Abstract
The epidermal growth factor receptor (EGFR) gene encodes four alternatively spliced mRNA, variants 1, 2, 3 and 4, respectively, encoding the whole isoform a (EGFR) and truncated isoforms b, c and d, all of which lack the receptor’s intracellular domain. In addition, a mutant EGFRvIII differs from isoform a in a truncated extracellular domain. The expression pattern of these isoforms is unknown in adult diffuse gliomas. Thus, we investigated in 47 cases: i) EGFR protein expression by immunohistochemistry using an extracellular domain-recognizing antibody (Ext-Ab) and an intracellular domain specific one (Int-Ab), ii) mRNA expression of EGFRv1, -v2, -v3, -v4 and -vIII by RT-PCR and iii) EGFR amplification by fluorescent in situ hybridization. The relation of these data with histological criteria and patient outcome was studied. The immunostaining was stronger with the Ext-Ab than with the Int-Ab. EGFRv1, -v2, -v3 and -v4 mRNA expression were highly correlated. They were expressed in all tumors, with highest levels in glioblastomas. EGFRv1 strong levels and the presence of vIII mRNAs were more closely associated with Int-Ab staining. EGFR gene amplification concerned only glioblastomas and was associated with the presence of EGFRvIII and high levels of EGFRv2, -v3 and -v4 transcripts. A pejorative outcome was associated with: histology (glioblastomas), EGFR amplification, strong Int-Ab labeling and high levels of variant mRNAs. Our results indicated that the full-length EGFR and mutant EGFRvIII are not the sole EGFR isoform expressed in diffuse gliomas. This could explain discordant immunohistochemical results reported in the literature and may have therapeutic implications.
Keywords: gliomas, glioblastoma, epidermal growth factor receptor, EGFR isoforms, EGFR mRNA variants
Introduction
According to the World Health Organization classification (WHO), adult diffuse gliomas include astrocytomas, glioblastomas, oligodendrogliomas and oligoastrocytomas (1). Despite therapeutic advances, these tumors remain incurable and the prognosis for patients afflicted with anaplastic tumors and glioblastomas is still very poor (2).
The epidermal growth factor receptor (EGFR) belongs to HER family, a group of four receptors. Many reports have shown that EGFR contribute to diffuse glioma oncogenesis (3–8). The most prevalent EGFR pathway modifications involved are EGFR gene amplification and receptor overexpression, the latter of which remains controversial (9–15).
The EGFR gene is located in 7p12 and generates a first type of mRNA transcript, referred to as EGFR variant 1 (EGFRv1), which encodes the full-length receptor, known as isoform a, EGFR or HER1. Isoform a is a transmembrane protein with an intracellular tyrosine kinase domain. Ligand binding on the extracellular domain leads to stimulation of cellular proliferation and cell survival pathways. Glioblastomas harboring EGFR gene amplification frequently contain a genetic variant, EGFRvIII, which encodes a mutant receptor with an altered extracellular domain that renders it constitutively active (16–18).
In addition to variant v1, the EGFR gene generates three other alternatively spliced transcripts, referred to as variants 2–4 (EGFRv2-v4). The corresponding mRNAs are shorter than the EGFRv1 transcript and, respectively, encode truncated forms of the receptor (isoforms b, c and d), which lack the cytoplasmic tyrosine kinase domain. Soluble isoforms have been reported (19–21). The role of these truncated EGFR isoforms remains poorly known. In vitro, they have been shown to decrease cellular proliferation (22,23). The hypotheses for this cellular growth inhibition include the competitive binding of EGFR ligands by the truncated isoforms (24,25) and formation of catalytically inactive heterodimers of different isoforms, which interfere with the formation of functional holoreceptor dimers. This consequently prevents intracellular kinase activity (26). To our knowledge, expressions of truncated EGFR isoforms and their transcripts have never been studied in gliomas.
To assess whether EGFR protein isoforms and their corresponding transcripts are expressed in diffuse gliomas, we performed an immunohistochemical analysis and determined the expression patterns of EGFRv1, -v2, -v3, -v4 and mutant EGFRvIII mRNAs. Results were analyzed with respect to the clinical data, patient outcome, histological tumor type and presence or absence of EGFR gene amplification.
Patients and methods
Patients and tissue samples
Tumors were obtained from 47 adult patients diagnosed with infiltrating glioma who were undergoing surgery at Limoges Dupuytren University Hospital between 1995 and 2011. Clinical and survival data were obtained by a retrospective query and all samples were used in accordance with French bioethics laws regarding patient information and con-sent. No patients received EGFR-targeted therapeutics. Tumor samples were fixed in 4% formalin at the time of resection. They were then embedded in paraffin and tumor sections were stained with hemalum phloxine saffron. Part of the surgical specimen was snap-frozen and conserved at −80°C. The histological type and grade of gliomas were determined according to the WHO classification (1). Non-tumor tissue was used as a control.
Immunohistochemistry
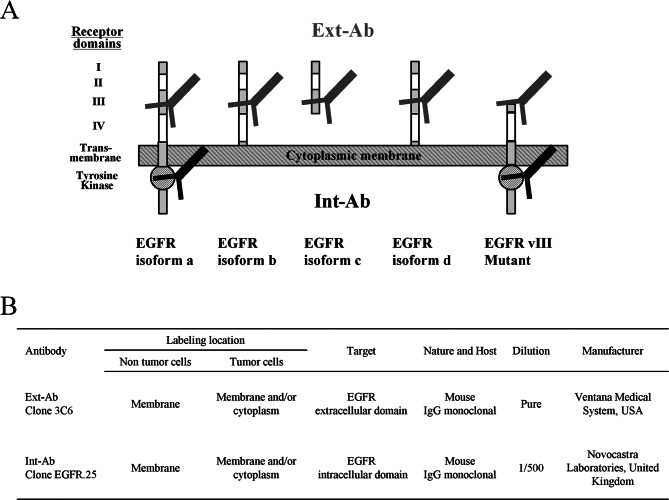
Sections (5 μm) were cut from paraffin-embedded tumors and stained with two different anti-EGFR antibodies (Fig. 1). One antibody targeted the extracellular domain (Ext-Ab) and the other intracellular domain (Int-Ab). Sample slides were processed automatically (BenchMark XT ICH/ISH, Ventana Medical Systems, Tucson, AZ, USA) according to protocols supplied by the manufacturers. EGFR staining was quantified according to a semiquantitative method proposed by Hirsch et al (27), as previously described (28). Staining was scored for labeling intensity (1, negative or trace; 2, weak; 3, moderate; 4, intense), percentage of positive cells per slide (0%–100%) and for the Hirsch score resulting from multiplication of these two parameters (absolute values ranging from 0 to 400). For certain analyses, the level of expression in samples was scored as: negative or low, intermediate, and high, which corresponded to Hirsch values of 0–200, 201–300, and 301–400, respectively.
Figure 1.
Characteristics of antibodies used in immunohistochemistry. (A), Ext-Ab (grey) and Int-Ab (black) targeting different domains of EGFR isoforms a, b, c, d and the EGFRvIII mutant; (B), Ext-Ab and Int-Ab characteristics.
We also tested the following antibodies: Ki67 (clone MiB-1, DakoCytomation, Glostrup, Denmark; 1/200e), p53 (Dako Cytomation; 1/50e), Olig2 (Immuno-Biological Laboratories, Gunma, Japan; 1/200e), and glial fibrillar acidic protein (Dako Cytomation; 1/1600e). The percentages of cells labeled with these antibodies were determined by studying at least five hundred cells for each antibody in tumor areas of highest positivity.
Total RNA extraction
Tumor tissue (30 mg) was incubated with 1 ml Qiazol® solution (Qiagen, Courtaboeuf, France) and CK14 ceramic beads (Ozyme) and then pulverized two times for 40 sec each at 6500 rpm in a Precellys 24 homogenizer (Bertin Technologies). Homogenized tissues were then lysed and RNA purification was performed according to the manufacturer’s protocol (RNeasy Lipid Tissue Mini kit, Qiagen). RNA concentration and purity was estimated by spectrophotometry (NanoDrop ND1000, Labtech, France). RNA quality was assessed by capillary electrophoresis (Bioanalyzer 2100, Agilent Technologies) and only RNA with an Integrity Number (R.I.N) >6 was used for analysis.
Quantitative and qualitative RT-PCR
Complementary DNA (cDNA) was synthesized from 2 μg of total RNA using the Transcriptor First Strand cDNA Synthesis® kit (Roche) and hexamer primers, according to the manufacturer’s protocol. For PCR, primers were designed using Amplify 1.2 or Primer 3 (http://fokker.wi.mit.edu/primer3/input.html/) software and their specificity was determined by BLASTn in the NCBI database (http://www.ncbi.nlm.nih.gov/). Primer characteristics are listed in Table I. Amplicon size and specificity were initially determined by 4% NuSieve agarose gel electrophoresis. Quantitative PCR [EGFRv1, -v2, -v3, -v4 and hypoxanthine phosphoribosyl transferase (HPRT)] and qualitative PCR (EGFRvIII) were performed on a Rotor Gene thermocycler (Corbett Research) using the Light Cycler Fast Start DNA Master SYBR Green I kit (Roche). All targets were amplified twice in duplicate in the presence of 3 mM MgCl2 and 0.5 μM primers. Relative quantification of mRNA content was performed using the ΔΔCt method [(Ctsample-Ctcalibrator)interest gene - (Ctsample-Ctcalibrator)reference gene], modified according to Pfaffl (29), with efficiency correction by Rotor Gene software. mRNA content was expressed in relative arbitrary units (R.A.U.).
Table I.
Characteristics of the primer used for quantitative and qualitative RT-PCR.
| Target | Primer | Location | Sequence 5′-3′ | Amplicon size (bp) | Primer temperature (°C) |
|---|---|---|---|---|---|
| EGFR variant 1 | Forward | Exon 29–30 | CTCCCAGTGCCTGAATACATA | 83 | 58 |
| Reverse | Exon 30 | GGCTGATTGTGATAGACAGGA | |||
| EGFR variant 2 | Forward | Exon 15 | TGCACCTACGGGTCCTAAT | 97 | 58 |
| Reverse | Exon 16 | TGAAGCAAAGGGAGAAATTG | |||
| EGFR variant 3 | Forward | Exon 10 | AAGGAAATCACAGGTTTGAGC | 99 | 58 |
| Reverse | Exon 10bis | TCCAAGGGAACAGGAAATATG | |||
| EGFR variant 4 | Forward | Exon 15 | CTACGGGCCAGGAAATGAG | 86 | 62 |
| Reverse | Exon 17 | CGCTGCCATCATTACTTTGA | |||
| HPRT | Forward | Exon 6 | CTTTCCTTGGTCAGGCAGTA | 90 | 58 |
| Reverse | Exon 7 | TGGCTTATATCCAACACTTCG | |||
| EGFRvIII | Forward | Exon 1 | GCTCTGGAGGAAAAGAAAGGTAAT | 90 | 62 |
| Reverse | Exon 8 | TCCTCCATCTCATAGCTGTCG |
Fluorescent in situ hybridization
EGFR gene amplification and 1p36 and 19q13 losses were analyzed by double fluorescent in situ hybridization with the ‘LSI EGFR SpectrumOrange/CEP 7 SpectrumGreen Probe’, or with the ‘LSI 1p36 spectrum orange/LSI 1q25 spectrum green probe’ and the ‘LSI 19q13 spectrum orange/LSI 19p13 spectrum green probe’ (Abbott Molecular Inc., IL, USA) kits, respectively. They were investigated on consecutive paraffin sections from the same blocks used in immunohistochemical analyses. This technique was a modification of the method previously described (28): briefly, 4 μm paraffin sections were incubated 16 h at 56°C, submitted to deparaffinising, digested with pepsin (Abbott Molecular Inc.) at 37°C during 45 min and dehydrated in successive ethanol baths. Slides were incubated with 10 μl of each probe for 5 min at 73°C to denature DNA and 16 h at 37°C to ensure hybridization. Sections were washed in 2X SSC/0.3% NP40 solution, once for 1 min at room temperature, once for 2 min at 73°C, and dehydrated in successive ethanol baths. Counterstaining and microscopic observation of EGFR amplification were performed as previously described (28). EGFR gene amplification was considered to have occurred if more than 10% of the cells analyzed produced a red (corresponding to the EGFR-specific probe) to green (centromeric region of chromosome 7) signal ratio ≥2, as recommended previously (30,31). Eight sequential focus stacks with 0.3 μm were acquired and then integrated into a single image in order to reduce thickness related artifacts. Preparations were considered as valid when more of 80% of the cells showed bright signals.
Statistical analyses
StatView® 5.0 software (SAS Institute, Inc., Cary, NC, USA) was used for statistical analyses. Fisher’s exact test was used to assess differences between nominal variables. Means were compared with the non-parametric Mann-Whitney test for pairs of variables and with the Kruskall-Wallis tests for comparisons of more than two variables. Correlation Spearman test was used to compare quantitative variables. Overall survival (OS) and progression-free survival (PFS) were analyzed by Kaplan-Meier and median OS or PFS medians were compared with the non-parametric log-rank or Breslow tests. Results for which p<0.05 were considered to be statistically significant.
Results
Patient and tumor characteristics
Patient characteristics are summarized in Table II. For the series, median follow-up was 23.3 (0.5–240) months. Ki67 labeling index, Olig2 and p53 protein expression, and presence or absence of a 1p36–19q13 loss were consistent with histological typing. Thus, oligodendrogliomas were characterized by a 1p36/19q13 deletion, stronger olig2 and weaker p53 expressions compared to other tumor types (Table III).
Table II.
Demographic, pathological and clinical features.
| No. | Grade | Sex (male/female) | Age (median) | Tumor status (primary/recurrent) | Radiotherapy (yes/no) | Chemotherapy (yes/no) | |
|---|---|---|---|---|---|---|---|
| All | 47 | 18/19 | 50.6 | 36/11 | 42/5 | 37/10 | |
| A | 3 | 1 II, 2 III | 1/2 | 51.3 | 2/1 | 2/1 | 2/1 |
| GBM | 21 | 21 IV | 10/11 | 59.7 | 19/2 | 20/1 | 18/3 |
| O | 14 | 5 II, 9 III | 3/11 | 48.1 | 10/4 | 13/1 | 13/1 |
| OA | 9 | 6 II, 3 III | 4/5 | 43.2 | 5/4 | 7/2 | 4/5 |
A, astrocytoma; GBM, glioblastoma; O, oligodendroglioma; OA, oligoastrocytoma.
Table III.
Molecular characterization of glioma histological types.
| Ki67 (n=45) | p53 (n=45) | Olig2 (n=45) | 1p36-19q13 loss (n=46) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| % Mean ± SD | p-value | % Mean ± SD | p-value | % Mean ± SD | p-value | Yes | No | p-value | |
| All patients | 19±14 | 46.3±31.1 | 58.6±23.9 | 17 | 29 | ||||
| Histological type | |||||||||
| A | 13.7±10 | 0.06 | 80±10 | 0.0004 | 51.7±27.5 | 0.04 | 0 | 3 | <0.0001 |
| GBM | 26.9±13.5 | 49.7±28.3 | 48.7±25.8 | 2 | 18 | ||||
| O | 14.8±19 | 20.6±18.4 | 71.8±18.3 | 14 | 0 | ||||
| OA | 10.8±11.2 | 67.8±28.5 | 61.1±19 | 1 | 8 | ||||
A, astrocytoma; GBM, glioblastoma; O, oligodendroglioma; OA, oligoastrocytoma.
Immunohistochemical detection of EGFR isoforms and EGFRvIII mutant
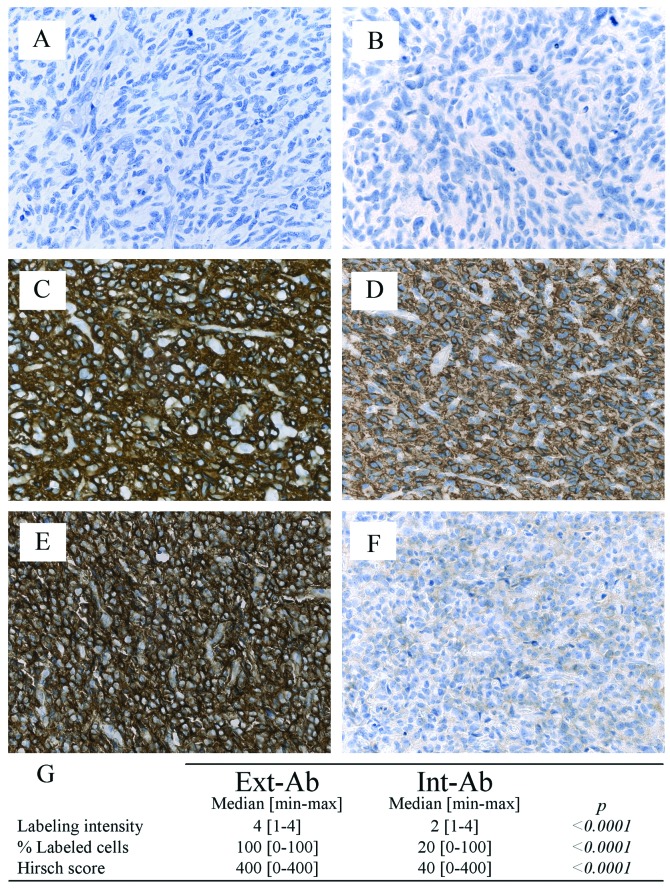
The percentage of gliomas stained by Ext-Ab was 98% (44/45), whereas only 78% (35/45) of the gliomas were stained by Int-Ab. The Ext-Ab and Int-Ab antibodies generated very different staining patterns in the gliomas (Fig. 2A-F). Glioma staining by Int-Ab was significantly lower than that of Ext-Ab in terms of intensity, percentage of positive cells and Hirsch score (p<0.0001; Fig. 2G).
Figure 2.
Immunohistochemical staining. Absence of labeling with both Ext-Ab (A) and Int-Ab (B) in a glioblastoma. Strong EGFR expression with both Ext-Ab (C) and Int-Ab (D) in a glioblastoma. Discordant staining: strong with the Ext-Ab (E) and very weak with the Int-Ab (F) in a grade III oligodendroglioma. (G), table of statistical analysis.
Neither antibody detected any significant differences in EGFR staining with respect to patient sex or age (data not shown). Staining intensities, percentages of labeled cells and Hirsch scores obtained with Ext-Ab did not significantly differ according to histological types. In contrast, Int-Ab scores were significantly higher in glioblastomas, astrocytomas or oligodendrogliomas compared to oligoastrocytomas (Table IV). In non-tumor tissue, we found no or very weak EGFR expression whatever the antibody used.
Table IV.
Association between Ext-Ab and Int-Ab labeling and pathological parameters.
| Ext-Ab | Int-Ab | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| No. | Labeling intensity | p-value | Percentage of labeled cells | p-value | Hirsch score | p-value | Labeling intensity | p-value | Percentage of labeled cells | p-value | Hirsch score | p-value | |
| All | 45 | 4 [1–4] | 100 [0–100] | 400 [0–400] | 2 [1–4] | 20 [0–100] | 40 [0–400] | ||||||
| Histological type | |||||||||||||
| A | 3 | 4 [3–4] | 0.73 | 70 [20–90] | 0.33 | 360 [60–400] | 0.69 | 2 [2–3] | 0.006 | 30 [5–40] | 0.01 | 60 [10–120] | 0.01 |
| GBM | 19 | 4 [2–4] | 100 [10–100] | 400 [20–400] | 3 [1–4] | 30 [0–100] | 90 [0–400] | ||||||
| O | 14 | 4 [1–4] | 100 [0–100] | 380 [0–400] | 2 [1–3] | 45 [0–70] | 90 [0–210] | ||||||
| OA | 9 | 4 [2–4] | 100 [20–100] | 300 [80–400] | 1 [1–2] | 0 [0–10] | 0 [0–20] | ||||||
Ext-Ab, antibody targeting the extracellular domain; Int-Ab, antibody targeting the intracellular domain; A, astrocytoma; GBM, glioblastoma; O, oligodendroglioma; OA, oligoastrocytoma.
Quantitation of EGFR variants 1, 2, 3, 4 and EGFRvIII mRNAs
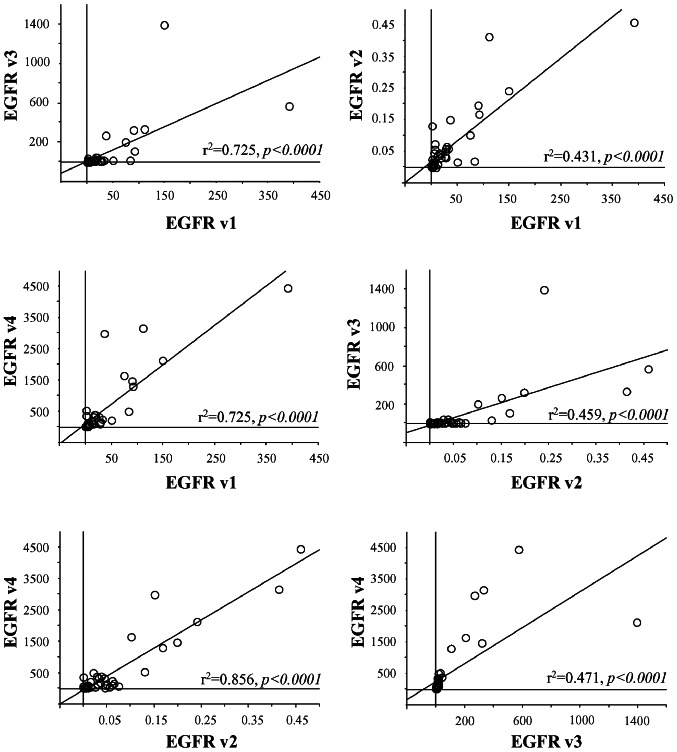
EGFR mRNA levels varied widely among the tumor samples. Median R.A.U. values were 7.3 (0.4–390.2) for EGFRv1+vIII mRNA, 0.02 (0–0.5) for EGFRv2 mRNA, 6.2 (0.1–1396.8) for EGFRv3 mRNA and 94.6 (2.1–4445.2) for EGFRv4 mRNA. Straight correlations were found for all comparisons between EGFRv1+vIII, -v2, -v3 and -v4 mRNA levels (p<0.0001 for all, Fig. 3). EGFRvIII mutant expression was qualitatively detected in 26% (12/47) of the gliomas. In non-tumor tissue mRNA variant were very weakly expressed with 0.7 R.A.U. for EGFRv1+vIII and EGFRv3, 6.6 R.A.U. for EGFRv4 and no EGFRvIII and EGFRv2 expression.
Figure 3.
Correlation for EGFRv1+vIII, -v2, -v3 and -v4 mRNA levels.
Association of EGFR variant and EGFRvIII mRNA expression with other parameters
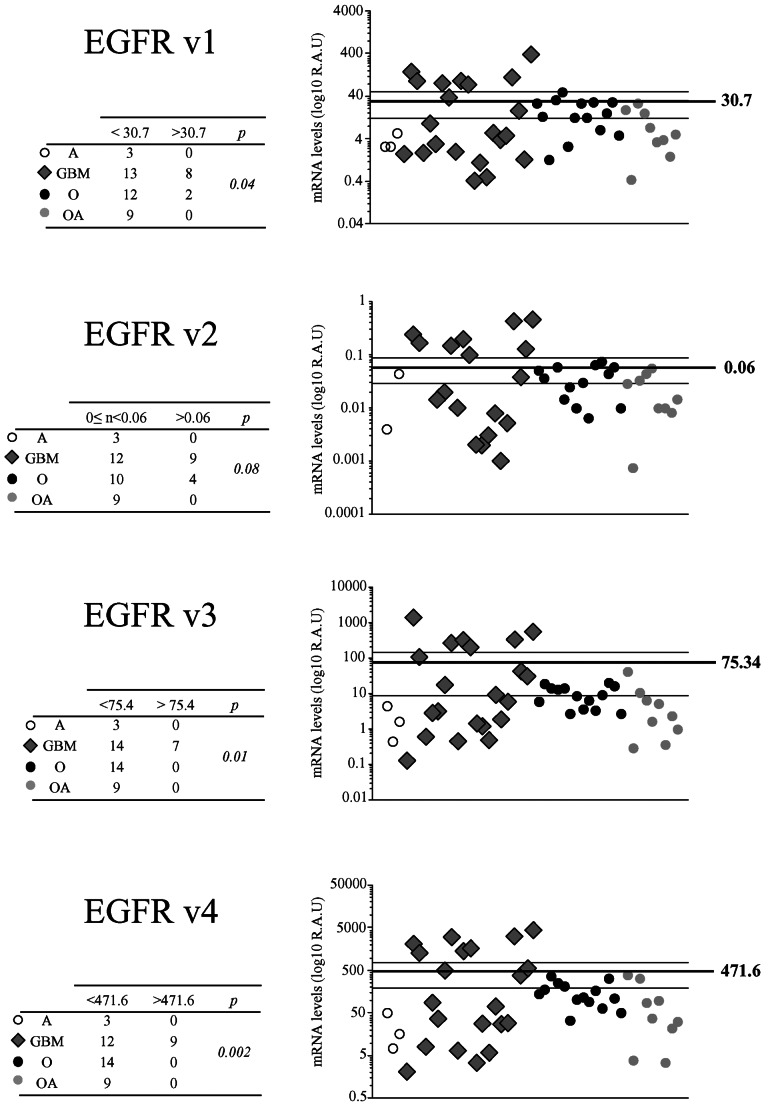
EGFRv1+vIII, -v2, -v3, and -v4 mRNA levels were not influenced by patient sex and age (data not shown). EGFRv1+vIII, -v3 and -v4 mRNA levels were higher in glioblastomas than in other tumor types when mean values were taken as a cut-off (p=0.04, 0.01 and 0.002, respectively) (Fig. 4). EGFRvIII mRNA was significantly associated with histological type, it was found in one astrocytoma, ten glioblastomas and one oligoastrocytoma, but in none oligodendroglioma (p=0.01, data not shown). We also observed that EGFRv1+vIII mRNA levels more straightly correlated with Int-Ab staining than with Ext-Ab staining (Fig. 5).
Figure 4.
Relation between EGFR variant mRNA levels and histological types. EGFRv1+vIII, -v2, -v3 and -v4 mRNA levels were expressed in Log10 relative arbitrary unit (Log10 R.A.U). Mean value was taken as a cut-off to determine the number of tumors under (< mean) and above (> mean) it.
Figure 5.
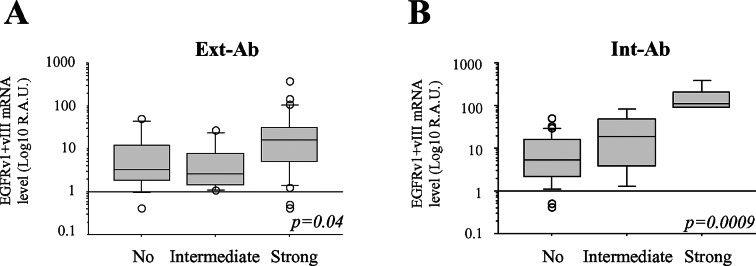
Relation between EGFRv1+vIII mRNA levels and immunohistochemical data. Ext-Ab (A) and Int-Ab (B) labeling were expressed as no vs. intermediate vs. strong Hirsch score.
EGFR gene amplification in gliomas
We detected EGFR gene amplification in 8 out of the 20 glioblastomas, but not in the other glioma types (p=0.007, Table V). Glioblastomas with EGFR gene amplification expressed significantly stronger EGFRv1, -v2, -v3 and -v4 mRNA levels than gliomas with no EGFR amplification (Table V). The presence of mutant EGFRvIII mRNA was significantly associated with EGFR amplification (p<0.0001).
Table V.
EGFR amplification.
| EGFR amplification | |||
|---|---|---|---|
|
|
|||
| No. | Yes | p-value | |
| Histological type | |||
| A | 3 | 0 | 0.007 |
| GBM | 12 | 8 | |
| O | 13 | 0 | |
| OA | 9 | 0 | |
| EGFRv1+vIII mRNA | |||
| < mean | 34 | 2 | 0.0001 |
| > mean | 3 | 6 | |
| EGFRv2 mRNA | |||
| < mean | 32 | 2 | 0.001 |
| > mean | 5 | 6 | |
| EGFRv3 mRNA | |||
| < mean | 36 | 1 | <0.0001 |
| > mean | 3 | 5 | |
| EGFRv4 mRNA | |||
| < mean | 36 | 1 | <0.0001 |
| > mean | 1 | 7 | |
| EGFRvIII mRNA | |||
| Present | 4 | 7 | <0.0001 |
| Absent | 33 | 1 | |
A, astrocytoma; GBM, glioblastoma; O, oligodendroglioma; OA, oligoastrocytoma.
Weak Ext-Ab staining was more closely associated with the absence of EGFR gene amplification than with its presence (p=0.09), whereas strong Int-Ab staining was significantly associated with gene amplification (p=0.01; Fig. 6).
Figure 6.
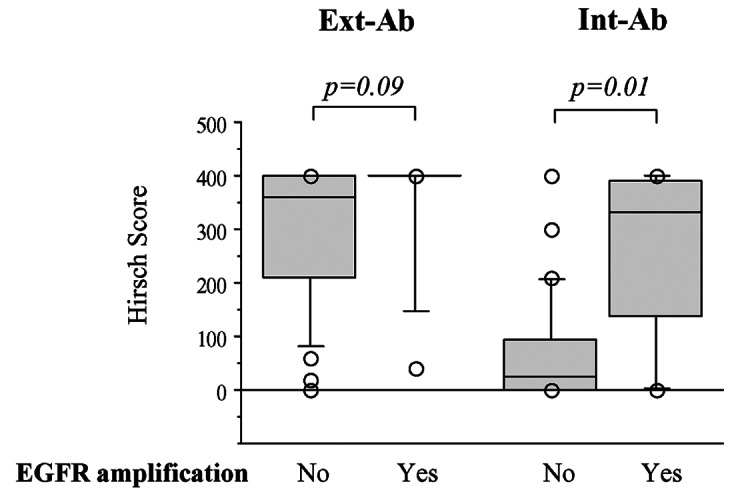
Association between EGFR gene amplification and immunohistochemical staining. Ext-Ab (A) and Int-Ab (B) staining expressed as Hirsch scores was compared in tumors exhibiting or lacking EGFR gene amplification.
Prognostic values
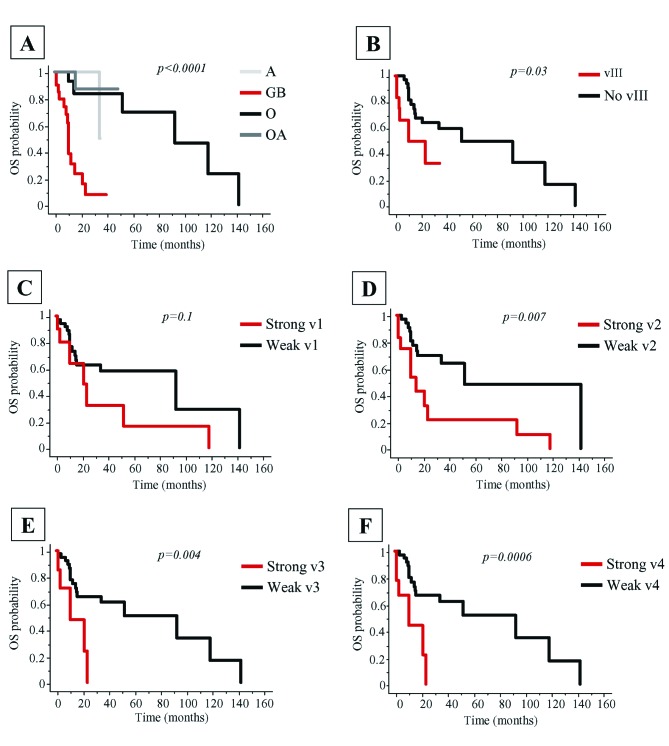
PFS and OS were significantly shorter for patients diagnosed with glioblastoma and astrocytoma than for those with oligodendroglioma and oligoastrocytoma (p<0.0001 for both) (Table VI, Fig. 7A). OS and PFS were better for patients with tumors showing a 1p36–19q13 loss and an absence of EGFR amplification (Table VI).
Table VI.
PFS and OS according to histological and molecular parameters.
| PFS | OS | |||
|---|---|---|---|---|
|
|
|
|||
| Median (months) | p-value | Median (months) | p-value | |
| Histological types | ||||
| A | 16.4 | <0.0001 | 34.9 | <0.0001 |
| GBM | 5.4 | 10.9 | ||
| O | 45.1 | 93.3 | ||
| OA | 41 | nr | ||
| EGFR amplification | ||||
| Yes | 4.7 | <0.0001 | 8.8 | 0.0003 |
| No | 21.6 | 93.3 | ||
| 1p36-19q13 loss | ||||
| Yes | 21.9 | 0.04 | 93.3 | 0.09 |
| No | 16.4 | 24 | ||
| Ext-Ab | ||||
| No/intermediate | 22.7 | 0.25 | 52.8 | 0.7 |
| Strong | 16.4 | 93.3 | ||
| Int-Ab | ||||
| No/intermediate | 21.4 | 0.006 | 93.3 | 0.07 |
| Strong | 4.7 | 21.6 | ||
| EGFRv1 mRNA | ||||
| Weak | 21 | 0.02a | 93.3 | 0.1 |
| Strong | 5.4 | 21.6 | ||
| EGFRv2 mRNA | ||||
| Weak | 21.4 | 0.01a | 52.8 | 0.007 |
| Strong | 9 | 15.1 | ||
| EGFRv3 mRNA | ||||
| Weak | 21 | 0.0007 | 93.3 | 0.004 |
| Strong | 4.7 | 10.9 | ||
| EGFRv4 mRNA | ||||
| Weak | 21.4 | <0.0001 | 93.3 | 0.0006 |
| Strong | 4.7 | 10.9 | ||
| EGFRvIII mRNA | ||||
| No | 21.4 | 0.0007 | 52.8 | 0.03 |
| Yes | 4.7 | 10.9 | ||
The difference was not significant using log-rank test but was significant with Breslow-Gehan-Wilcoxon test.
OS, overall survival; PFS, progression-free survival; Nr, not reached; A, astrocytoma; GBM, glioblastoma; O, oligodendroglioma; OA, oligoastrocytoma.
Figure 7.
Patient overall survival (OS). Differences in OS according to histological types (A), mutant EGFRvIII expression (B), EGFRv1+vIII (C), -v2 (D), -v3 (E) and -v4 (F) mRNA levels were assessed by log-rank test.
No/intermediate or strong tumor labeling with EGFR Ext-Ab did not influence OS and PFS times whereas no/intermediate labeling with EGFR Int-Ab was associated with longer OS and PFS (Table VI). In addition, PFS and OS were longer when gliomas expressed weak EGFRv1+vIII, -v2, v3, or -v4 mRNA levels and showed no mutant EGFRvIII mRNA expression (Table VI, Fig. 7B-F).
In glioblastomas (data not shown), PFS was significantly better for patients with no EGFR amplification (5.4 vs. 8.4 months, p=0.01), no expression of EGFRvIII mutant mRNA (3.7 vs. 8.4 months, p=0.04), or weak EGFRv2 (3.3 vs. 5.6 months, p=0.04) or -v4 mRNA levels (8.4 vs. 4.7 months, p=0.05). OS did not change according to these parameters.
Discussion
Based on the present results, we report that diffuse gliomas expressed truncated EGFR protein isoforms, based on: i) immunohistochemical data and ii) EGFRv1, -v2, -v3, -v4 variants and EGFRvIII mutant mRNA detection.
Immunohistochemical results varied according to the antibody used and favored the hypothesis of an expression of the truncated isoforms. The stronger EGFR staining obtained with Ext-Ab reflected their expression since, in addition to functional EGFR and the EGFRvIII mutant targeted by both antibodies, Ext-Ab also recognized the truncated EGFR isoforms b, c, and d whereas Int-Ab did not. The detection of truncated isoforms, depending on the antibody used, could explain some of the discrepancies found in literature regarding EGFR expression in gliomas (9–15). In our series, glioblastomas and oligodendrogliomas were strongly labeled by both Ext-Ab and Int-Ab, whereas oligoastrocytomas were moderately or strongly labeled by Ext-Ab but weakly by Int-Ab. Thus, in combination with other markers such as Olig2, p53 or 1p19q deletion, the study of EGFR expression might be useful to further characterize the diffuse gliomas (32–36).
The transcriptomic analysis showed that alternatively spliced EGFRv2, -v3 and -v4 transcript variants, encoding EGFR isoforms b, c and d, respectively, were expressed in addition to the EGFRv1 and EGFRvIII mutant mRNAs. In accordance with immunohistochemistry, detection of EGFRv1+vIII mRNA associated more closely with Int-Ab staining than with Ext-Ab staining. However, we also found an association between Int-Ab staining and EGFRv2, -v3 and -v4 transcript expressions, although Int-Ab did not detect the isoform they encode. This association is probably the consequence of the strong link existing between the expressions of each EGFR transcript (Fig. 3). Nevertheless, alternatively spliced EGFR transcript variants and EGFRvIII mRNA were produced at different levels according to the histological type of glioma. The glioblastomas had a peculiar profile. They expressed the highest levels of EGFRv3 and -v4 mRNA transcripts and, in addition, EGFRvIII mRNA expression was related to this tumor type.
EGFR gene amplification was detected in eight glioblastomas. As previously reported (28), weak Ext-Ab staining was associated with the lack of EGFR amplification. In contrast, Int-Ab staining intensity directly correlated with EGFR gene amplification, as already shown (37). This suggests that EGFR gene amplification is tightly associated with high expression of EGFR receptor isoforms that contain the intracellular tyrosine kinase domain, i.e., EGFR isoform a and the EGFRvIII mutant. Regarding the relationships with the prognosis in our series, the histological type (astrocytoma and glioblastoma), a strong staining with Int-Ab and the presence of EGFR amplification and of mutant vIII were associated with shorter PFS and OS times. High levels of EGFRv2, -v3 and -v4 transcript expression were also related to a shortened OS and PFS.
Our data tend to indicate that the role of EGFR pathway in glioblastoma oncogenesis is more complex than expected. In our series, in addition to the known molecular alterations i.e., EGFR gene amplification and expression of vIII mutant, we observed a strong staining with Int-Ab and high levels of EGFR mRNA variants. Actually, the functional roles of the truncated EGFR isoforms are poorly known, particularly in vivo. In vitro, it has been described that soluble isoforms may regulate EGFR signaling in normal and tumor cells (26,38). Paradoxically, these isoforms have been shown to decrease cellular proliferation (22,23). The known mechanisms responsible for growth inhibition include competitive binding of ligands to soluble isoforms and formation of inactive heterodimers which inhibit the formation of holoreceptor dimers and/or intracellular kinase activity (24–26).
The presence of truncated EGFR isoforms in adult infiltrating gliomas must be considered in therapeutic management. The interactions between truncated EGFR isoforms and EGFR-targeted therapeutics are not well understood. The presence of non-functional receptors could contribute to the failure of therapeutics which target the EGFR extracellular domain (39). Conversely, it has been reported that the presence of truncated EGFR isoforms may be predictive of the therapeutic response to gefitinib in endometrious adenocarcinomas (40). Lafky and coworkers speculated that the soluble vascular endothelial growth factor receptor (sVEGFR) represents a paradigm for understanding the function and potential application of EGFR isoforms as novel therapeutic molecules (41). Soluble VEGFR isoforms have been presented as effective therapeutic molecules (42) and a similar application for certain EGFR truncated isoforms may be possible.
To our knowledge, this is the first report that gliomas express EGFR transcripts other than EGFRv1 mRNA, which encodes the full-length and functional EGFR isoform a. The role of EGFR isoforms in glioma pathogenesis remains to be clarified, but their expression makes them potential targets of future diagnostic and therapeutic strategies.
Acknowledgments
This work was supported by grants from the ‘Comité Départemental de la Ligue contre le Cancer de la Corrèze’. We thank Mrs. Marion Porcheron for technical assistance, the ‘Tumorothèque du Limousin’ for providing all tissue samples and the ‘Plateforme d’Oncologie Moléculaire’ of the Limoges Dupuytren University Hospital.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 3.Andersson U, Guo D, Malmer B, Bergenheim AT, Brannstrom T, Hedman H, Henriksson R. Epidermal growth factor receptor family (EGFR, ErbB2-4) in gliomas and meningiomas. Acta Neuropathol. 2004;108:135–142. doi: 10.1007/s00401-004-0875-6. [DOI] [PubMed] [Google Scholar]
- 4.Bredel M, Pollack IF, Hamilton RL, James CD. Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clin Cancer Res. 1999;5:1786–1792. [PubMed] [Google Scholar]
- 5.McLendon RE, Turner K, Perkinson K, Rich J. Second messenger systems in human gliomas. Arch Pathol Lab Med. 2007;131:1585–1590. doi: 10.5858/2007-131-1585-SMSIHG. [DOI] [PubMed] [Google Scholar]
- 6.Potti A, Forseen SE, Koka VK, Pervez H, Koch M, Fraiman G, Mehdi SA, Levitt R. Determination of HER-2/neu overexpression and clinical predictors of survival in a cohort of 347 patients with primary malignant brain tumors. Cancer Invest. 2004;22:537–544. doi: 10.1081/cnv-200026523. [DOI] [PubMed] [Google Scholar]
- 7.Schlegel J, Stumm G, Brandle K, Merdes A, Mechtersheimer G, Hynes NE, Kiessling M. Amplification and differential expression of members of the erbB-gene family in human glioblastoma. J Neurooncol. 1994;22:201–207. doi: 10.1007/BF01052920. [DOI] [PubMed] [Google Scholar]
- 8.Tabori U, Rienstein S, Dromi Y, Leider-Trejo L, Constantini S, Burstein Y, Dvir R, Amariglio N, Toren A, Rechavi G, Izraeli S, Aviram A. Epidermal growth factor receptor gene amplification and expression in disseminated pediatric low-grade gliomas. J Neurosurg. 2005;103:357–361. doi: 10.3171/ped.2005.103.4.0357. [DOI] [PubMed] [Google Scholar]
- 9.Gil-Benso R, Lopez-Gines C, Benito R, Lopez-Guerrero JA, Callaghan RC, Pellin A, Roldan P, Cerda-Nicolas M. Concurrent EGFR amplification and TP-53 mutation in glioblastomas. Clin Neuropathol. 2007;26:224–231. doi: 10.5414/npp26224. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura JL. The epidermal growth factor receptor in malignant gliomas: pathogenesis and therapeutic implications. Expert Opin Ther Targets. 2007;11:463–472. doi: 10.1517/14728222.11.4.463. [DOI] [PubMed] [Google Scholar]
- 11.Okada Y, Ohno C, Ueki K, Ogino M, Kawamoto S, Kim P. Comparison of numerical change of epidermal growth factor receptor gene among pre- and postradiation glioma, and gliosis, and its clinical use. Brain Tumor Pathol. 2007;24:15–18. doi: 10.1007/s10014-007-0213-5. [DOI] [PubMed] [Google Scholar]
- 12.Schober R, Bilzer T, Waha A, et al. The epidermal growth factor receptor in glioblastoma: genomic amplification, protein expression, and patient survival data in a therapeutic trial. Clin Neuropathol. 1995;14:169–174. [PubMed] [Google Scholar]
- 13.Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, Oka K, Ishimaru Y, Ushio Y. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–6970. [PubMed] [Google Scholar]
- 14.Torp SH, Helseth E, Dalen A, Unsgaard G. Epidermal growth factor receptor expression in human gliomas. Cancer Immunol Immunother. 1991;33:61–64. doi: 10.1007/BF01742530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torp SH, Helseth E, Ryan L, Stolan S, Dalen A, Unsgaard G. Amplification of the epidermal growth factor receptor gene in human gliomas. Anticancer Res. 1991;11:2095–2098. [PubMed] [Google Scholar]
- 16.Gan HK, Kaye AH, Luwor RB. The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci. 2009;16:748–754. doi: 10.1016/j.jocn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Lal A, Glazer CA, Martinson HM, Friedman HS, Archer GE, Sampson JH, Riggins GJ. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62:3335–3339. [PubMed] [Google Scholar]
- 18.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, II, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maihle NJ, Baron AT, Barrette BA, Boardman CH, Christensen TA, Cora EM, Faupel-Badger JM, Greenwood T, Juneja SC, Lafky JM, Lee H, Reiter JL, Podratz KC. EGF/ErbB receptor family in ovarian cancer. Cancer Treat Res. 2002;107:247–258. doi: 10.1007/978-1-4757-3587-1_11. [DOI] [PubMed] [Google Scholar]
- 20.Reiter JL, Maihle NJ. Characterization and expression of novel 60-kDa and 110-kDa EGFR isoforms in human placenta. Ann NY Acad Sci. 2003;995:39–47. doi: 10.1111/j.1749-6632.2003.tb03208.x. [DOI] [PubMed] [Google Scholar]
- 21.Reiter JL, Threadgill DW, Eley GD, Strunk KE, Danielsen AJ, Sinclair CS, Pearsall RS, Green PJ, Yee D, Lampland AL, Balasubramaniam S, Crossley TD, Magnuson TR, James CD, Maihle NJ. Comparative genomic sequence analysis and isolation of human and mouse alternative EGFR transcripts encoding truncated receptor isoforms. Genomics. 2001;71:1–20. doi: 10.1006/geno.2000.6341. [DOI] [PubMed] [Google Scholar]
- 22.Flickinger TW, Maihle NJ, Kung HJ. An alternatively processed mRNA from the avian c-erbB gene encodes a soluble, truncated form of the receptor that can block ligand-dependent transformation. Mol Cell Biol. 1992;12:883–893. doi: 10.1128/mcb.12.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber W, Gill GN, Spiess J. Production of an epidermal growth factor receptor-related protein. Science. 1984;224:294–297. doi: 10.1126/science.6324343. [DOI] [PubMed] [Google Scholar]
- 24.Cadena DL, Gill GN. Expression and purification of the epidermal growth factor receptor extracellular domain utilizing a polycistronic expression system. Protein Expr Purif. 1993;4:177–186. doi: 10.1006/prep.1993.1024. [DOI] [PubMed] [Google Scholar]
- 25.Greenfield C, Hiles I, Waterfield MD, Federwisch M, Wollmer A, Blundell TL, McDonald N. Epidermal growth factor binding induces a conformational change in the external domain of its receptor. EMBO J. 1989;8:4115–4123. doi: 10.1002/j.1460-2075.1989.tb08596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu A, Raghunath M, Bishayee S, Das M. Inhibition of tyrosine kinase activity of the epidermal growth factor (EGF) receptor by a truncated receptor form that binds to EGF: role for interreceptor interaction in kinase regulation. Mol Cell Biol. 1989;9:671–677. doi: 10.1128/mcb.9.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, Di Maria MV, Veve R, Bremmes RM, Baron AE, Zeng C, Franklin WA. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 28.Guillaudeau A, Durand K, Pommepuy I, Robert S, Chaunavel A, Lacorre S, De Armas R, Bourtoumieux S, El Demery M, Moreau JJ, Labrousse F. Determination of EGFR status in gliomas: usefulness of immunohistochemistry and fluorescent in situ hybridization. Appl Immunohistochem Mol Morphol. 2009;17:220–226. doi: 10.1097/pai.0b013e31818db320. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korshunov A, Sycheva R, Golanov A. Molecular stratification of diagnostically challenging high-grade gliomas composed of small cells: the utility of fluorescence in situ hybridization. Clin Cancer Res. 2004;10:7820–7826. doi: 10.1158/1078-0432.CCR-04-0710. [DOI] [PubMed] [Google Scholar]
- 31.Marks RA, Zhang S, Montironi R, McCarthy RP, MacLennan GT, Lopez-Beltran A, Jiang Z, Zhou H, Zheng S, Davidson DD, Baldridge LA, Cheng L. Epidermal growth factor receptor (EGFR) expression in prostatic adenocarcinoma after hormonal therapy: a fluorescence in situ hybridization and immunohistochemical analysis. Prostate. 2008;68:919–923. doi: 10.1002/pros.20715. [DOI] [PubMed] [Google Scholar]
- 32.Durand K, Guillaudeau A, Pommepuy I, Mesturoux L, Chaunavel A, Gadeaud E, Porcheron M, Moreau JJ, Labrousse F. Alpha-internexin expression in gliomas: relationship with histological type and 1p, 19q, 10p and 10q status. J Clin Pathol. 2011;64:793–801. doi: 10.1136/jcp.2010.087668. [DOI] [PubMed] [Google Scholar]
- 33.Durand KS, Guillaudeau A, Weinbreck N, De Armas R, Robert S, Chaunavel A, Pommepuy I, Bourthoumieu S, Caire F, Sturtz FG, Labrousse FJ. 1p19q LOH patterns and expression of p53 and Olig2 in gliomas: relation with histological types and prognosis. Mod Pathol. 2010;23:619–628. doi: 10.1038/modpathol.2009.185. [DOI] [PubMed] [Google Scholar]
- 34.Hirose T, Ishizawa K, Shimada S. Utility of in situ demonstration of 1p loss and p53 overexpression in pathologic diagnosis of oligodendroglial tumors. Neuropathology. 2010;30:586–596. doi: 10.1111/j.1440-1789.2010.01116.x. [DOI] [PubMed] [Google Scholar]
- 35.Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, Louis DN, Stiles CD, Rowitch DH. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63:499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- 36.Okada M, Yano H, Hirose Y, Nakayama N, Ohe N, Shinoda J, Iwama T. Olig2 is useful in the differential diagnosis of oligodendrogliomas and extraventricular neurocytomas. Brain Tumor Pathol. 2011;28:157–161. doi: 10.1007/s10014-011-0017-5. [DOI] [PubMed] [Google Scholar]
- 37.Coulibaly B, Nanni I, Quilichini B, Gaudart J, Metellus P, Fina F, Boucard C, Chinot O, Ouafik L, Figarella-Branger D. Epidermal growth factor receptor in glioblastomas: correlation between gene copy number and protein expression. Hum Pathol. 2010;41:815–823. doi: 10.1016/j.humpath.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 38.Ilekis JV, Stark BC, Scoccia B. Possible role of variant RNA transcripts in the regulation of epidermal growth factor receptor expression in human placenta. Mol Reprod Dev. 1995;41:149–156. doi: 10.1002/mrd.1080410205. [DOI] [PubMed] [Google Scholar]
- 39.Voelzke WR, Petty WJ, Lesser GJ. Targeting the epidermal growth factor receptor in high-grade astrocytomas. Curr Treat Options Oncol. 2008;9:23–31. doi: 10.1007/s11864-008-0053-5. [DOI] [PubMed] [Google Scholar]
- 40.Albitar L, Pickett G, Morgan M, Wilken JA, Maihle NJ, Leslie KK. EGFR isoforms and gene regulation in human endometrial cancer cells. Mol Cancer. 2010;9:166. doi: 10.1186/1476-4598-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafky JM, Wilken JA, Baron AT, Maihle NJ. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochim Biophys Acta. 2008;1785:232–265. doi: 10.1016/j.bbcan.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Shibata MA, Ambati J, Shibata E, Albuquerque RJ, Morimoto J, Ito Y, Otsuki Y. The endogenous soluble VEGF receptor-2 isoform suppresses lymph node metastasis in a mouse immunocompetent mammary cancer model. BMC Med. 2010;8:69. doi: 10.1186/1741-7015-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]