcam | ab18461 | IHC | 1:5,000 |
| IF | 1:1,000 | ||||
| Mouse | Abcam | ab10988 | IHC | 1:4,000 | |
| IF | 1:1,000 | ||||
| Anti-insulin | Guinea pig | Abcam | ab7842 | IHC | 1:500 |
| IF | 1:2,000 | ||||
| Mouse | Abcam | ab7760 | IHC | 1:3,000 | |
| IF | 1:2,000 | ||||
| Anti-LGR5 | Rabbit | Abcam | ab75732 | IHC | 1:300 |
| IF | 1:100 | ||||
| Anti-NEUROG3 | Rabbit | Abcam | ab38548 | IHC | 1:100 |
| IF | 1:100 | ||||
| Anti-SSEA3 | Rat | Abcam | ab16286 | IHC | 1:100 |
| IF | 1:100 | ||||
| Anti-mouse Zscan4 | Rabbit | NIH | IHC | 1:2,000 | |
| IF | 1:500 | ||||
| Anti-human ZSCAN4 | Mouse | Abnova | H00201516-B01P | IHC | 1:200 |
| IF | 1:100 | ||||
| Alexa Fluor 488 anti-mouse | Goat | Invitrogen | A11017 | IF | 1:500 |
| Alexa Fluor 488 anti-rabbit | Goat | Invitrogen | A11070 | IF | 1:500 |
| Alexa Fluor 488 anti-rat | Chicken | Invitrogen | A21470 | IF | 1:500 |
| Alexa Fluor 594 anti-mouse | Goat | Invitrogen | A11020 | IF | 1:500 |
| Alexa Fluor 594 anti-rabbit | Goat | Invitrogen | A11072 | IF | 1:500 |
| Alexa Fluor 594 anti-guinea pig | Goat | Invitrogen | A11076 | IF | 1:500 |
ALDH, aldehyde dehydrogenase 1; AQP1, aquaporin 1; αSMA, α-smooth mucle actin; BMI1, B lymphoma Mo-MLV insertion region 1 homolog; CAII, carbonic anhydrase II; DCAMKL-1, doublecortin and CaM kinase-like-1; GFAP, glial fibrillary acidic protein; LGR5, leucine-rich repeat-containing, G protein-coupled receptor 5; NEUROG3, neurogenin 3; SSEA3, stage-specific embryonic-antigen 3; ZSCAN4, zinc finger and SCAN domain containing 4; IHC, immunohistochemistry; IF, immunofluorescent.
RT-PCR analysis of ZSCAN4 expression in human tissues.
Human multiple tissue cDNA panel (catalog no. 636742; Takara Bio, Shiga, Japan) was subjected to the analysis of ZSCAN4 expression at the RNA level. ZSCAN4-specific primers for PCR were designed based on the nucleotide sequence of NCBI reference sequence no. NM_152677.2.
Materials.
All of the reagents in molecular biology grade were obtained from Sigma-Aldrich unless otherwise stated.
Statistical analysis.
Statistical analysis was performed with the Mann-Whitney test. Differences with a P value of <0.05 were considered statistically significant. All values were expressed as means ± SD.
RESULTS
Presence of ZSCAN4+ cells in adult human pancreas.
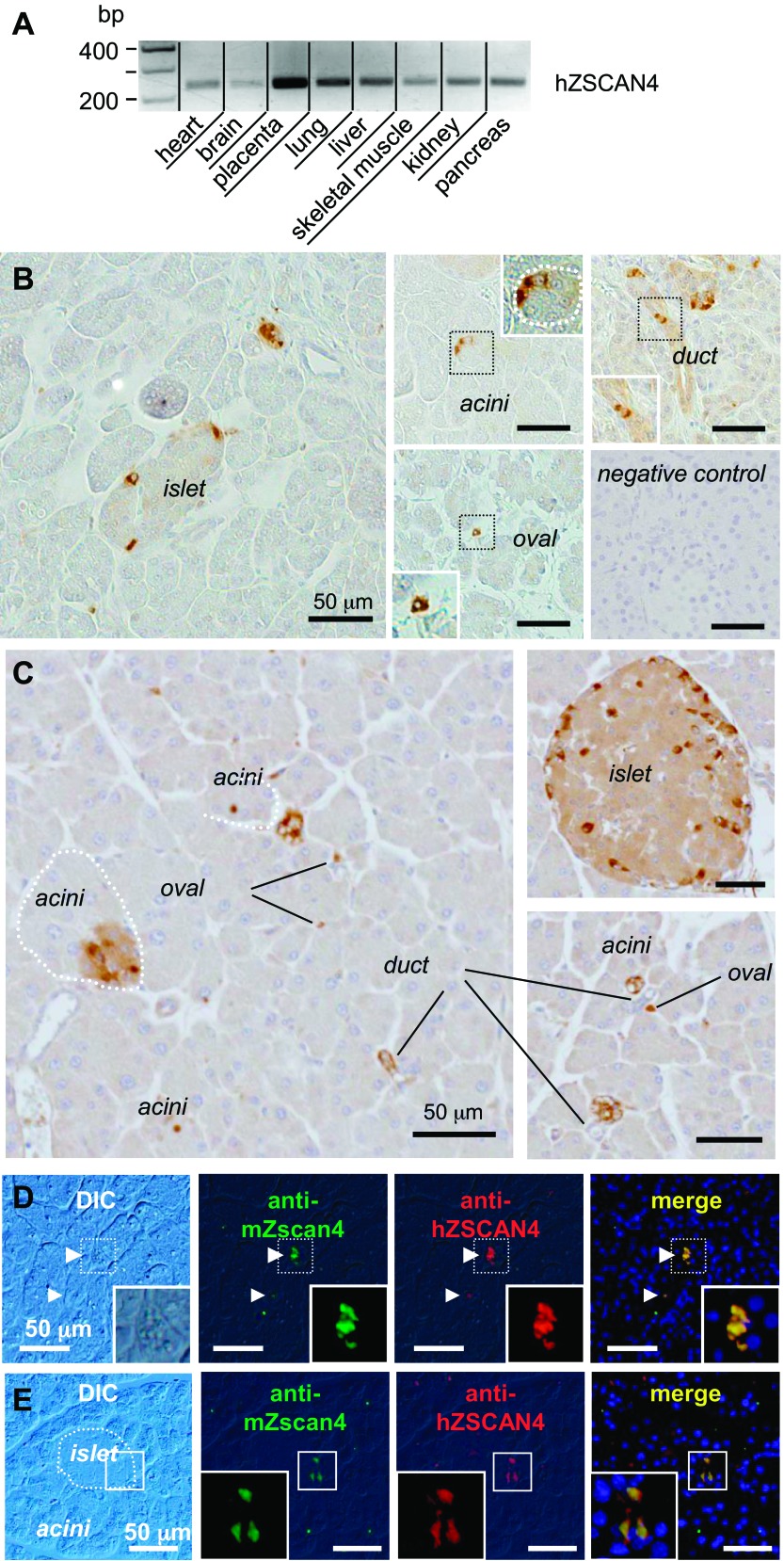
Although public database searches did not reveal any human tissues with high ZSCAN4 expression, RT-PCR analysis detected low levels of expression of ZSCAN4 in all the major organs examined, including the pancreas and placenta with relatively high expression (Fig. 1A). We reasoned that such a low level of expression of ZSCAN4 can be explained by its transient expression in a relatively small number of cells in adult tissues. Indeed, immunohistochemical analyses of the human pancreas with antibodies against human ZSCAN4 revealed that, although the majority of human pancreatic tissues were negative for ZSCAN4 staining, a very small number of cells showed strong nuclear and cytoplasmic staining for ZSCAN4 in all the major compartments (i.e., the islets of Langerhans, acini, and ducts) of the adult human pancreas (Fig. 1B) and mouse pancreas (Fig. 1C). As validation for the human ZSCAN4 antibody, we confirmed that both antibodies against human ZSCAN4 and mouse Zscan4 used in our earlier studies (28) marked almost identical cells on either human (Fig. 1D) or mouse pancreas (Fig. 1E) sections.
Fig. 1.
Dynamic control of zinc finger and SCAN domain containing 4 (ZSCAN4) expression in adult human (h) pancreas. A: ZSCAN4 expression at RNA levels in various human organs. Low level of ZSCAN4 expression was detected in all of the human tissues examined. B: immunolocalization of ZSCAN4 protein in adult human pancreas. A very small number of cells showed strong nuclear and cytoplasmic staining for ZSCAN4 in all of the major compartments: the islet of Langerhans, acini, ducts, and oval-shaped cells (referred to as oval cells: putative quiescent pancreatic stellate cells). Immunostaining without a primary antibody against ZSCAN4 is shown as a negative control. C: immunolocalization of mouse Zscan4 in the pancreas. Most of the cells are negative for Zscan4, but a subset of cells in acini, ducts, the islet of Langerhans are positive for mouse Zscan4. Oval-shaped cells (see text) are also positive for Zscan4. Note that oval-shaped cells between acini appear as a single cell or sometimes a cluster of a few cells. D: an anti-mouse (m) Zscan4 antibody and an anti-human ZSCAN4 antibody stained identical cells in the adult human pancreas. E: an anti-mouse Zscan4 antibody and an anti-human ZSCAN4 antibody stained identical cells in a subset of cells in the islet of Langerhans of mouse pancreas. DIC, differential interference contrast microscopy.
ZSCAN4+ cells express differentiated cell markers in the pancreas.
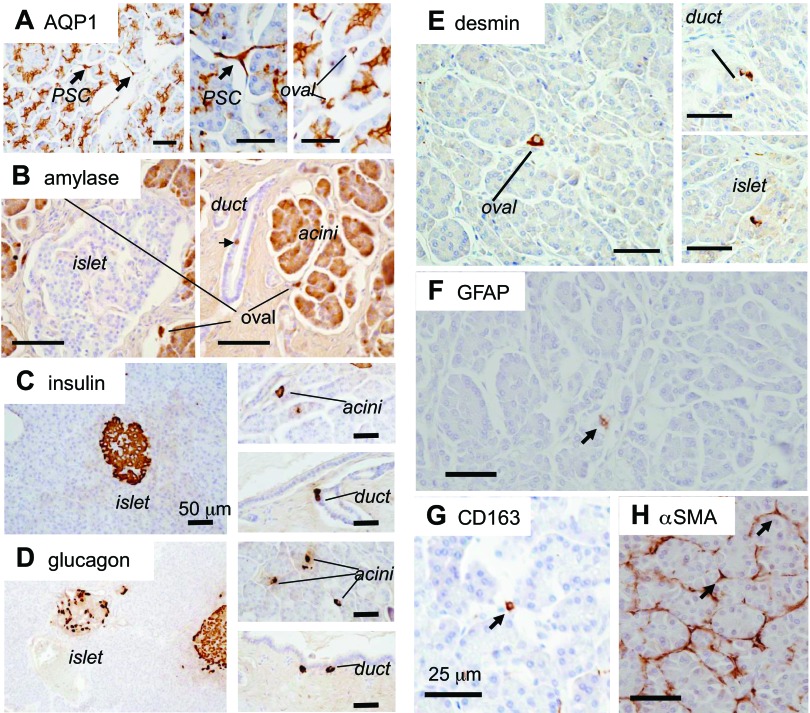
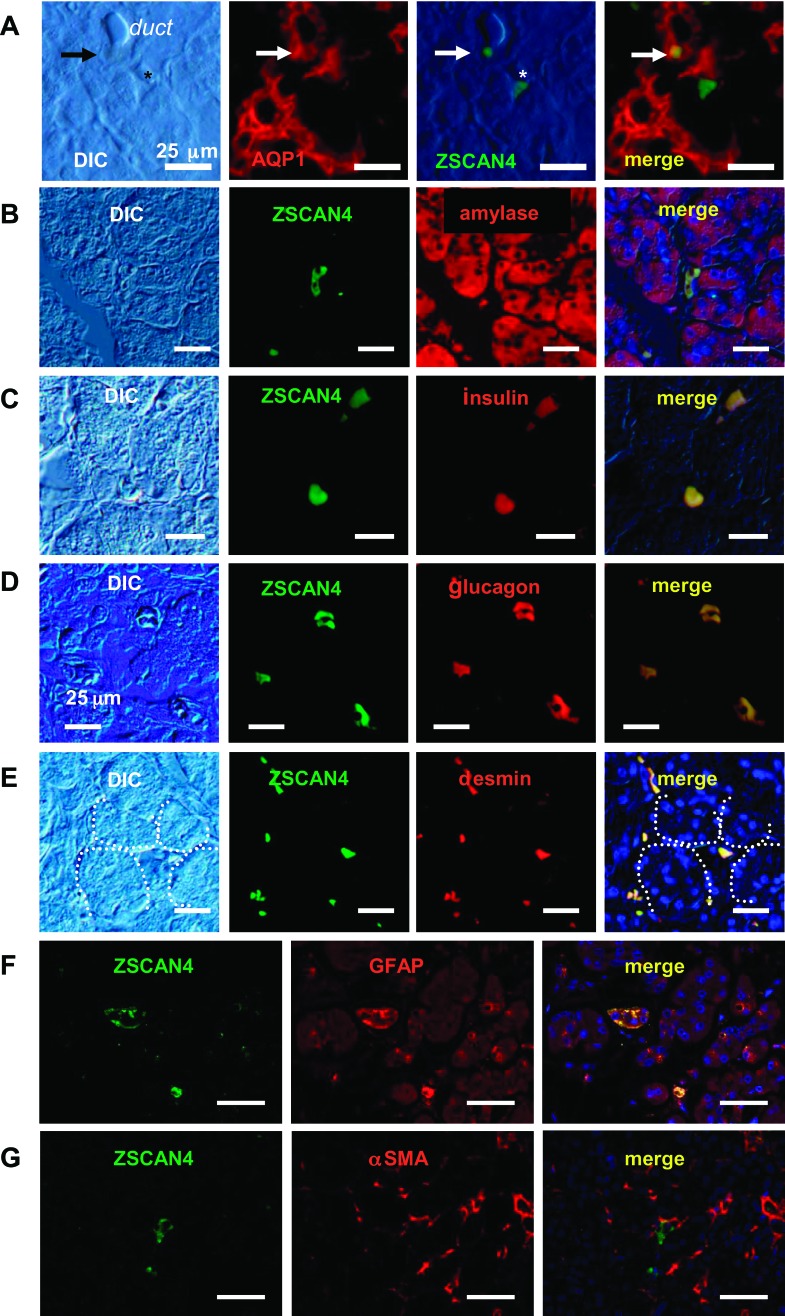
These ZSCAN4+ cells also expressed markers of differentiated cells specific to cells in each compartment: the expression of a duct marker, AQP1, was detected in ZSCAN4+ cells in the duct (Figs. 2A and 3A); the expression of an acinar marker, amylase, in ZSCAN4+ cells in the acinar cells (Figs. 2B and 3B); the expression of endocrine markers, insulin (Figs. 2C and 3C) and glucagon (Figs. 2D and 3D), in ZSCAN4+ cells in the islet; and the expression of pancreatic stellate cell markers, desmin (Figs. 2E and 3E), GFAP (Figs. 2F and 3F), and CD163 (Fig. 2G), but not α-smooth muscle actin (Figs. 2H and 3G), in ZSCAN4+ cells in the subset of oval-shaped cells (see below for the details). Our earlier work showed that undifferentiated ES cells oscillate between the Zscan4− state and the Zscan4+ state (28). Therefore, these data suggest that these differentiated cells in each compartment also oscillate between the ZSCAN4− state and ZSCAN4+ state while retaining their differentiated cell markers. The extremely rare presence of these ZSCAN4+ cells in the adult human pancreas indicates that the differentiated cells rarely take the ZSCAN4+ state, as is also the case in mouse ES cells.
Fig. 2.
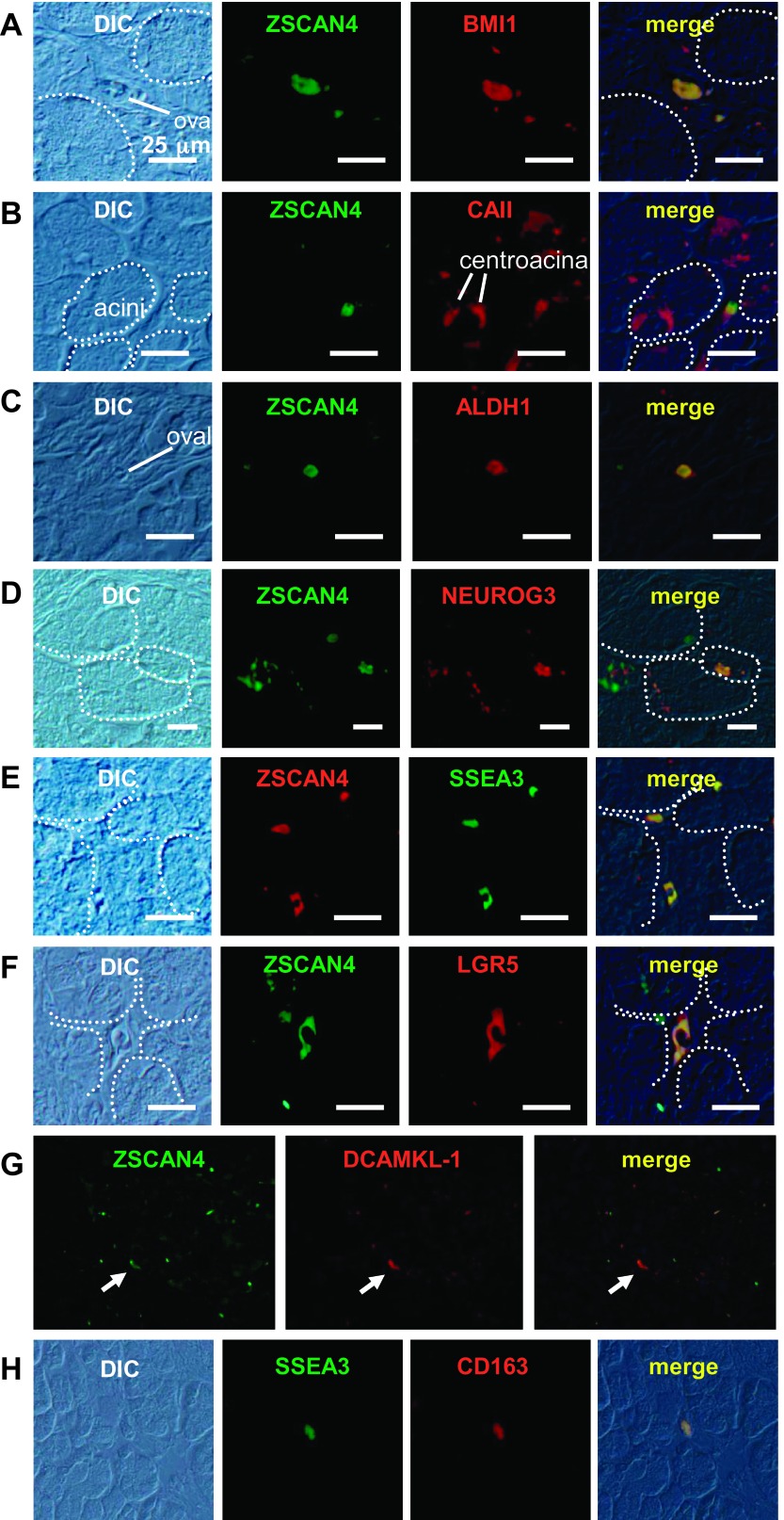
Immunolocalization of differentiated cell markers in adult human pancreas. A: aquaporin 1 (AQP1) water channel is expressed in the plasma membrane of duct cells from centroacinar cells to the medium-sized ducts. Note that AQP1 is also expressed in pancreatic stellate cells (PSC) with cytoplasmic protrusions and round oval cells between adjacent acini (see text). B: acinar cells are positive for the digestive enzyme amylase. Note that amylase is also positive in a subset of cells in ducts (arrow), oval-shaped cells between acini. C: insulin. D: glucagon. All of these hormone-expressing cells are not only confined inside the islet of Langerhans but also in a subset of acinar cells and oval-shaped cells in the human pancreas. Note that some of the cells at the peripheral region of human pancreatic ducts are also positive for endocrine hormones. E: immunolocalization of desmin in the pancreas. Desmin is expressed not only in a subset of oval cells but in a subset of cells in ducts and the islet of Langerhans. F: immunolocalization of glial fibrillary acidic protein (GFAP) in the pancreas. GFAP is expressed in rare cells in the human pancreas, probably in a subset of oval-shaped cells. G: immunolocalization of CD163. CD163 is localized in oval cells. H: immunolocalization of α-smooth muscle actin (αSMA) in the pancreas. αSMA is localized at the peripheral region of acini. Some of the pancreatic stellate cells with cytoplasmic protrusions (black arrows) are positive for αSMA. Note that none of the oval cells (most likely quiescent stellate cells) was positive for αSMA. Bars, 50 μm.
Fig. 3.
Coexpression of ZSCAN4 and differentiated cell markers in the adult human pancreas. A: a ZSCAN4+ cell is located among pancreatic duct cells, which are positive for the duct cell marker AQP1. Note that there is a ZSCAN4+ cell without immunoreactivity for AQP1 (*). B: a subpopulation of amylase-positive cells expresses ZSCAN4. These cells are located between adjacent acini. C: an oval-shaped single cell located between acini expresses both insulin and ZSCAN4. D: glucagon and ZSCAN4 expressions were completely overlapped in a few cells scattered in the adult human pancreas. Note that these cells are easily identifiable in DIC images. E: oval-shaped cells located between adjacent acini coexpress ZSCAN4. F: a subset of oval-shaped cells (putative quiescent pancreatic stellate cells) is positive for both ZSCAN4 and GFAP. G: ZSCAN4 is not colocalized with αSMA-positive fibrotic cells in the human pancreas. Bars, 25 μm.
ZSCAN4+ cells coexpress stem/progenitor cell markers.
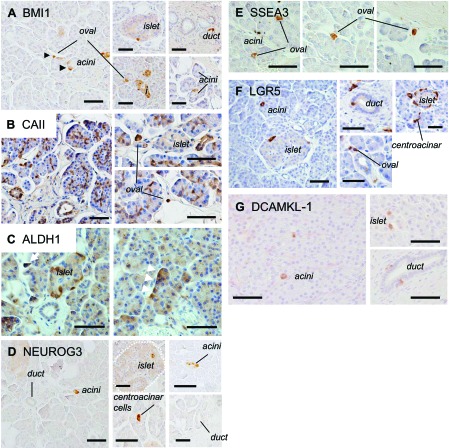
To further characterize these rare ZSCAN4+ cells in the pancreas, we examined the expression of well-established stem/progenitor markers for each compartment of the human and mouse pancreas: polycomb ring finger oncogene BMI1 for the stem/progenitor cells in acinar cells, CAII and aldehyde dehydrogenase 1 (ALDH1) for duct progenitors, and NEUROG3 for endocrine progenitors (6, 25). We found that rare ZSCAN4+ cells and rare cells marked with the respective stem/progenitor markers largely overlapped in each compartment of the pancreas (Figs. 4 and 5). Interestingly, we found cells marked with BMI1 (Figs. 4A and 5A), as well as LGR5 (Figs. 4F and 5F), a marker for tissue stem cells in the mouse small intestine, colon, and skin (2), were present not only in acinar cells but also in a subset of all the cell types in the pancreas, i.e., islet and ducts. Moreover, we found that DCAMKL-1, a recently identified intestinal (15) and pancreatic (16) stem/progenitor cell marker, was also expressed in very rare cells in a subset of both exocrine and endocrine pancreatic cells (Fig. 4G) and was coexpressed with ZSCAN4 (Fig. 5G). The population of rare cells marked with BMI1+ and LGR5+ was largely overlapped, and a fraction (∼10%) of BMI1+/LGR5+ cells was also ZSCAN4+ (Fig. 6, A and B). The expression patterns of marker genes in each cell type are summarized in Table 2. Taken together, coexpression of ZSCAN4 and progenitor cell markers of each compartment (i.e., CAII in Figs. 4A and 5B, ALDH1 in Figs. 4C and 5C, and NEUROG3 in Figs. 4D and 5D) as well as across compartments (i.e., BMI1 in Fig. 4A, LGR5 in Fig. 4F, and DCAMKL-1 in Fig. 4G) suggests that Zscan4 marks a portion of pancreatic cells that have been previously ascribed as progenitor cells (7, 9, 14, 17, 21, 23, 25, 26).
Fig. 4.
Immunolocalization of stem/progenitor cell markers in the adult human pancreas. A: B lymphoma Mo-MLV insertion region 1 homolog (BMI1) is expressed in a subset of cells in every compartment of the pancreas, i.e., acinar cells (arrowhead), the islet of Langerhans, ducts, and oval cells (arrowhead). B: carbonic anhydrase II (CAII) is expressed in ducts from centroacinar cells to small-sized ducts. Note that CAII is also positive in oval cells. C: aldehyde dehydrogenase 1 (ALDH1) is localized in a subset of acinar cells and oval cells (white arrow). D: neurogenin 3 (NEUROG3) is not only localized in a subset of acinar cells but also in the islet of Langerhans and centroacinar cells. E: stage-specific embryonic antigen 3 (SSEA3) is exclusively expressed in a subset of oval cells. F: leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) is expressed in a subset of cells in every compartment of the pancreas as well as BMI1: acinar cells, the islet of Langerhans, duct and centroacinar cells, and oval cells. G: rare Doublecortin and CaM kinase-like-1 (DCAMKL-1)-positive cells are localized in a subset of pancreatic acinar cells, islets of Langerhans, and duct cells. Bars, 50 μm.
Fig. 5.
Coexpression of ZSCAN4 with stem/progenitor cell markers in the adult human pancreas. A: coexpression of ZSCAN4 and BMI1 is shown in an oval cell between acini. Note that these oval cells exist as a single cell or sometimes a cluster of a few cells. White dotted lines indicate the margin of acini. B: CAII is expressed in centroacinar cells and oval cells. ZSCAN4 is colocalized with CAII in an oval cell. C: ZSCAN4 and ALDH1 are colocalized in an oval cell between acini. D: NEUROG3 is colocalized with ZSCAN4 in a subpopulation of acinar cells. White dotted lines indicate the margin of pancreatic acini. E: both ZSCAN4 and SSEA3 are expressed in the same oval cells. F: coexpression of ZSCAN4 with LGR5 in an oval cell with some cytoplasmic protrusions. White dotted lines indicate the margin of pancreatic acini. G: DCAMKL-1 is colocalized with ZSCAN4 in the pancreas (white arrows). H: SSEA3-positive oval cells are also positive for CD163. Bars, 25 μm.
Fig. 6.
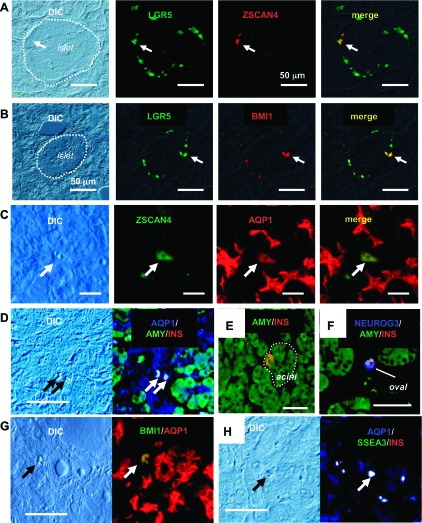
ZSCAN4 expression in pancreatic cells in adult human pancreas. A: colocalization of LGR5 and ZSCAN4 in the islet of Langerhans. LGR5+ cells are located at the peripheral region of the islet. Only a small portion of LGR5+ cells are positive for ZSCAN4 in the islet of Langerhans. White dashed line indicates the margin of the islet. B: LGR5 and BMI1 expression in the islet of Langerhans. Some of the LGR5+ cells are also positive for BMI1. C: ZSCAN4 is expressed in a mature AQP1+ cell apart from pancreatic ducts. Note that ZSCAN4+ cells are clearly identifiable in a DIC image. D: AQP1, amylase, and insulin (INS) triple-positive intermediate mixed exocrine and endocrine cells located in the interstitium of the human pancreas. Note that these cells are clearly identifiable in a DIC image. E: an insulin-positive cell in pancreatic acini. F: amylase and insulin double-positive oval-shaped cell is also positive for the undifferentiated endocrine cell marker NEUROG3. G: a BMI1 and AQP1 double-positive cell at the interstitium of the human pancreas. H: AQP1 and insulin double-positive oval cells also express SSEA3. Bars, 50 μm.
Table 2.
Summary of marker-gene expression patterns of cells in adult human pancreas
| Stem/Progenitor Cell Markers |
Differentiated Cell Markers |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZSCAN4 | BMI1 | CAII | ALDH1 | NEUROG3 | SSEA3 | DCAMKL-1 | LGR5 | AQP1 | Desmin | GFAP | CD163 | Amylase | Insulin | Glucagon | |
| Acinar cells | Z− | − | − | − | − | − | − | − | − | − | − | − | + | − | − |
| Z+ | + | − | + | + | − | + | + | − | − | − | − | + | + | + | |
| Duct cells | Z− | − | ± | ± | − | − | − | − | + | − | − | − | − | − | − |
| Z+ | + | ± | ± | − | − | + | + | + | ± | − | − | ± | + | + | |
| Endocrine cells | Z− | − | − | − | − | − | − | − | − | − | − | − | − | + | + |
| Z+ | + | − | + | + | − | + | + | − | ± | − | − | − | + | + | |
| Stellate cells | Z− | − | − | − | − | − | − | − | + | + | − | + | − | − | − |
| Oval cells | Z− | − | − | − | − | − | − | − | + | + | − | + | − | − | − |
| Z+ | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
A subset of oval-shaped cells express ZSCAN4 along with progenitor cell markers and differentiated cell markers.
In addition to cells in the duct, acini, or islet, we noticed that the expression of ZSCAN4 was detected in oval-shaped cells (tentatively referred to as oval cells), which exist as a single cell or sometimes a cluster of a few cells between adjacent pancreatic acini (Fig. 1, B and C). In the human pancreas, the oval cells seemed to be the only cell type that could be stained with a SSEA3 antibody (Fig. 4E), a marker for adult human stem cells recently identified in skin fibroblasts and bone marrow stromal cells (13). In addition, these oval cells were also positive for CD163 (Fig. 2G), a marker for monocytes and macrophages. From their location, cell morphology, and the expression of desmin (1, 3) (Figs. 2E and 3E) and GFAP (Figs. 2F and 3F), oval cells are most likely a quiescent form of pancreatic stellate cells (3). It is worth pointing out that only a small portion of oval cells were ZSCAN4+. Furthermore, pancreatic stellate cells of the typical morphology with protrusions, so-called “activated pancreatic stellate cells (8),” were not stained with either ZSCAN4 (Fig. 1B) or SSEA3 (Fig. 4E). Interestingly, these rare ZSCAN4+ oval cells expressed both differentiated and undifferentiated cell markers such as AQP1 (Fig. 6, C, D, F, and G), amylase (Figs. 3B, 6D, and 6E), insulin (Figs. 3C, 6D, 6E, and 6G), glucagon (Fig. 3D), BMI1 (Figs. 5A and 6F), SSEA3 (Figs. 5E and 6G), and LGR5 (Fig. 5F). We note that a possibility of bone marrow origin of the ZSCAN4+ oval cells cannot be excluded at this point, since they expressed both SSEA3 and CD163 (Fig. 5G). This notion may be consistent with previous reports that at least some of the pancreatic stellate cells originate from bone marrow (see Refs. 14 or 26 for review).
Chronic inflammation increases the number of ZSCAN4+ cells in adult human pancreas.
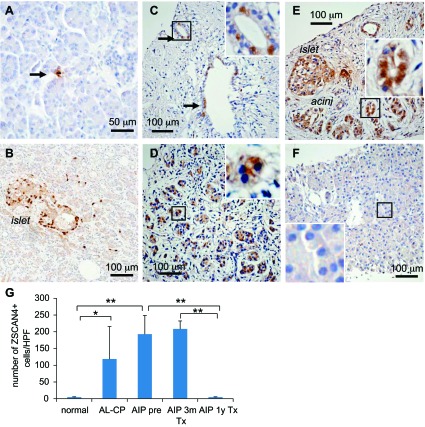
We further noticed that ZSCAN4 expression levels varied among tissues from different subjects. In sharp contrast to the normal pancreas of unaffected individuals, where ZSCAN4+ cells were very rare and rather difficult to spot (Fig. 7A), a dramatic increase of ZSCAN4 expression was observed in tissues with chronic alcoholic pancreatitis (Fig. 7B) and autoimmune pancreatitis (Fig. 7, C and D). Such an increase of ZSCAN4+ cells was particularly notable in some areas (the acinar cells and the islet) that appear to be intact among the tissues damaged severely by alcoholic pancreatitis (Fig. 7B). ZSCAN4+ cells were even more abundant in the pancreatic tissues with autoimmune pancreatitis, where pancreatic cells were actively regenerating after a 3-mo corticosteroid treatment (Fig. 7E). Interestingly, the number of ZSCAN4+ cells returned to a basal level after 1 yr of maintenance corticosteroid treatment for autoimmune pancreatitis (Fig. 7F). To assess these changes more quantitatively, we counted the number of ZSCAN4+ cells per high-power field in each condition (Fig. 7G). These data indicate that the inflammation, destruction, and restoration of pancreatic tissues are accompanied with the increase of ZSCAN4+ cells, suggesting the involvement of the ZSCAN4+ state in tissue damage (inflammatory insult) and subsequent regeneration (differentiation).
Fig. 7.
Dynamic control of ZSCAN4 expression in chronic pancreatitis under inflammation and regeneration. ZSCAN4 expression patterns in tissues taken from patients with chronic alcoholic pancreatitis and autoimmune pancreatitis. A: a representative ZSCAN4 immunostaining of the pancreas from an unaffected individual. Arrow, a ZSCAN4+ cell. B: a representative ZSCAN4 immunostaining of the pancreas from a patient with chronic alcoholic pancreatitis. ZSCAN4+ cells are scattered in the pancreas. A number of ZSCAN4+ cells in the islet of Langerhans are dramatically increased in sections of alcoholic pancreatitis. C: a representative ZSCAN4 immunostaining of the duct region of the pancreas from a patient with untreated autoimmune pancreatitis. ZSCAN4+ cells increase in tissues on chronic inflammation. Arrows, a small-sized pancreatic duct containing a few ZSCAN4+ cells. D: a representative ZSCAN4 immunostaining of the acinar region of the pancreas from a patient with untreated autoimmune pancreatitis. ZSCAN4+ cells are scattered throughout the pancreas with autoimmune pancreatitis. E: a representative ZSCAN4 immunostaining of the pancreas from a patient with autoimmune pancreatitis under the maintenance corticosteroid therapy for 3 mo. A large number of ZSCAN4+ cells are seen in pancreatic ducts, the islet of Langerhans, and regenerating pancreatic acinar cells. F: a representative ZSCAN4 immunostaining of the pancreas from a patient with autoimmune pancreatitis under the maintenance corticosteroid therapy for 12 mo. Note that immunoreactivity for ZSCAN4 almost disappeared and returned to a basal level at fully regenerated tissues. G: the no. of ZSCAN4+ cells per high-power field (HPF) in each condition is summarized (n = 3–5, mean ± SD). Normal subjects, 1.8 ± 1.3; alcoholic pancreatitis (AL-CP), 118.8 ± 97.0; autoimmune pancreatitis pretreatment (AIP pre), 193.0 ± 56.0; autoimmune pancreatitis 3 mo after the initiation of corticosteroid treatment (AIP 3m Tx), 208.7 ± 23.4; autoimmune pancreatitis 1 yr after the initiation of corticosteroid treatment (AIP 1y Tx), 0.6 ± 0.9. *P < 0.01 and **P < 0.05.
Acute inflammation increases the number of ZSCAN4+ cells in mouse caerulein pancreatitis.
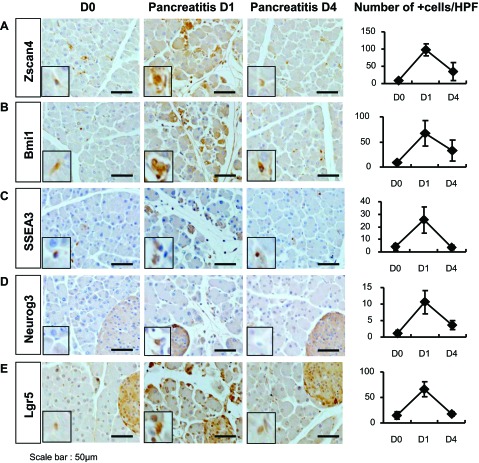
To experimentally validate our observations in human alcoholic chronic pancreatitis and autoimmune pancreatitis, we used a well-established caerulein-induced acute pancreatitis model in mouse. In control mice, the number of Zscan4+ cells was very low, as expected (Fig. 8A). The number of Zscan4+ cells dramatically increased 1 day after caerulein injections six times at hourly intervals, but returned to the basal level as early as the 4th day after the injection. Essentially, the same trends were observed for BMI1, SSEA3, NEUROG3, and LGR5 (Fig. 8, B-E). The rapid increase and subsequent decrease of Zscan4+ cells without noticeable structural changes indicate that the increase of Zscan4+ cells was not caused by the proliferation of Zscan4+ cells but rather caused by the rapid upregulation of Zscan4 expression, which is accompanied with the rapid upregulation of other progenitor markers. These observations in mouse acute pancreatitis are quite consistent with the finding that ZSCAN4 in the human pancreas is remarkably upregulated in chronic inflammation irrespective of etiology.
Fig. 8.
Increased expression of Zscan4 and other progenitor cell markers in mouse acute pancreatitis. All of the progenitor cell markers examined are upregulated in caerulein-induced mouse acute pancreatitis at day 1 (D1) but returned to the basal levels at as early as day 4 (D4) (see text). D0, control day. A: Zscan4; B: Bmi1; C: SSEA3; D: Neurog3; E: Lgr5. The no. of marker positive cells per HPF is summarized (mean ± SD, n = 3) in the panels on the right.
DISCUSSION
In the current study, we have identified a rare population of cells that express ZSCAN4 in adult human pancreas. We have also found that the number of ZSCAN4+ cells dramatically increases during inflammation associated with alcoholic or autoimmune pancreatitis, which is subsequently reduced to the basal level after the regeneration of pancreatic tissues and the restoration of their function by corticosteroid hormone treatment. An increase of ZSCAN4+ cells by inflammation has also been confirmed by using experimental pancreatitis models in mouse. It is worth mentioning that the current study is indeed the first report of the expression of human ZSCAN4 in any human cells and tissues. Considering the demonstrated function of Zscan4 in mouse ES cells (28) and during reprogramming (8), it is tempting to consider that ZSCAN4+ cells in the adult human pancreas may undergo similar processes, such as telomere elongation (28) and epigenetic reprogramming (8). However, such a possibility remains to be investigated.
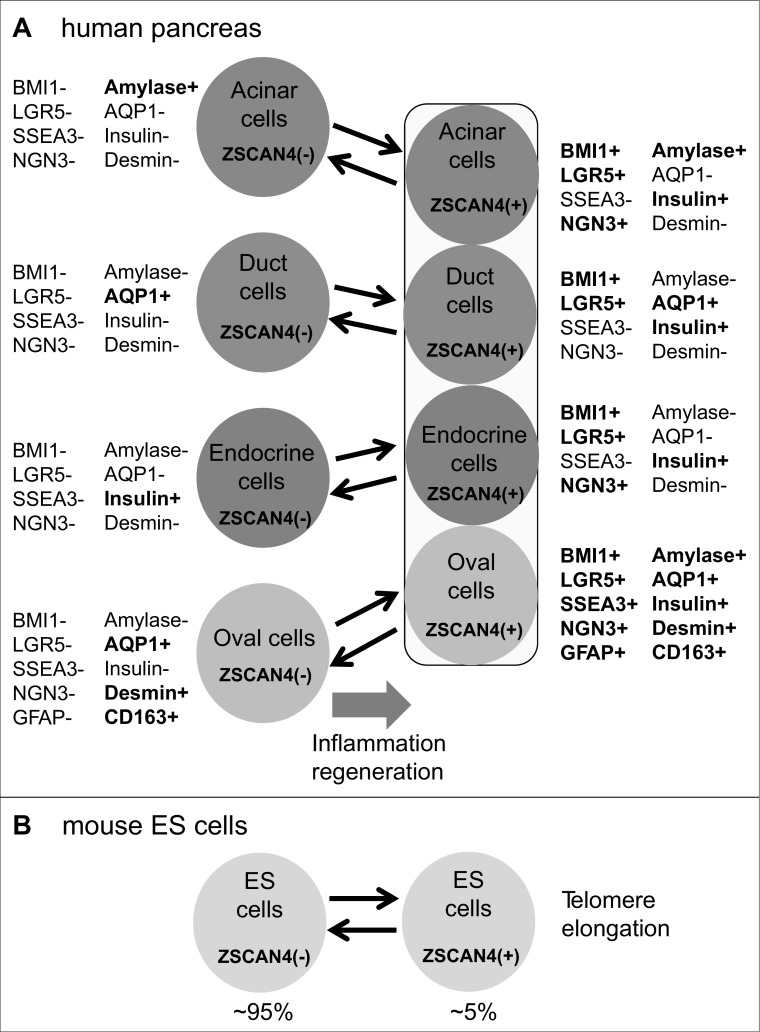
Although the current study is limited primarily to the descriptive observational study of human pancreas in normal and diseased states as well as mouse-induced pancreatitis, the detailed immunohistochemical studies have revealed the presence of rare and unique populations of cells that coexpress ZSCAN4, stem/progenitor markers, and markers specific to the differentiated cells in distinct compartments of pancreas: ducts, acini, islets of Langerhans, and oval cells. Considering all the data together, we propose that cells in each compartment (e.g., acinar cells), which usually do not express ZSCAN4, infrequently transition to the ZSCAN4+ state while retaining the expression of specific markers (e.g., amylase) (Fig. 9A). The transition of differentiated pancreatic cells to the ZSCAN4+ state can be stimulated by inflammation (Fig. 9A). Importantly, this model is consistent with unique expression patterns of mouse Zscan4 in mouse ES cells: ES cells oscillate between the Zscan4− state and Zscan4+ state, resulting in the mixed population of ∼95% Zscan4− cells and ∼5% Zscan4+ cells in undifferentiated ES cell cultures (Fig. 9B) (28). Interestingly, when the differentiated cells in each compartment infrequently become ZSCAN4+, these cells also express stem/progenitor cell markers, such as BMI1 and LGR5 (Fig. 9A). The coexpression of stem/progenitor cell markers and differentiation markers in the same cell suggests that there are no distinct stem/progenitor cell pools that are set aside from the differentiated cells in the pancreas but does suggest the existence of differentiated cells that transiently possess a stem/progenitor cell character (Fig. 9A). The infrequent presence of ZSCAN4+ cells in the unaffected pancreatic tissues is also consistent with the notion that the pancreas is an organ that does not have active tissue turnover/regeneration without inflammation (4).
Fig. 9.
Summary diagram. A: summary diagram showing the expression of various markers in each compartment of adult human pancreas at the ZSCAN4− state and the ZSCAN4+ state. B: diagram showing the demonstrated oscillation between the Zscan4− state and the Zscan4+ state in mouse ES cells (redrawn from the Ref. 28).
The model shown in Fig. 9A has been further extended by the following additional intriguing observation: when the differentiated cells in each compartment become ZSCAN4+, these cells express not only stem/progenitor cells markers but also differentiation markers of other cell types (Fig. 9A). For example, the expression of insulin was detected in ZSCAN4+ acinar cells, duct cells, and oval cells; the expression of amylase was detected in ZSCAN4+ in duct cells and oval cells. These data suggest that these ZSCAN4+ cells may be able to take phenotypes that are different from the original cell types and further suggest that these mixed endocrine-exocrine cells are progenitors for each compartment of adult human pancreas. These data seem to be consistent with previous reports: the existence of intermediate endocrine-acinar pancreatic cells in the pancreatic duct ligation condition (5); the commonality of the origin of exocrine and endocrine cells in the pancreas or transdifferentiation from exocrine cells to insulin-positive endocrine cells in a specific condition (12, 26, 41). Overall, our findings are consistent with the notion that facultative stem/progenitor cells (40) are present in the pancreas. Although further study will be required to obtain evidence that these ZSCAN4+ cells can indeed differentiate to mature exocrine and endocrine cells in vivo and in vitro, the concept of facultative stem/progenitor cells is not only consistent with our data but may also help to explain some of the controversial issues regarding the stem/progenitor cells in adult pancreas: the presence of functional stem/progenitor cells that reside in each compartment [i.e., duct cells (10, 12, 16, 29, 31, 32), acinar cells (14, 26, 30, 33, 39, 44), endocrine cells (34, 35), and pancreatic stellate cells (23)] of the adult human and mouse pancreas.
A possibility of ZSCAN4+ cells being facultative stem/progenitor cells may also have important implications for the treatment of type 1 diabetes, which is caused by a specific immunological destruction of pancreatic β-cells. Major efforts and progress have been made to produce functional β-cells for transplantation therapy (1) from human ES cells (7, 17, 21) and iPS cells (2, 37) in vitro. It has also been reported that mature differentiated pancreatic acinar cells were successfully reprogrammed to insulin-secreting β-cells in vivo by the forced expression of several transcription factors with adenoviruses (45). However, the production of sufficient quantity of mature pancreatic β-cells has not been reported. Considering the presence of a rare population of ZSCAN4+ cells in each compartment of the pancreas, use of these cells as alternative sources for producing β-cells might be an attractive option for further study.
GRANTS
This work was supported in part by grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology; the Ministry of Health, Labor and Welfare for intractable pancreatic diseases; Nagono Medical foundation; and Japan Medical Association. This research was supported in part by the Intramural Research Program of the National Institute on Aging.
DISCLOSURES
None of the authors have any conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: S.B.K. and M.S.K. conception and design of research; S.B.K., S.A., Y.Y., A.Y., K.K., S.N., and H.I. performed experiments; S.B.K. and M.S.K. analyzed data; S.B.K. and M.S.K. interpreted results of experiments; S.B.K. prepared figures; S.B.K. drafted manuscript; S.B.K. and M.S.K. edited and revised manuscript; S.B.K., S.A., Y.Y., A.Y., K.K., S.N., H.I., and M.S.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Y. Sawa and K. Kamimura (Nagoya University) for technical assistance and N. Mizuno (Aichi Cancer Center Hospital) for providing autoimmune pancreatitis sections.
Glossary
- ALDH1
Aldehyde dehydrogenase 1
- αSMA
α-smooth muscle actin
- AQP1
Aquaporin 1
- BMI1
B lymphoma Mo-MLV insertion region 1 homolog
- CAII
Carbonic anhydrase II
- DCAMKL-1
Doublecortin and CaM kinase-like-1
- ES cells
Embryonic stem cells
- GFAP
Glial fibrillary acidic protein
- LGR5
Leucine-rich repeat-containing, G protein-coupled receptor 5
- NEUROG3
Neurogenin 3
- SSEA3
Stage-specific embryonic-antigen 3
- ZSCAN4
Zinc finger and SCAN domain containing 4
REFERENCES
- 1. Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 43: 128–133, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138: 1681–1696, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, Esposito I, Friess H, Gress TM, Habisch HJ, Hwang RF, Jaster R, Kleeff J, Kloppel G, Kordes C, Logsdon CD, Masamune A, Michalski CW, Oh J, Phillips PA, Pinzani M, Reiser-Erkan C, Tsukamoto H, Wilson J. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut 61: 172–178, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falco G, Lee SL, Stanghellini I, Bassey UC, Hamatani T, Ko MS. Zscan4: a novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Dev Biol 307: 539–550, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43: 34–41, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129: 2447–2457, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Hao E, Tyrberg B, Itkin-Ansari P, Lakey JR, Geron I, Monosov EZ, Barcova M, Mercola M, Levine F. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med 12: 310–316, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Hirata T, Amano T, Nakatake Y, Amano M, Piao Y, Hoang HG, Ko MS. Zscan4 transiently reactivates early embryonic genes during the generation of induced pluripotent stem cells (Abstract). Sci Rep 2: 208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA 105: 19915–19919, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, Okumura F, Nishikawa T, Kobayashi K, Ichiya T, Takatori H, Yamakita K, Kubota K, Hamano H, Okamura K, Hirano K, Ito T, Ko SB, Omata M. Standard steroid treatment for autoimmune pancreatitis. Gut 58: 1504–1507, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Ko SB, Mizuno N, Yatabe Y, Yoshikawa T, Ishiguro H, Yamamoto A, Azuma S, Naruse S, Yamao K, Muallem S, Goto H. Corticosteroids correct aberrant CFTR localization in the duct and regenerate acinar cells in autoimmune pancreatitis. Gastroenterology 138: 1988–1996, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ko SB, Naruse S, Kitagawa M, Ishiguro H, Furuya S, Mizuno N, Wang Y, Yoshikawa T, Suzuki A, Shimano S, Hayakawa T. Aquaporins in rat pancreatic interlobular ducts. Am J Physiol Gastrointest Liver Physiol 282: G324–G331, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A, Shigemoto T, Nabeshima Y, Nakahata T, Fujiyoshi Y, Dezawa M. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci USA 107: 8639–8643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mato E, Lucas M, Petriz J, Gomis R, Novials A. Identification of a pancreatic stellate cell population with properties of progenitor cells: new role for stellate cells in the pancreas. Biochem J 421: 181–191, 2009 [DOI] [PubMed] [Google Scholar]
- 15. May R, Sureban SM, Hoang N, Riehl TE, Lightfoot SA, Ramanujam R, Wyche JH, Anant S, Houchen CW. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells 27: 2571–2579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. May R, Sureban SM, Lightfoot SA, Hoskins AB, Brackett DJ, Postier RG, Ramanujam R, Rao CV, Wyche JH, Anant S, Houchen CW. Identification of a novel putative pancreatic stem/progenitor cell marker DCAMKL-1 in normal mouse pancreas. Am J Physiol Gastrointest Liver Physiol 299: G303–G310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, Oyama K, Kawaguchi M, Ishizuka N, Iwanaga T, Seino S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci USA 102: 15116–15121, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mizuno N, Bhatia V, Hosoda W, Sawaki A, Hoki N, Hara K, Takagi T, Ko SB, Yatabe Y, Goto H, Yamao K. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: a comparison study with EUS-FNA. J Gastroenterol 44: 742–750, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Okazaki K, Kawa S, Kamisawa T, Shimosegawa T, Tanaka M. Japanese consensus guidelines for management of autoimmune pancreatitis: I. Concept and diagnosis of autoimmune pancreatitis. J Gastroenterol 45: 249–265, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology 132: 720–732, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci USA 107: 75–80, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sangiorgi E, Capecchi MR. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proc Natl Acad Sci USA 106: 7101–7106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smukler SR, Arntfield ME, Razavi R, Bikopoulos G, Karpowicz P, Seaberg R, Dai F, Lee S, Ahrens R, Fraser PE, Wheeler MB, van der Kooy D. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 8: 281–293, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell 17: 849–860, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132: 197–207, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Yatoh S, Dodge R, Akashi T, Omer A, Sharma A, Weir GC, Bonner-Weir S. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes 56: 1802–1809, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 90: 5002–5012, 1997 [PubMed] [Google Scholar]
- 28. Zalzman M, Falco G, Sharova LV, Nishiyama A, Thomas M, Lee SL, Stagg CA, Hoang HG, Yang HT, Indig FE, Wersto RP, Ko MS. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 464: 858–863, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 457: 603–607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]