Abstract
Although past studies demonstrate that altered serotonin (5-HT) signaling is present in adults with idiopathic pulmonary arterial hypertension, whether serotonin contributes to the pathogenesis of persistent pulmonary hypertension of the newborn (PPHN) is unknown. We hypothesized that 5-HT contributes to increased pulmonary vascular resistance (PVR) in a sheep model of PPHN and that selective 5-HT reuptake inhibitor (SSRI) treatment increases PVR in this model. We studied the hemodynamic effects of 5-HT, ketanserin (5-HT2A receptor antagonist), and sertraline, an SSRI, on pulmonary hemodynamics of the late gestation fetal sheep with PPHN caused by prolonged constriction of the ductus arteriosis. Brief intrapulmonary infusions of 5-HT increased PVR from 1.0 ± 0.07 (baseline) to 1.4 ± 0.22 mmHg/ml per minute of treatment (P < 0.05). Ketanserin decreased PVR from 1.1 ± 0.15 (baseline) to 0.82 ± 0.09 mmHg/ml per minute of treatment (P < 0.05). Sertraline increased PVR from 1.1 ± 0.17 (baseline) to 1.4 ± 0.17 mmHg/ml per minute of treatment (P = 0.01). In addition, we studied 5-HT production and activity in vitro in experimental PPHN. Compared with controls, pulmonary artery endothelial cells from fetal sheep with PPHN exhibited increased expression of tryptophan hydroxylase 1 and 5-HT production by twofold and 56%, respectively. Compared with controls, 5-HT2A R expression was increased in lung homogenates and pulmonary artery smooth muscle cell lysates by 35% and 32%, respectively. We concluded that increased 5-HT contributes to high PVR in experimental PPHN through activation of the 5-HT2A receptor and that SSRI infusion further increases PVR in this model.
Keywords: serotonin, selective serotonin reuptake inhibitors, pulmonary hypertension, newborn, ketanserin
in utero, pulmonary vascular resistance (PVR) is greater than systemic vascular resistance (1, 62). At birth, PVR rapidly falls, allowing for an 8–10-fold increase in pulmonary blood flow (8, 11). Failure of PVR to decrease at birth causes hypoxemia due to extrapulmonary shunting of blood at the ductus arteriosis and foramen ovale, resulting in the clinical syndrome known as pulmonary hypertension of the newborn (PPHN) (41). Although the pathogenesis of PPHN is poorly understood, it is believed to be multifactorial in origin and includes altered NO-cGMP, endothelin (ET), and Rho kinase (ROCK) signaling, resulting in abnormal vascular reactivity, remodeling, and growth (2, 20, 21, 24, 58).
PPHN is a clinical syndrome, associated with a number of neonatal conditions, including meconium aspiration, sepsis, and abnormalities in lung structure (10, 54). In addition to these neonatal conditions, the use of maternal medications has been linked to the development of PPHN (6, 64, 65). Recently, maternal use of selective serotonin (5-hydroxytryptamine; 5-HT) reuptake inhibitors (SSRIs) after the 20th wk of gestation has been associated with the development of PPHN (9, 33, 37). Despite this association, little is known about serotonin signaling in the fetal and newborn pulmonary circulation. Although it is not completely clear how SSRIs may cause PPHN, SSRIs are known to readily cross the placenta, which leads to the possibility that SSRIs can directly affect the fetus independent of maternal effects (28, 59). We have previously shown that acute intrapulmonary infusions of SSRIs in chronically instrumented fetal sheep cause potent and sustained pulmonary vasoconstriction (12). However, several studies demonstrate that SSRI treatment attenuates pulmonary vascular remodeling and improves survival in adult rodent models of pulmonary hypertension (25, 42, 48, 63). Whether or not SSRI treatment causes pulmonary vasodilation or further elevates PVR in the setting of neonatal pulmonary hypertension is unknown.
5-HT is primarily produced in the enterochromaffin cells of the intestine. Circulating levels of 5-HT are extremely low, as 5-HT is primarily stored in platelets, which pool and release 5-HT via the serotonin transporter (SERT). Recently, it has been recognized that the lung synthesizes 5-HT in pulmonary neuroendocrine and pulmonary artery endothelial cells (PAECs) from tryptophan via the enzyme tryptophan hydroxylase 1 (Tph1) (17). Serotonin levels are increased in patients with idiopathic pulmonary arterial hypertension (iPAH) as well as in rodent models of PAH (7, 17, 29, 38). To date, 14 receptors for 5-HT have been identified. The 5-HT 1B, 2A, and 2B receptors, as well as the SERT are all expressed in the adult lung, and alterations in their signaling have been linked to the development of PAH (26, 36, 40, 44, 45, 49). Serotonin intracellular signaling is complex and incompletely understood but is known to include activation of the mitogen activated protein kinase pathway, generation of reactive oxygen species, and ROCK activation (23, 43, 66).
We have previously shown that the 5-HT2A receptor is expressed in fetal ovine lung and that intrapulmonary infusions of ketanserin, the 5-HT2A receptor antagonist, increases pulmonary blood flow in chronically instrumented fetal sheep. These findings indicate that 5-HT contributes to high PVR in the normal fetus. Based on our prior work and the contribution of serotonin to the development of PAH in adults, we hypothesized that 5-HT and SSRIs would increase PVR and that blockade of the 5-HT2A receptor may increase pulmonary blood flow in fetal sheep with experimental PPHN. We further hypothesized that increased pulmonary serotonin production and activity contribute to the pathogenesis of PPHN in this model.
MATERIALS AND METHODS
Pregnant, mixed-breed (Colombia-Rambouillet) ewes were used in this study. All procedures and protocols were reviewed and approved by the Animal Care and Use Committee of the University of Colorado Denver and followed the Guide for the Care and Use of Laboratory Animals established by the National Research Council.
Fetal Surgical Preparation
Surgery was performed between 124 and 129 days gestation (full term = 147 days) according to previously published methods (3). Under isofluorane inhalational anesthesia, the left fetal forelimb was exposed through a hysterotomy and a left thoracotomy was performed. Polyvinyl catheters (20 gauge) were placed in the left axillary artery and vein and advanced in the ascending aorta and superior vena cava, respectively. Using a 16-gauge intravenous placement unit (Angiocath; Travenol, Deerfield, IL), a 22-gauge catheter was placed through purse-string sutures in the left pulmonary artery (LPA) to allow for selective drug infusions. A 14-gauge intravenous placement unit (Angiocath) was used to place 20-gauge catheters in the main pulmonary artery (MPA) and left atrium. After gentle, blunt dissection of the bifurcation of the MPA, a flow transducer (Transonic Systems, Ithaca, NY) was placed around the LPA to measure blood flow to the left lung (QLPA). A cotton umbilical tie was placed around the ductus arteriosus and tied to cause constriction.
Western Blot Analysis
Western blot analysis for pulmonary artery smooth muscle cell (PASMC) and PAEC expression of Tph1 and 5-HT 2A R was performed by standard methods. Membranes were incubated overnight at 4°C with antibodies raised against the 5-HT2A receptor (catalog no. sc-32538; Santa Cruz Biotechnology, Santa Cruz, CA; dilution 1:200), or Tph1 (catalog no. ab-78969; Abcam, Cambridge, MA; dilution 1:1,000). The membranes for 5-HT2A R were washed and incubated for 1 h at room temperature with donkey anti-goat IgG-horseradish peroxidase (HRP) (catalog no. sc-2033; Santa Cruz Biotechnology; 1:4,000 dilution). The membranes for Tph1 were washed and incubated for 1 h at room temperature with goat anti rabbit HRP (catalog no. Biorad 1706515; Bio-Rad, Hercules, CA; 1:2000 dilution). Immunocomplexes were visualized using the Enhanced Chemiluminescence Plus kit and identified by molecular weight as designated by the manufacturer. Membranes were stripped and reprobed with an antibody to β-actin (catalog no. A5316; Sigma, St. Louis, MO). Densitometry was performed using NIH Image J software. Changes in protein expression were analyzed after normalization for β-actin expression.
Serotonin ELISA Assay
ELISA was performed using the GenWay 5-HT ELISA kit (catalog no. 40–371-25002; GenWay Biotech, San Diego, CA), according to the manufacturer's instructions. Briefly, PAECs from control (N = 3) and PPHN (N = 4) lambs were grown on 150-mm dishes in DMEM supplemented with 10% fetal bovine serum to 80–90% confluence. The supernatant was collected and stored in −20°C and cell number was recorded. The 5-HT ELISA assay was performed in triplicate, and 5-HT signal was determined by measurement of absorbance at 405 nm using a microplate spectrophotometer. Differences in absorbance between normal and PPHN PAECs were measured and quantified.
Drug Preparation
A solution of 5-HT, serotonin creatinine sulfate monohydrate complex (3 μg/ml, Sigma H7752) was made immediately before each study by dissolving the drug in normal saline. Ketanserin (50 mg/ml DMSO, Sigma S006) solution was made immediately before each experiment. Sertraline hydrochloride (20 mg/ml DMSO, Sigma S6319) was made and stored at −20°C.
Study Design
Physiological studies were performed at least 5 days after surgery. During each study, pulmonary arterial, aortic, and left atrial pressures were measured by connecting externalized catheters to computer-driven pressure transducers (model MP100A; Biopac Systems, Santa Barbara, CA). Pressure measurements were referenced to simultaneously recorded amniotic pressure. The flow transducer was connected to an internally calibrated flowmeter (Transonics Systems) to measure blood flow to the left lung (QLPA). Before infusion of study drugs, a 30-min period of stable baseline hemodynamics was established. Hemodynamic variables, including main pulmonary artery pressure (MPAP), left atrial pressure (LAP), aortic pressure (AoP), and left pulmonary artery blood flow (QLPA), were measured continuously for the duration of each study protocol and recorded every 10 min. Left lung PVR was calculated as follows: PVR = (MPAP − LAP)/QLPA. Heart rate (HR) was determined from phasic pressure traces. Arterial blood gas measurements were obtained before and after each drug infusion and included pH, Pco2, and Po2 (model ABL 800; Radiometer, Copenhagen, Denmark).
Experimental Design
Protocol 1: pulmonary hemodynamic effects of 5-HT in experimental PPHN.
The purpose of this study was to investigate the pulmonary effects of selective 5-HT infusions. Baseline hemodynamic measurements were recorded every 10 min for QLPA, MPAP, AoP, LAP, and HR. After baseline measurements were stable for a 30-min period, 5-HT (12–20 μg) was infused into the LPA over 20 min. Hemodynamic measurements were recorded every 10 min for 40 min after the infusion concluded. Arterial blood gas tensions were obtained at baseline and at the conclusion of each infusion.
Protocol 2: pulmonary hemodynamic effects of ketanserin, the 5-HT2A receptor blocker.
The purpose of these studies was to investigate whether blockade of the 5-HT2A receptor decreases PVR and increases pulmonary blood flow. Baseline hemodynamic measurements were recorded every 10 min for QLPA, MPAP, AoP, and HR. After baseline measurements were stable for a 30-min period, ketanserin (20 mg) was infused into the LPA over 20 min. Hemodynamic measurements were recorded every 10 min for 40 min after the infusion concluded. Arterial blood gas tensions were obtained at baseline and at the conclusion of each infusion.
Protocol 3: pulmonary hemodynamic effects of the SSRI sertraline in experimental PPHN.
The purpose of these studies was to determine the pulmonary hemodynamic effects of SSRIs in a model of PPHN. Baseline hemodynamic measurements were recorded every 10 min for QLPA, MPAP, AoP, LAP, and HR. After baseline measurements were stable for a 30-min period, sertraline (10 mg) was infused into the LPA over 40 min. Arterial blood gas tensions were obtained at baseline and at the conclusion of each infusion.
Protocol 4: effects of ROCK inhibition on the hemodynamic response of exogenous 5-HT administration.
The purpose of this protocol was to determine whether in this model of PPHN serotonin induces pulmonary vasoconstriction through ROCK activation. The hemodynamic response of 5-HT (20 μg) infusion alone was compared with the infusion of fasudil (100 μg) alone and the combination of fasudil (100 μg) and 5-HT (20 μg).
Protocol 5: 5-HT production and Tph1 protein content in fetal PAEC isolated from control and PPHN fetal sheep.
The purpose of this study was to determine whether 5-HT synthesis is increased in PAEC isolated from fetal sheep with experimental PPHN. Using ELISA, we measured 5-HT levels in the supernatant from control (N = 3) and PPHN (N = 4) PAECs grown to 80–90% confluence in 150-mm dishes. 5-HT content was normalized to cell number. To determine whether Tph1 protein is increased in experimental PPHN, PAEC lysates were collected from 80–90% confluent cells grown between passages 2 and 4 from control (N = 9) animals and animals with experimental PPHN (N = 9). Tph1 protein content was measured using Western blot, and the values were normalized to β-actin.
Protocol 6: expression of 5-HT2A R in whole lung and isolated PASMC from control and PPHN fetal sheep.
Whole lung lysates were collected from the ovine fetus (gestational age 136–143 days) with (N = 4) and without (N = 4) experimental PPHN. PASMC cell lysates were collected from 80–90% confluent cells between passages 2 and 4 from control animals (N = 6) and animals with experimental PPHN (N = 6). 5-HT2A R protein expression was measured by Western blot analysis, and values were normalized for β-actin expression.
Statistical Analysis
Statistical analysis for hemodynamic variables was performed using SAS version 9.2 (SAS Institute). Hemodynamic variables over time were compared using repeated-measures ANOVA with Fishers least-squares difference for individual means comparison. Data measured once were analyzed using unpaired t-tests and paired t-tests. Data are presented as means ± SE. The significance level was set at P < 0.05.
RESULTS
Protocol 1: Pulmonary Hemodynamic Effects of Brief Serotonin Infusion in Experimental PPHN
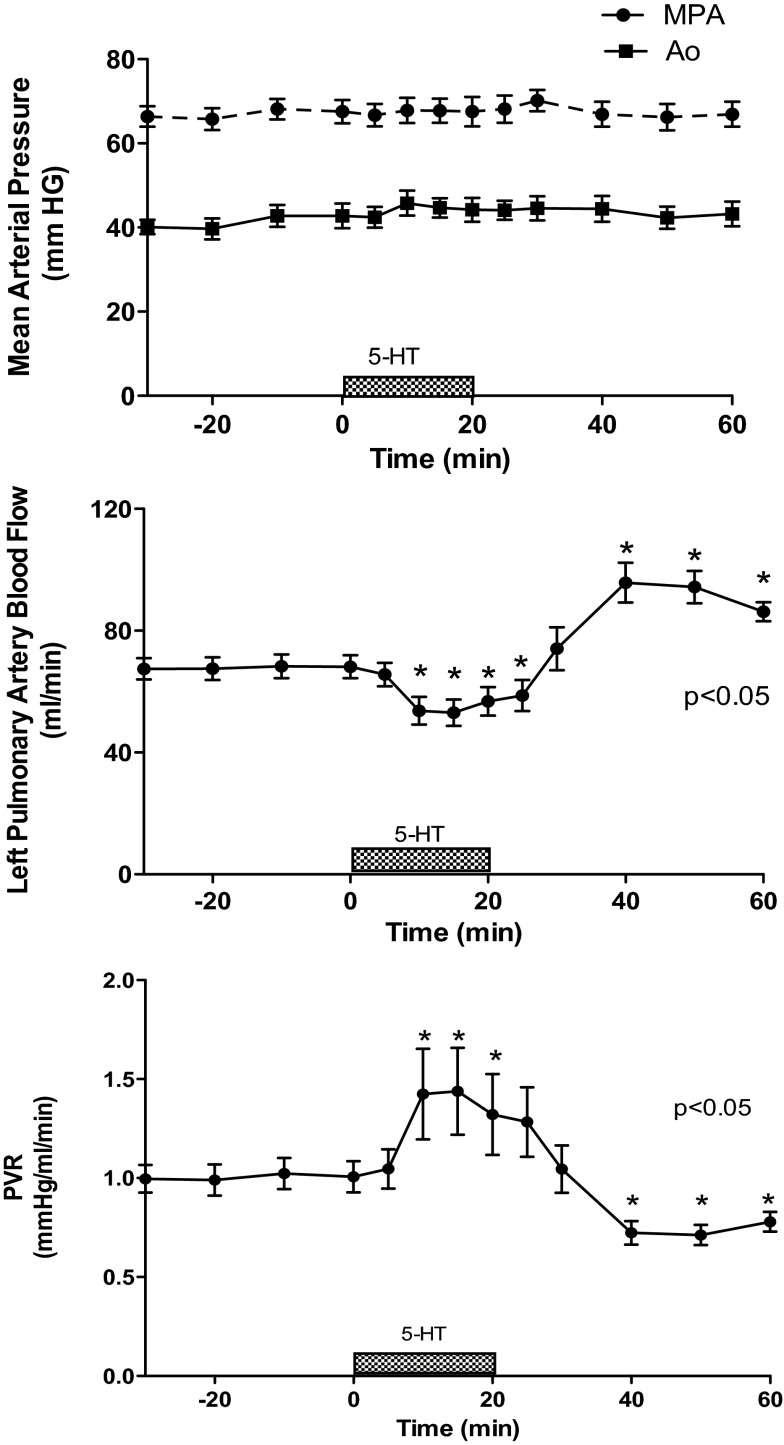
Brief intrapulmonary infusions of 5-HT (12–20 μg) had no effect on MPAP, 67 ± 2.7 mmHg (baseline) to 67 ± 2.8 mmHg, or AoP, 42 ± 2.9 mmHg (baseline) to 44 ± 2.2 mmHg (treatment). Infusions of 5-HT decreased pulmonary blood flow (QLPA) by 15% (67 ± 4 vs. 57 ± 5 ml/min, P < 0.05) and increased PVR by 40% from baseline (1.0 ± 0.07 vs. 1.4 ± 0.22 mmHg/ml per min, P < 0.05) (Fig. 1). The effects of 5-HT were short-lived as QLPA and PVR returned to baseline shortly after the end of the infusion. HR decreased from 176 ± 4 (baseline) to 159 ± 4 beats per minute (bpm) at the end of the infusion (P < 0.05), and pH decreased from 7.37 ± 0.02 to 7.36 ± 0.02, P < 0.05. PaO2 and PaCO2 did not change in response to 5-HT.
Fig. 1.
Serotonin (5-HT) causes pulmonary vasoconstriction in experimental persistent pulmonary hypertension of the newborn (PPHN). Hemodynamic response to 5-HT (12–20 μg). MPA, mean pulmonary artery; Ao, aortic system; PVR, pulmonary vascular resistance. *P < 0.05 vs. baseline (N = 13).
Protocol 2: Pulmonary Hemodynamic Effects of the 5-HT2A Receptor Blocker, Ketanserin
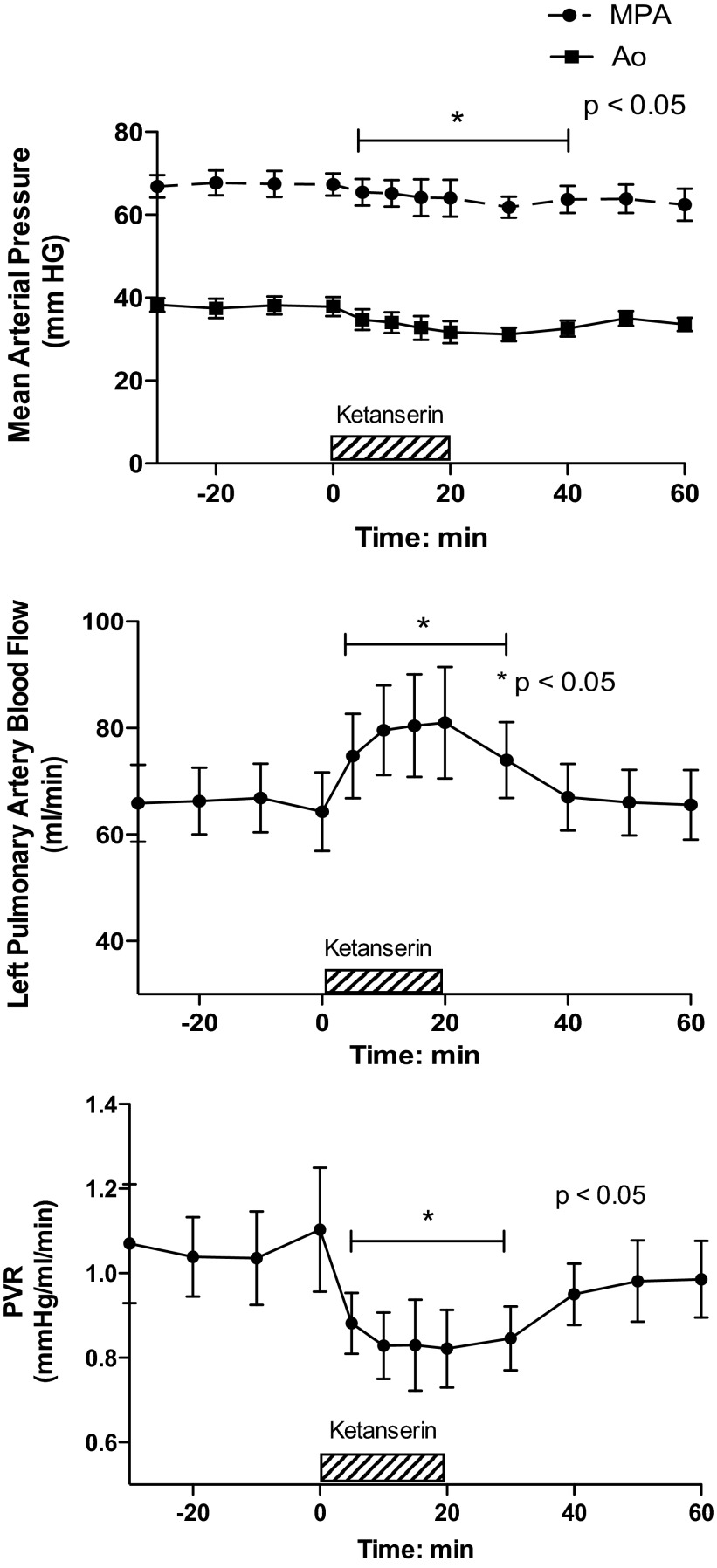
Brief intrapulmonary infusions of ketanserin (20 mg), a 5-HT2A receptor antagonist, decreased MPAP from 67 ± 2.7 to 65 ± 3.2 mmHg, P < 0.05 (Fig. 2). AoP decreased during the infusion (from 38 ± 2.3 to 34 ± 2.5 mmHg, P < 0.05) but returned to baseline values for the remainder of the study. Ketanserin increased pulmonary blood flow (QLPA) by 27% (64 ± 7 vs. 81 ± 10 ml/min, P < 0.05, Fig. 2) and decreased PVR by 26%, from 1.1 ± 0.15 at baseline to 0.82 ± 0.09 mmHg/ml per min (P = 0.05 Fig. 2). Ketanserin did not change HR or arterial blood gas tensions.
Fig. 2.
Ketanserin decreases PVR in experimental PPHN. Hemodynamic response to ketanserin, 5-HT 2A receptor antagonist. *P < 0.05 vs. baseline (N = 7).
Protocol 3: Pulmonary Hemodynamic Effects of the SSRI Sertraline in Experimental PPHN
Pulmonary artery pressure (67 ± 5.4 to 75 ± 4 mmHg; P < 0.05) and systemic pressures (39 ± 3.1 to 44 ± 3.1 mmHg; P < 0.05) were significantly increased following the infusion of sertraline (Fig. 3). Infusions of sertraline decreased QLPA by 7% (65 ± 5.9 vs. 61 ± 4.6 ml/min, P < 0.05) (Fig. 3) and increased PVR by 30% (Fig. 3) from 1.08 ± 0.17 (baseline) to 1.40 ± 0.17 mmHg/ml per min (40 min), P < 0.05. The pulmonary vasoconstrictor response to sertraline was sustained for at least 80 min after the completion of the infusion. Acute infusions of sertraline did not alter HR (169 ± 9 at baseline vs. 187 ± 13 bpm). Direct intrapulmonary infusions of sertraline decreased pH (7.4 ± 0.008 vs. 7.2 ± 0.016; P < 0.001) and increased Pco2 (48 ± 2 vs. 55 ± 2.2 Torr; P < 0.05). Acute infusions of sertraline did not alter Po2 (18 ± 1.4 vs. 16 ± 1.1 Torr).
Fig. 3.
Sertraline increases PVR in experimental PPHN. Hemodynamic response to the selective serotonin reuptake inhibitor sertraline. *P < 0.05 vs. baseline (N = 7).
Protocol 4: Effects of ROCK Inhibition on the Hemodynamic Response of Exogenous 5-HT Administration
The hemodynamic response of 5-HT (20 μg) infusion alone was compared with the infusion of fasudil (100 μg) alone and the combination of fasudil (100 μg) and 5-HT (20 μg). The infusion of 5-HT alone increased PVR by 30% (Fig. 4). The infusion of fasudil alone decreased PVR by 30% (Fig. 4). However, in combination with 5-HT, fasudil had no effect on PVR. Acute intrapulmonary infusions of 5-HT in combination with fasudil resulted in a 38% increase in PVR (Fig. 4).
Fig. 4.
Fasudil does not inhibit 5-HT-induced pulmonary vasoconstriction. Pulmonary vascular response to the infusion of 5-HT alone, fasudil (Rho kinase inhibitor) alone, or 5-HT in combination with fasudil, expressed as percentage of change from baseline PVR. Infusions of 5-HT resulted in a 30% increase in PVR; this was not attenuated by the coadministration of fasudil.
Protocol 5: Tph1 Expression and 5-HT Content in Fetal PAECs Isolated from Normal and PPHN Sheep
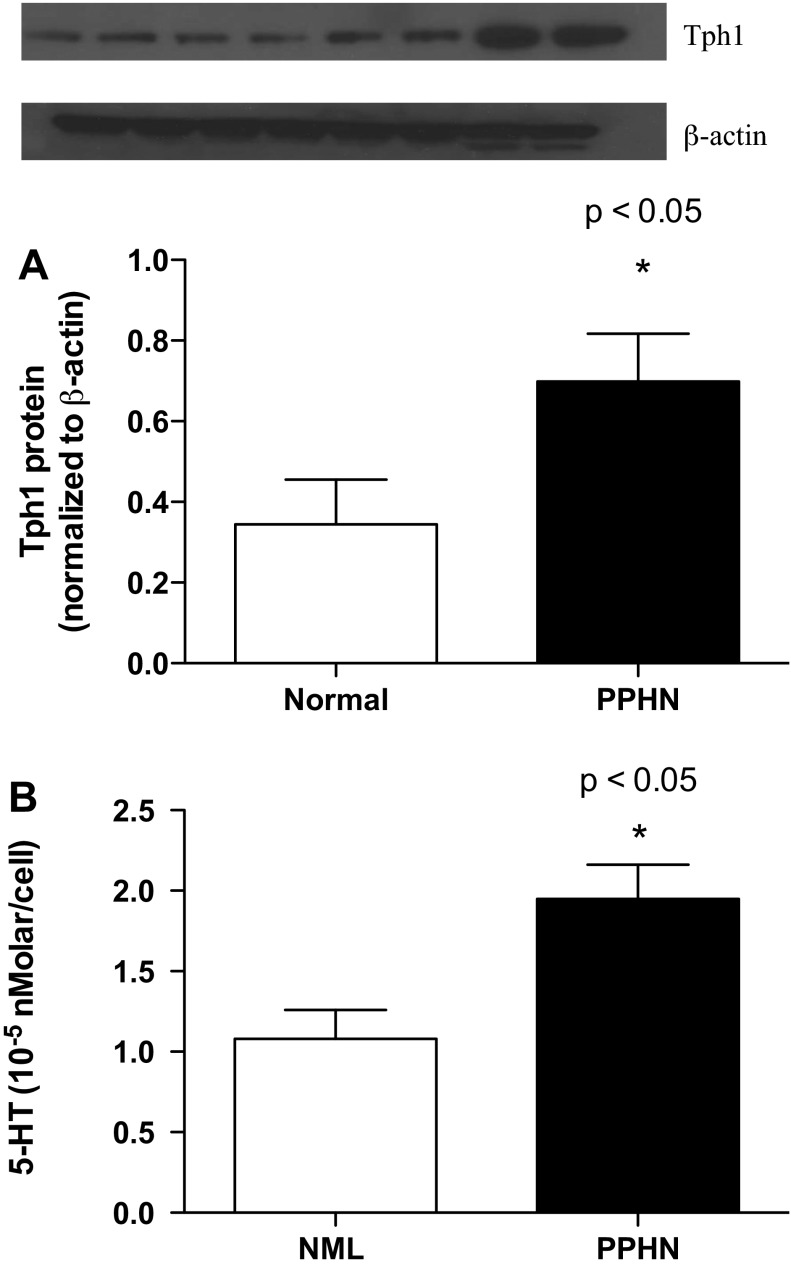
Compared with controls, PAECs from fetal sheep with PPHN exhibited increased expression of Tph1 and increased production of 5-HT. Western blot analysis of PAEC lysates from normal and PPHN fetal sheep demonstrated a doubling of Tph1 protein in PAECs from PPHN animals (P < 0.05; Fig. 5A). As determined by ELISA, 5-HT content in the media of PAECs grown to 80–90% confluence was increased 56% in PPHN PAECs compared with control PAECs (P < 0.05; Fig. 5B).
Fig. 5.
Tryptophan hydroxylase 1 (Tph1) expression and 5-HT content are increased in experimental PPHN. Compared with controls, pulmonary artery epithelial cells (PAECs) from PPHN lambs exhibited increased expression of Tph1 and increased production of 5-HT. Western blot analysis of PAEC lysates from control and PPHN PAECs demonstrated a doubling in Tph1 protein in PAECs from PPHN lambs (P < 0.5; A). As determined by ELISA, 5-HT synthesis was increased 56% in PPHN PAECs (P < 0.05; B). NML, normal.
Protocol 6: Expression of 5-HT 2A R in Whole Lung Homogenates and Isolated PASMC from Control and PPHN Fetal Sheep
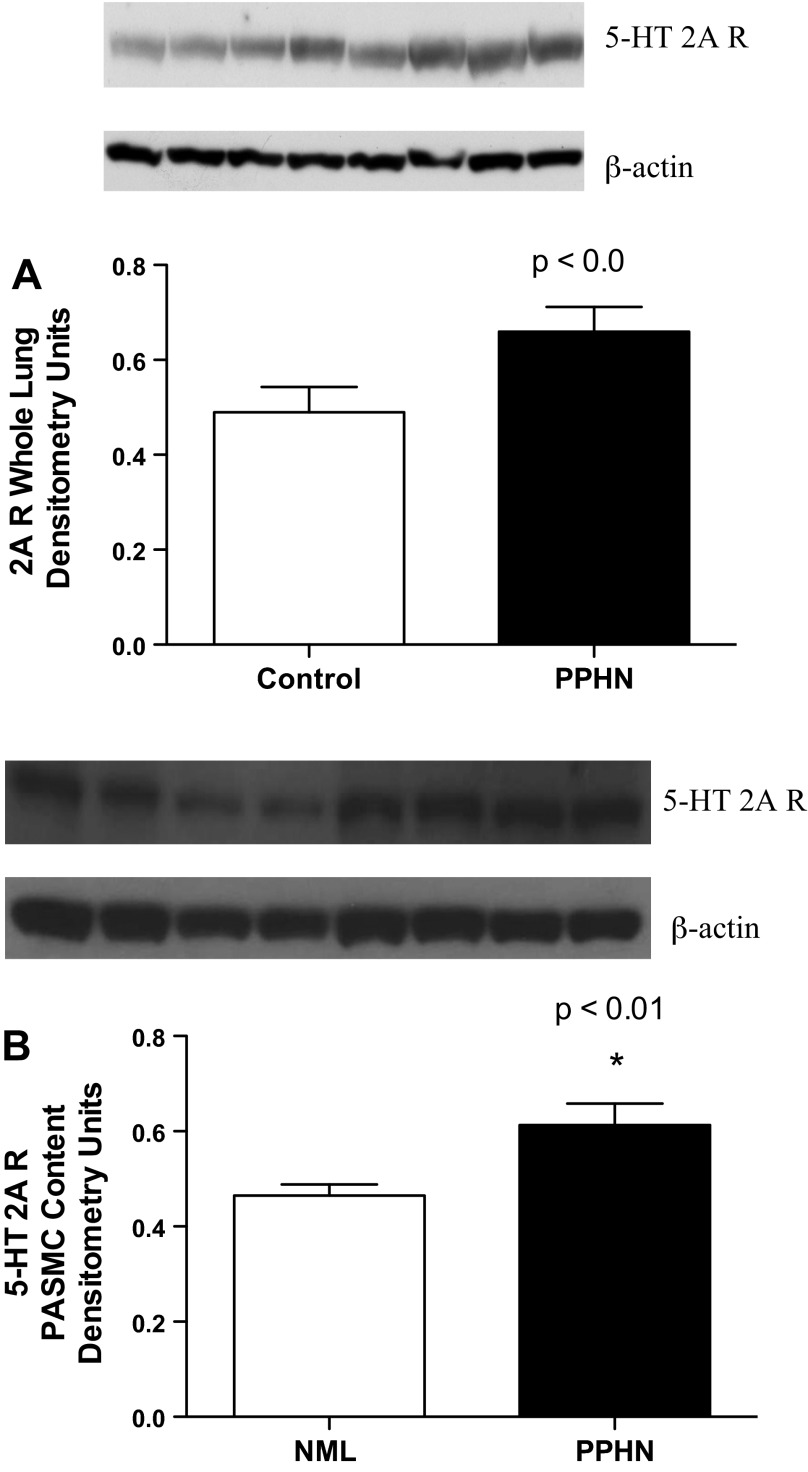
Compared with those isolated from control fetal sheep, whole lung lysates (Fig. 6A; P < 0.01) and PASMC lysates (Fig. 6B; P < 0.01) isolated from PPHN fetal sheep had increased expression of the 5-HT 2A R by 35% and 32%, respectively.
Fig. 6.
5-HT2A receptor expression is increased in PPHN. Increased protein expression of the 5-HT 2A R in whole lung lysates (A; P < 0.05) and pulmonary artery smooth muscle cells (PASMCs) (B; P < 0.01) isolated from fetal sheep with experimental PPHN.
DISCUSSION
Maternal SSRI use is associated with an increased risk for the development of PPHN, yet SSRIs protect against the development of pulmonary hypertension in adults (9, 33, 35, 37, 63). The mechanisms through which altered 5-HT signaling causes PPHN remain unclear, and little is known about the role of serotonin in the developing lung. Based on our previously published findings that serotonin and SSRIs are potent pulmonary vasoconstrictors in the normal fetus and that serotonin contributes to the development of PAH in adults, we hypothesized that 5-HT and SSRIs increase fetal PVR and that blockade of the 5-HT2A receptor may increase pulmonary blood flow in fetal sheep with experimental PPHN. We further hypothesized that increased pulmonary serotonin production and activity contributes to the pathogenesis of PPHN in this model. We report that acute intrapulmonary infusions of serotonin and SSRIs further increase fetal PVR and that blockade of the 5-HT2A receptor causes pulmonary vasodilation in experimental PPHN. In contrast to our findings in control animals, infusions of the ROCK inhibitor, fasudil, did not attenuate 5-HT-mediated pulmonary vasoconstriction. In addition, we found that PAEC synthesis of 5-HT is increased in experimental PPHN and that both Tph1 and 5-HT2A receptor expressions are increased in this model. These findings provide evidence that serotonin contributes to elevated pulmonary vascular tone seen in experimental PPHN and, in striking contrast with adult models of pulmonary hypertension, demonstrates that SSRI infusion does not cause pulmonary vasodilation and further elevates PVR.
These findings are interesting, as this is the first study to show that acute and selective pulmonary infusions of the SSRI sertraline cause potent and sustained pulmonary vasoconstriction in animals with experimental PPHN. The novel finding that fetal PAECs synthesize serotonin and that serotonin synthesis is increased in experimental PPHN suggests that serotonin signaling may contribute to altered pulmonary vascular tone seen in this model of experimental PPHN.
Serotonin is a signaling molecule with a number of diverse biological functions. Although it is mainly studied for its role in the central nervous system, the majority of serotonin is located in the systemic circulation, where it has numerous physiological roles including the regulation of intestinal motility, vascular tone, and platelet aggregation. The serotonin receptors are classified into seven different subfamilies, which have many different subtypes, accounting for the diverse physiological effects of serotonin (34). In addition to the 5-HT receptors, the SERT is also known to contribute to the biological effects of 5-HT (25, 45, 50). Previous studies have shown that the 5-HT receptors 1B, 2A, and 2B as well as the SERT mediate serotonin signaling in the adult lung (26, 36, 40, 44, 45, 49).
Previous laboratory and clinical studies have strongly implicated 5-HT in the pathogenesis of adult pulmonary hypertension (15, 16, 26, 45). Specifically, clinical studies demonstrate that patients with iPAH have increased plasma levels of 5-HT (29) and that 5-HT production and Tph1 expression are increased in PAEC from patients with iPAH compared with controls (17). Animal models of PAH reveal that 5-HT promotes the development of hypoxic PAH by stimulating PASMC proliferation (16), and pharmacological inhibition of the 5-HT 1B, 2A, 2B receptor attenuates hypoxia and monocrotaline-induced PH (13, 30, 36, 40). Furthermore, overexpression of the SERT is associated with more severe PAH (15, 26, 45), and SERT inhibition, with SSRIs and SERT knockout mice, attenuates PAH in adult animal models (25, 30, 42, 48). In addition, clinical studies suggest that SSRI use in patients with PAH is associated with a reduction in the risk of death (35, 63).
However, in striking contrast, epidemiological studies report a sixfold increase in the incidence of PPHN in infants exposed to SSRIs, particularly when ingested during the second half of gestation (9, 33, 37). We have previously shown that acute intrapulmonary infusions of SSRIs result in profound pulmonary vasoconstriction, and Fornaro et al. (12, 18) have reported that treatment of pregnant rats results in increased mortality in rat pups and pulmonary vascular remodeling. In this study, we found that, in contrast to findings in adult models of PAH, treatment with SSRIs in a model of PPHN results in further increases in pulmonary vascular tone, with no evidence for vasodilation.
Although we have demonstrated that SSRIs induce pulmonary vasoconstriction in an experimental model of PPHN, the mechanisms underlying these findings are not clear. Investigators have proposed that SSRIs induce ductal constriction, resulting in PPHN; our previous findings, however, do not support this hypothesis (12). SSRIs do increase extracellular levels of serotonin, and we have previously reported that pretreatment with ketanserin abolishes SSRI-mediated vasoconstriction, leading us to speculate that SSRI-induced vasoconstriction occurs via increased extracellular serotonin and activation of the 5-HT2A R (12).
Pharmacological blockade of the 5-HT2A R blocks the development of PAH in rodents with monocrotaline-induced pulmonary hypertension (30). The use of the 5-HT2A R antagonist, ketanserin, in adults with pulmonary hypertension had modest effects on lowering PVR, buts its use has been limited by systemic hypotension (47). Few studies have been performed in the fetus or neonate to elucidate which serotonin receptor subtype is involved in serotonin-mediated vasoconstriction at this developmental stage. In 1998, Morecroft et al. (51) reported that the predominant receptor subtype that mediates vasoconstriction in isolated rabbit pulmonary arteries is the 5-HT2A receptor. Recently Goyal (22) reported that the 5-HT2A receptor is the predominant 5-HT receptor involved in 5-HT-induced contraction in isolated ovine fetal pulmonary arteries exposed to maternal long-term hypoxia. Our findings, as well as findings by MacLean and Goyal, support that the 5-HT2A R is the predominant receptor that regulates serotonin-induced vasoconstriction in the perinatal circulation.
Serotonin induces pulmonary vasoconstriction and vascular remodeling via activation of several downstream cellular targets, including MAP Kinase 67, the generation of reactive oxygen species, and ROCK activation (23, 31, 43, 27, 46). We have previously shown that ROCK inhibition attenuates 5-HT-mediated pulmonary vasoconstriction in the normal fetus. However, in this model of PPHN, fasudil did not block 5-HT-induced vasoconstriction. However, when infused alone, fasudil did decrease basal PVR by 30%. We speculate that, although ROCK activation contributes to high PVR in PPHN, 5-HT-induced constriction is not mediated through ROCK in this experimental model of PPHN. This may be due to already enhanced 5-HT-ROCK activity or other mechanisms that will be addressed in future studies.
One potential limitation of this study is the use of fetal PAECs harvested from proximal vessels and the possibility that the behavior of these cells may differ from PAECs harvested from more distal vessels. Future studies are needed to evaluate serotonin production and signaling in the pulmonary microvascular circulation.
In conclusion, 5-HT and SSRIs further increase PVR in an ovine model of PPHN, and blockade of the 5-HT2A R decreases PVR in this model. In addition, experimental PPHN results in increased pulmonary endothelial cell production of serotonin and increased pulmonary expression of Tph1 and the 5-HT2A R. We speculate that altered serotonin signaling contributes to the pathogenesis of PPHN and that manipulation of serotonin signaling may be an attractive therapeutic target for the treatment of newborns with pulmonary hypertension.
GRANTS
C. Delaney is supported by NIH-NICHD K12HD068072 and an unrestricted grant from Gilead Sciences (Gilead Sciences Young Investigator Award in Pulmonary Hypertension). J. Gien is supported by NIH grant 1K08HL102261. S. Abman is supported by NIH grants T32 HL007670-23, RO1 HL085703-01A1, and R01 HL068702-08.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.D. and S.H.A. conception and design of research; C.D., J.G., G.B.R., N.I., and J.K. performed experiments; C.D. and J.G. analyzed data; C.D. and J.G. interpreted results of experiments; C.D. and J.G. prepared figures; C.D. drafted manuscript; C.D., J.G., G.B.R., and S.H.A. edited and revised manuscript; C.D. and S.H.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful for statistical advice from Dr. Paul Rozance.
REFERENCES
- 1.Abman SH. Abnormal vasoreactivity in the pathophysiology of persistent pulmonary hypertension of the newborn. Pediatr Rev 20: e103–e109, 1999 [PubMed] [Google Scholar]
- 2.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transitin of pulmonary circulation at birth. Am J Physiol Heart Circ Physiol 259: H1921–H1927, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Abman SH, Shanley PF, Accurso FJ. Failure of postnatal adaptation of the pulmonary circulation after chronic intrauterine pulmonary hypertension in fetal lambs. J Clin Invest 83: 1849–1858, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Obstetrics and Gynecology. Treatment with selective serotonin reuptake inhibitors during pregnancy. Obstet Gynecol 108: 1601–1603, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Anderson GM, Czarkowski K, Ravski N, Epperson CN. Platelet serotonin in newborns and infants: ontogeny, heritability, and effect of in utero exposure to selective serotonin reuptake inhibitors. Pediatr Res 56: 418–422, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Bearer C, Emerson RK, O'Riordan MA, Roitman E, Shackleton C. Maternal tobacco smoke exposure and persistent pulmonary hypertension of the newborn. Environ Health Perspect 105: 202–206, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callebert J, Esteve JM, Herve P, Peoc'h K, Tournois C, Drouet L, Launay JM, Maroteaux L. Evidence for control of plasma serotonin levels by 5-hydroxytryptamine (2B) receptors in mice. J Pharmacol Exp Ther 317: 724–731, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Cassin S, Dawes GS, Mott JC, Ross BB, Strang LB. The vascular resistance of the foetal and newly ventilated lung of the lamb. J Physiol 171: 61–79, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers C, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchell AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 354: 579–587, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Dakshinamurti S. Pathophysiologic mechanisms of persistent pulmonary hypertension of the newborn. Pediatr Pulmonol 39: 492–503, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Dawes GS, Mott JC. The vascular tone of the foetal lung. J Physiol 164: 465–477, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaney C, Gien J, Grover TR, Roe G, Abman SH. Pulmonary vascular effects of serotonin and selective serotonin reuptake inhibitors in the late-gestation ovine fetus. Am J Physiol Lung Cell Mol Physiol 301: L937–L944, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dempsie Y, Morecroft I, Welsh DJ, MacRitchie NA, Herold N, Loughlin L, Nilsen M, Peacock AJ, Harmar A, Bader M, MacLean MR. Converging evidence in support of the serotonin hypothesis of dexfenfluramine-induced pulmonary hypertension with novel transgenic mice. Circulation 117: 2928–2937, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Dunn JA, Lorch V, Sinha SN. Responses of small intrapulmonary arteries to vasoactive compounds in the fetal and neonatal lamb: norepinephrine, epinephrine, serotonin, and potassium chloride. Pediatr Res 25: 360–363, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest 108: 1141–1150, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eddahibi S, Raffestin B, Pham I, Launay JM, Aegerter P, Sitbon M, Adnot S. Treatment with 5-HT potentiates development of pulmonary hypertension in chronically hypoxic rats. Am J Physiol Heart Circ Physiol 272: H1173–H1181, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Eddahibi S, Guignabert C, Barlier-Mur AM, Dewachter L, Fadel E, Dartevelle P, Humbert M, Simonneau G, Hanoun N, Saurini F, Hamon M, Adnot S. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle cell hyperplasia. Circulation 113: 1857–1864, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Fornaro E, Li D, Pan J, Belik J. Prenatal exposure to fluoxetine induces fetal pulmonary hypertension in the rat. Am J Respir Crit Care Med 176: 1035–1040, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 106: 1071–1083, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Geggel RL, Reid LM. The structural basis of PPHN. Clin Perinatol 11: 525–549, 1984 [PubMed] [Google Scholar]
- 21.Gien J, Seedorf GJ, Balasubramaniam V, Markham N, Abman SH. Chronic intrauterine pulmonary hypertension increase endothelial cell Rho kinase activity and impairs angiogenesis in vitro. Am J Physiol Lung Cell Mol Physiol 295: L680–L687, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal R, Papamatheakis DG, Loftin M, Vrancken K, Dawson AS, Osman NJ, Blood AB, Pearce WJ, Longo LD, Wilson SM. Long-term maternal hypoxia: the role of extracellular Ca2+ entry during serotonin-mediated contractility in fetal ovine pulmonary arteries. Reprod Sci 18: 948–962, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greene E, Houghton O, Collinsworth G, Garnovskaya M, Nagai T, Sajjad T, Bheemanathini V, Grewal J, Paul R, Raymond J. 5-HT 2A receptors stimulate mitogen activated protein kinase via H2O2 generation in rat renal mesangial cells. Am J Physiol Renal Physiol 278: F650–F658, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Grover T, Parker T, Zenge J, Markham N, Kinsella J, Abman S. Intrauterine hypertension decreases lung VEGF expression and VEGF inhibition causes pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 284: L508–L517, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Guignabert C, Raffestin B, Benferhat R, Raoul W, Zadigue P, Rideau D, Hamon M, Adnot S, Eddahibi S. Serotonin transporter inhibition prevents and reverses monocrotaline-induced pulmonary hypertension in rats. Circulation 111: 2812–2819, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Guignabert C, Izikki M, Tu Ll Li Z, Zadigue P, Barlier-Mur AM, Hanoun N, Rodman D, Hamon M, Adnot S, Eddahibi S. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res 98: 1323–1330, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Guilluy C, Eddahibi S, Agard C, Guignabert C, Izikki M, Tu L, Savale L, Humbert M, Fadel E, Adnot S, Loirand G, Pacaud P. RhoA and rho kinase activation in human pulmonary hypertension Role of 5-HT signaling. Am J Respir Crit Care Med 179: 1151–1158, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Hendrick V, Smith LM, Suri R, Hwang S, Haynes D, Altshuler L. Birth outcomes after prenatal exposure to antidepressant medication. Am J Obstet Gynecol 188: 812–815, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Herve P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, Poubeau P, Cerrina J, Duroux P, Drouet L. Increased plasma serotonin in primary pulmonary hypertension. Am J Med 99: 249–254, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Hironaka E, Hongo M, Sakai A, Mawatari E, Terasawa R, Okumura N, Yamazaki A, Ushiyama Y, Yazaki Y, Kinoshita O. Serotonin receptor antagonist inhibits monocrotaline-induced pulmonary hypertension and prolongs survival in rats. Cardiovasc Res 60: 692–629, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Homma N, Nagaoka T, Morio Y, Ota H, Gebb A, Karoor V, McMurtry I, Oka M. Endothelin-1 and serotonin are involved in activation of rhoA/rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharmacol 50: 697–702, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Kallen B, Olausson PO. Maternal use of selective serotonin re-uptake inhibitors and persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf 17: 801–806, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Kaumann AJ, Levy FO. 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther 111: 674–706, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Kawut SM, Horn EM, Berekashvili KK, Lederer DJ, Widlitz AC, Rosenzweig EB, Barst RJ. Selective serotonin reuptake inhibitor use and outcomes in pulmonary arterial hypertension. Pulm Pharmacol Ther 19: 370–374, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Keegan A, Morecroft I, Smillie D, Hicks M, MacLean M. Contribution of the 5-HT1B receptor to hypoxia-induced pulmonary hypertension: converging evidence using 5-HT 1B-receptor knockout mice and the 5-HT 1B/1D receptor antagonist GR127935. Circ Res 89: 1231–1239, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Kieler H, Aratma M, Engeland A, Ericsso O, Furu K, Gissler M, Nielsen RB, Norgaard M, Stephansson O, Valdimarsdottir U, Zoega H, Haglund B. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension of the newborn: population based cohort study from the five Nordic countries. Br Med J 344: d8012, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Kereveur A, Callebert J, Humbert M, Herve P, Simonneau G, Launay JM, Drouet L. High plasma serotonin levels in primary pulmonary hypertension. Effect of long-term epoprostenol (prostacyclin) therapy. Arterioscler Thromb Vasc Biol 20: 2233–2239, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Laine K, Heikkinen T, Ekblad U, Kero P. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns, and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry 60: 720–726, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Launay J, Herve P, Peoch K, Tournois C, Callebert J, Nebigil CG, Etienne N, Drouet L, Humbert M, Simonneau G, Maroteaux L. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med 8: 1129–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Levin DL, Heymann MA, Kitterman JA, Gregory GA, Phibbs RH, Rudolph AM. Persistent pulmonary hypertension of the newborn infant. J Pediatr 89: 626–630, 1976 [DOI] [PubMed] [Google Scholar]
- 42.Li XQ, Hong Y, Wang Y, Zhang XH, Wang HL. Sertraline protects against monocrotaline-induced pulmonary hypertension in rats. Clin Exp Pharmacol Physiol 33: 1047–1051, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Suzuki Y, Day R, Fanburg B. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res 95: 579–586, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Maclean MR, Sweeney G, Baird M, McCulloch KM, Houslay M, Morecroft I. 5-Hydroxytryptamine receptors mediating vasoconstriction in pulmonary arteries from control and pulmonary hypertensive rats. Br J Pharmacol 119: 917–930, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacLean MR, Deuchar G, Hicks MN, Morecroft I, Shen S, Sheward J, Colston J, Loughlin L, Nilsen M, Dempsie Y, Harmar A. Overexpression of the 5-hydroxytryptamine transporter gene: effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation 109: 2150–2155, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Mair KM, MacLean MR, Morecroft I, Dempsie Y, Palmer TM. Novel interactions between the 5-HT transporter, 5-HT 1B receptors and rho kinase in vivo and in pulmonary fibroblasts. Br J Pharmacol 155: 606–616, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGoon MD, Vlietsra RE. Acute hemodynamic response to the S2-serotonergic receptor antagonist, ketanserin, in patients with primary pulmonary hypertension. Int J Cardiol 14: 303–309, 1987 [DOI] [PubMed] [Google Scholar]
- 48.Marcos E, Adnot S, Pham MH, Nosjean A, Raffestin B, Hamon M, Eddahibi S. Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. Am J Respir Crit Care Med 168: 487–493, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Morecroft I, Heeley R, Prentice H, Kirk A, MacLean MR. 5-Hydroxytryptamine receptors mediating contraction in human small muscular pulmonary arteries: importance of the 5-HT1B receptor. Br J Pharmacol 128: 730–734, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morecroft I, Loughlin L, Nilsen M, Colston J, Dempsie Y, Sheward J, Harmar A, MacLean MR. Functional interactions between 5-Hydroxytryptamine receptors and the serotonin transporter in pulmonary arteries. J Pharmacol Exp Ther 313: 539–548, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Morecroft I, MacLean MR. 5-hydroxytryptamine receptors mediating vasoconstriction and vasodilation in perinatal and adult rabbit small pulmonary arteries. Br J Pharmacol 125: 69–78, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrison JL, Chien C, Riggs KW, Gruber N, Rurak D. Effect of maternal fluoxetine administration on uterine blood flow, fetal blood gas status, and growth. Pediatr Res 4: 433–442, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Morrison JL, Riggs KW, Chien C, Gruber N, McMillen IC, Rurak DW. Chronic maternal fluoxetine infusion in pregnant sheep: effects on the maternal and fetal hypothalamic-pituitary-adrenal axes. Pediatr Res 56: 40–46, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Murphy JD, Vawter GF, Reid LM. Pulmonary vascular disease in fatal meconium aspiration. J Pediatr 104: 758–762, 1984 [DOI] [PubMed] [Google Scholar]
- 55.Nagaoka T, Gebb SA, Karoor V, Homma N, Morris KG, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in pulmonary hypertension of the fawn-hooded rat. J Appl Physiol 100: 996–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Ni W, Watts SW. 5-hydroxytryptamine in the cardiovascular system: Focus on the serotonin transporter. Clin Exp Pharmacol Physiol 33: 575–583, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Oka M, Homma N, Tarasevicience-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Parker TA, Roe G, Grover TR, Abman SH. Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 291: L976–L982, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Rampano J, Proud S, Hackett LP, Kristensen JH, Ilett KF. A pilot study of newer antidepressant concentrations in cord and maternal serum and possible effects in the neonate. Int J Neuropsychopharmcol 7: 329–334, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Rondelet B, Van Beneden R, Kerbaul F, Motte S, Fesler P, McEntee K, Brimioulle S, Ketelslegers JM, Naeije R. Expression of the serotonin 1B receptor in experimental pulmonary hypertension. Eur Respir J 22: 408–412, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Rudolph AM, Paul MH. Pulmonary and systemic vascular response to continuous infusion of 5-hydroxytryptamine (serotonin) in the dog. Am J Physiol 189: 263–268, 1957 [DOI] [PubMed] [Google Scholar]
- 62.Rudolph AM. Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ Res 57: 811–821, 1985 [DOI] [PubMed] [Google Scholar]
- 63.Shah SJ, Gomberg-Maitland M, Thenappan T, Rick S. Selective serotonin reuptake inhibitors and the incidence and outcome of pulmonary hypertension. Chest 136: 694–700, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Socol ML, Manning FA, Murata Y, Druzin ML. Maternal smoking causes fetal hypoxia: Experimental evidence. Am J Obstet Gynecol 142: 214–218, 1982 [DOI] [PubMed] [Google Scholar]
- 65.Van Marter LJ, Leviton A, Allred EN, Pagano M, Sullivan KF, Cohen A, Epstein MF. Persistent pulmonary hypertension of the newborn and smoking and aspirin and nonsteroidal anti-inflammatory drug consumption during pregnancy. Pediatrics 97: 658–663, 1996 [PubMed] [Google Scholar]
- 66.Watts SW, Yang P, Banes AK, Baez M. Activation of Erk mitogen-activated protein kinase proteins by vascular serotonin receptors. J Cardiovasc Pharmacol 38: 539–551, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Wilson KL, Zelig CM, Harvey JP, Cummingham BS, Dolinsky BM, Napolitano PG. Persistent pulmonary hypertension is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol 28: 19–24, 2011 [DOI] [PubMed] [Google Scholar]