Abstract
Actin-based remodelling underlies spine structural changes occurring during synaptic plasticity, the process that constantly reshapes the circuitry of the adult brain in response to external stimuli, leading to learning and memory formation. A positive correlation exists between spine shape and synaptic strength and, consistently, abnormalities in spine number and morphology have been described in a number of neurological disorders. In the present study, we demonstrate that the actin-regulating protein, Eps8, is recruited to the spine head during chemically induced long-term potentiation in culture and that inhibition of its actin-capping activity impairs spine enlargement and plasticity. Accordingly, mice lacking Eps8 display immature spines, which are unable to undergo potentiation, and are impaired in cognitive functions. Additionally, we found that reduction in the levels of Eps8 occurs in brains of patients affected by autism compared to controls. Our data reveal the key role of Eps8 actin-capping activity in spine morphogenesis and plasticity and indicate that reductions in actin-capping proteins may characterize forms of intellectual disabilities associated with spine defects.
Keywords: actin-capping activity, activity-dependent plasticity, Eps8, learning and memory defects, spine morphogenesis
Introduction
The establishment of synaptic contacts between appropriate neurons is the basis for the formation of neural networks. Filopodia are thought to play an active role in synaptogenesis, guiding the co-ordinated growth of pre- and postsynaptic partners and functioning as initial bridges between neurons (Dailey and Smith, 1996; Ziv and Smith, 1996; Fiala et al, 1998; Dunaevsky et al, 1999; Okabe et al, 2001; Evers et al, 2006). Subsequently, through the actions of synapse-inducing factors and neuronal activity, filopodia switch to more stable structures, the dendritic spines, which gradually gain a typical mushroom-like structure with a prominent head and a thin neck, and ultimately become the dominant forms in adults (Harris et al, 1992; Fiala et al, 1998; Jontes and Smith, 2000; Bhatt et al, 2009; Hotulainen et al, 2009; Hotulainen and Hoogenraad, 2010). This process is associated with the assembly of pre- and postsynaptic components (Craig et al, 2006; Arikkath and Reichardt, 2008; Yoshihara et al, 2009; Hotulainen and Hoogenraad, 2010). There is a positive correlation between spine shape and dimensions and synaptic strength (Yuste and Bonhoeffer, 2001; Kasai et al, 2003); also, abnormalities in spine number and morphology have been observed in a number of neurological disorders (van Spronsen and Hoogenraad, 2010), thus linking spine morphogenesis with plasticity processes eventually leading to memory formation. Consistently, spine abnormalities and excessive synaptic growth have been reported in subjects with autism (Hutsler and Zhang, 2010; Toro et al, 2010; Penzes et al, 2011).
The process of spinogenesis is controlled by actin, which, besides providing the structural basis for spine formation and elimination, also regulates spine shape (Matus, 2000; Cingolani and Goda, 2008). During development and plasticity, the stabilization of filopodia to form new synaptic contacts is based on a rapid and persistent reorganization of the spine actin cytoskeleton (Luscher et al, 2000; Jourdain et al, 2003; Nikonenko et al, 2003; Honkura et al, 2008). This mainly consists of a reduced depolymerization rate from the pointed end of the filament at the core of the spine, with polymerization continuing at the barbed end in the spine periphery (Fukazawa et al, 2003; Okamoto et al, 2004; Ramachandran and Frey, 2009).
It has been proposed recently that spine formation and enlargement of spine heads may rely on actin remodelling processes similar to those occurring in lamellipodia (Hotulainen and Hoogenraad, 2010). The latter process mainly involves the activity of the branched actin filament nucleator Arp2/3 complex, working in concert with actin-capping proteins. These latter proteins, by binding to the barbed ends of densely packed, plasma membrane-localized actin filaments, control not only their lifetime but also the architecture of the resulting meshwork (Akin and Mullins, 2008). Indeed, when capping activity is high, newly nucleated branched filaments become rapidly capped; this also causes a local increase in the concentration of available actin monomers, which further feeds Arp2/3 nucleation/branching activity, ultimately promoting the generation of a dense and highly branched dendritic array of short actin filaments. Conversely, when capping activity is low, local monomer availability is reduced, as G-actin becomes incorporated into long and uncapped actin filaments (Mogilner and Rubinstein, 2005; Akin and Mullins, 2008; Korobova and Svitkina, 2008).
In support of this possibility, a branched actin filament network is detectable in the distal regions of the spine head (Korobova and Svitkina, 2010); furthermore, the Arp2/3 complex is concentrated within spines (Racz and Weinberg, 2008), where it appears to be an important molecular signal for regulating spine size and synaptic plasticity (Wegner et al, 2008; Nakamura et al, 2011). Platinum replica electron microscopy analysis has revealed that spine heads also contain large amounts of capping proteins (Korobova and Svitkina, 2010). Also, the actin-capping protein CP has been found to be essential for spine development (Fan et al, 2011). However, since CP forms a complex with other proteins, such as twinfilin (Falck et al, 2004), and also mediates membrane attachment of actin (Amatruda et al, 1992; Schafer et al, 1992), the direct demonstration that the actin-capping activity is, in fact, a crucial function required for spine formation is still missing.
Eps8 is a multimodular protein involved in actin remodelling through several activities, including regulation of Rac, a pivotal GTPase involved in the control of actin dynamics and direct interaction with actin. Through the latter property, in particular, Eps8 exerts both actin barbed end capping and actin bundling activities (Disanza et al, 2004, 2006). Eps8 is reported to be expressed at elevated levels in a range of human malignancies (Welsch et al, 2010; Abdel-Rahman et al, 2012), while loss of Eps8 causes intestinal defects and improved metabolic status in mice (Tocchetti et al, 2010). Notably, Eps8 plays a unique and nonredundant role in the polarized migration of dendritic cells. Consequently, Eps8 KO dendritic cells are delayed in reaching the draining lymph node after inflammatory challenge and Eps8 KO mice are unable to mount a contact hypersensitivity response (Frittoli et al, 2011). In brain, Eps8 has been localized postsynaptically in the dendritic articulations of cerebellar granule neurons (Offenhäuser et al, 2006; Sekerková et al, 2007) and in axons of cultured hippocampal neurons, where it controls filopodia formation (Menna et al, 2009). Here we show that the actin-capping protein, Eps8, is recruited to the spine head during chemically induced LTP and that inhibition of its capping activity impairs spine enlargement and plasticity. Accordingly, mice lacking Eps8 display immature spines, are impaired in cognitive function and show an abnormal EEG profile characterized by spike activity. Finally, we show that reduced levels of Eps8 are present in the brain of patients affected by autism.
Results
Eps8 knockout (Eps8 KO) mice are impaired in learning and memory
Eps8 KO mice were subjected to a series of behavioural tests to evaluate learning and memory.
Radial maze performance, in terms of mean total number of errors, days to reach the criterion and percentage of animals that reached the criterion over 30 days, is shown in Figure 1A.
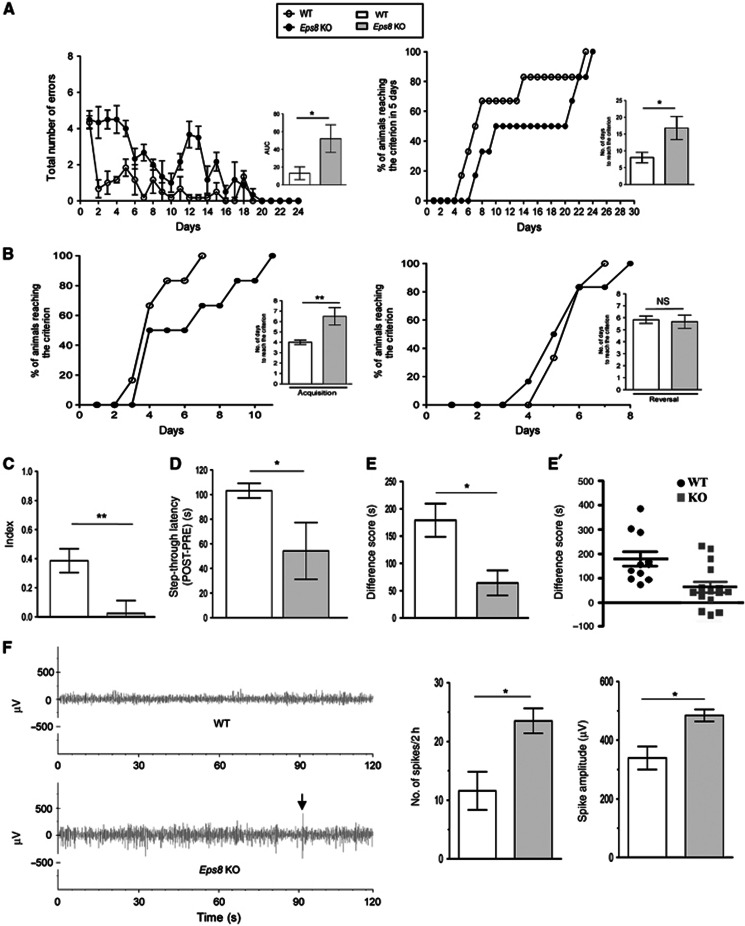
Figure 1.
Eps8 KO mice are impaired in learning and sociability. (A and B) Eps8 KO mice are impaired in spatial learning. (A) Eight-arm radial maze. (Left) Eps8 KO mice show a delayed learning in terms of increased number of errors statistically evaluated as the area under the curve (AUC); (Right) A lower number of Eps8 KO mice reaches the criterion within 5 days evaluated as number of days taken to reach the criterion. (B) T-maze task. During the acquisition phase (left), Eps8 KO mice exhibit a delayed learning in terms of increased number of days to reach the criterion but normal learning during reversal phase (right). The number of days to reach the criterion during both phases is illustrated in the flanking graph. (C) Novel object recognition test. Eps8 KO mice show no net preference between novel and familiar objects, as shown by the lower discrimination index. (D) Passive avoidance task. A reduced step-through latency is detected in Eps8 KO mice compared to wt animals. (E, E′) Sociability test. Eps8 KO mice spend significantly less time exploring a conspecific than an empty cage in a social choice paradigm, as shown by the significantly lower difference score. (F) EEG. Eps8 KO mice display abnormal EEG profile. (Left) EEG recordings of two representative mice (one for each genotype) for 120 s. KO mouse shows higher spike activity. The mean number of spikes recorded for 2 h in Eps8 KO mice is higher compared to wt (centre) and the spike amplitude was larger than wt (right). Increments above a threshold determined according to the increments distribution through an unsupervised approach (Manfredi et al, 2009) and whose amplitude was greater than twice the background were considered as spikes. Data are shown as mean±s.e.m. of ten animals for each genotype and each test. Statistical assessments were performed by Student’s t-test comparing wt and KO mice (*P<0.05, **P<0.01). n.s., not significant.
Eps8 KO mice exhibited a worse performance, in comparison with the wild-type (wt) group, as indicated by the higher number of errors and by the calculated area under the curve (AUC), which revealed a significant increase of this parameter. Consistently, Eps8 KO mice needed significantly more days than controls to reach the criterion (Figure 1A, right).
In the T-maze task, wt mice performed statistically better during acquisition compared to Eps8 KO mice (Figure 1B, left). Conversely, no significant difference was detected in the reversal phase (Figure 1B, right).
When tested for novel object recognition (Figure 1C), no significant difference was detected in the amount of time that the mice spent exploring the two objects during the familiarization (T1) phase, indicating that both genotypes had the same motivation to explore the object. However, during T2 (novel object recognition phase), Eps8 KO mice spent significantly less time exploring the novel object compared to the familiar one, as shown by a significant decrease in the discrimination index (Figure 1C). This was not due to altered sensorial parameters, as all mice appeared healthy, displaying normal motor activity and sensory abilities (Supplementary Table 1). Long-term memory was altered in Eps8 KO mice, as shown by the significant reduction of the mean value of step through latency compared to wt mice in the passive avoidance task (Figure 1D).
Furthermore, when tested for sociability, differently from wt mice, which spent longer time to explore the compartment with the stranger mouse than the empty cage, Eps8 KO mice were significantly less social and spent the same amount of time in the two compartments (Figure 1E).
Finally, 2-h cortical EEG recordings revealed that Eps8 KO mice displayed frequent spikes of high amplitude (Figure 1F), which, however, did not lead to spontaneous seizures, either spontaneously or even after mice handling. The mean number and the mean amplitude of spikes were significantly higher than in wt mice (Figure 1F).
These data indicate that Eps8 KO mice show defects in learning and memory, social behaviour and EEG.
No alterations of the gross anatomy were observed in the brain of Eps8 KO mice. Indeed, the cortex, hippocampus and cerebellum displayed normal architecture and all layers were preserved (Supplementary Figure 1A–L). Therefore, excluding developmental defects (e.g., cortical displacement of neurons, lamination defects), no compensatory elevation of Eps8L family members has ever been detected in different tissues of Eps8-null mice (Frittoli et al, 2011; Zampini et al, 2011) and, accordingly, was not detected in the hippocampus (Supplementary Figure 1M).
Excessive synaptic growth and abnormal spine morphology in the hippocampus of Eps8 KO mice
We have previously shown that Eps8 controls filopodia formation during neuronal development and that the lack of Eps8 results in increased formation of protrusions from both the axon and dendrites (Menna et al, 2009). Since filopodia represent the precursors of pre- and postsynaptic compartments during the process of hippocampal synaptogenesis (Fiala et al, 1998), we investigated whether Eps8 KO adult brain is characterized by a higher number of synaptic contacts compared to wt. Figure 2A shows the CA1 hippocampal region of wt and Eps8 KO mouse brain, stained for the synaptic vesicle protein synaptobrevin/VAMP2 and the glutamatergic postsynaptic protein PSD-95. A significantly higher number of both pre- and postsynaptic puncta were detected in the hippocampi of Eps8 KO mice relative to control (Figure 2B).
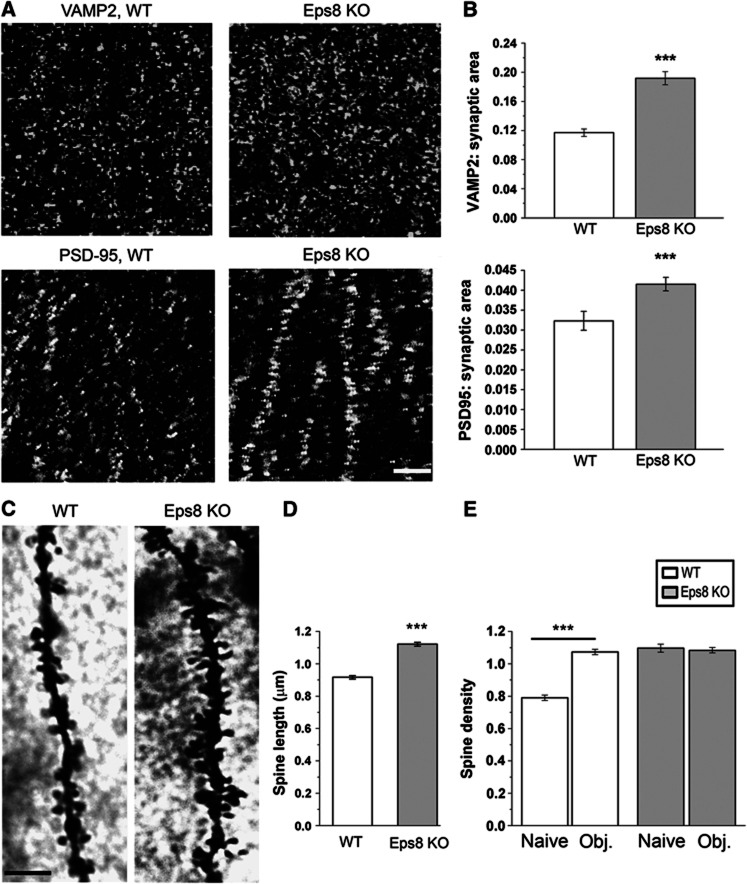
Figure 2.
Excessive synaptic growth and spine abnormalities in the hippocampus of Eps8 KO mice. (A, B) Representative fields of the CA1 hippocampal region of a wt and Eps8 KO mouse brains, stained for the synaptic vesicle protein synaptobrevin/VAMP2 and the glutammatergic postsynaptic protein, PSD-95. Quantitation of either pre- or postsynaptic areas reveals a larger number of synaptic contacts in Eps8 KO hippocampus. Scale bar, 5 μm. (C) Details of CA1 apical dendrites from wt and Eps8 KO hippocampi stained with Golgi-Cox technique. Scale bars, 10 μm. (D, E) Quantitation of spine length and density in wt or KO animals under control conditions (naïve) or after application of the novel object recognition test (obj) (length, D, wt spines=0.91±0.010 μm; KO spines=1.12±0.012 μm; total number of examined spines: 551, wt and 506, Eps8 KO; number of independent experiments: 3) (density, E, wt naive=0.789±0.017 spines per μm of parent dendrite; wt obj.=1.07±0.016 spines per μm; KO naive=1.09±0.024 spines per μm; KO obj.=1.08±0.016 spines per μm total number of examined dendritic branches: 321 wt ctr, 242 wt obj, 105 KO ctr, 361 KO obj; number of independent experiments: 3). Note that Eps8 KO mice display denser and longer spines, which fail in undergoing further increase in number after the object recognition test. Mann–Whitney rank sum test P<0.001. All data are expressed as mean±s.e.m. Six animals for each condition have been analysed.
Ultrastructural analysis of synaptic terminals revealed normal numbers and dimensions of synaptic vesicles (SVs) and a normal size of synaptic boutons (Supplementary Figure 1N–P), thus indicating that lack of Eps8, although affecting synapse number, does not prominently impact the structural organization of the presynaptic compartment. Conversely, analysis of dendritic spines by Golgi-Cox staining revealed that Eps8 KO mice displayed a clear alteration in the morphology of spines, which appeared thinner and were significantly longer relative to wt (Figure 2C and D). A significantly higher number of protrusions per unit length was detected on secondary branches of CA1 neuronal dendrites in the Eps8 KO group compared with the wt (Figure 2E, compare the first and third columns).
Given that mutant mice are impaired in learning and memory and in consideration of the abnormal spine features, we investigated whether learning-dependent spinogenesis processes occur properly in Eps8 KO mice. We found that Eps8 KO mice lacked structural dendritic plasticity, i.e., increases in spine density, which typically develop in the hippocampus during memory formation (Restivo et al, 2006). Figure 2E shows that mutant mice, trained for object recognition and processed for Golgi-Cox 24 h after training, did not display any increase in spine number, which was instead clearly detectable in wt mice. These results indicated that Eps8-null mice have a defect in spine formation and learning-dependent spinogenesis in the hippocampus.
Excessive synaptic growth and abnormal spine morphology in Eps8 KO hippocampal cultures
To gain insights into the cellular and molecular mechanisms at the basis of abnormal spine morphology and plasticity defects occurring in Eps8 KO mice, we analysed synapse density and dendritic morphology in primary hippocampal cultures established from E18 wt or mutant mice. Quantification of pre- and postsynaptic puncta in 21 DIV cultures revealed that, similar to what occurred in vivo, Eps8 KO cultures displayed a significantly higher synapse density than wt cultures, measured as number of vGlut1 or PSD-95 positive contacts per unit length of tubulin-positive dendrites (Supplementary Figure 2A). Similarly to the in vivo situation (Supplementary Figure 1), ultrastructural analysis of 21DIV old wt and Eps8 KO cultures did not reveal any gross alteration of the presynaptic compartment, including SV size and number (not shown). Transfection of cultures with a vector coding for red fluorescence protein, which fills all neuronal processes and allows a direct examination of dendritic morphology, revealed that Eps8 KO neurons, similar to Eps8 KO brain sections, are characterized by a higher density of spines, which appeared significantly longer than wt (Figure 3A). Indeed, the morphology of spines changes, as the number of thin spines is significantly increased while the number of mushroom type decreases (Figure 3A). Notably, however, most of these protrusions, although appearing immature and filopodia like, displayed PSD-95 and bassoon staining, thus indicating that they represent bona fide synaptic contacts (Figure 3A).
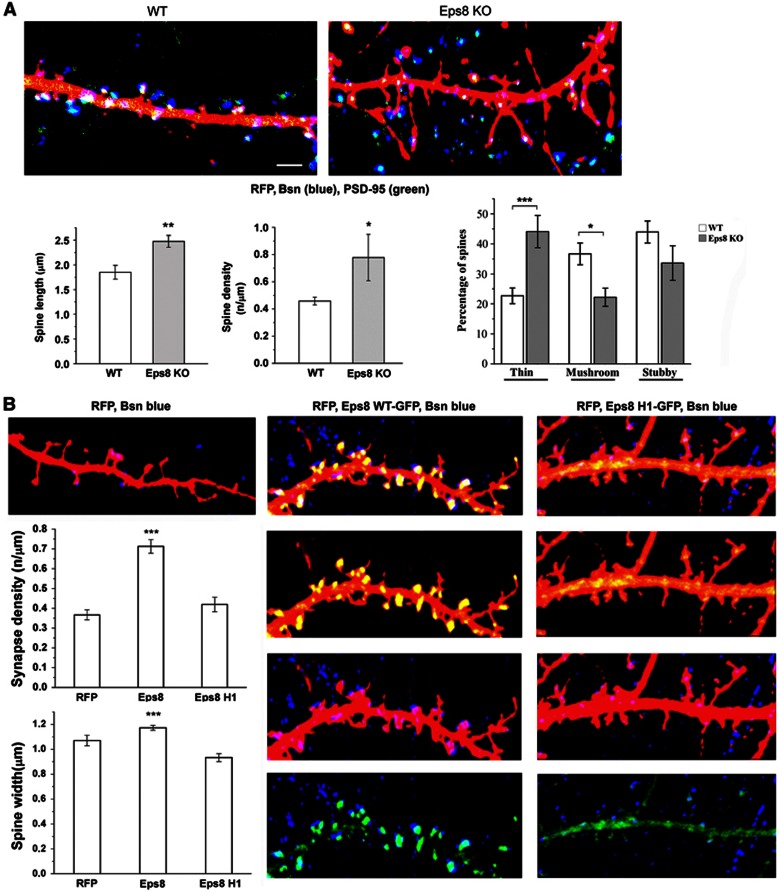
Figure 3.
The actin-capping activity of Eps8 is required for spine formation. (A) Eps8 wt and KO neurons transfected with RFP and stained for the presynaptic protein Bassoon (Bsn, blue) and for the postsynaptic marker PSD-95 (green). Eps8 KO neurons display more abundant and longer protrusions (total number of examined protrusions: 260 wt, 146 Eps8 KO; number of independent experiments: 3). (B) Analysis of spine width and density in neurons transfected with RFP, with the cDNA for wt Eps8 (Eps8WT-EGFP) or with the construct for Eps8 devoid of capping activity (Eps8 H1-EGFP) reveals that Eps8wt but not Eps8H1 increases spine number and size (total number of examined neurons: 15 for RFP, 29 for Eps8 wt and 18 for Eps8 H1; total number of examined spines: 145 for RFP, 553 for Eps8 wt and 105 for Eps8 H1; number of independent experiments: 5). Scale bars depict 5 μm in all panels.
Fluorescence recovery after photobleaching (FRAP) measurements of PSD-95-GFP in wt or mutant neurons revealed a significantly higher PSD-95 mobile fraction in Eps8 KO spines with respect to wt, thus indicating that the dynamics of PSD-95 are altered in mutant neurons (Supplementary Figure 2B). Consistent with the possibility of a functional defect, miniature excitatory postsynaptic currents (mEPSCs), recorded in the presence of 1 μM TTX, displayed a significantly reduced amplitude in Eps8-null neurons with respect to wt (Supplementary Figure 2C). Despite the increase in synapse density, no changes in mEPSC frequency were detected (Supplementary Figure 2C). This could result from the reduced mEPSC amplitude, which would cause many of the events falling below detection. We cannot, however, exclude a role of Eps8 in reducing presynaptic release probability.
A branched actin filament network containing the Arp2/3 complex and capping proteins, the conventional lamellipodial markers, is a dominant feature of spine heads (Svitkina et al, 2010). The possibility, therefore, has been raised that high capping and branching activity may be required for spine head enlargement during development and plasticity (Hotulainen and Hoogenraad, 2010). We, therefore, hypothesized that the ability of Eps8 to cap actin filaments in the spine head may be required for spine formation. It has been previously shown that the capping activity of Eps8 is primarily mediated by the amphipathic H1 helix, while the globular H2–H5 core is responsible for bundling (Hertzog et al, 2010). We then took advantage of the Eps8 capping mutant Eps8H1, in which the hydrophobic residues in the amphipathic helix, H1, critical for actin capping, were mutated while leaving intact the actin bundling activity (Hertzog et al, 2010). A total of 10–11 DIV hippocampal cultures were transfected with constructs expressing either the Eps8 wt protein or its actin-capping mutant, Eps8H1. Figure 3B shows that overexpression of Eps8 induced a potent increase in mature spine density, also promoting the formation of larger spines. Conversely, expression of the H1 actin-capping mutant did not result in spine enlargement, clearly indicating that the actin-capping activity is required for the process. No changes of spine length are observed (data not shown). Notably, Eps8-induced spines appeared positive for the presynaptic active zone protein Bassoon (Bsn) (Figure 3B) and displayed significantly larger PSD-95 puncta compared to neurons transfected with either RFP or the H1 mutant, as indicated by immunofluorescence staining and by IMARIS reconstruction (Supplementary Figure 3A and A′). These results indicate that the actin-capping activity of Eps8 is required for proper mushroom-type spine formation. However, we cannot exclude that the bundling activity of Eps8 might play a role in the filopodia protrusion from the dendritic shaft, a step that precedes the transition from filopodia to mature spines.
Lack of Eps8 precludes synaptic potentiation in hippocampal cultures
We then aimed to define whether Eps8 KO neurons in culture are able to undergo synaptic potentiation, or, like their in vivo counterpart, show defects in structural plasticity. To address this issue, a chemically-induced form of LTP was applied to cultures. Selective activation of synaptic NMDA receptors was achieved by briefly (3 min) elevating the concentration of the NMDA receptor co-agonist glycine in the perfusion solution to suprasaturating levels (100 μM, Lu et al, 2001). The potential activation of glycine receptors was avoided by including strychnine in all of the solutions. Following washout of glycine, insertion of AMPA receptors in the spine head accompanied by LTP of mEPSCs occurs (Lu et al, 2001). In line with previous reports, application of the protocol to wt neurons resulted in a significant increase in both density and size of the PSD-95 positive puncta and density of synaptic contacts (Figure 4A and B). Furthermore, a significant increase in the extent of colocalization between PSD-95 and vGlut1 staining was detected, in line with synaptic strengthening occurring during potentiation phenomena (Fortin et al, 2010). Notably, in neurons devoid of Eps8, application of the same protocol did not induce any significant increase in either the density or the size of PSD-95 positive puncta, or any increase in the colocalization extent of pre- and postsynaptic markers (Figure 4A and B). Both wt and Eps8 KO cultures displayed normal input resistance before and after LTP protocol application, thus confirming that the protocol did not impact neuronal health (input resistance: wt=224±7 MΩ; KO=210±3 MΩ; access resistance: wt=15±2 MΩ; KO=13±2 MΩ; membrane capacitance: wt=42±3 pF; KO=41±10 pF. Number of cells examined, 12 wt and 10 KO).
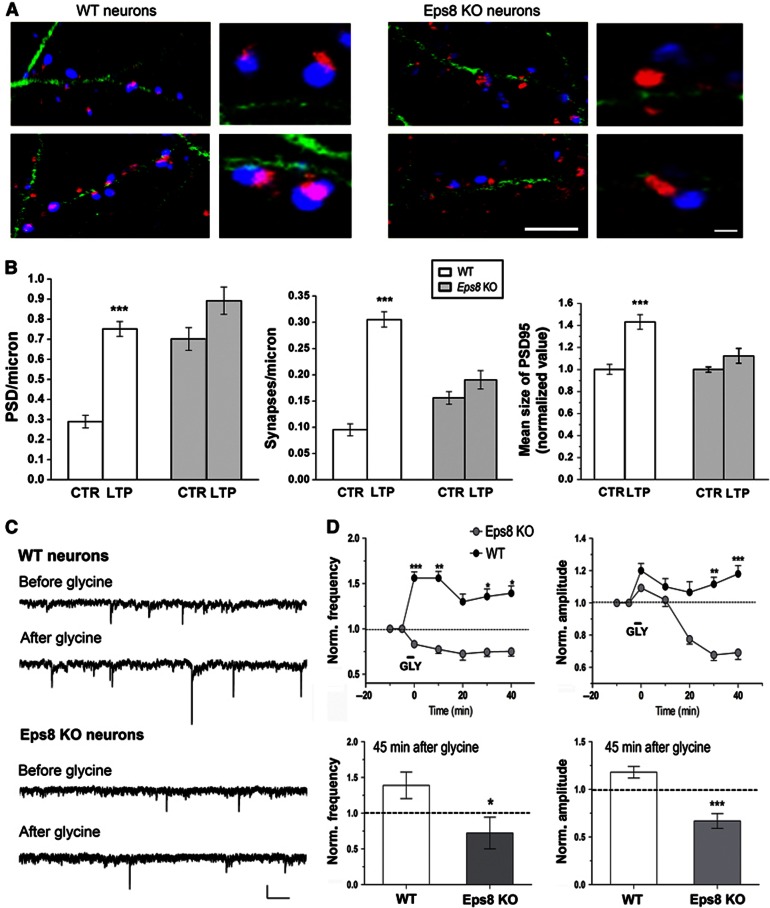
Figure 4.
Lack of Eps8 impairs long-term potentiation. (A) Representative images of wt and KO cultured neurons before and after application of chemical LTP. Neurons are stained for tubulin (green), PSD-95 (red) and v-Glut1 (blue). Scale bars 10 and 2 μm for higher-magnification images. (B) Quantification of potentiation, as represented by number per unit length of PSD-95 (left), synapse density (middle), mean size of PSD-95 (right). Potentiation occurs in wt but not Eps8 KO cultures (Mann–Whitney rank sum test, P<0.001). Data are expressed as mean±s.e.m.; normalized values (total number of examined neurons for analysis of PSD-95 or synapse density: 35 wt ctr and 36 wt LTP; 33 KO ctr and 75 KO LTP; total number of fields for analysis of PSD-95: 46 wt ctr and 59 wt LTP; 72 KO ctr and 90 KO LTP; size number of independent experiments 5). (C, D) Electrophysiological analysis of LTP. (C) Representative mEPSCs traces before and after the induction of chemical LTP, in wt and KO neurons. (D) Analysis of mEPSC frequency and amplitude at different recording times (5 min before LTP application, 5, 10, 20, 30 and 40 min after LTP application) shows that KO neurons are unable to undergo LTP. Graphs indicate the mEPSC mean frequency and amplitude 45 min after the application of LTP. Scale bars, 10 pA and 250 ms (total number of examined neurons: 13 wt and 16 KO; number of independent experiments: 5).
The lack of potentiation in Eps8 KO neurons was also confirmed by electrophysiological recordings of mEPSCs after the application of the glycine protocol. Figure 4C and D show that, differently from wt neurons, which display a significant increase of mEPSCs frequency and amplitude (Lu et al, 2001), mutant neurons fail to undergo potentiation (mEPSC frequency, during GLY: wt=1.561±0.1054, n=13 Eps8, KO=0.8319±0.07001, n=16, ***P<0.001; 10-min wash: wt=1.559±0.1604, n=9 Eps8, KO=0.7738±0.1193, n=8, **P=0.0016; 20-min wash: wt=1.300±0.1449, n=5 Eps8, KO=0.7250±0.1750, n=6, P=0.217; 30-min wash: wt=1.356±0.1410, n=5 Eps8, KO=0.7460±0.3230, n=5, *P=0.036; 40-min wash: wt=1.390±0.1860, n=5 EPS8, KO=0.7220±0.2215, n=5, *P=0.0491; mEPSC amplitude, during GLY: wt=1.200±0.07783, n=13 Eps8, KO=1.093±0.04924, n=15, P=0.2418; 10-min wash: wt=1.100±0.0773, n=9 Eps8, KO=1.019±0.1084, n=7, P=0.5414; 20-min wash: wt=1.066±0.1054, n=7 Eps8, KO=0.7750±0.075, n=5, P=0.0893; 30-min wash: wt=1.116±0.0457, n=5 Eps8, KO=0.6775±0.08664, n=5, **P=0.0021; 40-min wash: wt 1.180±0.06042, n=5 Eps8, KO=0.6680±0.07761, n=5, ***P=0.0008). The lack of potentiation was also confirmed by two independent paired recordings experiments, where stimulation of the presynaptic neuron (three 50-Hz, 2-s trains of depolarizations at 20-s intervals) during brief perfusion with Mg2+-free solution (Arancio et al, 1996) induced potentiation of the excitatory current in wt but not Eps8 KO neurons (eEPSC amplitude, wt=1.350±0.05 Eps8, KO=0.7750±0.0125).
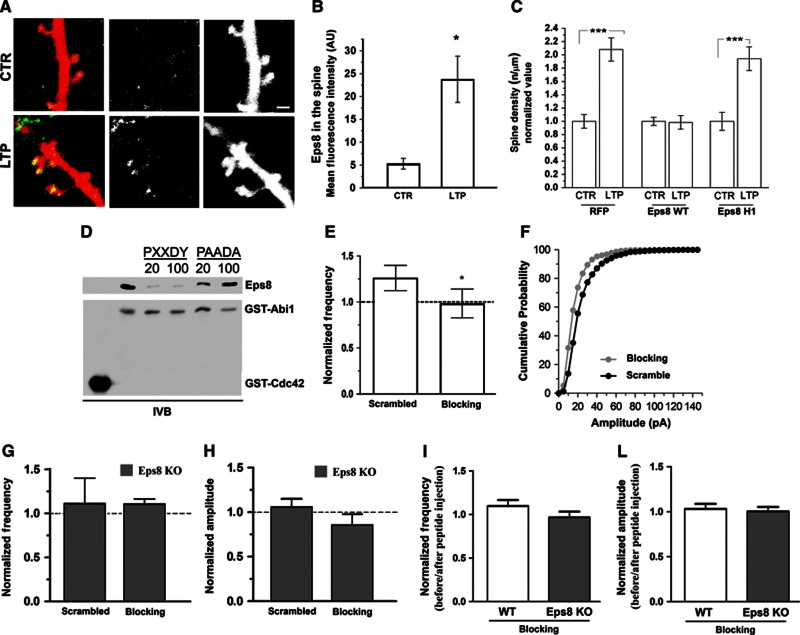
Acute downregulation of Eps8 expression by siRNA similarly prevented synaptic potentiation (Supplementary Figure 3B and B′). These data indicate that Eps8 is required for LTP expression in hippocampal cultures and suggest that the protein may play a role in stabilizing the actin cytoskeleton during spine remodelling. In further support of this hypothesis, endogenous Eps8 is recruited to the spine head upon application of LTP, as indicated by increased protein localization in RFP-labelled dendritic protrusions (Figure 5A and B).
Figure 5.
The acute inhibition of Eps8 actin-capping activity precludes potentiation. (A) Representative examples of dendrites of mice hippocampal neurons transfected with RFP, exposed to chemical LTP and stained for Eps8. Note that Eps8 immunoreactivity at the spine head increases after potentiation. Scale bar, 2 μm. (B) Quantitation of Eps8 immunofluorescence at the spine head in vehicle-treated and glycine-treated (100 μM) neurons (Mann–Whitney Rank Sum Test, P=0.013) (total number of examined neurons: 16 untreated neurons and 12 gly-treated neurons; number of independent experiments: 3). (C) Quantitation of spine density under the different experimental conditions shows that potentiation is prevented by overexpression of Eps8 but not by its actin-capping mutant (total number of examined neurons: 15 ctr and 18 LTP for RFP, 29 ctr and 25 LTP for Eps8 wt and 18 ctr and 24 LTP for Eps8 H1; number of independent experiments: 5). (D) The proline rich consensus site of Abi1 (PXXDY) competes with Abi1 for binding to Eps8. Equal amounts of His-Eps8 (0.2 μM) were incubated with 0.2 μM immobilized GST-Abi1 in the absence or in the presence of 20 and 100 μM of either PXXDY or PAADA synthesized peptides. 2 μM GST-Cdc42 was used as a control. Proteins were analysed by immunoblotting with the indicated antibodies. (E, F) mEPSC frequency (E) and amplitude (F) in neurons exposed to chemical LTP and intracellularly perfused via the patch pipette with either the scrambled or the blocking peptide (the blocking peptide competes with Abi1 for binding to Eps8 and therefore inhibits the Eps8 capping activity). Note that neurons intracellularly perfused with the blocking peptide (grey column, white dots) are defective in potentiation, measured as mEPSC frequency or amplitude. Synaptic potentiation occurs in neurons intracellularly perfused with a scramble peptide (black dots) (total number of examined neurons: 6 for both conditions; number of independent experiments: 3). (G, H) mEPSC frequency (G) and amplitude (H) in Eps8 KO neurons exposed to chemical LTP and intracellularly perfused with either the scrambled or the blocking peptide. Note that mEPSC frequency and amplitude of KO neurons do not change upon glycine administration with or without injection of the blocking peptide. (I–L) Normalized mEPSC frequency (I) and amplitude (L) in WT and KO neurons before and after blocking peptide injection. Note that injection of the blocking peptide does not affect per se basal synaptic activity.
Inhibition of Eps8 capping activity impairs spine enlargement and plasticity
Since Eps8 is recruited to the spine head after chemical LTP induction (Figure 5A and B) and the actin-capping activity of Eps8 is required for proper spine formation during neuronal development (Figure 3B), one could hypothesize that the capping activity of Eps8 is crucial for the process of structural plasticity. The Eps8 wt protein or its actin-capping mutant, Eps8H1, was then exogenously expressed in hippocampal neurons and cultures were exposed to chemical LTP. Representative images of this experiment are shown in Supplementary Figure 4A–C. Quantitative analysis (Figure 5C) demonstrates that high Eps8 capping activity impaired actin cytoskeleton remodelling and spine formation, possibly due to the blockade of actin barbed ends and altered actin dynamics. Indeed, exogenous expression of the Eps8 actin-capping-deficient mutant, Eps8H1, which has no effect on actin polymerization (Menna et al, 2009), did not prevent spine remodelling (Figure 5C).
To demonstrate more directly that the actin-capping activity of Eps8 is required for plasticity, we injected the postsynaptic neuron with a synthetic peptide (blocking peptide), which prevents Eps8 from capping actin filaments by competing with Abi1 for binding to Eps8 (Mongioví et al, 1999). Direct competition could be observed in in vitro binding assay using recombinant purified proteins (Figure 5D). LTP was induced 10 min after injection, while mEPSCs were recorded during the entire procedure. Injection of the blocking, but not of a scrambled peptide, impaired synaptic potentiation induced by glycine treatment (Figure 5E and F, mEPSC frequency, 40-min wash: wt=1.260±0.1060, blocking peptide=0.9833±0.2215 unpaired t-test, *P=0.0486; mEPSC amplitude, 40-min wash: wt=1.180±0.06042, blocking peptide=0.6680±0.07761, Kolmogorov–Smirnov test **P=0.0028; number of cells examined: 6 for both conditions; number of independent experiments: 3). Injection of either the blocking or the scrambled peptides in Eps8 KO neurons following glycine administration does not have any effect (Figure 5G and H). Furthermore, injection of these peptides does not change per se the mEPSC frequency and amplitude in either wt or Eps8 KO neurons (Figure 5I and L).
Notably, the lack of Eps8 had no effect on rac activation in 15 DIV hippocampal neurons (Supplementary Figure 4D) and in the brain (Menna et al, 2009), suggesting that Eps8 primarily functions as a capper in this system (Vaggi et al, 2011) and ruling out the possibility that the LTP impairment in Eps8 KO neurons could be due to a deregulation of rac activity or its downstream pathway, WAVE/SCAR and Arp2/3.
Altogether, these results univocally demonstrate that the capping activity of Eps8 is essential for LTP-mediated synapse formation and strengthening.
Eps8 is expressed at lower levels in brains of patients affected by autism
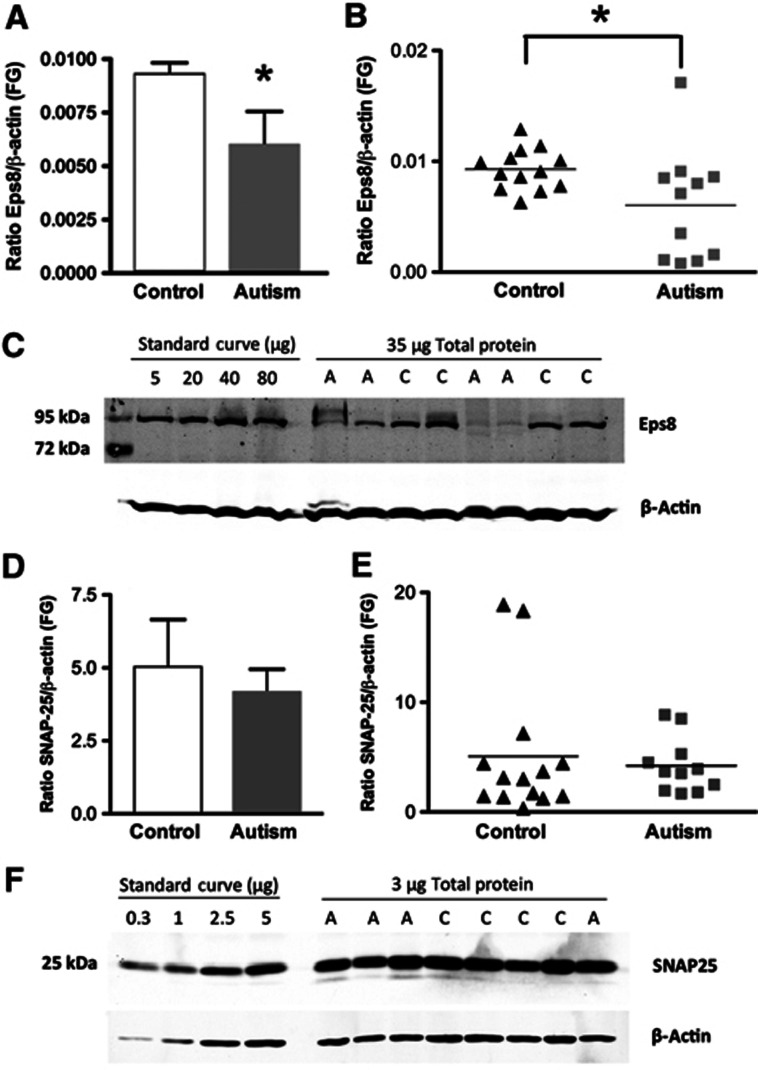
Eps8 capping activity is regulated by the neurotrophic factor BDNF (Menna et al, 2009). BDNF has been demonstrated to be required for spine maturation and dendritic LTP (An et al, 2008; Tanaka et al, 2008), and is critical for synaptogenesis, synaptic plasticity and memory formation (Chapleau et al, 2009; Cunha et al, 2010). Furthermore, the balance between the BDNF precursor, proBDNF, and mature BDNF, which controls spine formation (Koshimizu et al, 2009), has been found to be disrupted in the brain tissue of autism patients (Garcia et al, 2012). Given the established spine pathology in autism spectrum disorder (Hutsler and Zhang, 2010; Penzes et al, 2011), we examined Eps8 levels by quantitative western blotting in postmortem fusiform gyrus tissues from 11 patients with autism and 13 controls. We found a substantial reduction of Eps8 in autism patients compared to controls (Figure 6A–C). No differences in the expression of the SNARE protein, SNAP-25, were detected in the same samples (Figure 6D–F), supporting the specificity of the Eps8 deficit. By leading to changes in spine density and dynamics, a decrease in Eps8 expression in the brain of autism patients could contribute to the morphological, cognitive and behavioural defects of this disorder. The cognitive and social impairments and the alterations in spine number and morphology we observed in Eps8 KO mice support this hypothesis.
Figure 6.
Eps8 expression levels are reduced in brains of patients affected by Autism. (A, B) Quantification of Eps8 protein expression in fusiform gyrus (FG) of autism and control samples by Western blotting. Each sample was normalized to β-actin. *P<0.05, two-tailed Student’s t-test. Bars indicate mean±s.e. Autism, n=11; control, n=13. (C) Representative western blot of fusiform gyrus showing autism (A) and control (C) cases. Lanes 2–5: standard curve consisting of different amounts of total protein from a single normal human cortex sample. Lanes 6–13: 35 μg of total protein from each autism and control sample. Blots were run twice with two different Eps8 antibodies and gave similar results. (D, E) No change in SNAP-25 levels in fusiform gyrus of autism subjects compared to controls. Quantification of SNAP-25 protein expression in fusiform gyrus (FG) of autism and control samples determined by western blotting. Each sample was normalized to β-actin. P=0.67, two-tailed t-test. Bars indicate mean±s.e. Autism, n=11; control, n=13. (F) Representative western blot of fusiform gyrus showing autism (A) and control (C) cases. Lanes 1–4: standard curve consisting of different amounts of total protein from a single normal human cortex sample. Lanes 5–12: 3 μg of total protein from each autism and control sample.
Discussion
There is substantial evidence that actin-based remodelling underlies spine structural changes and memory stabilization (Lamprecht and LeDoux, 2004; Hotulainen and Hoogenraad 2010). For instance, blockers of actin polymerization suppress LTP (Krucker et al, 2000; Fukazawa et al, 2003; Okamoto et al, 2004; Ramachandran and Frey, 2009); also, LIMK1 knockout mice, which do not regulate the activity of the actin-severing protein cofilin, have enhanced hippocampal LTP (Meng et al, 2002); finally, mice lacking the actin-regulating protein WAVE-1 displayed changes in spine density and abnormalities in synaptic plasticity (Soderling et al, 2007), while Abi2 knockout mice exhibit deficits in learning and memory (Grove et al, 2004). Here, we provide a direct demonstration that the actin-regulating protein Eps8, required for optimal actin-based motility and intestinal morphogenesis (Croce et al, 2004; Disanza et al, 2004; Disanza et al, 2006), critically involved in the formation of axonal and dendritic actin filopodia during neuronal development (Menna et al, 2009; Vaggi et al, 2011), is required for the process of spine morphogenesis during neuronal development and synaptic plasticity.
Dendritic protrusions vary in shape and length. Filopodia, thin and mobile structures, are more abundant at early developmental stages, when they sample potential presynaptic partners, eventually mediating the formation of synaptic contacts. This process coincides with the transition from filopodia to spiny protrusions occurring during development (Ziv and Smith, 1996; Yoshihara et al, 2009; Hotulainen and Hoogenraad, 2010). Further modifications of spine size and shape occur during synaptic plasticity, a process that constantly reshapes the circuitry of the adult brain in response to external stimuli, leading to learning and memory formation. It is known that the morphology of dendritic protrusions is directly linked to their function, with the spine head size being directly correlated with the density of glutamate AMPA receptors, and, therefore, with synaptic strength. Accordingly, immature spines are characterized by highly dynamic and less stable PSD-95 clusters (Tsuriel et al, 2009; Zheng et al, 2010). Notably, Eps8 knockdown leads to the formation of thinner and longer spines, characterized by a decreased synaptic strength and less stable PSD-95 dynamics, thus suggesting that the capping protein Eps8 is required for spine maturation and function involving both scaffolding proteins and receptors. Given that immature spines have impaired synaptic signalling and display defects in synaptic plasticity, the ratio of mature to immature spines could play a crucial role in neuronal function and connectivity. In line with this, dendritic spines in Eps8 KO neurons are unable to undergo potentiation.
It has been recently proposed that the mechanisms underlying spine head expansion during synaptic plasticity may share similarities with the process controlling lamellipodia formation, which involves a concomitant action of actin-capping proteins and Arp2/3-mediated actin nucleation and branching, eventually leading to spine head expansion (Hotulainen and Hoogenraad, 2010). The only evidence suggesting that actin-capping proteins may regulate synaptic plasticity comes from the study of Kitanishi and coworkers, who identified the F-actin-capping protein CapZ (Schafer et al, 1995), previously shown to regulate growth cone morphology and neurite outgrowth (Davis et al, 2009), among the proteins whose expression is regulated by neuronal activity (Kitanishi et al, 2010). Activity-dependent CapZ accumulation at the spine supported the possible role of the protein in the regulation of actin dynamics in response to synaptic inputs and eventually in synaptic plasticity. Despite this evidence, a direct demonstration that the capping activity of actin-regulating protein is in fact required for the process of spine expansion occurring during synaptic plasticity was totally missing.
In the present study, we demonstrate that Eps8, through its actin-capping activity, controls spine morphogenesis and synaptic potentiation. Eps8 is the founding member of a unique family of capping proteins capable of side binding and bundling actin filaments. The protein has been detected in many regions of the grey matter, including the olfactory bulb, anterior olfactory nuclei, basal forebrain, cerebral cortex, hippocampus, septal nuclei, amygdala, thalamus, hypothalamus, colliculi, pontine nuclei, cerebellum, cochlear nuclear complex and inferior olive, while the white matter was generally unstained (Sekerková et al, 2007). Besides the cerebellum, Eps8 is expressed at higher levels in neurons in layers II and III of the cerebral cortex and in the hippocampus, two areas classically implicated in higher cognitive functions (Offenhäuser et al, 2006). Eps8 has been detected by western blotting in synaptosomal fractions from hippocampus (Menna et al, 2009) and cerebellum (Offenhäuser et al, 2006). By immunoelectron microscopy, Eps8 was localized postsynaptically in the dendritic articulations of cerebellar granule neurons (Offenhäuser et al, 2006; Sekerková et al, 2007), although the protein expression in axons of cultured hippocampal neurons (Menna et al, 2009) and of granule cells in situ (Sekerková et al, 2007) suggests probable multiplicity of Eps8 functions at the synapse. In line with a role of Eps8 in spine morphogenesis and plasticity, mice lacking Eps8 display immature spines and are impaired in cognitive functions.
The identification of Eps8 capping activity as necessary for the process of spine morphogenesis comes from two lines of evidence. The first relies on the demonstration that a well-characterized Eps8H1 mutant, specifically devoid of actin-capping activity, is unable to support proper spine formation. The Eps8H1 mutant allowed us to dissociate the Eps8 capping from bundling activity: indeed, while the Eps8 bundling activity is mainly mediated by a compact four-helix bundle, which contacts three actin subunits along the filament, the actin-capping activity of Eps8 is mainly mediated by a amphipathic helix that binds within the hydrophobic pocket at the barbed ends of actin, thus blocking further addition of actin monomers (Hertzog et al, 2010). In the Eps8H1 mutant, the hydrophobic residues critical for actin capping were mutated, while leaving intact the actin bundling activity. As a further support, acute inhibition of the protein capping activity, obtained through neuronal intracellular perfusion with a blocking peptide, resulted in impairment of plasticity phenomena, thus univocally demonstrating that Eps8 controls spine formation and activity-driven potentiation through the capping of actin filaments. While the present manuscript was under revision, a paper has been published (Stamatakou et al, 2013) showing that acute Eps8 reduction in primary cultures impacts spine formation and plasticity, the former, in particular, through the protein capping activity. Stamatakou et al did not observe any changes in mEPSC amplitude upon LTP, leading them to conclude that Eps8 is required for LTP-mediated synapse formation, but not LTP-induced synaptic strengthening. Our in vitro and in vivo data support that Eps8 is needed for LTP-induced synaptic strengthening. The differences observed may be due to residual Eps8 levels in the experimental conditions of the Stamatakou’s study.
End capping of cytoskeletal filaments is a key mechanism for regulating filaments’ elongation and disassembly, as well as the organization of the cytoskeletal architecture. It is therefore conceivable that the lack of Eps8, either genetic or consequent to siRNA knockdown, impairs actin organization and remodelling in the dendritic spines. As a further support to the view that a proper process of spine morphogenesis is required both during development and during plasticity phenomena, alterations of the spine actin structure and its dynamic regulation have been observed in a number of neurological disorders characterized by intellectual disability (ID), such as autism spectrum disorder (ASD), mental retardation and fragile X syndrome (van Spronsen and Hoogenraad, 2010; Penzes et al, 2011). Mutations in specific synaptic genes including the Akt/mTOR pathway (Kelleher and Bear, 2008; Bourgeron, 2009) involved in regulation of spine protein synthesis, the Neurexin–Neuroligin–Shank pathway (Jamain et al, 2003; Durand et al, 2007) associated with synaptogenesis and excitation-inhibition imbalance, and Ras/Rho GTPase pathway (Pinto et al, 2010) implicated in spine formation and stabilization have been identified in subjects with ASD. Furthermore, overabundant and immature spines have been reported in ASD (Hutsler and Zhang, 2010). Taken together, these findings suggest a major role for dendritic spine abnormalities in the pathogenesis of these diseases. We show here that reduced levels of the actin-capping protein, Eps8, occur in brains of patients affected by autism. Together with previous evidence that capping protein (CP) levels are significantly lower in fetal brains of Down syndrome than in controls (Gulesserian et al, 2002), our data suggest that reduction in actin-capping proteins may characterize cognitive impairments associated with spine defects. Interestingly, alterations in BDNF isoform levels have been found in autism patients (Garcia et al, 2012). Since the actin-capping activity of Eps8 in neurons is regulated by BDNF (Menna et al, 2009), the possibility arises that BDNF controls actin-capping and spine morphogenesis via Eps8 during synaptic plasticity and learning and that defects in this network may be involved in the pathogenesis of autism.
Materials and methods
See also Supplementary data for ‘In vitro binding assay’, ‘Immunofluorescent staining on sections’ and ‘Golgi staining’.
Animals
All the experimental procedures followed the guidelines established by the Italian Council on Animal Care and were approved by the Italian Government Decree No. 27/2010. All efforts were made to minimize the number of subjects used and their suffering. Eps8 wild-type (WT) and Eps8 knockout (KO) mice (Croce et al, 2004) were housed in cages with free access to food and water at 22°C and with a 12-h alternating light/dark cycle. Genotyping was performed by PCR.
Behavioural tests
All behavioural tests are shortly described below. Full details are available in details in the Supplementary Methods.
T-maze. Animals (10 Eps8 KO and 10 Eps8 wt) were food deprived until reaching 85–90% of their free-feeding body weight. Mice were habituated to a black wooden T-maze (stem length 41 cm; arm length 91 cm) and processed as described in Braida et al (2004).
Radial maze. Working memory was studied in 10 Eps8 KO and 10 Eps8 wt mice using a computerized wooden eight-arm radial maze according to Braida et al (2004).
Passive avoidance. Passive avoidance task was carried out in 10 Eps8 KO and 10 Eps8 wt mice as previously described (Braida et al, 2004) and described in detail in Supplementary data.
Object recognition. The test was conducted on 10 Eps8 KO and 10 Eps8 WT over a two-day period in an open plastic arena (60 × 50 × 30 cm), as previously described (Pan et al, 2008; Corradini et al, 2012 (see Supplementary data for details).
Sociability test. The test was performed on 11 wt and 15 Eps8 KO animals as described in Sala et al, 2011.
EEG
Electroencephalogram (EEG) activity was recorded, in a Faraday chamber, using a PowerLab digital acquisition system (AD Instruments, Bella Vista, Australia; sampling rate 100 Hz) in freely moving mice (n=10 mice per genotype) previously submitted to surgical implantation of electrodes (See for details Supplementary data).
Cell cultures
Primary cultures of mouse hippocampal neurons were established from E18 fetal, Eps8 KO or wild type (wt) littermates C57BL/6 mice as described by Banker and Cowan (1977) and Bartlett and Banker (1984) with slight modifications. Briefly, hippocampi were dissociated by treatment with trypsin (0.125% for 15 min at 37°C), followed by trituration with a polished Pasteur pipette. The dissociated cells were plated onto glass coverslips coated with poly-L-lysine at density of 400 cells/mm2. The cells were maintained in Neurobasal (Invitrogen, San Diego, CA) with B27 supplement and antibiotics, 2 mM glutamine and 12.5 μM glutamate (neuronal medium).
cDNA constructs and expression
Neuronal cultures were transfected at 10DIV with pEGFP-C1 (Clontech, Palo Alto, CA, USA) or pSUPER-DsRed plasmid (obtained from pSUPER-GFP, Oligoengine, Seattle, USA). The Eps8 WT or the capping mutant H1 are cloned in pEGFP vector as described (Disanza et al, 2006; Hertzog et al, 2010). Two different double-strand small interfering RNA (siRNA) oligonucleotides (Stealth RNAi; called 1525 and 1158) against mouse Eps8 were used according to Menna et al (2009). Hippocampal neurons were transfected by using Lipofectamine 2000 (Invitrogen).
Immunofluorescence staining of dissociated neurons
Neuronal cultures were fixed with 4% paraformaldehyde and 4% sucrose or with 100% cold methanol. The following antibodies were used: mouse anti-VAMP2 (1:1000; Synaptic System, Goettingen, Germany), guinea pig anti-Bassoon (1:300; Synaptic System, Goettingen, Germany), guinea pig anti-vGLUT1 (1:1000; Synaptic System, Germany), mouse anti-PSD-95 (1:400; UC Davis/NIH NeuroMab Facility, CA, USA), rabbit anti-GFP (1:400; Invitrogen, San Diego, CA), mouse anti-beta III tubulin (1:400; Promega Corporation, Madison, USA). Secondary antibodies were conjugated with Alexa-488, Alexa-555 or Alexa-633 fluorophores (Invitrogen, San Diego, CA, USA). Images were acquired using a Zeiss LSM 510 META confocal microscope producing image stacks. Pixel size was 110 nm × 110 nm, and acquisition parameters (i.e., laser power, gain and offset) were kept constant among different experimental settings. For the analysis of synaptic puncta only clusters lying along secondary dendritic branches were counted. The detection threshold was set to 2.5-fold the level of background fluorescence referring to diffuse fluorescence within dendritic shafts. The minimum puncta size was set at four pixels (0.048 μm2). Colocalization of two or three selected markers was measured using the boolean function ‘and’ for the selected channels. The resulting image was binarized and used as a colocalization mask to be subtracted to single channels. The number of the puncta resulting from colocalization mask subtraction were measured for each marker. A colocalization ratio was set as colocalizing puncta/total puncta number. The total area of the measured synaptic puncta represents synaptic area. For each cell, three or four dendrites were analysed from maximum projection images. Filopodia were defined as thin protrusions without a distinguishable head, stubby spines as short protrusions without a neck, and mushroom spines as protrusions with a short neck and a distinguishable head. Synapses were defined by the apposition of presynaptic and postsynaptic markers, such as vGlut1 or Bsn and PSD-95. Fluorescence images processing and analyses were performed with ImageJ Software (National Institutes of Health).
Cell culture electrophysiology
Whole-cell voltage-clamp recordings were performed on rat embryonic hippocampal neurons or on wt and Eps8 mice null hippocampal neurons maintained in culture for 13–15 DIV. Miniature activity was recorded as described in Antonucci et al (2012) (see Supplementary data for details).
For glycine-induced LTP experiments, recordings from each neuron lasted at least 60 min and each cell was continuously perfused (1 ml/min) from a computer-controlled perfusion system with a solution containing (in mM) 125 NaCl, 5 KCl, 1.2 KH2PO4, 2 CaCl2, 6 glucose, and 25 HEPES-NaOH, TTX 0.001, Strychnine 0.001 and bicuculline methiodide 0.02 (pH 7.4). Solution with glycine (100 μM) was applied for 3 min and then washed out for at least 45 min. The patch pipette electrode contained the following solution (in mM): 130 CsGluconate, 8 CsCl, 2 NaCl, 10 HEPES, 4 EGTA, 4 MgATP and 0.3 Tris-GTP.
The Eps8-capping inhibitor peptide (blocking peptide) was dissolved in the intracellular solution and injected into neurons via the patch pipette. Glycine was applied at least 10 min after the injection of inhibitor peptide or its inactive control.
Analysis of human brain tissue samples
Eleven postmortem brain samples from subjects with autism and thirteen control brain samples were provided to us by the Autism Speaks’ Tissue Program (Princeton, NJ, USA) via the Harvard Brain Bank (Belmont, MA, USA) and the University of Maryland Brain and Tissue Bank (Baltimore, MD, USA). Clinical information about each tissue sample was obtained through the Autism Tissue Program online portal (http://www.atpportal.org). There were no statistically significant differences between groups for age at death or PMI. Fusiform gyrus brain tissue was chosen, because this area is hypoactivated during face discrimination tasks in subjects with autism (Schultz et al, 2000). The diagnosis of autistic disorder was confirmed using the Autism Diagnostic Interview-Revised (Lord et al, 1994) postmortem through interviews with the parents and/or caregivers. Samples were stored at −80°C before use. Protein extraction was performed as previously described with minor modifications (Fahnestock et al, 2001; Garcia et al, 2012). Approximately 100 mg of tissue was homogenized on ice without thawing using a sonic dismembrator in homogenization buffer (HB) (0.05 M Tris pH 7.5, 0.5% Tween-20, 10 mM EDTA, 1 complete, Mini, EDTA-free tablet (Roche, Cat. no. 11 836 170 001) per 10 ml of HB, 2 μg/ml pepstatin, 2 μg/ml aprotinin, 50 mM sodium fluoride, 2 mM sodium orthovanadate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 0.5% sodium deoxycholate). The homogenate was incubated for 15 min on ice and then centrifuged at 12 000 × g for 20 min at 4°C. Supernatants containing solubilized protein were aliquoted and stored at −80°C before use. Protein concentrations were determined using a DC protein assay kit as described by the manufacturer (Bio-Rad Laboratories, Mississauga, Ontario, Canada).
Western blotting analysis on human brain tissue samples
Western blotting was carried out as previously described with slight modifications (Fahnestock et al, 2001; Kawaja et al, 2011; Garcia et al, 2012). Samples containing 35 μg protein were resolved in 10% sodium dodecyl sulphate-polyacrylamide gels under reducing conditions. After transfer onto polyvinylidene diflouride membranes for 2 h at 250 mA at 4°C, blots were blocked for 1 h at room temperature in a 1:1 solution of phosphate-buffered saline (PBS) pH 7.4 and Odyssey Blocking Buffer (BB) (Cedarlane, Burlington, Ontario, Canada) and then incubated with rabbit polyclonal or mouse monoclonal Eps8 primary antibodies (dilution 1:1000) and β-actin antibodies (Sigma, diluted 1:5000) at 4°C overnight in BB:PBS (1:1), 0.5% Tween-20 (PBS-T). Subsequently, membranes were washed and incubated for 1 h at room temperature in PBS-T with the secondary antibodies IRDye 680-conjugated goat anti-rabbit and IRDye 800CW-conjugated goat anti-mouse (LI-COR Biosciences, Lincoln, NE, USA; diluted 1:8000). All blots were scanned using an Odyssey Infrared Imaging System (LI-COR Biosciences). Blots were run twice with two different Eps8 antibodies. Each western blot contained a standard curve consisting of different amounts of protein per lane (from 5 to 80 μg) to ensure that the sample loading amount was in the linear range of detection for Eps8 (Fahnestock et al, 2001; Kawaja et al, 2011; Garcia et al, 2012). The intensities of immunoreactive bands were measured using LI-COR Odyssey Software, version 2.0 with local background subtracted. Eps8 pixel values were normalized to β-actin values for each sample.
Statistical analysis
Morphological analysis of spine parameters and synapse density was performed using ImageJ software (NIH, Bethesda, MD, USA).
n refers to the number of elements analysed. Statistical analysis was performed using SigmaStat 3.5 (Jandel Scientific) or PRISM 5 software (GraphPad, Software Inc., San Diego, CA, USA). After testing whether data were normally distributed or not, the appropriate statistical test has been used, see figure legends. Data are presented as mean±s.e.m. from the indicated number of elements analysed. For behaviour, the continuous data were analysed using a paired Student’s t-test and the categorical data were analysed using Fisher’s exact probability test. The AUC was calculated for the total number of errors in completing the maze. The differences were considered to be significant if P<0.05 and are indicated by an asterisk; those at P<0.01 are indicated by double asterisks; those at P<0.001 are indicated by triple asterisks.
Supplementary Material
Acknowledgments
We wish to acknowledge Dr Noam Ziv, Technion, Haifa for the gift of PSD-95-GFP construct and Dr Carlo Sala (CNR, Milano) for the gift of antibodies against PSD-95. The research leading to these results has received funding by the European Union Seventh Framework Programme under grant agreement no. HEALTH-F2-2009-241498 (‘EUROSPIN’ project), by Telethon GGP12115 and by PRIN 2010–2011 to MM.
Author contributions: Conceived and designed the experiments: EM, MM, MS, MF; performed the experiments: EM, SZ, RM, AD, AD, DC, DB, CN, MO, GF, CR, CF; analysed the data: EM, SZ, RM; contributed reagents/materials/analysis tools: GS, MF, MF; wrote the paper: EM, MM.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abdel-Rahman WM, Ruosaari S, Knuutila S, Peltomäki P (2012) Differential roles of EPS8 in carcinogenesis: loss of protein expression in a subset of colorectal carcinoma and adenoma. World J Gastroenterol 18: 3896–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin O, Mullins RD (2008) Capping protein increases the rate of actin-based motility by promoting filament nucleation by the Arp2/3 complex. Cell 133: 841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda JF, Gattermeir DJ, Karpova TS, Cooper JA (1992) Effects of null mutations and overexpression of capping protein on morphogenesis, actin distribution and polarized secretion in yeast. J Cell Biol 119: 1151–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B (2008) Distinct role of long 3' UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 134: 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci F, Alpár A, Kacza J, Caleo M, Verderio C, Giani A, Martens H, Chaudhry FA, Allegra M, Grosche J, Michalski D, Erck C, Hoffmann A, Harkany T, Matteoli M, Härtig W (2012) Cracking down on inhibition: selective removal of GABAergic interneurons from hippocampal networks. J Neurosci 32: 1989–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD (1996) Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell 87: 1025–1035 [DOI] [PubMed] [Google Scholar]
- Arikkath J, Reichardt LF (2008) Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci 31: 487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker GA, Cowan WM (1977) Rat hippocampal neurons in dispersed cell culture. Brain Res 126: 397-442. [DOI] [PubMed] [Google Scholar]
- Bartlett WP, Banker GA (1984) An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. I. Cells which develop without intercellular contacts. J Neurosci 4: 1944–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DH, Zhang S, Gan WB (2009) Dendritic spine dynamics. Annu Rev Physiol 71: 261–282 [DOI] [PubMed] [Google Scholar]
- Bourgeron T (2009) A synaptic trek to autism. Curr Opin Neurobiol 19: 231–234 [DOI] [PubMed] [Google Scholar]
- Braida D, Sacerdote P, Panerai AE, Bianchi M, Aloisi AM, Iosuè S, Sala M (2004) Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav Brain Res 153: 423–429 [DOI] [PubMed] [Google Scholar]
- Chapleau CA, Larimore JL, Theibert A, Pozzo-Miller L (2009) Modulation of dendritic spine development and plasticity by BDNF and vesicular trafficking: fundamental roles in neurodevelopmental disorders associated with mental retardation and autism. J Neurodev Disord 1: 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y (2008) Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci 9: 344–356 [DOI] [PubMed] [Google Scholar]
- Corradini I, Donzelli A, Antonucci F, Welzl H, Loos M, Martucci R, De Astis S, Pattini L, Inverardi F, Wolfer D, Caleo M, Bozzi Y, Verderio C, Frassoni C, Braida D, Clerici M, Lipp HP, Sala M, Matteoli M (2012) Epileptiform activity and cognitive deficits in SNAP-25+/− mice are normalized by antiepileptic drugs. Cereb Cortex (advance online publication, 12 October 2012) [DOI] [PubMed] [Google Scholar]
- Craig AM, Graf ER, Linhoff MW (2006) How to build a central synapse: clues from cell culture. Trends Neurosci 29: 8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce A, Cassata G, Disanza A, Gagliani MC, Tacchetti C, Malabarba MG, Carlier MF, Scita G, Baumeister R, Di Fiore PP (2004) A novel actin barbed-end-capping activity in EPS-8 regulates apical morphogenesis in intestinal cells of Caenorhabditis elegans. Nat Cell Biol 6: 1173–1179 [DOI] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL (2010) A simple role for BDNF in learning and memory? Front Mol Neurosci 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey ME, Smith SJ (1996) The dynamics of dendritic structure in developing hippocampal slices. J Neurosci 16: 2983–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DA, Wilson MH, Giraud J, Xie Z, Tseng HC, England C, Herscovitz H, Tsai LH, Delalle I (2009) Capzb2 interacts with beta-tubulin to regulate growth cone morphology and neurite outgrowth. PLoS Biol 7: e1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanza A, Carlier MF, Stradal TE, Didry D, Frittoli E, Confalonieri S, Croce A, Wehland J, Di Fiore PP, Scita G (2004) Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat Cell Biol 6: 1180–1188 [DOI] [PubMed] [Google Scholar]
- Disanza A, Mantoani S, Hertzog M, Gerboth S, Frittoli E, Steffen A, Berhoerster K, Kreienkamp HJ, Milanesi F, Di Fiore PP, Ciliberto A, Stradal TE, Scita G (2006) Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8-IRSp53 complex. Nat Cell Biol 8: 1337–1347 [DOI] [PubMed] [Google Scholar]
- Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R (1999) Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci USA 96: 13438–13443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsäter H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Rogé B, Héron D, Burglen L et al. (2007) Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 39: 25–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers JF, Muench D, Duch C (2006) Developmental relocation of presynaptic terminals along distinct types of dendritic filopodia. Dev Biol 297: 214–227 [DOI] [PubMed] [Google Scholar]
- Falck S, Paavilainen VO, Wear MA, Grossmann JG, Cooper JA, Lappalainen P (2004) Biological role and structural mechanism of twinfilin-capping protein interaction. EMBO J 23: 3010–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD (2001) The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci 18: 210–220 [DOI] [PubMed] [Google Scholar]
- Fan Y, Tang X, Vitriol E, Chen G, Zheng JQ (2011) Actin capping protein is required for dendritic spine development and synapse formation. J Neurosci 31: 10228–10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM (1998) Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci 18: 8900–8911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DA, Davare MA, Srivastava T, Brady JD, Nygaard S, Derkach VA, Soderling TR (2010) Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J Neurosci 30: 11565–11575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frittoli E, Matteoli G, Palamidessi A, Mazzini E, Maddaluno L, Disanza A, Yang C, Svitkina T, Rescigno M, Scita G (2011) The signaling adaptor Eps8 is an essential actin capping protein for dendritic cell migration. Immunity 35: 388–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K (2003) Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron 38: 447–460 [DOI] [PubMed] [Google Scholar]
- Garcia KLP, Guanhua Y, Nicolini C, Michalski B, Garzon D, Chiu VS, Tongiorgi E, Szatmari P, Fahnestock M (2012) Altered balance of proteolytic forms of pro-brain-derived-neurotrophic factor in autism. J Neuropathol Exp Neurol 71: 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KL, Yu G, Nicolini C, Michalski B, Garzon DJ, Chiu VS, Tongiorgi E, Szatmari P, Fahnestock M (2012) Altered balance of proteolytic isoforms of pro-brain-derived neurotrophic factor in autism. J Neuropathol Exp Neurol 71: 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove M, Demyanenko G, Echarri A, Zipfel PA, Quiroz ME, Rodriguiz RM, Playford M, Martensen SA, Robinson MR, Wetsel WC, Maness PF, Pendergast AM (2004) ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol Cell Biol 24: 10905–10922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulesserian T, Kim SH, Fountoulakis M, Lubec G (2002) Aberrant expression of centractin and capping proteins, integral constituents of the dynactin complex, in fetal down syndrome brain. Biochem Biophys Res Commun 291: 62–67 [DOI] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B (1992) Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci 12: 2685–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog M, Milanesi F, Hazelwood L, Disanza A, Liu H, Perlade E, Malabarba MG, Pasqualato S, Maiolica A, Confalonieri S, Le Clainche C, Offenhauser N, Block J, Rottner K, Di Fiore PP, Carlier MF, Volkmann N, Hanein D, Scita G (2010) Molecular basis for the dual function of Eps8 on actin dynamics: bundling and capping. PLoS Biol 8: e1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkura N, Matsuzaki M, Noguchi J, Ellis-Davies GC, Kasai H (2008) The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron 57: 719–729 [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Hoogenraad CC (2010) Actin in dendritic spines: connecting dynamics to function. J Cell Biol 189: 619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P, Llano O, Smirnov S, Tanhuanpaa K, Faix J, Rivera C, Lappalainen P (2009) Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J Cell Biol 185: 323–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsler JJ, Zhang H (2010) Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res 1309: 83–94 [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T (2003) Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Paris Autism Research International Sibpair Study. Nat. Genet 34: 27–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jontes JD, Smith SJ (2000) Filopodia, spines and the generation of synaptic diversity. Neuron 27: 11–14 [DOI] [PubMed] [Google Scholar]
- Jourdain P, Fukunaga K, Muller D (2003) Calcium/calmodulin-dependent protein kinase II contributes to activity-dependent filopodia growth and spine formation. J Neurosci 23: 10645–10649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H (2003) Structure-stability-function relationships of dendritic spines. Trends Neurosci 26: 360–368 [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Smithson LJ, Elliot J, Trinh G, Crotty AM, Michalski B, Fahnestock M (2011) Nerve growth factor promoter activity revealed in mice expressing enhanced green fluorescent protein. J Comp Neurol 519: 2522–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, Bear MF (2008) The autistic neuron: troubled translation? Cell 135: 401–406 [DOI] [PubMed] [Google Scholar]
- Kitanishi T, Sakai J, Kojima S, Saitoh Y, Inokuchi K, Fukaya M, Watanabe M, Matsuki N, Yamada MK (2010) Activity-dependent localization in spines of the F-actin capping protein CapZ screened in a rat model of dementia. Genes Cells 15: 737–747 [DOI] [PubMed] [Google Scholar]
- Korobova F, Svitkina T (2008) Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell 19: 1561–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Svitkina T (2010) Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell 21: 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, Ogura A, Lu B, Kojima M (2009) Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain 2: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S (2000) Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci USA 97: 6856–6861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J (2004) Structural plasticity and memory. Nat Rev Neurosci 5: 45–54 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A (1994) Autism diagnostic interview-revised. A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Autism Dev Dis 24: 659–685 [DOI] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT (2001) Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29: 243–254 [DOI] [PubMed] [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC, Muller D (2000) Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci 3: 545–550 [DOI] [PubMed] [Google Scholar]
- Manfredi I, Zani AD, Rampoldi L, Pegorini S, Bernascone I, Moretti M, Gotti C, Croci L, Consalez GG, Ferini-Strambi L, Sala M, Pattini L, Casari G (2009) Expression of mutant beta2 nicotinic receptors during development is crucial for epileptogenesis. Hum Mol Genet 18: 1075–1088 [DOI] [PubMed] [Google Scholar]
- Matus A (2000) Actin-based plasticity in dendritic spines. Science 290: 754–758 [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M, Lu WY, MacDonald JF, Wang JY, Falls DL, Jia Z (2002) Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron 35: 121–133 [DOI] [PubMed] [Google Scholar]
- Menna E, Disanza A, Cagnoli C, Schenk U, Gelsomino G, Frittoli E, Hertzog M, Offenhauser N, Sawallisch C, Kreienkamp HJ, Gertler FB, Di Fiore PP, Scita G, Matteoli M (2009) Eps8 regulates axonal filopodia in hippocampal neurons in response to brain-derived neurotrophic factor (BDNF). PLoS Biol 7: e1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilner A, Rubinstein B (2005) The physics of filopodial protrusion. Biophys J 89: 782–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongioví AM, Romano PR, Panni S, Mendoza M, Wong WT, Musacchio A, Cesareni G, Di Fiore PP (1999) A novel peptide-SH3 interaction. EMBO J. 18: 5300–5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Wood CL, Patton AP, Jaafari N, Henley JM, Mellor JR, Hanley JG (2011) PICK1 inhibition of the Arp2/3 complex controls dendritic spine size and synaptic plasticity. EMBO J 30: 719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonenko I, Jourdain P, Muller D (2003) Presynaptic remodeling contributes to activity-dependent synaptogenesis. J Neurosci 23: 8498–8505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenhäuser N, Castelletti D, Mapelli L, Soppo BE, Regondi MC, Rossi P, D'Angelo E, Frassoni C, Amadeo A, Tocchetti A, Pozzi B, Disanza A, Guarnieri D, Betsholtz C, Scita G, Heberlein U, Di Fiore PP (2006) Increased ethanol resistance and consumption in Eps8 knockout mice correlates with altered actin dynamics. Cell 127: 213–226 [DOI] [PubMed] [Google Scholar]
- Okabe S, Miwa A, Okado H (2001) Spine formation and correlated assembly of presynaptic and postsynaptic molecules. J Neurosci 21: 6105–6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y (2004) Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci 7: 1104–1112 [DOI] [PubMed] [Google Scholar]
- Pan D, Sciascia A 2nd, Vorhees CV, Williams MT (2008) Progression of multiple behavioral deficits with various ages of onset in a murine model of Hurler syndrome. Brain Res 1188: 241–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14: 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Almeida J, Bacchelli E, Bader GD, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bölte S, Bolton PF et al. (2010) Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466: 368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Weinberg RJ (2008) Organization of the Arp2/3 complex in hippocampal spines. J Neurosci 28: 5654–5659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran B, Frey JU (2009) Interfering with the actin network and its effect on long-term potentiation and synaptic tagging in hippocampal CA1 neurons in slices in vitro. J Neurosci 29: 12167–12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo L, Roman FS, Ammassari-Teule M, Marchetti E (2006) Simultaneous olfactory discrimination elicits a strain-specific increase in dendritic spines in the hippocampus of inbred mice. Hippocampus 16: 472–479 [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B (2011) Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry 69: 875–882 [DOI] [PubMed] [Google Scholar]
- Schafer DA, Hug C, Cooper JA (1995) Inhibition of CapZ during myofibrillogenesis alters assembly of actin filaments. J Cell Biol 128: 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA, Mooseker MS, Cooper JA (1992) Localization of capping protein in chicken epithelial cells by immunofluorescence and biochemical fractionation. J Cell Biol 118: 335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC (2000) Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry 57: 331–340 [DOI] [PubMed] [Google Scholar]
- Sekerková G, Diño MR, Ilijic E, Russo M, Zheng L, Bartles JR, Mugnaini E (2007) Postsynaptic enrichment of Eps8 at dendritic shaft synapses of unipolar brush cells in rat cerebellum. Neuroscience 145: 116–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Guire ES, Kaech S, White J, Zhang F, Schutz K, Langeberg LK, Banker G, Raber J, Scott JD (2007) A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J Neurosci 27: 355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakou E, Marzo A, Gibb A, Salinas PC (2013) Activity-dependent spine morphogenesis: a role for the actin-capping protein Eps8. J Neurosci 33: 2661–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina T, Lin WH, Webb DJ, Yasuda R, Wayman GA, Van Aelst L, Soderling SH (2010) Regulation of the postsynaptic cytoskeleton: roles in development, plasticity, and disorders. J Neurosci 30: 14937–14942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H (2008) Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 319: 1683–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocchetti A, Soppo CB, Zani F, Bianchi F, Gagliani MC, Pozzi B, Rozman J, Elvert R, Ehrhardt N, Rathkolb B, Moerth C, Horsch M, Fuchs H, Gailus-Durner V, Beckers J, Klingenspor M, Wolf E, Hrabé de Angelis M, Scanziani E, Tacchetti C et al. (2010) Loss of the actin remodeler Eps8 causes intestinal defects and improved metabolic status in mice. PLoS One 5: e9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Konyukh M, Delorme R, Leblond C, Chaste P, Fauchereau F, Coleman M, Leboyer M, Gillberg C, Bourgeron T (2010) Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet 26: 363–372 [DOI] [PubMed] [Google Scholar]
- Tsuriel S, Fisher A, Wittenmayer N, Dresbach T, Garner CC, Ziv NE (2009) Exchange and redistribution dynamics of the cytoskeleton of the active zone molecule bassoon. J Neurosci 29: 351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaggi F, Disanza A, Milanesi F, Di Fiore PP, Menna E, Matteoli M, Gov NS, Scita G, Ciliberto A (2011) The Eps8/IRSp53/VASP network differentially controls actin capping and bundling in filopodia formation. PLoS Comput Biol 7: e1002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spronsen M, Hoogenraad CC (2010) Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep 10: 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ (2008) N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J Biol Chem 283: 15912–15920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch T, Younsi A, Disanza A, Rodriguez JA, Cuervo AM, Scita G, Schmidt J (2010) Eps8 is recruited to lysosomes and subjected to chaperone-mediated autophagy in cancer cells. Exp Cell Res 316: 1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, De Roo M, Muller D (2009) Dendritic spine formation and stabilization. Curr Opin Neurobiol 19: 146–153 [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T (2001) Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci 24: 1071–1089 [DOI] [PubMed] [Google Scholar]
- Zampini V, Rüttiger L, Johnson SL, Franz C, Furness DN, Waldhaus J, Xiong H, Hackney CM, Holley MC, Offenhauser N, Di Fiore PP, Knipper M, Masetto S, Marcotti W (2011) Eps8 regulates hair bundle length and functional maturation of mammalian auditory hair cells. PLoS Biol 9: e1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng CY, Petralia RS, Wang YX, Kachar B, Wenthold RJ (2010) SAP102 is a highly mobile MAGUK in spines. J Neurosci 30: 4757–4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ (1996) Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17: 91–102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.