Abstract
Members of the Apicomplexa phylum possess an organelle surrounded by four membranes, originating from the secondary endosymbiosis of a red alga. This so-called apicoplast hosts essential metabolic pathways. We report here that apicoplast inheritance is an actin-based process. Concordantly, parasites depleted in either profilin or actin depolymerizing factor, or parasites overexpressing the FH2 domain of formin 2, result in loss of the apicoplast. The class XXII myosin F (MyoF) is conserved across the phylum and localizes in the vicinity of the Toxoplasma gondii apicoplast during division. Conditional knockdown of TgMyoF severely affects apicoplast turnover, leading to parasite death. This recapitulates the phenotype observed upon perturbation of actin dynamics that led to the accumulation of the apicoplast and secretory organelles in enlarged residual bodies. To further dissect the mode of action of this motor, we conditionally stabilized the tail of MyoF, which forms an inactive heterodimer with endogenous TgMyoF. This dominant negative mutant reveals a central role of this motor in the positioning of the two centrosomes prior to daughter cell formation and in apicoplast segregation.
Keywords: apicomplexa, apicoplast, centrosome, myosin, Toxoplasma gondii
Introduction
The apicomplexan parasites are responsible for a wide range of diseases in humans and animals. This large group of obligate intracellular parasites includes Plasmodium spp., the causative agent of malaria responsible for more than one million deaths annually (Murray et al, 2012), and Toxoplasma gondii, a life-threatening pathogen in immunocompromised individuals and also a risk factor for severe congenital defects (Montoya and Liesenfeld, 2004). This phylum is unified by the conservation of an apical complex composed of secretory organelles (micronemes and rhoptries) that participate in active host cell entry and in the establishment of the parasitophorous vacuole membrane inside which the parasite safely replicates (Carruthers and Sibley, 1997; Bradley and Sibley, 2007; Dubremetz, 2007; Carruthers and Tomley, 2008). The Apicomplexa also possess an inner membrane complex (IMC) composed of flattened vesicular sacs residing directly beneath the plasma membrane and opposed to the subpellicular microtubules (MTs) (Figure 1A). The apicoplast, a plastid-like organelle, is present in nearly all members of the phylum except for Cryptosporidium spp. (Zhu et al, 2000). This organelle is surrounded by four membranes and originates from a secondary endosymbiotic event when the ancestor of chromalveolates engulfed a red alga (McFadden et al, 1996; Kohler et al, 1997; Lang-Unnasch et al, 1998; Roos et al, 1999; Janouskovec et al, 2010). The apicoplast possesses several copies of a circular 35 kb DNA that constitutes the plastome, mainly dedicated to its own replication (Wilson et al, 1996; Gleeson, 2000; Matsuzaki et al, 2001; Reiff et al, 2012). The majority of apicoplast proteins are encoded by the nuclear genome and targeted presumably through the endoplasmic reticulum (ER) to the plastid via a bipartite N-terminal targeting signal. This signal is composed of a leader signal peptide and a larger transit peptide (SPTP) cleaved upon reaching the organelle (Waller et al, 1998; DeRocher et al, 2000; Waller et al, 2000; He et al, 2001b; van Dooren et al, 2002). This organelle has lost the capacity to perform photosynthesis but hosts three major metabolic pathways leading to the generation of isoprenoids, fatty acids and heme. It is also involved in lipoic acid biosynthesis and contains an iron-sulphur cluster biosynthesis pathway (Brooks et al, 2010; Lim et al, 2010; Seeber and Soldati-Favre, 2010; Yeh and DeRisi, 2011). In T. gondii and Plasmodium spp., a perturbation of apicoplast functions by either pharmacological compounds or molecular genetic manipulation results in parasite death. Parasites lacking this organelle are only compromised upon entry into the next lytic cycle, a phenomenon described as a ‘delayed death’ phenotype (Fichera and Roos, 1997; He et al, 2001a; Dahl and Rosenthal, 2007). The evolutionary origin of the apicoplast implies that it is inherited and not formed de novo and thus requires a mechanism for its segregation during the course of parasite division.
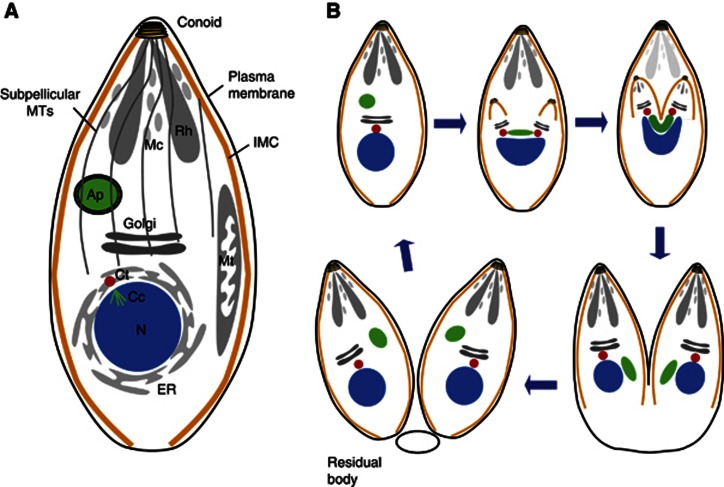
Figure 1.
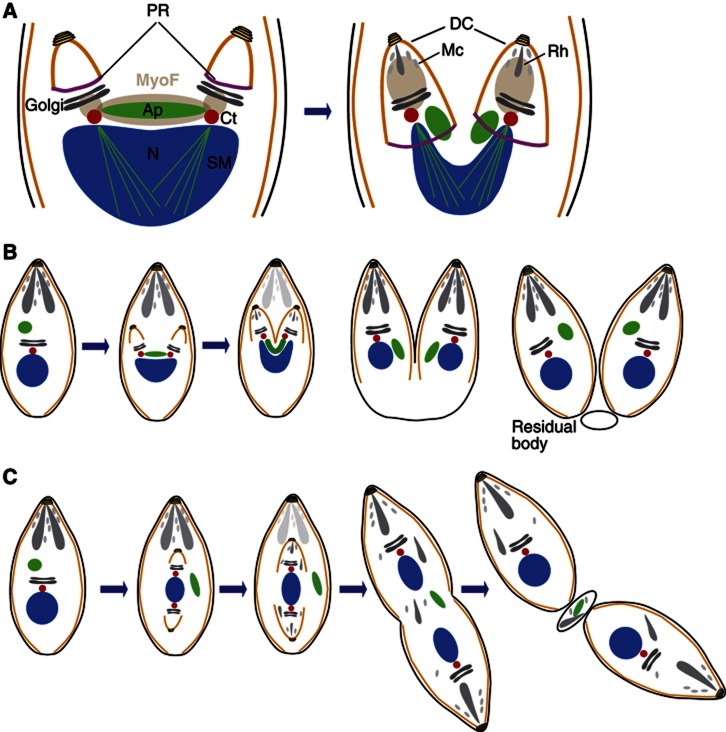
Schematic model of apicoplast division in T. gondii. (A) Apical secretory organelles, rhoptries (Rh) and micronemes (Mc); Ap, apicoplast surrounded by four membranes; Ct, centrosome; Cc, centrocone; Mt, mitochondrion; ER, endoplasmic reticulum; IMC, inner membrane complex; MTs, microtubules; N, nucleus. (B) Schematic representation of T. gondii tachyzoite division. Endodyogeny starts with duplication of the centrosomes and fission of the Golgi apparatus. Meanwhile, the apicoplast elongates with both extremities closely associated with the centrosomes. During nuclear division, the daughter cells emerge while the apicoplast remains associated to the centrosomes and adopts a U-shaped structure. Following constriction, the apicoplast segregates in two. Rhoptries and micronemes are made de novo and at the end of endodyogeny. Following division, residual mother cell constituents are disposed off in the residual body. ER, Mt, Cc and MTs are not represented.
Cell division in Apicomplexa is characterized by the formation of two or more daughter cells within the mother by processes collectively referred to as schizogony, endopolygeny or endodyogeny (Speer and Dubey, 1999; Bannister et al, 2000; Vaishnava et al, 2005; Vaishnava and Striepen, 2006; Striepen et al, 2007; Ferguson et al, 2008). In T. gondii, this process involves the synchronous geometric expansion of two daughter cells within a mature mother cell (endodyogeny) while maintaining the highly polarized organization required for invasion (Figure 1B) (Gubbels et al, 2006; Nishi et al, 2008; Lorestani et al, 2010). Division is initiated by duplication of the centrosomes and lateral elongation and fission of the Golgi apparatus (Pelletier et al, 2002; Hartmann et al, 2006). Concurrently, the apicoplast begins to elongate and associates with the centrosomes (Striepen et al, 2000). The scaffold of the daughters, comprising the conoid, the nascent IMC and the subpellicular MTs, starts to emerge slightly before the completion of nuclear DNA replication (Hu et al, 2002; Nishi et al, 2008). Mitosis and cytokinesis progress in concert with daughter cell growth and result in the encapsulation of the Golgi (Morrissette and Sibley, 2002b; Nishi et al, 2008; Agop-Nersesian et al, 2009, 2010). During nuclear division, the ends of the elongated apicoplast insert into the growing daughter cells while the organelle adopts a U-shape with both ends remaining associated with the centrosomes (Striepen et al, 2000; van Dooren et al, 2009). A dynamin-related protein (DrpA) is recruited at the base of the U-shaped apicoplast to further constrict the organelle and ensure successful fission (van Dooren et al, 2009). Next, the nucleus, the ER and finally the mitochondrion are packed into the developing daughter cells (Nishi et al, 2008). The apical secretory organelles (rhoptries and micronemes) are made de novo by vesicular budding from the Golgi (Sheffield and Melton, 1968; Hoppe et al, 2000; Ngo et al, 2003; Nishi et al, 2008; Breinich et al, 2009; Sloves et al, 2012). Ultimately, the mother apical organelles degenerate and the plasma membrane is incorporated into the daughter cells. What remains from the division is left behind in a residual body (Hu et al, 2002; Nishi et al, 2008).
Eukaryotic cell division typically involves the spatial and temporal coordination of both actin and microtubule cytoskeletons (Rogers, 2010; Vaughan and Dawe, 2011). In T. gondii, the centrosome is associated with the centrocone (also named centriolar plaque, spindle pole body/plaque) that organizes the mitotic spindle during division (Figure 1A) (Morrissette and Sibley, 2002a; Gubbels et al, 2006; Striepen et al, 2007). Treatment of T. gondii with drugs affecting microtubules leads to a catastrophic breakdown of the coordination between nuclear division and budding (Shaw et al, 2000; Striepen et al, 2000; Morrissette and Sibley, 2002b). In contrast, drugs perturbing the actin cytoskeleton (cytochalasin D, latrunculin A and jasplakinolide) were reported to have limited impact on parasite replication. Actin antagonists do not affect daughter formation and nuclear division, but disrupt the orderly turnover of the mother cell organelles (Shaw et al, 2000). Based on these observations, actin dynamics was thought to be dispensable for parasite growth, maintenance of cell polarity and daughter cell budding (Shaw et al, 2000). In sharp contrast, actin dynamics play a central role in parasite motility implicating the key contribution of the class XIV myosin A, TgMyoA (Dobrowolski and Sibley, 1996; Dobrowolski et al, 1997; Meissner et al, 2002; Wetzel et al, 2003). Several key players of actin dynamics including profilin (PRF) (Plattner et al, 2008), formins (FRMs) (Baum et al, 2008; Daher et al, 2010) and actin depolymerization factor (ADF) (Mehta and Sibley, 2011) have been reported to have a profound impact on parasite motility.
In the present study, we report the essential contribution of actin dynamics in apicoplast inheritance. We have identified the class XXII T. gondii myosin F (TgMyoF), a broadly conserved motor across the Apicomplexa phylum, as a key player implicated in this process. The rapid and tightly controlled stabilization of a dominant negative mutant form of TgMyoF was an instrumental tool to dissect motor functioning. TgMyoF participates in the correct positioning of the daughter cells scaffold presumably by maintaining the two centrosomes in close proximity. This defect in centrosome positioning is likely responsible for apicoplast loss in the progeny and their death in the subsequent cycle. Segregation of the Golgi is not affected; however, some secretory organelles accumulate together with the apicoplast in enlarged residual bodies.
Results
Actin dynamics are critical for apicoplast inheritance
Treatment of intracellular parasites in the presence of 0.2 μM cytochalasin D (CD) for 14 h led to abnormal segregation of the apicoplast in the progeny (Figure 2A), while the host cells retained normal morphology. Western blot analysis revealed a significant accumulation of Cpn60 pro-protein in CD-treated parasites (Figure 2B). Cpn60 is a nuclear-encoded apicoplast protein targeted to the plastid via its bipartite signal, cleavage of which occurs upon import into the organelle (Agrawal et al, 2009). CD treatment also led to the accumulation of rhoptries and to a lesser extent to micronemes and mitochondrial fragments in the residual bodies (Figure 2C). Within the parasitophorous vacuole (PV), parasites were disorganized and failed to adopt the typical rosette arrangement in the presence of CD (Figure 2A and C). Same treatment with 0.2 μM jasplakinolide (Jas) did not reveal any phenotypes in apicoplast inheritance. Increased drug concentration up to 1 μM led to severe host cell damage and parasite death, hampering proper assessment of the specific contribution of actin polymerization.
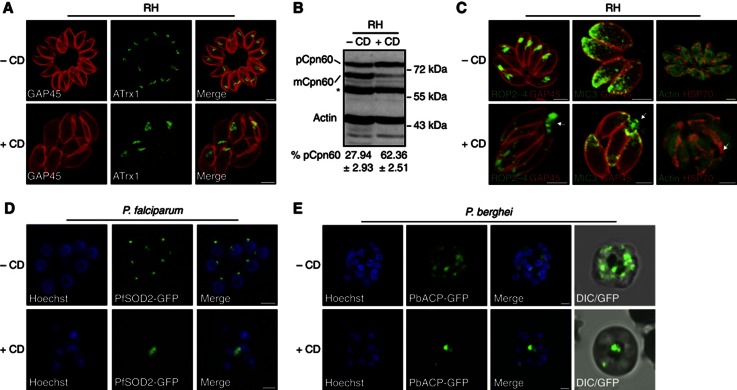
Figure 2.
Actin dynamics are implicated in apicoplast inheritance. (A) Indirect immunofluorescence analysis (IFA) of T. gondii RH parasites (wild type) showed abnormal segregation of the apicoplast-associated thioredoxin family protein (ATrx1) after 14 h±0.2 μM CD treatment. The Gliding-Associated Protein 45 (GAP45) was used to stain the periphery of the parasites. Scale bar, 2 μm. (B) Western blot analysis of RH parasites treated 14 h±0.2 μM CD showed accumulation of the Cpn60 precursor and the concomitant reduction of the processed (mature) form. Detection of T. gondii actin (TgACT1) was used as loading control. The percentage of Cpn60 precursor was quantified. Data are mean values±s.d. from three independent experiments. A representative western blot is presented. pCpn60, Cpn60 precursor; mCpn60, Cpn60 mature; *nonspecific binding. (C) RH parasites treated 14 h±0.2 μM CD showed accumulation of rhoptries (ROP2-4), micronemes (MIC3) and mitochondria (HSP70) in residual bodies (arrows). The micronemes and mitochondrion were only slightly affected. Scale bar, 2 μm. (D) Live fluorescence microscopy of a synchronized P. falciparum strain expressing PfSOD2-GFP treated from trophozoite to schizont stage (12 h)±0.3 μM CD. In the presence of CD, apicoplast inheritance was impaired in newly formed schizonts and parasites were blocked in egress. Scale bar, 8 μm. (E) Live fluorescence microscopy of P. berghei expressing PbACP-GFP treated 14 h±0.3 μM CD in vitro. Interference with actin polymerization resulted in aberrant plastid segregation. Accumulation of apicoplast staining was observed in close proximity to the food vacuole. Scale bar, 5 μm.
A similar CD-induced perturbation of apicoplast inheritance was observed in Plasmodium falciparum intraerythrocytic stages expressing the apicoplast enzyme superoxide dismutase 2 fused to GFP (PfSOD2-GFP) (Pino et al, 2007). Double sorbitol-synchronized parasites treated with 0.3 μM CD were able to form segmented schizonts and no morphological defects were visible by Giemsa staining under light microscopy. In untreated parasites, each merozoite contained an apicoplast, whereas upon CD treatment, the apicoplast appeared as an enlarged single structure and was not properly segregated in the developed schizonts blocked in egress (Figure 2D). Western blot analyses of CD-treated parasites from ring to trophozoite or from trophozoite to schizont stages showed correct PfSOD2-GFP maturation (Supplementary Figure S1A). This result contrasts with the accumulation of apicoplast pro-protein in T. gondii; however, P. falciparum merozoites only form after the final round of nuclear division and hence apicoplast proteins are still able to target to the organelle until schizogony is completed. Actin-dependent apicoplast inheritance was also confirmed in the rodent malaria parasite expressing the SPTP of the Plasmodium berghei acyl carrier protein fused to GFP (PbACP-GFP) (Stanway et al, 2009). Drug-treated parasites displayed abnormal apicoplast segregation with PbACP-GFP accumulating in a single spot in close proximity to the food vacuole, while in nontreated parasites each nucleus was associated with an apicoplast (Figure 2E and Supplementary Figure S1B and C). In both Plasmodium spp., the typical tubular appearance of the apicoplast previously observed during schizogony was significantly reduced following CD treatment (van Dooren et al, 2005; Stanway et al, 2009). These data suggest a conserved role for actin in apicoplast inheritance across the phylum.
In light of these observations, we revisited a series of characterized mutants defective in genes coding for regulators of actin dynamics in T. gondii. Analysis of the Tet-inducible knockout of profilin (TgPRF-iKO) (Plattner et al, 2008) revealed a progressive loss of the apicoplast upon PRF depletion in the presence of anhydrotetracycline (ATc). After a short-term treatment (48 h±ATc), the apicoplast appeared elongated (Figure 3A), and in a few vacuoles, the parasites lacked the organelle (Supplementary Figure S2A). After prolonged treatment (72–80 h), organellar morphology was severely affected (Figure 3A) and up to 90% of the vacuoles contained parasites devoid of apicoplast (Supplementary Figure S2A). No phenotype was observed with the parental strain TATi-1 or in PRF-iKO complemented with wt PRF (PRF-iKO+WTc) (Figure 3A). The Tet-inducible knockout of actin depolymerization factor (TgADF-iKO) has been previously shown to cause abnormal accumulation of actin filaments, resulting in severe defects in gliding motility, host cell invasion and egress (Mehta and Sibley, 2011). Conditional depletion in ADF also led to apicoplast loss similar to that observed upon PRF depletion (Figure 3B).
Figure 3.
Regulators of actin dynamics are essential for apicoplast inheritance in T. gondii. (A) Long-term depletion of TgPRF leads to abnormal apicoplast (Cpn60) inheritance. In the absence of ATc, PRF-iKO parasites showed normal plastid segregation. Following 48h depletion of PRF, the apicoplast adopted an elongated structure (arrowhead) and is lost in some parasites. After 80 h of ATc treatment, most of the vacuoles contained parasites lacking an apicoplast and Cpn60 staining was often found outside of the parasites (arrows), presumably in residual bodies. Functionally complemented parasites expressing wild-type PRF (PRF-iKO+WTc) showed no phenotype after 80 h of ATc treatment. (B) ADF-iKO parasites treated for 48 h with ATc loss the apicoplast as shown by anti-ATrx1 staining that accumulated outside of the parasites (arrow). (C) Apicoplast inheritance was affected 36 h after stabilization of DDFH2/2. (D–F) Western blot analysis revealed the accumulation of the Cpn60 precursor form and reduction of the mature form after 80h depletion of TgPRF, 48h depletion of ADF or after 36h stabilization of DDFH2/2. Complemented and parental strain as well as untreated parasites showed proper processing of Cpn60. Regulation of Myc-tagged inducible copy of PRF in PRF-iKO and the presence of the Ty-tagged complemented wild-type copy (PRF-iKO+WTc) was analysed using the corresponding antibodies. Anti-HA was used to assess the regulation of ADF-iKO. Detection of TgACT1 was used as loading control. The percentage of Cpn60 precursor was quantified. Data are mean values±s.d. from three independent experiments. A representative western blot is presented. pCpn60, Cpn60 precursor; mCpn60, Cpn60 mature; *nonspecific binding. Scale bar, 2 μm.
T. gondii possesses three formins that act as key regulators of actin dynamics. These large proteins contain a formin-homology 2 (FH2) domain and assemble into homodimers to polymerize actin in a processive manner (Daher et al, 2010). The FH2 domain of TgFRM1 (FH2/1) and TgFRM2 (FH2/2) are potent actin nucleators in vitro (Skillman et al, 2012) and in vivo (Daher et al, 2010). Uncontrolled actin polymerization is deleterious to parasite survival and hence the FH2 domains were fused to a FKBP destabilization domain (DD) for targeting to proteasomal degradation in the absence of the stabilization ligand Shld-1 (Herm-Gotz et al, 2007). We observed here that overexpression of DDFH2/2 but not DDFH2/1 caused a dramatic loss of the apicoplast (Figure 3C and Supplementary Figure S2B). In contrast, the actin binding site mutant DDFH2/2-R/A, which fails to polymerize actin and exclusively poisons the endogenous TgFRM2 by assembly into an inactive heterodimer, affects parasite motility and invasion only (Daher et al, 2010) (Supplementary Figure S2C). Taken together, these results indicate that the loss of the apicoplast in the presence of DDFH2/2 is due to uncontrolled actin polymerization and not necessarily linked directly to the function of TgFRM2.
Western blot analyses using anti-Cpn60 antibodies showed an accumulation of the precursor and a concomitant decrease in the mature form of the protein in all situations where actin dynamics were perturbed and led to loss of the apicoplast (Figure 3D–F). By IFA, Cpn60 and the apicoplast-associated thioredoxin family protein (ATrx1) accumulated in small vesicles (Figure 3A–C). Under all conditions of actin perturbation tested, the parasites failed to adopt the typical arrangement in rosettes and the apicoplast is frequently found outside of the parasite, that is, in residual bodies (Figure 3A–C). Micronemes and mitochondrion were not affected by TgPRF or TgADF depletion or by stabilization of DDFH2/2. In a few cases, some rhoptries were found in residual bodies (Supplementary Figure S2D).
T. gondii myosin F localizes around the apicoplast during parasite division
The contribution of actin dynamics to apicoplast inheritance in both T. gondii and Plasmodium spp. suggests the implication of a conserved myosin motor present in all Apicomplexa harbouring this plastid. Among the 11 myosins present in T. gondii, TgMyoA and TgMyoF are the only two motors conserved across the phylum (Foth et al, 2006). Conditional deletion of TgMyoA is known to exhibit a strong invasion phenotype, but this motor is not implicated in apicoplast inheritance (Supplementary Figure S3A and B).
TgMyoF belongs to the class XXII myosins and contains seven WD40 domains, six putative IQ motifs and a predicted coiled-coil domain suggesting a function as a dimer (Peckham, 2011) (Figure 4A). The WD40 domains are present in the other members of this class of myosins and restricted to the phylum of Apicomplexa (Foth et al, 2006) (Supplementary Figure S3C). To localize this motor, a C-terminal triple epitope-tag was inserted to the open reading frame by single homologous recombination at the endogenous TgMyoF locus in Ku80-KO strain (Huynh and Carruthers, 2009). The resulting transgenic parasites expressed MyoF-3Ty at the expected mass by western blot (Figure 4B) and accumulated to the vicinity of the apicoplast by IFA (Figure 4C). TgMyoF-3Ty was also found associated with the growing daughter cells and at the pellicle in nondividing parasites (Figure 4C). In extracellular parasites, TgMyoF-3Ty was found on top of the nucleus in addition to its spread signal in the cytoplasm and at the pellicle (Supplementary Figure S3D). Short-time treatment with 1 μM CD or 1 μM Jas did not reveal any change in the localization of the motor (Supplementary Figure S3D and E).
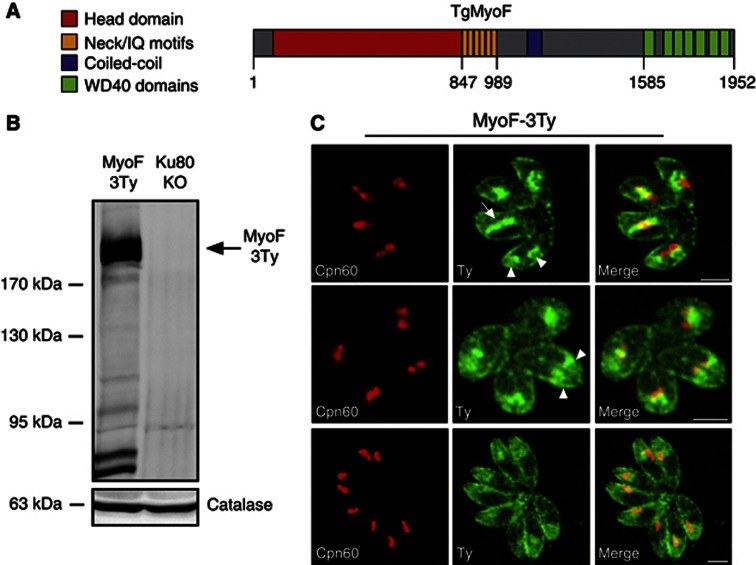
Figure 4.
T. gondii myosin F is associated with the apicoplast during parasite division. (A) TgMyoF (DQ131541) contains six IQ motifs, a coiled-coil domain and seven WD40 domains. All domains were predicted using SMART EMBL. (B) TgMyoF-3Ty is found at the expected molecular mass by western blot (216 kDa). Detection of T. gondii catalase was used as loading control. (C) MyoF-3Ty concentrates at the extremities of the apicoplast during division (arrow, upper panel) and to the newly formed daughter cells after apicoplast fission (arrowhead, upper and middle panels). In nondividing parasites (lower panel), TgMyoF is concentrated in the apical region, on top of the nucleus and at the pellicle. Scale bar, 2 μm.
TgMyoF is essential for parasite survival
The function of TgMyoF was investigated by generation of an inducible knockdown (MyoF-iKO) in the Ku80-KO strain using a one-step approach. The strategy is based on double homologous recombination in the TgMyoF locus leading to the insertion of the transactivator TATi-1 and replacement of the TgMyoF promoter with the 7-tet-OpSag1-inducible promoter (Meissner et al, 2001). Concomitant with the promoter swap, a Myc epitope-tag was inserted at the N-terminus of the inducible copy of TgMyoF (Supplementary Figure S4A). PCR analysis confirmed correct integration solely in the 3′ end, suggesting that a single homologous recombination event took place (Supplementary Figure S4B and C). This integration event is reversible as observed by loss of the Myc tag by IFA in 1–2% of the population after several weeks of propagation in the absence of selection. This mutant was analysed phenotypically in depth here and a second independent clone was subsequently generated where double recombination occurred (Supplementary Figure S4D) that exhibited the same phenotypes.
Quantitative RT–PCR analysis confirmed that the inducible MyoF (iMycMyoF) is tightly regulated (Supplementary Figure S4E). The expression of iMycMyoF dropped to undetectable levels by western blot after 48 h of ATc treatment (Figure 5A). Importantly, iMycMyoF localized in the vicinity of the apicoplast as observed with MyoF-3Ty (Figure 5B). Upon iMycMyoF depletion, a defect in apicoplast inheritance was already detectable 24 h post ATc treatment (Figure 5C) and accumulation of the Cpn60 precursor was confirmed after 48 h in the presence of ATc (Figure 5D). In the absence of MyoF, antibodies specific to mitochondrion, rhoptries or micronemes contents were staining enlarged residual bodies (Figure 5E), yet did not affect the parasite ability to invade or egress from infected cells (Supplementary Figure S4F and G). In contrast, the Golgi apparatus partitioned accurately between the daughter cells (Figure 5E). The plaque assay, which recapitulated all the steps of the lytic cycle, revealed that TgMyoF is critical for parasite survival (Supplementary Figure S4H). The MyoF-iKO parasites formed slightly smaller plaques than the Ku80-KO recipient strain and formed no visible plaques in the presence of ATc. Intracellular growth assay at 48 h showed no defect whereas a severe block at the stages of 2 to 4 parasites per vacuole was observed in the subsequent lytic cycle (78 h of ATc treatment; Figure 5F).
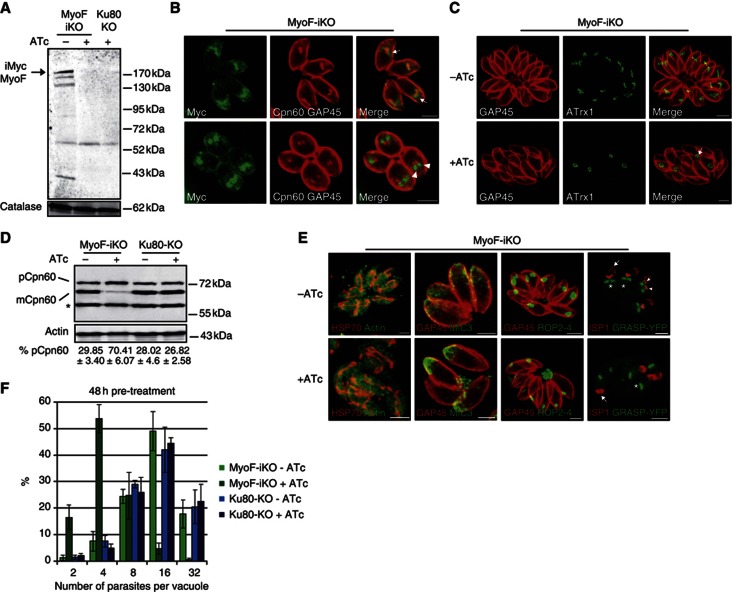
Figure 5.
Knockdown of TgMyoF is deleterious for parasite survival and affects apicoplast inheritance. (A) Inducible MyoF (iMycMyoF) migrates at the predicted molecular size (216 kDa) by western blot and is downregulated following 48 h of ATc treatment. Detection of T. gondii catalase was used as a loading control. (B) The iMycMyoF localized in the vicinity of the apicoplast and accumulated at the extremities of the dividing organelle (Cpn60; arrow). After apicoplast fission, iMycMyoF localized in the nascent daughter cells (arrowhead). (C) IFA showed abnormal apicoplast inheritance in parasites treated 32 h with ATc. The apicoplast was found in residual bodies (arrow). (D) Accumulation of Cpn60 precursor and the concomitant decrease in processed Cpn60 were observed upon 48 h of ATc treatment. Parental Ku80-KO strain and MyoF-iKO parasites grown in the absence of ATc showed proper maturation of Cpn60. The percentage of Cpn60 precursor was quantified. Data are mean values±s.d. from three independent experiments. A representative western blot is presented. pCpn60, Cpn60 precursor; mCpn60, Cpn60 mature; *nonspecific binding. (E) Except for a few cases, the mitochondrion stained with anti-HSP70 was not affected by TgMyoF depletion and no or very little accumulation in residual bodies was observed. In contrast, some rhoptry and microneme contents stained with anti-ROP2-4 and anti-MIC3 antibodies respectively were accumulated in residual bodies in TgMyoF-depleted parasites. The inheritance of the Golgi (*) was unaffected. Division was visualized using anti-ISP1 antibodies that stained the apical cap of the mother cell (arrow) and the nascent daughter cells (arrowhead). Parasites were treated 32 h±ATc. (F) Intracellular growth of MyoF-iKO and Ku80-KO grown 78 h±ATc. Parasites per vacuole were counted 30 h after inoculation of new host cells. TgMyoF-depleted parasites were blocked at 2–4 parasites. Ku80-KO strain and nontreated MyoF-iKO displayed normal growth. Data are mean values±s.d. from three biological independent experiments where 200 vacuoles were counted for each condition. Scale bar, 2 μm.
Dissection of the role of TgMyoF via the expression of a dominant negative mutant
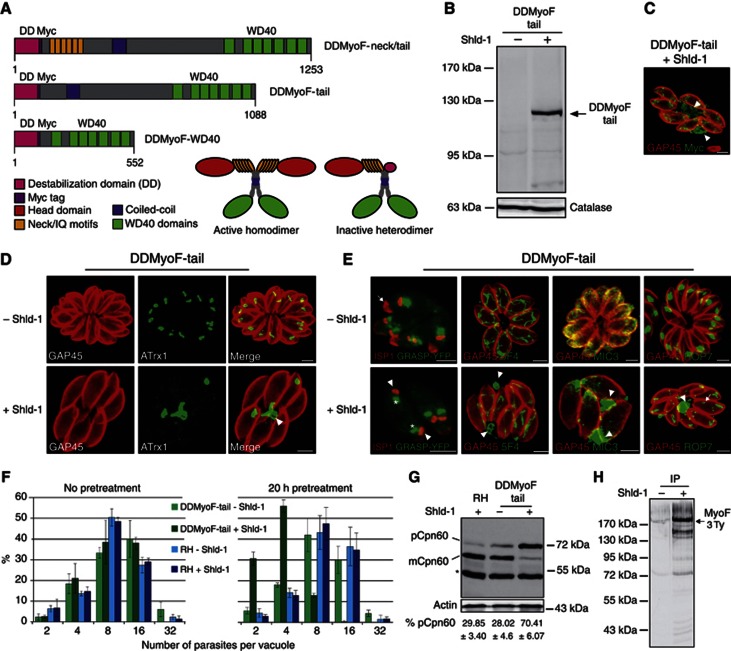
The presence of a coiled-coil domain in the tail of TgMyoF suggests that this motor acts as a dimer and hence offers the potential to create a dominant negative mutant by expressing the tail domain only, which could form a defective single-headed dimer and hence poison endogenous TgMyoF (Figure 6A). Given the essential nature of this motor, TgMyoF-tail was fused to DD for tight and fast control of its expression. Parasites expressing the neck (IQ motifs spanning region) and tail domain (DDMyoF-neck/tail), the tail only (DDMyoF-tail) or the seven WD40 domains (DDMyoF-WD40) were generated (Figure 6A) and their Shld-1-dependent expression assessed by western blot (Figure 6B and Supplementary Figure S5A). Upon stabilization, DDMyoF-neck/tail caused a dramatic morphological defect with a loss of pellicle integrity and the complete disintegration of the rhoptries and apicoplast (Supplementary Figure S5B). The parasites failed to progress beyond 1–2 division cycles in the presence of Shld-1 (Supplementary Figure S5C). Since overexpression of IQ motifs might sequester MLCs and could impact on other myosins sharing the same light chains (Polonais et al, 2011), no further experiments were conducted with this strain. In sharp contrast, the overexpression of DDMyoF-WD40 did not result in detectable growth defects as recorded by plaque assay (Supplementary Figure S5D). Stabilization of DDMyoF-tail produced an intermediate phenotype leading to the formation of enlarged residual bodies where this protein accumulated (Figure 6C). Parasites failed to form rosettes, and lost the apicoplast (Figure 6D). The Golgi apparatus was not affected (Figure 6E), whereas mitochondrial fragments were found in residual bodies. Rhoptries were found detached from the apex and accumulating together with micronemes in the residual bodies (Figure 6E) but without significant impact on invasion or egress (Supplementary Figure S5E and F). Intracellular parasites treated during 30 h with Shld-1 showed no defect in replication (Figure 6F, left) but exhibited a block in replication in the subsequent cycle of infection indicative of a delayed death phenotype (Figure 6F, right). DDMyoF-tail-expressing parasites failed to form plaques of lysis (Supplementary Figure S5G) and accumulated the unprocessed form of Cpn60 (Figure 6G). Taken together, these results confirmed a role for TgMyoF in apicoplast inheritance, this time based on the selective inactivation of the motor by formation of a defective heterodimer formed between endogenous MyoF and DDMyoF-tail. Co-immunoprecipitation experiments confirmed the formation of these heterodimers in the presence of Shld-1 (Figure 6H and Supplementary Figure S5H).
Figure 6.
Overexpression of TgMyoF tail acts as a dominant negative mutant. (A) Schematic representation of the FKBP destabilization domain (DD) constructs and dominant negative effect of DDMyoF-tail caused by the formation of an inactive heterodimer that poisons the endogenous TgMyoF. (B) Western blot analysis using anti-Myc showed the stabilization of DDMyoF-tail at the predicted size (118 kDa) after Shld-1 treatment for 48 h. Catalase serves as loading control. (C) IFA (anti-Myc) detected DDMyoF-tail in the cytosol and in enlarged residual bodies (arrowhead). (D) The 24h stabilization of DDMyoF-tail led to a defect in apicoplast (anti-ATrx1) inheritance with the majority found outside the parasites (arrowhead). (E) Golgi (*) was not affected by stabilization of DDMyoF-tail. Dividing parasite were visualized with anti-ISP1. Arrow, mother cell; arrowhead, nascent daughter cell. In the presence of Shld-1, few mitochondrial fragments stained with anti-F1-ATPase (5F4) were observed in residual bodies (arrowhead). Microneme (anti-MIC3) and rhoptry contents (anti-ROP7) were found accumulating in residual bodies (arrowhead) and some rhoptries were dispersed in the cytosol (arrow). (F, left) Intracellular growth assay was performed on parental (RH) and DDMyoF-tail parasites grown for 30 h±Shld-1 before fixation and no alteration of growth was observed. (F, right) RH and DDMyoF-tail parasites were pretreated±Shld-1 during 20 h. Host cells were inoculated and the pretreated parasites were grown for 30 h±Shld-1 before fixation. Stabilization of DDMyoF-tail led to a severe arrest at 2–4 parasites per vacuole. Data are mean values±s.d. from three independent experiments. (G) The 48h stabilization of DDMyoF-tail led to the accumulation of Cpn60 precursor and decrease of the processed Cpn60 by western blot. TgACT1 was used as a loading control. The percentage of Cpn60 precursor was quantified. Data are mean values±s.d. from three independent experiments. pCpn60, Cpn60 precursor; mCpn60, Cpn60 mature; *nonspecific binding. (H) DDGFPMyoF-tail was stably expressed in MyoF-3Ty parasites. Immunoprecipitation (IP) was performed with anti-GFP coupled beads. Bound fractions were analysed by western blot and revealed the presence of MyoF-3Ty only when DDGFPMyoF-tail is stabilized (+Shld-1). Scale bar, 2 μm.
Perturbation of MyoF function leads to accumulation of intact organelles in residual bodies
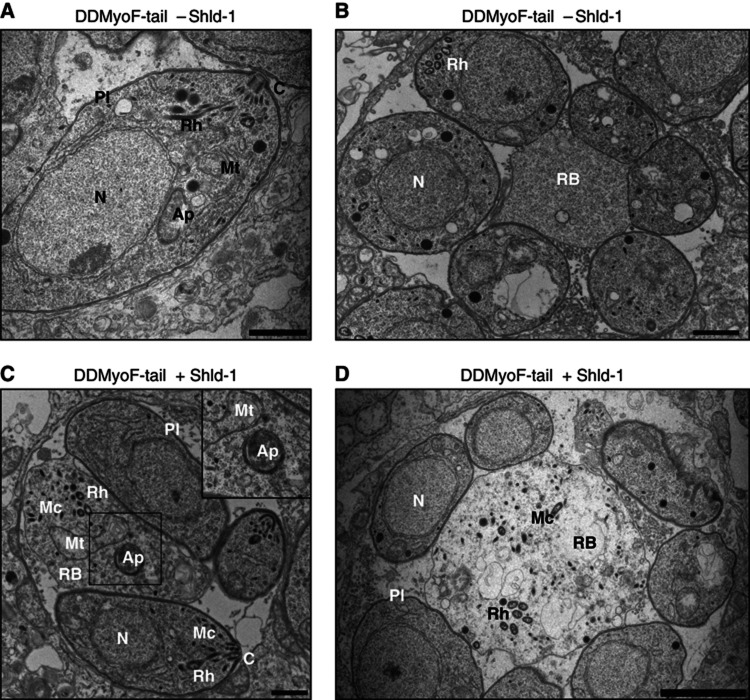
Electron microscopy (EM) was utilized to further examine the organellar contents observed by IFA in the residual bodies of parasites overexpressing DDMyoF-tail and revealed the presence of intact organelles within these structures. In the absence of Shld-1, rhoptries and micronemes are properly positioned at the apex, while the apicoplast is apical and closer to the nucleus (Figure 7A). Only small residual bodies are identified, surrounded by a single membrane and contrasting with the triple membrane of the parasite pellicle (Figure 7B) (Shaw et al, 2000). In parasites treated with Shld-1, the apicoplast, fragments of the mitochondrion, and morphologically intact micronemes and rhoptries are found within considerably enlarged residual bodies (Figure 7C and D). Despite this, some secretory organelles are positioned to the apical pole of the parasite (Figure 7C).
Figure 7.
Electron microscopy analysis of parasites expressing DDMyoF-tail. (A) In the absence of Shld-1, DDMyoF-tail parasites show a typical morphology, with the apicoplast positioned near the nucleus and the rhoptries and micronemes at the apical pole. Scale bar, 1 μm. (B) A vacuole showing a residual body devoid of organelles and surrounded by parasites. Scale bar, 1 μm. (C) Stabilization of DDMyoF-tail led to an accumulation of intact micronemes, rhoptries and mitochondrial fragments in enlarged residual bodies. An intact apicoplast surrounded by four membranes was also detected. Some rhoptries and micronemes were also found correctly localized to the parasite apical pole. Scale bar, 1 μm. (D) A vacuole showing a residual body containing rhoptries and micronemes and undefined membranous material. Scale bar, 2 μm. Ap, apicoplast; C, conoid; N, nucleus; Mc, micronemes; Mt, mitochondria; RB, residual body; Pl, pellicle; Rh, rhoptries.
TgMyoF governs the positioning of centrosomes during parasite division
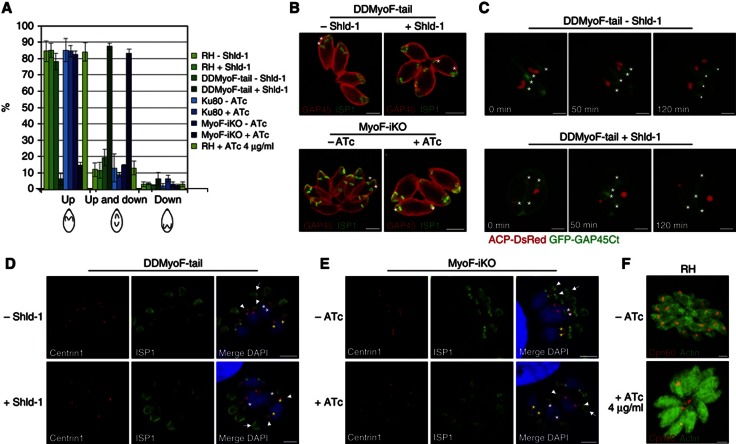
As early as 6 h post treatment with Shld-1, a defect in apicoplast segregation was noticeable in parasites expressing DDMyoF-tail. In order to gain further insight into the mechanism leading to apicoplast loss, several markers of parasite division were analysed by IFA on these transgenic parasites. T. gondii IMC subcompartment protein (TgISP1) is a marker of the apical cap portion of the IMC used to visualize daughter cell formation (Beck et al, 2010). In wild-type parasites, most of the daughter cells (84.8%±3.7) grow in the same orientation, towards the apical pole of the mother cell (up). Under these conditions, only few parasites (11.3%±3.9) were observed with opposite dividing daughter cells (up/down) or with the daughter cells growing in the same direction but towards the basal end of the mother cell (down) (3.8%±2.1). Intriguingly, in parasites overexpressing DDMyoF-tail and in MyoF-depleted parasites, the ratio between up and up/down was inverted, with most of the parasites dividing in opposite directions (Figure 8A and B). These observations on fixed cells were confirmed by time-lapse video microscopy using the DDMyoF-tail-expressing parasites transiently transfected with a vector expressing ACP-DsRed that targets to the apicoplast (Pino et al, 2007) and with GFP-GAP45Ct that localizes to the IMC of nascent daughter cells (Frenal et al, 2010). In the absence of Shld-1, parasites divided normally (Supplementary Movie S1 and Figure 8C). Daughter cells emerged in the same orientation towards the apical pole of the mother cell, and growth resulted in the formation of a U-shaped apicoplast, which ultimately underwent fission and partitioned between the two daughter cells (van Dooren et al, 2009). In contrast, in presence of Shld-1, the daughter cells formed on opposite sides of the nucleus and the apicoplast failed to segregate between the daughter cells and ended up in the residual body (Figure 8C and Supplementary Movies S2–4). Regardless of the position of the mitotic centre, the forming daughter cells were always closely associated with the centrosomes, along the axis formed by the nucleus, the centrosome and the Golgi (Figure 8D and E).
Figure 8.
MyoF contributes to the close positioning of centrosomes during division. (A) Scoring of daughter cell orientation during parasite division by IFA using anti-ISP1 antibodies. Interference with TgMyoF function led to up/down topology whereas wild-type or untreated parasites adopted an up topology. Loss of apicoplast by antibiotic treatment (RH+ATc 4 μg/ml) did not affect the orientation of division. Data are mean values±s.d. from three independent experiments where 200 vacuoles were counted for each condition. (B) IFA of representative parasites scored in (A). Daughter cells (ISP1) are marked with *. (C) Time-lapse video microscopy images of DDMyoF-tail transiently transfected with ACP-DsRed (apicoplast) and GFP-GAP45Ct (daughter cells, IMC). In the presence of Shld-1, daughter cells (*) emerged in an up/down orientation and the apicoplast failed to be encapsulated in contrast with nontreated parasites that divided in up orientation. (D–E) Regardless of MyoF functional impairment, the centrosomes (centrin1, *) were always found in association with the forming daughter cell. Arrow, mother cell; arrowhead, nascent daughter cell. DDMyoF-tail parasites were treated 24 h±Shld-1 and MyoF-iKO 48 h±ATc. (F) Wild-type parasites treated with 4 μg/ml ATc for 14 h lost the apicoplast but are still able to form rosette. Scale bar, 2 μm.
To determine whether the association of the apicoplast with the centrosomes might be responsible for their close localization, that is, serving as a connecting bridge, wild-type parasites were transiently treated with high doses of ATc (4 μg/ml) in order to eliminate the apicoplast by blocking its protein biosynthesis as previously reported for tetracycline (Dahl et al, 2006; Wiesner et al, 2008). The ATc-induced loss of apicoplast did not affect rosette formation and parasite division still occurred in a polarized fashion as observed in nontreated parasites (Figure 8A and F).
Discussion
This study establishes that the actomyosin system of Apicomplexa plays a crucial role in the inheritance of the apicoplast. CD treatment provokes the loss of this double endosymbiotic organelle upon division of T. gondii tachyzoites and schizogony in human and rodent malaria parasites. Consistent with these observations, the recent knockout of T. gondii actin 1 by conditional gene excision via dimerizable Cre recombinase was reported to cause the loss of the apicoplast (Andenmatten et al, 2013). Perturbation of actin dynamics not only severely impacts the inheritance of a single apicoplast, but also alters proper segregation of rhoptries, and to a lesser extent the micronemes and fragments of the mitochondrion that accumulate in enlarged residual bodies as previously reported by EM investigations (Shaw et al, 2000).
Both TgPRF and TgADF were previously described as key regulators of actin dynamics in T. gondii, critically contributing to motility, host cell invasion and egress. When revisiting the phenotypes of parasites conditionally depleted in TgPRF or TgADF, we observed a dramatic defect in apicoplast inheritance that recapitulated the effects observed in the presence of CD. Recently, TgPRF was shown to function primarily in the sequestration of globular actin (G-actin) when assessed in vitro using purified T. gondii actin (Skillman et al, 2012). If this holds true in vivo, depletion in TgPRF is anticipated to increase the pool of G-actin. TgADF has been characterized as a strong G-actin sequestering protein with weak severing activity on filaments (Mehta and Sibley, 2010). Consequently, depletion of either TgPRF or TgADF likely results in a similar increase in the pool of free G-actin and hence boosts the formation of filaments. In parallel, the FH2 domain of TgFRM2, which nucleates and polymerizes actin, also interferes with apicoplast inheritance. Intriguingly, the FH2 domain of TgFRM1 was also reported to exhibit a deleterious effect due to uncontrolled actin polymerization (Daher et al, 2010), but unlike the FH2/2, FH2/1 does not compromise apicoplast inheritance. The differential effect observed with these two formins is not understood but might indicate a specific role of TgFRM2 in this process. The stabilization of DDFH2/2-R/A that specifically blocks TgFRM2 function is not affecting apicoplast inherence; however, we cannot exclude that its overexpression fails to neutralize all the endogenous molecules of TgFRM2 and that residual actin nucleator activity of this formin explains the lack of impact on this organelle.
The actin dependency of apicoplast inheritance led us to postulate the participation of a myosin motor. Besides MyoA, MyoF is the only other myosin motor conserved across the Apicomplexa and hence was considered as a primary candidate.
In T. gondii tachyzoites, the apicoplast elongates with both extremities apparently associated to the closely positioned centrosomes (Striepen et al, 2000). TgMyoF localizes to the parasite periphery as well as in close proximity with the elongating apicoplast, and subsequently also with the nascent daughter cells (Figure 9A). The dynamic changes in TgMyoF subcellular distribution during parasite division potentially reflect multiple functions of the motor.
Figure 9.
Contribution of TgMyoF to apicoplast inheritance. (A) Schematic representation of TgMyoF localization (grey area) during parasite division. In the early steps, TgMyoF localizes along the apicoplast and accumulates at the extremities of the organelle. After apicoplast fission, TgMyoF localizes inside the growing daughter cells (DCs). PR, posterior ring; N, nucleus; Ct centrosome; SM, spindle microtubules; Ap, apicoplast; Rh, rhoptries; Mc, micronemes. (B) In the wild-type situation, division starts with the duplication of the centrosomes, fission of the Golgi and elongation of the apicoplast, which associates with both centrosomes. Slightly before the end of DNA replication, daughter cells start to emerge and engulf the apicoplast. During daughter bud extension the apical organelles (rhoptries and micronemes) are made de novo and anchored at the apical pole. Meanwhile, the mother apical organelles are degraded. The large majority of daughter cells were found to bud in the same orientation; towards the apical end of the mother cell. (C) Alteration of MyoF function does not affect the duplication of the centrosomes or fission of the Golgi. However, daughter cells emerge in opposite or random orientations and fail to encapsulate the apicoplast. MyoF participates in the correct positioning of the daughter cell likely by maintaining the centrosomes in close proximity. Not only the apicoplast but also rhoptries and micronemes accumulate in the residual bodies either as a result of alteration of centrosome positioning or another function of TgMyoF.
Downregulation of TgMyoF, based on the tet-inducible system, confirmed the involvement of this motor in apicoplast inheritance. Depletion of MyoF had no immediate impact on intracellular growth but resulted in a severe block in replication in the subsequent lytic cycle, which has previously been described as delayed death phenotype associated with the loss of the apicoplast (Fichera and Roos, 1997).
Since the tet-inducible system acts at the level of transcription, the response to ATc is slow and limits the dissection of TgMyoF function. Therefore, we opted for the generation of a dominant negative mutant of TgMyoF leading to the formation of inactive heterodimers with the endogenous TgMyoF. TgMyoF-tail lacks the neck domain and therefore does not titrate out other MLCs and thus selectively targets TgMyoF function. This dominant negative mutant is tightly controlled at the protein stability level via fusion with a DD, which provides with a fast responding experimental tool to investigate the role of this motor.
Time-lapse video microscopy and IFA using different division markers revealed that the daughter cells adopt an unusual orientation during endodyogeny. Most daughter cells grow on opposite sides of the nucleus in the absence of functional TgMyoF instead of developing side by side towards the apical tip of the mother cell as observed in wild-type parasites (Figure 9B and C). Such abnormal topology results in a failure of the apicoplast to be encapsulated and segregated in the nascent daughter cells. This miss-positioning of the centrosomes is therefore likely responsible for the apicoplast loss (Figure 9C).
In T. gondii, at the beginning of cell division, centrosomes were reported to migrate to the basal end of the nucleus, divide and move back to the apical end to re-associate with the Golgi (Hartmann et al, 2006). Since paired centrosomes were also observed when MyoF function was impaired, this motor is likely playing a role in a later stage, following re-association with the Golgi. Concordantly, the inheritance of the Golgi was not affected and the organelle was always found associated with the centrosome and the daughter cells regardless of their orientation during division.
Plasmodium spp. lack a centrosome structure but possess a mitotic microtubule-organizing centre (MTOC, also known as spindle pole body or centriolar plaques) that resembles yeast spindle pole bodies (Mahajan et al, 2008; Arnot et al, 2011; Gerald et al, 2011; Reininger et al, 2011). This structure consists of electron-dense plaques that appear to be embedded in the nuclear membrane with one face exposed to the nuclear side and the other facing the cytoplasm (Bannister et al, 2000; Gerald et al, 2011). During schizogony in P. falciparum and in P. berghei liver stage development, the apicoplast undergoes massive morphological changes, starting from a single discrete organelle to a giant tubular structure. Association of the apicoplast with the mitotic MTOC has been proposed as the strategy by which the parasite senses how many apicoplast should be inherited in accordance with the number of nuclei (Striepen et al, 2007). However, in both parasites, live imaging studies failed to show a constant interaction of the growing apicoplast with the dividing nuclei (van Dooren et al, 2005; Stanway et al, 2009, 2011). In Sarcocystis neurona, the development of the apicoplast mirrors the nucleus with a continuous tubular plastid growing alongside the nucleus in tight association with paired, newly divided centrosomes (Vaishnava et al, 2005). Our data on T. gondii tachyzoites strongly support a critical role for centrosome positioning in the mechanism of apicoplast inheritance. TgMyoF appears to maintain the two centrosomes in close proximity, allowing the apicoplast to associate with both structures. At this point, we cannot exclude that MyoF not only holds the centrosomes in close proximity but also participate in the association or the recruitment of the apicoplast with the centrosomes. In contrast, we were able to exclude a role for the apicoplast itself in maintaining the two centrosomes in close proximity by treating parasites with high doses of ATc. The antibiotic action of ATc led to apicoplast loss without interfering with the apical growth of the daughter cells.
The mechanism by which TgMyoF maintains the position of the centrosomes remains to be elucidated. Irrespective of the positions of the centrosomes around the nucleus, the parasites are able to divide and grow normally during one lytic cycle. This suggests that the centrosome acts as a ‘super-organizing centre’ (Morrissette and Sibley, 2002a) to organize both the nuclear division and daughter formation independent of its position. In accordance with this model, it was recently demonstrated that daughter cell formation depend on the formation of a striated rootlet fibre that emerge from the centrosomes immediately after their duplication (Francia et al, 2012).
There is a considerable diversity of centrosome movements in biology and all require the actin or microtubule cytoskeletons or both (Manneville and Etienne-Manneville, 2006; Kunda and Baum, 2009; Ou et al, 2010; Vaughan and Dawe, 2011). Notably, the repositioning of centrosomes is a critical event at the immunological synapse, where centrosomal docking at the plasma membrane is implicated in the creation of a focal point of exocytosis and endocytosis with the recruitment of both Golgi and recycling endosomes (Griffiths et al, 2010). During synapse formation, there is a dramatic polarization of both the actin and microtubule cytoskeletons. This model of centrosome polarization involves microtubules tethered to actin, with the consequent stabilization of the microtubules imposing tension on the network, pulling the centrosomes forward (Stinchcombe and Griffiths, 2007).
It is also known that plant organelle movement and positioning is predominantly mediated by the actin cytoskeleton (Wada and Suetsugu, 2004). Drugs affecting actin dynamics inhibit chloroplast movement in various green plant species but the requirement of a myosin in this process remains controversial (Suetsugu and Wada, 2007). Furthermore, in tobacco-derived cells, chloroplasts redistribute from cortical to perinuclear regions in an actin-dependent process. Perturbation of actin dynamics with CD leads to an imbalanced organelle inheritance (Sheahan et al, 2004). In Apicomplexa as well as in organisms that possess red alga-derived plastids (e.g., Cryptomonads, Haptophytes, Stramenopiles, Dinoflagellates), the segregation of the plastid is complicated by the presence of additional membranes originating from the secondary endosymbiotic events and likely differs from the chloroplasts. However, the actin-dependent mechanism described here might be conserved across the alveolates.
The accumulation of rhoptry contents in the residual bodies suggests an involvement of TgMyoF in either anchoring or trafficking of this organelle. In Saccharomyces cerevisiae, the vacuoles are transported to the bud by a motor composed of the class V myosin Myo2, and two accessory proteins Vac17 and Vac8 (Weisman, 2006). In Apicomplexa, the recently characterized Armadillo Repeats-Only protein (ARO) might correspond to a homologue of Vac8 at the surface of the rhoptries (Cabrera et al, 2012). Consistently, the conditional disruption of TgARO leads to a random dispersion of mature rhoptries within the cytosol (Beck et al, 2013; Mueller et al, 2013). Moreover, TgMyoF has been identified as one partner of TgARO potentially ensuring the anchorage and/or transport of the rhoptries to the apical pole (Mueller et al, 2013). Regardless of the accumulation of organelles in enlarged residual bodies, conditional perturbation of TgMyoF function did not alter on host cell invasion, suggesting that enough organelles reach the apical pole to fulfil the function. This lack of phenotype contrasts with the severe effect on invasion observed when TgARO is depleted. The partial disruption of TgMyoF might be more dramatic on a single organelle that cannot be made de novo such as the apicoplast.
TgMyoF is a clear example of an actin-based motor playing a critical role in the correct positioning of the centrosomes at the onset of parasite division. This motor is essential for long-term survival of the parasite by insuring apicoplast inheritance. TgMyoF also appears to fulfil additional roles such as the apical transport and/or anchoring of secretory organelles. Identification of TgMyoF interacting partners will be instrumental to dissect mechanistically the mode of action of this central motor for the Apicomplexa, a group of important pathogens causing significant threats for human and animal health.
Materials and methods
T. gondii culture
T. gondii RH strains HXGPRT-KO (RH) (Donald et al, 1996) and Ku80-KO (Huynh and Carruthers, 2009) were grown in confluent human foreskin fibroblasts (HFFs) maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; GIBCO, Invitrogen) supplemented with 10% fetal calf serum, 2 mM glutamine and 25 μg/ml gentamicin. Conditional expression of the different constructs was performed with 0.5 μM Shld-1 for DD-fusion stabilization (Herm-Gotz et al, 2007) and with 1 μg/ml anhydrotetracycline (ATc) for the Tet-inducible system (Meissner et al, 2001).
Parasite transfection and selection of stable transgenic parasites
Parasite transfections were performed as previously reported (Soldati and Boothroyd, 1993). Selections of transgenic parasites were performed with either mycophenolic acid and xanthine for HXGPRT selection (Donald et al, 1996) or pyrimethamine for DHFR selection (Donald and Roos, 1993) or phleomycin for ble selection (Messina et al, 1995). All strains were cloned by limited dilution.
The RH strain was transfected with 70 μg of the plasmids: pT8DDmycTgMyoF-neck/tail-HX (linearized with NotI), pT8DDmycTgMyoF-tail-HX (linearized with NotI) or pT8DDMycHisTgMyoF-WD40-HX (linearized with SacI). Ku80-KO (Fox et al, 2009; Huynh and Carruthers, 2009) strain was transfected with 40 μg of the plasmids: 5’MyoF-TATi1-HX-tetS1MycNtMyoF (linearized with NcoI/NotI) or TgMyoF-3Ty (linearized with NcoI). TgMyoF-3Ty was transfected with 40 μg of pT8DDmycGFPTgMyoF-tail-HX-Ble vector (linearized with EcoNI).
Plaque assay
A confluent monolayer of HFFs was infected with freshly egressed parasites and treated±ATc or ±Shld-1 for 7 days before the cells were fixed with PFA/GA. The host cell layer was then stained for 10 min at RT with Giemsa (Sigma-Aldrich GS500).
Intracellular growth assay
TgMyoF-iKO parasites were pretreated for 48 h with or without ATc. DDMyoF-tail-expressing parasites were pretreated for 20 h with or without Shld-1. Parasites were then allowed to grow for 30 h (±ATc or Shld-1) prior to fixation with PFA/GA. IFA using anti-GAP45 antibodies was performed and the number of parasites per vacuole was scored. For each condition, 200 vacuoles were counted. Data are mean values±s.d. from three independent biological experiments.
Daughter cell orientation assay
Parasites were treated±ATc for 48 h or ±Shld-1 for 24 h prior to IFAs using anti-ISP1 and anti-GAP45 antibodies. Formation of rosette structures and daughter cell orientation was assessed. Data are mean values±s.d. from three independent biological experiments. For each condition, 200 parasites were observed.
Loss of apicoplast by antibiotic treatment
Parasites were treated for 30 h with 4 μg/ml of ATc or with EtOH as a control before fixation with PFA/GA. IFA using anti-GAP45 and anti-ISP1 antibodies was performed. For each condition, 200 vacuoles were counted. Data are mean values±s.d. from three independent biological experiments.
Transmission electron microscopy
Freshly egressed parasites were allowed to invade HFF monolayers for 10 h and were treated±Shld-1 for an additional 14 h prior to fixation. Infected host cells were washed with 0.1 M phosphate buffer pH 7.4 and were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer pH 7.4, postfixed in osmium tetroxide, dehydrated in ethanol and treated with propylene oxide prior to embedding in Spurr’s epoxy resin. Thin sections were stained with uranyl acetate and lead citrate prior to examination using a Technai 20 electron microscope (FEI Company). Samples for EM were prepared twice independently and multiple thin sections for each sample were examined.
P. falciparum culture and CD treatment
P. falciparum strain 3D7 was grown in human blood (A+) and RPMI-1640 medium with glutamine (Life Technologies), 0.2% sodium bicarbonate, 25 mM HEPES, 0.2% glucose, 5% human serum and 0.1% Albumax II (Life Technologies). Parasites were synchronized by a double sorbitol treatment as previously described (Lambros and Vanderberg, 1979). To the culture, ±0.3 μM CD was added for 12 h. For western blot analysis, parasites were extracted with saponin lysis following the standard procedure.
P. berghei culture and CD treatment
Transgenic parasites expressing ACP-GFP (Stanway et al, 2009) were derived from clone 2.34 of the ANKA strain. Parasites were gown in CD1 mice. Parasites were matured in vitro O/N±0.3 μM CD in shaking flasks at 37 °C with the same medium as described for P. falciparum. Mice were treated according to the Swiss Federal Act on Animal Protection 1026/3604/2.
Supplementary Material
Acknowledgments
We acknowledge Fabienne Plattner for her initial, critical observation on the actin dependence of apicoplast inheritance. We are grateful to Paco Pino and Arnault Graindorge for their support with the P. falciparum and P. berghei experiments. We thank Thierry Soldati and Jean-Baptiste Marq for their assistance at the confocal and electron microscopy, Hayley Bullen for critical reading of the manuscript and members of the laboratory for constructive discussions. We thank Peter Bradley for providing the anti-ISP1, anti-α-F1-ATPase β-subunit and anti-ATrx1 antibodies, Boris Striepen for the anti-Cpn60 and anti-centrin1, Rebecca Stanway for the P. berghei transgenic ACP-GFP and David Sibley for the T. gondii ADF-iKO strain. WD was supported by the Swiss National Foundation (FN3100A0-116722), and DJ is supported by the Indo-Swiss Joint Research Programme. The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 242095– EVIMalaR.
Author contributions: WD performed the IFAs on PRF-iKO, and the DDFH2-expressing parasites. DJ performed all the other experiments. DS-F and DJ conceived and designed the experiments and wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Agop-Nersesian C, Egarter S, Langsley G, Foth BJ, Ferguson DJ, Meissner M (2010) Biogenesis of the inner membrane complex is dependent on vesicular transport by the alveolate specific GTPase Rab11B. PLoS Pathog 6: e1001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agop-Nersesian C, Naissant B, Ben Rached F, Rauch M, Kretzschmar A, Thiberge S, Menard R, Ferguson DJ, Meissner M, Langsley G (2009) Rab11A-controlled assembly of the inner membrane complex is required for completion of apicomplexan cytokinesis. PLoS Pathog 5: e1000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S, van Dooren GG, Beatty WL, Striepen B (2009) Genetic evidence that an endosymbiont-derived endoplasmic reticulum-associated protein degradation (ERAD) system functions in import of apicoplast proteins. J Biol Chem 284: 33683–33691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andenmatten N, Egarter S, Jackson AJ, Jullien N, Herman JP, Meissner M (2013) Conditional genome engineering in Toxoplasma gondii uncovers alternative invasion mechanisms. Nat Methods 10: 125–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnot DE, Ronander E, Bengtsson DC (2011) The progression of the intra-erythrocytic cell cycle of Plasmodium falciparum and the role of the centriolar plaques in asynchronous mitotic division during schizogony. Int J Parasitol 41: 71–80 [DOI] [PubMed] [Google Scholar]
- Bannister LH, Hopkins JM, Fowler RE, Krishna S, Mitchell GH (2000) A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol Today 16: 427–433 [DOI] [PubMed] [Google Scholar]
- Baum J, Tonkin CJ, Paul AS, Rug M, Smith BJ, Gould SB, Richard D, Pollard TD, Cowman AF (2008) A malaria parasite formin regulates actin polymerization and localizes to the parasite-erythrocyte moving junction during invasion. Cell Host Microbe 3: 188–198 [DOI] [PubMed] [Google Scholar]
- Beck JR, Fung C, Straub KW, Coppens I, Vashisht AA, Wohlschlegel JA, Bradley PJ (2013) A Toxoplasma palmitoyl acyl transferase and the palmitoylated Armadillo Repeat protein TgARO govern apical rhoptry tethering and reveal a critical role for the rhoptries in host cell invasion but not egress. PLoS Pathog 9: e1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JR, Rodriguez-Fernandez IA, Cruz de Leon J, Huynh MH, Carruthers VB, Morrissette NS, Bradley PJ (2010) A novel family of Toxoplasma IMC proteins displays a hierarchical organization and functions in coordinating parasite division. PLoS Pathog 6: e1001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PJ, Sibley LD (2007) Rhoptries: an arsenal of secreted virulence factors. Curr Opin Microbiol 10: 582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breinich MS, Ferguson DJ, Foth BJ, van Dooren GG, Lebrun M, Quon DV, Striepen B, Bradley PJ, Frischknecht F, Carruthers VB, Meissner M (2009) A dynamin is required for the biogenesis of secretory organelles in Toxoplasma gondii. Curr Biol 19: 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CF, Johnsen H, van Dooren GG, Muthalagi M, Lin SS, Bohne W, Fischer K, Striepen B (2010) The toxoplasma apicoplast phosphate translocator links cytosolic and apicoplast metabolism and is essential for parasite survival. Cell Host Microbe 7: 62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera A, Herrmann S, Warszta D, Santos JM, John Peter AT, Kono M, Debrouver S, Jacobs T, Spielmann T, Ungermann C, Soldati-Favre D, Gilberger TW (2012) Dissection of minimal sequence requirements for rhoptry membrane targeting in the malaria parasite. Traffic 13: 1335–1350 [DOI] [PubMed] [Google Scholar]
- Carruthers VB, Sibley LD (1997) Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol 73: 114–123 [PubMed] [Google Scholar]
- Carruthers VB, Tomley FM (2008) Microneme proteins in apicomplexans. Subcell Biochem 47: 33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher W, Plattner F, Carlier MF, Soldati-Favre D (2010) Concerted action of two formins in gliding motility and host cell invasion by Toxoplasma gondii. PLoS Pathog 6: e1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl EL, Rosenthal PJ (2007) Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob Agents Chemother 51: 3485–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl EL, Shock JL, Shenai BR, Gut J, DeRisi JL, Rosenthal PJ (2006) Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum. Antimicrob Agents Chemother 50: 3124–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRocher A, Hagen CB, Froehlich JE, Feagin JE, Parsons M (2000) Analysis of targeting sequences demonstrates that trafficking to the Toxoplasma gondii plastid branches off the secretory system. J Cell Sci 113(Pt 22): 3969–3977 [DOI] [PubMed] [Google Scholar]
- Dobrowolski JM, Carruthers VB, Sibley LD (1997) Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol Microbiol 26: 163–173 [DOI] [PubMed] [Google Scholar]
- Dobrowolski JM, Sibley LD (1996) Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84: 933–939 [DOI] [PubMed] [Google Scholar]
- Donald RG, Carter D, Ullman B, Roos DS (1996) Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J Biol Chem 271: 14010–14019 [DOI] [PubMed] [Google Scholar]
- Donald RG, Roos DS (1993) Stable molecular transformation of Toxoplasma gondii: a selectable dihydrofolate reductase-thymidylate synthase marker based on drug-resistance mutations in malaria. Proc Natl Acad Sci USA 90: 11703–11707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubremetz JF (2007) Rhoptries are major players in Toxoplasma gondii invasion and host cell interaction. Cell Microbiol 9: 841–848 [DOI] [PubMed] [Google Scholar]
- Ferguson DJ, Sahoo N, Pinches RA, Bumstead JM, Tomley FM, Gubbels MJ (2008) MORN1 has a conserved role in asexual and sexual development across the apicomplexa. Eukaryot Cell 7: 698–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichera ME, Roos DS (1997) A plastid organelle as a drug target in apicomplexan parasites. Nature 390: 407–409 [DOI] [PubMed] [Google Scholar]
- Foth BJ, Goedecke MC, Soldati D (2006) New insights into myosin evolution and classification. Proc Natl Acad Sci USA 103: 3681–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox BA, Ristuccia JG, Gigley JP, Bzik DJ (2009) Efficient gene replacements in Toxoplasma gondii strains deficient for nonhomologous end joining. Eukaryot Cell 8: 520–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia ME, Jordan CN, Patel JD, Sheiner L, Demerly JL, Fellows JD, de Leon JC, Morrissette NS, Dubremetz JF, Striepen B (2012) Cell division in Apicomplexan parasites is organized by a homolog of the striated rootlet fiber of algal flagella. PLoS Biol 10: e1001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenal K, Polonais V, Marq JB, Stratmann R, Limenitakis J, Soldati-Favre D (2010) Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe 8: 343–357 [DOI] [PubMed] [Google Scholar]
- Gerald N, Mahajan B, Kumar S (2011) Mitosis in the human malaria parasite Plasmodium falciparum. Eukaryot Cell 10: 474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson MT (2000) The plastid in Apicomplexa: what use is it? Int J Parasitol 30: 1053–1070 [DOI] [PubMed] [Google Scholar]
- Griffiths GM, Tsun A, Stinchcombe JC (2010) The immunological synapse: a focal point for endocytosis and exocytosis. J Cell Biol 189: 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels MJ, Vaishnava S, Boot N, Dubremetz JF, Striepen B (2006) A MORN-repeat protein is a dynamic component of the Toxoplasma gondii cell division apparatus. J Cell Sci 119: 2236–2245 [DOI] [PubMed] [Google Scholar]
- Hartmann J, Hu K, He CY, Pelletier L, Roos DS, Warren G (2006) Golgi and centrosome cycles in Toxoplasma gondii. Mol Biochem Parasitol 145: 125–127 [DOI] [PubMed] [Google Scholar]
- He CY, Shaw MK, Pletcher CH, Striepen B, Tilney LG, Roos DS (2001a) A plastid segregation defect in the protozoan parasite Toxoplasma gondii. EMBO J 20: 330–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CY, Striepen B, Pletcher CH, Murray JM, Roos DS (2001b) Targeting and processing of nuclear-encoded apicoplast proteins in plastid segregation mutants of Toxoplasma gondii. J Biol Chem 276: 28436–28442 [DOI] [PubMed] [Google Scholar]
- Herm-Gotz A, Agop-Nersesian C, Munter S, Grimley JS, Wandless TJ, Frischknecht F, Meissner M (2007) Rapid control of protein level in the apicomplexan Toxoplasma gondii. Nat Methods 4: 1003–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe HC, Ngo HM, Yang M, Joiner KA (2000) Targeting to rhoptry organelles of Toxoplasma gondii involves evolutionarily conserved mechanisms. Nat Cell Biol 2: 449–456 [DOI] [PubMed] [Google Scholar]
- Hu K, Mann T, Striepen B, Beckers CJ, Roos DS, Murray JM (2002) Daughter cell assembly in the protozoan parasite Toxoplasma gondii. Mol Biol Cell 13: 593–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh MH, Carruthers VB (2009) Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot Cell 8: 530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janouskovec J, Horak A, Obornik M, Lukes J, Keeling PJ (2010) A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids. Proc Natl Acad Sci USA 107: 10949–10954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Delwiche CF, Denny PW, Tilney LG, Webster P, Wilson RJ, Palmer JD, Roos DS (1997) A plastid of probable green algal origin in Apicomplexan parasites. Science 275: 1485–1489 [DOI] [PubMed] [Google Scholar]
- Kunda P, Baum B (2009) The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol 19: 174–179 [DOI] [PubMed] [Google Scholar]
- Lambros C, Vanderberg JP (1979) Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65: 418–420 [PubMed] [Google Scholar]
- Lang-Unnasch N, Reith ME, Munholland J, Barta JR (1998) Plastids are widespread and ancient in parasites of the phylum Apicomplexa. Int J Parasitol 28: 1743–1754 [DOI] [PubMed] [Google Scholar]
- Lim L, Linka M, Mullin KA, Weber AP, McFadden GI (2010) The carbon and energy sources of the non-photosynthetic plastid in the malaria parasite. FEBS Lett 584: 549–554 [DOI] [PubMed] [Google Scholar]
- Lorestani A, Sheiner L, Yang K, Robertson SD, Sahoo N, Brooks CF, Ferguson DJ, Striepen B, Gubbels MJ (2010) A Toxoplasma MORN1 null mutant undergoes repeated divisions but is defective in basal assembly, apicoplast division and cytokinesis. PLoS One 5: e12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan B, Selvapandiyan A, Gerald NJ, Majam V, Zheng H, Wickramarachchi T, Tiwari J, Fujioka H, Moch JK, Kumar N, Aravind L, Nakhasi HL, Kumar S (2008) Centrins, cell cycle regulation proteins in human malaria parasite Plasmodium falciparum. J Biol Chem 283: 31871–31883 [DOI] [PubMed] [Google Scholar]
- Manneville JB, Etienne-Manneville S (2006) Positioning centrosomes and spindle poles: looking at the periphery to find the centre. Biol Cell 98: 557–565 [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Kikuchi T, Kita K, Kojima S, Kuroiwa T (2001) Large amounts of apicoplast nucleoid DNA and its segregation in Toxoplasma gondii. Protoplasma 218: 180–191 [DOI] [PubMed] [Google Scholar]
- McFadden GI, Reith ME, Munholland J, Lang-Unnasch N (1996) Plastid in human parasites. Nature 381: 482. [DOI] [PubMed] [Google Scholar]
- Mehta S, Sibley LD (2010) Toxoplasma gondii actin depolymerizing factor acts primarily to sequester G-actin. J Biol Chem 285: 6835–6847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S, Sibley LD (2011) Actin depolymerizing factor controls actin turnover and gliding motility in Toxoplasma gondii. Mol Biol Cell 22: 1290–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner M, Brecht S, Bujard H, Soldati D (2001) Modulation of myosin A expression by a newly established tetracycline repressor-based inducible system in Toxoplasma gondii. Nucleic Acids Res 29: E115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner M, Schluter D, Soldati D (2002) Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298: 837–840 [DOI] [PubMed] [Google Scholar]
- Messina M, Niesman I, Mercier C, Sibley LD (1995) Stable DNA transformation of Toxoplasma gondii using phleomycin selection. Gene 165: 213–217 [DOI] [PubMed] [Google Scholar]
- Montoya JG, Liesenfeld O (2004) Toxoplasmosis. Lancet 363: 1965–1976 [DOI] [PubMed] [Google Scholar]
- Morrissette NS, Sibley LD (2002a) Cytoskeleton of apicomplexan parasites. Microbiol Mol Biol Rev 66: 21–38table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissette NS, Sibley LD (2002b) Disruption of microtubules uncouples budding and nuclear division in Toxoplasma gondii. J Cell Sci 115: 1017–1025 [DOI] [PubMed] [Google Scholar]
- Mueller C, Klages N, Jacot D, Santos JM, Cabrera A, Gilberger TW, Dubremetz JF, Soldati-Favre D (2013) The Toxoplasma protein ARO mediates the apical positioning of rhoptry organelles, a prerequisite for host cell invasion. Cell Host Microbe 13: 289–301 [DOI] [PubMed] [Google Scholar]
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379: 413–431 [DOI] [PubMed] [Google Scholar]
- Ngo HM, Yang M, Paprotka K, Pypaert M, Hoppe H, Joiner KA (2003) AP-1 in Toxoplasma gondii mediates biogenesis of the rhoptry secretory organelle from a post-Golgi compartment. J Biol Chem 278: 5343–5352 [DOI] [PubMed] [Google Scholar]
- Nishi M, Hu K, Murray JM, Roos DS (2008) Organellar dynamics during the cell cycle of Toxoplasma gondii. J Cell Sci 121: 1559–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou G, Stuurman N, D'Ambrosio M, Vale RD (2010) Polarized myosin produces unequal-size daughters during asymmetric cell division. Science 330: 677–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham M (2011) Coiled coils and SAH domains in cytoskeletal molecular motors. Biochem Soc Trans 39: 1142–1148 [DOI] [PubMed] [Google Scholar]
- Pelletier L, Stern CA, Pypaert M, Sheff D, Ngo HM, Roper N, He CY, Hu K, Toomre D, Coppens I, Roos DS, Joiner KA, Warren G (2002) Golgi biogenesis in Toxoplasma gondii. Nature 418: 548–552 [DOI] [PubMed] [Google Scholar]
- Pino P, Foth BJ, Kwok LY, Sheiner L, Schepers R, Soldati T, Soldati-Favre D (2007) Dual targeting of antioxidant and metabolic enzymes to the mitochondrion and the apicoplast of Toxoplasma gondii. PLoS Pathog 3: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, Soldati-Favre D (2008) Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe 3: 77–87 [DOI] [PubMed] [Google Scholar]
- Polonais V, Javier Foth B, Chinthalapudi K, Marq JB, Manstein DJ, Soldati-Favre D, Frenal K (2011) Unusual anchor of a motor complex (MyoD-MLC2) to the plasma membrane of Toxoplasma gondii. Traffic 12: 287–300 [DOI] [PubMed] [Google Scholar]
- Reiff SB, Vaishnava S, Striepen B (2012) The HU protein is important for apicoplast genome maintenance and inheritance in Toxoplasma gondii. Eukaryot Cell 11: 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininger L, Wilkes JM, Bourgade H, Miranda-Saavedra D, Doerig C (2011) An essential Aurora-related kinase transiently associates with spindle pole bodies during Plasmodium falciparum erythrocytic schizogony. Mol Microbiol 79: 205–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GC (2010) Spindle assembly: more than just microtubules. Curr Biol 20: R364–R366 [DOI] [PubMed] [Google Scholar]
- Roos DS, Crawford MJ, Donald RG, Kissinger JC, Klimczak LJ, Striepen B (1999) Origin, targeting, and function of the apicomplexan plastid. Curr Opin Microbiol 2: 426–432 [DOI] [PubMed] [Google Scholar]
- Seeber F, Soldati-Favre D (2010) Metabolic pathways in the apicoplast of apicomplexa. Int Rev Cell Mol Biol 281: 161–228 [DOI] [PubMed] [Google Scholar]
- Shaw MK, Compton HL, Roos DS, Tilney LG (2000) Microtubules, but not actin filaments, drive daughter cell budding and cell division in Toxoplasma gondii. J Cell Sci 113(Pt 7): 1241–1254 [DOI] [PubMed] [Google Scholar]
- Sheahan MB, Rose RJ, McCurdy DW (2004) Organelle inheritance in plant cell division: the actin cytoskeleton is required for unbiased inheritance of chloroplasts, mitochondria and endoplasmic reticulum in dividing protoplasts. Plant J 37: 379–390 [DOI] [PubMed] [Google Scholar]
- Sheffield HG, Melton ML (1968) The fine structure and reproduction of Toxoplasma gondii. J Parasitol 54: 209–226 [PubMed] [Google Scholar]
- Skillman KM, Daher W, Ma CI, Soldati-Favre D, Sibley LD (2012) Toxoplasma gondii profilin acts primarily to sequester G-actin while formins efficiently nucleate actin filament formation in vitro. Biochemistry 51: 2486–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloves PJ, Delhaye S, Mouveaux T, Werkmeister E, Slomianny C, Hovasse A, Dilezitoko Alayi T, Callebaut I, Gaji RY, Schaeffer-Reiss C, Van Dorsselear A, Carruthers VB, Tomavo S (2012) Toxoplasma sortilin-like receptor regulates protein transport and is essential for apical secretory organelle biogenesis and host infection. Cell Host Microbe 11: 515–527 [DOI] [PubMed] [Google Scholar]
- Soldati D, Boothroyd JC (1993) Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science 260: 349–352 [DOI] [PubMed] [Google Scholar]
- Speer CA, Dubey JP (1999) Ultrastructure of shizonts and merozoites of Sarcocystis falcatula in the lungs of budgerigars (Melopsittacus undulatus). J Parasitol 85: 630–637 [PubMed] [Google Scholar]
- Stanway RR, Mueller N, Zobiak B, Graewe S, Froehlke U, Zessin PJ, Aepfelbacher M, Heussler VT (2011) Organelle segregation into Plasmodium liver stage merozoites. Cell Microbiol 13: 1768–1782 [DOI] [PubMed] [Google Scholar]
- Stanway RR, Witt T, Zobiak B, Aepfelbacher M, Heussler VT (2009) GFP-targeting allows visualization of the apicoplast throughout the life cycle of live malaria parasites. Biol Cell 101: 415–430415 p following 430 [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Griffiths GM (2007) Secretory mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol 23: 495–517 [DOI] [PubMed] [Google Scholar]
- Striepen B, Crawford MJ, Shaw MK, Tilney LG, Seeber F, Roos DS (2000) The plastid of Toxoplasma gondii is divided by association with the centrosomes. J Cell Biol 151: 1423–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepen B, Jordan CN, Reiff S, van Dooren GG (2007) Building the perfect parasite: cell division in apicomplexa. PLoS Pathog 3: e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N, Wada M (2007) Chloroplast photorelocation movement mediated by phototropin family proteins in green plants. Biological chemistry 388: 927–935 [DOI] [PubMed] [Google Scholar]
- Vaishnava S, Morrison DP, Gaji RY, Murray JM, Entzeroth R, Howe DK, Striepen B (2005) Plastid segregation and cell division in the apicomplexan parasite Sarcocystis neurona. J Cell Sci 118: 3397–3407 [DOI] [PubMed] [Google Scholar]
- Vaishnava S, Striepen B (2006) The cell biology of secondary endosymbiosis--how parasites build, divide and segregate the apicoplast. Mol Microbiol 61: 1380–1387 [DOI] [PubMed] [Google Scholar]
- van Dooren GG, Marti M, Tonkin CJ, Stimmler LM, Cowman AF, McFadden GI (2005) Development of the endoplasmic reticulum, mitochondrion and apicoplast during the asexual life cycle of Plasmodium falciparum. Mol Microbiol 57: 405–419 [DOI] [PubMed] [Google Scholar]
- van Dooren GG, Reiff SB, Tomova C, Meissner M, Humbel BM, Striepen B (2009) A novel dynamin-related protein has been recruited for apicoplast fission in Toxoplasma gondii. Curr Biol 19: 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dooren GG, Su V, D'Ombrain MC, McFadden GI (2002) Processing of an apicoplast leader sequence in Plasmodium falciparum and the identification of a putative leader cleavage enzyme. J Biol Chem 277: 23612–23619 [DOI] [PubMed] [Google Scholar]
- Vaughan S, Dawe HR (2011) Common themes in centriole and centrosome movements. Trends Cell Biol 21: 57–66 [DOI] [PubMed] [Google Scholar]
- Wada M, Suetsugu N (2004) Plant organelle positioning. Curr Opin Plant Biol 7: 626–631 [DOI] [PubMed] [Google Scholar]
- Waller RF, Keeling PJ, Donald RG, Striepen B, Handman E, Lang-Unnasch N, Cowman AF, Besra GS, Roos DS, McFadden GI (1998) Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc Natl Acad Sci USA 95: 12352–12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller RF, Reed MB, Cowman AF, McFadden GI (2000) Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J 19: 1794–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman LS (2006) Organelles on the move: insights from yeast vacuole inheritance. Nat Rev Mol Cell Biol 7: 243–252 [DOI] [PubMed] [Google Scholar]
- Wetzel DM, Hakansson S, Hu K, Roos D, Sibley LD (2003) Actin filament polymerization regulates gliding motility by apicomplexan parasites. Mol Biol Cell 14: 396–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner J, Reichenberg A, Heinrich S, Schlitzer M, Jomaa H (2008) The plastid-like organelle of apicomplexan parasites as drug target. Curr Pharm Des 14: 855–871 [DOI] [PubMed] [Google Scholar]
- Wilson RJM, Denny PW, Preiser PR, Rangachari K, Roberts K, Roy A, Whyte A, Strath M, Moore DJ, Moore PW, Williamson DH (1996) Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. J Mol Biol 261: 155–172 [DOI] [PubMed] [Google Scholar]
- Yeh E, DeRisi JL (2011) Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol 9: e1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Marchewka MJ, Keithly JS (2000) Cryptosporidium parvum appears to lack a plastid genome. Microbiology 146(Pt 2): 315–321 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.