Abstract
The process by which proteins are secreted without entering the classical endoplasmic reticulum (ER)–Golgi complex pathway, in eukaryotic cells, is conveniently called unconventional protein secretion. Recent studies on one such protein called Acb1 have revealed a number of components involved in its secretion. Interestingly, conditions that promote the secretion of Acb1 trigger the biogenesis of a new compartment called CUPS (Compartment for Unconventional Protein Secretion). CUPS form near the ER exit site but lack ER-specific proteins. Other proteins that share some of the features common with the secretion of Acb1 are interleukin-1β and tissue transglutaminase. Here I will review recent advances made in the field and propose a new model for unconventional protein secretion.
Keywords: autophagy, IL-1β, MVB, protein secretion
See the Glossary for abbreviations used in this article.
Glossary.
- CUPS
Compartment for Unconventional Protein Secretion
- IL-1
Interleukin-1
- AcbA
Acyl-CoA binding protein
- GrpA
Golgi-associated protein GRASP
- Grh1
Grasp homology 1
- ESCRT
Endosomal Sorting Complex Required for Transport
- MVB
MultiVesicular Body
- Sso1
plasma membrane specific t-SNARE
- Sso2
plasma membrane specific t-SNARE
- Tlg2
endosomal specific t-SNARE
- COPII
coat protein complex II
- COPI
coat protein complex I
- Vps23
ESCRT-I
- Vps4
ATPase vacuolar protein sorting 4
- PAS
Pre-Autophagosomal Structure
- tTG
tissue transglutaminase
- NSF
N-ethylmaleimide sensitive factor
- CARTS
CARriers from the Trans golgi network to the cell Surface
- ILV
IntraLumenal Vesicles
- Ypt6
Yeast RabGTPase-6
- VSV-G
Vesicular Stomatitis Virus-Glycoprotein
- GRASP55
Golgi Reassembly And Stacking Protein 55
- Atg proteins
Autophagy related proteins
Introduction
The cloning and sequencing of the secreted protein interleukin (IL)-1 revealed that it lacked a signal sequence for entering the classical endoplasmic reticulum (ER)–Golgi complex pathway of protein secretion (Auron et al, 1984). This was a surprising finding and the authors cautiously quoted ‘it is entirely possible that appearance of IL-1 from stimulated monocytes is predominantly the result of cell leakage rather than active transport. Such a model is clearly consistent with the idea that IL-1 is synthesized in response to tissue injury and inflammation’ (Auron et al, 1984). Soon thereafter, Sitia and colleagues (Rubartelli et al, 1990) reported the stress-induced release of IL-1β from activated human monocytes and, importantly, the release was unaffected by chemical inhibition of the conventional secretory pathway. They also found a pool of IL-1β in the activated human monocytes encased in a membrane compartment and insensitive to protease treatment. The authors proposed that IL-1β is released from activated monocytes by a novel pathway, which may involve intracellular membranes. Since then, a large number of signal sequence-lacking proteins have been identified in the extracellular space of eukaryotic cells (Table I), but an understanding of the pathway and the mechanism of their release has remained a challenge. The major reasons are the difficulties in reconstituting this secretory pathway. It is not clear whether these secretory proteins follow a common pathway of export from the cytoplasm across the plasma membrane (Rabouille et al, 2012). How many of the total cytoplasmic—signal sequence lacking—proteins are secreted and are they secreted at some basal rate, which is enhanced in a signal-dependent manner?
Table 1. Signal sequence-lacking proteins.
A large number of signal sequence-lacking proteins have been identified in the extracellular space of eukaryotic cells. But for the sake of clarity—and based on the published data—the above proteins are most likely to be similar to Acb1 with respect to their mode of secretion. The list does not include other well-characterized unconventionally secreted proteins, such as FGF2 and a-factor, that are directly translocated across the plasma membrane without involving an intracellular membrane-bounded compartment.
Discovery of the unconventional secretion of Acb1
Nutrient starvation triggers the secretion of the signal sequence-lacking acyl-CoA binding protein (AcbA) by Dictyostelium discoideum, and the secreted protein is cleaved to generate a small peptide called SDF-2, which is required for spore viability (Anjard and Loomis, 2005). In D. discoideum <5% of total AcbA was released in a single burst about 2 h after the onset of starvation (Kinseth et al, 2007). This strongly indicates that the release of AcbA is not a result of cell lysis. Interestingly, the secretion of AcbA was found to require the Golgi-associated protein GRASP (GrpA) and not the ABC transporter activity (Kinseth et al, 2007). The secretion of AcbA is therefore different from the Ste6p (an ABC transporter)-mediated unconventional secretion of a-factor in yeast (Kuchler et al, 1989). Chemically blocking the membrane fusion inhibited secretion of AcbA, and about 1% of the total cellular pool was found in membrane-bound compartments (Cabral et al, 2010). This strongly suggested that AcbA was secreted unconventionally, most likely through a vesicular intermediate. AcbA is a highly conserved protein of about 10 kDa and studies on the secretion of its yeast orthologue (Acb1) in Saccharomyces cerevisiae and Pichia pastoris have revealed the involvement of the GRASP protein Grasp homology 1 (Grh1), autophagy-related proteins, proteins required for the fusion of membranes with the endosomes, proteins of the endosomal sorting complex required for transport (ESCRT)–machinery involved in the formation of multivesicular bodies (MVBs)—and one of the two plasma membrane-specific t-SNAREs, Sso1 (Duran et al, 2010; Manjithaya et al, 2010). It has also been established that secretion of Acb1 is independent of proteins required for the biogenesis of coat protein complex (COP)II-coated vesicles at the ER and proteins required for trafficking into, across, and from the Golgi membranes (Duran et al, 2010). The secretion of Acb1 is triggered by nutrient starvation, and this condition causes the appearance of a new compartment conveniently called CUPS (compartment for unconventional protein secretion) near the ER exit sites in S. cerevisiae (Bruns et al, 2011). The biogenesis of CUPS is specifically induced by glucose starvation, but not by rapamycin treatment or by nitrogen starvation (unpublished data). However, the secretion of Acb1 in Pichia pastoris can be triggered by rapamycin treatment and nitrogen starvation (Manjithaya et al, 2010). It is likely that the two signalling pathways triggered independently by glucose and nitrogen starvation converge to promote Acb1 secretion.
What is the relevance of CUPS in unconventional protein secretion? CUPS contain phosphatidylinositol 3-phosphate (PI(3)P), Atg9, Atg8, Grh1, and Vps23 (ESCRT-I). Although the role of PI(3)P in secretion of Acb1 is not known, all other components contained in CUPS are essential for Acb1 secretion (Duran et al, 2010; Bruns et al, 2011). Starvation is also known to promote autophagosome biogenesis. Are CUPS involved in autophagy-related events? At the level of fluorescence microscopy, Ape1-containing autophagosomes are clearly separated from the CUPS. Ape1 is a specific cargo of the Cvt pathway under growth condition, and upon induction of macroautophagy by rapamycin treatment or by starvation it is captured into general autophagosomes (Shintani and Klionsky, 2004). The autophagy-inducing drug rapamycin also did not elicit the biogenesis of CUPS (Bruns et al, 2011). Moreover, Grh1 is not required for autophagy (Duran et al, 2010). It is therefore reasonable to suggest that CUPS are not like the pre-autophagosomal structure, but are rather an intermediate in the process by which the cytoplasmic Acb1 is delivered to the extracellular space. The involvement of Atg-related proteins in the secretion of Acb1 and the presence of Atg8 and Atg9 in the CUPS has led to the suggestion that an autophagosome-like vesicle forms at the CUPS and is required for subsequent events leading to the secretion of Acb1 (Duran et al, 2010; Bruns et al, 2011). This autophagosome has to be different from the degradative autophagosome, as it should contain cargo destined for secretion (Duran et al, 2010; Manjithaya et al, 2010; Bruns et al, 2011).
What other compartments are linked to the secretion of Acb1? The endosome-specific t-SNARE Tlg2 is required for Acb1 release from the cell (Duran et al, 2010), which suggests that a subpopulation of endosomes serve as intermediates in Acb1 secretion. The involvement of ESCRT proteins suggests the biogenesis of MVBs as an essential step in Acb1 secretion. Finally, of the two plasma membrane-specific t-SNAREs, Sso1 and Sso2, only Sso1 is required for Acb1 secretion (Duran et al, 2010). On the basis of these published data, it has been proposed that an autophagosome-like vesicle containing Acb1 forms at the CUPS. The vesicle fuses with the endosomes/MVB to release a vesicle into the lumen, which is then somehow processed for fusion with the plasma membrane to secrete Acb1 (Duran et al, 2010; Bruns et al, 2011; Subramani and Malhotra, 2013). Another well-characterized protein secreted unconventionally is the tissue transglutaminase (tTG), which regulates the cellular interactions with the extracellular matrix. The secretion of tTG is independent of the ER–Golgi complex, but is mediated instead by a pathway that involves recycling endosomes, MVB, and the N-ethylmaleimide-sensitive factor (Zemskov et al, 2011). Secretion of IL-1β by primary murine bone marrow-derived macrophages is dependent upon GRASP55 and Atg5, and it has been proposed that IL-1β is secreted by an autophagosome-like vesicular intermediate (Dupont et al, 2011).
Is the traffic of Acb1 mediated by an autophagosome-like vesicle?
The following reasons suggest that CUPS are not directly converted to produce an autophagosome-like vesicle.
Live-cell imaging has revealed that CUPS are not consumed during unconventional secretion; therefore, CUPS are not directly converted into an autophagosome-like vesicle during unconventional protein secretion (Bruns et al, 2011). Moreover, if the CUPS were converted into an autophagosome by membrane fusion, how would Acb1 be sorted from the rest of the cytoplasmic material?
If an autophagosome fuses with an endosome, it should, in principle, generate an MVB. Why does Acb1 secretion then require ESCRT proteins?
On the basis of these observations, I suggest that an autophagosome-like vesicle (double-bilayered vesicle) is not the intermediate, which would form at the CUPS and be processed by endosome–membrane fusion events to finally release Acb1 to the extracellular space.
A new model of unconventional protein secretion
On the basis of the published data, I propose the following alternative scheme and steps in the secretion of Acb1 (see Figure 1):
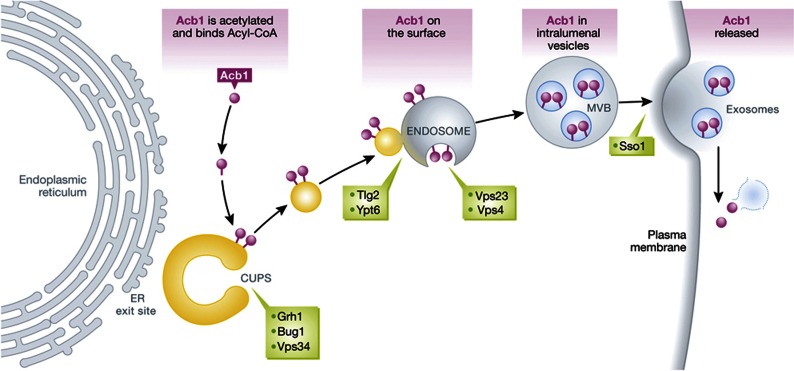
Figure 1.
The mechanism of unconventional protein secretion of Acb1. CUPS assemble in response to starvation near the ER exit sites in yeast. Acb1 is acetylated and binds acyl-CoA, and these reactions assist its targeting to CUPS. The biogenesis of CUPS requires Grh1, Bug1, and Vps34 (in green). A new class of vesicular intermediates is generated at the CUPS by a COPII-independent process. These vesicles containing Acb1 on their cytoplasmic face fuse with the endosomes by a Tlg2- and Ypt6-dependent process. Acb1, which is deposited on the cytoplasmic face of an endosome is then internalized by an ESCRT (Vps23 and Vps4)-dependent process. The t-SNARE Sso1 mediates the fusion of resulting MVB with the plasma membrane to release Acb1-containing exosome-like vesicles into the extracellular space where they lyse to release Acb1.
Step 1. Biogenesis of CUPS
Nutrient starvation triggers the assembly of a new compartment, CUPS, near the ER exit sites. The formation of CUPS (one to three per cell) is protein synthesis-independent. Although CUPS do not contain Sec13 and other ER proteins (e.g., Sec63, unpublished data), we cannot rule out the possibility that CUPS are a specialized extension of the ER. CUPS contain Grh1, PI(3)P, Atg8, and Atg9. Atg9 is a transmembrane protein synthesized at the ER and transported to the Golgi apparatus, and exists in cytosolic mobile vesicles termed Atg9 reservoirs (Yamamoto et al, 2012). How Atg9 is incorporated into the CUPS is not known; it could be via fusion of Atg9-containing vesicles or by lateral migration of newly synthesized Atg9 to the CUPS, which is possible if one assumes that CUPS are a (contiguous) specialization of the ER. The biogenesis of CUPS does not require Sec18, which suggests that membrane fusion events are not involved or the CUPS form by some novel membrane fusion reaction. GRASP and PI(3)P are required for the biogenesis of CUPS; however, the exact role of these components remains unclear. Do CUPS contain secretory cargo (such as Acb1)? Are CUPS found in all cells that secrete proteins like Acb1? Is the signal for the biogenesis of CUPS conserved? CUPS are stable during the time the yeast is cultured in starvation medium. CUPS are consumed, upon return to growth medium, by a process that is independent of new protein synthesis (Bruns et al, 2011). But what is the fate of the membranes of the CUPS upon return of the cells to the growth medium? These questions will get addressed in due course, but for the time being it is important to note that CUPS are not consumed into an autophagosome-like vesicle during starvation.
Step 2. Recruitment and sorting of Acb1 at the CUPS
I suggest that a portion of the total cytoplasmic Acb1 is modified post translationally in a signal-dependent manner at the onset of starvation or a more specific extracellular signal. Knudsen and colleagues (Hansen et al, 2008) have shown that acyl-CoA binding is required for the attachment of Acb1 to membranes. The hydrophobic acyl-CoA could provide the means to target and anchor Acb1 to the CUPS. Grh1 in yeast is known to bind to membranes by an acetylated N-terminal amphipathic helix (Behnia et al, 2007), and it is possible that acetylation of Acb1 might, in addition to acyl-CoA binding, also be required to recruit Acb1 to the CUPS. This step, therefore, provides the possibility to sort Acb1 from other cytoplasmic proteins and might be a general mechanism for the attachment of unconventionally secreted proteins to the CUPS, and could therefore mediate their entry into the unconventional mode of protein secretion.
Step 3. Formation of vesicles containing Acb1
A vesicle containing Acb1 forms at and dissociates from the CUPS. The vesicle does not have a double membrane—similar to an autophagosome—but rather a single lipid bilayer, as other classes of vesicles, such as Clathrin, COPI, COPII and CARTS (CARriers from the Trans golgi network to the cell Surface) do. How are Acb1-containing vesicles formed at the CUPS? Acb1 attaches to the cytoplasmic side of CUPS and its capture into vesicular intermediates provides a second level of sorting for cargo en route to secretion from the cells.
Step 4. Delivery of Acb1 to the endosomes (Golgi bypass)
The Acb1-containing vesicular intermediate fuses with a special population of endosomes and thus target Acb1 to the cytoplasmic face of the endosomes. Acb1, at this stage, will therefore be susceptible to proteolysis. Tlg2 and Ypt6 are required for the fusion of membranes with the endosomes (Nichols et al, 1998; Luo and Gallwitz, 2003) and deletion of either TLG2 or YPT6 blocks the secretion of Acb1 (Duran et al, 2010). Therefore, Acb1-containing intermediates should accumulate in TLG2 and YPT6 deletion strains and, in principle, provide a means to identify, characterize, and isolate them.
Step 5. Formation of Acb1-containing intralumenal vesicles within MVBs
Vps23 of the ESCRT-I complex and Vps4 (ATPase vacuolar protein sorting 4) of the ESCRT-disassembly machinery are required for Acb1 secretion (Duran et al, 2010). It is very likely that these proteins promote the internalization of Acb1 from the cytoplasmic face of the endosomes into lumenal vesicles, thus producing an MVB. As a result, Acb1 at this stage will be protease-insensitive. This could be the membrane-enclosed pool of IL-1β and Acb1 reported previously (Rubartelli et al, 1990; Cabral et al, 2010).
Step 6. Fusion of MVB with the plasma membrane
The t-SNARE Sso1 is required for the secretion of Acb1 (Duran et al, 2010). I suggest it is also responsible for the fusion of Acb1-containing MVB with the plasma membrane. This results in the release of an exosome or an exosome-like vesicle containing Acb1 into the extracellular space. SSO1 mutants should accumulate Acb1-containing MVBs if those are unable to fuse with the cell surface.
Step 7. Release of Acb1 into the extracellular space
The Acb1-containing exosome lyses in the extracellular space to release Acb1. The biogenesis of exosomes, their release into the extracellular space, and the mechanism of lysis remain largely unknown. This assay based on the secretion of Acb1 could help address the issues of exosome biogenesis and secretion.
The function of autophagy-related proteins and Grh1 in unconventional protein secretion
As reviewed recently, many Atg-related proteins are required for processes not associated with autophagy (Subramani and Malhotra, 2013). Atg-related proteins might be involved in the early stages of the biogenesis of CUPS, processing of the cargoes, and other events occurring during Acb1 secretion. However, CUPS are not directly converted into an autophagosome-like vesicle. GRH1 deletion blocks Acb1 secretion, but its exact role or site of action in unconventional protein secretion, steps 1–7, remain unknown. One possibility is that Grh1 chaperones cargoes and components at the level of the CUPS. It could bind cargoes through its PDZ domain, dissociate by phosphorylation, and thus provide a mechanism for the events leading to the capturing and processing of cargoes destined for secretion.
Progress and future prospects
More than two decades after the first description of IL-1β secretion, we are now beginning to understand how proteins that cannot enter the ER–Golgi pathway are secreted. We are also learning that cells utilize numerous compartments and components to modify the secretory routes, so they are commensurate with the cargo. This has been a difficult problem to crack and it is worth highlighting some of the technical issues:
A number of signal sequence-lacking proteins are secreted by eukaryotic cells; notable examples include insulin-degrading enzyme, IL-1β, and galectin-3, tTG (Table I; Subramani and Malhotra, 2013). These proteins lack a signal sequence for entering the ER and are devoid of N- or O-linked glycosylation modifications. They do not share any obvious specific domains or features that could potentially help identify their binding partners and/or provide insights into the mechanism of their intracellular location en route to their release from the cells.
The use of VSV-G protein in Hela cells, secretion of invertase in yeast, and the signal sequence (ss)-HRP in Drosophila tissue culture cells have been very useful to address the mechanism of conventional protein secretion. Proteins secreted unconventionally are released in a cell- and signal-specific manner, which makes it difficult to use a generic cell line and a marker protein to address the mechanism. In other words, the cargo of choice is not available to investigate the mechanism of unconventional protein secretion.
Imaging the trafficking of Acb1-like proteins has been difficult, because tagging with proteins such as GFP affects their intracellular dynamics. Less than 5% of the total cellular content of these proteins is secreted, which also makes it difficult to visualize any specific intermediate over and above the remaining (bulk) cytoplasmic pool.
Cell lysis during experimental manipulations would mask the small signal due to secretion. This has been a key issue in designing an assay to monitor secretion of these kinds of proteins.
There is likely more than one mode of unconventional protein secretion (Rabouille et al, 2012). For example, the cytoplasmic protein fibroblast growth factor 2 (FGF2) is secreted unconventionally, potentially without the use of a vesicular intermediate. FGF2 is assembled into oligomers by phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), which is then suggested to be translocated across the plasma membrane through a lipidic pore (Steringer et al, 2012). The delivery of αPS1 integrin from the ER to the plasma membrane in Drosophila at a specific early developmental stage is also reported to bypass the Golgi membrane and requires the Drosophila GRASP orthologue dGRASP (Schotman et al, 2008). However, little else is known about the requirement for the trafficking of the αPS1 integrin. There are many excellent reviews on the overall process of unconventional protein secretion (Deretic et al, 2012; Ding et al, 2012; Prydz et al, 2013), and for the sake of simplicity I have focused the discussion only on the issues related to proteins, such as the Acb1.
In summary, I have outlined a (relatively simple) scheme to explain an unconventional mode of protein secretion. This scheme will undergo changes, as we learn more, but I hope that some of the proteins identified thus far for Acb1 secretion and the model proposed here will reveal the mechanism and the significance of unconventional protein secretion in cell and tissue physiology.
Acknowledgments
I thank Caroline Bruns and Juan Duran for helpful discussion. V Malhotra is an Institució Catalana de Recerca i Estudis Avançats (ICREA) professor at the Center for Genomic Regulation, and the work in his laboratory is funded by grants from the Plan Nacional (BFU2008-00414), Consolider (CSD2009-00016), Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) Grups de Recerca Emergents (SGR2009-1488; AGAUR-Catalan Government), and the European Research Council (268692).
Footnotes
The author declares that he has no conflict of interest.
References
- Anjard C, Loomis WF. (2005) Peptide signaling during terminal differentiation of Dictyostelium. Proc Natl Acad Sci USA 102: 7607–7611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, Dinarello CA (1984) Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci USA 24: 7907–7911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia R, Barr FA, Flanagan JJ, Barlowe C, Munro S (2007) The yeast orthologue of GRASP65 forms a complex with a coiled-coil protein that contributes to ER to Golgi traffic. J Cell Biol 176: 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns C, McCaffery JM, Curwin AJ, Duran JM, Malhotra V (2011) Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J Cell Biol 195: 979–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral M, Anjard C, Malhotra V, Loomis WF, Kuspa A (2010) Unconventional secretion of AcbA in Dictyostelium discoideum through a vesicular intermediate. Eukaryot Cell 9: 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Jiang S, Dupont N (2012) Autophagy intersections with conventional and unconventional secretion in tissue development, remodeling and inflammation. Trends Cell Biol 22: 397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Wang J, Wang J, Stierhof YD, Robinson DG, Jiang L (2012) Unconventional protein secretion. Trends Plant Sci 17: 606–615 [DOI] [PubMed] [Google Scholar]
- Dupont N, Jiang S, Pilli M, Ornatowski W, Bhattacharya D, Deretic V (2011) Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1beta. EMBO J 30: 4701–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V (2010) Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol 188: 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebov K, Schütze S, Walter J (2011) Functional relevance of a novel SlyX motif in non-conventional secretion of insulin-degrading enzyme. J Biol Chem 286: 22711–22715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JS, Faergeman NJ, Kragelund BB, Knudsen J (2008) Acyl-CoA-binding protein (ACBP) localizes to the endoplasmic reticulum and Golgi in a ligand-dependent manner in mammalian cells. Biochem J 410: 463–472 [DOI] [PubMed] [Google Scholar]
- Hughes RC (1999) Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta 1473: 172–185 [DOI] [PubMed] [Google Scholar]
- Kakkar R, Hei H, Dobner S, Lee RT (2012) Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem 287: 6941–6948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinseth MA, Anjard C, Fuller D, Guizzunti G, Loomis WF, Malhotra V (2007) The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell 130: 524–534 [DOI] [PubMed] [Google Scholar]
- Kuchler K, Sterne RE, Thorner J (1989) Sachhraomyces cerevisiae DTE6 gene product; a novel pathway for protein export in eukaryotic cells. EMBO J 8: 3973–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Gallwitz D (2003) Biochemical and genetic evidence for the involvement of yeast Ypt6-GTPase in protein retrieval to different Golgi compartments. J Biol Chem 278: 791–799 [DOI] [PubMed] [Google Scholar]
- Manjithaya R, Anjard C, Loomis WF, Subramani S (2010) Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J Cell Biol 188: 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BJ, Holthuis JC, Pelham HR (1998) The Sec1p homologue Vps45p binds to the syntaxin Tlg2p. Eur J Cell Biol 77: 263–268 [DOI] [PubMed] [Google Scholar]
- Prydz K, Tveit H, Vedeler A, Saraste J (2013) Arrivals and departures at the plasma membrane: direct and indirect transport routes. Cell Tissue Res 352: 1409–1415 [DOI] [PubMed] [Google Scholar]
- Rabouille C, Malhotra V, Nickel W (2012) Diversity in unconventional protein secretion. J Cell Sci 125: 5251–5255 [DOI] [PubMed] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, Sitia R (1990) A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J 9: 1503–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotman H, Karhinen L, Rabouille C (2008) dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev Cell 14: 171–182 [DOI] [PubMed] [Google Scholar]
- Shintani T, Klionsky DJ (2004) Cargo proteins facilitate the formation of transport vesicles in the cytoplasm to vacuole targeting pathway. J Biol Chem 279: 29889–29894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons D, Grieb G, Hristov M, Pallua N, Weber C, Bernhagen J, Steffens G (2011) Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment. J Cell Mol Med 15: 668–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steringer JP, Bleicken S, Andreas H, Zacherl S, Laussmann M, Temmerman K, Contreras FX, Bharat TA, Lechner J, Muller HM, Briggs JA, Garcia-Saez AJ, Nickel W (2012) Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)-dependent oligomerization of fibroblast growth factor 2 (FGF2) triggers the formation of a lipidic membrane pore implicated in unconventional secretion. J Biol Chem 287: 27659–27669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S, Malhotra V (2013) Non-autophagic roles of autophagy-related proteins. EMBO Rep 14: 143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kakuta S, Watanabe TM, Kitamura A, Sekito T, Kondo-Kakuta C, Ichikawa R, Kinjo M, Ohsumi Y (2012) Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J Cell Biol 198: 219–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemskov EA, Mikhailenko I, Hsia RC, Zaritskaya L, Belkin AM (2011) Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. PLoS One 6: 19414. [DOI] [PMC free article] [PubMed] [Google Scholar]