Abstract
Maintenance of genomic stability during eukaryotic cell division relies on the spindle assembly checkpoint (SAC) that prevents mitotic exit until all chromosomes are properly attached to the spindle. Polo is a mitotic kinase proposed to be involved in SAC function, but its role has remained elusive. We demonstrate that Polo and Aurora B functional interdependency comprises a positive feedback loop that promotes Mps1 kinetochore localization and activity. Expression of constitutively active Polo restores normal Mps1 kinetochore levels even after Aurora B inhibition, highlighting a role for Polo in Mps1 recruitment to unattached kinetochores downstream of Aurora B. We also show that Mps1 kinetochore localization is required for BubR1 hyperphosphorylation and formation of the 3F3/2 phosphoepitope. This is essential to allow recruitment of Cdc20 to unattached kinetochores and the assembly of anaphase-promoting complex/cyclosome-inhibitory complexes to levels that ensure long-term SAC activity. We propose a model in which Polo controls Mps1-dependent BubR1 phosphorylation to promote Cdc20 kinetochore recruitment and sustained SAC function.
Keywords: BubR1 phosphorylation, Cdc20-BubR1 complex, Mps1, Polo, SAC
Introduction
Genomic stability during cell division relies on the ability of chromosomes to establish proper interactions with the mitotic spindle, a highly dynamic process monitored by the spindle assembly checkpoint (SAC). The SAC operates as a surveillance mechanism that restrains anaphase onset until all chromosomes are correctly attached to microtubules from opposite spindle poles. Unattached kinetochores provide a platform that generates a ‘wait-anaphase’ signal that inhibits the activity of the anaphase-promoting complex/cyclosome (APC/C) thereby preventing degradation of Securin and Cyclin B (Musacchio and Salmon, 2007). Models for SAC molecular framework suggest that Mad1/Mad2 heterodimers associate with unattached kinetochores and catalyse the conformational activation of open Mad2 (O-Mad2) to a closed conformer (C-Mad2) that has higher affinity for APC/C activator Cdc20 (De Antoni et al, 2005; Yu, 2006). This transient Mad2-Cdc20 interaction promotes binding of Cdc20 to BubR1-Bub3 to generate the final mitotic checkpoint complex (MCC) that efficiently inhibits APC/C activity (Kulukian et al, 2009; Luo and Yu, 2012).
Mps1 and Aurora B kinases are required for SAC function by acting at multiple points along the pathway. Mps1 checkpoint function is attributed to its upstream role in the recruitment of Mad1, Mad2, BubR1, Bub1 and Bub3 to unattached kinetochores (Lan and Cleveland, 2010). Its activity was also shown to be required for Mad2 conformational activation by maintaining the recruitment of O-Mad2 to the kinetochore-associated Mad1-Mad2 heterodimer (Hewitt et al, 2010; Zich et al, 2012) and for the formation or stability of APC/C inhibitory complexes (Maciejowski et al, 2010). Recent data also support a direct role for Aurora B in SAC signalling by acting in concert with the Ndc80 complex to promote Mps1 kinetochore recruitment and activation at the onset of mitosis (Santaguida et al, 2011; Saurin et al, 2011) and by maintaining the stability of APC/C inhibitory complexes (Maldonado and Kapoor, 2011).
The role of Polo kinases in SAC signalling remains elusive and controversial. Plk1 was initially linked to SAC function due to its role in the generation of the 3F3/2 phosphoepitope at kinetochores of chromosomes that are not under tension (Ahonen et al, 2005). Subsequent work revealed that phosphorylation of BubR1 by Plx1 generated the 3F3/2 epitope and was required for SAC arrest in Xenopus egg extracts (Wong and Fang, 2007). However, Plk1 and 3F3/2 phosphoepitope appear to be dispensable for SAC function in human cells (Sumara et al, 2004; Lénárt et al, 2007). Rather, Plk1-mediated phosphorylation of BubR1 was shown to be important for the stability of kinetochore–microtubule attachments (Elowe et al, 2007; Matsumura et al, 2007). Moreover, several reports have shown that Plk1 inhibition or RNAi-mediated silencing causes a SAC-dependent prometaphase arrest with accumulation of Mad2 and BubR1 at kinetochores (Sumara et al, 2004; Lénárt et al, 2007; Petronczki et al, 2008). This contrasts with previous results showing a significant decrease of Mad2 and Cdc20 accumulation at unattached kinetochores upon inhibition of Plk1 expression (Ahonen et al, 2005). Accordingly, Plk1 was shown to phosphorylate Mad1 to allow its localization at unattached kinetochores and consequently Mad2 accumulation and SAC activity (Chi et al, 2008).
Here, we demonstrate that Drosophila Polo is required to allow mitotic exit but also for SAC signalling by promoting the recruitment of Mps1 to unattached kinetochores. Kinetochore-associated Mps1 controls BubR1 hyperphosphorylation and 3F3/2 phosphoepitope formation at kinetochores that are not under tension. Although commonly used as read out for low kinetochore tension during mitosis (Gorbsky and Ricketts, 1993), the molecular output of 3F3/2 accumulation at kinetochores and its physiological relevance remain undefined. Here we have uncoupled 3F3/2 formation from kinetochore tension. Overexpressing Mps1 in Drosophila S2 cells results in accumulation of 3F3/2 at kinetochores that are correctly attached to spindle microtubules and under tension. Our results strongly suggest that the molecular outcome of Mps1-dependent 3F3/2 formation is to promote the association of Cdc20 with BubR1 allowing proper kinetochore recruitment of Cdc20 and MCC formation. These findings redefine the functional and hierarchical interplay between several mitotic kinases to generate the 3F3/2 phosphoepitope and indicate that BubR1 and Cdc20 kinetochore association and regulation are essential to promote the efficient assembly of the MCC and sustained SAC activity.
Results
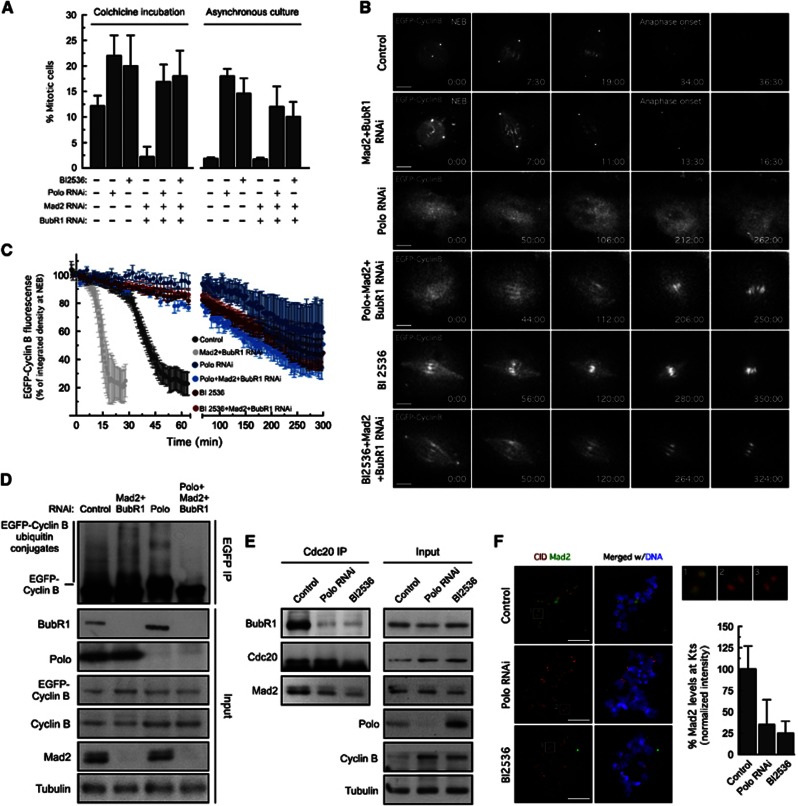
Loss of Polo activity leads to a spindle checkpoint-independent mitotic arrest with decreased levels of the mitotic checkpoint complex
As reported for cultured human cells, Drosophila S2 cells depleted of Polo or treated with the Polo-specific inhibitor, BI2536, arrested in mitosis as suggested by the increase of mitotic indices (Figure 1A). To determine whether this arrest was due to SAC activation, we co-depleted Mad2 and BubR1 by RNAi. Surprisingly, loss of both SAC components did not prevent the mitotic arrest. Likewise, whereas control asynchronous cells co-depleted of Mad2 and BubR1 degraded Cyclin B prematurely and exited mitosis (Figures 1B and C;Supplementary Movies S1 and S2), Mad2 and BubR1 co-depletion in cells treated with BI2536 or depleted of Polo failed to induce premature degradation of Cyclin B or to override the mitotic arrest (Figures 1B and C Supplementary Movies S3, S4, S5, S6). In accordance, the amount of EGFP–Cyclin B–ubiquitin conjugates was found to be significantly reduced in EGFP–Cyclin B immunoprecipitates from Polo-depleted cells, even upon co-depletion of Mad2 and BubR1 to relieve APC/C inhibition by the MCC (Figure 1D). Thus, contrasting with what has been reported for human cells (Sumara et al, 2004; Lénárt et al, 2007; Santamaria et al, 2007), these findings indicate that the mitotic arrest resulting from loss of Polo activity is not due to SAC activation. Instead, we speculate that it may arise from a potential requirement for Polo in APC/C activation (Kotani et al, 1998; Golan et al, 2002; Kraft et al, 2003), which precludes the assessment of SAC function in Polo-impaired cells by simple monitoring of the kinetics of mitotic exit. For that reason, and given that the SAC operates through MCC production, we assessed the ability of cells to assemble the MCC upon colchicine incubation. Consistent with a role of Polo in SAC signalling, the levels of BubR1 and Mad2 detected in Cdc20 immunoprecipitates were severely reduced in Polo-depleted cells or in cells treated with BI2536 (Figure 1E). Moreover, Polo depletion or inhibition led to a dramatic decrease of Mad2 association with unattached kinetochores (Figure 1F). We conclude that the SAC-independent mitotic arrest in Polo-inhibited cells masks a requirement of Polo activity for SAC signalling, as revealed by the impaired Mad2 kinetochore recruitment and the significantly decreased levels of the MCC.
Figure 1.
Loss of Polo activity results in a SAC-independent mitotic arrest with decreased MCC levels. (A) Mitotic index quantification based on H3Ser10Ph staining in asynchronous-cultured cells and after 24-h colchicine incubation. Data represent mean±s.d., n>3000 cells for each condition, from three independent experiments. (B) Mitotic progression of S2 cells expressing EGFP–Cyclin B was monitored by time-lapse microscopy. Selected stills of live-cell imaging for the indicated conditions are shown. Time is in min:sec. (C) EGFP–Cyclin B degradation profiles of experiments in (B). Data represent the mean±s.d. of at least 10 cells for each experimental condition. EGFP–Cyclin B fluorescence at NEB was set to 100% except in cells treated with BI2536 or depleted of Polo. Due to technical constraints resulting from loss of Polo activity, we were not able to detect NEB in those cells and, therefore set EGFP–Cyclin B fluorescence in the first frame to 100%. (D) Ubiquitination analysis of EGFP–Cyclin B immunoprecipitates from total cell lysates obtained from control and cells depleted of the indicated proteins. Cultured cells were treated with MG132 for 2 h. Immunoprecipitates (IP) were probed by immunoblotting for conjugated ubiquitin and corresponding total cell lysates (Input) for the indicated proteins. (E) Immunoprecipitation of Cdc20 from total cell lysates obtained from control cells, cells depleted of Polo and cells treated with BI2536. IP and corresponding inputs were probed by immunoblotting for the indicated proteins. (F) Immunolocalization of Mad2. Insets show higher-magnification views of kinetochore pairs in the boxed areas. Graphs display the quantification of Mad2 kinetochore levels. Mad2 fluorescence intensities were determined relative to CID. Data represent mean±s.d. and mean values obtained for control cells were set to 100%. n>20 cells for each condition. (E, F) Cells were treated with MG132 for 1 h followed by 2 h of colchicine incubation. BI2536 was added 30 min prior to microtubule depolymerization with colchicine. Scale bars correspond to 5 μm.
Source data for this figure is available on the online supplementary information page.
Polo is required for kinetochore localization of Mps1 checkpoint kinase
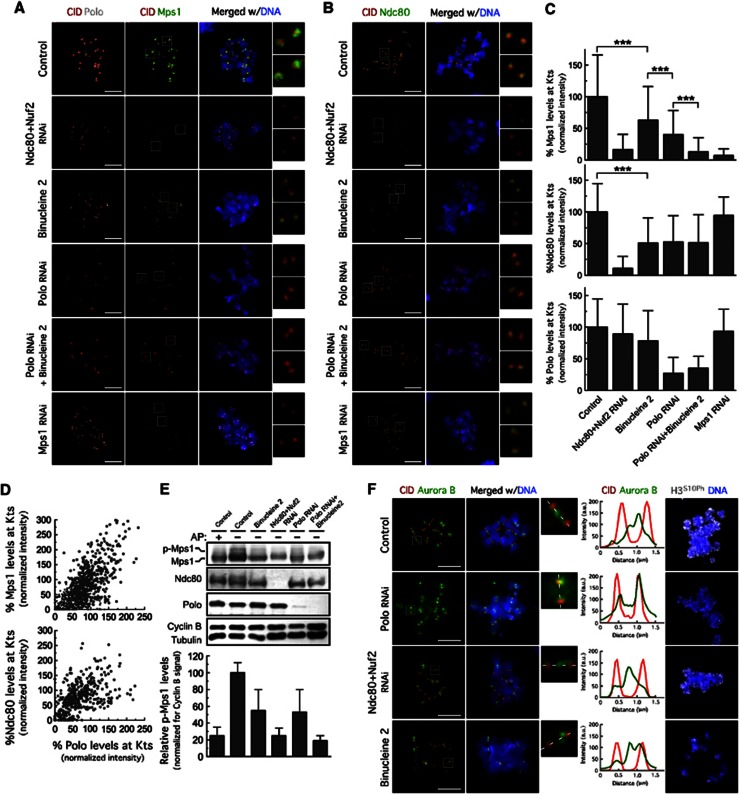
Having established that Polo is required for Mad2 kinetochore localization and to promote MCC formation, we next asked whether Polo depletion affects the kinetochore localization of Mps1 checkpoint kinase, previously shown to regulate both these events (Vleugel et al, 2012). We found that Polo depletion, both in S2 cells (Figures 2A, C and D) or in polo9 (Donaldson et al., 2001) mutant neuroblasts (Supplementary Figures S1A, B), resulted in a significant reduction of Mps1 at unattached kinetochores. Kinetochore accumulation of Mps1 potentiates its auto-activation by trans-phosphorylation, which is necessary to achieve full kinase activity (Kang et al, 2007; Jelluma et al, 2008). We, therefore, examined Mps1 hyperphosphorylation as a read-out of its activity as previously described (Jelluma et al, 2008; Sliedrecht et al, 2010; Saurin et al, 2011). Consistent with Mps1 hyperphosphorylation, western blotting of lysates from colchicine-treated S2 cells revealed a second Mps1 isoform with lower electrophoretic mobility, which disappeared upon phosphatase treatment (Figure 2E). Concomitantly with the observed reduction of Mps1 levels at kinetochores, depletion of Polo resulted in a significant decrease of Mps1 hyperphosphorylation (Figure 2E). These results indicate that the role of Polo in SAC signalling involves kinetochore targeting and possibly activation of the essential checkpoint kinase Mps1.
Figure 2.
Polo is required for Mps1 and Ndc80 recruitment to unattached kinetochores. Immunolocalization of Mps1, Polo (A) and Ndc80 (B). Insets show higher-magnification views of kinetochore pairs in the boxed areas. (C) Quantification of experiments in (A) and (B). Mps1, Polo and Ndc80 fluorescence intensities were determined relative to CID. Data represent mean±s.d. and mean values obtained for control cells were set to 100%. ***P<0.001, one-way ANOVA Turkey’s multiple comparison test. n>20 cells for each condition. (D) Variation of Mps1 and Ndc80 kinetochore levels with Polo levels at kinetochores from control and Polo-depleted cells in (A) and (B). (E) Immunoblot showing Mps1 phosphorylation status (top) and quantification of p-Mps1 (phosphorylated Mps1) normalized to Cyclin B signals. Data represent mean±s.d. and were obtained from four independent experiments. AP: alkaline phosphatase. (F) Immunolocalization of Aurora B and p-Ser10-Histone3 (H3S10Ph). Kinetochore pairs in boxed areas are represented magnified and the respective intensity profiles of Aurora B and CID labelling along the dotted line shown. (A–F) Cells were treated with MG132 for 1 h followed by 2 h of colchicine incubation. When required, binucleine 2 was added to the cultures 30 min before treatment with colchicine. Scale bars represent 5 μm.
Source data for this figure is available on the online supplementary information page.
Polo regulates Mps1 kinetochore recruitment by controlling outer kinetochore assembly
Similarly to that reported for human cells (Santaguida et al, 2011; Saurin et al, 2011), co-depletion of Ndc80 and Nuf2 completely prevented Mps1 localization at unattached kinetochores (Figures 2A and C) and severely reduced Mps1 hyperphosphorylation (Figure 2E). In budding yeast, Mps1 was shown to bind directly to the Ndc80 complex (Kemmler et al, 2009). To determine if Mps1 and Ndc80 can interact in vivo when the SAC is active, we immunoprecipitated Mps1 from lysates of S2 cells treated with colchicine. We were able to detect Ndc80 in Mps1 immunoprecipitates (Figure 4F), which supports the idea that the Ndc80 complex may mediate kinetochore binding of Mps1 in metazoans. We found that Polo depletion caused a 50% reduction of Ndc80 levels at kinetochores (Figures 2B–D) and inhibition of Aurora B with the specific inhibitor Binucleine 2 (Smurnyy et al, 2010) led to a similar decrease in Ndc80 kinetochore association and impaired to the same extent the recruitment of Mps1 to unattached kinetochores (Figures 2A–C).
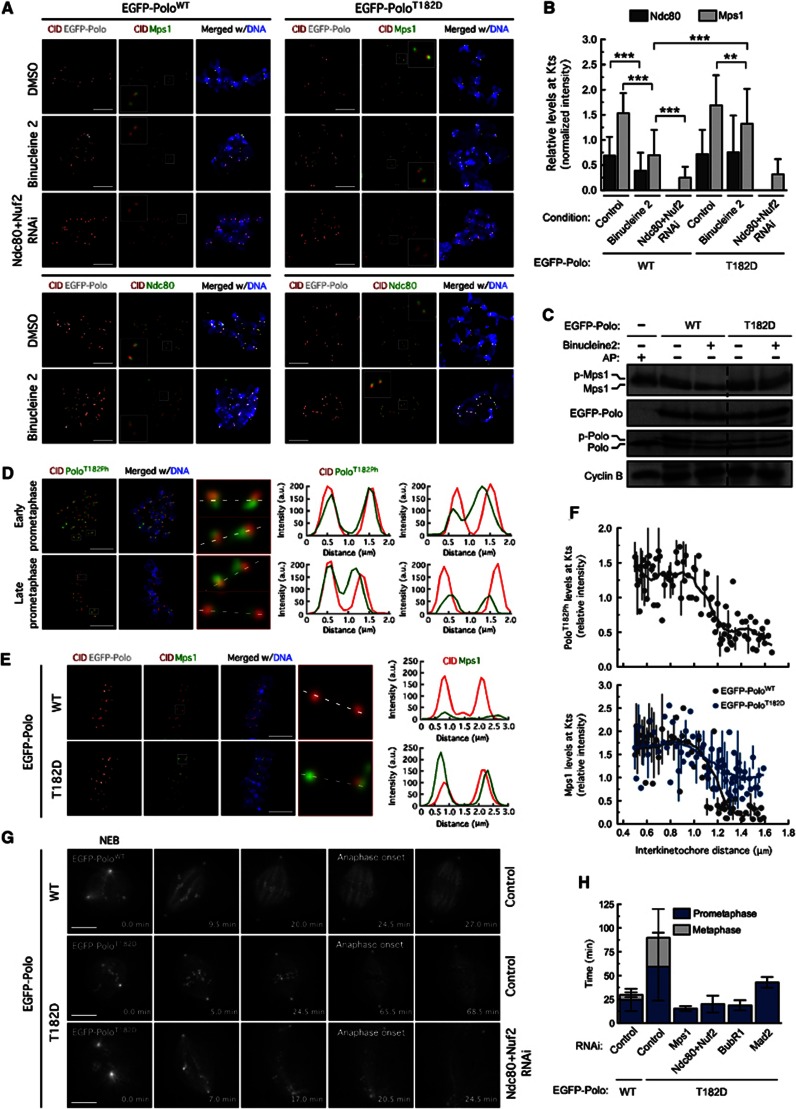
We recently showed that Polo is required for Aurora B centromeric localization and activity (Moutinho-Santos et al, 2012). Consistent with these results, depletion of Polo de-localized Aurora B from the inner centromere and reduced Aurora B activity as judged by the decrease of Histone-H3 phosphorylation (Figure 2F) (Moutinho-Santos et al, 2012). However, it was recently shown that Aurora B phosphorylates Polo on its activation T-loop (T182) in early mitosis to promote Polo activation at kinetochores/centromeres (Carmena et al, 2012). Given this functional interdependency, we reasoned that Aurora B-activated Polo could play a more direct role in Ndc80 kinetochore recruitment. To test this, we generated S2 cell lines expressing either wild-type Polo (EGFP-PoloWT) or constitutively active Polo carrying the phosphomimetic T182D mutation (EGFP-PoloT182D) at identical, near endogenous levels. Similar to what we observed in S2 cells without transgenes (Figures 2A–C), inhibition of Aurora B in cells expressing EGFP-PoloWT caused a pronounced reduction (50%) of Ndc80 and Mps1 levels at unattached kinetochores (Figures 3A and B). By contrast, expression of EGFP-PoloT182D rescued to control levels Ndc80 kinetochore localization and recovered to some extent Mps1 kinetochore recruitment and hyperphosphorylation in the absence of Aurora B activity (Figures 3A–C). As expected, the ability of constitutively active Polo to restore Mps1 kinetochore recruitment in Aurora B-inhibited cells was dependent on the Ndc80 complex (Figures 3A and B). These data are consistent with a role for Polo in outer kinetochore assembly downstream of Aurora B activity.
Figure 3.
Constitutively active Polo promotes Ndc80 and Mps1 kinetochore recruitment independently of Aurora B activity. (A) Immunolocalization of Mps1 (top) and Ndc80 (bottom) in cells expressing EGFP–PoloWT and EGFP–PoloT182D upon treatment with DMSO, binucleine 2 or Ndc80 and Nuf2 RNAi. Insets show higher-magnification views of kinetochore pairs in the boxed areas. (B) Quantification of experiment in (A). Mps1 and Ndc80 fluorescence intensities at kinetochores were determined relative to CID. Data represent mean±s.d. **P<0.01; ***P<0.001, One-way ANOVA Turkey’s multiple comparison test. n>20 cells for each condition. (C) Cell lysates were analysed by immunoblotting to assess Mps1 phosphorylation status. AP: alkaline phosphatase. (D) Immunofluorescence analysis of asynchronous S2 cells stained for Polo–T182 phosphorylation (PoloT182Ph) and CID. An early prometaphase cell with unaligned chromosomes and a late prometaphase cell with a discernible metaphase plate and one unaligned chromosome (yellow *) are shown. Kinetochore pairs in boxed areas are magnified and the respective fluorescence intensity profiles of PoloT182Ph and CID are shown. (E) Immunolocalization of Mps1 in metaphase cells expressing EGFP–PoloWT and EGFP–PoloT182D treated with MG132 for 2 h. Kinetochore pairs in boxed areas are shown in higher magnification and the respective intensity profiles of Mps1 and CID labelling are represented. (F) Quantification of PoloT182Ph and Mps1 kinetochore levels from experiments in (D) and (E), respectively, plotted against interkinetochore distance. PoloT182Ph and Mps1 fluorescence intensities at kinetochores were determined relative to CID. (G) Mitotic progression of S2 cells expressing EGFP–PoloWT or EGFP–PoloT182D was monitored by time-lapse microscopy. Selected stills of live-cell imaging of control and RNAi-treated cells are shown. Time is in min. (H) Quantification of the mitotic timing — from nuclear envelope breakdown to anaphase onset — for cells expressing EGFP–PoloWT or EGFP–PoloT182D. (A–C) Cells were treated with MG132 for 1 h followed by 2-h incubation with colchicine. Binucleine 2 was added to cultures 30 min prior to colchicine treatment. Scale bars represent 5 μm.
Source data for this figure is available on the online supplementary information page.
Constitutively active Polo promotes Mps1 recruitment to microtubule-attached kinetochores
Results so far suggest that Polo regulates Mps1 kinetochore localization indirectly by controlling the levels of Ndc80 at kinetochores. However, we found that the combination of Aurora B inhibition with Binucleine 2 and Polo depletion dramatically impaired Mps1 recruitment to unattached kinetochores reducing its levels to 15%, whereas the observed effect on Ndc80 was less pronounced, with levels remaining at 50% (Figures 2A–C). Although one cannot exclude further effects, though independent of Ndc80, this observation argues in favour of an additional role for Polo in the recruitment of Mps1 to kinetochores beyond its requirement for kinetochore assembly. We reason that the cooperative regulation of Ndc80 phosphorylation both by Polo and Aurora B kinases during prometaphase might play a key role in the recruitment of Mps1 to unattached kinetochores. Current models suggest that phosphorylation of Ndc80 by Aurora B is spatially regulated by tension (Liu et al, 2009; Welburn et al, 2010; Lampson and Cheeseman, 2011). To determine if Polo phosphorylation by Aurora B is also tension sensitive, we assessed Polo–T-loop phosphorylation in asynchronous-cultured cells with a phosphospecific antibody that was previously shown to recognize Drosophila PoloT182Ph (Carmena et al, 2012). In prometaphase cells, PoloT182Ph was abundantly present at kinetochores and could also be detected at centromeres of some chromosomes (Figure 3D). As chromosomes aligned at the metaphase plate and centromeric tension was exerted (evaluated by the increase in interkinetochore distance), the amount of active Polo at kinetochores dropped significantly and was no longer present at centromeres (Figures 3D and F). Given that total levels of Polo at kinetochores remained unaltered from prometaphase to metaphase (our unpublished observation; Moutinho-Santos et al, 1999), this suggests that Polo activation by Aurora B might be spatially regulated by tension similarly to Ndc80. Establishment of tension upon chromosome biorientation limits the access of Aurora B to Polo and to the Ndc80 complex thus minimizing their phosphorylation and consequently, prevent the recruitment of Mps1 to kinetochores of metaphase chromosomes. However, expression of EGFP-PoloT182D, but not EGFP-PoloWT, was able to promote Mps1 localization to bi-oriented kinetochores of congressed chromosomes that have established interkinetochore tension (Figures 3E and F). Accordingly, live-cell imaging revealed that expression of EGFP-PoloT182D caused a pronounced metaphase delay that was abolished upon removal of Mps1 from kinetochores through Ndc80 and Nuf2 co-depletion or by depleting Mps1, hence indicating that the resulting delay is SAC-dependent (Figures 3G and H; Supplementary Movies S7, S8, S9). We conclude that a constitutively active Polo mutant largely bypasses the requirement for Aurora B in recruiting and activating Mps1 at unattached kinetochores and promotes abnormal accumulation of Mps1 at bi-oriented kinetochores of congressed chromosomes when Aurora B-mediated phosphorylation at kinetochores is known to be minimal (Welburn et al, 2010). Cumulatively, these results suggest that in addition to its contribution for proper loading of Ndc80 on kinetochore, Polo has a role in controlling Mps1 kinetochore recruitment downstream of Aurora B.
Drosophila Mps1 is dispensable for kinetochore recruitment of Bub1, Bub3, and BubR1
The observation that Polo controls Mps1 recruitment to kinetochores prompted us to further explore the role of Mps1 in SAC function in Drosophila. Not surprisingly, Mps1 depletion in S2 cells overrides the mitotic arrest caused by microtubule depolymerization with colchicine (Supplementary Figures S2A–C) and in asynchronous-cultured cells led to a premature anaphase onset that correlates with an increased rate of Cyclin B degradation (Supplementary Figures S2D, E; Supplementary Movies S10, S11, S12). These observations show that Mps1 is essential for SAC signalling and normal mitotic timing in our experimental system, which is consistent with initial observations in Mps11 mutant embryos (Fischer et al, 2004).
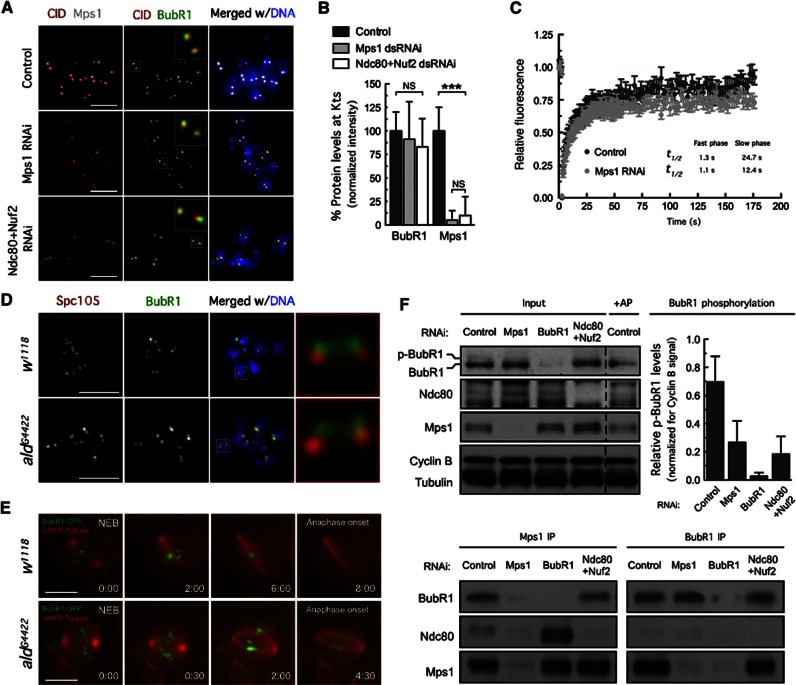
Mps1 activity has been shown to contribute to the SAC by promoting kinetochore localization of checkpoint proteins, but the extent to which different SAC components rely on Mps1 activity remains contentious (Lan and Cleveland, 2010). To assess the role of Drosophila Mps1 in the kinetochore recruitment of SAC components, we analysed by immunofluorescence the levels of several SAC proteins at unattached kinetochores upon Mps1 depletion, excluding cells in which residual Mps1 could be detected. This single cell analysis revealed that in Drosophila, Mps1 is dispensable for recruitment of BubR1, Bub3, Bub1 or CENP-meta (one of two Drosophila CENP-E paralogues) to unattached kinetochores (Figures 4A and B and Supplementary Figures S3A and B). This was further confirmed in aldG4422 (Mps1 Drosophila locus) mutant neuroblasts (Figure 4D and Supplementary Figures S3C and D). To examine whether Mps1 depletion affected BubR1 dynamic exchange at unattached kinetochores, we performed FRAP analysis in S2 cell expressing EGFP–BubR1 treated with colchicine. Similarly to that observed on prometaphase kinetochores of PtK2 cells (Howell et al, 2004), EGFP–BubR1 displayed biphasic recovery after photobleaching. Mps1 depletion had no noticeable effect on the kinetic parameters of the fast component but caused a two-fold decrease in the half-life of recovery of EGFP–BubR1 slow component. Thus, although Mps1 is not required for the recruitment of BubR1 to unattached kinetochores, its absence increases the rate at which the slow component of BubR1 exchanges on kinetochores.
Figure 4.
Mps1 kinetochore localization controls BubR1 hyperphosphorylation. (A) Immunolocalization of BubR1 and Mps1. Insets show higher-magnification views of kinetochore pairs in the boxed areas. (B) Quantification of the experiment in (A). BubR1 and Mps1 fluorescence intensities at kinetochores were determined relative to CID. Data represent mean±s.d. and the mean values obtained for control cells were set to 100%. ***P<0.001, Student’s t-test. n >20 cells for each condition. (C) FRAP analysis of EGFP–BubR1 in control and Mps1-depleted cells after bleaching of single kinetochores. Graph shows average fluorescence intensities±s.e., n=18 cells/condition. Recovery half-life for both the slow and fast phase is depicted. (D) Kinetochore localization of BubR1 in third-instar larvae neuroblasts from w1118 and aldG4422homozygous. Insets show higher-magnification views of selected kinetochore pairs. (E) Selected stills of live-cell imaging showing the mitotic progression of control w1118 and mutant aldD4422 neuroblasts expressing mRFP-Tubulin and BubR1-GFP. Time is shown in min:sec. (F) Immunoprecipitation of BubR1 and Mps1 from total cell lysates obtained from control cells, Mps1-depleted cells and cells co-depleted of Ndc80 and Nuf2. Immunoprecipitates (IP) and corresponding total cell lysates (Input) were probed by immunoblotting for the indicated proteins. The phosphorylation status of BubR1 was examined (Input) and the graph displays the quantification of p-BubR1 blots normalized for Cyclin B signals. AP: alkaline phosphatase. Data are mean±s.d. of three independent experiments. (A and F) Cultured cells were treated with MG132 for 1 h followed by a 2-h period of colchicine incubation. Scale bars represent 5 μm.
Source data for this figure is available on the online supplementary information page.
Next, we evaluated by time-lapse microscopy the requirement of Mps1 for BubR1 kinetochore localization during unperturbed mitosis in neuroblasts and in S2 cells expressing tagged BubR1 under control of its natural promoter (Buffin et al, 2005; Matos et al, 2009). Not surprisingly, Mps1 depletion in aldG4422 mutant neuroblasts (Figure 4E, Supplementary Movies S13 and S14), or in S2 cells (Supplementary Movies S15 and S16), resulted in premature anaphase onset in the presence of misaligned chromosomes but had no visible effect on BubR1 localization pattern during prometaphase. We conclude that, in contrast to vertebrates and yeast, Drosophila Mps1 is dispensable for kinetochore localization of Bub1, Bub3 and BubR1.
Kinetochore localization of Mps1 promotes BubR1 hyperphosphorylation
BubR1 hyperphosphorylation was shown to correlate with mitotic progression and to be induced by microtubule depolymerization (Chan et al, 1999; Taylor et al, 2001; Chen, 2002; Huang et al, 2008; Elowe et al, 2010). Multiple mitotic kinases (Cdk1, Plk1, Mps1 and Aurora B) and CENP-E have been implicated as important regulators of BubR1 phosphorylation in human cells (Elowe et al, 2007; Matsumura et al, 2007; Huang et al, 2008; Santaguida et al, 2011; Guo et al, 2012). As in other systems, the hyperphosphorylated form of Drosophila BubR1 is readily detected as a slow migrating band on immunoblots (Figure 4F). A previous study implicating Mps1 in phosphorylation of BubR1 (Huang et al, 2008) was performed in human cells, where it was subsequently shown that Mps1 is required for BubR1 localization to kinetochores (Elowe et al, 2010; Maciejowski et al., 2010). Therefore, loss of BubR1 hyperphosphorylation upon Mps1 inhibition was interpreted as an indirect effect resulting from BubR1 de-localization from kinetochores. By contrast, we established that Mps1 in Drosophila is dispensable for recruitment of BubR1 to unattached kinetochores. Nevertheless, depletion of Mps1 from S2 cells treated with MG132 and colchicine resulted in a dramatic reduction of hyperphosphorylated BubR1 on immunoblots (Figure 4F). Co-depletion of Ndc80 and Nuf2 to displace Mps1 from kinetochores similarly reduced BubR1 hyperphosphorylation (Figure 4F) without affecting kinetochore levels of BubR1 (Figures 4A and B), suggesting that Mps1-dependent BubR1 phosphorylation requires Mps1 kinetochore localization. To assess whether Mps1 and BubR1 could interact in vivo when the SAC is active, we performed reciprocal co-immunoprecipitation experiments using lysates of cells treated with colchicine and MG132. Mps1 could be readily detected in BubR1 immunoprecipitates, and, conversely, BubR1 was present in Mps1 immunoprecipitates (Figure 4F). Interestingly, the complex was also detected in immunoprecipitates from lysates of Ndc80 and Nuf2 co-depleted cells (Figure 4F), indicating that the BubR1–Mps1 interaction might also occur outside kinetochores. Taken together, these results indicate that Mps1 is present in a complex with BubR1 in vivo and its kinetochore localization, which has been associated with its activation, has a major role in the mitotic phosphorylation of BubR1.
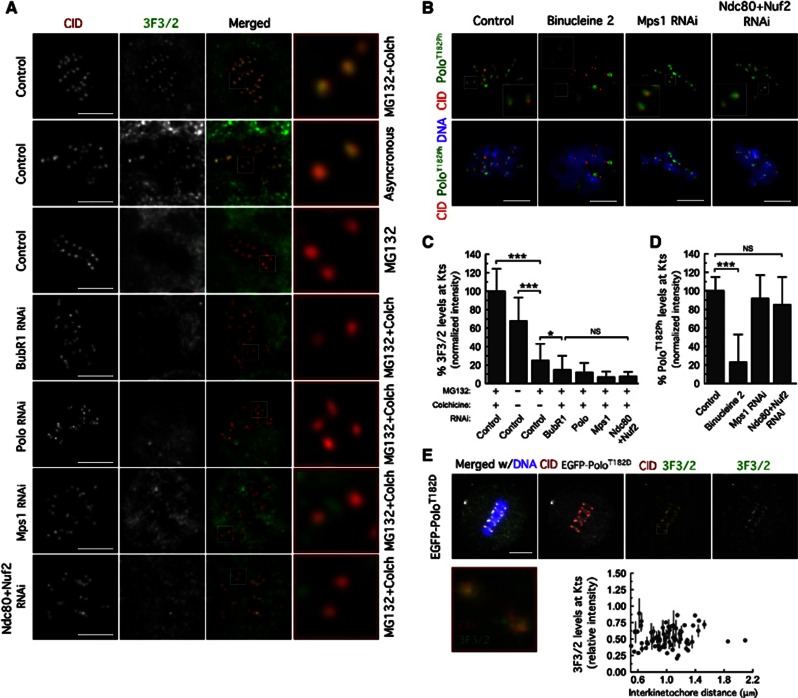
Kinetochore localization of Mps1 is required for 3F3/2 phosphoepitope formation
Kinetochores of chromosomes that are not under tension are phosphorylated at an epitope recognized by the 3F3/2 monoclonal antibody. BubR1 has been pointed as the likely 3F3/2 phosphoepitope (Wong and Fang, 2006, 2007) and its formation at tensionless kinetochores proposed to be critical for proper SAC signalling (Gorbsky and Ricketts, 1993; Maresca and Salmon, 2009). Thus, we evaluated the levels of 3F3/2 phosphoepitope as a read-out of BubR1 phosphorylation at kinetochores (Figures 5A and C). In control S2 cells, the 3F3/2 antibody strongly labelled kinetochores of unaligned or unattached chromosomes, whereas the staining was greatly reduced at kinetochores of aligned chromosomes. As predicted, BubR1 depletion severely impaired the accumulation of the 3F3/2 phosphoepitope at unattached kinetochores. Likewise, Mps1 depletion or co-depletion of Ndc80 and Nuf2 resulted in a severe reduction of 3F3/2 phosphoepitope levels revealing a requirement for kinetochore-associated Mps1 in the establishment of the 3F3/2 phosphoepitope.
Figure 5.
Mps1 kinetochore localization is required to promote 3F3/2 phosphoepitope formation. (A) Immunolocalization of 3F3/2 phosphoepitope. Kinetochore pairs in boxed areas are shown at higher magnification. (B) Immunofluorescence analysis of Polo-T182 phosphorylation. The insets show higher-magnification views of kinetochore pairs in the boxed areas. Cultured cells were treated with MG132 for 1 h followed by 2 h of colchicine incubation. Binucleine 2 was added to cultures 30 min before microtubule depolymerization with colchicine. (C) Quantification of experiment in (A). 3F3/2 signal intensities at kinetochores were determined relative to CID. Data represent mean±s.d. and the mean values obtained for control cells were set to 100%. 0.01<*P<0.05; ***P<0.001, One-way ANOVA, Turkey’s multiple comparison test. n>20 cells for each condition. (D) Quantifications of experiment in (B). PoloT182Ph fluorescence intensities at kinetochores were determined relative to CID. Data represent mean±s.d. and mean values obtained for control cells were set to 100%. ***P<0.001, One-way ANOVA, Turkey’s multiple comparison test. n>20 cells for each condition. (E) Immunolocalization of 3F3/2 phosphoepitope in S2 cells expressing EGFP–PoloT182D treated with MG132 for 2 h. Kinetochore pair in boxed area is shown at higher magnification. 3F3/2 kinetochore levels were determined relative to CID and plotted against interkinetochore distance. Scale bars represent 5 μm.
In Xenopus egg extracts and human cells, Plx1 and Plk1 have been implicated as kinases responsible for 3F3/2 formation (Ahonen et al, 2005; Wong and Fang, 2005). Importantly, and contrasting with what was reported in those systems (Wong and Fang, 2005; Maciejowski et al, 2010), we show that kinetochore targeting of Polo was unperturbed in Mps1-depleted S2 cells and in aldG4422 mutant neuroblasts (Figures 2A and C; Supplementary Figures S1C and D). Moreover, Polo T-loop activation at kinetochores remained unaffected upon depletion of Mps1 or co-depletion of Ndc80 and Nuf2 from S2 cells as assayed by quantification of Polo T182 phosphorylation (Figures 5B and D). Therefore, given that Mps1 depletion, or preventing its kinetochore localization through Ndc80 and Nuf2 co-depletion, resulted in a severe reduction of 3F3/2 phosphoepitope at unattached kinetochores without affecting kinetochore levels of BubR1 or active PoloT182ph, we reason that Drosophila Polo contributes indirectly for generation of 3F3/2 phosphoepitope by targeting Mps1 to kinetochores that are not under tension.
To further test this, we evaluated the levels of 3F3/2 on kinetochores of cells expressing EGFP–PoloT182D. In agreement with the capacity to promote Mps1 recruitment to kinetochores of congressed chromosomes, expression of constitutively active Polo resulted in the accumulation of 3F3/2 phosphoepitope at kinetochores of chromosomes that have established interkinetochore tension (Figure 5 E). Whether Mps1 is directly responsible for the 3F3/2 phosphoepitope through BubR1 phosphorylation, or controls a downstream event that catalyses the formation of the phosphoepitope remains to be clarified. Nevertheless, these results imply kinetochore-associated Mps1 as an important regulator of BubR1 mitotic phosphorylation and of 3F3/2 formation downstream of Polo activity.
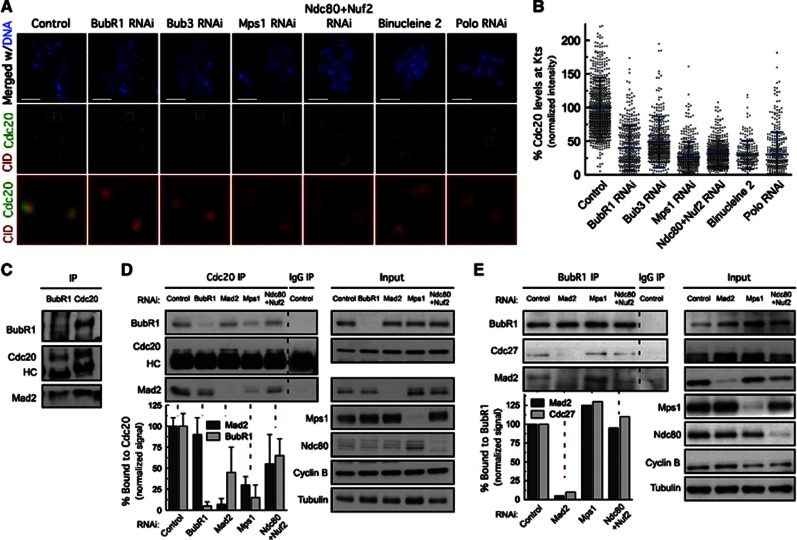
Mps1-dependent BubR1 hyperphosphorylation is required for recruitment of Cdc20 to kinetochores and MCC formation.
We then wanted to determine the molecular outcome of Mps1-dependent BubR1 hyperphosphorylation and the importance of 3F3/2 phosphoepitope for SAC function. Recent work in Drosophila neuroblasts and syncytial embryos showed that BubR1 recruits the APC/C activator Cdc20 to unattached kinetochores independently of Mad2 (Li et al, 2010). We confirmed that depletion of BubR1, or preventing its kinetochore localization through depletion of Bub3, severely compromised the recruitment of Cdc20 to unattached kinetochores in S2 cells (Figures 6A and B). A similar reduction of Cdc20 kinetochore levels was observed in cells depleted of Mps1 (Figures 6A and B). Given that Mps1 is dispensable for BubR1 kinetochore localization in Drosophila, impairment of Cdc20 kinetochore recruitment resulting from Mps1 depletion may be attributed to loss of BubR1 hyperphosphorylation. Moreover, co-depletion of Ndc80 and Nuf2, Polo depletion or inhibition of Aurora B activity, resulted in a reduction of kinetochore-associated Cdc20 to similar levels as depletion of Mps1 (Figures 6A and B), suggesting that BubR1-dependent recruitment of Cdc20 parallels Mps1-dependent BubR1 hyperphosphorylation. Interestingly, western blotting revealed the presence of two BubR1 isoforms in BubR1 immunoprecipitates from colchicine-treated cells. However, only the isoform with lower electrophoretic mobility was found to co-immunoprecipitate with Cdc20 further indicating that in vivo, Cdc20 forms a complex, preferentially with hyperphosphorylated BubR1 (Figure 6C).
Figure 6.
Mps1-dependent BubR1 hyperphosphorylation is required for recruitment of Cdc20 to kinetochores and MCC formation. (A) Immunolocalization of Cdc20. Selected kinetochore pairs in the boxed areas are shown at higher-magnification. (B) Distribution of Cdc20 levels at unattached kinetochores for the experiments in (A). Cdc20 fluorescence intensities at kinetochores were determined relative to CID. n>20 cells for each condition. (C) Immunoprecipitation of Cdc20 and BubR1 from control cells lysates were probed by immunoblottimg for the indicated proteins. (D, E) Immunoprecipitation of Cdc20 (D) and BubR1 (E) from total cell lysates obtained from cells depleted of the indicated proteins. Immunoprecipitates (IP) and corresponding total cell lysates (Input) were probed by immunoblotting for the indicated proteins. Quantification of relative levels of protein bound to Cdc20 (C) or BubR1 (D) is shown. Co-immunoprecipitated protein signals were adjusted to the corresponding input signal and normalized for immunoprecipitated Cdc20 or BubR1. Graph bars in Cdc20 IP represent mean±s.d. and were obtained from three independent experiments. Values obtained for Cdc20 and BubR1 IP in control cells were set to 100%. Graph bars in BubR1 IP result from a single IP experiment. (A–E) Cultured cells were treated with MG132 for 1 h followed by 2 h of colchicine incubation. Binucleine 2 was added to cultures 30 min before microtubule depolymerization. Scale bars represent 5 μm.
Source data for this figure is available on the online supplementary information page.
Although Mad2 is dispensable for kinetochore recruitment of Cdc20 (Li et al, 2010), its kinetochore localization and conformational activation are still essential for proper SAC activity in Drosophila (Supplementary Figures S2A–D; Buffin et al, 2005, 2007; Li et al, 2010). To understand how Mad2, BubR1 and Cdc20 intersect to establish the MCC in Drosophila, we determined the levels of Mad2 and BubR1 that co-immunoprecipitated with Cdc20 by quantitative immunoblotting (Figure 6D). Mad2 depletion caused a reduction in the levels of Cdc20–BubR1 complex indicating that although Mad2 is not required for kinetochore recruitment of Cdc20 (Li et al, 2010), the BubR1–Cdc20 interaction at kinetochores is likely transient in the absence of C-Mad2 and therefore insufficient for sustained inhibition of APC/C activity. In contrast, depletion of BubR1 had no significant impact in the amount of Cdc20–Mad2 complex (Figure 6D) corroborating previous findings (Nilsson et al, 2008; Li et al, 2010). Mps1 depletion caused Cdc20 to dissociate from Mad2 and BubR1 (Figure 6D), whereas in cells depleted of the Ndc80 complex, the Cdc20–BubR1 interaction was restored to 60% and a modest increase in Cdc20–Mad2 complex formation was detected compared to Mps1-depleted cells (Figure 6D). This indicates that cytosolic Mps1 is able to promote the formation or stability of MCC, as previously proposed (Maciejowski et al, 2010) but to levels that only allow a partial rescue of SAC function (our unpublished observation). However, and contrasting with recent data from fission yeast (Zich et al, 2012), the ability of BubR1 to interact with the APC/C remained unaffected upon Mps1 depletion as revealed by the capacity of BubR1 to co-immunoprecipitate the APC/C subunit Cdc27 (Figure 6E). Collectively, these findings strongly suggest that BubR1 mitotic hyperphosphorylation controlled by Mps1 activity at kinetochores is important to allow proper kinetochore recruitment of Cdc20 and MCC assembly to levels that support a fully functional mitotic checkpoint.
Mps1-dependent 3F3/2 phosphoepitope promotes Cdc20 kinetochore localization and MCC assembly
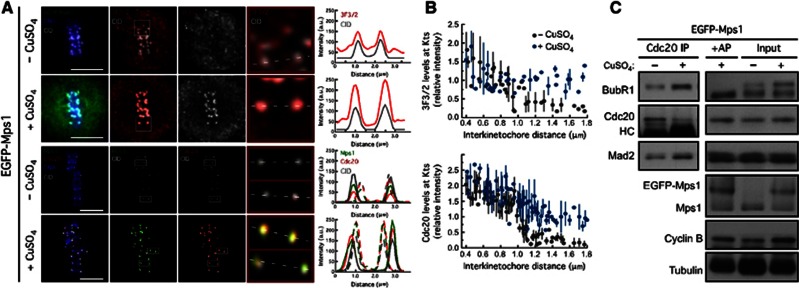
We reasoned that the role of Mps1-dependent BubR1 phosphorylation might be to promote the initial binding of Cdc20 to BubR1 at kinetochores. To test this, we overexpressed Mps1 in S2 cells. Live-cell imaging and immunofluorescence analysis revealed that in contrast to endogenous Mps1 in non-induced control cells, the EGFP–Mps1 signal persisted at kinetochores of congressed chromosomes (Figure 7A, Supplementary Figure S4A and Supplementary Movie S17). Consistent with a bi-orientated state, Mps1-decorated kinetochores were under normal interkinetochore tension (Supplementary Figures S5A and B) and were stably attached to spindle microtubules, as revealed by the presence of cold-stable kinetochore-fibers (Supplementary Figures S5C and D). These observations indicate that overexpressed EGFP–Mps1 enforced persistent accumulation of Mps1 at kinetochores regardless of microtubule attachment. This promoted to some extent the recruitment of Mad1 to metaphase-aligned chromosomes (Supplementary Figures S5E and F) similarly to that observed in previous studies when Mps1 dissociation from kinetochores was prevented by fusions with constitutive kinetochore proteins (Jelluma et al, 2010; Ito et al, 2012). In accordance, live-cell imaging revealed that these cells arrest in mitosis for extended periods even after biorientation of all chromosomes and depletion of Mad2 or BubR1 prevented the arrest, demonstrating that it was due to persistent SAC activity (Supplementary Figures S4A, B; Supplementary Movies S17, S18, S19). These results parallel those we observed in cells expressing constitutively active PoloT182D (Figures 3E–H), which similarly caused retention of Mps1 at bi-oriented kinetochores.
Figure 7.
Overexpression of Mps1 promotes accumulation of 3F3/2 phosphoepitope and Cdc20 at kinetochore regardless of chromosome biorientation. (A) 3F3/2, Cdc20 and Mps1 kinetochore localization in metaphase chromosomes of non-induced (−CuSO4) and in overexpressing EGFP–Mps1 cells (+CuSO4). CID signal was used as kinetochore reference. Selected kinetochore pairs are shown at higher magnification and the respective intensities profiles represented. (B) Variation of 3F3/2 and Cdc20 kinetochore levels with the interkinetochore distance in control cells and in cells overexpressing Mps1. (C) Immunoprecipitation of Cdc20 from total cell lysates obtained from control cells and cells overexpressing Mps1. Immunoprecipitates and corresponding total cell lysates were probed by immunoblotting for the indicated proteins. AP: alkaline phosphatase. For (C) cultured cells were treated with MG132 for 1 h followed by 2 h of colchicine incubation. Scale bars represent 5 μm.
Source data for this figure is available on the online supplementary information page.
Overexpression of Mps1 resulted in a significant increase of BubR1 hyperphosphorylation as detected on immunoblots (Figure 7C). Likewise, immunofluorescence analysis showed that cells overexpressing Mps1 retained high levels of 3F3/2 phosphoepitope at kinetochores despite normal attachment and centromeric tension (Figures 7A and B), which correlated with the capacity to recruit Cdc20 to kinetochores of metaphase chromosomes (Figures 7A and B). This markedly contrasts with non-induced cells, in which establishment of tension upon chromosome biorientation, precluded Mps1 localization at kinetochores and consequently resulted in a significant reduction of 3F3/2 and Cdc20 kinetochore levels (Figures 7A and B). These results strongly suggest that the molecular outcome of Mps1-dependent 3F3/2 formation is to promote the association of Cdc20 with BubR1 at kinetochores thus allowing proper recruitment of Cdc20. Strikingly, cells overexpressing Mps1 and treated with colchicine, co-immunoprecipitated significantly higher levels of BubR1 with Cdc20 than non-induced control cells (Figure 7C) further supporting a model in which Mps1-regulated phosphorylation of BubR1 is instrumental for the formation of the Cdc20-BubR1 complex, which in turn is required for MCC assembly and sustained SAC function.
Discussion
Polo inhibition causes a SAC-independent mitotic arrest
Polo and its orthologues are key mitotic regulators. Early work showing that Polo kinases become enriched at kinetochores in prometaphase cells (Golsteyn et al, 1995; Arnaud et al, 1998) led to the hypothesis that it could play an important role in SAC function. However, subsequent studies failed to demonstrate a clear role for Polo kinases in SAC signalling. Plx1 was shown to be required for SAC function in Xenopus as the kinase responsible for 3F3/2 formation at tensionless kinetochores (Wong and Fang, 2007). However, in human cells, Plk1 seems to be dispensable for SAC activity as its inhibition or depletion resulted in a SAC-dependent prometaphase arrest with accumulation of Mad2 and BubR1 at kinetochores (Sumara et al, 2004; Lénárt et al, 2007; Petronczki et al, 2008). This contrasts with other studies that reported a substantial reduction of kinetochore-associated levels of Mad1, Mad2 and Cdc20, upon downregulation of Plk1 suggesting a role for Plk1 in SAC regulation (Ahonen et al, 2005; Chi et al, 2008).
In this study, we demonstrate that inhibition or depletion of Polo in S2 cells results in a prometaphase arrest that fails to be abolished by co-depletion of Mad2 and BubR1. This conflicts with that reported for human cells and implies that SAC unlikely explains the mitotic arrest observed in the absence of Polo activity in Drosophila. Although at this point the mechanisms that act downstream of Polo inhibition to impair mitotic progression in Drosophila remain unclear, studies in budding yeast, Xenopus, murine and human cells have indicated a role for Polo orthologues in APC/C activation (Charles et al, 1998; Descombes and Nigg, 1998; Kotani et al, 1998; Shirayama et al, 1998; Golan et al, 2002). Nevertheless, although Plk1 was shown to synergise with Cdk1/Cyclin B in phosphorylating and activating the APC/C (Kotani et al, 1998; Golan et al, 2002; Kraft et al, 2003), activation can still occur in the absence of Plk1 (Kraft et al, 2003; Lénárt et al, 2007), indicating that Plk1-mediated phosphorylation of APC/C contributes marginally for its activation in human cells. However, it is possible that APC/C activation in Drosophila involves a more stringent requirement of Polo activity. Nonetheless, our results clearly demonstrate that the mitotic arrest in Polo-inhibited cells is not due to SAC activation, which masks a requirement of Polo in SAC signalling, as revealed by significantly decreased levels of the MCC and failure to recruit Mad2 to unattached kinetochores.
Polo is required for SAC signalling by acting as an upstream kinase that controls the recruitment of Mps1 to unattached kinetochores
In this study, we demonstrate that Polo is required for mitotic exit and proper SAC function in Drosophila. Polo depletion led to a severe reduction of Mad2 kinetochore levels and MCC formation in cells challenged with colchicine. Efficient assembly of MCC and Mad2 kinetochore recruitment have been previously shown to depend on Mps1 kinase (Maciejowski et al, 2010b; Sliedrecht et al, 2010), whose accumulation at unattached kinetochores is essential for full activation and to ensure competent SAC engagement (Santaguida et al, 2011; Saurin et al, 2011; Heinrich et al, 2012). Here, we show that Polo is an important upstream regulator of Mps1 recruitment to unattached kinetochores. Depletion of Polo, both in S2 cells and neuroblasts, markedly reduced Mps1 kinetochore accumulation and activation. As described for human cells (Santaguida et al, 2011; Saurin et al, 2011), in Drosophila, the Ndc80 complex is critical for Mps1 kinetochore localization. Mps1 forms a complex with Ndc80 in vivo and its depletion abolished the recruitment of Mps1 to kinetochores thus supporting Ndc80 as an attractive candidate to mediate kinetochore binding of Mps1. It has been proposed that Aurora B activity is required for outer kinetochore assembly (Emanuele et al, 2008). We found that inhibition of Aurora B or Polo depletion reduces to a similar extent the loading of Ndc80 onto kinetochores. The observation that Polo is required for Aurora B centromeric localization and activity suggests the possibility that Polo contributes to outer kinetochore assembly indirectly through the control exerted over Aurora B. On the other hand, it was recently shown that in early mitosis, Aurora B activates Polo at kinetochores/centromeres by phosphorylation of its T-loop (Carmena et al, 2012). Expression of a constitutively active Polo mutant (T182D) allowed us to bypass this functional interdependency and revealed the ability of active Polo to restore correct Ndc80 kinetochore localization and Mps1 recruitment in the absence of Aurora B activity. The effectors that elicit outer kinetochore assembly in early mitosis remain unclear. Here, we demonstrate that Polo acts downstream of Aurora B to regulate proper outer kinetochore organization in Drosophila and therefore Mps1 recruitment to unattached kinetochores.
The observation that simultaneous depletion of Polo and Aurora B inhibition virtually impaired Mps1 kinetochore association without further compromising outer kinetochore organization, argues in favour of an additional role for Polo and Aurora B in the control of Mps1 kinetochore recruitment beyond their requirement for Ndc80 kinetochore localization. Although the underlying molecular mechanism is not known, it is tempting to speculate that Aurora B-mediated phosphorylation of Ndc80 generates the docking sites that potentiate direct interaction with Mps1. Our results also suggest that Polo activation by Aurora B is spatially regulated by tension similarly to Ndc80 phosphorylation. Therefore, the levels of active Polo- and Aurora B-mediated phosphorylation of Ndc80 at kinetochores drop upon chromosomes biorientation and consequently Mps1 recruitment is impaired. However, the presence of PoloT182D at kinetochores was able to promote the recruitment of Mps1 to metaphase-aligned chromosomes that are under centromeric tension, resulting in a SAC-dependent metaphase delay. These results support a role for active Polo in the recruitment of Mps1 to kinetochores that is independent of Aurora B, further substantiating the requirement of Polo activity for proper SAC signalling in Drosophila. Interestingly, in Xenopus and human cells, the opposite interdependency takes place (Wong and Fang, 2006; Maciejowski et al, 2010), highlighting the existence of species-specific kinetochore assembly pathways. Our data show that Polo and Aurora B are engaged in a positive feedback loop so that activities of both kinases are amplified during prometaphase to ensure prompt and efficient recruitment of Mps1 to unattached kinetochores through the Ndc80 complex.
Mps1 kinetochore localization is required for BubR1 phosphorylation that results in 3F3/2 phosphoepitope formation
As mentioned above, kinetochore localization of Mps1 is required to render the kinase fully active and consequently, orchestrate a sustained SAC response. As expected, Mps1 depletion abolished Mad1 and Mad2 kinetochore association (our unpublished observation; Althoff et al, 2012). However, and contrasting with most organisms, Drosophila Mps1 is not required for the recruitment of BubR1, Bub3, Bub1 or CENP-meta/CENP-E to unattached kinetochores. This may reflect species specificities of kinetochores as scaffolds for SAC. In fungi and in human cells, Mps1 promotes Bub1/Bub3 and BubR1/Bub3 kinetochore localization by phosphorylating Knl-1 on several of its MELT motifs (London et al, 2012; Shepperd et al, 2012; Yamagishi et al, 2012). However, Drosophila Spc105/Knl-1 has been previously shown to be largely dispensable for BubR1 kinetochore localization (Schittenhelm et al, 2009). At present, we do not fully understand the reason for this divergence, but it is plausible that it might reflect an optimization to fly mitosis. Mitotic progression in Drosophila can be remarkably fast, taking less than 3 min from the start of mitosis until anaphase onset during the syncytial cycles (Althoff et al, 2012). This likely demands a prompt and efficient SAC response to small perturbation that may occur. It is therefore possible that recruitment of BubR1, Bub1 and Bub3 to kinetochores in the absence of Mps1 reflects an evolutionary adaptation of SAC speed and efficiency.
Although BubR1 targeting to kinetochores is independent of Mps1 activity in Drosophila, its hyperphosphorylation does require Mps1 at kinetochores. BubR1 hyperphosphorylation correlates with mitotic progression and is induced by microtubule depolymerization (Chan et al, 1999; Taylor et al, 2001; Chen, 2002; Huang et al, 2008; Elowe et al, 2010). However, its molecular relevance for SAC function has remained elusive. In human cells, Mps1 has been previously suggested as a major kinase for BubR1 in vivo (Huang et al, 2008). Nevertheless, in vitro studies failed to provide evidence for a direct phosphorylation so that loss of BubR1 hyperphosphorylation observed upon Mps1 inhibition was attributed to BubR1 mislocalization (Elowe et al, 2010). Cdk1 and Plk1 phosphorylate BubR1 at several residues to maintain proper kinetochore–microtubule attachments and chromosome congression. These phosphorylations appear nonetheless to be largely dispensable for SAC functions of BubR1 (Elowe et al, 2007, 2010). It has been reported that CENP-E interaction with BubR1 at unattached kinetochores promotes its autophosphorylation to enhance SAC function in human cells (Guo et al, 2012). However, recent findings have shown that vertebrate BubR1 displays degenerated kinase motifs resulting in lack of catalytic activity (Suijkerbuijk et al, 2012). Vertebrate BubR1 orthologues seem to represent pseudokinases that retained the kinase domain through evolution for the sake of conformational stability. Interestingly, Drosophila BubR1 retains intact kinase features essential to maintain sister-chromatid cohesion through meiosis but that are dispensable for mitotic SAC function (Malmanche et al, 2007). Given that depletion of Mps1 or impairment of its kinetochore localization through Ndc80 depletion resulted in a dramatic reduction of BubR1 hyperphosphorylation without affecting the levels of kinetochore-associated BubR1, CENP-meta/CENP-E or active Polo, we reason that in Drosophila, Mps1 is a major kinase responsible for BubR1 hyperphosphorylation. In addition, we show that Mps1 and BubR1 are able to co-immunoprecipitate in mitotic cell extracts. Whether Mps1 directly phosphorylates BubR1 in flies, will be critical to address.
Hyperphosphorylation of BubR1 was initially linked to SAC signalling through accumulation of 3F3/2 phosphoepitope at tensionless kinetochores. Phosphorylation of BubR1 by Plx1 generates the 3F3/2 phosphoepitope required for checkpoint-mediated mitotic arrest in Xenopus (Wong and Fang, 2007). Our results, strongly suggest that in Drosophila, Mps1 acts downstream of Polo to generate the 3F3/2 phosphoepitope at kinetochores. (1) Depletion of Polo or Mps1 prevented to the same extent 3F3/2 formation at unattached kinetochores. (2) Inhibition or depletion of Polo resulted in a dramatic reduction of Mps1 levels at kinetochores, whereas Mps1 depletion had no effect on kinetochore association of Polo or on its activation. (3) Importantly, persistent kinetochore localization of Mps1 was able to retain high levels of 3F3/2 phosphoepitope at metaphase kinetochores despite normal kinetochore–microtubule attachments and establishment of centromeric tension. We propose that Polo contributes indirectly to the generation of 3F3/2 phosphoepitope by targeting Mps1 to kinetochores that are not under tension. Whether Mps1 is directly responsible for the 3F3/2 phosphoepitope through BubR1 phosphorylation, or controls a downstream event that actually catalyses the formation of the phosphoepitope needs to be clarified. Nevertheless, our results imply kinetochore-associated Mps1 as an important regulator of BubR1 mitotic phosphorylation and of 3F3/2 formation downstream of Polo activity.
Mps1-dependent BubR1 hyperphosphorylation promotes Cdc20 kinetochore recruitment and MCC formation
Although unattached kinetochores are known to be hyperphosphorylated (Gorbsky and Ricketts, 1993; Nicklas et al, 1998) and the 3F3/2 phosphoepitope established as a reliable read out for lack of kinetochore tension, the precise mechanisms translating changes in tension and microtubule attachment at kinetochores into biochemical signals that regulate SAC activity have remained unknown. Here we demonstrate that the molecular outcome of BubR1 mitotic hyperphosphorylation and 3F3/2 formation controlled by Mps1 activity at kinetochores is to promote the association of Cdc20 with BubR1 to allow proper kinetochore accumulation of Cdc20 and MCC assembly to levels that support a sustained SAC activity. Current models propose that C-Mad2 generated at unattached kinetochores interacts with Cdc20 and promotes its binding to the BubR1/Bub3 sub-complex to form the final MCC. Whether this requires Cdc20 and BubR1 at kinetochores remains controversial. Nevertheless, in human cells, efficient recruitment of Cdc20 to kinetochores seems to be necessary for proper APC/C inhibition (Elowe et al, 2010). It was recently shown that kinetochore recruitment of Cdc20 in Drosophila requires BubR1 but not Mad2 (Li et al, 2010). Our results provide further insight by demonstrating that phosphorylation of BubR1 promoted by Mps1 at kinetochores is crucial to promote Cdc20 recruitment. Firstly, depletion of Mps1 or preventing its kinetochore targeting led to a comparable reduction of Cdc20 levels at unattached kinetochores, without affecting BubR1 kinetochore localization, but that paralleled the resultant decrease in its hyperphosphorylation. Secondly, persistent localization of Mps1 at kinetochores, resulting from Mps1 overexpression, allowed the recruitment of Cdc20 despite normal attachment and alignment, and this was concomitant with the presence of 3F3/2 phosphoepitope at these kinetochores. Importantly, Mps1 overexpression allowed us to uncouple 3F3/2 formation from interkinetochore tension, resulting in 3F3/2 accumulation at kinetochores that are correctly attached to spindle microtubules and under tension. Moreover, BubR1 and Cdc20 exhibit two dynamic populations at unattached kinetochores with both slow phases exchanging through kinetochores at similar rates (Howell et al, 2004). Interestingly, Mps1 depletion resulted in a 2-fold decrease in BubR1 slow component half-life recovery at unattached kinetochores. A possible interpretation for this is that loss of Mps1-mediated BubR1 phosphorylation increases the exchange rate of BubR1 at kinetochores, thus affecting its interaction with Cdc20. Although further evidences are required to draw such conclusions, our results clearly demonstrate that Mps1 regulates BubR1 dynamics at unattached kinetochores.
These findings come to challenge the view that kinetochore localization of BubR1 and Mps1 might not be required for their checkpoint functions (Malureanu et al, 2009; Maciejowski et al, 2010b). Although expression of N-terminal BubR1 was shown to be sufficient to inhibit APC/C in vitro, recent studies indicate that in a cellular context, kinetochore localization of BubR1 is required for efficient SAC response (Elowe et al, 2010; Lara-Gonzalez et al, 2012). Likewise, cytosolic Mps1 is able to promote MCC assembly but to levels that are unable to support long-term SAC function (our unpublished observation) suggesting that kinetochore recruitment of Mps1 is critical for maximal Mps1 SAC function.
We speculate that kinetochore binding of BubR1 and Mps1 brings them into close proximity so that phosphorylation of BubR1 promoted by Mps1 is favoured. This then promotes the local association of Cdc20 with BubR1 thereby increasing the concentration of BubR1–Cdc20 complex at the site where C-Mad2 is being generated. Given that Mad2 is not required for recruitment of Cdc20 to kinetochores but in its absence the levels of BubR1-Cdc20 complex found in vivo are drastically reduced, we believe that instead of the proposed role of Mad2 in priming Cdc20 for BubR1 binding, the main function of Drosophila Mad2 might be to increase the stability of BubR1–Cdc20 interaction to allow the efficient assembly of the MCC.
Conclusion
Taken together, our data support a model in which SAC signalling is tightly controlled through a kinase network that also actively contributes for chromosome biorientation (Figure 8). We propose that Polo regulates SAC signalling as an upstream kinase required for correct outer kinetochore assembly and for Aurora B centromeric localization and activity. In early mitosis, when chromosomes lack centromeric tension, Polo and Aurora B are engaged in a positive feedback loop that synergizes with Ndc80 to potentiate prompt kinetochore recruitment of Mps1. At kinetochores, fully active Mps1 controls BubR1 phosphorylation to promote kinetochore localization of Cdc20 and together with C-Mad2 allow the formation of MCC, which delays mitotic exit until all kinetochores are properly attached to the mitotic spindle and chromosomes bi-oriented.
Figure 8.
Schematic representation of the proposed model for SAC signalling controlled through a kinase network. At kinetochores/centromeres, Polo and Aurora B functional interdependency creates a positive feedback loop that promotes efficient recruitment of Mps1 kinase to unattached kinetochores allowing its prompt activation. At kinetochores, Mps1 activity is responsible for conformational exchange of soluble O-Mad2 to C-Mad2 (Hewitt et al, 2010) and controls BubR1 hyperphosphorylation. This then promotes the local association of Cdc20 with BubR1 thereby increasing the concentration of BubR1–Cdc20 complex at the site where C-Mad2 is being generated. The incorporation of C-Mad2 in the complex increases the stability of BubR1—Cdc20 interaction to allow efficient MCC assembly and sustained SAC function.
Materials and methods
Immunofluorescence analysis in S2 cells and neuroblasts
For immunofluorescence analysis of S2 cells, 105 cells were centrifuged onto slides for 5 min, at 1000, rpm (Cytospin 2, Shandon), and simultaneously fixed and extracted in 3.7% formaldehyde (Sigma), 0.5% Triton X-100 in PBS for 10 min followed by three washing steps in PBS-T (PBS with 0.05% Tween 20) for 5 min each. Alternatively, cells were fixed 3.7% formaldehyde (Sigma) in PHEM (60 mM PIPES, 25 mM HEPES, pH 7.0, 10 mM EGTA, 4 mM MgSO4) for 12 min and then detergent extracted with 0.5% Triton X-100 in PBS three times for 5 min each. For immunofluorescence with Mad2 antibody, cells were fixed in 4% paraformaldehyde in PBS for 12 min and further extracted for 8 min with 0.1% Triton X-100 in PBS. For immunofluorescence with the monoclonal antibody 3F3/2, cells were processed and fixed as described in Coelho et al (2008). Immunostaining was performed as described previously (Orr et al, 2007). For immunofluorescence analysis of Drosophila neuroblasts, third-instar larval brains homozygous for aldG4422were dissected in PBS and incubated with 50 μM colchicine for 1.5 h at 25°C before a 20 min fixation step in 3.7% formaldehyde. Larval brains were then washed three times for 10 min in PBS with 0.1% Triton X-100 and immediately incubated overnight with primary antibodies. Brains were again washed three times in PBS with 0.1% Triton X-100 and incubated with secondary antibodies for 1.5 h, after which three final washing steps were performed. Larval brains were left in 50% glycerol in PBS at 4°C before mounting on a slide. Images were collected in a Zeiss Axio Imager microscope (Carl Zeiss, Germany) or in a Leica TCS II scanning confocal microscope (Leica Microsystems). When required, deconvolution of data stacks was done with Huygens Essential, version 3.0.2p1 (Scientific Volume Imaging, Hilversum, The Netherlands). Data stacks were analysed and projected using ImageJ 1.42 m software (http://rsb.info.nih.gov/ij/). For immunofluorescence quantification of kinetochore proteins, the mean fluorescence intensity was quantified for individual kinetochores, selected manually by CID staining. The size of the region of interest was predefined so that each single kinetochore could fit into it. After subtraction of background intensities, estimated from regions of the cell with no kinetochores, the intensity was determined relative to CID reference and averaged over multiple kinetochores. Fluorescence intensity profiles of Aurora B and 3F3/2 staining were traced using an RGB profiler plugin of ImageJ1.45 h Software (NIH). To quantify the relative levels of p-H3, DAPI staining was used to define a selected area with the freehand tool. The integrated intensities of the projected sum of stacks were calculated for p-H3 relative to DAPI after background subtraction.
FRAP
FRAP experiments were performed in S2 cells expressing EGFP–BubR1 treated with MG132 for 1 hr followed by a 2-h treatment with colchicine. Data sets were collected at 25°C with a Leica TCS SP5II scanning confocal microscope (Leica Microsystems, Germany). The EGFP tag of BubR1 was excited using the 488-nm laser line set to 8% and bleached with the 405-nm laser line. Single kinetochores were bleached for two iterations once the fluorescence signal of EGFP–BubR1 had become stable. Fluorescence intensity in the bleached area was acquired every 529 ms before and after bleaching. FRAP data analysis was performed with ImageJ. For each measurement, the average fluorescence intensity in the bleached area was corrected for background and the ratio to average fluorescence in the cytoplasm determined. Average values before bleaching were set to 100%. The exponential kinetics of FRAP were analysed by calculating the normalized unrecovered fluorescence at each time point (Finf−F(t))/(Finf−F(0)) where Finf is the value reached at the plateau, F(0) is the value observed in the first frame after bleaching and F(t) is the value at a given time point. FRAP kinetics parameters were determined by double-exponential curve fitting to normalized data using GraphPad Prism software
Time-lapse fluorescence imaging
Live analysis of mitosis was done in S2 cell lines expressing the indicated constructs. 4D data sets were collected at 25°C with a spinning disc confocal system (Revolution; Andor) equipped with an electron multiplying charge-coupled device camera (iXonEM+; Andor) and a CSU-22 unit (Yokogawa) based on an inverted microscope (IX81; Olympus). Two laser lines (488 and 561 nm) were used for near-simultaneous excitation of EGFP and mCherry/mRFP. The system was driven by iQ software (Andor). Time-lapse imaging of z stacks with 0.8 μm steps covering the entire volume of the cell were collected every 30, 60 or 120 s. Image sequence analysis and video assembly were done with ImageJ. For quantification of EGFP–Cyclin B fluorescence, the area to be measured and corresponding to the whole cell was drawn on the first image of a series and reproduced on each successive image. The mean intensity was calculated within each area and corrected for background. The changes in fluorescence intensity with time were plotted as normalized signal relative to the signal measured at NEB.
Statistical analysis
All statistical analysis was performed with GraphPad Prism V5 (GraphPad Software, Inc.). Values were considered statistically different whenever P<0.05.
Supplementary Material
Acknowledgments
The authors would like to thank Ana Rita Maia, André Maia and Reto Gassmann for critical reading of the manuscript and helpful suggestions. Work in Claudio Sunkel laboratory is supported by grants PTDC/BIA-BCM/100305/2008 and PTDC/BIA-BCM/120366/2010 from Fundação para a Ciência e Tecnologia of Portugal (FCT) – COMPETE-FEDER. Carlos Conde holds an FCT postdoctoral fellowship (SFRH/BPD/34998/2007), Tatiana Moutinho-Santos holds an FCT postdoctoral fellowship (SFRH/BPD/41820/2007), Sofia Guimarães holds an FCT doctoral fellowship (SFRH/BD/39865/2007). Work in the laboratory of Helder Maiato is funded by grants PTDC/SAU-GMG/099704/2008 and PTDC/SAU-ONC/112917/2009 from FCT (COMPETE-FEDER), the Human Frontier Research Program, and the seventh framework program grant PRECISE from the European Research Council.
Author contributions: Conceived and designed the experiments: CC, MO, CES. Performed the experiments: CC, MO, JB, TM-S, DP, SG. Analysed the data: CC, MO, TM-S, HM, CES. Contributed with reagents/material: IM, HM. Wrote the paper: CC CES.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahonen LJ, Kallio MJ, Daum JR, Bolton M, Manke IA, Yaffe MB, Stukenberg PT, Gorbsky GJ (2005) Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr Biol 15: 1078–1089 [DOI] [PubMed] [Google Scholar]
- Althoff F, Karess RE, Lehner CF (2012) Spindle checkpoint-independent inhibition of mitotic chromosome segregation by Drosophila Mps1. Mol Biol Cell 23: 2275–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud L, Pines J, Nigg EA (1998) GFP tagging reveals human Polo-like kinase 1 at the kinetochore/centromere region of mitotic chromosomes. Chromosoma 107: 424–429 [DOI] [PubMed] [Google Scholar]
- Buffin E, Lefebvre C, Huang J, Gagou ME, Karess RE (2005) Recruitment of Mad2 to the kinetochore requires the Rod/Zw10 complex. Curr Biol 15: 856–861 [DOI] [PubMed] [Google Scholar]
- Buffin E, Emre D, Karess RE (2007) Flies without a spindle checkpoint. Nat Cell Biol 9: 565–572 [DOI] [PubMed] [Google Scholar]
- Carmena M, Pinson X, Platani M, Salloum Z, Xu Z, Clark A, Macisaac F, Ogawa H, Eggert U, Glover DM, Archambault V, Earnshaw WC (2012) The chromosomal passenger complex activates Polo kinase at centromeres. PLoS Biol 10: e1001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GK, Jablonski SA, Sudakin V, Hittle JC, Yen TJ (1999) Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J Cell Biol 146: 941–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles JF, Jaspersen SL, Tinker-Kulberg RL, Hwang L, Szidon A, Morgan DO (1998) The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr Biol 8: 497–507 [DOI] [PubMed] [Google Scholar]
- Chen RH (2002) BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J Cell Biol 158: 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y-H, Haller K, Ward MD, Semmes OJ, Li Y, Jeang K-T (2008) Requirements for protein phosphorylation and the kinase activity of polo-like kinase 1 (Plk1) for the kinetochore function of mitotic arrest deficiency protein 1 (Mad1). J Biol Chem 283: 35834–35844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho PA, Queiroz-Machado J, Carmo AM, Moutinho-Pereira S, Maiato H, Sunkel CE (2008) Dual role of topoisomerase II in centromere resolution and aurora B activity. PLoS Biol 6: e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Antoni A, Pearson CG, Cimini D, Canman JC, Sala V, Nezi L, Mapelli M, Sironi L, Faretta M, Salmon ED, Musacchio A (2005) The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr Biol 15: 214–225 [DOI] [PubMed] [Google Scholar]
- Descombes P, Nigg EA (1998) The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J 17: 1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson MM, Tavares AA, Ohkura H, Deak P, Glover DM (2001) Metaphase arrest with centromere separation in polo mutants of Drosophila. J Cell Biology 153: 663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowe S, Dulla K, Uldschmid A, Li X, Dou Z, Nigg EA (2010) Uncoupling of the spindle-checkpoint and chromosome-congression functions of BubR1. J Cell Sci 123: 84–94 [DOI] [PubMed] [Google Scholar]
- Elowe S, Hümmer S, Uldschmid A, Li X, Nigg EA (2007) Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev 21: 2205–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele MJ, Lan W, Jwa M, Miller SA, Chan CSM, Stukenberg PT (2008) Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. J Cell Biol 181: 241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MG, Heeger S, Häcker U, Lehner CF (2004) The mitotic arrest in response to hypoxia and of polar bodies during early embryogenesis requires Drosophila Mps1. Curr Biol 14: 2019–2024 [DOI] [PubMed] [Google Scholar]
- Golan A, Yudkovsky Y, Hershko A (2002) The cyclin-ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdk1-cyclin B and Plk. J Biol Chem 277: 15552–15557 [DOI] [PubMed] [Google Scholar]
- Golsteyn RM, Mundt KE, Fry AM, Nigg EA (1995) Cell cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J Cell Biol 129: 1617–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky GJ, Ricketts WA (1993) Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J Cell Biol 122: 1311–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Kim C, Ahmad S, Zhang J, Mao Y (2012) CENP-E-dependent BubR1 autophosphorylation enhances chromosome alignment and the mitotic checkpoint. J Cell Biol 198: 205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich S, Windecker H, Hustedt N, Hauf S (2012) Mph1 kinetochore localization is crucial and upstream in the hierarchy of spindle assembly checkpoint protein recruitment to kinetochores. J Cell Sci 125(Part 20): 4720–4727 [DOI] [PubMed] [Google Scholar]
- Hewitt L, Tighe A, Santaguida S, White AM, Jones CD, Musacchio A, Green S, Taylor SS (2010) Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J Cell Biol 190: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BJ, Moree B, Farrar EM, Stewart S, Fang G, Salmon ED (2004) Spindle checkpoint protein dynamics at kinetochores in living cells. Curr Biol 14: 953–964 [DOI] [PubMed] [Google Scholar]
- Huang H, Hittle J, Zappacosta F, Annan RS, Hershko A, Yen TJ (2008) Phosphorylation sites in BubR1 that regulate kinetochore attachment, tension, and mitotic exit. J Cell Biol 183: 667–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Saito Y, Matsumoto T (2012) Centromere-tethered Mps1 pombe homolog (Mph1) kinase is a sufficient marker for recruitment of the spindle checkpoint protein Bub1, but not Mad1. Proc Natl Acad Sci USA 109: 209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelluma N, Brenkman AB, McLeod I, Yates JR, Cleveland DW, Medema RH, GJPL Kops (2008) Chromosomal instability by inefficient Mps1 auto-activation due to a weakened mitotic checkpoint and lagging chromosomes. PLoS ONE 3: e2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelluma N, Dansen TB, Sliedrecht T, Kwiatkowski NP, GJPL Kops (2010) Release of Mps1 from kinetochores is crucial for timely anaphase onset. J Cell Biology 191: 281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Chen Y, Zhao Y, Yu H (2007) Autophosphorylation-dependent activation of human Mps1 is required for the spindle checkpoint. Proc Natl Acad Sci USA 104: 20232–20237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmler S, Stach M, Knapp M, Ortiz J, Pfannstiel J, Ruppert T, Lechner J (2009) Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J 28: 1099–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S, Tugendreich S, Fujii M, Jorgensen PM, Watanabe N, Hoog C, Hieter P, Todokoro K (1998) PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol Cell 1: 371–380 [DOI] [PubMed] [Google Scholar]
- Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, Peters J-M (2003) Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J 22: 6598–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulukian A, Han JS, Cleveland DW (2009) Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev Cell 16: 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson MA, Cheeseman IM (2011) Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol 21: 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Cleveland DW (2010) A chemical tool box defines mitotic and interphase roles for Mps1 kinase. J Cell Biol 190: 21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Gonzalez P, Scott MIF, Diez M, Sen O, Taylor SS (2012) BubR1 blocks substrate recruitment to the APC/C in a KEN-box-dependent manner. J Cell Sci 124: 4332–4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lénárt P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters J-M (2007) The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol 17: 304–315 [DOI] [PubMed] [Google Scholar]
- Li D, Morley G, Whitaker M, Huang JY (2010) Recruitment of Cdc20 to the kinetochore requires BubR1 but not Mad2 in Drosophila melanogaster. Mol Cell Biol 30: 3384–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Vader G, Vromans MJM, Lampson MA, Lens SMA (2009) Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 323: 1350–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N, Ceto S, Ranish JA, Biggins S (2012) Phosphoregulation of Spc105 by Mps1 and PP1 Regulates Bub1 Localization to Kinetochores. Curr Biol 22: 900–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Yu H (2012) Mitosis: short-circuiting spindle checkpoint signaling. Curr Biol 22: R128–R130 [DOI] [PubMed] [Google Scholar]
- Maciejowski J, George KA, Terret M-E, Zhang C, Shokat KM, Jallepalli PV (2010) Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J Cell Biol 190: 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado M, Kapoor TM (2011) Constitutive Mad1 targeting to kinetochores uncouples checkpoint signalling from chromosome biorientation. Nat Cell Biol 13: 475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmanche N, Owen S, Gegick S, Steffensen S, Tomkiel JE, Sunkel CE (2007) Drosophila BubR1 is essential for meiotic sister-chromatid cohesion and maintenance of synaptonemal complex. Curr Biol 17: 1489–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malureanu LA, Jeganathan KB, Hamada M, Wasilewski L, Davenport J, van Deursen JM (2009) BubR1 N terminus acts as a soluble inhibitor of Cyclin B degradation by APC/CCdc20 in interphase. Dev Cell 16: 118–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca TJ, Salmon ED (2009) Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol 184: 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos I, Pereira AJ, Lince-Faria M, Cameron LA, Salmon ED, Maiato H (2009) Synchronizing chromosome segregation by flux-dependent force equalization at kinetochores. J Cell Biol 186: 11–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura S, Toyoshima F, Nishida E (2007) Polo-like kinase 1 facilitates chromosome alignment during prometaphase through BubR1. J Biol Chem 282: 15217–15227 [DOI] [PubMed] [Google Scholar]
- Moutinho-Santos T, Conde C, Sunkel CE (2012) POLO ensures chromosome bi-orientation by preventing and correcting erroneous chromosome-spindle attachments. J Cell Sci 125: 576–583 [DOI] [PubMed] [Google Scholar]
- Moutinho-Santos T, Sampaio P, Amorim I, Costa M, Sunkel CE (1999) In vivo localisation of the mitotic Polo kinase shows a highly dynamic association with the mitotic apparatus during early embryogenesis. Biol Cell 91: 582–596 [PubMed] [Google Scholar]
- Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8: 379–393 [DOI] [PubMed] [Google Scholar]
- Nicklas RB, Campbell MS, Ward SC, Gorbsky GJ (1998) Tension-sensitive kinetochore phosphorylation in vitro. J Cell Sci 111: Pt 21 3189–3196 [DOI] [PubMed] [Google Scholar]
- Nilsson J, Yekezare M, Minshull J, Pines J (2008) The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat Cell Biol 10: 1411–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr B, Bousbaa H, Sunkel CE (2007) Mad2-independent spindle assembly checkpoint activation and controlled metaphase-anaphase transition in Drosophila S2 cells. Mol Biol Cell 18: 850–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M, Lénárt P, Peters J-M (2008) Polo on the rise-from mitotic entry to cytokinesis with Plk1. Dev Cell 14: 646–659 [DOI] [PubMed] [Google Scholar]
- Santaguida S, Vernieri C, Villa F, Ciliberto A, Musacchio A (2011) Evidence that Aurora B is implicated in spindle checkpoint signalling independently of error correction. EMBO J 30: 1508–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria A, Neef R, Eberspächer U, Eis K, Husemann M, Mumberg D, Prechtl S, Schulze V, Siemeister G, Wortmann L, Barr FA, Nigg EA (2007) Use of the novel Plk1 inhibitor ZK-thiazolidinone to elucidate functions of Plk1 in early and late stages of mitosis. Mol Biol Cell 18: 4024–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurin AT, van der Waal MS, Medema RH, Lens SMA, GJPL Kops (2011) Aurora B potentiates Mps1 activation to ensure rapid checkpoint establishment at the onset of mitosis. Nat Commun 2: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittenhelm RB, Chaleckis R, Lehner CF (2009) Intrakinetochore localization and essential functional domains of Drosophila Spc105. EMBO J 28: 2374–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd LA, Meadows JC, Sochaj AM, Lancaster TC, Zou J, Buttrick GJ, Rappsilber J, Hardwick KG, Millar JBA (2012) Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr Biol 22: 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M, Zachariae W, Ciosk R, Nasmyth K (1998) The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J 17: 1336–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliedrecht T, Zhang C, Shokat KM, GJPL Kops (2010) Chemical genetic inhibition of Mps1 in stable human cell lines reveals novel aspects of Mps1 function in mitosis. PLoS ONE 5: e10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smurnyy Y, Toms AV, Hickson GR, Eck MJ, Eggert US (2010) Binucleine 2, an isoform-specific inhibitor of Drosophila Aurora B kinase, provides insights into the mechanism of cytokinesis. ACS Chem Biol 5: 1015–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suijkerbuijk SJE, van Dam TJP, Karagöz GE, Castelmur E, Hubner NC, Duarte AMS, Vleugel M, Perrakis A, Rüdiger SGD, Snel B, Kops GJPL (2012) The vertebrate mitotic checkpoint protein BUBR1 is an unusual pseudokinase. Dev Cell 22: 1321–1329 [DOI] [PubMed] [Google Scholar]
- Sumara I, Giménez-Abián JF, Gerlich D, Hirota T, Kraft C, la Torre de C, Ellenberg J, Peters J-M (2004) Roles of polo-like kinase 1 in the assembly of functional mitotic spindles. Curr Biol 14: 1712–1722 [DOI] [PubMed] [Google Scholar]
- Taylor SS, Hussein D, Wang Y, Elderkin S, Morrow CJ (2001) Kinetochore localisation and phosphorylation of the mitotic checkpoint components Bub1 and BubR1 are differentially regulated by spindle events in human cells. J Cell Sci 114: 4385–4395 [DOI] [PubMed] [Google Scholar]
- Vleugel M, Hoogendoorn E, Snel B, GJPL Kops (2012) Evolution and function of the mitotic checkpoint. Dev Cell 23: 239–250 [DOI] [PubMed] [Google Scholar]
- Welburn JPI, Vleugel M, Liu D, Yates JR III, Lampson MA, Fukagawa T, Cheeseman IM (2010) Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell 38: 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong OK, Fang G (2005) Plx1 is the 3F3/2 kinase responsible for targeting spindle checkpoint proteins to kinetochores. J Cell Biol 170: 709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong OK, Fang G (2006) Loading of the 3F3/2 antigen onto kinetochores is dependent on the ordered assembly of the spindle checkpoint proteins. Mol Biol Cell 17: 4390–4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong OK, Fang G (2007) Cdk1 phosphorylation of BubR1 controls spindle checkpoint arrest and Plk1-mediated formation of the 3F3/2 epitope. J Cell Biol 179: 611–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y, Yang C-H, Tanno Y, Watanabe Y (2012) MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nat Cell Biol 14: 746–752 [DOI] [PubMed] [Google Scholar]
- Yu H (2006) Structural activation of Mad2 in the mitotic spindle checkpoint: the two-state Mad2 model versus the Mad2 template model. J Cell Biol 173: 153–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zich J, Sochaj AM, Syred HM, Milne L, Cook AG, Ohkura H, Rappsilber J, Hardwick KG (2012) Kinase activity of fission yeast Mph1 is required for Mad2 and Mad3 to stably bind the anaphase promoting complex. Curr Biol 22: 296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.