Abstract
Previous studies reported that diets high in simple carbohydrates could increase blood pressure in rodents. We hypothesized that the converse, a low-carbohydrate/high-fat diet, might reduce blood pressure. Six-week-old spontaneously hypertensive rats (SHR; n = 54) and Wistar-Kyoto rats (WKY; n = 53, normotensive control) were fed either a control diet (C; 10% fat, 70% carbohydrate, 20% protein) or a low-carbohydrate/high-fat diet (HF; 20% carbohydrate, 60% fat, 20% protein). After 10 wk, SHR-HF had lower (P < 0.05) mean arterial pressure than SHR-C (148 ± 3 vs. 159 ± 3 mmHg) but a similar degree of cardiac hypertrophy (33.4 ± 0.4 vs. 33.1 ± 0.4 heart weight/tibia length, mg/mm). Mesenteric arteries and the entire aorta were used to assess vascular function and endothelial nitric oxide synthase (eNOS) signaling, respectively. Endothelium-dependent (acetylcholine) relaxation of mesenteric arteries was improved (P < 0.05) in SHR-HF vs. SHR-C, whereas contraction (potassium chloride, phenylephrine) was reduced (P < 0.05). Phosphorylation of eNOSSer1177 increased (P < 0.05) in arteries from SHR-HF vs. SHR-C. Plasma glucose, insulin, and homoeostatic model of insulin assessment were lower (P < 0.05) in SHR-HF vs. SHR-C, whereas peripheral insulin sensitivity (insulin tolerance test) was similar. After a 10-h fast, insulin stimulation (2 U/kg ip) increased (P < 0.05) phosphorylation of AktSer473 and S6 in heart and gastrocnemius similarly in SHR-C vs. SHR-HF. In conclusion, a low-carbohydrate/high-fat diet reduced blood pressure and improved arterial function in SHR without producing signs of insulin resistance or altering insulin-mediated signaling in the heart, skeletal muscle, or vasculature.
Keywords: heart, blood pressure, diet, arterial function, hypertrophy
the american heart association reports that ∼75 million Americans have hypertension (44). Despite the efficacious pharmacological treatments that currently are available, a need exists for other treatment options due to issues such as noncompliance to medication, prevalence of side effects, and resistance to commonly used drugs. Accordingly, diet and lifestyle interventions including lower sodium intake (56), increased fruit and vegetable consumption (45), and weight loss (50) have been shown to reduce blood pressure.
The spontaneously hypertensive rat (SHR) is a model of evolving hypertension, utilized for understanding the causes of hypertension and the development of more effective strategies for its treatment. Recently, it was shown that restricting total caloric intake by 40% reduced blood pressure in the SHR (17). However, altering the ratio of macronutrient intake may also impact blood pressure. Animal studies indicate that diets high in simple carbohydrates, such as sucrose, can promote hypertension in rodents (19, 23, 38, 59), whereas consumption of very high-fat diets (≥60% energy from fat) can reduce blood pressure in the SHR (14, 57). Blood pressure in humans may also be responsive to alterations in macronutrient intake. For example, a clinical study by Appel et al. (2) reported that hypertensive patients had a 2.9-mmHg drop in systolic pressure when consuming a 48% carbohydrate, 37% fat diet (total energy intake) compared with a 58% carbohydrate, 27% fat diet. The mechanism(s) for the blood pressure changes evoked by low-carbohydrate/high-fat diets in the aforementioned animal and human studies remain unknown.
Studies reporting the cardiovascular benefit of low-carbohydrate/high-fat diets have been understandably controversial because high-fat diets have traditionally been associated with obesity and metabolic syndrome in some rodent models [for review see Buettner et al. (9)]. In the present study we sought to clarify the observation that a low-carbohydrate/high-fat diet attenuates hypertension, and we therefore hypothesized that such a diet would reduce blood pressure and improve arterial function without producing insulin resistance in SHR, a clinically relevant model of hypertension.
Our main findings are that SHR fed low-carbohydrate/high-fat diets have lower blood pressure and improved endothelial function of mesenteric arteries. Importantly, systemic glucose homeostasis and insulin-mediated signal transduction in the heart and skeletal muscle were similar between SHR fed low-carbohydrate/high-fat or control diets. Furthermore, mesenteric arterial function was improved in SHR that consumed diets relatively higher in fat content and might contribute, at least in part, to lower blood pressure that was observed in these animals. These studies provide important evidence that the ratio of carbohydrate to fat intake influences blood pressure during the condition of hypertension and provide mechanistic insight into the clinical observation that low-carbohydrate/high-fat diets can reduce blood pressure.
MATERIALS AND METHODS
Animals and diets.
All protocols were approved by the University of Utah Institutional Animal Care and Use Committee. Fifty-four SHR (SHR/NCrl) and 53 control Wistar-Kyoto normotensive rats (WKY) were obtained from Charles River Laboratories at 5–6 wk of age and randomized to have free access to either a low-carbohydrate/high fat (HF; 20% carbohydrate, 20% protein, 60% fat by calories) or control diet (C; 70% carbohydrate, 20% protein, 10% fat by calories) (Research Diets, New Brunswick, NJ) for 10 wk (Table 1).
Table 1.
Macronutrient and sodium content of diets
| Source | Control | Low Carbohydrate-High Fat |
|---|---|---|
| Carbohydrate | 34.5% Sucrose | 6.8% Sucrose |
| 31.1% Starch | 0% Starch | |
| 3.5% Maltodextrin | 12.3% Maltodextrin | |
| Fat | 5.5% Soybean oil | 5.5% Soybean oil |
| 4.5% Lard | 54.5% Lard | |
| Protein | 19.7% Casein, 80 mesh | 19.7% Casein, 80 mesh |
| 0.3% l-Cystine | 0.3% l-Cystine | |
| Sodium | 1 g/kg Diet | 1 g/kg Diet |
Carbohydrate, fat, and protein are expressed as percentages of total calorie content.
Blood pressure and sampling.
At 15–16 wk of age, after a 2 ± 1-h fast, systemic arterial blood pressure was assessed in conscious rats (n = 13–15/group), as previously described, using a fluid-filled catheter inserted into the caudal artery (52). After blood pressure was measured, a caudal artery blood sample was taken from a subgroup of rats to measure plasma glucose directly (n = 8–10/group; One Touch glucometer; LifeScan) as well as insulin (n = 8–10/group; ELISA; Crystal Chem) (53), leptin and homomultimer adiponectin (n = 5–7/group; RIA; Millipore, Billerica, MA) (54). The homeostasis model assessment (HOMA) index was calculated [fasting glucose (mmol/l) × fasting insulin (mU/l)/22.5] as previously described (30).
Peripheral insulin sensitivity.
An insulin tolerance test was performed in a cohort of rats (n = 6 for all groups). After an 11 ± 1-h fast from 2300 to 0900 baseline (i.e., 0 min), glucose was assessed using a blood sample obtained from a caudal artery catheter. Next, insulin (2 U/kg body wt) was injected via the caudal artery, and glucose was sampled after 10, 20, 30, and 60 min.
Insulin-mediated signal transduction in heart, gastrocnemius, and vessels.
After an 11 ± 1-h fast (2300 to 0900), rats (n = 10–12/group) were injected intraperitoneally with insulin (2 U/kg body wt) or vehicle (saline), and 5 min later tissue was obtained for analysis.
Vascular function.
Isometric tension techniques were used to evaluate arterial function of mesenteric arteries (n = 6/group) in the absence of neural, humoral, metabolic, and mechanical influences as previously described (26, 53, 54). After Lmax tension was determined for each artery (100 mM KCl), vasocontractile responses to phenylephrine (PE; 10−8–10−5 M) and KCl (10–100 mM) were performed to assess receptor- and non-receptor-mediated contraction, respectively. Responses to acetylcholine (ACh; 10−8–10−4 M) and sodium nitroprusside (SNP; 10−9–10−4 M) were performed on vessels precontracted to ∼65% of maximal PE-induced tension development to determine endothelium-dependent and endothelium-independent vasorelaxation, respectively. Vasocontractile responses to NG-monomethyl-l-arginine (l-NMMA; 10−3 M) were evaluated in PE-precontracted arteries to estimate basal NO production. All concentration-response curves were separated by 30–40 min.
Immunoblotting.
All tissues were homogenized at 4°C, and Western blotting was performed using standard methods we have previously described (3, 12, 26). Membranes were probed with antibodies directed against p-AktSer473, p-AktThr308, Akt, p-S6Ser235/236, S6, p-ERKThr202/204, ERK, p-AMPKThr172, AMP-activated protein kinase (AMPK), p-LKB1Ser428, S6KThr389, S6K, p-mTORSer2448, mammalian target of rapamycin (mTOR) (Cell Signal Technology, Beverly, MA), LKB1, tubulin (Santa Cruz Biotechnology, Santa Cruz, CA), p-eNOSSer1177, and endothelial nitric oxide synthase (eNOS) (BD Transduction Laboratories, Franklin Lakes, NJ). Cardiac LKB1 and p70S6K were measured in hearts that were not exposed to insulin. Relative band densities of immunoblots after chemiluminescent detection were measured using a Kodak Gel Logic 1500 imaging system. All immunoblots were done in triplicate to confirm results, and band densities were normalized to tubulin as a loading control when required.
Statistical analysis.
A two-way analysis of variance (ANOVA) and Tukey's post hoc tests were utilized to determine significant differences for all parameters except where noted as follows (SPSS version 16). For insulin-stimulated signaling experiments, t-tests for independent samples were used to determine differences between each genotype × diet × vehicle group and the corresponding genotype × diet × insulin stimulation group. Vascular function dose-response curves were analyzed using a two-way repeated-measures ANOVA followed by Tukey's post hoc test when a significant main effect was obtained. Data are means ± SE. Significance was accepted at P < 0.05.
RESULTS
Blood pressure, cardiac hypertrophy, and body weight.
As expected, arterial blood pressure was greater in SHR vs. WKY rats regardless of the feeding condition (Table 2 and Fig. 1, A and C). Consistent with our hypothesis, hypertension was less severe in SHR that consumed HF vs. C chow. Compared with WKY, cardiac hypertrophy developed to a similar extent in SHR despite the relative reduction in blood pressure observed in HF vs. C rats (Table 2 and Fig. 1, B and C).
Table 2.
Morphometric and metabolic characteristics of rats
| WKY-C | WKY-HF | SHR-C | SHR-HF | |
|---|---|---|---|---|
| n = 25–29/group | ||||
| Body wt, g | 332 ± 4 | 342 ± 4 | 333 ± 4 | 345 ± 4 |
| Heart wt, mg | 1128 ± 20 | 1163 ± 19 | 1284 ± 19* | 1318 ± 18* |
| n = 13–15/group | ||||
| Systolic blood pressure, mmHg | 136 ± 4 | 136 ± 2 | 183 ± 3* | 172 ± 3† |
| Diastolic blood pressure, mmHg | 111 ± 4 | 112 ± 2 | 147 ± 4* | 136 ± 3† |
| Heart rate, beats/min | 370 ± 6 | 353 ± 9 | 385 ± 6 | 390 ± 8* |
| n = 11–13/group | ||||
| Gonadal left and right fat pad, g | 6.35 ± 0.20 | 8.70 ± 0.39* | 5.10 ± 0.16* | 6.11 ± 0.24 |
| n = 11–13/group | ||||
| Kidney wt/tibia length, mg/mm | 49.4 ± 0.6 | 49.5 ± 0.9 | 54.3 ± 0.8* | 54.3 ± 0.9* |
| n = 5–8/group | ||||
| Plasma insulin, ng/ml | 5.92 ± 0.39 | 4.52 ± 0.38 | 5.55 ± 0.51 | 3.81 ± 0.33* |
| Plasma glucose, mg/dl | 111 ± 2 | 106 ± 3 | 106 ± 3 | 100 ± 3* |
| HOMA-IR index | 37.0 ± 3.5 | 25.5 ± 2.9* | 30.2 ± 3.2 | 21.5 ± 2.7* |
| Plasma leptin, ng/ml | 5.78 ± 0.49 | 9.75 ± 1.54* | 5.07 ± 0.84 | 5.88 ± 0.38 |
| Plasma adiponectin, μg/ml | 7.15 ± 0.29 | 6.82 ± 0.53 | 6.15 ± 0.33 | 5.33 ± 0.31 |
Values are means ± SE in Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHR) fed a control (C) or a low-carbohydrate/high-fat (HF) diet.
P < 0.05, significant difference compared with WKY-C.
P < 0.05, significant difference compared with WKY-C and SHR-C. HOMA-IR, homeostasis model assessment of insulin resistance.
Fig. 1.

A: mean arterial blood pressure of Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHR) fed control (C) and low-carbohydrate/high-fat (HF) diets. B: cardiac hypertrophy as measured by the ratio of heart weight (mg) to tibia length (mm) of WKY and SHR fed C and HF diets for 10 wk. C: index of cardiac hypertrophy vs. blood pressure in WKY and SHR fed C and HF diets. All feeding protocols were carried out for 10 wk. Data are means ± SE from 13–16 animals per group. *P < 0.05 vs. WKY-C, WKY-HF, and SHR-HF. **P < 0.05 vs. WKY-C and WKY-HF.
LKB1, and S6K are important regulators of cardiac hypertrophy in SHR (16, 51). The phosphorylation of cardiac LKB1, the upstream activating kinase of AMPK, as represented by its phosphorylation on serine 428, was reduced in all SHR vs. all WKY (Fig. 2A). However, feeding HF diets to WKY or SHR did not affect LKB1 phosphorylation compared with the C diet (Fig. 2A). Phosphorylation of the downstream target of mTOR, S6 kinase, was markedly increased in the hearts of SHR-C vs. all WKY and was further augmented in the SHR-HF (Fig. 2B).
Fig. 2.
LKB1 (A) and S6K (B) in hearts of WKY and SHR fed C and HF diets. Data are means ± SE from 6–8 animals per group. *P < 0.05.
Metabolic characterization.
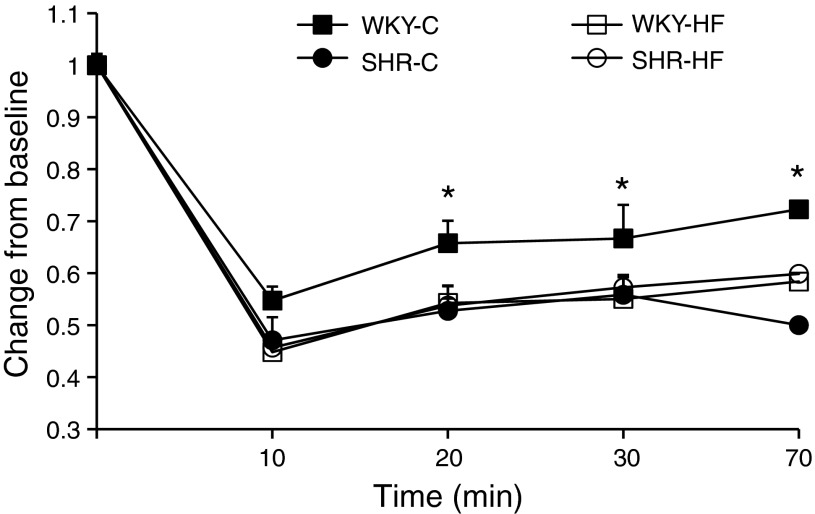
Plasma insulin, plasma glucose, and the HOMA index were lower in SHR-HF vs. SHR-C, whereas only HOMA was lower in WKY-HF vs. WKY-C (Table 2). Insulin sensitivity as determined by an insulin tolerance test (ITT) was similar between SHR-HF vs. SHR-C (Fig. 3). Interestingly, WKY-C had significantly reduced insulin sensitivity (higher blood glucose at all time intervals after insulin injection) compared with all other groups (Fig. 3). Leptin levels increased ∼70% in WKY-HF vs. WKY-C but were similar in SHR-C vs. SHR-HF (Table 2). Plasma adiponectin (all forms except monomeric adiponectin) was similar between WKY and SHR regardless of which diet they consumed (Table 2).
Fig. 3.
Insulin tolerance test (ITT) of WKY and SHR fed C and HF diets for 10 wk and challenged with 2 units of insulin/kg body wt. Glucose levels are expressed as a percentage decrease from baseline immediately before injection of insulin. Data are means ± SE from 6 animals per group. *P < 0.05 vs. WKY-HF, SHR-C, and SHR-HF.
Insulin-mediated signal transduction in the heart and skeletal muscle.
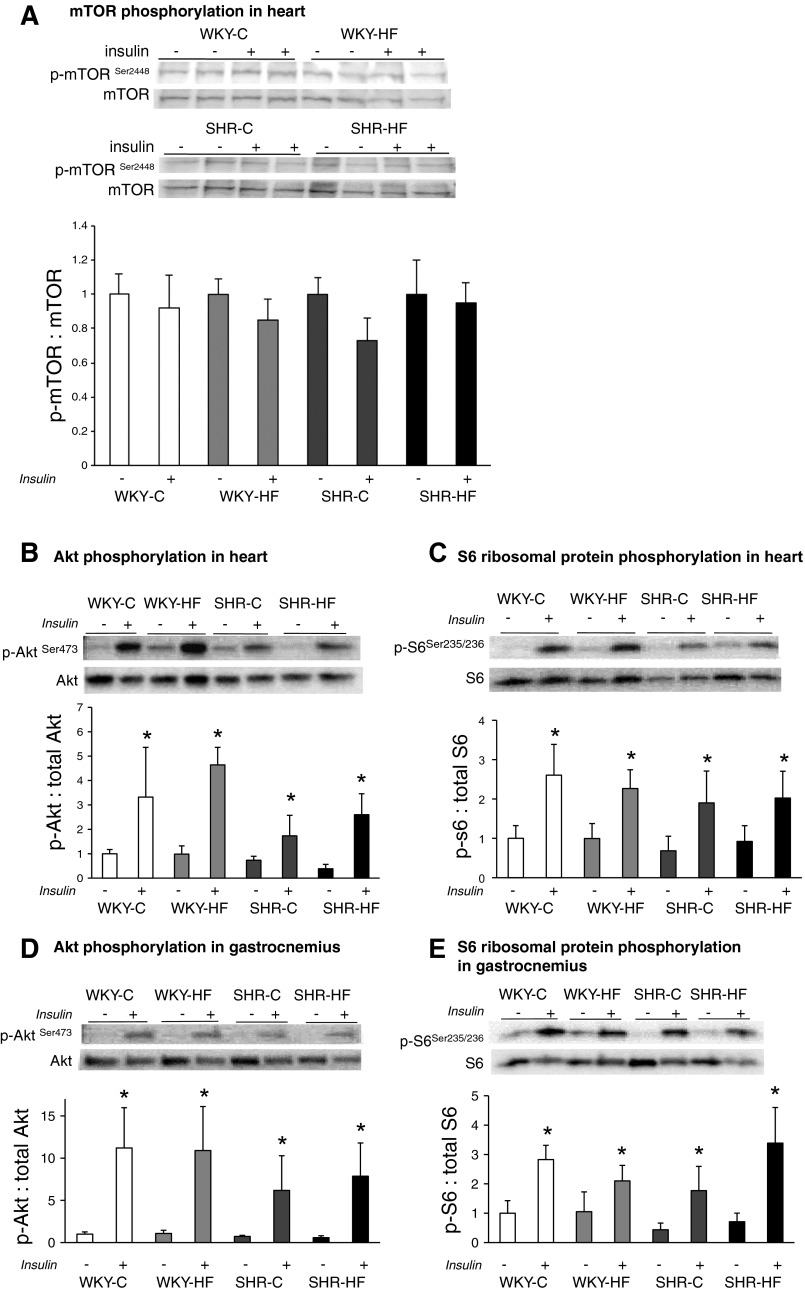
After 5 min of insulin stimulation, mTOR phosphorylation was not altered in heart (Fig. 4A) or skeletal muscle (data not shown) of WKY and SHR. There was no dietary effect on basal Akt expression in heart or skeletal muscle, but insulin stimulation increased the ratio of p-AktSer473 to total Akt in all groups of SHR and WKY (Fig. 4, B and D). In skeletal muscle, but not heart, insulin treatment led to a slight reduction of total Akt levels across all groups. Insulin stimulation also led to a robust phosphorylation of S6 in these same tissues, resulting in similar elevation of p-S6-to-total S6 elevated across all groups, regardless of diet (Fig. 4, C and E).
Fig. 4.
Basal (−) and insulin-stimulated (+) mTORSer2448, AktSer473, and S6Ser235/236 phosphorylation in heart (A, B, and C) and gastrocnemius (D and E) of WKY and SHR fed C and HF diets. Data are means ± SE from 6–8 animals per group. *P < 0.05. mTOR, mammalian target of rapamycin.
Arterial function.
Receptor- and non-receptor-mediated vasocontraction was less severe in arteries from SHR-HF vs. SHR-C (Fig. 5, A and B). Endothelium-dependent and -independent relaxation was greater in vessels from SHR-HF vs. SHR-C (Fig. 5, E and F). l-NMMA-evoked vasocontraction in precontracted arteries was higher in vessels from SHR-HF (50 ± 2%) vs. SHR-C (23 ± 6%). Vasocontraction in mesenteric arteries was evoked using KCl and PE. No differences in arterial function were observed in vessels from WKY rats regardless of diet (Fig. 5, C, D, G, H), except for an increase in developed tension in response to PE (Fig. 5D, log 10−5.5) in WKY-HF vs. WKY-C.
Fig. 5.
Vascular function in vehicle-treated SHR fed C and HF diets for 10 wk. Non-receptor-mediated (A) and receptor-mediated vasocontraction (B) was less in mesenteric arteries from SHR-HF vs. SHR-C and largely unchanged (C and D) in WKY-C vs. WKY-HF. Endothelium-dependent (E) and endothelium-independent vasorelaxation (F) was significantly greater in vessels from SHR-HF vs. SHR-C and similar (G and H) between WKY-C and WKY-HF. Data are means ± SE from 6 rats per group, 2 vessels per animal. *P < 0.05.
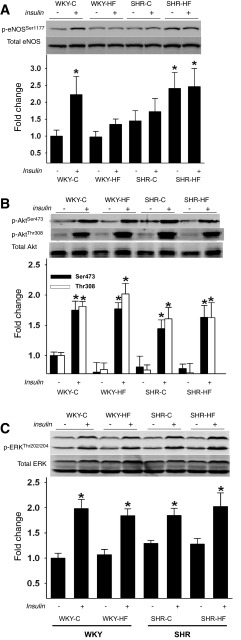
Using arteries from the same animals, we sought to determine whether blunted hypertension, improved vasorelaxation, and less severe vasoconstriction might be secondary to phosphorylation of eNOS and the upstream signaling kinases that phosphorylate eNOS at both positive (i.e., Akt, AMPK) and negative (i.e., ERK) regulatory sites. Under basal conditions p-eNOSSer1177 was similar among WKY-C, WKY-HF, and SHR-C; however, SHR-HF had an almost 2.5-fold increase in p-eNOSSer1177 vs. WKY-C. After insulin stimulation, p-eNOSSer1177 increased in WKY-C but not in WKY-HF or SHR-C. Insulin stimulation did not further augment the high level of p-eNOSSer1177 already present in SHR-HF (Fig. 6A). The differential response in p-eNOSSer1177 was not secondary to alterations in upstream signaling via Akt, ERK (Fig. 6, B and C), or AMPK (data not shown). Although both Akt and ERK demonstrated increased phosphorylation after insulin stimulation (Fig. 6, B and C), there were no differences in phosphorylation level between groups regardless of diet.
Fig. 6.
Aortas were used to assess basal (−) and insulin-stimulated (+) ERKThr202/204 phosphorylation (A), AktSer473 and AktThr308 phosphorylation (B), and eNOSSer1177 phosphorylation (C). Data are means ± SE from 6 animals per treatment (i.e., vehicle or insulin) × 4 groups (i.e., 48 rats). *P < 0.05.
DISCUSSION
In the present study we provide data that supports three main findings. First, a low-carbohydrate/high-fat diet attenuates hypertension in the SHR. Second, these beneficial effects can be achieved without causing insulin resistance or detriment to insulin-stimulated signaling in heart and skeletal muscle. Finally, a low-carbohydrate diet might attenuate blood pressure in SHR by improving reactivity of resistance-sized arteries. These results provide important proof of principle that in the setting of experimental hypertension, limiting the carbohydrate intake (at the expense of increasing fat intake) reduces blood pressure but does not disrupt insulin sensitivity or vascular function.
Although our findings are clinically relevant given that low-carbohydrate/high-fat diets have been reported to reduce blood pressure in diabetic humans (41, 48), it remains unknown if attenuated blood pressure in SHR-HF was a function of a reduction in overall carbohydrate or the sucrose component of the diet. It is known that SHR fed very high-sugar diets have greater blood pressure than chow-fed SHR (28, 38, 40). These studies support a hypertensive effect from excess sugar. The diets used in our study contained 34% sucrose, 31% starch, 4% maltodextrin in the control chow and 7% sucrose, 0% starch and 14% maltodextrin in the low-carbohydrate/high-fat chow (Table 1). Even though the amount of sucrose present in the control diet was higher than in “typical” control diets, the observed blood pressure in SHR-C was same level as we have previously observed using the same strain and age of SHR fed control diets with 10% sucrose (12, 51). Since the differential sucrose content between the control and HF diet is a limitation to the present study, we cannot rule out reduced sucrose consumption by SHR-HF as a factor in blood pressure reduction. Similarly, the kidney-specific effect of the low-carbohydrate/high-fat vs. control diet was also not evaluated in this study; however, Preuss et al. (40) previously reported no significant or consistent differences in creatinine clearance and excretion of sodium, potassium, and protein in SHR and Wistar rats fed high-sugar diets vs. high-fat diets.
Previous studies have indicated that low-carbohydrate/high-fat diets reduce cardiac hypertrophy in other rat models. For example, the laboratory of Stanley et al. reported that 60% fat, 20% carbohydrate in the diet of Dahl salt-sensitive and pressure-overloaded rats (aortic constriction) reduces cardiac hypertrophy, independent of any change in blood pressure (18, 34, 35, 49). In contrast, we found a reduction in blood pressure in SHR but no change in cardiac hypertrophy. It is possible that the variable effects produced by the seemingly similar diets employed in our study and the aforementioned ones may be due to strain-specific differences in rat models that were used.
It is known that cardiac hypertrophy can be attenuated in SHR by reducing blood pressure (1, 10, 11, 13, 20, 37, 47, 58). Our data are in contrast to these findings because despite the 11-mmHg reduction in mean arterial blood pressure experienced by SHR-HF, the extent of hypertrophy was similar between SHR-C and SHR-HF. Others have also reported that low-carbohydrate/high-fat diets lower blood pressure in SHR, but without any change in cardiac hypertrophy (14, 15). Since previous studies using antihypertensive agents have reported that lowering SHR blood pressure by >30 mmHg attenuates cardiac hypertrophy (1, 10, 11, 13, 20, 37, 47, 58), one could argue that the 11-mmHg reduction of mean arterial pressure in SHR-HF was not sufficient to attenuate cardiac hypertrophy. Along these lines, it is also possible that hypertrophic signaling pathways may have remained active to engage cardiac growth. For example, we found that both SHR-C and SHR-HF hearts had attenuated LKB1 activation coupled with increased S6K phosphorylation, suggestive of signaling through the mTOR pathway, and a pattern that has been associated with hypertrophy in SHR (16). We have previously reported that mTOR regulates hypertrophy in the SHR and that repression of mTOR, independent of any change in blood pressure, can attenuate hypertrophy (51). Hypertrophic pathways that are independent of blood pressure, such as endonuclease G (ENDOG), may also play a role in SHR. McDermott-Roe et al. (31) demonstrated that inhibition of ENDOG leads to cardiomyocyte hypertrophy in vitro and in vivo. Interestingly, SHR posses a frame-shift insertion in exon 1 of ENDOG that leads to reduced cardiac ENDOG expression (31). Taking these findings together, we speculate that the reported lack of ENDOG expression in SHR, along with our observed elevation of cardiac p-S6K, suggests a sustained activation of hypertrophic signaling that could explain the continued presence of hypertrophy in SHR-HF, despite the reduction in blood pressure.
Our original rationale for assessing indexes of arterial reactivity was to determine whether any adverse effects of low-carbohydrate/high-fat diets might be observed in vessels from SHR-HF vs. SHR-C. Unexpectedly, mesenteric arteries from SHR-HF displayed greater ACh-evoked vasorelaxation, blunted PE- and KCl-evoked vasocontraction, and elevated l-NMMA-evoked tension development in precontracted arteries vs. vessels from SHR-C. Furthermore, basal p-eNOSSer1177 was significantly increased in segments of thoracic aorta from HF vs. C SHR animals. While these functional and biochemical alterations are consistent with greater nitric oxide bioavailability in the vasculature of SHR-HF vs. SHR-C, we cannot discount the possibility that vascular smooth muscle sensitivity might have been improved. In this regard, SNP-evoked vasorelaxation was greater in arteries from SHR-HF vs. SHR-C. Therefore, it is difficult to precisely identify whether improved vasorelaxation in resistance-sized vessels from SHR-HF is precipitated via endothelium-dependent or -independent sources. What is without doubt, however, is that the low-carbohydrate/high-fat diet did not impact arterial function in a negative manner. Moreover, it is not unreasonable to speculate that improved vasorelaxation of resistance-sized arteries might be partly responsible for the observation that systemic hypertension was less severe in SHR-HF vs. SHR-C. However, a limitation of our analysis is the lack of a secondary measure, such are peripheral vascular resistance, that would correlate with reductions in mean arterial pressure. Previous studies have shown that SHR have reductions in systemic vascular resistance that occur in conjunction with reduced blood pressure (21, 27).
There have been many contrasting reports over the years whether SHR are insulin resistant and if they can maintain normal glucose homeostasis. On one hand, there is a body of evidence demonstrating insulin resistance (4, 25, 32, 46) and diminished glucose uptake in skeletal muscle and adipocytes of SHR (33, 42), whereas others report normal, or enhanced, glucose uptake and similar insulin sensitivity in SHR vs. WKY (7, 8, 22, 55). There may be strain-specific variations in SHR insulin sensitivity and glucose uptake. A limitation of the historical literature is that many previous studies did not specify which SHR strain was used. We believe that a subset of the aforementioned studies, along with our present data, indicates that the WKY have poorer insulin sensitivity than the SHR strain used in the present study (NIH/Crl). The exact mechanism responsible for improved insulin sensitivity after feeding low-carbohydrate/high-fat diet to WKY remains unknown. Differences in hepatic and skeletal muscle insulin receptor density and/or GLUT4 expression could be explored as potential mechanisms in future studies. Given these contrasting reports, we quantified systemic glucose concentrations over time in response to insulin administration. The most important finding in the context of our study was that no differences existed concerning insulin-stimulated glucose response between SHR fed low-carbohydrate/high-fat or control chow. Interestingly, insulin-stimulated reductions in blood glucose were more profound in SHR vs. WKY fed a control diet, and both basal insulin concentrations and the HOMA index were lower in SHR and WKY fed low-carbohydrate/high-fat diets. Some of the controversy regarding glucose homeostasis in SHR may be linked to polymorphisms in CD36 that can affect fat uptake in organs such as liver and skeletal muscle in some strains of SHR. The model used in this study, SHR/NCrl, has been shown to express CD36 in all major organs (5) and, as our data demonstrate, may respond differently than other SHR strains to dietary challenges that influence glucose homeostasis. Overall, the data from our present study do not support the notion that SHR have insulin resistance or that a low-carbohydrate/high-fat diet impairs insulin sensitivity. We recognize that the present data are in contrast to other studies in the literature (4, 7, 8, 22, 25, 32, 33, 42, 46, 55); however, it is possible that some of these contrasting observations may be due to the experimental conditions employed with respect to state of consciousness and mode of restraint experienced by rats. For example, during the insulin tolerance test conducted in our study, we used unrestrained and conscious rats with catheters implanted in the caudal artery to administer insulin and monitor glucose levels. Therefore, the influence of this technique compared with others on glucose and insulin levels is unclear.
In the present study we observed that SHR had lower fat pad weight compared with diet-matched WKY, suggesting a resistance to weight gain. Furthermore, whereas adiponectin was similar among all groups, the low-carbohydrate/high-fat diets increased plasma leptin levels in WKY but not in SHR. Similar finding have been reported in SHR fed high-fat diets (6, 14, 15, 36). These results may be explained by the presence of a segment of chromosome 20 in SHR that differentially regulates obesity, glucose intolerance, and leptin levels in response to a high-fat diet compared with other rat strains (6, 36). For example, high-fat diets fed to congenic SHR containing a segment of chromosome 20 from Brown Norway rats resulted in increased fat pad weight and elevated leptin, whereas the same diet did not produce those effects in SHR with endogenous chromosome 20 (6).
Current dietary American Heart Association recommendations for management of blood pressure include consuming a diet rich in fruits, vegetables, whole grains, fiber, and fish while limiting dietary sodium. Recently, specific advice on limiting simple carbohydrate intake in the form of dietary sugar was also advocated by the American Heart Association. Studies using SHR and normotensive rat models provide rationale for the potential deleterious effects of dietary sugar on blood pressure (23, 24, 38–40, 43). However, there is sparse data regarding the effect that the overall proportion of dietary carbohydrate may have on blood pressure. Our study provides proof of principle that reducing carbohydrate intake in the setting of hypertension can reduce blood pressure, and we propose that the mechanism may be related to improved arterial function and enhanced eNOS phosphorylation. Although we do not have data in the present study regarding the long-term effect of the observed 11-mmHg reduction in blood pressure on the SHR mortality, it is known that this magnitude of reduction is clinically relevant in humans over the long term. It has been estimated that for each increase of 20 mmHg systolic and 10 mmHg diastolic, there is a doubling of mortality from stroke and ischemic heart disease (29). Furthermore, even individuals with systolic blood pressure of 130–139 mmHg have twice the risk of cardiovascular disease mortality than individuals with blood pressure of 115/75 mmHg (29). While replacing carbohydrate with fat as we did in our study is controversial, it does provide a tool to tease out the impact that dietary carbohydrate may have on blood pressure. Although it is premature to consider long-range clinical implications from this study, these data do give one pause when contemplating the standard advice of a general “low-fat/high-carbohydrate” diet that focuses on sodium intake for patients with hypertension. Data from the present study raise many new questions and warrant further research using other models of hypertension, varying levels of carbohydrate intake, and the potential contribution of substituting macronutrients such as protein or fat to replace carbohydrate.
GRANTS
Statistical analysis for this research was supported by the University of Utah Study Design and Biostatistics Center National Institutes of Health (NIH) Grants UL1-RR025764 and C06-RR11234. T. Jalili was supported by a University of Utah College of Health Grant and by NIH Grant HL085226. J. D. Symons was supported by American Diabetes Association Research Grant 7-08-RA-164 and NIH Grant R15-HL091493-01. V. W. Dolinsky was supported by grants from the Children's Hospital Foundation of Manitoba and the Manitoba Institute of Child Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.B., H.-Y.L., C.S., Q.-J.Z., T.J.P., V.W.D., J.D.S., and T.J. performed experiments; J.D.B., V.W.D., J.D.S., and T.J. analyzed data; J.D.B. and T.J. interpreted results of experiments; J.D.B., J.D.S., and T.J. prepared figures; J.D.B., E.D.A., V.W.D., J.D.S., and T.J. edited and revised manuscript; J.D.B., H.-Y.L., C.S., Q.-J.Z., E.D.A., V.W.D., J.D.S., and T.J. approved final version of manuscript; T.J. conception and design of research; T.J. drafted manuscript.
REFERENCES
- 1. Antonaccio MJ, Rubin B, Horovitz ZP, Laffan RJ, Goldberg ME, High JP, Harris DN, Zaidi I. Effects of chronic treatment with captopril (SQ 14,225), an orally active inhibitor of angiotensin I-converting enzyme, in spontaneously hypertensive rats. Jpn J Pharmacol 29: 285–294, 1979 [DOI] [PubMed] [Google Scholar]
- 2. Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 294: 2455–2464, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Avelar E, Jalili T, Dong L, Arvizo J, Hu P, Litwin SE, Mattson JP. PKC translocation and ERK1/2 activation in compensated right ventricular hypertrophy secondary to chronic emphysema. BMC Physiol 5: 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baumann R, Moritz V, Godicke W, Postnow JW, Ziegler M. Insulin kinetic, glucose tolerance, and lipid metabolism in genetically spontaneous-hypertensive rats. Acta Biol Med Ger 35: K33–K39, 1976 [PubMed] [Google Scholar]
- 5. Bonen A, Han XX, Tandon NN, Glatz JF, Lally J, Snook LA, Luiken JJ. FAT/CD36 expression is not ablated in spontaneously hypertensive rats. J Lipid Res 50: 740–748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourdon C, Hojna S, Jordan M, Berube J, Kren V, Pravenec M, Liu P, Arab S, Pausova Z. Genetic locus on rat chromosome 20 regulates diet-induced adipocyte hypertrophy: a microarray gene expression study. Physiol Genomics 38: 63–72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchanan TA, Sipos GF, Madrilejo N, Liu C, Campese VM. Hypertension without peripheral insulin resistance in spontaneously hypertensive rats. Am J Physiol Endocrinol Metab 262: E14–E19, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Buchanan TA, Youn JH, Campese VM, Sipos GF. Enhanced glucose tolerance in spontaneously hypertensive rats. Pancreatic beta-cell hyperfunction with normal insulin sensitivity. Diabetes 41: 872–878, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 15: 798–808, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Burrell LM, Droogh J, Man in't Veld O, Rockell MD, Farina NK, Johnston CI. Antihypertensive and antihypertrophic effects of omapatrilat in SHR. Am J Hypertens 13: 1110–1116, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Canby CA, Tomanek RJ. Role of lowering arterial pressure on maximal coronary flow with and without regression of cardiac hypertrophy. Am J Physiol Heart Circ Physiol 257: H1110–H1118, 1989 [DOI] [PubMed] [Google Scholar]
- 12. Carlstrom J, Symons JD, Wu TC, Bruno RS, Litwin SE, Jalili T. A quercetin supplemented diet does not prevent cardiovascular complications in spontaneously hypertensive rats. J Nutr 137: 628–633, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Choi SM, Seo MJ, Kang KK, Kim JH, Ahn BO, Yoo M. Beneficial effects of the combination of amlodipine and losartan for lowering blood pressure in spontaneously hypertensive rats. Arch Pharm Res 32: 353–358, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Contreras RJ, King S. High fat/sucrose feeding attenuates the hypertension of spontaneously hypertensive rats. Physiol Behav 46: 285–291, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Contreras RJ, Williams VL. Dietary obesity and weight cycling: effects on blood pressure and heart rate in rats. Am J Physiol Regul Integr Comp Physiol 256: R1209–R1219, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Dolinsky VW, Chan AY, Robillard Frayne I, Light PE, Des Rosiers C, Dyck JR. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation 119: 1643–1652, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Dolinsky VW, Morton JS, Oka T, Robillard-Frayne I, Bagdan M, Lopaschuk GD, Des Rosiers C, Walsh K, Davidge ST, Dyck JR. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension 56: 412–421, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Duda MK, O'Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Low-carbohydrate/high-fat diet attenuates pressure overload-induced ventricular remodeling and dysfunction. J Card Fail 14: 327–335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. el Zein M, Areas JL, Knapka J, MacCarthy P, Yousufi AK, DiPette D, Holland B, Goel R, Preuss HG. Excess sucrose and glucose ingestion acutely elevate blood pressure in spontaneously hypertensive rats. Am J Hypertens 3: 380–386, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Farina NK, Johnston CI, Burrell LM. Reversal of cardiac hypertrophy and fibrosis by S21402, a dual inhibitor of neutral endopeptidase and angiotensin converting enzyme in SHRs. J Hypertens 18: 749–755, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Flaim SF, Stranieri MT, Gill A, Carson JR, Brannan MD. Effects of the novel calcium channel blocker, McN-5691, on cardiocirculatory dynamics and cardiac output distribution in conscious spontaneously hypertensive rat. J Cardiovasc Pharmacol 11: 489–500, 1988 [DOI] [PubMed] [Google Scholar]
- 22. Frontoni S, Ohman L, Haywood JR, DeFronzo RA, Rossetti L. In vivo insulin action in genetic models of hypertension. Am J Physiol Endocrinol Metab 262: E191–E196, 1992 [DOI] [PubMed] [Google Scholar]
- 23. Gondal JA, MacArthy P, Myers AK, Preuss HG. Effects of dietary sucrose and fibers on blood pressure in hypertensive rats. Clin Nephrol 45: 163–168, 1996 [PubMed] [Google Scholar]
- 24. Hulman S, Falkner B. The effect of excess dietary sucrose on growth, blood pressure, and metabolism in developing Sprague-Dawley rats. Pediatr Res 36: 95–101, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Hulman S, Falkner B, Chen YQ. Insulin resistance in the spontaneously hypertensive rat. Metabolism 40: 359–361, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Jalili T, Carlstrom J, Kim S, Freeman D, Jin H, Wu TC, Litwin SE, Symons JD. Quercetin-supplemented diets lower blood pressure and attenuate cardiac hypertrophy in rats with aortic constriction. J Cardiovasc Pharmacol 47: 531–541, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Jazbutyte V, Arias-Loza PA, Hu K, Widder J, Govindaraj V, von Poser-Klein C, Bauersachs J, Fritzemeier KH, Hegele-Hartung C, Neyses L, Ertl G, Pelzer T. Ligand-dependent activation of ERβ lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized spontaneously hypertensive rats. Cardiovasc Res 77: 774–781, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Kopilas MA, Dang LN, Anderson HD. Effect of dietary chromium on resistance artery function and nitric oxide signaling in the sucrose-fed spontaneously hypertensive rat. J Vasc Res 44: 110–118, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 31. McDermott-Roe C, Ye J, Ahmed R, Sun XM, Serafin A, Ware J, Bottolo L, Muckett P, Canas X, Zhang J, Rowe GC, Buchan R, Lu H, Braithwaite A, Mancini M, Hauton D, Marti R, Garcia-Arumi E, Hubner N, Jacob H, Serikawa T, Zidek V, Papousek F, Kolar F, Cardona M, Ruiz-Meana M, Garcia-Dorado D, Comella JX, Felkin LE, Barton PJ, Arany Z, Pravenec M, Petretto E, Sanchis D, Cook SA. Endonuclease G is a novel determinant of cardiac hypertrophy and mitochondrial function. Nature 478: 114–118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mondon CE, Reaven GM. Evidence of abnormalities of insulin metabolism in rats with spontaneous hypertension. Metabolism 37: 303–305, 1988 [DOI] [PubMed] [Google Scholar]
- 33. Mondon CE, Reaven GM, Azhar S, Lee CM, Rabkin R. Abnormal insulin metabolism by specific organs from rats with spontaneous hypertension. Am J Physiol Endocrinol Metab 257: E491–E498, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Okere IC, Chess DJ, McElfresh TA, Johnson J, Rennison J, Ernsberger P, Hoit BD, Chandler MP, Stanley WC. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive Dahl salt-sensitive rat. Clin Exp Pharmacol Physiol 32: 825–831, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low-carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension 48: 1116–1123, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Pausova Z, Sedova L, Berube J, Hamet P, Tremblay J, Dumont M, Gaudet D, Pravenec M, Kren V, Kunes J. Segment of rat chromosome 20 regulates diet-induced augmentations in adiposity, glucose intolerance, and blood pressure. Hypertension 41: 1047–1055, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Pfeffer JM, Pfeffer MA, Mirsky I, Braunwald E. Regression of left ventricular hypertrophy and prevention of left ventricular dysfunction by captopril in the spontaneously hypertensive rat. Proc Natl Acad Sci USA 79: 3310–3314, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Preuss HG, el Zein M, Knapka J, MacArthy P, Yousufi AK, Gleim GW, Glace B, Zukowska-Grojec Z. Blood pressure responses to sucrose ingestion in four rat strains. Am J Hypertens 5: 244–250, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Preuss HG, Jarrell ST, Scheckenbach R, Lieberman S, Anderson RA. Comparative effects of chromium, vanadium and gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr 17: 116–123, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Preuss HG, Zein M, MacArthy P, Dipette D, Sabnis S, Knapka J. Sugar-induced blood pressure elevations over the lifespan of three substrains of Wistar rats. J Am Coll Nutr 17: 36–47, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Rasmussen OW, Thomsen C, Hansen KW, Vesterlund M, Winther E, Hermansen K. Effects on blood pressure, glucose, and lipid levels of a high-monounsaturated fat diet compared with a high-carbohydrate diet in NIDDM subjects. Diabetes Care 16: 1565–1571, 1993 [DOI] [PubMed] [Google Scholar]
- 42. Reaven GM, Chang H, Hoffman BB, Azhar S. Resistance to insulin-stimulated glucose uptake in adipocytes isolated from spontaneously hypertensive rats. Diabetes 38: 1155–1160, 1989 [DOI] [PubMed] [Google Scholar]
- 43. Reil TD, Barnard RJ, Kashyap VS, Roberts CK, Gelabert HA. Diet-induced changes in endothelial-dependent relaxation of the rat aorta. J Surg Res 85: 96–100, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25–e146, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 344: 3–10, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Sechi LA, Griffin CA, Giacchetti G, Zingaro L, Catena C, Bartoli E, Schambelan M. Abnormalities of insulin receptors in spontaneously hypertensive rats. Hypertension 27: 955–961, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Sen S, Tarazi RC, Bumpus FM. Effect of converting enzyme inhibitor (SQ14,225) on myocardial hypertrophy in spontaneously hypertensive rats. Hypertension 2: 169–176, 1980 [DOI] [PubMed] [Google Scholar]
- 48. Shah M, Adams-Huet B, Bantle JP, Henry RR, Griver KA, Raatz SK, Brinkley LJ, Reaven GM, Garg A. Effect of a high-carbohydrate versus a high–cis-monounsaturated fat diet on blood pressure in patients with type 2 diabetes. Diabetes Care 28: 2607–2612, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Sharma N, Okere IC, Duda MK, Johnson J, Yuan CL, Chandler MP, Ernsberger P, Hoit BD, Stanley WC. High fructose diet increases mortality in hypertensive rats compared to a complex carbohydrate or high fat diet. Am J Hypertens 20: 403–409, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Siebenhofer A, Jeitler K, Berghold A, Waltering A, Hemkens LG, Semlitsch T, Pachler C, Strametz R, Horvath K. Long-term effects of weight-reducing diets in hypertensive patients. Cochrane Database Syst Rev CD008274, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Soesanto W, Lin HY, Hu E, Lefler S, Litwin SE, Sena S, Abel ED, Symons JD, Jalili T. Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension 54: 1321–1327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stebbins CL, Symons JD, Hageman KS, Musch TI. Endogenous prostaglandins limit angiotensin-II induced regional vasoconstriction in conscious rats. J Cardiovasc Pharmacol 42: 10–16, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res 104: 1085–1094, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tanner JM, Kearns DT, Kim BJ, Sloan C, Jia Z, Yang T, Abel ED, Symons JD. Fasting-induced reductions in cardiovascular and metabolic variables occur sooner in obese versus lean mice. Exp Biol Med (Maywood) 235: 1489–1497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsutsu N, Takata Y, Nunoi K, Kikuchi M, Takishita S, Sadoshima S, Fujishima M. Glucose tolerance and insulin secretion in conscious and unrestrained normotensive and spontaneously hypertensive rats. Metabolism 38: 63–66, 1989 [DOI] [PubMed] [Google Scholar]
- 56. Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG, Conlin PR, Svetkey LP, Erlinger TP, Moore TJ, Karanja N. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med 135: 1019–1028, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Wexler BC. Inhibition of the pathogenesis of spontaneous hypertension in spontaneously hypertensive rats by feeding a high fat diet. Endocrinology 108: 981–989, 1981 [DOI] [PubMed] [Google Scholar]
- 58. Yokoyama H, Averill DB, Brosnihan KB, Smith RD, Schiffrin EL, Ferrario CM. Role of blood pressure reduction in prevention of cardiac and vascular hypertrophy. Am J Hypertens 18: 922–929, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Young JB, Landsberg L. Effect of oral sucrose on blood pressure in the spontaneously hypertensive rat. Metabolism 30: 421–424, 1981 [DOI] [PubMed] [Google Scholar]