Abstract
Nitric oxide (NO) is the principal mediator of penile erection, and soluble guanylate cyclase (sGC) is the receptor for NO. In pathophysiological conditions when sGC is inactivated and not responsive to NO or sGC stimulators a new class of agents called sGC activators increase the activity of NO-insensitive sGC and produce erection. The aim of this study was to investigate erectile responses to BAY 60-2770, a sGC activator, under physiological and pathophysiological conditions. In the present study increases in intracavernosal pressure (ICP) in response to intracavernosal (ic) injections of BAY 60-2770 were investigated under baseline conditions, when sGC was inhibited by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), when nitric oxide synthase (NOS) was inhibited by N-nitro-l-arginine methyl ester (l-NAME), and after cavernosal nerve crush injury. Under baseline conditions ic injections of BAY 60-2770 increase ICP, ICP/mean arterial pressure (MAP), and area under the ICP curve (AUC) and produce small decreases in MAP at the highest doses studied. BAY 60-2770 was very potent in its ability to induce erection and responses to BAY 60-2770 were enhanced by ODQ which attenuates erectile responses to sodium nitroprusside (SNP), diethylamine NONOate (DEA/NO), and cavernosal nerve stimulation. Responses to BAY 60-2770 were not altered by l-NAME or cavernosal nerve crush injury. These data indicate that BAY 60-2770 has potent erectile activity that is enhanced by ODQ and show that responses to BAY 60-2770 are not attenuated by NOS inhibition or cavernosal nerve injury. These results suggest that BAY 60-2770 would be effective in the treatment of erectile dysfunction when NO bioavailability is reduced, after pelvic nerve injury, and when sGC is oxidized.
Keywords: erectile dysfunction, oxidative stress, impaired cavernosal nerve function
nitric oxide (NO) is the principal mediator of penile erection, and a decrease in NO formation or bioavailability will result in erectile dysfunction (ED) (2, 11, 15). The treatment of ED with phosphodiesterase type 5 (PDE-5) inhibitors is effective in most patients; however, some patients do not respond to these agents because of very low endogenous NO formation and increased oxidative stress which can inactivate NO and inhibit the activity of soluble guanylate cyclase (sGC) (Fig. 1) (1, 14). In situations with increased reactive oxygen species formation, sGC can be oxidized and rendered nonresponsive to stimulation by NO or agents that are sGC stimulators (28). A new class of agents called sGC activators has been shown to increase the catalytic activity of oxidized or heme-free sGC and promote vasodilation in experimental animals when responses to NO donors and sGC stimulators are severely attenuated (Fig. 1) (23, 27). BAY 60-2770 is a sGC activator that increases the activity of oxidized sGC; however, the effects of BAY 60-2770 on erectile function have not been determined. It is our hypothesis that the sGC activator BAY 60-2770 will induce erection in rats. It is also our hypothesis that erectile responses to BAY 60-2770 will be enhanced by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) in a dose that inhibited erectile responses to the NO donors sodium nitroprusside (SNP) and diethylamine NONOate (DEA/NO) and to the sGC stimulator BAY 41-8543 (29). The purpose of the present study was to investigate erectile responses to the sGC activator BAY 60-2770 under baseline conditions, when sGC is inactivated by ODQ, when nitric oxide synthase (NOS) is inhibited by N-nitro-l-arginine methyl ester (l-NAME), and after cavernosal nerve crush injury. The results of these studies indicate that BAY 60-2770 has very potent erectile activity that is enhanced by treatment with ODQ and is not decreased by treatment with a NOS inhibitor or by cavernosal nerve crush injury.
Fig. 1.
Diagram showing the activation of normally reduced soluble guanylate cyclase (sGC) by nitric oxide (NO), CO, the sGC stimulators YC-1, BAY 41–2272, BAY 41-8543, BAY 60–4552, A-350619, and CFM 1571, and the sGC activators BAY 60-2770, BAY 58–2667. The activation of sGC results in increased conversion of GTP to cGMP and erection. The catalytic activity of the normally reduced enzyme is increased by NO, CO, sGC stimulators, and sGC activators, whereas only sGC activators can increase the catalytic activity of oxidized sGC which is not responsive to NO, CO or sGC stimulators as shown in Figs. 4, 5, 6, and 8. sGC is inactivated by ODQ, which oxidizes the heme iron on the beta subunit of the enzyme and renders it insensitive to NO. The heme oxygenase pathway, which induces erection by generating CO, is also shown.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee of Tulane University School of Medicine approved the experimental protocol used in these studies, and all procedures were conducted in accordance with institutional guidelines. For these experiments, adult male Sprague-Dawley rats, weighing 318–419 g, were anesthetized with Inactin (thiobutabarbital), 100 mg/kg ip. Supplemental doses of Inactin were given intraperitoneally as needed to maintain a uniform level of anesthesia. Body temperature was maintained with a heating lamp. The trachea was cannulated with a short segment of PE-240 tubing to maintain a patent airway, and the left carotid artery was catheterized with PE-50 tubing for measurement of systemic arterial pressure. Intracavernosal pressure (ICP) was measured with a 25-gauge needle inserted into the left crura of the penis connected to PE-50 tubing filled with heparin. Systemic arterial pressure and ICP were measured with Namic Perceptor DT pressure transducers and a data acquisition system (Biopac MP 100A-CE, Santa Barbara, CA). Intracavernosal pressure, systemic arterial pressure, and mean systemic arterial pressure (MAP) obtained by electronic averaging were continuously recorded and were displayed and stored on a Dell PC. The left jugular vein was catheterized with PE-50 tubing for the systemic administration of drugs and fluids. A 25-gauge needle connected to PE-50 tubing was placed in the right crura of the penis for administration of drugs. Maximal ICP in response to intracavernosal (ic) injection of the vasodilator agents or in response to cavernosal nerve stimulation was measured at the peak of the ICP pressure increase. The area under the ICP curve (AUC) and duration of the increase in ICP were measured to characterize the total erectile response.
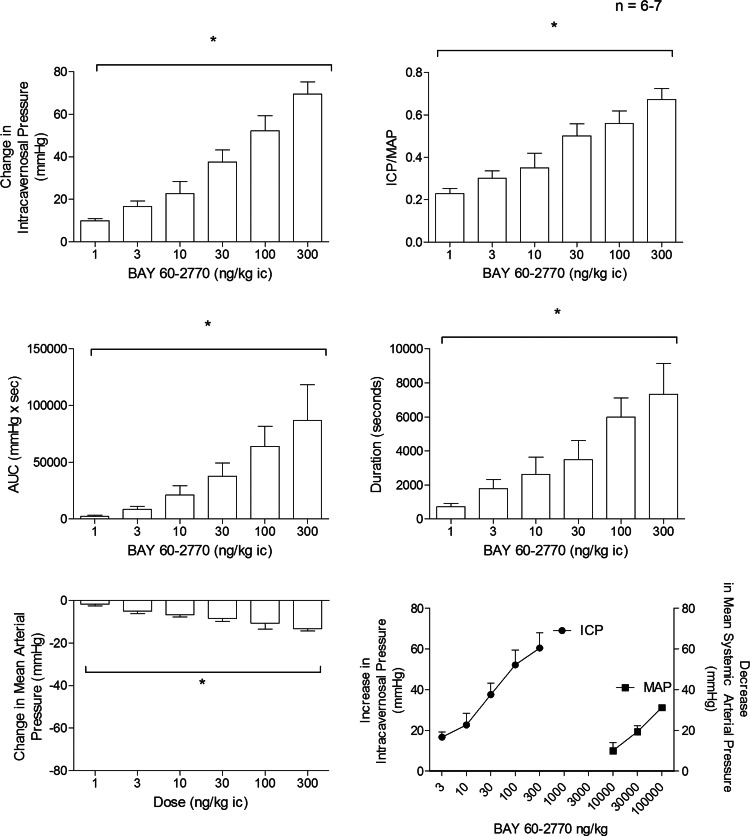
The ic injection of BAY 60-2770 at the highest doses studied produced small decreases in MAP. To further characterize the effects of BAY 60-2770 on MAP responses to intravenous injections of BAY 60-2770, 10, 30, and 100 μg/kg were investigated and the data are shown in Fig. 2, bottom right. The results of these experiments indicate that BAY 60-2770 is far more potent (more than 4 orders of magnitude) in its ability to increase ICP when injected ic compared with its ability to decrease MAP when injected intravenously.
Fig. 2.
Bar graphs showing the dose-dependent response to intracavernosal (ic) injections of BAY 60-2770 on intracavernosal pressure (ICP), ICP/mean arterial pressure (MAP), area under the ICP curve (AUC), duration, and MAP in the anesthetized rat. Line graph in the bottom right panel shows a comparison of responses to ic injections and iv injections of BAY 60-2770 on intracavernosal and mean systemic arterial pressure. n = no. of experiments; *P < 0.05 with ANOVA.
Cavernosal nerve stimulation was carried out as previously described in the literature (16). For nerve stimulation, the bladder and prostate were exposed through a midline abdominal incision. The cavernosal nerve was identified posterolateral to the prostate on one side, and a stainless steel bipolar stimulating electrode was placed on the nerve. The cavernosal nerve was stimulated with square wave pulses at frequencies of 2, 4, 8, 10, and 16 Hz, at 5 V and a pulse width of 5 ms for a duration of 60 s with a Grass Instruments SD9 Stimulator. A rest period of at least 3 min was allowed between nerve stimulation trials and similar results were obtained with 5- and 10-min rest periods. Acute nerve crush injury experiments were performed using three 15-s applications of a 3-in. forcep to the cavernosal nerve 5 mm distal from the major pelvic ganglion. For chronic crush experiments, rats were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg), the prostate was exposed through a midline laparotomy, and the cavernosal nerves were identified. Bilateral nerve crush injury was performed by applying three 15-s applications of a 3-in. forcep to the cavernosal nerves 5 mm distal from the major pelvic ganglion. A two-layered closure was performed on the animals with 4–0 Vicryl suture. Thirty days after bilateral cavernosal nerve injury erectile function was measured as described above.
Methemoglobin levels were measured in 85-μl arterial blood samples removed from the carotid artery catheter and were analyzed with a Radiometer NPT7 series analyzer (Copenhagen).
BAY 60-2770 and BAY 41-8543 were provided by Dr. Johannes-Peter Stasch of the Institute of Cardiovascular Research, Pharma Research Centre, Bayer AG, Wuppertal, Germany, and were dissolved in Transcutol-Cremophor EL-0.9% NaCl solution (10:10:80). ODQ (Cayman) was also dissolved in the Transcutol-Cremophor vehicle. The dose of ODQ used in these experiments was determined from pilot experiments and reports in the literature (17). No financial support was provided by Bayer. Sodium nitroprusside, DEA/NO, l-NAME, isoproterenol hydrochloride (Sigma-Aldrich) and fasudil (LC Laboratories) were dissolved in 0.9% NaCl, and solutions were made on a frequent basis. Cromakalim (TOCRIS Biosciences) was dissolved in 1.0% DMSO solution. Imatinib mesylate (Novartis), avanafil (Vivus), and pinacidil monohydrate (Research Biochemicals International) were dissolved in deionized water titrated to a pH of 5. For ic injections the doses of BAY 60-2770, BAY 41-8543, SNP, DEA/NO, pinacidil, cromakalim, imatinib, fasudil, and isoproterenol were prepared in a total volume of 200 μl and were injected through the 25-gauge needle in the right crura. The data are expressed as means ± SE and were analyzed using a one-way ANOVA and a Student's t-test for paired data. A P value of <0.05 was used as the criterion for statistical significance.
RESULTS
Effect of BAY 60-2770 on erectile activity.
A diagram illustrating the effects of sGC stimulators (YC-1, BAY 41–2272, BAY 41-8543, BAY 60–4552, A-350619, and CFM 1571), of NO, and of the sGC activators (BAY 60-2770, BAY 58–2667, and HMR 1766) on sGC activity and erectile function is shown in Fig. 1. sGC stimulators, NO, and sGC activators all increase the catalytic activity of normally reduced sGC, increase cGMP formation, and induce vasodilation and erection (15). In contrast, only sGC activators will have the capacity to increase the catalytic activity of oxidized or heme free sGC as shown by Schmidt et al. (24) and induce vasodilation and erection (Fig. 1). The heme oxygenase pathway is also shown in Fig. 1. Heme oxygenase converts heme to biliverdin, iron, and CO which can induce penile erection by activating the reduced form of sGC as well as by opening Ca2+ activated potassium channels (KCa) (25).
The effect of BAY 60-2770 on erectile function was investigated in the anesthetized rat, and these data are summarized in Fig. 2. The ic injection of BAY 60-2770 in doses of 1–300 ng/kg produced dose-related increases in ICP, ICP/MAP, AUC, and response duration (Fig. 2). The increases in ICP in response to ic injections of BAY 60-2770 were rapid in onset with values ranging from 15–30 s and MAP was only reduced with ic injections of the higher doses of BAY 60-2770 which decreased MAP by 9 ± 2, 11 ± 3, and 14 ± 1 mmHg at doses of 30, 100, and 300 ng/kg ic, respectively (Fig. 2). The results with ic injections of BAY 60-2770 indicate that the sGC activator has very potent erectile activity and produces only small decreases in MAP at the higher doses injected ic in the anesthetized rat. A comparison of the effects of ic injection of BAY 60-2770 on ICP and of intravenous injection of BAY 60-2770 on MAP is shown in Fig. 2, bottom right. The comparison of the dose-response curves for the increase in ICP and the decrease in MAP in response to BAY 60-2770 indicates the sGC activator was more than 4 orders of magnitude more potent in its ability to increase ICP when injected ic compared with its ability to decrease MAP when injected intravenously. These data indicate that BAY 60-2770 is far more potent in its ability to increase ICP than in its ability to produce vasodilation in the systemic vascular bed and decrease MAP in the rat.
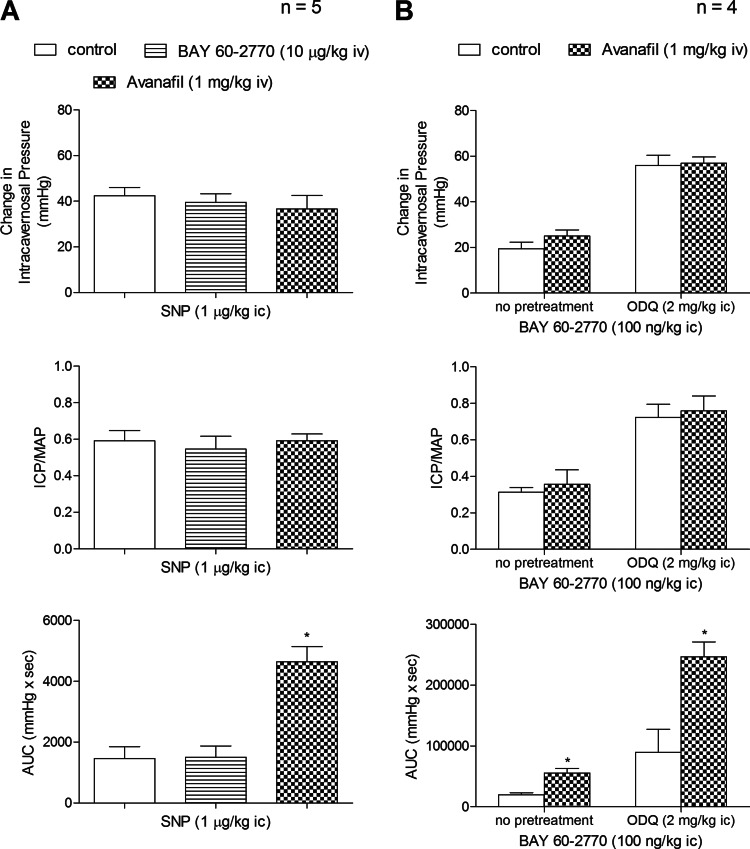
The potential role of phosphodiesterase inhibition in contributing to the erectile response to BAY 60-2770 was investigated, and these results are presented in Fig. 3. The administration of the PDE-5 inhibitor avanafil increased the AUC (total erectile response) to the NO donor SNP whereas BAY 60-2770 had no significant effect on the AUC of the response to SNP (Fig. 3). These data indicate that BAY 60-2770 had no apparent effect on response duration whereas the PDE-5 inhibitor avanafil increased the AUC of the response to the NO donor. Avanafil increased the AUC of the response to BAY 60-2770 under control conditions and after treatment with ODQ. These data provide support for the hypothesis that cyclic guanosine monophosphate (cGMP) mediates responses to BAY 60-2770 when sGC is normally reduced or oxidized by ODQ and suggest that BAY 60-2770 does not inhibit the degradation of cGMP.
Fig. 3.
A: bar graphs showing the effect of treatment with the PDE-5 inhibitor avanafil (1 mg/kg iv) or BAY 60-2770 (10 μg/kg iv) on the change in ICP, ICP/MAP, and AUC in response to ic injection of the NO donor sodium nitroprusside (SNP). B: bar graphs showing the effect of treatment with avanafil (1 mg/kg iv), ODQ (2 mg/kg ic) or combination treatment of avanafil (1 mg/kg iv) + ODQ (2 mg/kg ic) on the change in ICP, ICP/MAP. and AUC in response to ic injection of BAY 60-2770 (100 ng/kg). n = no. of experiments; *P < 0.05, paired comparison.
Effect of ODQ.
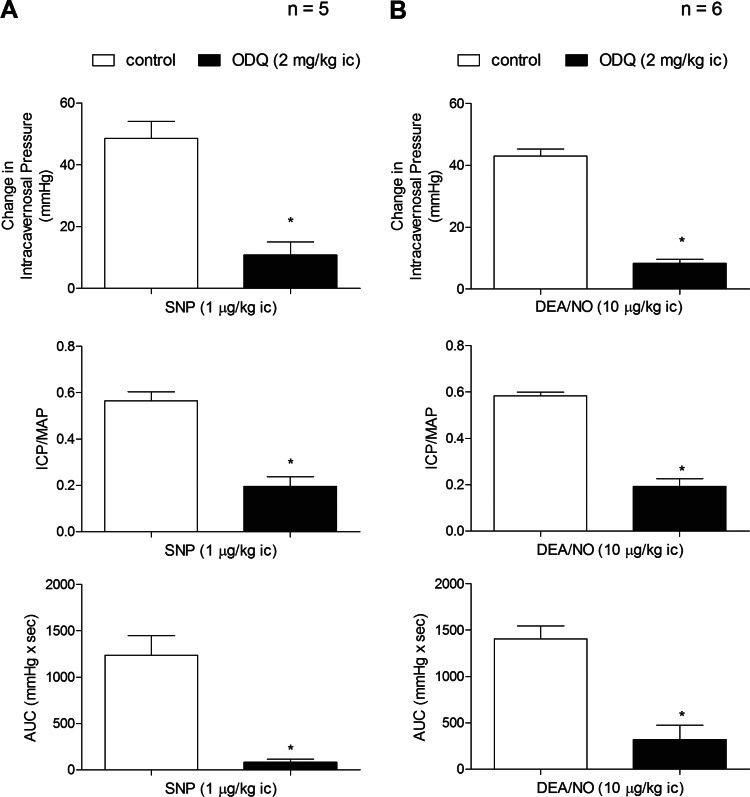
ODQ is an agent that has been shown to inhibit the activation of sGC by NO by oxidizing the heme iron on the beta subunit of the enzyme (22, 28, 29). In the present study, the administration of ODQ in a dose of 2 mg/kg ic attenuated the increase in ICP in response to ic injections of the NO donors SNP and DEA/NO and the sGC stimulator BAY 41-8543 (Figs. 4 and 6).
Fig. 4.
Bar graphs showing the effect of treatment with ODQ (2 mg/kg ic) on the change in ICP, ICP/MAP, and AUC in response to ic injection of the NO donors SNP (A) and DEA/NO (B). n = no. of experiments; *P < 0.05, paired comparison.
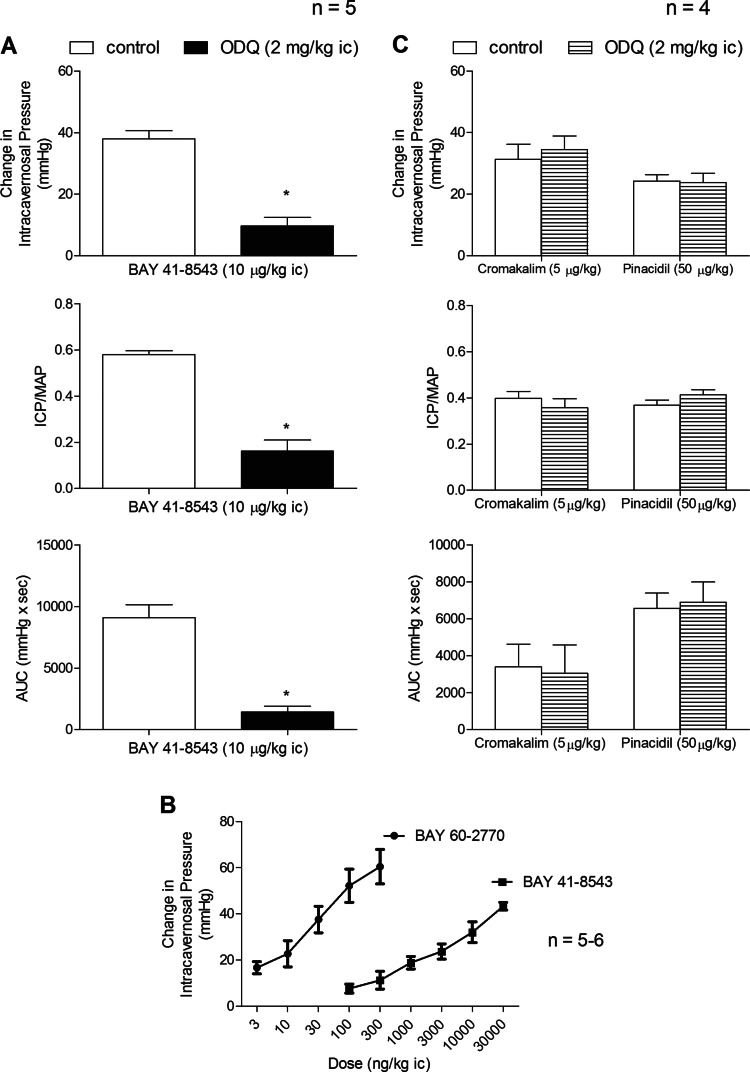
Fig. 6.
Bar graphs comparing the effect of ODQ treatment (2 mg/kg ic) on the change in ICP, ICP/MAP, and AUC in response to ic injection of BAY 41-8543 (A) and ic injection of the K+ ATP channel agonists cromakalim and pinacidil (C). Line graphs comparing change in ICP in response to ic injections of BAY 60-2770 and BAY 41-8543 (B). n = no. of experiments; *P < 0.05, paired comparison.
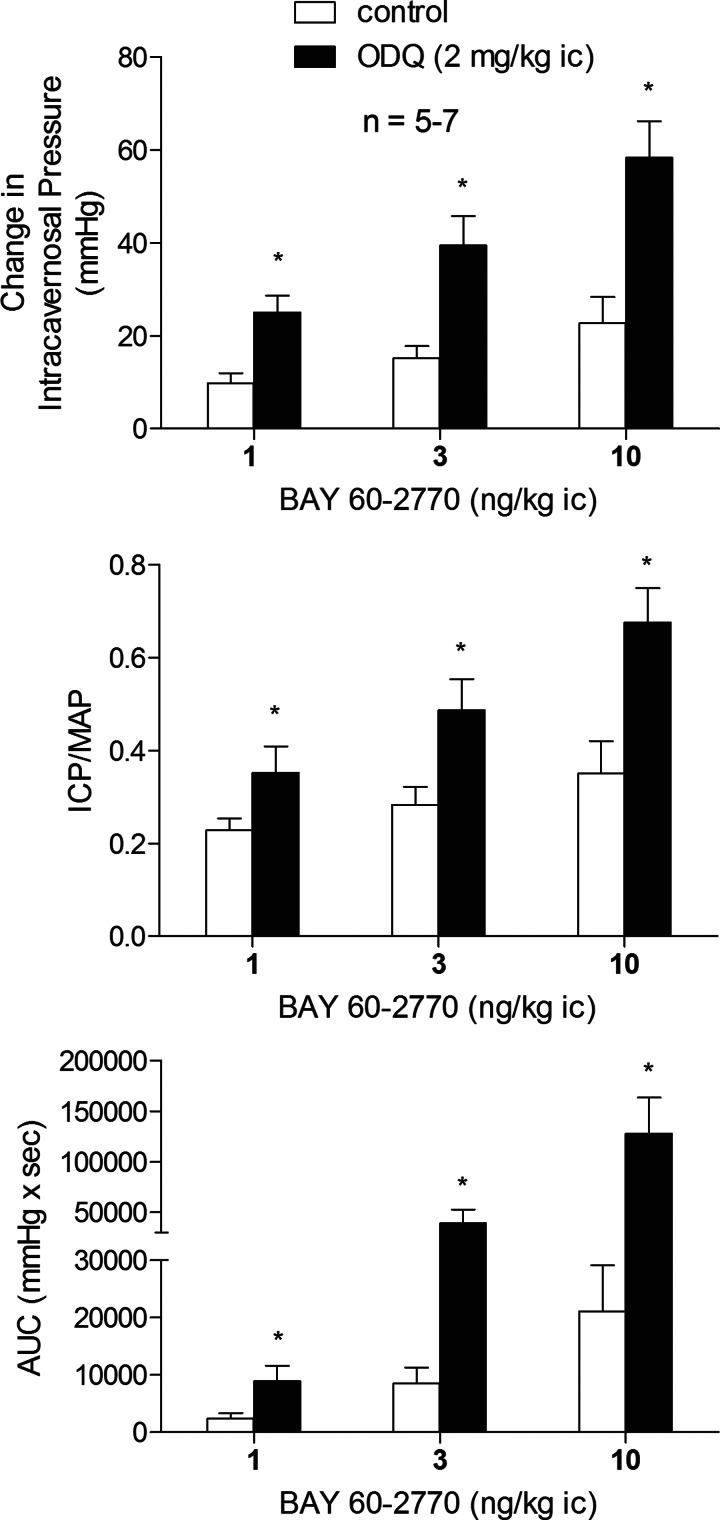
The sGC activators like BAY 60-2770 can increase the catalytic activity of oxidized or heme-free sGC as reported by Schmidt et al. and illustrated in Fig. 1 (24, 28). The effect of ODQ treatment on the erectile response to BAY 60-2770 was investigated, and these data are shown in Fig. 5. After treatment with ODQ, 2 mg/kg ic, the increases in ICP, ICP/MAP, and AUC in response to ic injection of BAY 60-2770 were enhanced significantly (Fig. 5). In contrast to the effect of ODQ treatment on the erectile response to ic injection of the sGC activator BAY 60-2770, the increases in ICP, ICP/MAP, and AUC in response to ic injection of the sGC stimulator BAY 41-8543 were significantly reduced by ODQ treatment (Fig. 6A). The comparison of dose-response curves for ic injections of BAY 60-2770 and BAY 41-8543 show that the erectile activity of the sGC activator BAY 60-2770 is approximately 1,000-fold greater than the erectile activity of the sGC stimulator BAY 41-8543 under control conditions (Fig. 6B).
Fig. 5.
Bar graphs showing the effect of treatment with ODQ (2 mg/kg ic) on the increases in ICP, ICP/MAP, and AUC in response to ic injections of BAY 60-2770. n = no. of experiments; * P < 0.05, paired comparison.
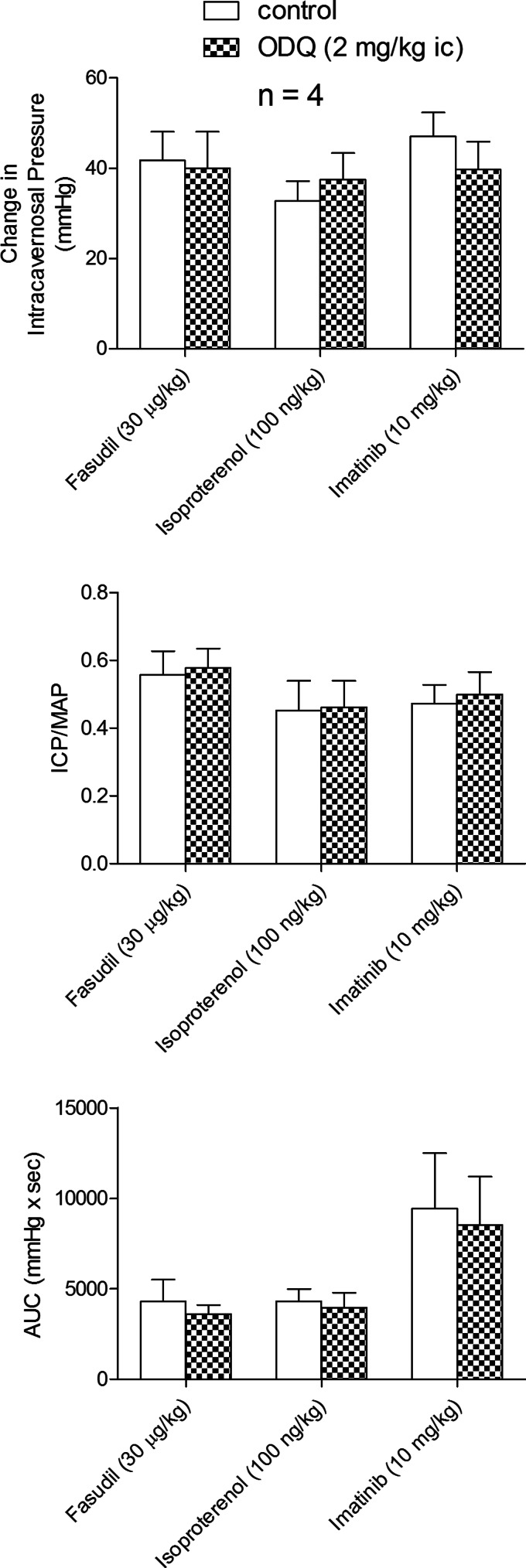
The selectivity of the inhibitory effect of ODQ on the erectile response to the NO donors was investigated in experiments with the K+ ATP channel agonists cromakalim and pinacidil which have been shown to have erectile activity (10, 20, 21). The ic injection of 5 μg/kg cromakalim or 50 μg/kg pinacidil produced increases in ICP, ICP/MAP, and AUC, and these responses were not changed by treatment with ODQ (2 mg/kg ic) (Fig. 6C). In addition the effect of ODQ treatment on erectile responses to fasudil, isoproterenol, and imatinib were investigated, and these data are summarized in Fig. 7. The ic injection of 30 μg/kg fasudil, 100 ng/kg isoproterenol, or 10 mg/kg imatinib produced significant increases in ICP, ICP/MAP, and AUC, and these increases were not changed by treatment with ODQ, 2 mg/kg ic (Fig. 7). These data indicate that the inhibitory effect of ODQ on NO-mediated erectile responses is specific or not off-target since the erectile response to the K+ ATP activators cromakalim or pinacidil which induce vasodilation and erection by hyperpolarizing smooth muscle was not changed by the sGC inhibitor ODQ. In addition the effect of ODQ treatment on responses to agents that increase ICP by diverse mechanisms was examined and these results indicate that ODQ did not alter erectile responses mediated by inhibition of Rho-kinase (fasudil), activation of beta adrenergic receptors (isoproterenol), or inhibition of tyrosine kinase signaling (imatinib).
Fig. 7.
Bar graphs comparing the effect of ODQ treatment (2 mg/kg ic) on the change in ICP, ICP/MAP, and AUC in response to ic injections of the Rho-kinase inhibitor fasudil, the beta-adrenergic agonist isoproterenol, and the tyrosine kinase inhibitor imatinib. n = no. of experiments; *P < 0.05, paired comparison.
Since ODQ can oxidize other heme proteins including hemoglobin the effect of ODQ treatment on methemoglobin levels was investigated and the results show that ODQ treatment in a dose that enhanced responses to BAY 60-2770 and inhibited responses to NO donors and the sGC stimulator BAY 41-8543 had no significant effect on methemoglobin values in arterial blood (control metHb = 1.77 ± 0.42% vs. ODQ treated metHb = 2.32 ± 0.47%, P > 0.05, n = 6).
Effect of ODQ on the response to cavernosal nerve stimulation.
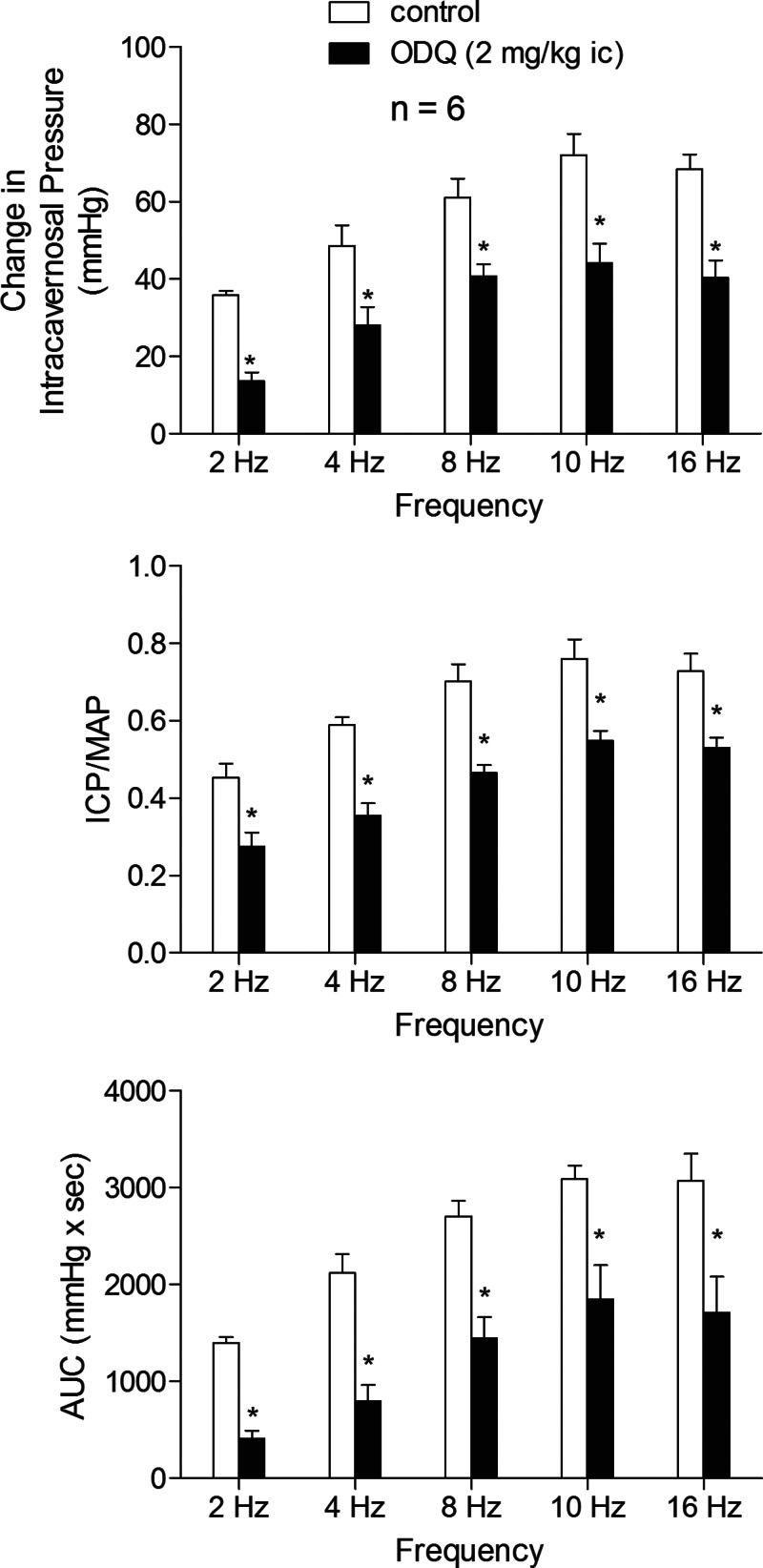
The effect of ODQ treatment on the erectile response to cavernosal nerve stimulation was investigated and these data are summarized in Fig. 8. Cavernosal nerve stimulation at 2–16 Hz increased ICP, ICP/MAP, and AUC in a stimulus frequency-dependent manner (Fig. 8). Treatment with ODQ, 2 mg/kg ic, significantly reduced the increases in ICP, ICP/MAP, and AUC at all stimulus frequencies of cavernosal nerve stimulation studied (Fig. 8). These data indicate that the responsiveness of sGC in penile vascular and corporal tissue to endogenous (neurogenically released) NO is attenuated by ODQ treatment which inhibits sGC by oxidizing the heme iron on the enzyme.
Fig. 8.
Bar graphs illustrating the effect of ODQ treatment (2 mg/kg iv) on the change in ICP, ICP/MAP, and AUC in response to cavernosal nerve stimulation at 2–16 Hz. n = no. of experiments; *P < 0.05, paired comparison.
Effect of nerve crush injury.
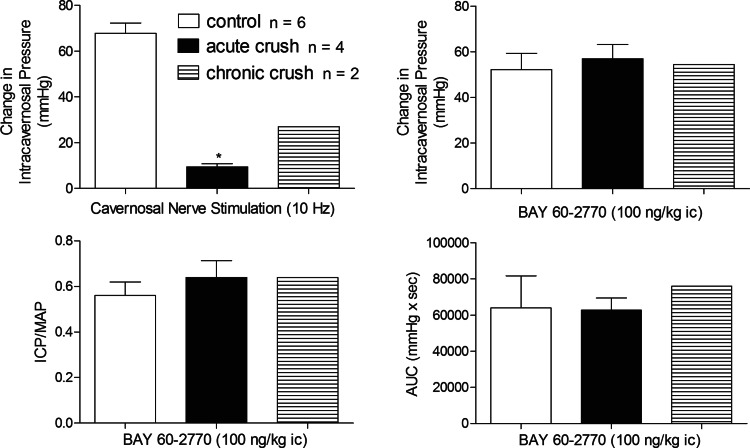
The effect of nerve crush injury on the response to BAY 60-2770 was investigated, and the data are summarized in Fig. 9. Acute (n = 4) and chronic (n = 2) nerve crush injury caused a significant attenuation in the increase in ICP, ICP/MAP, and AUC in the response to cavernosal nerve stimulation. The increases in ICP, ICP/MAP, or AUC in response to ic injection of BAY 60-2770 after nerve crush injury were not different from responses obtained in the control period or in control animals (Fig. 9).
Fig. 9.
Bar graphs illustrating the effect of cavernosal nerve crush injury (solid bars) on the change in ICP in response to cavernosal nerve stimulation at 10 Hz and the effect of acute (n = 4) and chronic (n = 2) cavernosal nerve crush injury on the responses to BAY 60-2770 (100 ng/kg ic). n = no. of experiments; *P < 0.05, paired comparison.
Effect of l-NAME on the response to BAY 60-2770.
The effect of the NOS inhibitor l-NAME on the response to BAY 60-2770 was investigated and treatment with l-NAME 50 mg/kg iv significantly attenuated the increases in ICP, ICP/MAP, and AUC in response to cavernosal nerve stimulation at all stimulus frequencies tested (Fig. 10A). The ic injection of 100 ng/kg BAY 60-2770 in animals treated with l-NAME produced an increase in ICP, ICP/MAP, and AUC that was not different from the response to the sGC activator observed in control animals (Fig. 10B). These data indicate that the erectile response to BAY 60-2770 is independent of NO release in the rat penis.
Fig. 10.
Bar graphs illustrating the effect of l-NAME treatment, 50 mg/kg iv, on the change in ICP, ICP/MAP, and AUC in response to cavernosal nerve stimulation at 2–16 Hz (A). Bar graphs showing the effect of l-NAME treatment on the change in ICP, ICP/MAP, and AUC in response to ic injection of BAY 60-2770 100 ng/kg ic (B). n = no. of experiments; *P < 0.05, paired comparison.
DISCUSSION
NO is the principal mediator of penile erection (2, 6, 11). NO is released from the nerves innervating the corpora cavernosa and from the endothelium of small arteries and the corpora cavernosa, and the response to cavernosal nerve stimulation is reduced by treatment with the NOS inhibitor l-NAME (3). Once released from nerve endings or the endothelium NO binds to the normally reduced heme iron group on sGC and increases the formation of cGMP, which induces vasodilation and penile erection (11). Oxidation of the heme iron on the enzyme reduces the sensitivity of sGC to NO and ODQ inhibits sGC by oxidizing the heme iron on the enzyme and attenuates responses to NO and sGC stimulators like BAY 41-8543 (22, 28, 29). The activity of oxidized or heme-free sGC can be restored with a new class of agents called sGC activators, of which BAY 60-2770 is a member (Fig. 1) (8, 12, 22). Erectile responses to sGC activators have not been reported, and new findings in the present study are that BAY 60-2770 has potent erectile activity in the anesthetized rat and is about 1,000-fold more potent than the sGC stimulator BAY 41-8543 in its ability to increase ICP, ICP/MAP, and AUC under control conditions. Although BAY 60-2770 had potent erectile activity in the rat it has very modest hypotensive activity and was more than 4 orders of magnitude less potent in its ability to decrease MAP which suggests that the activator has selectivity for sGC in cavernosal and vascular smooth muscle in the penis.
In addition to having potent erectile activity in the rat, the present data show that erectile responses to BAY 60-2770 were enhanced by treatment with ODQ which attenuated responses to the NO donors DEA/NO and SNP and to the sGC stimulator BAY 41-8543 by inhibiting sGC by oxidizing the heme iron on the enzyme as reported by Zhao et al. (29). The observation that increases in ICP, ICP/MAP, and AUC in response to BAY 60-2770 were enhanced by ODQ suggests that sGC activators would be effective in conditions where ROS production is increased, NO is inactivated, and sGC is not responsive to NO or sGC stimulators. The administration of ODQ attenuates erectile responses to cavernosal nerve stimulation and to the NO donors DEA/NO and SNP and the sGC stimulator BAY 41-8543 providing support for the hypothesis that sGC stimulators and NO may share a similar sGC activating mechanism (4, 26). The selectivity of the inhibitory effect of ODQ on the response to the NO donors was investigated in experiments with cromakalim and pinacidil which induce erection by opening K+ ATP channels and hyperpolarizing smooth muscle of the corpora cavernosa (13, 20). In addition the effect of ODQ on erectile responses to fasudil, isoproterenol, and imatinib was investigated. The observation that erectile responses to the K+ ATP channel activators and the beta-adrenergic receptor agonist isoproterenol or agents which inhibit Rho-kinase or tyrosine kinase signaling are not altered by ODQ in a dose that attenuated responses to NO donors SNP and DEA/NO suggests that the actions of ODQ are selective for NO and are not nonspecific or off-target. The observation that methemoglobin levels were not altered suggests the effect of ODQ on the heme iron of sGC is not nonselective in that hemoglobin is not oxidized by ODQ in the present study.
Inasmuch as ODQ has been reported to elicit sGC-independent effects future experiments should be performed to confirm the results of the present experiments using nonpharmacological means such as siRNA or shRNA in the intact rat penis (7, 18, 19). These experiments are beyond the scope of the present studies, and it would be interesting to investigate responses to sGC activators like BAY 60-2770 in cGKI knockout mice.
The effect of ODQ treatment on the response to electrical stimulation of the cavernosal nerves was investigated, and the results of these experiments show that increases in ICP, ICP/MAP, and AUC in response to nerve stimulation are attenuated indicating that ODQ treatment decreases the response of sGC in penile vascular and corporal smooth muscle to neurogenically released NOS by inhibiting sGC. The effect of NOS inhibition with l-NAME was investigated, and treatment with l-NAME in a dose that inhibited the response to cavernosal nerve stimulation by more than 90% did not attenuate the erectile response to BAY 60-2770. The results with ODQ and l-NAME treatment could be interpreted to suggest that BAY 60-2770 would enhance erectile function in pathological conditions where NO production and bioavailability are diminished or when sGC is inactivated by oxidative stress. The effect of acute and chronic nerve crush injury on the response to BAY 60-2770 was investigated and the ability of BAY 60-2770 to increase ICP, ICP/MAP, and AUC was not altered by nerve injury that reduced the response to cavernosal nerve stimulation by more than 80%. The results with acute and chronic nerve crush injury suggest that responses to BAY 60-2770 are not dependent on tonic cavernosal nerve activity and that agents in this class may be useful in the treatment of ED resulting from surgical procedures such as prostatectomy.
Conclusions.
In summary and as illustrated in Fig. 1, sGC activators increase the catalytic activity of normally reduced or oxidized sGC (22, 27). In the present study the sGC activator BAY 60-2770 induces vasodilation and penile erection. BAY 60-2770 had very potent erectile activity compared with the sGC stimulator BAY 41-8543 and had very modest hypotensive activity in the rat suggesting that the agent has selectivity for sGC in penile tissues. Erectile responses to BAY 60-2770 were enhanced by treatment with ODQ, which inhibits NO-stimulated sGC activity by oxidizing the heme iron on the enzyme and reduces responses to NO donors and sGC stimulators. The potent erectile activity of BAY 60-2770 was not attenuated by treatment with the NOS inhibitor l-NAME or cavernosal nerve crush injury. These results suggest that agents such as BAY 60-2770, which increase the catalytic activity of oxidized or heme-free sGC, would potentially be useful in the treatment of pathological conditions where NO is inactivated by ROS and sGC is oxidized and not responsive to endogenously released or exogenous NO or sGC stimulators. It is known that sGC is present in cavernosal smooth muscle and endothelium and it is hypothesized that agents that target the NO/sGC/cGMP pathway may improve signaling and reduce oxidative stress (9). Therefore the use of such therapies can improve NO bioavailability and improve erectile function (5).
GRANTS
These studies were supported in part by National Heart, Lung, and Blood Institute Grants HL-62000 and HL-77421.
AUTHOR CONTRIBUTIONS
Author contributions: G.F.L., E.A.P., and P.J.K. conception and design of research; G.F.L., E.A.P., T.J.F., J.R.Z., and K.A.W. performed experiments; G.F.L., E.A.P., K.A.W., and P.J.K. analyzed data; G.F.L., E.A.P., K.A.W., and P.J.K. interpreted results of experiments; G.F.L. and E.A.P. prepared Figs.; G.F.L., E.A.P., and P.J.K. drafted manuscript; G.F.L., E.A.P., T.J.F., K.A.W., and P.J.K. edited and revised manuscript; G.F.L., E.A.P., T.J.F., J.R.Z., K.A.W., and P.J.K. approved final version of manuscript.
REFERENCES
- 1. Agarwal A, Nandipati KC, Sharma RK, Zippe CD, Raina R. Role of oxidative stress in the pathophysiological mechanism of erectile dysfunction. J Androl 27: 335–347, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Andersson KE. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol Rev 63: 811–859, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev 75: 191–236, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Badejo AM, Jr, Nossaman VE, Pankey EA, Bhartiya M, Kannadka CB, Murthy SN, Nossaman BD, Kadowitz PJ. Pulmonary and systemic vasodilator responses to the soluble guanylyl cyclase stimulator, BAY 41-8543, are modulated by nitric oxide. Am J Physiol Heart Circ Physiol 299: H1153–H1159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bivalacqua TJ, Sussan TE, Gebska MA, Strong TD, Berkowitz DE, Biswal S, Burnett AL, Champion HC. Sildenafil inhibits superoxide formation and prevents endothelial dysfunction in a mouse model of secondhand smoke induced erectile dysfunction. J Urol 181: 899–906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science 257: 401–403, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Chen H, Levine YC, Golan DE, Michel T, Lin AJ. Atrial natriuretic peptide-initiated cGMP pathways regulate vasodilator-stimulated phosphoprotein phosphorylation and angiogenesis in vascular endothelium. J Biol Chem 283: 4439–4447, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 5: 755–768, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gebska MA, Stevenson BK, Hemnes AR, Bivalacqua TJ, Haile A, Hesketh GG, Murray CI, Zaiman AL, Halushka MK, Krongkaew N, Strong TD, Cooke CA, El-Haddad H, Tuder RM, Berkowitz DE, Champion HC. Phosphodiesterase-5A (PDE5A) is localized to the endothelial caveolae and modulates NOS3 activity. Cardiovasc Res 90: 353–363, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giraldi A, Wagner G. Effects of pinacidil upon penile erectile tissue, in vitro and in vivo. Pharmacol Toxicol 67: 235–238, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Gratzke C, Angulo J, Chitaley K, Dai YT, Kim NN, Paick JS, Simonsen U, Uckert S, Wespes E, Andersson KE, Lue TF, Stief CG. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med 7: 445–475, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Gur S, Kadowitz PJ, Hellstrom WJ. Exploring the potential of NO-independent stimulators and activators of soluble guanylate cyclase for the medical treatment of erectile dysfunction. Curr Pharm Des 16: 1619–1633 [DOI] [PubMed] [Google Scholar]
- 13. Hellstrom WJG, Wang R, Kadowitz PJ, Domer FR. Potassium channel agonists cause penile erection in cats. Int J Imp Res 4: 27–35, 1992 [PMC free article] [PubMed] [Google Scholar]
- 14. Hidalgo-Tamola J, Chitaley K. Review type 2 diabetes mellitus and erectile dysfunction. J Sex Med 6: 916–926, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Lasker GF, Maley JH, Kadowitz PJ. A review of the pathophysiology and novel treatments for erectile dysfunction. Adv Pharmacol Sci 2010: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lasker GF, Pankey EA, Allain AV, Dhaliwal JS, Stasch JP, Murthy SN, Kadowitz PJ. Analysis of erectile responses to BAY 41-8543 and muscarinic receptor stimulation in the rat. J Sex Med 10: 704–718, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lasker GF, Pankey EA, Allain AV, Murthy SN, Stasch JP, Kadowitz PJ. The selective Rho-kinase inhibitor azaindole-1 has long-lasting erectile activity in the rat. Urology 81: 465.e7–e14, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin G, Hayashi N, Carrion R, Chang LJ, Lue TF, Lin CS. Improving erectile function by silencing phosphodiesterase-5. J Urol 174: 1142–1148, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Magee TR, Kovanecz I, Davila HH, Ferrini MG, Cantini L, Vernet D, Zuniga FI, Rajfer J, Gonzalez-Cadavid NF. Antisense and short hairpin RNA (shRNA) constructs targeting PIN (Protein Inhibitor of NOS) ameliorate aging-related erectile dysfunction in the rat. J Sex Med 4: 633–643, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Minkes RK, Kvamme P, Higuera TR, Nossaman BD, Kadowitz PJ. Analysis of pulmonary and systemic vascular responses to cromakalim, an activator of K+ATP channels. Am J Physiol Heart Circ Physiol 260: H957–H966, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Moon DG, Byun HS, Kim JJ. A KATP-channel opener as a potential treatment modality for erectile dysfunction. BJU Int 83: 837–841, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Pankey EA, Bhartiya M, Badejo AM, Jr, Haider U, Stasch JP, Murthy SN, Nossaman BD, Kadowitz PJ. Pulmonary and systemic vasodilator responses to the soluble guanylyl cyclase activator, BAY 60-2770, are not dependent on endogenous nitric oxide or reduced heme. Am J Physiol Heart Circ Physiol 300: H792–H802, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt HH, Schmidt PM, Stasch JP. NO- and haem-independent soluble guanylate cyclase activators. Handb Exp Pharmacol 309–339, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Schmidt P, Schramm M, Schroder H, Stasch JP. Receptor binding assay for nitric oxide- and heme-independent activators of soluble guanylate cyclase. Anal Biochem 314: 162–165, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Shamloul R. The potential role of the heme oxygenase/carbon monoxide system in male sexual dysfunctions. J Sex Med 6: 324–333, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Stasch JP, Dembowsky K, Perzborn E, Stahl E, Schramm M. Cardiovascular actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41-8543: in vivo studies. Br J Pharmacol 135: 344–355, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, HSA, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Muller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Muller-Esterl W, Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao Y, Brandish PE, DiValentin M, Schelvis JP, Babcock GT, Marletta MA. Inhibition of soluble guanylate cyclase by ODQ. Biochemistry 39: 10848–10854, 2000 [DOI] [PubMed] [Google Scholar]