Abstract
Barrington's nucleus, in the pons, regulates micturition through spinal projections to preganglionic parasympathetic neurons. The stress neuropeptide CRF is prominent in these projections and has an inhibitory influence. Social stress in rats causes urinary retention and abnormal urodynamics resembling those produced by partial bladder outlet obstruction (pBOO), and this is associated with CRF upregulation in Barrington's nucleus. Here, we examined the role of CRF in social stress- and pBOO-induced urodynamic dysfunction by assessing the ability of a CRF1 receptor antagonist to alter these effects. Male rats exposed to repeated resident-intruder stress were administered vehicle or a CRF1 antagonist (NBI-30775) daily prior to the stress. Urodynamic function was recorded in the unanesthetized state 72 h after the final stress. NBI-30775 prevented the increased intermicturition interval, micturition volume, and bladder capacity produced by social stress, but not the increase in CRF expression in Barrington's nucleus neurons. The urinary dysfunction was also partly prevented by shRNA targeting of CRF in Barrington's nucleus, suggesting that stress-induced urinary dysfunction results, in part, from CRF upregulation in Barrington's nucleus and enhanced postsynaptic effects in the spinal cord. Finally, NBI-30775 improved urodynamic function of rats that had pBOO of 2-wk duration when administered daily during the second week but did not block the increase in CRF expression in Barrington's nucleus neurons. These findings implicate a role for Barrington's nucleus CRF in stress- and pBOO-induced urodynamic changes and suggest that CRF1 antagonists may be useful therapeutic agents for the treatment of urinary dysfunction.
Keywords: cystometry, resident-intruder, Barrington's nucleus, urinary
barrington's nucleus in the pons regulates the descending limb of the micturition reflex through its axonal projections to preganglionic neurons of the lumbosacral spinal cord that provide the parasympathetic input to the detrusor muscle (21). Electrical and chemical stimulation of Barrington's nucleus elicits detrusor contraction, and conversely, lesions disrupt the micturition reflex (1, 27, 29). More recent anatomical and physiological evidence suggests that Barrington's nucleus plays a key role in coordinating central and visceral responses during micturition (39) (for review, see Ref. 41). In addition to its connections to the bladder, Barrington's nucleus neurons are transsynaptically linked to the distal colon and other pelvic viscera, suggesting a broader role for this nucleus in the regulation of pelvic visceral functions (23, 24, 33, 40, 44). Elucidating the function of neuromodulators expressed by Barrington's nucleus neurons could lead to a better understanding of central control of pelvic visceral functions and how these can be modulated by pharmacological interventions to treat pelvic visceral disorders.
The stress-related neuropeptide, corticotropin-releasing factor, or CRF, is prominently expressed in Barrington's nucleus neurons and in its spinal projections (36, 37, 43). CRF is the major neurohormone that initiates adrenocorticotropin release from the anterior pituitary in response to stress (38). Additionally, CRF is present in extrahypophyseal brain circuits involved in autonomic and behavioral responses to stress (37). CRF release in different brain regions is hypothesized to coordinate the many aspects of the stress response. In vivo cystometry studies examining the effects of CRF agonists and/or antagonists have suggested conflicting roles for CRF in the regulation of micturition (16, 17). However, a study of the specific effect of CRF in Barrington's nucleus spinal projections suggested an inhibitory influence on parasympathetic input to the bladder (29). Thus, discrete chemical activation of Barrington's nucleus neurons elicited bladder contractions that were increased by intrathecal administration of a CRF antagonist, and conversely, decreased by intrathecal CRF. An inhibitory role for CRF in Barrington's nucleus regulation of the bladder is consistent with reports that social stress in rodents leads to urinary retention, abnormal urodynamics, and bladder hypertrophy (4, 6, 12, 13, 45), and this is associated with increased expression of CRF in Barrington's nucleus neurons (45). These findings suggest that pharmacological manipulation of CRF may improve bladder dysfunctions associated with stress or other conditions.
The present study used in vivo cystometry in unanesthetized rats to determine whether treatment with a CRF antagonist, NBI-30775, could prevent social stress-induced changes in urodynamics and/or CRF expression in Barrington's nucleus neurons. Because some of the urodynamic changes produced by social stress mimic those seen in rats with partial bladder outlet obstruction (pBOO), the effects of NBI-30775 on the bladder dysfunction associated with a 2-wk pBOO were also investigated.
MATERIALS AND METHODS
Animals.
The experimental subjects were male Sprague-Dawley rats (Charles River, ∼300 g). Male Long-Evans retired breeders (Charles River, 650–850 g) were used as residents in the social stress study. All rats were singly housed in a 12:12-h light-dark cycle (lights on at 7:00 AM), climate-controlled room and were given free access to food and water. All studies were approved by the Children's Hospital of Philadelphia's Institutional Animal Care and Use Committee and conformed to the Principles of Laboratory Animal Care.
Social stress.
The social stressor used in these studies was modified from the resident-intruder model originally described by Miczek (26). Rats were randomly assigned to either social stress or control exposure for 30 min on seven consecutive days. During each social stress exposure, an intruder rat was placed into the home cage territory of an unfamiliar Long-Evans resident, previously screened for high aggression (2, 3, 47). A typical social stress exposure resulted in intruder subordination, termed defeat, and was operationally defined by the Sprague-Dawley intruder, assuming a supine posture. Following defeat, a wire mesh partition was placed in the cage to prevent physical contact between the resident and intruder, but allowing auditory, olfactory, and visual contact to continue for the remainder of the 30-min social stress session. Control rats were placed in a novel cage behind a wire partition for 30 min daily. Rats were returned to their home cage after each session.
Partial bladder outlet obstruction.
Surgery for pBOO was identical to that previously described (31). Rats were anesthetized with isoflurane, and an incision was made along the midline of the lower abdomen to access the bladder and prostates. Connective tissue was dissected between the bladder and prostates to provide a clear view of the bladder neck and ureters. An 18-gauge needle was placed parallel to the urethra, and suture was laced inside of the ureters around the bladder neck and needle. The needle was removed leaving the ligature around the bladder neck, and the abdominal muscles and skin were then sutured closed. This ligature remained throughout the experiment. Sham rats had the same surgery up to the point of exposing the bladder neck and base of the urethra.
Drug treatment.
One hour prior to each of the seven social stress or control exposures, rats were treated with vehicle (10% solution of 0.1 M tartaric acid in sterile water, sc) or NBI-30775 (10 mg/kg sc). For the partial bladder outlet obstruction (PBOO) study, PBOO rats were treated with vehicle or NBI-30775 (10 mg/kg sc) on seven consecutive days (days 7–14 postsurgery). Sham rats were administered vehicle (1 ml/kg sc) daily 7–14 days postsurgery. This dose of NBI-30775 has a half-life of 130 min in vivo and has been shown to result in 75% occupancy of brain CRF1 (8, 9, 11). Additionally, this has been demonstrated to prevent stress-induced ACTH release and behavioral and cardiovascular consequences of social stress (14, 46).
shRNA vector design and construction.
Adeno-associated viral vectors (AAV2/1) containing short-hairpin RNAs were produced in order to knock down CRF expression. shRNAs were targeted against the 3′ coding region of CRF mRNA or a scrambled control sequence. The CRF shRNA (gift from Dr. Alon Chen, Weizmann Institute of Science, Rehovot, Israel) was previously shown to dramatically reduce expression in 293T cells (30). The scrambled shRNA sequence was generated using siRNA Wizard V3.1 and synthesized de novo. shRNA sequences (sense and antisense in italics, hairpin in bold): shRNA-CRF: 5′-AGATTATCGGGAAATGAAATCTCTTGAATTTCATTTCCCGATAATCT and shRNA-CRFscramble: 5′-GTGAAATATCGAAGGTAAATCTCTTGAATTTACCTTCGATATTTCAC. The CRF and scrambled shRNAs were ligated into the AAV2 vector plasmid pZAC2.1 under the control of the H1 promoter. The packaging and purification of vector were performed by the University of Pennsylvania Vector Core, as previously described (28). Viral titers were determined by the Vector Core by running vector preps on an SDS-PAGE gel and comparing the intensity of VP3 bands to a reference standard. Viral titers were as follows: AAV-shRNA-CRF, 4 × 1012 and AAV-shRNA-CRFscramble, 7.5 × 1012.
Surgery and vector injection.
Surgery and injection of AAV vectors into Barrington's nucleus were as previously described (25). Rats were anesthetized with a mixture of isoflurane-in-air, positioned in a stereotaxic and surgically prepared for placement of a double-barrel glass micropipette into Barrington's nucleus. The center barrel was filled with 2% pontamine sky blue in 0.5 M sodium acetate and served as the recording pipette. The ejection barrel was filled with a solution containing a mixture (1:1) of AAV-containing green fluorescent protein (GFP) cDNA and either AAV-shRNA-CRF or AAV-shRNA-CRFscramble. The AAV-GFP served to help visualize the injections in sections and verify the localization in Barrington's nucleus. Barrington's nucleus was localized through electrophysiological recordings, as previously described (34). Once the pipette was positioned into the region considered to be Barrington's nucleus, 200 nl of the vector solution was microinfused by application of pressure pulses (20–40 psi, 30 ms) using a picospritzer. The ejection pipette was calibrated to deliver known volumes. Bilateral injections were done in every animal. After the skin over the skull was sutured, rats were observed until ambulatory and placed back into their home cage. One cohort of rats was exposed to social stress on days 8–14 after AAV-shRNA infusion. Twenty-four hours after the final social stress catheters were surgically implanted for quantification of urodynamics, as described below. Rats were decapitated after cystometry (17 days after AAV injection), and brains were removed for in situ hybridization of CRF mRNA at this time after injection. Another cohort of unstressed rats was just decapitated, and the brain was removed 9 days following AAV-shRNA injection for in situ hybridization of CRF mRNA in Barrington's nucleus.
Quantification of urodynamics.
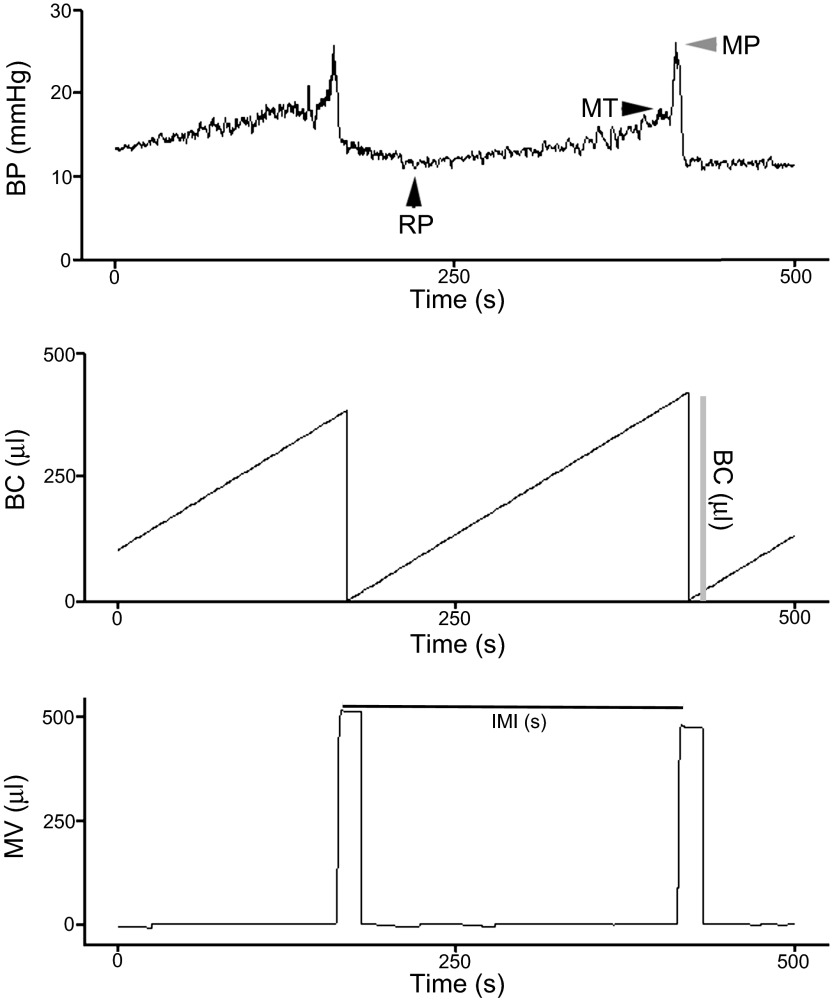
Twenty-four hours after the last stress or control manipulation, a catheter (5-French umbilical artery catheter) was surgically inserted into the bladder dome and tunneled subcutaneously from the bladder to the scapulas to an incision between the scapulas, as in our previous study (16). Forty-eight hours after this surgery, rats were placed into a cystometry chamber (Medical Associates, St. Albans, VT), the catheter was connected to a swivel device, and urodynamic function was recorded for 1 h in the unanesthetized, unrestrained (no jacket) state using cystometry equipment and software (Medical Associates, St. Albans, VT), as previously described (16). Sterile saline was continuously infused into the bladder (100 μl/min) through a closed circuit system to monitor intermicturition interval (IMI), bladder capacity (BC), and voided volume. Figure 1 shows how data were calculated from the cystometry records. The top trace shows the pressure recording and the points at which resting pressure (RP), micturition threshold (MT), and micturition pressure (MP) were determined. Urine was collected in a pan situated on a scale under the cage. The micturition volume was derived from the weight of the urine that fell into the pan during each cycle. Intermicturition interval was defined as the time between the end of one micturition cycle and the beginning of another (Fig. 1, bottom). The recording pen reset for each trace after each micturition event. This allows for an estimate of BC as the volume of saline infused during one micturition cycle (Fig. 1, middle). This is only an estimate because it assumes no residual volume. However, there was no reason to assume residual volumes in these cases. Values were averaged for at least three stable micturition cycles. Rats were euthanized by decapitation, and the brains were dissected and flash frozen for CRF mRNA or immunohistochemistry.
Fig. 1.
Sample cystometry indicating how endpoints were determined. Bladder pressure (BP; mmHg) (top), bladder capacity (BC; μl) (middle), and micturition volume (MV; μl) (bottom). Top: arrowheads show the points at which resting pressure (RP), micturition threshold (MT), and micturition pressure (MP) were determined. Middle: vertical line labeled “BC (μl)” indicates how bladder capacity was determined. Bottom: horizontal line labeled “IMI (s)” indicates how intermicturition was derived. See materials and methods for details.
CRF mRNA quantification.
Brain sections collected on slides were postfixed by incubation in 10% formalin and processed to quantify CRF mRNA, as previously described (42). Sections containing Barrington's nucleus were hybridized with an antisense riboprobe to CRF mRNA (Dr. Audrey F. Seasholtz, University of Michigan) and coated with Kodak NTB2 liquid autoradiographic emulsion (7-day exposure at 4°C) identical to that previously published (45). The tissue was lightly stained with cresyl violet, and bright-field images were captured and magnified using Open Lab software to identify cresyl violet-stained cells containing silver grains greater than 2–4 times background. The number of CRF-expressing cells from two Barrington's nucleus sections was averaged for each rat by an observer blinded to the treatment conditions.
CRF immunofluoresence.
Brains were cut into 14-μm-thick sections and collected on charged slides (ProbeOnPlus, Fisher Scientific). Sections were postfixed in 4% paraformaldahyde for 1 h and then rinsed in PBS. Sections were then incubated with rabbit anti-CRF (C-70, 1:2,000, Wylie Vale, Salk Institute) for 48 h at 4°C. Following rinses, slides were incubated with rhodamine-conjugated donkey anti-rabbit IgG antibody (1:200; Jackson Laboratories) for 90 min. Sections from rats of all experimental groups were processed at the same time using the same solutions. CRF immunoreactivity was visualized with a Leica DMRX-fluorescent microscope. Images were captured using a Hamamatsu ORCA-ER digital camera and Open Lab software (Perkin-Elmer) using the identical exposure times for all sections, so that immunoreactivity could be compared between groups. For quantification of CRF-immunoreactive cells in Barrington's nucleus, sections were photographed at the same exposure, and the grayscale images were inverted using ImageJ (rsbweb.nih.gov/ij/), as previously reported (45). Images were magnified, and inverted grayscale photomicrographs resulted in black cells on a white background, allowing for unambiguous distinction between staining within a cell and background. An oval region of interest was drawn to encompass Barrington's nucleus at its largest extent, and this was superimposed on all images. CRF-immunoreactive cells within this region of interest were quantified by an individual blinded to experimental groups. CRF cells were counted in two or three nonserial sections from an individual rat and averaged as the value for that rat. The mean determined from all individual rats was averaged for the group mean.
Statistical analysis.
All data are presented as means ± SE. Two-way ANOVAs followed by Holm-Sidak's multiple-comparison method were used within the social stress-CRF antagonist study to identify the effects of drug on stress-induced changes in urodynamic parameters. Separate one-way ANOVAs followed by Student-Newman-Keuls method were used to compare bladder parameters between stressed rats injected with AAV-shRNAs and rats in the stress-CRF antagonist study. Likewise, separate one-way ANOVAs followed by Student-Newman-Keuls method were used to identify differences in urodynamic parameters between sham/vehicle, pBOO/vehicle, and pBOO/NBI-30775 rats. A one-way ANOVA was also used to identify differences in the number of CRF-expressing cells within Barrington's nucleus between control and vehicle, stress and vehicle, and stress and NBI-30775 rats, as well as in CRF mRNA-expressing cell count between sham, pBOO/vehicle, and pBOO/NBI-30775. A Student's t-test was used to identify differences in the number of CRF mRNA-expressing cells in Barrington's nucleus in defeated rats treated with AAV-shRNA-CRFscramble vs. AAV-shRNA-CRF. For all analyses, two-tailed P values of <0.05 were considered significant. All post hoc significance is reported in the figure legends.
RESULTS
NBI-30775 improves stress-induced urodynamic dysfunction.
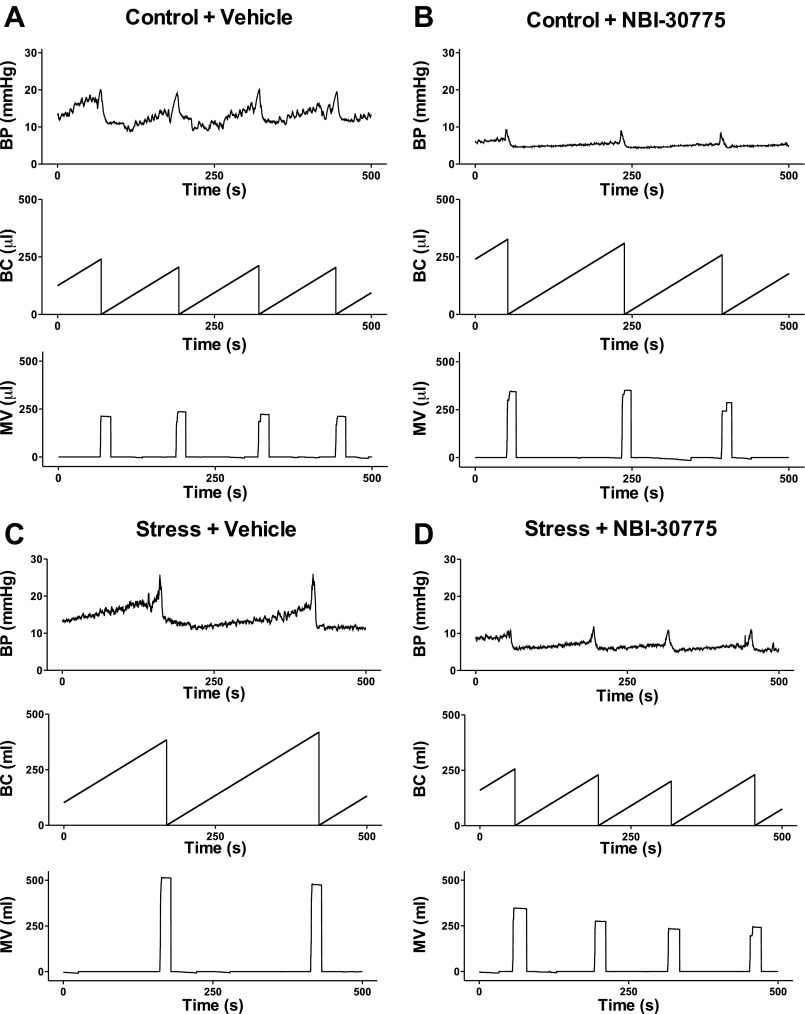
Consistent with previous reports (45), social stress resulted in an abnormal urodynamic profile. Figure 2 shows representative examples of cystometry traces from control and stressed rats treated with vehicle or NBI-30775 before each manipulation. Intermicturition interval (IMI), BC, and micturition volume (MV) were all elevated in socially stressed rats administered vehicle compared with control rats administered vehicle (Figs. 2, A and C and 3, A–C). Notably, pretreatment with NBI-30775 prior to each stress prevented the effects of stress on all urodynamic measures but had no effect of its own in control rats (n = 5) (Figs. 2, B and D and 3, A–C). MT, MT, and RP were unaffected by social stress (Fig. 3D). A two-way ANOVA for IMI revealed significant effects of stress (F1,29 = 4.9; P < 0.05), treatment (F1,29 = 4.5; P < 0.05), and a stress × treatment interaction [IMI; F1,29 = 4.6; P = 0.041 < 0.05]. In stressed rats administered vehicle (n = 9), the IMI was greater compared with control rats administered vehicle (n = 10; P < 0.005). The effect of stress on IMI was significantly prevented by treatment with NBI-30775 (n = 9; P < 0.005). For BC, there was a significant effect of stress (F1,29 = 4.9; P < 0.05), treatment (F1,29 = 4.5; P < 0.05), and a stress × treatment interaction (F1,29 = 4.6; P < 0.05). Bladder capacity was significantly greater in socially stressed rats pretreated with vehicle compared with control rats administered vehicle (P < 0.005). Stress-induced increases in BC were significantly prevented by treatment with NBI-30775 (P < 0.005). For MV, there was a trend toward a significant effect of treatment (P = 0.051) and a significant stress × treatment interaction (F1,29 = 4.2; P < 0.05). Stressed rats administered vehicle had a larger mean MV compared with control rats administered vehicle (P < 0.01). NBI-30775 significantly prevented the stress-induced increases in MV (P < 0.005).
Fig. 2.
Representative cystometry traces from different experimental groups. A–D: in all traces, the abscissae indicate time from 0 to 500 s. The top trace in each part shows BP (mmHg); the middle trace shows BC (μl), as defined by the volume of saline infused into the bladder between micturition cycles, and the bottom trace shows MV (μl). For comparison, the ordinates were scaled to be equivalent for all groups.
Fig. 3.
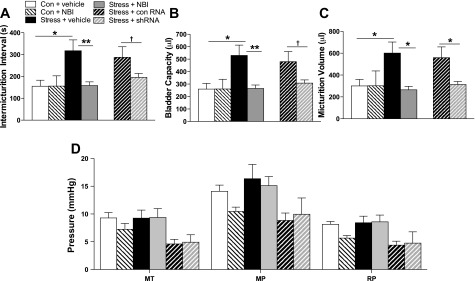
Quantification of the effects of different treatments on urodynamics of control and stressed rats. A–C: micturition interval, bladder capacity, and micturition volume, respectively. D: MT, MP, and RP for all groups in one graph. Results of a two-way ANOVA comparing stress and drug treatment are presented in the text. A one-way ANOVA was also performed comparing all groups, including the AAV-shRNA groups, and symbols in the figure refer to results of post hoc tests from this analysis. *P < 0.05, **P < 0.01, †P < 0.06. Control+vehicle (n = 10), control/NBI (n = 5), stress+vehicle (n = 9), stress+NBI (n = 9), stress+shRNA (n = 8), and stress+control RNA (n = 7).
Social stress-induced bladder hypertrophy was also observed in vehicle-treated social stress rats, consistent with previous findings (45). The bladder-to-body weight ratio in stressed rats treated with vehicle (stress+vehicle: 0.60 ± 0.02; n = 9) was greater than control rats treated with vehicle (0.48 ± 0.02; n = 10). This effect was prevented in stressed rats treated with NBI-30775 (stress+NBI-30775; 0.50 ± 0.02; n = 9), which had no effect on bladder-to-body weight ratio in control rats (0.52 ± 0.03; n = 5). A two-way ANOVA revealed a significant stress × treatment interaction (F1,27 = 7.4; P < 0.05). Post hoc analysis revealed a significant effect of stress within vehicle-treated rats (P < 0.001; control+vehicle vs. stress+vehicle). There was also a significant effect of treatment within stressed rats (P < 0.01; stress+vehicle vs. stress+NBI-30775). There was no effect of stress (P = 0.2) or treatment (P = 0.4) on body weight [mean body wt (g) ± SE; control+vehicle: 331 ± 8, control+NBI: 346 ± 10, stress+vehicle: 329 ± 8, and stress+NBI: 328 ± 8].
Role of CRF upregulation in Barrington's nucleus in stress-induced urodynamic dysfunction.
We previously reported that social stress increases CRF mRNA and the number of CRF-immunolabeled neurons in Barrington's nucleus (45). Figure 4A shows representative photomicrographs of CRF immunolabeling in the core of Barrington's nucleus in vehicle-treated control (top) and social-stressed (bottom) rats. Consistent with our previous report, social stress increased the mean number of CRF-immunolabeled Barrington's nucleus neurons (n = 9; P < 0.05) compared with vehicle-treated control rats (n = 8) and NBI-30775 pretreatment did not prevent this effect (n = 7; P < 0.05) (Fig. 4B; F2,21 = 5.5; P < 0.05).
Fig. 4.
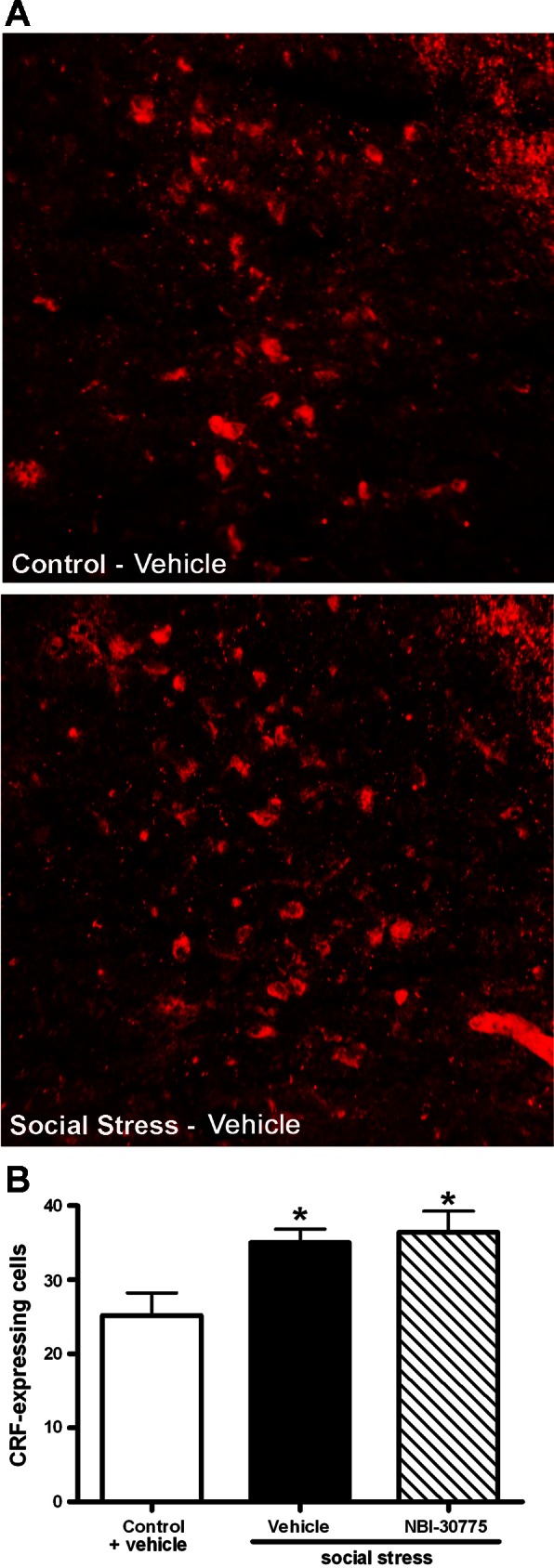
NBI-30775 had no effect on the stress-induced increase in the number of CRF-immunoreactive neurons in Barrington's nucleus. A: fluorescent photomicrographs of CRF-immunoreactive Barrington's nucleus neurons of representative control (top) and stressed (bottom) rats treated with vehicle. Top shows dorsal and medial appears to the left in all photomicrographs. B: bars represent the mean number of CRF-immunoreactive Barrington's nucleus neurons for controls treated with vehicle and stressed rats treated with vehicle or NBI-30775. Newman-Keuls post hoc tests: *P < 0.05 vs. control/vehicle.
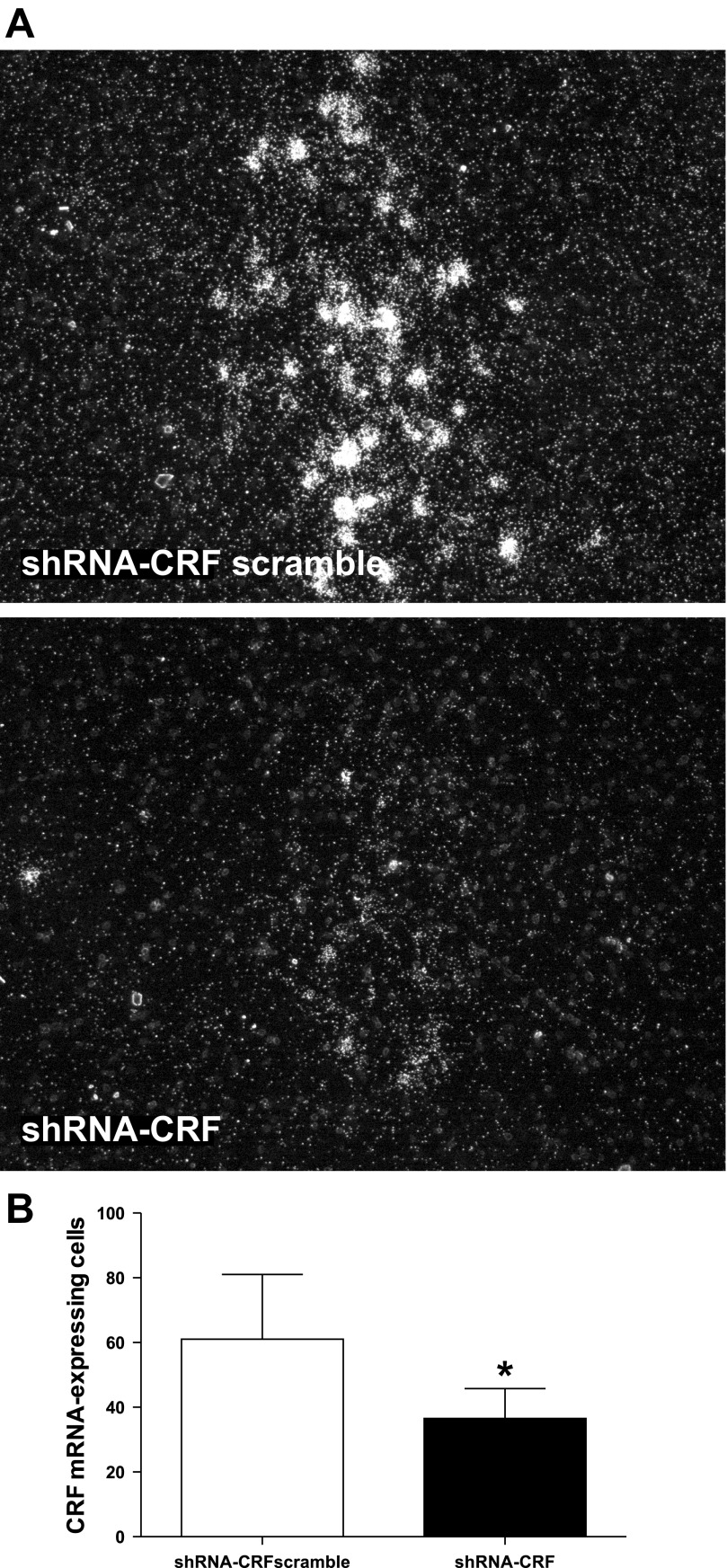
To evaluate the role of CRF upregulation in Barrington's nucleus neurons in the urodynamic consequences of social stress, local injections of AAV-shRNA-CRF were used to inhibit CRF expression in Barrington's nucleus neurons. By 17 days after injection (72 h after the last stress exposure), the mean number of CRF immunoreactive Barrington's nucleus neurons was less in rats administered AAV-shRNA-CRF (27 ± 3; n = 7 rats; P = 0.03) compared with those administered AAV-shRNA-CRFscramble (40 ± 4, n = 7). Notably, the number of CRF-immunolabeled Barrington's nucleus neurons in rats that were stressed and received AAV-shRNA-CRFscramble was comparable to those that were stressed and received vehicle or NBI-30775, whereas stressed rats that received AAV-shRNA-CRF were similar to nonstressed controls (Fig. 4B). In contrast to the immunohistochemical findings, there were no statistically significant differences in the number of Barrington's nucleus neurons expressing CRF mRNA in AAV-shRNA-CRFscramble (57 ± 6) compared with AAV-shRNA-CRF (45 ± 6) groups (P = 0.18). Because recovery of mRNA may precede protein, the number of Barrington's nucleus neurons expressing CRF mRNA was examined at an earlier time after injection that would correspond to an early point in the stress procedure (9 days after injection). Figure 5A shows representative dark-field photomicrographs of CRF mRNA expression in Barrington's nucleus of rats treated with AAV-shRNA-CRFscramble or AAV-shRNA-CRF 9 days after injection. shRNA targeting of CRF (n = 6) significantly reduced the number of CRF mRNA-expressing cells compared with the scrambled RNA at this time (n = 4) [treatment: t8 = 2.7; P = 0.029, Fig. 5B].
Fig. 5.
CRF mRNA expression in Barrington's nucleus is reduced in stressed rats with infusion of AAV-shRNA-CRF into Barrington's nucleus. A: darkfield photomicrographs showing the CRF mRNA hybridization signal in Barrington's nucleus of stressed rats that were infused with AAV-shRNA-CRFscrambled (top) and AAV-shRNA-CRF (bottom). The two photomicrographs were taken using identical exposure times, and brightness and contrast of the composite photograph were adjusted after the composite was flattened, so adjustments were identical for both examples. B: bar graph indicates the mean number of CRF mRNA-expressing Barrington's nucleus neurons for rats infused with shRNA-CRF scrambled (n = 4) and shRNA-CRF (n = 6). *P < 0.05.
The urodynamic parameters of stressed rats injected with AAV-shRNA-CRF or AAV-shRNA-CRFscramble were compared with those of control and stressed rats administered vehicle or NBI-30775 using a one-way ANOVA (Fig. 2). For IMI, a one-way ANOVA revealed a significant difference between groups (F5,41 = 4.3, P = 0.003). Post hoc analysis confirmed that social stress similarly increased IMI in rats treated with vehicle or AAV-shRNA-CRFscramble, and these effects were prevented by NBI-30775 and shRNA targeting of CRF in Barrington's nucleus resulted in a trend toward a decrease (P = 0.06) in IMI. For BC, there were also significant differences between groups [F5,41 = 4.2; P = 0.003]. BC was significantly greater in socially stressed rats treated with vehicle or AAV-shRNA-CRFscramble compared with control rats (Fig. 2B). Stress-induced increases in BC were significantly prevented by treatment with NBI-30775, and there was a trend for shRNA targeting of CRF in Barrington's nucleus to block increase in BC. For MV, there was also a significant difference between groups (F5,31 = 3.8; P = 0.006). Stressed rats administered vehicle or AAV-shRNA-CRFscramble had a larger mean MV compared with control rats administered vehicle (Fig. 2C). Both NBI-30775 treatment and AAV-shRNA-CRF infusion significantly prevented the stress-induced increases in MV (Fig. 2C). One-way ANOVA analysis revealed an overall significant difference between groups for MT (F5,41 = 3.5; P = 0.011), MP (F5,41 = 3.4; P = 0.011), and RP (F5,41 = 4.4; P = 0.003). These effects reflected lower pressures, for the AAV-injected rats and were not an effect of stress or treatment. The shRNA studies were conducted separately from the NBI studies, possibly contributing to the differences observed in MT, MP, and RP.
NBI-30775 improves pBOO-induced urodynamic dysfunction.
Fourteen days of partial bladder outlet obstruction resulted in abnormal urodynamics that were mitigated, in part, by daily treatment with NBI-30775 from days 7 to 14. Figure 6 shows representative cystometry records from a vehicle-treated sham rat and pBOO rats treated with vehicle or NBI-30775. Quantification of mean urodynamic parameters demonstrated a significant increase in IMI in pBOO rats treated with vehicle (n = 9) compared with pBOO rats administered NBI-30775 (n = 10; F2,22 = 4.0; P < 0.05) (Fig. 7A). Bladder capacity was also significantly greater in pBOO rats treated with vehicle compared with pBOO rats treated with NBI-30775 (BC) (F2,22 = 4.0; P < 0.05) (Fig. 7B). Micturition volume was also increased in pBOO rats treated with vehicle compared with sham rats treated with vehicle (n = 6) (F2,22 = 5.4; P < 0.05), and this was prevented by NBI-30775 (P < 0.05) (Fig. 7C). In contrast, NBI-30775 treatment did not affect the increased micturition pressure produced by pBOO (F2,21 = 3.6; P < 0.05) (Fig. 7D). MT and RP were similar between groups.
Fig. 6.
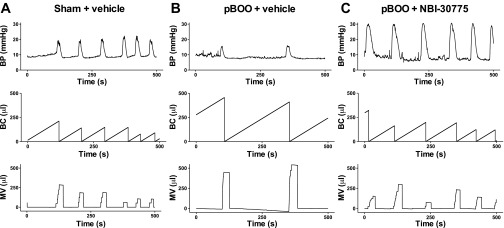
Representative cystometrograms from sham and partial bladder outlet obstruction (pBOO) rats. Shown are traces of a vehicle-treated sham rat (A), a vehicle-treated pBOO rat (B), and a pBOO rat treated with NBI-30775 (C). A–C: all traces of the abscissae indicate time from 0 to 500 s. The top trace of each part shows BP (mmHg), the middle trace shows BC (μl), as defined by the volume of saline infused into the bladder between micturition cycles, and the bottom trace shows MV (μl). For comparison, the ordinates were scaled to be equivalent for all groups.
Fig. 7.
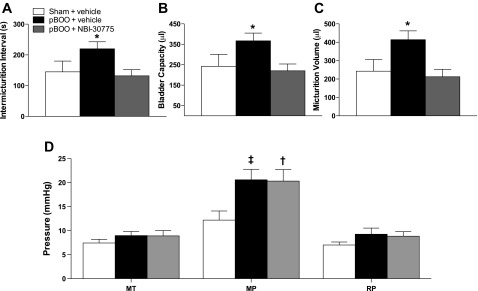
NBI-30775 attenuates the urodynamic effects of pBOO. Bars represent the mean values for sham rats treated with vehicle (n = 6), and pBOO rats treated with vehicle (n = 9) or NBI-30775 (n = 10) for intermicturition interval (IMI; s), BC (μl), MV (μl), MT (mmHg), MP (mmHg), and RP (mmHg). Student-Newman-Keuls post hoc test: *P < 0.05 vs. pBOO/NBI. †P < 0.05 vs. sham/vehicle. ‡P = 0.06 vs. sham/vehicle.
The bladder-to-body weight ratio tended to increase in the 2-wk pBOO rats regardless of pretreatment (F2,21 = 2.4; P = 0.1). Thus, in pBOO rats treated with vehicle (n = 9) and NBI-30775 (n = 10), the ratios were 0.72 ± 0.05 and 0.73 ± 0.07, respectively, compared with 0.54 ± 0.03 in sham rats treated with vehicle (n = 6) (P = 0.07 and P = 0.05, respectively). There was no difference in body weight between groups [mean body wt (g) ± SE; sham/vehicle: 366 ± 15, pBOO/vehicle: 371 ± 7, pBOO/NBI: 367 ± 11; P = 0.9].
pBOO increases CRF mRNA-expressing neurons in Barrington's nucleus.
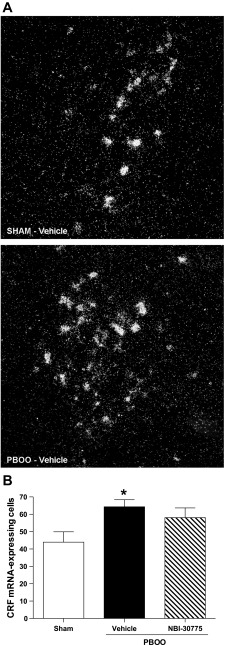
Given the inhibitory influence of CRF in Barrington's nucleus and the effects of NBI-30775, the effect of 2-wk pBOO on CRF mRNA was examined. Figure 8A shows representative dark-field photomicrographs of the hybridization signal for CRF mRNA in Barrington's nucleus of a sham rat (top) and a pBOO rat treated with vehicle (bottom). Two-week pBOO (vehicle treated: n = 9) increased the number of CRF mRNA-positive neurons in Barrington's nucleus compared with sham (n = 7), and this was unaffected by NBI-30775 treatment [n = 6; F2,19 = 0.1; P = 0.028; Fig. 8B].
Fig. 8.
CRF mRNA expression in Barrington's nucleus is increased in pBOO rats. A: dark-field photomicrographs showing the CRF mRNA hybridization signal in Barrington's nucleus of a sham (top) and a pBOO rat (bottom). The two photomicrographs were taken using identical exposure times and brightness, and contrast of the composite photograph was done after the composite was flattened, so adjustments were identical for both examples. B: bar graph indicates the mean number of CRF mRNA expressing Barrington's nucleus neurons for sham rats administered NBI-30775 (n = 7), pBOO rats administered vehicle (n = 9), and pBOO rats administered NBI-30775 (n = 6). *P < 0.05.
DISCUSSION
The present results confirmed previous studies showing that repeated social stress increases bladder mass and alters urodynamics in a manner that is consistent with urinary retention (45). The results also supported the previous finding that repeated social stress upregulates CRF in Barrington's nucleus neurons (45). The present demonstration that pretreatment with a CRF1 receptor antagonist prior to the stress prevents the abnormal urodynamic profile implicates CRF in the social stress-induced bladder pathology. That the urodynamic dysfunction produced by stress can also be attenuated by shRNA targeted against Barrington's nucleus CRF supports a role for CRF upregulation in Barringtons' nucleus neurons in these effects. The inability of the antagonist to alter stress-induced CRF upregulation in Barrington's nucleus neurons implies an action downstream from these neurons at the level of the spinal cord where their axons terminate (21). By preventing the inhibitory influence of excess CRF at the level of the spinal cord, the CRF antagonist can prevent the development of the urinary dysfunction associated with social stress. The CRF antagonist was also effective in reversing some of the same urodynamic consequences associated with pBOO. Together, the results support a role for CRF in urinary retention in certain bladder dysfunctions and suggest that CRF receptor antagonists may improve function in these conditions.
Social stress-induced bladder dysfunction.
Although fear has been anecdotally associated with micturition, numerous studies have documented that rodents that become subordinate by exposure to social stress develop urinary retention that is sufficient to increase bladder mass (4, 6, 12, 22, 45). In some cases, this can be sufficiently severe as to result in death due to nephritis (12). Cystometry studies in mice and rats exposed to repeated social stress demonstrated that the voiding dysfunction was characterized by increased IMI, BC, and MV (4, 45). Importantly, it was not associated with increased MP, consistent with decreased central drive to the detrusor, as opposed to increased outlet resistance as occurs in pBOO. Notably, social stress can induce some of the same molecular changes as produced by pBOO, and these changes are thought to lead to bladder remodeling (4). The urodynamic effects of social stress have been documented in males only because rodent models of confrontational social stress are generally limited to males. Special conditions (exposure to lactating females) are required to produce a comparable confrontational stress for female rats, so that the effects of this stress on urodynamic dysfuntion in females have yet to be studied (10).
Role of CRF.
CRF in Barrington's nucleus neurons was hypothesized to be a mediator of social stress-induced voiding dysfunction based on evidence that it has an inhibitory influence in Barrington's nucleus spinal projections. For example, localized chemical activation of Barrington's nucleus elicits bladder contractions that are increased by intrathecal administration of CRF antagonists and conversely, intrathecal CRF administration decreased bladder contractions elicited by Barrington's nucleus stimulation (29). Moreover, social stress upregulates CRF mRNA and protein in Barrington's nucleus neurons (45). Interestingly, repeated restraint stress, which does not alter CRF expression in Barrington's nucleus neurons, has no effect on urodynamics (45). These results led to the hypothesis that social stress increases CRF expression in Barrington's nucleus neurons and its spinal projections, resulting in decreased central drive to the detrusor and urinary retention. This may be an adaptive response to being in the presence of a dominant conspecific. However, if it persists, the voiding postponement and abnormal urodynamics can progress to bladder remodeling similar to that seen with pBOO.
In a recent study of the effects of NBI-30775 on cardiovascular consequences of social stress, the antagonist was demonstrated to prevent stress-induced increases in bladder mass (46). The present study confirmed that finding and further showed that repeated administration of NBI-30775 prevented the urodynamic consequences of repeated social stress. Acute administration of NBI-30775 and antalarmin, another CRF1 antagonist, have been reported to increase micturition frequency and decrease bladder capacity in control rats (16). However, the effects of repeated NBI-30775 in the present study were unrelated to these acute effects because cystometry was performed 72 h after the last dose, and repeated NBI-30775 had no effect on urodynamics in unstressed rats, as seen in control subjects of the social stress study (Fig. 3).
The receptor-ligand binding kinetics of CRF1 receptor antagonists at physiological temperatures have been extensively studied (8). NBI-30775 is a highly potent CRF1 receptor antagonist, possessing a KD of 0.36 nM and a half life of 130 min. Importantly, NBI-30775 is highly efficacious in vivo at the dose used in the present study (10 mg/kg). At this dose, NBI-30775 selectively occupied 75% of CRF1 receptors in the cortex 3 h after treatment and suppressed ACTH in adrenalectomized rats for more than 6 h (8, 9, 46). When administered daily, as in the present study, 10 mg/kg NBI-30775 blocked the behavioral, cardiovascular, and bladder hypertrophic effects of repeated social defeat stress (46).
Systemic NBI-30775 could act at multiple sites. However, given that CRF is a major neurotransmitter in the pontine micturition circuit extending from Barrington's nucleus to the preganglionic parasympathetic neurons of the lumbosacral spinal cord, this is its most likely site of action in affecting urodynamics (43). Other brain circuits in which CRF is prominent have not been directly associated with bladder regulation. Moreover, it is unlikely that the site of action of NBI-30775 is at the level of the bladder because neither CRF1 receptor nor CRF1 immunoreactivity are present in bladder (19). The finding that NBI-30775 prevented the urodynamic consequences of social stress without affecting CRF upregulation in Barrington's nucleus neurons indicates that it was acting at the level of the spinal cord where Barrington's nucleus neurons terminate so that the social stress-induced bladder dysfunction failed to occur in spite of elevated levels of CRF in this pathway.
Social stress had similar urodynamic consequences in rats injected with AAV-shRNA-CRFscrambled as in vehicle-injected rats in the drug study and the mean numbers of CRF-immunoreactive Barrington's nucleus neurons were similar in both groups. AAV-shRNACRF was effective in reducing the number of CRF-immunoreactive Barrington's nucleus neurons in stressed rats and alleviated some of the urodynamic consequences. For these experiments, the timing between the shRNA injection and examination of the urodynamic endpoints is critical to observe optimal effects of CRF knockdown. The number of CRF-immunoreactive Barrington's nucleus neurons (i.e., protein expression) was reduced 17 days after shRNA injection, consistent with the improved urodynamics. However, CRF mRNA recovered at this time. CRF mRNA was significantly decreased by 9 days after injection, which would correspond to the second day of social stress. Given that urodynamic endpoints are examined 3 days after 7 days of social stress, this suggests that the optimum timing of the injection may be closer to the beginning of the stress. However, having a short-recovery duration between surgical craniotomy and exposure to social stress is not optimal for animal welfare and so it was not tested here. Nonetheless, the results of these experiments remain consistent with the idea of a role for CRF upregulation in Barrington's nucleus neurons in social stress-induced bladder dysfunction.
Together, the CRF1 antagonist and shRNA results support a role for central CRF in social stress-induced bladder dysfunction and suggest that pharmacological manipulation of CRF actions may be a useful line of therapy in stress-induced voiding dysfunctions. Notably, CRF1 antagonists, such as NBI-30775, are being developed for treating diverse stress-related disorders, and some of these have been found to have a profile of ideal pharmacokinetic properties and minor side effects (15, 18).
pBOO-induced bladder dysfunction.
The abnormal urodynamic profile produced by repeated social stress has many similarities with that seen in rats with pBOO in relatively early stages (1–2 wk). A major difference between the two models is the increased micturition pressure with pBOO as a result of increased outlet resistance. Repeated administration of NBI-30775 during the second week of pBOO could not reverse the increased resistance due to the obstruction and, therefore, did not prevent increases in micturition pressure or resulting, bladder hypertrophy. Nonetheless, the IMI, BC, and MV were normalized by administration of the CRF antagonist during the second week. Interestingly, like social stress, pBOO was found to increase CRF expression in Barrington's nucleus neurons. It is tempting to speculate that this contributes to the initial urodynamic profile and that by removing the inhibitory influence of CRF in Barrington's nucleus spinal projections and increasing micturition frequency, chronic antagonism of CRF receptors may improve function in early stages of pBOO.
Perspectives and Significance
Although the treatment of urologic disorders has focused on the bladder, the present results underscore the potential role of the brain in these disorders and that neuromodulators within circuits linking the brain and bladder are potential therapeutic targets. Here, we provided evidence for a role of CRF, one neuromodulator expressed by Barrington's nucleus neurons, in social stress-induced urinary retention. In humans, voiding postponement has been linked with psychiatric comorbidity and childhood sexual or physical abuse (5, 20, 32). Traumatic social events in adulthood such as loss of a loved one or broken marriages have also been associated with urinary retention (7). Notably, Barrington's neurons in human brain also exhibit CRF immunoreactivity (35). Manipulation of CRF actions may be a rational therapeutic avenue for the treatment of these and other urinary disorders.
GRANTS
This work was supported by a National Institutes of Health George O'Brien Center Grant P50 DK-52620, R01-NS-38690, and T32-DK-007748.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.K.W., J.H.W., S.A.Z., and R.J.V. conception and design of research; S.K.W., K.M., and T.G. performed experiments; S.K.W. and K.M. analyzed data; S.K.W., S.A.Z., and R.J.V. interpreted results of experiments; S.K.W. and K.M. prepared figures; S.K.W., K.M., and R.J.V. drafted manuscript; S.K.W., K.M., S.A.Z., and R.J.V. edited and revised manuscript; S.K.W., K.M., T.G., J.H.W., S.A.Z., and R.J.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Dimitri Grigoriadis (Neurocrine Biosciences, La Jolla, CA) for the gift of NBI-30775. The authors also acknowledge Sandra Luz for technical assistance in situ hybridization studies.
REFERENCES
- 1. Barrington FJT. The effect of lesion of the hind- abnd mid-brain on micturition in the cat. Q J Exp Physiol 15: 81–102, 1925 [Google Scholar]
- 2. Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav 43: 158–165, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol 18: 13–24, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Chang A, Butler S, Sliwoski J, Valentino R, Canning D, Zderic S. Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am J Physiol Renal Physiol 297: F1101–F1108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davila GW, Bernier F, Franco J, Kopka SL. Bladder dysfunction in sexual abuse survivors. J Urol 170: 476–479, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science 182: 939–941, 1973 [DOI] [PubMed] [Google Scholar]
- 7. Fenster H, Patterson B. Urinary retention in sexually abused women. Can J Urol 2: 185–188, 1995 [PubMed] [Google Scholar]
- 8. Fleck BA, Hoare SR, Pick RR, Bradbury MJ, Grigoriadis DE. Binding kinetics redefine the antagonist pharmacology of the corticotropin-releasing factor type 1 receptor. J Pharmacol Exp Ther 341: 518–531, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Gutman DA, Owens MJ, Skelton KH, Thrivikraman KV, Nemeroff CB. The corticotropin-releasing factor1 receptor antagonist R121919 attenuates the behavioral and endocrine responses to stress. J Pharmacol Exp Ther 304: 874–880, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Haller J, Fuchs E, Halasz J, Makara GB. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res Bull 50: 33–39, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology 27: 194–202, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Henry JP, Meehan WP, Stephens PM. Role of subordination in nephritis of socially stressed mice. Contr Nephrol 30: 38–42, 1982 [DOI] [PubMed] [Google Scholar]
- 13. Hoshaw BA, Evans JC, Mueller B, Valentino RJ, Lucki I. Social competition in rats: cell proliferation and behavior. Behav Brain Res 175: 343–351, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jutkiewicz EM, Wood SK, Houshyar H, Hsin LW, Rice KC, Woods JH. The effects of CRF antagonists, antalarmin, CP154,526, LWH234, and R121919, in the forced swim test and on swim-induced increases in adrenocorticotropin in rats. Psychopharmacology 180: 215–223, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kehne J, De Lombaert S. Non-peptidic CRF1 receptor antagonists for the treatment of anxiety, depression and stress disorders. Curr Drug Targets CNS Neurol Disord 1: 467–493, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Kiddoo DA, Valentino RJ, Zderic S, Ganesh A, Leiser SC, Hale L, Grigoriadis DE. Impact of the state of arousal and stress neuropeptides on urodynamic function in the freely moving rat. Am J Physiol Regul Integr Comp Physiol 290: R1697–R1706, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Klausner AP, Streng T, Na YG, Raju J, Batts TW, Tuttle JB, Andersson KE, Steers WD. The role of corticotropin-releasing factor and its antagonist, astressin, on micturition in the rat. Auton Neurosci 123: 26–35, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Kunzel HE, Zobel AW, Nickel T, Ackl N, Uhr M, Sonntag A, Ising M, Holsboer F. Treatment of depression with the CRH-1-receptor antagonist R121919: endocrine changes and side effects. J Psychiatr Res 37: 525–533, 2003 [DOI] [PubMed] [Google Scholar]
- 19. LaBerge J, Malley SE, Zvarova K, Vizzard MA. Expression of corticotropin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 291: R692–R703, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Lettgen B, von Gontard A, Olbing H, Heiken-Lowenau C, Gaebel E, Schmitz I. Urge incontinence and voiding postponement in children: somatic and psychosocial factors. Acta Paediatr 91: 978–984; discussion 895–976, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Loewy AD, Saper CB, Baker RP. Descending projections from the pontine micturition center. Brain Res 172: 533–538, 1979 [DOI] [PubMed] [Google Scholar]
- 22. Lumley LA, Sipos ML, Charles RC, Charles RF, Meyerhoff JL. Social stress effects on territorial marking and ultrasonic vocalizations in mice. Physiol Behav 67: 769–775, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Marson L. Central nervous system neurons identified after injection of pseudorabies virus into the rat clitoris. Neurosci Lett 190: 41–44, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Marson L, McKenna KE. CNS cell groups involved in the control of the ischiocavernosus and bulbospongiosus muscles; a transneuronal tracing study using pseudorabies virus. J Comp Neurol 374: 161–179, 1996 [DOI] [PubMed] [Google Scholar]
- 25. McFadden K, Griffin T, Levy V, Wolfe J, Valentino R. Overexpression of corticotropin-releasing factor in Barrington's nucleus neurons by adeno-associated viral transduction: effects on bladder function and behavior. Eur J Neurosci 36: 3356–3364, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 60: 253–259, 1979 [DOI] [PubMed] [Google Scholar]
- 27. Noto H, Roppolo JR, Steers WD, De Groat WC. Excitatory and inhibitory influences on bladder activity elicited by electrical stimulation in the pontine micturition center in the rat. Brain Res 492: 99–115, 1989 [DOI] [PubMed] [Google Scholar]
- 28. Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL, Wolfe JH. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol 77: 7034–7040, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pavcovich LA, Valentino RJ. Central regulation of micturition in the rat by corticotropin- releasing hormone from Barrington's nucleus. Neurosci Lett 196: 185–188, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol Psychiatry 71: 317–326, 2012 [DOI] [PubMed] [Google Scholar]
- 31. Rickenbacher E, Baez MA, Hale L, Leiser SC, Zderic SA, Valentino RJ. Impact of overactive bladder on the brain: central sequelae of a visceral pathology. Proc Natl Acad Sci USA 105: 10589–10594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Romans S, Belaise C, Martin J, Morris E, Raffi A. Childhood abuse and later medical disorders in women. An epidemiological study. Psychother Psychosom 71: 141–150, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Rouzade-Dominguez ML, Miselis R, Valentino RJ. Central representation of bladder and colon revealed by dual transsynaptic tracing: substrates for pelvic visceral coordination. Eur J Neurosci 18: 3311–3324, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Rouzade-Dominguez ML, Pernar L, Beck S, Valentino RJ. Convergent responses of Barrington's nucleus neurons to pelvic visceral stimuli: a juxtacellular labeling study. Eur J Neurosci 18: 3325–3334, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Ruggiero DA, Underwood MD, Rice PM, Mann JJ, Arango V. Corticotropin-releasing hormone and serotonin interact in the human brainstem: behavioral implications. Neuroscience 91: 1343–1354, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Sakanaka M, Shibasaki T, Lederes K. Corticotropin-releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxide-diaminobenzidene method. J Comp Neurol 260: 256–298, 1987 [DOI] [PubMed] [Google Scholar]
- 37. Swanson LW, Sawchenko PE, Rivier J, Vale W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in rat brain: an immunohistochemical study. Neuroendocrinology 36: 165–186, 1983 [DOI] [PubMed] [Google Scholar]
- 38. Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213: 1394–1397, 1981 [DOI] [PubMed] [Google Scholar]
- 39. Valentino RJ, Chen S, Zhu Y, Aston-Jones G. Evidence for divergent projections of corticotropin-releasing hormone neurons of Barrington's nucleus to the locus coeruleus and spinal cord. Brain Res 732: 1–15, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Valentino RJ, Kosboth M, Winogren ML, Miselis RR. Transneuronal labeling from the rat distal colon: anatomical evidence for regulation of distal colon function by a pontine corticotropin-releasing factor system. J Comp Neurol 417: 399–414, 2000 [PubMed] [Google Scholar]
- 41. Valentino RJ, Wood SK, Wein AJ, Zderic SA. The bladder-brain connection: putative role of corticotropin-releasing factor. Nature Rev Urol 8: 19–28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Viau V, Chu A, Soriano L, Dallman MF. Independent and overlapping effects of corticosterone and testosterone on corticotropin-releasing hormone and arginine vasopressin mRNA expression in the paraventricular nucleus of the hypothalamus and stress-induced adrenocorticotropic hormone release. J Neurosci 19: 6684–6693, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vincent SR, Satoh K. Corticotropin-releasing factor (CRF) immunoreactivity in the dorsolateral pontine tegmentum: further studies on the micturition reflex system. Brain Res 308: 387–391, 1984 [DOI] [PubMed] [Google Scholar]
- 44. Vizzard MA, Erickson VL, Card JP, Roppolo JR, de Groat WC. Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J Comp Neurol 355: 629–640, 1995 [DOI] [PubMed] [Google Scholar]
- 45. Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol 296: R1671–R1678, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: a putative role for corticotropin-releasing factor. Psychopharmacology 222: 325–336, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology 151: 1795–1805, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]