Abstract
The modified Oxford maneuver is the reference standard for assessing arterial baroreflex function. The maneuver comprises a systemic bolus injection of 100 μg sodium nitroprusside (SNP) followed by 150 μg phenylephrine (PE). On the one hand, this results in an increase in oxyhemoglobin and total hemoglobin followed by a decrease within the cerebral sample volume illuminated by near-infrared spectroscopy (NIRS). On the other hand, it produces a decrease in cerebral blood flow velocity (CBFv) within the middle cerebral artery (MCA) during SNP and an increase in CBFv during PE as measured by transcranial Doppler ultrasound. To resolve this apparent discrepancy, we hypothesized that SNP dilates, whereas PE constricts, the MCA. We combined transcranial Doppler ultrasound of the right MCA with NIRS illuminating the right frontal cortex in 12 supine healthy subjects 18–24 yr old. Assuming constant O2 consumption and venous saturation, as estimated by partial venous occlusion plethysmography, we used conservation of mass (continuity) equations to estimate the changes in arterial inflow (ΔQa) and venous outflow (ΔQv) of the NIRS-illuminated area. Oxyhemoglobin and total hemoglobin, respectively, increased by 13.6 ± 1.6 and 15.2 ± 1.4 μmol/kg brain tissue with SNP despite hypotension and decreased by 6 ± 1 and 7 ± 1 μmol/kg with PE despite hypertension. SNP increased ΔQa by 0.36 ± .03 μmol·kg−1·s−1 (21.6 μmol·kg−1·min−1), whereas CBFv decreased from 71 ± 2 to 62 ± 2 cm/s. PE decreased ΔQa by 0.27 ± .2 μmol·kg−1·s−1 (16.2 μmol·kg−1·min−1), whereas CBFv increased to 75 ± 3 cm/s. These results are consistent with dilation of the MCA by SNP and constriction by PE.
Keywords: cerebral blood flow, middle cerebral artery, nitric oxide, near-infrared spectroscopy, vasodilation
the modified oxford maneuver comprises a systemic bolus injection of the nitric oxide (NO) donor sodium nitroprusside (SNP) followed by a systemic bolus injection of the α1-adrenergic vasoconstrictor phenylephrine (PE). It is a standard maneuver used to determine arterial baroreflex sensitivity (29). SNP decreases blood pressure (BP), which could reduce cerebral blood flow (CBF) despite cerebral vasodilation, whereas PE increases BP, which could increase CBF despite cerebral vasoconstriction. Indeed, transcranial Doppler ultrasound (TCD) measurements of CBF velocity (CBFv) in the middle cerebral artery (MCA) decrease with BP after a bolus injection of SNP and increases with BP after PE. However, the modified Oxford maneuver also produces an increase in oxyhemoglobin (HbO2) and total hemoglobin (THb) with SNP followed by a decrease in HbO2 and THb with PE within a corresponding cerebral sample volume illuminated by near-infrared spectroscopy (NIRS) (20, 34).
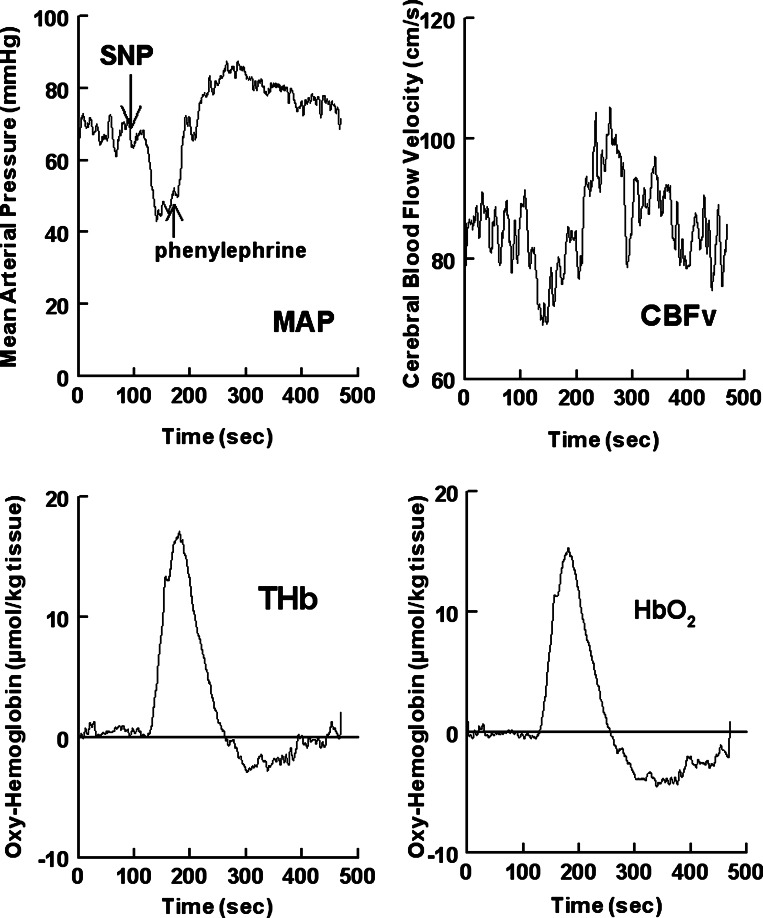
Therefore, TCD and NIRS measurements during the Oxford maneuver are apparently discrepant. Figure 1, provided for purposes of clarification, shows representative TCD and NIRS tracings during the modified Oxford maneuver. If the decrease in CBFv after SNP indicates a decrease in oxygenated arterial inflow (ΔQa), one would expect a decrease in cerebral HbO2 in the region of perfusion. Instead, NIRS HbO2 as well as THb increase. Conversely, if the increase in CBFv after PE indicates an increase in oxygenated ΔQa, one would expect an increase HbO2 in the region of perfusion. Instead, NIRS HbO2 and THb decrease, as shown in Fig. 1. We hypothesized that a transient increase in ΔQa via the MCA occurs during systemic SNP administration because MCA dilation occurs and accounts in large part for the increase in HbO2 and THb despite a reduction in CBFv. Conversely, we hypothesized that, despite an increase in CBFv, a transient decrease in ΔQa via the MCA occurs during systemic PE administration because MCA constriction occurs during the modified Oxford maneuver.
Fig. 1.
Top left: data from a representative subject. Mean arterial pressure (MAP) is shown during the modified Oxford method in which a bolus of sodium nitroprusside (SNP) was followed 1 min later by a bolus of phenylephrine (PE). Blood pressure decreased below baseline with SNP and increased above baseline with PE. Top right: cerebral blood flow velocity (CBFv) measured by transcranial Doppler ultrasound. Bottom left: increase in total hemoglobin (THb) per kilogram of brain tissue. Bottom right: increase in oxyhemoglobin (HbO2) per kilogram of brain tissue. CBFv decreased during SNP and increased during PE. Cerebral oxygenation and THb increased during SNP and decreased during PE.
These hypotheses follow from considerations of CBF regulation by systems comprising myogenic, neurogenic, and metabolic controls (9) at intrinsic (intracerebral) and extrinsic (extracerebral) levels. Intrinsic regulation takes place at the level of penetrating intracerebral vessels that form a neurovascular unit comprising the artery, NO-releasing nitrergic neurons, GABA-releasing interneurons, and glutamatergic pyramidal cells interacting with astrocytes apposed to the arterial adventitia (17). Intrinsic neurovascular coupling ensures that vasomotor activity remains synchronized to neuronal metabolic demands (16). Extrinsic regulation takes place at the level of extracerebral blood vessels, including pial and cerebral conduit arteries. These receive sympathetic nerves containing norepinephrine, ATP, and neuropeptide Y from the superior cervical ganglion, parasympathetic nerves containing NO, ACh, pituitary adenylate cyclase-activating polypeptide, and vasoactive intestinal polypeptide from the sphenopalatine ganglion, and trigeminal nerves that contribute neurokinins, serotonin, and calcitonin gene-related peptide (10). Extracerebral blood vessels are also further vasoregulated by substances crossing the blood-brain barrier, including endothelin-1 and endothelial NO (6).
Thus, NO contributes to both intrinsic and extrinsic CBF regulation. Endogenous or exogenous NO might be supposed to produce cerebral vasodilation at both intrinsic and extrinsic levels. This supposition is supported by literature showing NO-dependent dilation of all vessels, including conduit arteries (33), in primates (23, 30) and humans (35). Given the ease with which NO crosses the blood-brain barrier, a NO donor, such as SNP, should dilate all cerebral arteries regardless of size and independent of BPs changes.
Dilation of the MCA can explain the discrepancy between CBFv and NIRS measurements during the modified Oxford maneuver. TCD measures relative changes of CBFv in the MCA. Its utility depends on several assumptions: that peak velocity represents mean velocity and that the cross-sectional area (Area) of the vessel is constant along the MCA (such that blood flow = mean CBFv × Area), and thus changes in CBFv accurately reflect changes in flow. These assumptions have received support in experiments examining moderate orthostatic stress and changes in end-tidal CO2 (31) but may not be valid under other conditions (36). If Area increases sufficiently, then a fall of CBFv can occur during an increase in MCA inflow. If Area decreases sufficiently, then a rise of CBFv can occur during a decrease in MCA inflow.
To investigate our hypotheses, we derived equations to show that the conservation of mass applied to NIRS-measured HbO2 and THb quantifies changes of inflow and outflow from the NIRS-illuminated sample volume during the modified Oxford maneuver.
MATERIALS AND METHODS
Subjects
To test these hypotheses, we recruited 12 healthy volunteer subjects (6 men and 6 women) aged 18–24 yr. Subjects reported no clinical illness and had never fainted or suffered other forms of orthostatic intolerance. Exclusionary criteria for participation in this study included any infectious or systemic disease (including cardiovascular disease), competitive athletic training, and use of any medications or nicotine. Subjects were instructed to refrain from caffeine and xanthine-containing products for at least 72 h before the tests. All subjects were instructed to fast overnight before the tests. The study was approved by the Institutional Review Board of New York Medical College. All subjects provided informed consent.
Instrumentation
Subjects arrived at our center at 9:30 AM. Subjects were instructed about the procedures and were instrumented while supine. An intravenous catheter was placed in the left antecubital vein. Beat-to-beat BP was measured with a Finometer (FMS, Amsterdam, The Netherlands) on the forefinger or middle finger calibrated to the brachial artery. TCD (Multigon, Yonkers, NY) insonated the right MCA, and the signal was optimized for depth and signal strength. A custom headband held the 2-MHz probe in place over the right temporal window. Respiratory plethysmography (NIRS Respitrace, Miami Beach, FL) measured changes in respiration. We used a combined capnograph and integrated pulse oximeter (Smith Medical PM, Waukesha, WI) to measure end-tidal CO2 and arterial O2 saturation (Sa). An ECG measured heart rate from the beat-to-beat cardiac electrical intervals.
In addition to TCD, we used a continuous-wave spatially resolved near-infrared spectrometer (Oxymon MKII, Artinis) to monitor changes in HbO2 and deoxyhemoglobin (Hb) over a volume of cerebral tissue throughout the protocol. The theoretical underpinnings of this method have been well described in the literature (21). Subjects were instrumented with an emitter and a detector pair of NIRS probes on the forehead over the right frontal cortex to monitor absorption of light across the cerebral frontal area that is primarily perfused by the MCA (28). The spacing between optodes was 4.5 cm, and headset sizing and placement were adjusted to ensure optimal signal fidelity strength for each subject during each trial. Spatial resolution insured that only infrared signals from the brain were analyzed and cutaneous signals were excluded. The sampling rate of the Oxymon was 50 Hz, and an integrated digital-to-analog convertor provided a reconstructed analog signal. The modified Beer-Lambert law was used to calculate micromolar changes in tissue HbO2 and Hb across time using optical densities from near-infrared light at 780 and 850 nm. We used published differential path-length factors for each subject (5). Although illuminated cerebral volume may differ from subject to subject, the assumption is made that the same volume of the cerebrum is illuminated for a particular subject throughout a protocol performed on a given day. Changes in THb were also obtained by adding changes in HbO2 to changes in Hb so as to index changes in blood volume within the illuminated brain volume. Since only changes in Hb and HbO2 could be measured, the averages of HbO2 and Hb during quiet rest defined the resting baseline. Measurements were made in the dark with optodes shielded from light. It was assumed that changes in TCD CBFv correspond to changes in the flow velocity of conduit vessels feeding the volume of NIRS illumination.
All analog signals were digitized at 200 Hz with custom signal-processing software and analyzed offline.
Protocol
Response to bolus SNP followed by bolus PE.
We tested the hypothesis that dilation of the MCA occurs with SNP, whereas constriction occurs with PE. After instrumentation, subjects remained supine for 30 min to acclimate. Data were recorded throughout a 10-min supine baseline period.
An intravenous bolus injection of 100 μg SNP was followed 1 min later by an intravenous bolus injection of 150 μg PE (Fig. 1) with continuous recording. This constitutes the modified Oxford method (29) used for baroreflex assessment. SNP first decreases arterial pressure by ∼15–25 mmHg below baseline, and PE subsequently increases arterial pressure by ∼15–25 mmHg above baseline. Microvascular changes in the cerebral concentration of hemoglobin was measured by NIRS; MCA blood flow velocity was measured by TCD. The modified Oxford method was performed in duplicate for each subject with a resting period of at least 30 min between each series of bolus injections.
Methods of Analysis
Arterial pressure and CBFv.
Mean arterial pressure was calculated from systolic BP (SBP) and diastolic BP (DBP) as follows: mean arterial pressure = 1/3 × SBP + 2/3 × DBP. Mean CBFv was calculated from systolic CBFv and diastolic CBFv as follows: mean CBFv = 1/3 × systolic CBFv + 2/3 × diastolic CBFv and was verified by multibeat time averaging.
NIRS measurement of THb, HbO2, Hb, and CBF.
NIRS yields relative changes in concentrations of HbO2 and Hb that, when summed, yield THb. Hemoglobin mass-balance considerations for the sample volume can be summarized by equations for the conservation of THb, HbO2, and Hb per unit sampled mass of cerebral tissue (24). Thus, the change of THb (in μmol·kg tissue−1·s−1) within the illuminated sample volume is equal to the difference between the amount entering (Qa; in μmol·kg tissue−1·s−1) and the amount leaving the sample volume [venous outflow (Qv); in μmol·kg tissue−1·s−1], as follows:
| 1 |
Similarly, by applying mass-balance considerations to HbO2 and Hb, we can obtain the following:
| 2 |
The change of HbO2 (in μmol·kg tissue−1·s−1) within the sample volume is equal to the difference between the amount of HbO2 entering and the amount of HbO2 leaving the sample volume minus the cerebral metabolic O2 consumption–the rate of conversion of HbO2 to Hb. Sv is venous saturation and CMRo2 is the cerebral metabolic rate of O2 consumption within the sample volume of tissue interrogated by NIRS (22).
The change of Hb (in μmol·kg tissue−1·s−1) within the sample volume is equal to the rate of conversion of HbO2 to Hb by metabolism added to the amount of Hb entering minus the amount of Hb leaving the sample volume, as follows:
| 3 |
These three equations are redundant in that any one equation can be calculated from the other two equations. We will therefore focus on THb and HbO2 balance.
We assume that CMRo2 remains constant throughout drug administration (26). If Sa and Sv are known, we can calculate the change in Qa and Qv.
At baseline, on average, hemoglobin concentration does not change, i.e., both THb and HbO2 are constant, and their derivatives equal zero. Thus, from Eq. 1, Qa0 = Qv0, where subscript 0 denotes baseline. Equation 2 at baseline yields the following:
Since CMRo2 remains constant during transients, substitution for CMRo2 in Eq. 2 yields the following:
| 4 |
The literature indicates that Sv is unchanged by bolus administration of SNP and PE (1, 12). Also, we found no measureable changes in Sa during the Oxford maneuver; therefore, Sa = Sa0 (as confirmed by measurement) and Sv = Sv0: Sa and Sv do not change during transients. Substitution of Sv for Sv0 and use of Eq. 1 in the form Qv = Qa − (dTHb/dt) as well as substitution for Qv in Eq. 4 yields the following:
and therefore
| 5 |
Use of Eq. 1 in the form Qv = Qa − (dTHb/dt) and substitution for Qa and for Sv0 in Eq. 4 yields the following:
| 6 |
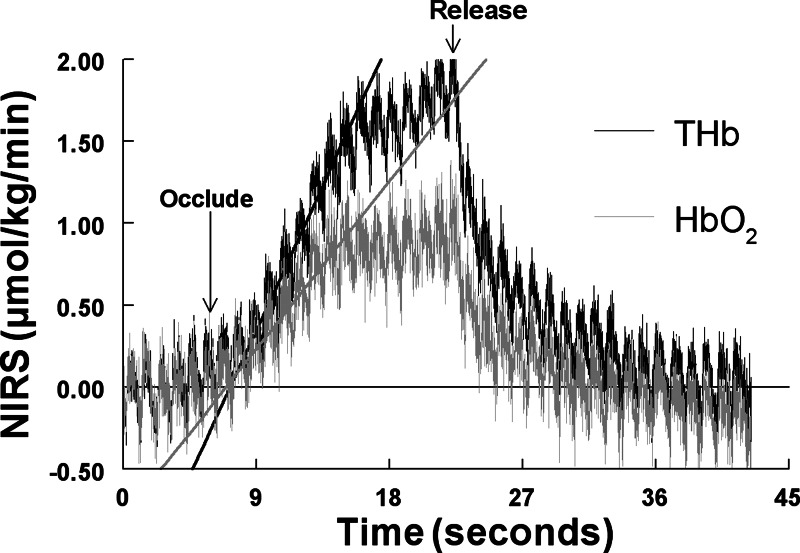
To estimate Sv, we used a brief partial right jugular venous occlusion (4, 38), a form of venous occlusion plethysmography, only during baseline conditions. Sv was assumed constant and used for all subsequent calculations. As shown in Fig. 2, this generated transient increases in THb and HbO2. Venous occlusion plethysmography assumes that Qa does not change early during occlusion and thus ΔQa = 0.
Fig. 2.
Representative near-infrared spectroscopy (NIRS) THb (black) and HbO2 (gray) responses to compressive partial venous occlusion of the external jugular vein. Both THb and HbO2 increased linearly with time. The slope of the increase was obtained by least-squares methods. The ratio of HbO2 to THb afforded an estimate of venous O2 saturation (Sv).
Therefore, during early occlusion, Eq. 5 reduces to the following:
or
Recent experimental evidence indicates that the ratio of the initial slopes of each curve gives the closest estimate of cerebral Sv (37). Therefore, we obtained slopes, as shown in Fig. 2, using linear least-squares analysis and singular value decomposition (27). Neck compressions were performed in triplicate, and the results for Sv were averaged for each subject. After subjects recovered from the compressions, SNP and PE boluses were given in sequence.
Rearranging Eq. 5 and solving for ΔQa yields the following:
| 7 |
while rearranging Eq. 6 and solving for ΔQv yields the following:
| 8 |
Derivatives of HbO2 and THb curves.
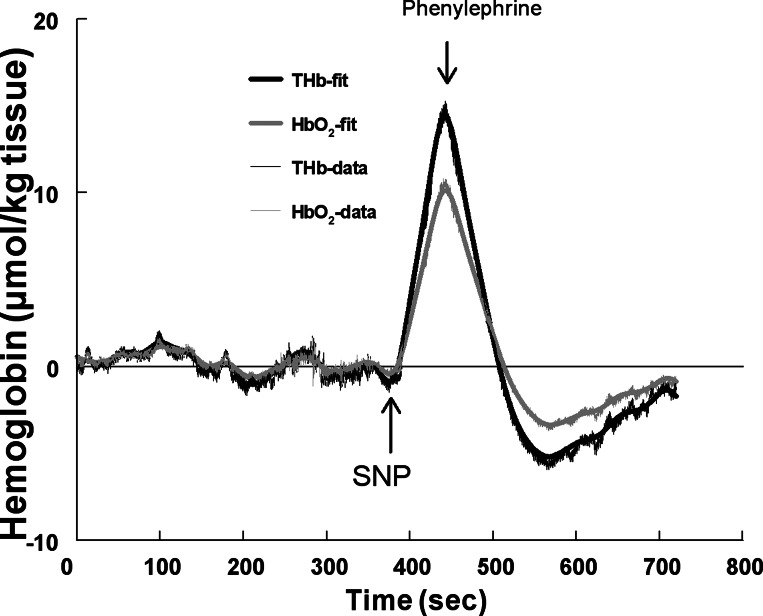
NIRS data were smoothed to remove beat-to-beat fluctuations. We used wavelet smoothing followed by a first derivative calculation using Savitzky-Golay polynomial filtering to compute the derivatives (27). A Dabauchies least asymmetric (LA12) mother (generating) wavelet function was used (3). The mother wavelet generated a dyadic orthonormal basis, where each term represents a doubling of scale. This provides a unique decomposition of each biological signal into subbands of wavelet coefficients, each representing the contribution of the scaled and translated wavelet function at the given scale. We used an extended version of the discrete wavelet transform to produce a “maximal overlap discrete wavelet transformation” (25). The maximal overlap discrete wavelet transformation fills all time points at each scale, allows precise alignment of the signal and its wavelets, allows for any sample size (not just a power of 2), and has zero phase-shifted “details” (representing each scale's contribution to the original signal as a function of time). Zero phase-shifted “smoothes” (representing averages over all scales larger than the scales of interest as a function of time) were found. Using this approach, we extracted a smooth corresponding to averages performed at a time scale of ∼10 s (0.1 Hz). Representative fitted THb and HbO2 responses to SNP and PE are shown in Fig. 3.
Fig. 3.
Data from a representative subject. The NIRS THb response (thick black line) was fit to raw THb data (light black line), and the NIRS HbO2 response (thick gray line) was fit to raw HbO2 data (light gray line).
Statistics
We sampled the data at 200 Hz, stored the digital data on a computer hard drive, and analyzed data offline. Because there were no discernible differences between the data from men and women, data from both groups were combined for analysis. For the modified Oxford maneuver, measurements of arterial pressure, heart rate, CBFv, changes in Hb, HbO2, and THb via NIRS, and NIRS-estimated changes in Qa and Qv (i.e., ΔQa and ΔQv) were tabulated before SNP, at the peak effect of SNP, and at the peak effect of PE and analyzed by one-way ANOVA. Representative results are shown in Figs. 1–4. Additional graphical results are shown in Figs. 5 and 6 showing averages over all subjects (n = 12) ± SE for THb, HbO2, ΔQa, ΔQv, and ΔCBFv at baseline and throughout the administration of bolus drugs. These complement data shown in Table 1 but were not used in statistical comparisons.
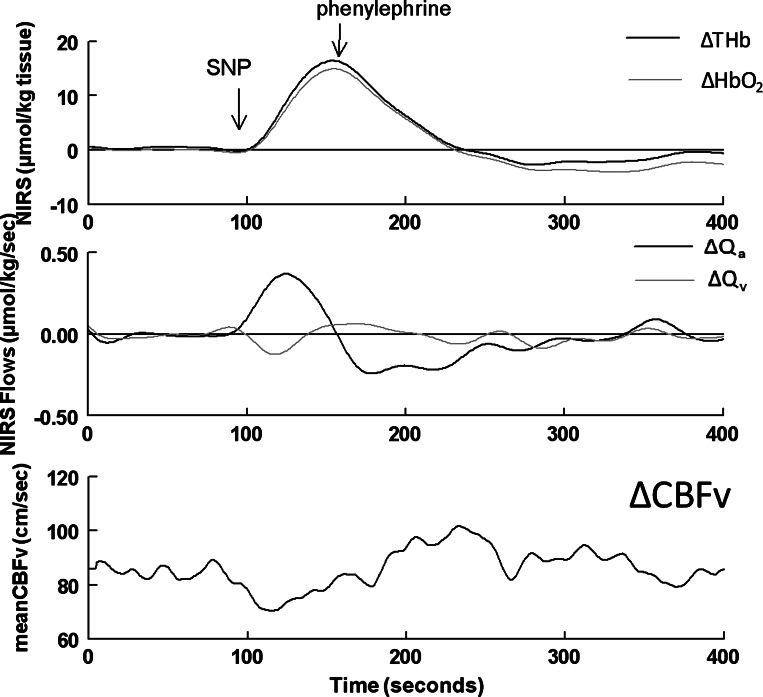
Fig. 4.
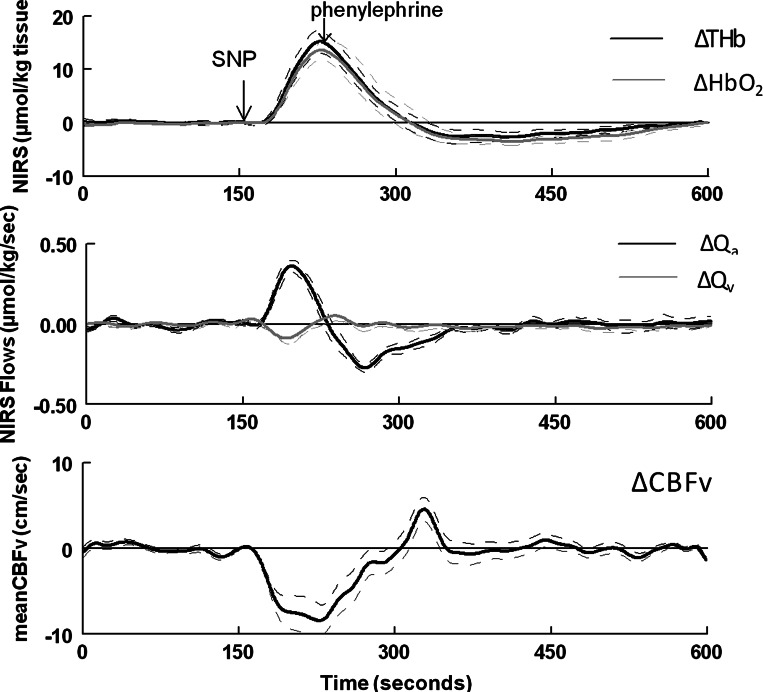
Data from a representative subject. Top: ΔTHb (black) and ΔHbO2 (gray) during the modified Oxford maneuver. Middle: corresponding changes in calculated values of cerebral arterial inflow (ΔQa; black) and venous outflow (ΔQv; gray) of the illuminated sample volume. Bottom: CBFv. Qa increased (ΔQa > 0) during the SNP bolus as THb and HbO2 rose, whereas Qv and CBFv decreased (ΔQv < 0). Qa decreased (ΔQa < 0) during PE as THb and HbO2 fell, whereas Qv and CBFv increased (ΔQv > 0).
Fig. 5.
Top: ΔTHb (black) and ΔHbO2 (gray) averaged over all 12 subjects during the modified Oxford maneuver, shown for illustrative purposes. Statistical comparisons are shown in Table 1. Dashed lines indicate SEs. Middle: corresponding averaged cerebral ΔQa (black) and ΔQv (gray) of the illuminated sample volume. Bottom: averaged ΔCBFv.
Fig. 6.
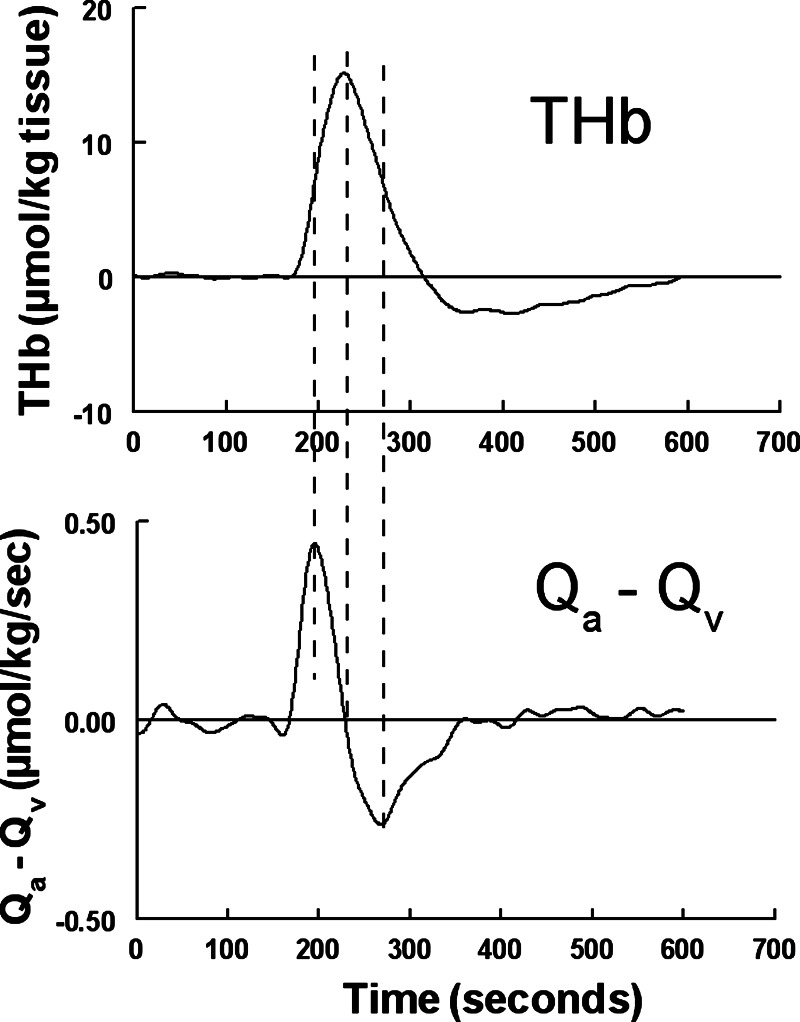
Top: ΔTHb averaged over all 12 subjects during the modified Oxford maneuver, shown for illustrative purposes. Statistical comparisons are shown in Table 1. Bottom: differences in NIRS-calculated inflow and outflow (Qa − Qv) of the illuminated sample volume, which should equal dTHb/dt. We used lines in the bottom graph to indicate the maximum of Qa − Qv, which correspond to the first inflection point of THb; the zero crossing of Qa − Qv, which corresponds to the maximum of THb; and minimum of Qa − Qv, which corresponds to the second inflection point of THb.
Table 1.
Supine hemodynamics measurements before the Oxford maneuver and after bolus injections of SNP and PE
| Measurement | Before Oxford Maneuver | SNP | PE |
|---|---|---|---|
| Heart rate, beats/min | 58 ± 2* | 81 ± 3 | 50 ± 2* |
| Systolic arterial pressure, mmHg | 116 ± 2* | 102 ± 4 | 135 ± 3*† |
| Diastolic arterial pressure, mmHg | 58 ± 3* | 41 ± 3 | 63 ± 4* |
| Mean arterial pressure, mmHg | 78 ± 2* | 61 ± 3 | 87 ± 3* |
| Respiration rate, breaths/min | 14 ± 2 | 15 ± 1 | 13 ± 1 |
| End-tidal CO2, Torr | 42 ± 1 | 39 ± 1 | 43 ± 1* |
| CBFv, cm/s | 71 ± 2* | 62 ± 2 | 75 ± 3* |
| Venous O2 saturation | 0.64 ± 0.02 | (0.64) | (0.64) |
| Arterial O2 saturation | 0.97 ± 0.01 | 0.97 ± 0.01 | 0.97 ± 0.01 |
| ΔNIRS arterial inflow, μmol•kg tissue−1•s−1 | 0 | 0.36 ± 0.03†* | −0.27 ± 0.2* |
| ΔNIRS venous outflow, μmol•kg tissue−1•s−1 | 0 | −0.09 ± 0.04† | 0.05 ± 0.03*† |
| ΔOxyhemoglobin, μmol/kg tissue | 0 | 13.6 ± 1.6† | −3.6 ± 0.6†* |
| ΔDeoxyhemoglobin, μmol/kg tissue | 0 | 1.5 ± 0.6† | −0.7 ± 0.3†* |
| ΔTotal hemoglobin, μmol/kg tissue | 0 | 15.2 ± 1.4† | −2.8 ± 1.0*† |
Values are mean ± SE. SNP, sodium nitroprusside; PE, phenylephrine; CBFv, cerebral blood flow velocity in the middle cerebral artery. NIRS flow and oxyhemoglobin and deoxyhemoglobin differences were set to zero before the Oxford recordings. Venous O2 saturation was assumed constant during the modified Oxford manuever, and therefore values during SNP and PE were estimated.
P < 0.05 compared with SNP;
P < 0.05 compared with before the Oxford maneuver.
When appropriate, post hoc comparisons were performed using Tukey's test. Differences were considered significant when P < 0.05. All values are reported as means ± SD. Results were calculated using SPSS software (version 11.5).
RESULTS
Hemodynamic Responses to Bolus SNP Followed by Bolus PE
Results are shown in Table 1. Heart rate increased significantly after SNP and decreased significantly after PE (P < 0.001). BP (mean, SBP, and DBP) decreased significantly (P < 0.001) after SNP and increased after PE (P < 0.001). Respiratory rate was unaffected, although end-tidal CO2 was increased after PE compared with SNP (P < 0.05).
Sv
Sv remained within a narrow range, 0.64 ± .02, for subjects at baseline. Sv was assumed to remain constant during the modified Oxford maneuver.
CBFv and NIRS Responses to Bolus SNP Followed by Bolus PE
Methods were used to calculate changes in THb, HbO2, Qa, and Qv (i.e., ΔTHb, ΔHbO2, ΔQa, and ΔQv) during the Oxford maneuver while absolute values of CBFv were obtained by TCD. These calculated values are shown in Table 1 and are shown for a representative subject in Fig. 4.
Absolute values for CBFv for each subject were converted to relative changes (ΔCBFv) by subtracting the mean baseline value for CBFv of each subject tracing. ΔCBFv was used for graphical comparison with ΔTHb, ΔHbO2, ΔQa, and ΔQv during the time course of the Oxford maneuver, as shown in Fig. 5. THb and HbO2 increased during SNP (P < 0.0001), decreased with PE to values below baseline (P < 0.001), and subsequently recovered to baseline. ΔQa was positive, and therefore Qa increased compared with baseline (P < 0.0001) during the upswing of THb; ΔQa was negative, and therefore Qa decreased compared with baseline (P < 0.001) during the downswing of THb. ΔQv was negative, and therefore Qv decreased compared with baseline during the upswing of THb (P < 0.001); ΔQv was positive, and therefore Qv increased compared with baseline (P < 0.01) during the downswing of THb with PE and thereafter returned to baseline. ΔCBFv decreased during the upswing of THb (P < 0.001), increased after PE (P < 0.001), and followed a prolonged course to return to baseline. Changes in CBFv were therefore directionally opposite to changes in Qa and directionally similar to changes in Qv. The absolute values of the maximum changes in Qa were always much larger than the absolute values of maximum changes in Qv for every subject.
Consistency of Qa and Qv Formulation With Fitted Data
Since Qa0 = Qv0, the difference between ΔQa and ΔQv is equal to the difference between Qa and Qv. Qa − Qv = ΔQa − ΔQv is shown as an average over all subjects in Fig. 6. An averaged fit to THb is also shown. The maximum and minimum of Qa − Qv corresponded to inflection points of THb, and the zero crossing of Qa − Qv corresponded to the maximum of THb, as required for consistency with Eq. 1.
Approximate Calculation of the Change in Conduit Vessel Diameter
For heuristic purposes, we calculated an estimate of the change in MCA radius (r) required to account for our CBFv and NIRS findings. This requires further assumptions about total cerebral blood volume and is therefore speculative.
Consistent with the ability of our NIRS equipment to measure only changes in Hb and HbO2, only changes in Qa and Qv could be estimated; an absolute value for Qa could not be calculated.
The increase in THb due to SNP lasts ∼1 min. The increase in THb above baseline due to an increase in Qa is equal to the area under the ΔQa curve from baseline (0) to peak THb, an increase of ∼14 μmol/kg. Thus,
where t is the time of peak THb (t = 1 min). Each integral is a time average over that minute, as follows:
where < > signify averaged values over the 1-min time that THb increases.
The fractional change on the left side of the equation is ΔTHb divided by absolute THb, which we estimate as 75 μmol/kg tissue (32). The fractional change on the right side of the equation is Area × <ΔCBFv> + CBFv × <ΔArea> divided by Area × CBFv where proportionality coefficients are normalized away. Then,
Estimating ΔCBFv = −9 cm/s and CBFv = 71 cm/s from the data shown in Table 1, ΔTHb = 14 μmol/kg and THb = 75 μmol. Then, (ΔArea/Area) = (14/75) − (−9/71) = 0.187 + 0.127 = 0.313, where the MCA is presumed cylindrical with Areanew = Area + ΔArea. If Area = πr2 and Areanew = πrnew2, then rnew2/r2 = 1.31. Taking the square root, rnew/r = 1.14 indicates that a 14% increase in r accounts for our findings.
DISCUSSION
We are the first to show that NIRS measured Qa increases with SNP, whereas NIRS measured Qv decreases with SNP. Also, Qa decreases, whereas Qv increases, with PE. Combined with MCA CBFv data, our results indicate dilation of the MCA during bolus SNP injection and constriction of the MCA during bolus PE injection. A NO donor dilates cerebral conduit arteries. The data are also consistent with venous dilation during SNP and venous constriction during PE: during SNP, NO reduces venous tone, resulting in venous pooling and reduced Qv, and during PE, α1-adrenergic stimulation increases venous tone, resulting in reduced venous pooling and expulsion of venous blood from the vasculature, thus increasing Qv. BP is decreased by SNP, whereas ΔQa is increased, and BP is increased by PE, whereas ΔQa is decreased: this indicates direct effects of SNP and phenyl on the cerebral vasculature since changes in BP and flow were directionally opposite.
Using equations that express the conservation of mass and continuity principles applied to HbO2, Hb, and THb and using previously validated assumptions of constant Sv and CMRo2 during bolus SNP and PE administration, we derived equations (Eqs. 7 and 8) to calculate ΔQa and ΔQv for a sample volume illuminated by NIRS. The continuous-wave spatially resolved near-infrared spectrometer as used here cannot be used to compute absolute sample volume blood flows. However, Eqs. 7 and 8 show that NIRS can be used to compute changes in flow, which depend only on the value of cerebral Sv obtained from partial venous occlusion plethysmography (4, 38). Computation based entirely on conservation of mass-continuity principles suffices to clearly demonstrate increased Qa (ΔQa > 0) and smaller decreased Qv (ΔQv < 0) during the period of increasing THb and HbO2 after SNP administration and decreased Qa (ΔQa < 0) and a smaller increased Qv (ΔQv > 0) during the period of decreasing THb and HbO2 after PE administration. Thus, referring to Eq. 1, the increase in THb, previously unexplained by TCD methods, is entirely accounted for by a larger increase in Qa and a smaller decrease in Qv. The same time course and same directional changes of Qa and Qv were consistently found across all subjects.
Increased NIRS Inflow Means Increased Conduit Artery Blood Flow
Qa and Qv represent inflow into and outflow from an illuminated sample volume. The sample volume contains brain parenchyma and microvessels in continuity with pial arteries and with larger conduit arteries, such as the MCA. Assuming blood is incompressible, then by fluid continuity (conservation of mass), inflow into the sample volume must reach the microvasculature by way of the conduit arteries and, conversely, outflow must exit the sample volume through venous drainage. Assuming no extravasation during the course of the modified Oxford maneuver, the results indicate increased MCA blood flow after SNP followed by decreased MCA blood flow and a return to baseline after PE.
Some investigators might propose that the increase in THb and HbO2 represents a change in (mostly) venous blood volume. This could be true. However, from the standpoint of conservation of mass (blood or hemoglobin), it does not matter whether increased delivery of blood within the sampling volume is contained in arteries or veins provided that it remains within the illuminated volume. Blood comprises a small volume fraction of the cerebral cortex, on the order of 4% (14, 18). Therefore, a change in blood volume of 10% will only change brain volume by at most 0.4%, negligibly affecting the size of the illuminated volume. From these arguments, we conclude that a transient increase in inflow (ΔQa > 0) signifies a transient increase in conduit inflow into the sample volume. The total amount of HbO2 within the sample volume of cerebral tissue increases with SNP in large part because of an increase in Qa, as shown in Fig. 5.
Increased MCA Blood Flow and Decreased Conduit Artery Blood Flow Velocity Implies Dilation of the MCA
The sample volume of NIRS illumination corresponds to the area of perfusion of the MCA (32). If molar Qa increases for the illuminated sample volume, and the sample volume corresponds to the perfusion zone for the MCA, it follows that despite a decrease in blood flow velocity (i.e., CBFv), blood flow increases in the MCA. Assuming uniform CBFv and uniform Area along the MCA and assuming that the maximum velocity profile measured by TCD is proportionate to the average velocity profile (19), then ΔQa > 0 implies that ΔMCA flow = Δ(CBFv × Area) > 0. Thus, Area × ΔCBFv + CBFv × ΔArea > 0 or ΔArea > −(Area ×ΔCBFv)/CBFv, which is greater than zero because ΔCBFv is negative.
Therefore, Area increases along with r.
The constancy of MCA diameter was an early assumption made in the development of TCD as a measure of CBF. While that constancy may apply under certain conditions (e.g., during lower body negative pressure), it cannot be assumed to apply under all conditions.
Comparison With the Literature
Our data agree with prior findings in which CBFv decreased during SNP and increased during PE (20). In this previous study (10), cerebral oxidation measured by NIRS increased during SNP infusion and was inversely related to CBFv, which was deemed “an unexpected finding” of that investigation because prior studies (13, 39) had indicated either unchanged or decreased CBF when Doppler methods were used to estimate MCA blood flow.
Exogenous SNP dilates cerebral arteries of all sizes, including the MCA, as verified in experimental animals, including primates and humans (23, 30, 35). Combined conduit vessel, pial artery, and penetrating artery dilation is consistent with hypotheses of a contribution of NO to cerebral vasodilation at the intrinsic (small artery) and extrinsic (large artery) level (2, 7, 23, 33). Other experiments offer a contrasting viewpoint: for example, Joshi et al. (15) administered SNP to humans via an intracarotid route and found no changes in CBF in normal subjects using the 133Xe method. However, to maintain BP, those investigators coadministered PE, which reduces CBF, consistent with our findings.
Other experiments have indicated a lack of effect of NO on CBFv measured in the MCA by TCD using systemic NO synthase inhibition. However, as we have shown, changes in Qa and CBFv can be directionally opposite and be consistent with vascular dilation from NO donors. This is evidenced by another study (35) using the nonspecific NO synthase inhibitor N-monomethyl-l-arginine and PET scanning, which showed a definitive decrease in CBF under similar experimental conditions, implying dilation by NO. Still another study (36) has shown that the MCA can dilate by up to 76% in response to high altitude. The regulation of CBF by conduit arteries is a long-known property of mammalian species (11).
Limitations
Our observations are limited by the use of indirect methods to estimate changes in CBF. However, our calculations are based on fundamental principles of conservation of mass and flow continuity, and our observations are supported by the literature in which investigators measured CBF as well as blood flow velocity. Our experimental arrangement and the rapid changes in CBFv and NIRS during bolus drug administration precluded the use of functional MRI. Direct measurement using functional MRI or other imaging modalities would indeed help to further validate the findings. Supportive findings using other imaging techniques have verified large cerebral vessel dilation with SNP (23, 30, 35).
Another problem is the use of derivative approximations. However, wavelet preprocessing and Savitzky-Golay polynomial filtering produce accurate fits and yield a smooth denoised signal that preserved major maxima and minima for each subject we investigated.
We assumed a uniform, cylindrical MCA with correlated changes in mean velocity and peak velocities. Uniformity of cylindrical Area and proportionality of mean and peak velocity profiles may not strictly apply but often works as a serviceable approximation in similar experiments. These assumptions underlie most studies using TCD to measure CBFv in conduit vessels.
We also assumed that the MCA perfused the sample volume of NIRS illumination. Alternatively, our assumptions would apply provided that other conduit arteries that might contribute to the perfusion of the sample volume are equivalently affected by SNP and PE.
As performed, our experiments are unable to determine which effects derive from a change of BP and which are direct effects of SNP and PE upon cerebral vessels. This can be clearly demonstrated in the equations where the fractional change in THb comprises contributions from ΔCBFv and from ΔArea.
Finally, we assumed constancy of CMRo2 and Sv during the modified Oxford maneuver. Under our experimental conditions, these are reasonable assumptions (1, 8).
Perspectives
Our data agree with previous findings of changing CBFv during bolus SNP- and PE-induced BP changes. Prior work deemed directionally opposite changes in HbO2 and THb “an unexpected finding.” The prior work suggested that these changes indicate a finite slope of cerebral autoregulation and that therefore a “paradigm shift in the concept of (autoregulation) might be required.” We found instead that pharmacologically induced BP changes cannot provide reliable stimuli to assess cerebral autoregulation using TCD CBFv alone.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-074873 and RO1-HL-087803.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.S. and C.T. conception and design of research; J.M.S., Z.R.M., and C.E.S. analyzed data; J.M.S., M.S.M., and C.E.S. interpreted results of experiments; J.M.S. prepared figures; J.M.S. drafted manuscript; J.M.S., M.S.M., A.D., Z.R.M., C.T., and C.E.S. approved final version of manuscript; M.S.M., A.D., and C.E.S. edited and revised manuscript; Z.R.M. and C.E.S. performed experiments.
REFERENCES
- 1. Brassard P, Seifert T, Wissenberg M, Jensen PM, Hansen CK, Secher NH. Phenylephrine decreases frontal lobe oxygenation at rest but not during moderately intense exercise. J Appl Physiol 108: 1472–1478, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Chen FY, Lee TJ. Role of nitric oxide in neurogenic vasodilation of porcine cerebral artery. J Pharmacol Exp Ther 265: 339–345, 1993 [PubMed] [Google Scholar]
- 3. Daubechies I. Orthonormal bases of compactly supported wavelets. Comm Pure Appl Math 41: 909–996, 1988 [Google Scholar]
- 4. Elwell CE, Matcher SJ, Tyszczuk L, Meek JH, Delpy DT. Measurement of cerebral venous saturation in adults using near infrared spectroscopy. Adv Exp Med Biol 411: 453–460, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Essenpreis M, Elwell CE, Cope M, van der ZP, Arridge SR, Delpy DT. Spectral dependence of temporal point spread functions in human tissues. Appl Opt 32: 418–425, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev 78: 53–97, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez C, Estrada C. Nitric oxide mediates the neurogenic vasodilation of bovine cerebral arteries. J Cereb Blood Flow Metab 11: 366–370, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Griffiths DP, Cummins BH, Greenbaum R, Griffith HB, Staddon GE, Wilkins DG, Zorab JS. Cerebral blood flow and metabolism during hypotension induced with sodium nitroprusside. Br J Anaesth 46: 671–679, 1974 [DOI] [PubMed] [Google Scholar]
- 9. Gros R, Afroze T, You XM, Kabir G, Van Wert R, Kalair W, Hoque AE, Mungrue IN, Husain M. Plasma membrane calcium ATPase overexpression in arterial smooth muscle increases vasomotor responsiveness and blood pressure. Circ Res 93: 614–621, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 100: 1059–1064, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Heistad DD, Marcus ML, Abboud FM. Role of large arteries in regulation of cerebral blood flow in dogs. J Clin Invest 62: 761–768, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henriksen L, Paulson OB, Lauritzen M. The effects of sodium nitroprusside on cerebral blood flow and cerebral venous blood gases. I. Observations in awake man during and following moderate blood pressure reduction. Eur J Clin Invest 12: 383–387, 1982 [DOI] [PubMed] [Google Scholar]
- 13. Ide K, Worthley M, Anderson T, Poulin MJ. Effects of the nitric oxide synthase inhibitor l-NMMA on cerebrovascular and cardiovascular responses to hypoxia and hypercapnia in humans. J Physiol 584: 321–332, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ito H, Kanno I, Kato C, Sasaki T, Ishii K, Ouchi Y, Iida A, Okazawa H, Hayashida K, Tsuyuguchi N, Ishii K, Kuwabara Y, Senda M. Database of normal human cerebral blood flow, cerebral blood volume, cerebral oxygen extraction fraction and cerebral metabolic rate of oxygen measured by positron emission tomography with 15O-labelled carbon dioxide or water, carbon monoxide and oxygen: a multicentre study in Japan. Eur J Nucl Med Mol Imaging 31: 635–643, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Joshi S, Young WL, Duong H, Aagaard BA, Ostapkovich ND, Connolly ES, Pile-Spellman J. Intracarotid nitroprusside does not augment cerebral blood flow in human subjects. Anesthesiology 96: 60–66, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci 32: 160–169, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 203: 47–59, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113: 27–47, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Lindegaard KF, Lundar T, Wiberg J, Sjoberg D, Aaslid R, Nornes H. Variations in middle cerebral artery blood flow investigated with noninvasive transcranial blood velocity measurements. Stroke 18: 1025–1030, 1987 [DOI] [PubMed] [Google Scholar]
- 20. Lucas SJ, Tzeng YC, Galvin SD, Thomas KN, Ogoh S, Ainslie PN. Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension 55: 698–705, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol 58: 541–560, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Nioka S, Kime R, Sunar U, Im J, Izzetoglu M, Zhang J, Alacam B, Chance B. A novel method to measure regional muscle blood flow continuously using NIRS kinetics information. Dyn Med 5: 5, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okamura T, Ayajiki K, Toda N. Basilar arterial construction caused by intracisternal NG-nitro-l-arginine in anesthetized monkeys. Cardiovasc Res 30: 663–667, 1995 [PubMed] [Google Scholar]
- 24. Olufsen MS, Peskin CS, Kim WY, Pedersen EM, Nadim A, Larsen J. Numerical simulation and experimental validation of blood flow in arteries with structured-tree outflow conditions. Ann Biomed Eng 28: 1281–1299, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Percival DB, Walden AT. Wavelet Methods for Time Series Analysis. Cambridge: Cambridge Univ. Press, 2000 [Google Scholar]
- 26. Pinaud M, Souron R, Lelausque JN, Gazeau MF, Lajat Y, Dixneuf B. Cerebral blood flow and cerebral oxygen consumption during nitroprusside-induced hypotension to less than 50 mmHg. Anesthesiology 70: 255–260, 1989 [DOI] [PubMed] [Google Scholar]
- 27. Press WH, Teukolsky WT, Vetterling SA, Flannery BP. Numerical Recipes in C. Cambridge: Cambridge Univ. Press, 1992, p. 59–70 [Google Scholar]
- 28. Rasmussen P, Dawson EA, Nybo L, van Lieshout JJ, Secher NH, Gjedde A. Capillary-oxygenation-level-dependent near-infrared spectrometry in frontal lobe of humans. J Cereb Blood Flow Metab 27: 1082–1093, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol 276: H1691–H1698, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Schumann-Bard P, Touzani O, Young AR, Toutain J, Baron JC, Mackenzie ET, Schmidt EA. Cerebrovascular effects of sodium nitroprusside in the anaesthetized baboon: a positron emission tomographic study. J Cereb Blood Flow Metab 25: 535–544, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Strangman G, Franceschini MA, Boas DA. Factors affecting the accuracy of near-infrared spectroscopy concentration calculations for focal changes in oxygenation parameters. Neuroimage 18: 865–879, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev 61: 62–97, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Tzeng YC, Willie CK, Atkinson G, Lucas SJ, Wong A, Ainslie PN. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension 56: 268–273, 2010 [DOI] [PubMed] [Google Scholar]
- 35. White RP, Hindley C, Bloomfield PM, Cunningham VJ, Vallance P, Brooks DJ, Markus HS. The effect of the nitric oxide synthase inhibitor l-NMMA on basal CBF and vasoneuronal coupling in man: a PET study. J Cereb Blood Flow Metab 19: 673–678, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Wilson MH, Edsell ME, Davagnanam I, Hirani SP, Martin DS, Levett DZ, Thornton JS, Golay X, Strycharczuk L, Newman SP, Montgomery HE, Grocott MP, Imray CH. Cerebral artery dilatation maintains cerebral oxygenation at extreme altitude and in acute hypoxia–an ultrasound and MRI study. J Cereb Blood Flow Metab 31: 2019–2029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong FY, Barfield CP, Campbell L, Brodecky VA, Walker AM. Validation of cerebral venous oxygenation measured using near-infrared spectroscopy and partial jugular venous occlusion in the newborn lamb. J Cereb Blood Flow Metab 28: 74–80, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Yoxall CW, Weindling AM, Dawani NH, Peart I. Measurement of cerebral venous oxyhemoglobin saturation in children by near-infrared spectroscopy and partial jugular venous occlusion. Pediatr Res 38: 319–323, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Zhang R, Wilson TE, Witkowski S, Cui J, Crandall GG, Levine BD. Inhibition of nitric oxide synthase does not alter dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 286: H863–H869, 2004 [DOI] [PubMed] [Google Scholar]