Abstract
Previous studies have demonstrated positive-inotropic effects of calcitonin gene-related peptide (CGRP), but the mechanisms remain unclear. Therefore, two experiments were performed to determine the physiological correlates of the positive-inotropic effects of CGRP. Treatments designed to antagonize the effects of physiologically active CGRP1–37 included posttreatment with CGRP8–37 and pretreatment with LY-294002 (LY, an inhibitor of phosphatidylinositol 3-kinase), 17β-estradiol (E), and progesterone (P) were also used to modulate the effects of CGRP1–37. Experiment 1 was in vitro studies on sarcomeres and cells of isolated adult rat cardiac myocytes. CGRP1–37, alone and in combination with E and P, decreased sarcomere shortening velocities and increased shortening percentages, effects that were antagonized by CGRP8–37, but not by LY. CGRP1–37 increased resting intracellular calcium ion concentrations and Ca2+ influxes, effects that were also antagonized by both CGRP8–37 and LY. Experiment 2 was in vivo studies on left ventricular pressure-volume (PV) loops. CGRP1–37 increased end-systolic pressure, ejection fraction, and velocities of contraction and relaxation while decreasing stroke volume, cardiac output, stroke work, PV area, and compliance. After partial occlusion of the vena cava, CGRP1–37 increased the slope of the end-systolic PV relationship. CGRP8–37 and LY attenuated most of the CGRP-induced changes. These findings suggest that CGRP-induced positive-inotropic effects may be increased by treatments with estradiol and progesterone and inhibited by LY. The physiological correlates of CGRP-induced positive inotropy observed in rat sarcomeres, cells, and intact hearts are likely to reveal novel mechanisms of heart failure in humans.
Keywords: calcitonin gene-related peptide, estrogen, progesterone, PI3K, cardiac
calcitonin gene-related peptide (CGRP) decreases blood pressure, increases heart rate, and has a positive-inotropic effect in humans (9, 14). CGRP is expressed in ventricular sensory nerves and cardiomyocytes (41), where it exerts positive chronotropic, inotropic, and pro-hypertrophic effects (3, 4, 39, 40). At low concentrations (pM range), CGRP may have a negative-inotropic effect (41). These effects are thought to be mediated mainly by the CGRP1 receptor (17, 39).
CGRP is also the most potent known vasodilator (43, 43, 44, 44), and administration of CGRP to normotensive animals and humans, as well as to spontaneously hypertensive rats, decreases blood pressure in a dose-dependent manner (25). The vasodilator and hypotensive effects of CGRP are amplified in the presence of sex steroid hormones (23, 38). A COOH-terminal fragment of human CGRP (CGRP8–37) is an effective CGRP receptor antagonist that can inhibit CGRP-induced vasodilation (22). CGRP-induced vasodilation might be responsible for ∼30% of basal coronary blood flow (24). Previous studies from our laboratory have demonstrated a predilection for hypertension in CGRP knockout mice (12). Moreover, acute administration of CGRP8–37 produces a hypertensive effect in NG-nitro-l-arginine methyl ester-treated rats during pregnancy when sex steroid hormone levels are increased, an effect not observed postpartum (11, 13).
Several second-messenger pathways, including cAMP, nitric oxide (NO), and K+ channel activation, are found to mediate the effects of CGRP on smooth muscle (24). The inotropic activity of CGRP is reported to be mediated mainly by cAMP/PKA or PKC signal transduction molecules (35, 41, 47). In human embryonic kidney-296 cells, CGRP appears to produce downstream activation of ERK by PKA and phosphatidylinositol 3-kinase (PI3K), as well as p38 MAPK activation via PKA signaling (35). These intracellular signal transduction pathways have recently been implicated in the regulation of cardiac contraction. Indeed, our laboratory has reported that ERK1/2 and PI3K inhibit K+ current and intermediate-conductance K+ current channels in normal and hypertrophied cardiomyocytes (42), which has depolarizing effects. In human cardiomyocytes, CGRP binding to a receptor juxtapositioned on L-type Ca2+ channels is reported to increase Ca2+ transport (5, 6); other researchers report decrement in the activity of transient outward current channels (41). Such increases in Ca2+ transport are known to increase the durations of cardiomyocyte action potentials and calcium transients and are important factors in cardiac hypertrophy and heart failure. Our laboratory has recently shown that the survival antiapoptotic PI3K signaling pathway is critical to cardiac mechanical function by calcium channel mechanisms, especially during cardiac hypertrophy (1). Moreover, increased plasma levels of CGRP are associated with these complications of cardiovascular disease (8, 14). Our laboratory has previously reported that estradiol and progesterone enhance CGRP-induced contractile effects in vascular smooth muscle, and we sought to consolidate these findings in the heart (10–12). The present study was, therefore, designed to determine the physiological correlates of CGRP-induced positive inotropy and to evaluate the roles of sex steroids and PI3K as mediators of CGRP signaling.
MATERIALS AND METHODS
Conformity Statement
All the procedures conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH), publication no. 85–23, revised 1996. The animal protocols have been independently approved by Howard University Institutional Animal Care and Use Committee.
Animal Preparation
Male Sprague-Dawley rats of 200–250 g body weight were purchased from Charles Rivers. The rats were allowed to recover and acquaint with their new environment upon arrival to the animal house of the Howard University College of Medicine for 1 wk. The animals were kept under secure, clean, and controlled room temperature (70–74°F) with a 600–1800 light cycle and were fed food and water ad libitum.
Isolation of Cardiomyocytes
All the reagents were purchased from Sigma Chemicals (St. Louis, MO). Double-distilled water from a MilliQ system (Millipore, MA) was used to prepare all solutions. Prepared solutions were oxygenated for 20 min before perfusion with 5% CO2-95% O2 and pH at 7.4 with HCl or NaOH.
Animals were injected with sodium heparin (1,000 U/kg ip) and anesthetized with pentobarbital sodium (40 mg/kg ip), 30 min before removal of the heart. After excision, the heart was quickly transferred to a Langendorff setup for 5-min retrograde coronary perfusion through the aorta (10 ml/min at 37°C) with normal Tyrode solution containing the following (in mM): 140 NaCl, 5.4 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 20 NaHCO3, 10 d-glucose, 0.1 EGTA, 0.4 NaH2PO4, 20 taurine, 10 creatine. This was followed by 20-min perfusion with nominal Ca2+-free Tyrode solution to which 25 mg/30 ml collagenase II (Sigma) and 3 mg/30 ml protease type XIV (Sigma) were added for the last 10 min. Then the heart was cut, minced, and filtered in a buffer solution containing the following (in mM): 113 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4.7H2O, 12 NaHCO3, 10 KHCO3. Calcium was introduced gradually to the freshly isolated cardiomyocytes through five 10-min step incubations to 1.2 mM CaCl2. Freshly isolated cardiomyocytes showing no signs of blebs or round edges were used for up to 12 h.
Inotropic Measurements In Vitro
The contractile properties of the cardiomyocytes were assessed at the cellular and sarcomere levels simultaneously, with the dynamic changes in intracellular calcium using the “Myocyte Calcium and Contractility Recording System” from Ionoptix (Milton, MA). A μStep light source allowing a sampling rate up to 1 kHz toggled between two excitation wavelengths of 360 and 380 μm, and a fluorescent emitted wavelength of 510 μm was filtered through a 480- to 520-μm filter and detected by a photomultiplier tube. Cells were first incubated with fura 2-AM (1 μM) for 15 min and then washed twice with Tyrode solution for 10 min before being loaded into the RC-27NE chamber from Warner Instruments (Hamden, CT) containing Tyrode solution with 1.5 mM calcium. The wired chamber was placed on an inverted IX70 Olympus microscope (Center Valley, PA) and connected to the Myopacer field stimulator (Ionoptix, MA), which was set to stimulate the cell at 1.5 × threshold for 3 ms (1 Hz). The cells were initially excited at 360 μm (0.5 s) and then switched to 380 μm for 30 s and then back to 360 μm for another 0.5 s. Emitted fluorescence of 510 μm was filtered though a 480- to 520-μm filter and captured by a photomultiplier tube connected to a Fluorescence System Interface (Ionoptic) relayed to a computer. Calcium levels were assessed by the ratio of 360/380 readings using IonWizard Software (Ionoptix), where 360-μm values were determined by extrapolation between the initial and final 360-μm acquisitions. Using Myocam-S connected to the same μStep excitation source, video images of the cells were recorded (60 Hz) simultaneously with the IonWizard software to measure the mechanical properties of sarcomeres and cells.
Pressure-Volume Loop Acquisition
Initially, rats were anesthetized using halothane inhalation for tracheal intubation. The endotracheal tube was then connected to a mechanical ventilator for continuous delivery of 1–2% halothane with oxygen-enriched room air. A dissection of the diaphragm exposed the apex of the heart where a 26-gauge needle was briefly inserted to introduce a conductance catheter (1.9 F from Scisense) in the left ventricle (LV) along the longitudinal axis toward the aortic valve. Once in the aorta, the catheter was retrieved back into the LV for pressure-volume (PV) measurements. The catheter was connected to the Advantage system by Scisense that acquired pressure, admittance, phase shift, and amplitude. Volume was derived in real time from admittance, phase, and amplitude data using an algorithm based on Wei's equation to compensate for the varying wall thickness during contraction and relaxation, as well as for the distance to the LV free wall, which helps optimum positioning of the catheter tip in the middle of the chamber. The Advantage box was connected to a computer where data were analyzed, and PV loops were displayed in real time using Labscribe 2 software from iWorx (Dover, NH). Real-time data comprised heart rate, end-diastolic and end-systolic pressures (ESP) and volumes, maximum and minimum pressures, change in pressure over time, stroke volume (SV), cardiac output, ejection fraction (EF), and stroke work. Labscribe 2 was also used for offline analysis of PV area (PVA), potential energy, cardiac efficiency, maximum power, preload-recruitable stroke work (PRSW), arterial elastance (Ea), τ, ESP vs. volume relationship (ESPVR), and the end-diastolic pressure vs. volume relationship (EDPVR). A 26-gauge catheter connected to a low-flow syringe pump (NE-1000, New Era Pump System, Farmindale, NY) was inserted in the jugular vein for slow steady and continuous delivery of test drugs, such as CGRP1–37, CGRP8–37, and LY-294002. The syringe concentrations were adjusted to the flow rate of 0.2 μl/min to achieve a steady level of the desired blood concentration in the rat within 5 min. Blood volume was calculated using the following Lee and Blaufox (26) equation, which was originally developed for determination of blood volume in the rat based on body weight: blood volume (ml) = 0.06 × body weight (mg) + 0.77. All measurements were performed during brief suspension of the mechanical ventilation (to avoid ventilation-related changes in pressures and volumes) and at steady state, where, at the beginning of the experiment, 15 min were allowed for stabilization of the acquired hemodynamic data for controls and for steady-state drug effects. Contractility measures for ESPVR and PRSW were performed during brief compression of the hepatic vein to decrease venous return and to measure the changes in pressure within sequential PV loops at different volumes.
Experimental Design
Experiment 1: effects of CGRP on cardiac myocytes, in vitro.
We tested the time course of CGRP effects in vitro on sarcomeres and cells associated with dynamic changes in intracellular calcium concentrations during contractions of ventricular myocytes. Freshly isolated myocytes were stimulated at 1 Hz, and the average of 30-s recordings were analyzed in control, 5 min, 15 min, 45 min, 1 h, 2 h, and 3 h of administering 1 nM CGRP1–37. The velocity and percentage of sarcomere shortening were increased by 32.4 ± 11.1% (P < 0.05) and 46.5 ± 22.9% (P < 0.05), respectively, after 3 h of exposure to CGRP1–37 (data not shown). A similar profile was depicted for effects of CGRP1–37 on intracellular Ca2+ concentrations. The CGRP1–37 effects reached steady state at 3 h after exposure. Treatment with 1 nM CGRP8–37 was used to antagonize the effects of CGRP1–37. LY-294002 (1 μM) was administered to determine whether the cell survival pathway signaling molecule PI3K was involved in the effects of CGRP1–37 and of CGRP8–37. 17β-Estradiol (10 nM) and progesterone (10 nM) treatments were employed to modulate the effects of the CGRP treatments.
Experiment 2: effects on heart function, in vivo.
Cardiac function in vivo was assessed by LV catheterization and acquisition of PV loop data. The same drugs as were used for cardiac myocyte treatments in experiment 1 were administered by bolus injection into the jugular vein. Compression of the vena cava was used to generate regressive PV loop data before and after the drug treatments, from which the ESPVR, EDPVR, and other inotropic properties were evaluated.
Statistical Analysis
All statistical analyses were performed using Prism 6.0 (Graphpad) software and verified using Microsoft Excel, which gave the same results. The paired Student's t-test was used to compare data before and after drug treatment of the same animal group. The heteroscedastic two-sample unpaired Student's t-test, assuming unequal variances, was used to compare the drug effects between two different animal groups. Using the null hypothesis, P ≤ 0.05 was significant.
RESULTS
Effects of CGRP on Cardiac Myocytes In Vitro
Mechanisms of the inotropic and lusitropic effects of CGRP on the sarcomere.
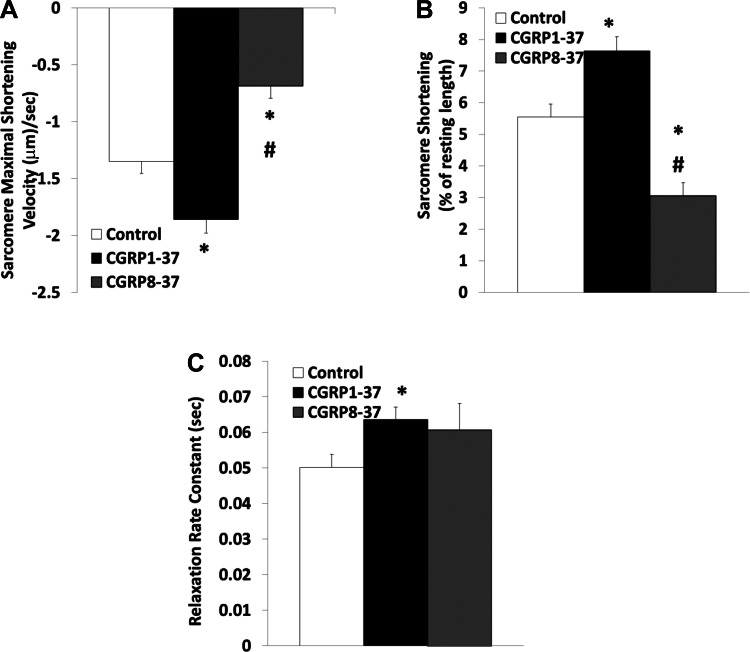
Baseline resting sarcomere length did not change significantly within the test time frame. However, addition of CGRP1–37 increased the baseline sarcomere length by 7.9 ± 0.2% (P < 0.01). Figure 1 shows that CGRP1–37 increased the sarcomeric velocity of contraction by 37.70 ± 7.88% (P < 0.01) and percent shortening by 37.57 ± 7.26% (P < 0.01). Posttreatment with CGRP8–37 decreased velocity and percent shortening to less than control levels (50.67 ± 16.43% and 55.07 ± 13.40% of control, respectively; P < 0.05).
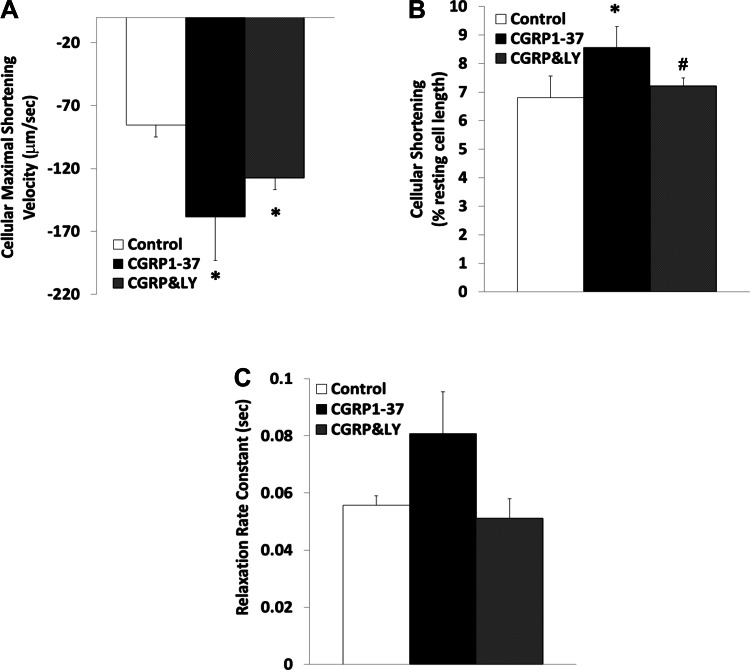
Fig. 1.
Effects of calcitonin gene-related peptide (CGRP)1–37 and CGRP8–37 on cardiomyocyte sarcomere contractile dynamics. Bars show effects of control, 1 nM CGRP1–37 treatments, and 1 nM CGRP8–37 posttreatments on maximal shortening velocity of sarcomeres during contraction (A), sarcomere shortening as a percentage of resting length (B), and sarcomere relaxation rate constant (C). Values are means ± SE; n = 20 from 4–5 animals for each group. *Significantly different from controls at P < 0.05. #Significantly different from CGRP1–37 treatments at P < 0.05.
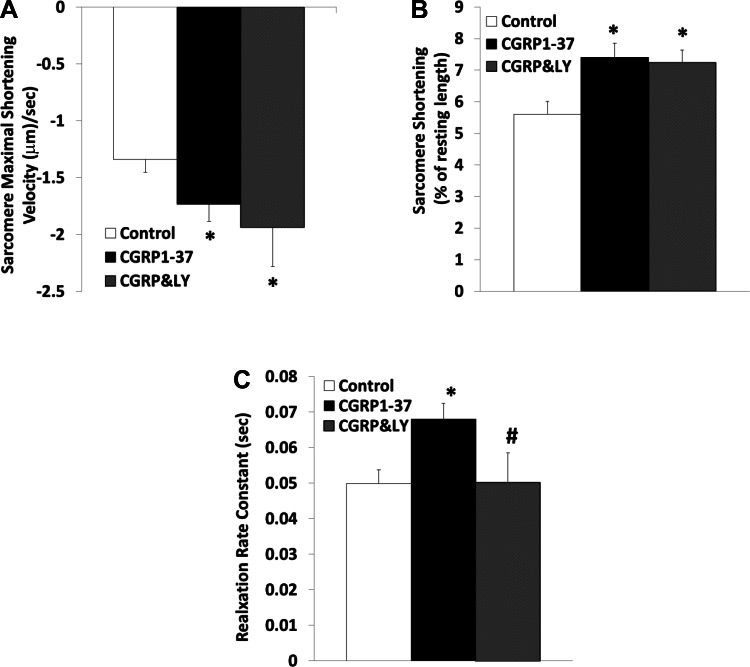
Figure 2 demonstrates that inhibition of PI3K by LY-294002 did not effectively antagonize the positive-inotropic effects of CGRP1–37 on the sarcomere. The effects of CGRP1–37 on the rate of sarcomeric relaxation were not significantly affected by CGRP8–37; however, this relaxation was blocked by inhibition of PI3K signaling using LY-294002.
Fig. 2.
Effects of CGRP1–37 and LY-294002 (LY) on cardiomyocyte sarcomere contractile dynamics. Bars show effects of control, 1 nM CGRP1–37 treatments, and 1 μM LY (CGRP&LY) posttreatments on maximal shortening velocity of sarcomeres during contraction (A), sarcomere shortening as a percentage of resting length (B), and sarcomere relaxation rate constant (C). Values are means ± SE; n = 20 from 4–5 animals for each group. *Significantly different from controls at P < 0.05. #Significantly different from CGRP1–37 treatments at P < 0.05.
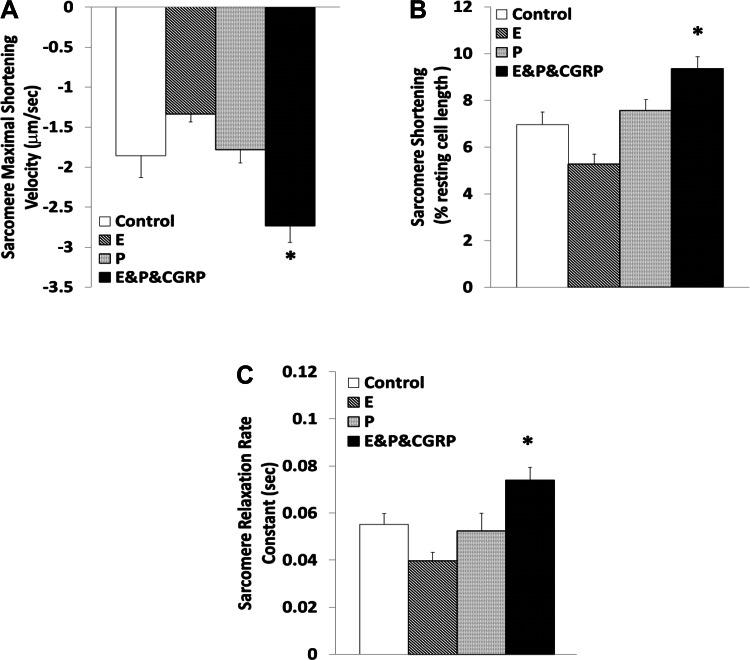
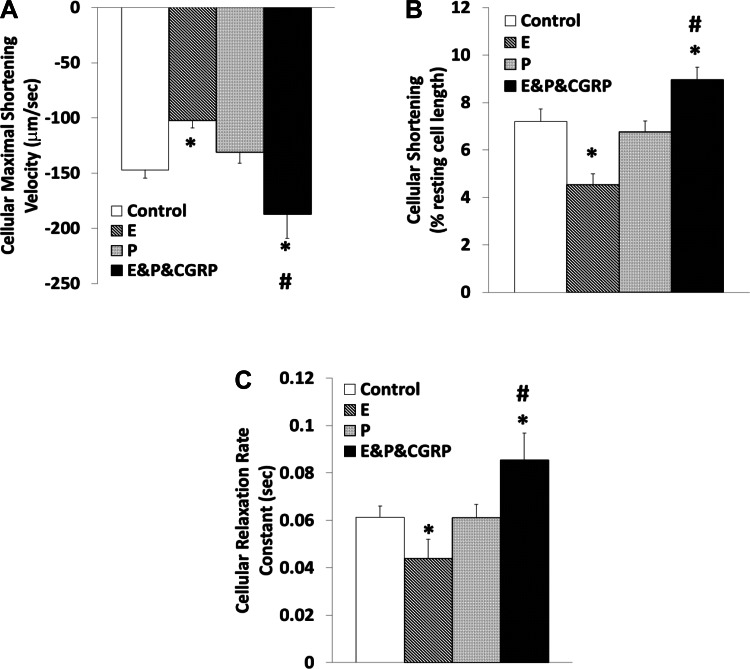
Figure 3 depicts the effects of 17β-estradiol and progesterone treatments, which did not change the sarcomere length significantly in the absence of CGRP1–37. 17β-Estradiol, but not progesterone, exhibited a tendency to reduce the maximal velocity of sarcomere contraction (27.94 ± 7.28%) and percent shortening (24.36 ± 8.52%) with marginal statistical significance (P = 0.07). The positive-inotropic effects of CGRP1–37 were enhanced in the presence of 17β-estradiol and progesterone (contraction by 47.33 ± 7.44% and percent shortening by 34.39 ± 5.48%, respectively; P < 0.05). Neither 17β-estradiol nor progesterone alone altered the relaxation rate constant. In the presence of both 17β-estradiol and progesterone, CGRP1–37 increased the relaxation rate constant by 34.30 ± 7.21% (P < 0.01).
Fig. 3.
Modulation of the contractile effects of CGRP1–37 by 17β-estradiol (E) and progesterone (P) at the sarcomere level. Bars show effects of control, 10 nM E, 10 nM P, and 1 nM CGRP1–37 with 10 nM of E and 10 nM of P (E&P&CGRP) treatments on maximal shortening velocity of sarcomeres during contraction (A), sarcomere shortening as a percentage of resting length (B), and sarcomere relaxation rate constant (C). Values are means ± SE; n = 20 from 4–5 animals for each group. *Significantly different from controls at P < 0.05.
Mechanisms of the Cellular Inotropic and Lusitropic Effects of CGRP
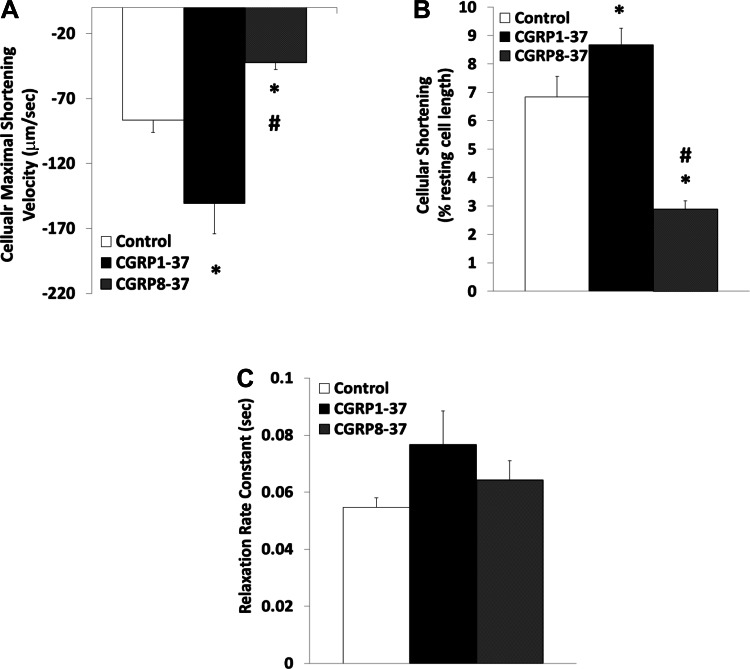
Figures 4 and 5 show that the cellular inotropic effects of CGRP were similar to those found at the sarcomeric level. CGRP1–37 increased the baseline myocyte length by 23.31 ± 2.74% (P < 0.01). The baseline cell length was decreased back to the control value by LY-294002; treatment with CGRP8–37 had no effect on baseline cell length. In parallel, CGRP1–37 increased the velocity of cell contraction (73.76 ± 15.5%; P < 0.05) and cell shortening (26.71 ± 6.78%; P < 0.05). Subsequent treatment with CGRP8–37 decreased the maximum velocity of cell contraction (48.85 ± 12.34%; P < 0.05) and the cellular shortening (42.12 ± 10.63% of control; P < 0.01) to below control levels. The inotropic effect of CGRP1–37 on the latter was antagonized by LY-294002. The tendency of CGRP to increase the rate of cellular relaxation was not statistically significant (P = 0.08).
Fig. 4.
Effects of CGRP1–37 and CGRP8–37 on cardiomyocyte contractile dynamics at the cellular level. Bars show effects of control, 1 nM CGRP1–37 treatments, and 1 nM CGRP8–37 posttreatments on maximal shortening velocity of cells during contraction (A), cell shortening as a percentage of resting length (B), and cell relaxation rate constant (C). Values are means ± SE; n = 20 from 4–5 animals for each group. *Significantly different from controls at P < 0.05. #Significantly different from CGRP1–37 treatments at P < 0.05.
Fig. 5.
Effects of CGRP1–37 and LY on cardiomyocyte contractile dynamics at the cellular level. Bars show effects of control, 1 nM CGRP1–37 treatments, and 1 μM LY (CGRP&LY) posttreatments on maximal shortening velocity of cells during contraction (A), cell shortening as a percentage of resting length (B), and cell relaxation rate constant (C). Values are means ± SE; n = 20 from 4–5 animals for each group. *Significantly different from controls at P < 0.05. #Significantly different from CGRP1–37 treatments at P < 0.05.
Figure 6 shows that 17β-estradiol decreased the maximal velocity of cellular contraction (30.36 ± 6.60%; P < 0.05), cellular shortening (36.87 ± 9.83%; P < 0.01), and the cellular relaxation rate constant (28.25 ± 18.63%; P < 0.05); progesterone had no effect. In the presence of 17β-estradiol, the positive-inotropic effects of CGRP1–37 were augmented (contraction velocity by 27.43 ± 11.67% and cell shortening by 24.31 ± 7.36%, respectively; P < 0.01), and the lusitropic effects of CGRP1–37 were attenuated (39.42 ± 13.25%; P < 0.01).
Fig. 6.
Modulation of cardiomyocyte contractile effects of CGRP1–37 by E and P at the cellular level. Bars show effects of control, 10 nM E, 10 nM P, and 1 nM CGRP1–37 with 10 nM of E and 10 nM of P (E&P&CGRP) on maximal shortening velocity of cells during contraction (A), cell shortening as a percentage of resting length (B), and cell relaxation rate constant (C). Values are means ± SE; n = 20 from 4–5 animals for each group. *Significantly different from controls at P < 0.05. #Significantly different from CGRP1–37 treatments at P < 0.05.
Mechanism of CGRP-induced Intracellular Calcium Dynamics
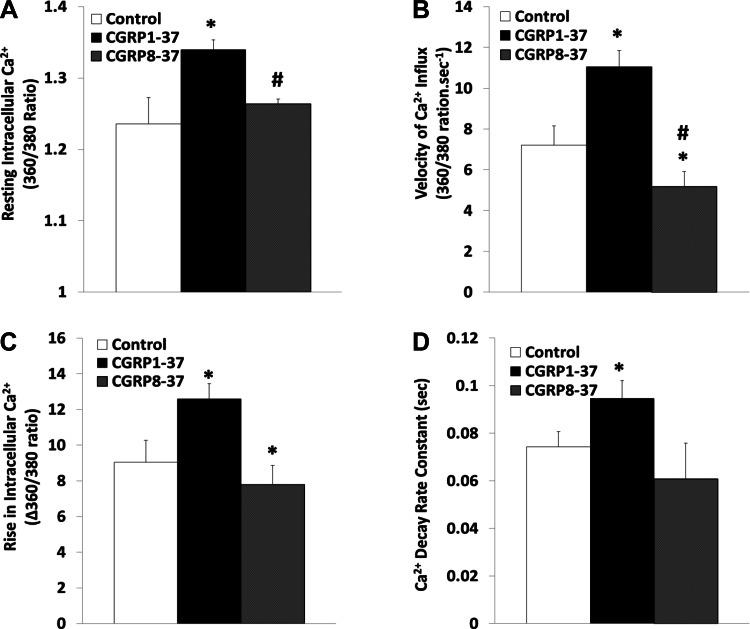
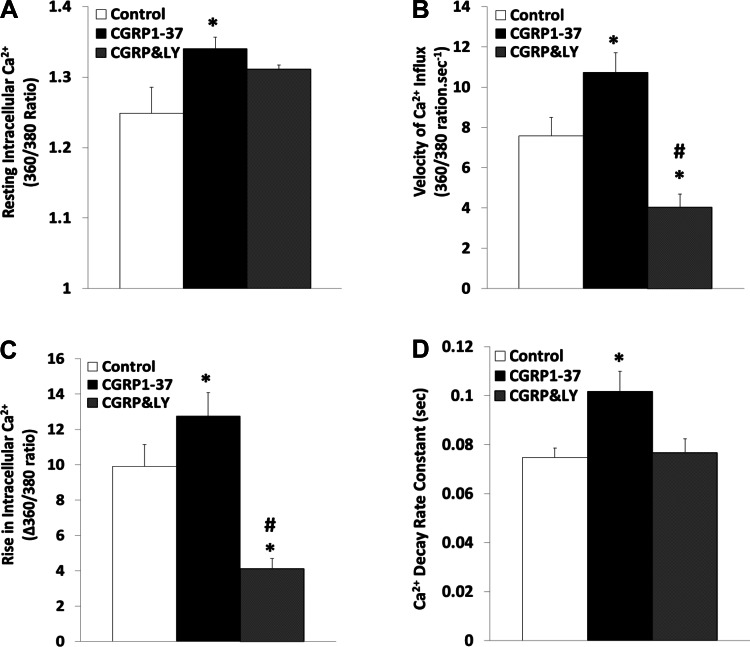
Figure 7 demonstrates that treatment of freshly isolated ventricular myocytes with CGRP1–37 increased the baseline level of intracellular Ca2+ by 8.06 ± 2.98% (P < 0.01), the amount of Ca2+ transients by 49.76 ± 13.71% (P < 0.05), and the velocity of Ca2+ rise by 57.28 ± 13.00% (P < 0.05) during contraction. CGRP1–37 also increased the rate constant of Ca2+ decay during relaxation by 40.79 ± 8.51% (P < 0.05); all of these CGRP1–37-induced changes in Ca2+ dynamics were inhibited by CGRP8–37. Figure 8 shows that treatment of the myocytes with LY-294002 reversed the CGRP1–37 effects; the maximum velocity of calcium influx and the rise in intracellular calcium were reverted to less than control values.
Fig. 7.
Effects of CGRP1–37 and CGRP8–37 on cardiomyocyte calcium dynamics. Bars show effects of control, 1 nM CGRP1–37 treatments, and 1 nM CGRP8–37 posttreatments on resting intracellular calcium ion concentration ([Ca2+]i; A), Ca2+ influx during cell contraction (B), [Ca2+]i increment during cell contraction (C), and Ca2+ decay rate constant during cell relaxation (D). Values are means ± SE; n = 20 from 4–5 animals for each group. *Significantly different from controls at P < 0.05. #Significantly different from CGRP1–37 treatments at P < 0.05.
Fig. 8.
Effects of CGRP1–37 and LY on cardiomyocyte calcium dynamics. Bars show effects of control, 1 nM CGRP1–37 pretreatments, and 1 μM LY posttreatments (CGRP&LY) on resting [Ca2+]i (A), Ca2+ influx during cell contraction (B), [Ca2+]i increment during cell contraction (C), and Ca2+ decay rate constant during cell relaxation (D). Values are means ± SE; n = 20 from 4–5 animals for each group. *Significantly different from controls at P < 0.05. #Significantly different from CGRP1–37 treatments at P < 0.05.
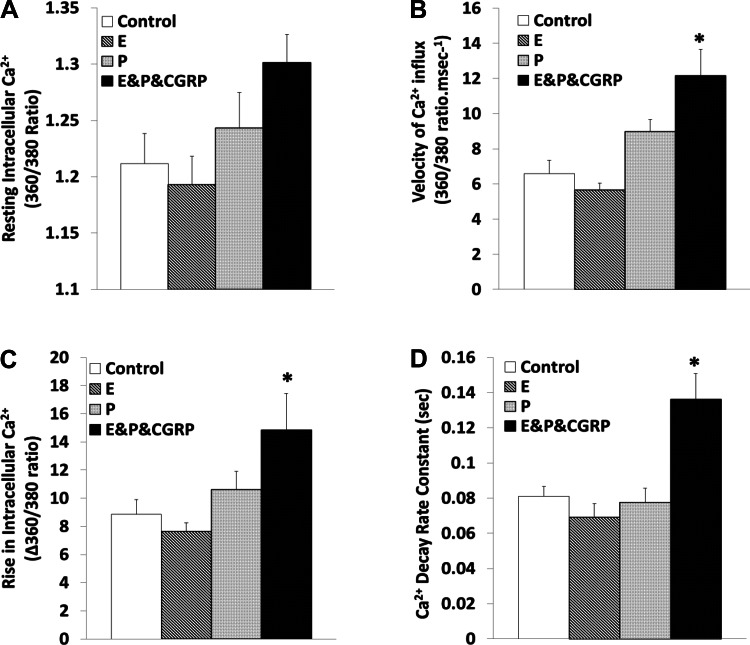
Figure 9 shows that combination treatment with 17β-estradiol plus progesterone augmented the calcium response to CGRP1–37. In the presence of CGRP1–37, percent increases in Ca2+ influx (77.72 ± 17.79%, P < 0.05), Ca2+ influx velocity (85.07 ± 12.14%, P < 0.01), and the rate constant of calcium removal during relaxation (67.77 ± 10.94%, P < 0.05) were detected. Neither 17β-estradiol nor progesterone treatments changed the baseline level of Ca2+ or the contraction-dependent calcium dynamics in the absence of CGRP1–37.
Fig. 9.
Modulation of cardiomyocyte calcium effects of CGRP1–37 by E and P. Bars show effects of control, 10 nM E, 10 nM P, and 1 nM CGRP1–37 with 10 nM of E and 10 nM of P (E&P&CGRP) on resting [Ca2+]i (A), Ca2+ influx during cell contraction (B), [Ca2+]i increment during cell contraction (C), and Ca2+ decay rate constant during cell relaxation (D). Values are means ± SE; n = 20 from 4–5 animals for each group. *Significantly different from controls at P < 0.05 on the rate of Ca2+ decay during relaxation.
Effects of CGRP on Heart Function In Vivo
Effects of CGRP on heart function on the heart's PV loop.
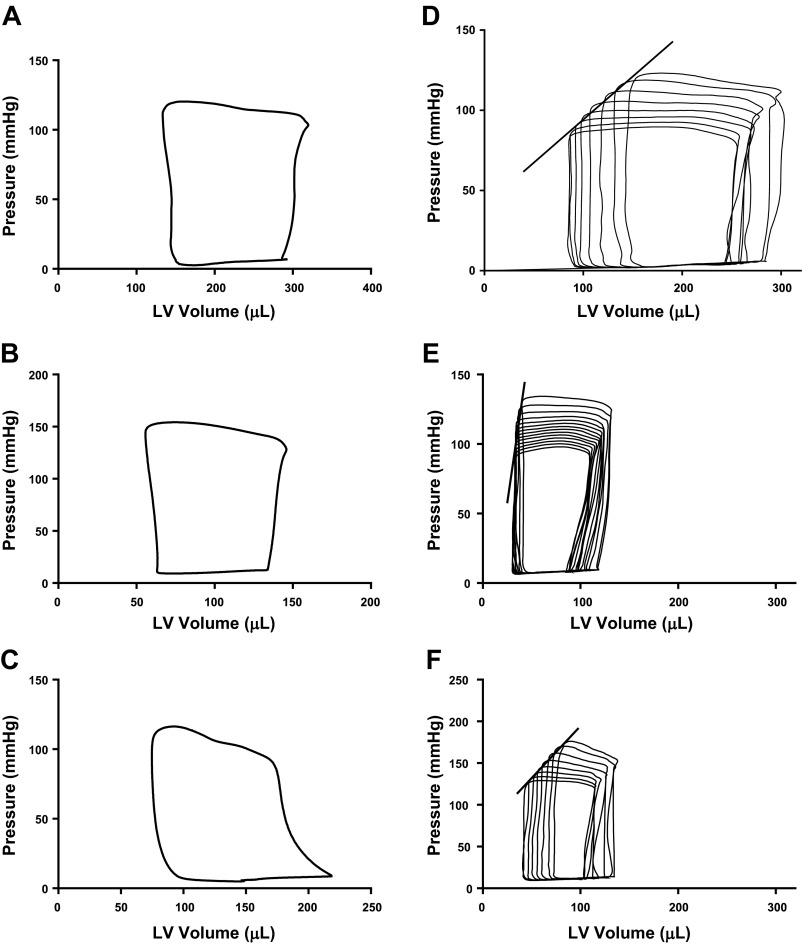
Cardiac function was assessed in vivo by LV catheterization and determination of the PV relationship from the PV loop shown in Fig. 10. CGRP1–37 increased LV ESP by 17.4% (116.0 ± 0.2 vs. 136.2 ± 1.2 mmHg; P < 0.001), velocity of LV contraction by 17% [maximum change in pressure (dPmax) 6,651 ± 26 vs. 7,767 ± 115 mmHg/s; P < 0.001] and velocity of LV relaxation by 12% [minimum change in pressure (dPmin) −6,223 ± 14 vs. −6,979 ± 101 mmHg/s; P < 0.001]. CGRP1–37 decreased the heart rate marginally (298 ± 1 vs. 288 ± 3 beats/min) and decreased the SV markedly, by 38% (170 ± 3 vs. 104 ± 1 μl; P < 0.001). This effect on SV resulted from decrements in both end-diastolic volume (EDV) (46 ± 2%; P < 0.001) and ESV (58 ± 2%; P < 0.001), which produced a 41% decrement in cardiac output (50,494 ± 741 vs. 29,930 ± 201 μl; P < 0.001). CGRP1–37 increased the EF (61.5 ± 0.6 vs. 70.1 ± 0.4%, P < 0.001) and decreased both the stroke work (34.2 ± 0.1%; P < 0.001) and the PVA (44.1 ± 0.2%, i.e., 3.6 ± 0.1 vs. 2.0 ± 0.1 mW/s; P < 0.001). PVA is the mechanical energy produced by LV contraction and thus an indirect measure of oxygen consumption per heartbeat. CGRP1–37 increased the effective relative Ea, an index of afterload derived by the quotient ESP/SV (0.77 ± 0.01 vs. 1.47 ± 0.02 mmHg/μl; P < 0.001) and decreased LV compliance measured by the quotient SV/pulse pressure from 1.60 ± 0.03 to 0.86 ± 0.01 mmHg/ml (P < 0.001). CGRP1–37 did not significantly change the isovolumic relaxation time constant, τ Glantz, a measure of diastolic function.
Fig. 10.
Effects of CGRP1–37 and CGRP8–37 on pressure-volume (PV) relationships of the left ventricle (LV) of adult male rats. Steady-state PV loop for control conditions (A), treatment with 1 nM CGRP1–37 (B), and pretreatment with 1 nM CGRP1–37 and posttreatment with CGRP8–37 (C) are shown. Regression of PV loop following occlusion of the vena cava where the slope of the regression line depicts the end-systolic PV relationship for control conditions (D), treatment with 1 nM CGRP1–37 (E), and pretreatment with 1 nM CGRP 1–37 and posttreatment with 1 nM CGRP8–37 (F) are shown.
Effects of CGRP on Regressive PV Loops
Figure 10 also depicts the effects of compression of the vena cava to reduce preload and thereby generate regressive PV loops before and after the addition of CGRP1–37. CGRP1–37 increased ESPVR, a measure of LV contractility (0.98 ± 0.13 to 2.75 ± 0.29; P < 0.001), increased PRSW, a direct measure of LV contractility, which is preload and afterload independent (84.98 ± 7.51 vs. 112.93 ± 5.52 mmHg; P < 0.001); EDPVR, a measure of LV stiffness (2.16 ± 0.64 to 3.63 ± 0.75; P = 0.15), increased marginally. CGRP1–37 had no significant effect on the slope of the PVA-EDV relationship (0.022 ± 0.001 vs. 0.020 ± 0.001).
Posttreatment with CGRP8–37 reversed the effects of CGRP1–37 on the PV loop as follows: LV ESP (106.03 ± 0.69 mmHg), dPmax (6,198 ± 49 mmHg/s), and dPmin (−4,875 ± 35 mmHg/s) decreased to less than control values (P < 0.001). CGRP8–37 also increased SV (180.47 ± 2.33 μl) and cardiac output (47,778 ± 963 μl) to control values associated with increased LV EDV by 53 ± 2% (P < 0.001 vs. CGRP1–37). EF was not decreased to control values. Posttreatment with CGRP8–37 also increased stroke work by 39.31 ± 0.05% (P < 0.001) and PVA by 22.55 ± 0.03% (P < 0.001) and decreased CGRP1–37-induced increments in the load-dependent and load-independent contractility parameters, ESPVR (Fig. 11A) and PRSW (1.40 ± 0.21 and 73.07 ± 12.63 mmHg, respectively; P < 0.05). CGRP8–37 returned Ea and LV compliance to control values (0.64 ± 0.01 mmHg/μl and 1.96 ± 0.04 mmHg/ml, respectively; P < 0.05 for both parameters vs. CGRP1–37).
Fig. 11.
Effects of CGRP1–37, CGRP8–37, and LY on the end-systolic PV relationship associated with vena cava occlusion. Regression lines of the end-systolic PV relationship during jugular intravenous infusions in control treatment, pretreatment with 1 nM CGRP1–37, and posttreatment with 1 nM CGRP8–37 (A) and in control, 1 μM LY, and 1 nM CGRP1–37 treatments (B), the latter with a 1 μM LY (CGRP+LY) posttreatment, are shown.
PI3K Dependence of CGRP-induced Inotropic and Lusitropic Effects In Vivo
To test whether the inotropic effects observed in vivo were mediated by PI3K/Akt signaling, PI3K was inhibited by pretreatment with LY-294002 and posttreatment with CGRP1–37. LY-294002 alone had no significant effect on the following PV loop parameters: ESP, EDP, dPmax, dPmin, SV, EF, and Ea. Pretreatment with LY-294002 increased the relaxation constant, τ Glantz, by 17.4 ± 0.6% (i.e., 21.6 ± 0.2 vs. 25.3 ± 0.4 ms; P < 0.05) and posttreatment with CGRP1–37 did not change the lusitropic effects previously observed with CGRP1–37 treatments alone. The LY-294002 pretreatment revealed a CGRP1–37-induced lusitropic effect, whereby the relaxation constant, τ Glantz, increased to 31.2 ms (i.e., 22.9 ± 1.1% vs. LY-294002 and 44.3 ± 0.9% vs. control, P < 0.001). Pretreatment with LY-294002 also blocked effects of CGRP1–37 on the load-dependent and load-independent contractility parameters, ESPVR (Fig. 11B) and PRSW, respectively, as well as the effects of CGRP1–37 on Ea.
Effects of CGRP1–37 on the Activation Level of Akt
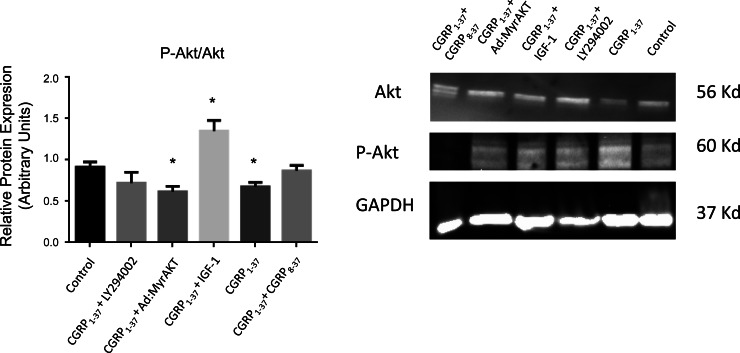
To confirm the relevance of Akt signaling to the effects of CGRP1–37, it was necessary to assess the activation level of Akt in the presence of CGRP1–37. As shown in Fig. 12, CGRP1–37 significantly reduced the p-Akt-to-total Akt ratio (0.913 ± 0.062 vs. 0.656 ± 0.053; P = 0.025), which was negated by cotreatment with CGRP8–37. Furthermore, inhibition of PI3K by LY-294002 prevented the CGRP1–37-dependent decrease in the Akt activation level, which indicates that PI3K is a necessary step between the CGRP1–37 receptor and Akt. Cotreatment with a known Akt agonist, insulin-like growth factor-I, reversed the CGRP1–37 negative effects on the Akt activation level (1.351 ± 0.128; P = 0.033); whereas, adenoviral transfection with a dominant negative construct for Akt (Ad.myrAkt) did not produce an additive effect to CGPR1–37 on Akt activation (0.616 ± 0.066; P = 0.024 vs. control).
Fig. 12.
Effects of CGRP1–37 on the expression and activation level of Akt in the LV of adult male rats. Right: representative Western blot gels demonstrating the effects of CGRP1–37 alone and with cotreatment using either the CGRP1–37 antagonist CGRP8–37, the phosphatidylinositol 3-kinase inhibitor LY, adenoviral transfection with negative Akt (Ad.myrAkt), or the phosphatidylinositol 3-kinase agonist insulin-like growth factor (IGF)-I, compared with control (untreated) ventricles. Left: bar graph showing the average effects of the aforementioned treatments on the activation level of Akt represented as the p-Akt-to-Akt ratio. Values are means ± SE; n = 6 for each group. *P < 0.05, compared with the control.
DISCUSSION
CGRP-induced Inotropy
This study is the first to show that CGRP1–37, independent of its known vasodilatory effects, has positive-inotropic effects on the heart, partially mediated by PI3K signaling. The positive-inotropic effects of CGRP1–37 were effectively antagonized by CGRP8–37, also partially mediated by PI3K signaling. The effects of CGRP at the cellular level were studied in vitro and were corroborated by LV PV loop measurements studied in vivo. The positive-inotropic effects of CGRP were also found to be load independent. It is noteworthy that CGRP8–37 decreased the effects of CGRP1–37 below control values, at both the sarcomeric and cellular levels. These findings suggest that, in the absence of an exogenous effect, basal activity of CGRP signaling might function to maintain normal cardiac contractility. PI3K inhibition was found to have comparable effects to CGRP8–37, which suggests that both CGRP and PI3K may activate the same intracellular signal transduction pathway. A comparable finding is reported for the role of PI3K/Akt signaling for CGRP-ergic mesenteric perivascular nerve functions in insulin-resistant rats (16).
Estrogenic Effects of CGRP-induced Inotropy
The results of this study show that 17β-estradiol decreased the heart's sarcomeric and cellular inotropy, whereas progesterone had no effect. 17β-Estradiol is also reported to decrease cellular contractility in human and mouse male cardiomyocytes, but not in female cardiomyocytes (21). This male sex-specific negative-inotropic effect is attributed to activity of estrogen-activated myosin regulatory light chain interacting protein (21). Increased cardiac contractility in ovariectomized female rats is attributed to low activity of 17β-estradiol (30). 17β-Estradiol treatments are also reported to increase progesterone receptor activity in female but not in male cardiomyocytes (21). These findings corroborate the data in this study, demonstrating negative-inotropic effects of 17β-estradiol on sarcomeres and cardiomyocytes from male rats, without effects of progesterone. 17β-Estradiol is reported to upregulate the vasodilatory effects of CGRP1–37 (45), with and without progesterone (10). In the present study, 17β-estradiol increased the positive-inotropic effects of CGRP in cardiomyocytes. This cellular effect should translate to decreased total peripheral resistance and afterload, while increasing cardiac contractility and improving cardiac function in the animal. These CGRP positive-inotropic effects on cardiomyocytes were found independent of the vascular ones. It is, therefore, likely that the CGRP-induced positive-inotropic effects that we observed in vivo did not result solely from decreased afterload, but also to an intrinsically increased cardiac contractility. As depicted in Fig. 1C, estrogen did not significantly reduce the sarcomeric relaxation rate constant. However, estrogen did enhance the positive effect of CGRP1–37, thereby prolonging the relaxation time. At the cellular level, estrogen, by itself, reduced the relaxation rate constant, but still enhanced the CGRP effect. This finding suggests that the negative effect of estrogen on the relaxation rate constant may be mediated by a separate mechanism compared with that of CGRP. Nevertheless, estrogen receptor activation may also cross-activate a CGRP-dependent signaling pathway, such as PI3K.
CGRP-induced Cardiac Inotropy and Calcium Dynamics
Intracellular calcium dynamics is one of the most important factors affecting cardiac contractility. In this study, we simultaneously recorded variables reflecting intracellular calcium dynamics associated with contraction-induced changes in sarcomeric and cellular lengths (Table 1). Although CGRP1–37 increased the calcium baseline, this effect was not abolished by PI3K inhibition. On the other hand, CGRP produced parallel increases in the amount and velocity of calcium release, as well as prolongation of Ca2+ sequestration, effects that were reversed by inhibition of PI3K. These findings are consistent with our laboratory's previous report that the PI3K/Akt pathway does not regulate the L-type calcium channels in normal rat cardiomyocytes, but does so in hypertrophied ones (1). The results of the present study imply that the CGRP-induced positive inotropy that we observed might be mediated by at least two parallel pathways, a PI3K-dependent as well as a calcium-dependent one. Accordingly, PI3K might regulate the positive-inotropic effects of CGRP in parallel with the calcium release events; whereas CGRP-induced calcium influx might be regulated by PI3K-independent mechanisms. Although neither 17β-estradiol nor progesterone affected calcium handling significantly, they did increase the effects of CGRP on intracellular calcium dynamics during cardiomyocyte contraction.
Table 1.
Effects of CGRP1–37 on baseline sarcomere and cellular lengths as well as intracellular Ca2+
| Sarcomere Length, μm | Cell Length, μm | Intracellular Ca2+ (340/380) | |
|---|---|---|---|
| Resting basal level | 1.64 ± 0.02 | 94.47 ± 1.91 | 1.24 ± 0.04 |
| CGRP1–37 | 1.77 ± 0.02* | 116.50 ± 3.20* | 1.34 ± 0.01* |
Values are means ± SE. CGRP, calcitonin gene-related peptide.
P < 0.01.
Effects of CGRP on the PV Loop
The in vivo PV loop measurements corroborated the in vitro measurements of this study, demonstrating that CGRP has positive-inotropic effects. These results imply that the in vivo effects of CGRP were not the consequence of its vasodilatory and afterload-reducing properties. CGRP improved the load-dependent and load-independent contractility parameters, ESPVR and PRSW, and decreased the EDV (preload). According to the force-length and force-tension relationships, decreasing the preload should decrease the SV. Indeed, decreased SVs associated with decreased cardiac outputs were found. However, the CGRP-induced interplay between the mechanically driven decrement in SV and the aforementioned increment in contractility was associated with increased EFs. This CGRP-induced increment in EF occurred at lower energy cost because both stroke work (which accounts for the mechanical work) and PVA (which includes the stroke work as well as the elastic potential energy accounted for by the PV reserve) were significantly reduced. The PVA is directly related to the amount of oxygen consumption by the LV per beat. Thus it appears that CGRP enabled the LV to produce greater EFs at lower energy costs. This is a significant finding in view of a report that CGRP is released from ex vivo pig hearts as a consequence of ischemia-reperfusion (33). Taken together, these findings suggest that CGRP promotes restoration of cardiac function with a small expenditure of energy. In the present study, all of the effects of CGRP occurred with no significant change in EDPVR or in the τ Glantz relaxation constant. Similar to the in vitro cellular findings, the CGRP1–37-induced increases in cardiac contractility and function were prevented by pretreatment with CGRP8–37 and by inhibition of PI3K signaling, indicative of PI3K-dependent, positive-inotropic effects for CGRP1–37. Positive-inotropic effects of CGRP1–37 antagonized by CGRP8–37 have also been reported in isolated adult rat cardiomyocytes, which were not prevented by inhibiting the production of cAMP or blocking L-type calcium channels (2). In contrast, a protein kinase A-dependent mechanism has been implicated in the positive-inotropic effects of CGRP at the cellular level (18). These positive-inotropic effects of CGRP were significantly greater than the ones we observed in the present study and also greater than others have reported (2). However, the cell contractility and intracellular calcium measurements performed in the present study are in agreement with both reports (2, 18). Moreover, the calcium measurements made in the present study are similar to previously reported CGRP-induced decrements in the calcium sequestration rate constant, attributed to increased calcium-induced calcium-release (18).
Interestingly, CGRP8–37 decreased LVESP, dPmin, and dPmax, as well as Vmax and percent shortening (sarcomere and cellular) below control levels. This indicates that CGRP1–37 is most likely to cross talk or interact with other active contractility-related signaling pathways or mechanical elements at basal levels. PI3K inhibition was effective in reversing the CGRP1–37 effects on the cellular and whole heart levels, but not effectively on the sarcomere. The CGRP-induced sarcomere effects on the relaxation rate constant were reversed by PI3K inhibition, similar to what we have found at the cellular and whole heart levels. However, the sarcomeric shortening was not PI3K dependent. The sarcomere being the basic elemental unit of contractility, it is not unexpected that differences between sarcomere and cell or whole heart preparations may exist due to other confounding factors found at higher architectural levels that can intensify and amplify parameters found relevant at the cellular and whole organ levels. Our group has also demonstrated and correlated the effects of CGRP on NO release by endothelial cells with its strong vasodilatory effects. Because the heart has an endothelial lining, it is expected that some CGRP-induced NO release may occur at the whole heart level, but not with the cardiomyocytes or sarcomere preparations. In view of the similarity we found in the inotropic effects of CGRP on cardiomyocytes and the whole heart, it is reasonable to assume that cardiac NO release does not significantly modulate the CGRP effects on the heart.
CGRP-induced Effects on Compliance and SV
One interesting aspect of the CGRP-induced positive inotropy described herein is its occurrence in conjunction with decrements in LV SV and compliance. The increase in elastance indicates the need for a greater ESP to produce the same SV and thus reflects an increased rather than a decreased afterload. This is consistent with the reduced compliance. Similarly, a previous report showed an increase in contractility with reduction in elastance (22). CGRP1–37 increases the relaxation rate constant at the sarcomere and cellular level, which indicates that the relaxation is prolonged. During such prolonged relaxation, calcium is, no doubt, accumulating in sarcomeres, thereby creating a state of prolonged contractility and contraction, which translates to greater LV stiffness and less compliance in the whole heart. There are several lines of evidence for involvement of CGRP in mechanisms relevant to this observation of heart stiffening. Myocardial fibrosis associated with cardiac remodeling and changes in ventricular compliance are complications of many common and uncommon heart failures, from collagen/elastin gene mutation syndromes (32, 34) to myocardial ischemia and infarctions (37). CGRP is reported to participate in isoprenaline-induced cardiac remodeling (27), cardiac fibroblast proliferation (20), and platelet aggregation (31). CGRP treatment is also reported to increase the expression of collagen mRNA and collagen production (48). CGRP appears to be involved in neurogenic inflammation (19), as well as in myocardial ischemia and preconditioning (28), wherein interferences with cardiac endothelial cell functions are likely to occur. Accordingly, treatment with the proinflammatory cytokine TNF-α is reported to increase CGRP gene promoter activity associated with stimulation of transcription factor NF-κB, Jun NH2-terminal kinase, and p38 mitogen-activated protein MAPKs (7).
As previously mentioned, increased production of cAMP and activation of PKA appear to play roles in some of the effects of CGRP (20, 31). Negative inotropy, mediated via phospholamban, a cAMP/PKA-sensitive protein, and activation of sarcoplasmic reticulum Ca2+-ATPase, have been associated with CGRP treatments (46). CGRP treatments are also shown to promote the synthesis and release of NO by endothelial cells (15). Intermedin, a vasoactive peptide derived from CGRP, is shown to have negative-inotropic effects in isolated LV papillary muscles in association with activation of NO/cGMP signaling and thin myofilament desensitization by increased phosphorylation of the troponin complex (36). In the present study, CGRP treatments produced decrements in ventricular compliance and SV in the absence of negative inotropy. The decrements in compliance and SV described in this laboratory animal model should be useful for elucidating important contributions to heart failure in humans.
Inotropic Effects of CGRP and Akt
Our data show that CGRP1–37 reduces the activation of Akt via PI3K. This was antagonized by CGRP8–37 and reversed by insulin-like growth factor-I. Thus it seems that CGRP1–37 downregulation of PI3K and its downstream effector Akt enhances intracellular calcium availability, as depicted by the enhanced intracellular calcium levels and calcium transients, as well as faster velocity in intracellular calcium rise during cardiomyocyte contraction. Our laboratory and others (1, 29) have previously shown a negative relationship of the slow calcium channel with Akt activation, which is in agreement with the current observation. So CGRP1–37-induced reduction of Akt activation would be expected to enhance calcium influx through slow calcium channel and the subsequent calcium-induced calcium-release mechanism. This does not preclude other mechanism being involved in the enhancement of intracellular calcium dynamics. Furthermore, these cellular CGRP1–37 effects are in concert with improved ventricular contractility with higher ESPVR and EF. Nonetheless, elevated basal intracellular calcium is also associated with reduced compliance (greater stiffness of the ventricle), as indicated by the PV-loop relationship with CGRP1–37.
Conclusion
This multilevel study shows that CGRP1–37 has positive inotropic but negative lusitropic effects on the heart. These findings were consistent in the sarcomere, cardiomyocyte, and LV in vivo and in vitro. The CGRP1–37 effects were enhanced by estrogen and progesterone and partly mediated by the PI3K/Akt signaling pathway. The CGRP1–37-dependent improvement in contractility led to an increase in the EF, despite a decrease in cardiac output. These effects were accompanied by an improvement in ESPVR, with a reduction in LV compliance. The inotropic effects of CGRP1–37 described herein were independent of the known vasodilatory effect of CGRP1–37, since in vivo whole heart measurements were corroborated with the in vitro cardiomyocytes/sarcomere load-independent measurements.
GRANTS
This work was supported in part by National Institutes of Health Grants 1 R15 AA-019816-01A1 and GM-08016-38, and Research Centers in Minority Institutions Grant 2G12 RR003048, Division of Research Infrastructure, to G. E. Haddad.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A.-R., P.R.G., R.M.M., V.M.C., and G.E.H. conception and design of research; M.A.-R., R.K.W., N.A.U., V.M.C., M.A.J., and G.E.H. performed experiments; M.A.-R., P.R.G., R.M.M., R.K.W., N.A.U., V.M.C., M.A.J., and G.E.H. analyzed data; M.A.-R., P.R.G., R.M.M., R.K.W., N.A.U., V.M.C., M.A.J., and G.E.H. interpreted results of experiments; M.A.-R., P.R.G., R.M.M., R.K.W., N.A.U., V.M.C., M.A.J., and G.E.H. prepared figures; M.A.-R., P.R.G., R.M.M., and G.E.H. drafted manuscript; M.A.-R., P.R.G., R.M.M., R.K.W., N.A.U., M.A.J., and G.E.H. edited and revised manuscript; M.A.-R., P.R.G., R.M.M., R.K.W., N.A.U., V.M.C., M.A.J., and G.E.H. approved final version of manuscript.
REFERENCES
- 1.Alvin Z, Laurence GG, Coleman BR, Zhao A, Hajj-Moussa M, Haddad GE. Regulation of L-type inward calcium channel activity by captopril and angiotensin II via the phosphatidyl inositol 3-kinase pathway in cardiomyocytes from volume-overload hypertrophied rat hearts. Can J Physiol Pharmacol 89: 206–215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell D, McDermott BJ. Calcitonin gene-related peptide stimulates a positive contractile response in rat ventricular cardiomyocytes. J Cardiovasc Pharmacol 23: 1011–1021, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Bell D, Schlnter KD, Zhou XJ, McDermott BJ, Piper MH. Hypertrophic effects of calcitonin gene-related peptide (CGRP) and amylin on adult mammalian ventricular cardiomyocytes. J Mol Cell Cardiol 27: 2433–2443, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Bell D, Tamamori M, Marumo F, Hiroe M, McDermott BJ, Ito H. Calcitonin gene-related peptide (CGRP) increases cell surface area and induces expression of skeletal [alpha]-actin and ANP mRNA in hypertrophying neonatal cardiomyocytes. Regul Pept 71: 1–7, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Bick RJ, Poindexter BJ, Davis RA, Schiess MC. Determination of the site of action of calcitonin gene-related peptide in the alteration of intracellular calcium levels in adult and neonatal rodent myocytes. Peptides 26: 2231–2238, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Bick RJ, Poindexter BJ, Schiess MC. Localization of calcitonin gene-related peptide in cardiomyocytes: comparison of neonatal and dedifferentiating cells to adult myocytes. Peptides 26: 331–336, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bowen EJ, Schmidt TW, Firm CS, Russo AF, Durham PL. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem 96: 65–77, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrari R, Panzali AF, Poole-Wilson PA, Anand IS. Plasma CGRP-like immunoreactivity in treated and untreated congestive heart failure. Lancet 338: 1084, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Franco-Cereceda A, Gennari C, Nami R, Agnusdei D, Pernow J, Lundberg JM, Fischer JA. Cardiovascular effects of calcitonin gene-related peptides I and II in man. Circ Res 60: 393–397, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Gangula PR, Chauhan M, Reed L, Yallampalli C. Age-related changes in dorsal root ganglia, circulating and vascular calcitonin gene-related peptide (CGRP) concentrations in female rats: effect of female sex steroid hormones. Neurosci Lett 454: 118–123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangula PR, Lanlua P, Bukoski RD, Wimalawansa SJ, Yallampalli C. Mesenteric arterial relaxation to calcitonin gene-related peptide is increased during pregnancy and by sex steroid hormones. Biol Reprod 71: 1739–1745, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Gangula PR, Lanlua P, Wimalawansa S, Supowit S, DiPette D, Yallampalli C. Regulation of calcitonin gene-related peptide expression in dorsal root ganglia of rats by female sex steroid hormones. Biol Reprod 62: 1033–1039, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol 292: G725–G733, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gennari C, Nami R, Agnusdei D, Fischer JA. Improved cardiac performance with human calcitonin gene related peptide in patients with congestive heart failure. Cardiovasc Res 24: 239–241, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Guiliano F, Rampin O, Benoit G, Jardin A. [The peripheral pharmacology of erection]. Prog Urol 7: 24–33, 1997 [PubMed] [Google Scholar]
- 16.Hashikawa-Hobara N, Hashikawa N, Zamami Y, Takatori S, Kawasaki H. The mechanism of calcitonin gene-related peptide-containing nerve innervation. J Pharm Sci 119: 117–121, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Hay DL, Conner AC, Howitt SG, Takhshid MA, Simms J, Mahmoud K, Poyner DR. The pharmacology of CGRP-responsive receptors in cultured and transfected cells. Peptides 25: 2019–2026, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Huang MH, Knight PR, III, Izzo JL., Jr Ca2+-induced Ca2+ release involved in positive inotropic effect mediated by CGRP in ventricular myocytes. Am J Physiol Regul Integr Comp Physiol 276: R259–R264, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa H, Matsuo S, Wakisaka S, Akai M. Fine structure of calcitonin gene-related peptide-immunoreactive nerve fibres in the rat temporomandibular joint. Arch Oral Biol 35: 727–730, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Jiang W, Yang JH, Wang SH, Pan CS, Qi YF, Zhao J, Tang CS. Effects of adrenomedullin on aldosterone-induced cell proliferation in rat cardiac fibroblasts. Biochim Biophys Acta 1690: 265–275, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kararigas G, Bito V, Tinel H, Becher E, Baczko I, Knosalla C, Albrecht-Kupper B, Sipido KR, Regitz-Zagrosek V. Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. J Am Coll Cardiol 59: 410–417, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Katori T, Hoover DB, Ardell JL, Helm RH, Belardi DF, Tocchetti CG, Forfia PR, Kass DA, Paolocci N. Calcitonin gene-related peptide in vivo positive inotropy is attributable to regional sympatho-stimulation and is blunted in congestive heart failure. Circ Res 96: 234–243, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kotchen JM, McKean HE, Kotchen TA. Blood pressure trends with aging. Hypertension 4: III128–III134, 1982 [DOI] [PubMed] [Google Scholar]
- 24.Kubota M, Moseley JM, Butera L, Dusting GJ, MacDonald PS, Martin TJ. Calcitonin gene-related peptide stimulates cyclic AMP formation in rat aortic smooth muscle cells. Biochem Biophys Res Commun 132: 88–94, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Lamon-Fava S, Barnett JB, Woods MN, McCormack C, McNamara JR, Schaefer EJ, Longcope C, Rosner B, Gorbach SL. Differences in serum sex hormone and plasma lipid levels in Caucasian and African-American premenopausal women. J Clin Endocrinol Metab 90: 4516–4520, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med 26: 72–76, 1985 [PubMed] [Google Scholar]
- 27.Li JZ, Peng J, Xiao L, Zhang YS, Liao MC, Li XH, Hu CP, Deng HW, Li YJ. Reversal of isoprenaline-induced cardiac remodeling by rutaecarpine via stimulation of calcitonin gene-related peptide production. Can J Physiol Pharmacol 88: 949–959, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Li YJ, Song QJ, Xiao J. Calcitonin gene-related peptide: an endogenous mediator of preconditioning. Acta Pharmacol Sin 21: 865–869, 2000 [PubMed] [Google Scholar]
- 29.Lu Z, Jiang YP, Ballou LM, Cohen IS, Lin RZ. Gαq inhibits cardiac L-type Ca2+ channels through phosphatidylinositol 3-kinase. J Biol Chem 280: 40347–40354, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Ma Y, Cheng WT, Wu S, Wong TM. Oestrogen confers cardioprotection by suppressing Ca2+/calmodulin-dependent protein kinase II. Br J Pharmacol 157: 705–715, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto Y, Ueda S, Matsushita S, Ozawa T, Yamaguchi H. Calcitonin gene-related peptide inhibits human platelet aggregation. Jpn Circ J 60: 797–804, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Miller AD, Tyagi SC. Mutation in collagen gene induces cardiomyopathy in transgenic mice. J Cell Biochem 85: 259–267, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Morrey C, Brazin J, Seyedi N, Corti F, Silver RB, Levi R. Interaction between sensory C-fibers and cardiac mast cells in ischemia/reperfusion: activation of a local renin-angiotensin system culminating in severe arrhythmic dysfunction. J Pharmacol Exp Ther 335: 76–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrone A, Morreau H, Zhou XY, Zammarchi E, Kleijer WJ, Galjaard H, d'Azzo A. Insertion of a T next to the donor splice site of intron 1 causes aberrantly spliced mRNA in a case of infantile GM1-gangliosidosis. Hum Mutat 3: 112–120, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Parameswaran N, Disa J, Spielman WS, Brooks DP, Nambi P, Aiyar N. Activation of multiple mitogen-activated protein kinases by recombinant calcitonin gene-related peptide receptor. Eur J Pharmacol 389: 125–130, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Pires AL, Pinho M, Sena CM, Seica R, Leite-Moreira AF. Intermedin elicits a negative inotropic effect in rat papillary muscles mediated by endothelial-derived nitric oxide. Am J Physiol Heart Circ Physiol 302: H1131–H1137, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Poulsen SH. Clinical aspects of left ventricular diastolic function assessed by Doppler echocardiography following acute myocardial infarction. Dan Med Bull 48: 199–210, 2001 [PubMed] [Google Scholar]
- 38.Randolph JF, Jr, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, McConnell DS, Finkelstein JS, Korenman SG, Matthews KA, Sternfeld B, Lasley BL. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab 88: 1516–1522, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Saetrum Opgaard O, Hasbak P, de Vries R, Saxena PR, Edvinsson L. Positive inotropy mediated via CGRP receptors in isolated human myocardial trabeculae. Eur J Pharmacol 397: 373–382, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Saetrum OO, de Vries R, Tom B, Edvinsson L, Saxena PR. Positive inotropy of calcitonin gene-related peptide and amylin on porcine isolated myocardium. Eur J Pharmacol 385: 147–154, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Schlier A, Schreckenberg R, Abdallah Y, Krasteva G, Piper HM, Pfeil U, Kummer W, Schlnter KD. CGRP-α responsiveness of adult rat ventricular cardiomyocytes from normotensive and spontaneously hypertensive rats. Eur J Cell Biol 88: 227–241, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Teos LY, Zhao A, Alvin Z, Laurence GG, Li C, Haddad GE. Basal and IGF-I-dependent regulation of potassium channels by MAP kinases and PI3-kinase during eccentric cardiac hypertrophy. Am J Physiol Heart Circ Physiol 295: H1834–H1845, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wimalawansa SJ. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev 17: 533–585, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Wimalawansa SJ, De Marco G, Gangula P, Yallampalli C. Nitric oxide donor alleviates ovariectomy-induced bone loss. Bone 18: 301–304, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Yallampalli C, Kondapaka SB, Lanlua P, Wimalawansa SJ, Gangula PR. Female sex steroid hormones and pregnancy regulate receptors for calcitonin gene-related peptide in rat mesenteric arteries, but not in aorta. Biol Reprod 70: 1055–1062, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q, Scholz PM, Pilzak A, Su J, Weiss HR. Role of phospholamban in cyclic GMP mediated signaling in cardiac myocytes. Cell Physiol Biochem 20: 157–166, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Zhao FP, Guo Z, Wang PF. Calcitonin gene related peptide (CGRP) inhibits norepinephrine induced apoptosis in cultured rat cardiomyocytes not via PKA or PKC pathways. Neurosci Lett 482: 163–166, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Zhao Z, Zhang G, Li Y, Wu M, Tan Y. The influence of RAMP1 overexpression on CGRP-induced osteogenic differentiation in MG-63 cells in vitro: an experimental study. J Cell Biochem 114: 314–322, 2013 [DOI] [PubMed] [Google Scholar]