Abstract
Using genomic microarray analysis, we sought to identify and annotate differences in the pretraining skeletal muscle transcriptomes among human subjects clustered as nonresponders (Non), modest responders (Mod), and extreme responders (Xtr) based on differential magnitudes of myofiber hypertrophy in response to progressive resistance training (RT) (Non −16 μm2, Mod 1,111 μm2, or Xtr 2,475 μm2). In prior work, we noted differences among clusters in the prevalence of myogenic stem cells prior to and during RT (35), and in the translational signaling responses to the first bout of resistance exercise (30). Here we identified remarkable differences in the pretraining transcript profiles among clusters (8,026 gene transcripts differentially expressed between Xtr and Non, 2,463 between Xtr and Mod, and 1,294 between Mod and Non). Annotated functions and networks of differentially expressed genes suggest Xtr were “primed” to respond to RT through transcriptional regulation, along with a uniquely expressed network of genes involved in skeletal muscle development, while the failed response in Non may have been driven by excessive proinflammatory signaling. Protein follow-up analysis revealed higher basal levels of acetylated histone H3 (K36) in the two responder clusters (Mod, Xtr) compared with Non, and only the responders experienced alterations in the muscle content of select proteins (e.g., α-tubulin, p27kip) in response to the first resistance exercise stimulus. Overall, the widely disparate transcriptomes identified prior to RT among the three clusters support the notion that at least some of the interindividual heterogeneity in propensity for RT-induced myofiber hypertrophy is likely predetermined.
Keywords: microarray, growth, exercise, response clusters
resistance exercise training (RT) induces skeletal muscle hypertrophy, increases muscle strength, power, and quality and is potentially the most promising method to regrow muscle in populations suffering from involuntary atrophy (e.g., aging, cancer) (7, 16, 17, 27, 35) or to prevent atrophy during unloading (4). However, we have observed a large degree of between-subjects variation for skeletal muscle myofiber hypertrophy in response to RT (5). The underlying mechanisms for this differential growth response are not completely understood but likely involve differential regulation of protein accretion and, perhaps, stem cell activity. Clearly myofiber hypertrophy is dependent on the accumulation of cellular proteins. And, although consensus has not been reached, it seems likely that at least later stages of hypertrophy are dependent on muscle satellite cell-mediated myonuclear addition.
Using K-means cluster analysis we previously grouped individuals, post hoc, into three nonbiased clusters (extreme responders, Xtr; modest responders, Mod; and nonresponders, Non) based on mean myofiber hypertrophy following 16 wk of 3 days/wk RT (2,475 ± 140 μm2 in Xtr, 1,111 ± 46 μm2 in Mod, and −16 ± 99 μm2 in Non) (5). Our previous findings suggest that intersubject variability in RT hypertrophy is due to intrinsic rather than extrinsic factors affecting these two fundamental processes (protein accretion and myonuclear addition) (5, 25, 35, 40). For example, we have shown that the prevalence of muscle satellite cells prior to training, proliferative capacity of these cells during RT, and the efficacy of myonuclear addition during RT all seem to confer growth advantages for Xtr and may be limiting factors in Non (35). We also found that specific components of the translation initiation signaling cascade responded differentially in Xtr vs. Non (30), as did the ability to induce myogenic growth factors and transcription factors (5, 25). On the other hand, wide-ranging magnitudes of myofiber hypertrophy occurred among clusters despite no differences in dietary intake, including no differences in daily consumption of protein, branched chain amino acids (e.g., leucine), and other nutrients thought to be important for muscle protein anabolism (40). It is important to note that each of the three clusters contained both men and women and both young (20–35 yr) and older (60–75 yr) adults (5).
A major knowledge gap in the field is the question of whether humans with an enhanced capacity for RT hypertrophy have a molecular advantage prior to the onset of training and, likewise, whether individuals resistant to RT hypertrophy share a distinct, pretraining molecular profile. It is plausible that the state of the skeletal muscle transcriptome, prior to RT, may play a significant role in predetermining trainability. This idea that unique, pretraining muscle molecular signatures differentiate responders and nonresponders is not without precedent, as Timmons et al. (41) recently applied this approach to endurance training, differentiating responder groups, based on gains in maximum oxygen uptake (V̇o2 max), by a pretraining molecular signature narrowed to 29 genes. Like increased V̇o2 max, myofiber hypertrophy is a complex and multifactorial physiological adaptation to exercise training. It thus seems likely that a pretraining gene expression “predictor” signature would be equally complex and require a broad-based approach to define it. Raue et al. (37) demonstrated that the skeletal muscle transcriptome is responsive to single and multiple bouts of resistance exercise, containing genes associated with muscle size and strength; however, whether the magnitude of hypertrophy is predetermined prior to the onset of exercise is unknown. The purpose of the current research was therefore to determine whether human cluster differences in RT-induced myofiber hypertrophy are associated with differential transcript profiles that may at least partially predetermine skeletal muscle trainability for myofiber hypertrophy. To accomplish this we performed genomic microarray analysis on pretraining muscle samples collected from the three myofiber hypertrophy response clusters (Xtr, Mod, Non) we previously defined and described in detail (5).
MATERIALS AND METHODS
Subjects.
Sixty-six untrained healthy men (n = 35) and women (n = 31) recruited from the Birmingham, Alabama, metropolitan area completed a 16-wk RT program and were subsequently clustered via K-means cluster analysis as Xtr (n = 17), Mod (n = 32), or Non (n = 17) based on vastus lateralis myofiber hypertrophy as described previously (5). For the current analysis, pretraining skeletal muscle transcript profiling was performed on 44 of the subjects (21 men, 23 women) in Xtr (n = 15), Mod (n = 14), and Non (n = 15). While by definition K-means clusters are distinct with no overlap, here we sought to maximize separation between clusters; thus we excluded the two Xtr at the bottom of the cluster (with the least hypertrophy) and the two Non at the top of the cluster (with the most hypertrophy). Among the 32 subjects in Mod, we focused on the 14 closest to the mean. As detailed in the original cluster paper (5), individuals were excluded for any musculoskeletal conditions or other disorders that might have affected their ability to complete RT and testing for the study: obesity (body mass index > 30.0 kg/m2), knee extensor RT within the past 5 yr, and for treatment with exogenous testosterone or other pharmacological agents known to influence muscle mass. The study was approved by the Institutional Review Boards of both the University of Alabama at Birmingham and the Birmingham Veterans Affairs Medical Center. All subjects provided written informed consent before participating. The phenotypic traits of the three clusters were previously documented (5). Resistance training sessions were fully supervised by study personnel, and training intensity, training volume, and adherence (∼90%) throughout the 16-wk program did not differ among the three response clusters (5).
RNA isolation and genechip processing.
RNA was isolated and purified using TriReagent (Molecular Research Center, Cincinnati, OH) and Qiagen RNeasy Mini Columns (QIAGEN, Valencia, CA), respectively. RNA concentration was determined with a spectrophotometer (NanoDrop ND-1000). An aliquot of purified RNA from each sample was sent to Beckman Coulter Genomics (Morrisville, NC) for genechip processing. Prior to sample processing, RNA quantity and size distribution of each sample were determined by spectrophotometry and an Agilent Bioanalyzer, respectively. Transcript profiles were determined using the standard single-color array protocol for the Agilent human 4 × 44K single-color array (Agilent Technologies, Santa Clara, CA), which contained probes for ∼41,000 gene transcripts. We converted 200 ng of total RNA into labeled cRNA with nucleotides coupled to fluorescent dye Cy3 using the Low Input Quick Amp Kit (Agilent Technologies, Palo Alto, CA) following the manufacturer's protocol. Cy3-labeled cRNA (1.65 μg) from each sample was hybridized to an Agilent Human Whole Genome 4 × 44K Microarray. The hybridized array was then washed and scanned to detect Cy3 intensities. Data were extracted from the scanned image using Feature Extraction version 10.7 (Agilent Technologies). All arrays passed the quality assessments conducted by Beckman Coulter Genomics.
Microarray and statistical analysis.
The gene transcript data were normalized using cubic spline and log transformed. Probes with low signal intensity were filtered out based on the detection P values. The filtering criterion for keeping a probe for further analyses was that the detection P value for the probe was <0.01 for ≥80% of the samples in at least one of the three groups. After filtering, 28,714 out of the 41,094 probes remained. Pair-wise comparisons (Xtr vs. Non, Xtr vs. Mod, and Mod vs. Non) were conducted by shrinkage-based t-tests. Permutation of samples was conducted to obtain P values and false discovery rates (FDR, 0.05).
Following statistical analysis, we used Ingenuity Pathways Analysis (IPA, Ingenuity Systems, http://www.ingenuity.com) and available gene ontology for functional annotation of differentially expressed genes among clusters. Functional annotation is a valuable means of interpreting large sets of differentially expressed genes, as genes are grouped into families for which the protein products are known participants in a given biological or biochemical function or process. The purpose herein was to identify candidate biological or biochemical processes in resting, untrained muscle that may be key in conferring an RT-induced myofiber hypertrophy advantage to Xtr or, equally important, a disadvantage to Non. IPA functional analysis identified genes linked to biological functions and/or diseases that were most significant to the data set. Differentially expressed genes that were associated with biological functions and/or diseases in Ingenuity's Knowledge Base were considered for the analysis. Right-tailed Fisher's exact test was used to calculate a P value determining the probability that each biological function and/or disease assigned to that data set is due to chance alone. IPA uses prediction calculations based on the direction and magnitude of differentially expressed genes and what is known in the literature. A z-score is determined for each differentially expressed gene; z-scores ≤ −2.00 are considered to have lower function, while z-scores ≥ 2.00 are considered to have greater function.
The data discussed in this publication have been deposited in National Center for Biotechnology Information's Gene Expression Omnibus (GEO) (11) and are accessible through GEO Series accession number GSE42507 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE42507).
Follow-up quantitative PCR and immunoblotting.
A few differentially expressed genes between the Xtr and Non groups in the skeletal muscle transcriptome were selected for follow-up validation via qRT-PCR (StepOne System; Applied Biosystems, Foster City, CA). Genes on the microarray that had a mean signal intensity of ≥10 were detected (i.e., signal intensity P value for the probe was <0.01) in all three clusters, and had ≥2.0-fold difference in expression between Xtr and Non clusters were used for validation. Among the genes that met these criteria, we validated the microarray with five: urocortin 2 (UCN2), corticotropin releasing hormone receptor 1 (CRHR1), forkhead box protein C2 (FOXC2), paxillin (PXN), and SRY-related HMG-box 8 (SOX8). TaqMan Probe-Based Gene Assays for these target genes were ordered from Life Technologies. cDNA was synthesized via reverse transcription using the SuperScript VILO cDNA Synthesis kit (Invitrogen, Carlsbad, CA). GAPDH (Hs02758991_g1) expression served as internal control. All samples were run in triplicate. Relative amounts of target mRNA (i.e., ΔCT values) were determined by the comparative threshold cycle method via StepOne software version 2.2.2 (Applied Biosystems), and results are described as the relative fold difference (i.e., 2−ΔΔCT) between Xtr and Non.
In addition to qPCR, protein levels of selected differentially expressed genes were determined across the three clusters from subjects at rest (n = 44, 14 Xtr, 15 Mod, and 15 Non) and 24 h following the first full bout of resistance exercise (RE) (n = 41, 13 Xtr, 15 Mod, and 13 Non). Target proteins were selected based on differentially expressed genes of interest that are either putative (HES6, MyoD, myogenin, cyclin D1, p27kip, NF-κB p50/105) or potentially novel [annexin A2, estrogen receptor 1 (ESR1), acetyl-histone H3 (Lys36), α-tubulin] regulators of myogenic processes. The rationale for this analysis was straightforward: cluster differences in resting, untrained mRNA levels of potentially important genes may influence protein expression responses to a mechanical load exposure. Potential regulators of muscle mass or factors that influence myogenic processes were selected for protein-level acute response analysis. Standard immunoblotting was performed by established methods in our laboratory (6, 26, 39). Muscle protein lysate was extracted from frozen muscle samples (average 30–35 mg) as detailed previously (6, 26, 27). Protein concentrations were determined by the bicinchoninic acid technique with bovine serum albumin as a standard. Samples were run on 4–12% Bis-Tris (Invitrogen) SDS-PAGE gel matrices with 25 μg total protein loaded into each well, which was determined as ideal by preliminary experiments. Samples within subjects across time (e.g., pre- to post-RE) were loaded in adjacent lanes, and samples from all three clusters were represented on each gel. Proteins were transferred to polyvinylidene difluoride membranes at 100 mA for 12 h. Based on microarray results and gene network analyses, the following proteins (and primary antibodies used) were assayed: HES6 (Santa Cruz, sc-133196), annexin A2 (BD Biosciences, 610068), myogenin (Santa Cruz, sc-576), NF-κB p50/105 (Cell Signaling, cs #3035), p27kip (Cell Signaling, cs #2552), cyclin D1 (Santa Cruz, sc-8396), MyoD (Santa Cruz, sc-760), estrogen receptor 1 (ESR1) (Santa Cruz, sc-787), acetyl-histone H3 (Lys36) (Millipore, 07–540), and α-tubulin (Cell Signaling, cs #2125). Appropriate primary antibody concentrations were determined in preliminary experiments and were 1:1,000 (vol/vol) for all but ESR1 (1:50), annexin-A2 (1:5,000), and acetyl-histone H3 (Lys36) (1:5,000). All membranes were preblocked in 5% goat serum (monoclonal Abs) or 2% milk/2% BSA (polyclonal Abs) for 1 h at room temperature. Membranes were then probed with primary antibodies in full block overnight at 4°C. Horseradish peroxidase-conjugated secondary antibody was used at 1:50,000 (wt/vol) followed by chemiluminescent detection in a Bio-Rad ChemiDoc imaging system with band densitometry performed using Bio-Rad Quantity One (software package 4.5.1; Bio-Rad Laboratories, Hercules, CA). Parameters for image development were consistent across all membranes according to predefined saturation criteria as described (6).
Statistical analysis of immunoblotting results.
Potential differences among clusters in resting, pretraining muscle protein levels were tested by one-way ANOVA. A 3 × 2 (cluster × time) repeated-measures ANOVA was used to test cluster, time, and cluster × time interaction effects. Fisher's least significant difference tests were performed post hoc as appropriate. Significance was accepted at P < 0.05 for all tests.
RESULTS
We found substantial differences in the skeletal muscle transcriptomes among response clusters, with the most notable differences between Xtr and Non. We found 8,026 gene transcripts to be differentially expressed between Xtr and Non, 2,463 between Xtr and Mod, and 1,294 between Mod and Non (FDR P < 0.05). Employing the selection criteria described in materials and methods, we used selected differentially expressed genes to validate the microarray analysis via qPCR. UCN2, CRHR1, and PXN had greater expression in the Xtr vs. Non by both microarray (2.9-, 2.4-, and 2.6-fold greater expression, respectively, P < 0.05) and qPCR (2.0-, 1.8-, and 1.5-fold greater expression, respectively, P < 0.05). FOXC2 and SOX8 were also more highly expressed in Xtr vs. Non by microarray (2.5- and 4.1-fold greater expression, respectively, P < 0.05), and similar, although nonsignificant, differences were seen via qPCR (1.4- and 1.7-fold, respectively).
IPA recognized 6,155, 1,651, and 605 genes in each of the three comparisons (Xtr vs. Non, Xtr vs. Mod, and Mod vs. Non, respectively) on which overrepresented functional and subfunctional categories were determined. Additionally, manually selected, differentially expressed genes from each comparison were annotated with available gene ontology.
Transcriptional regulation.
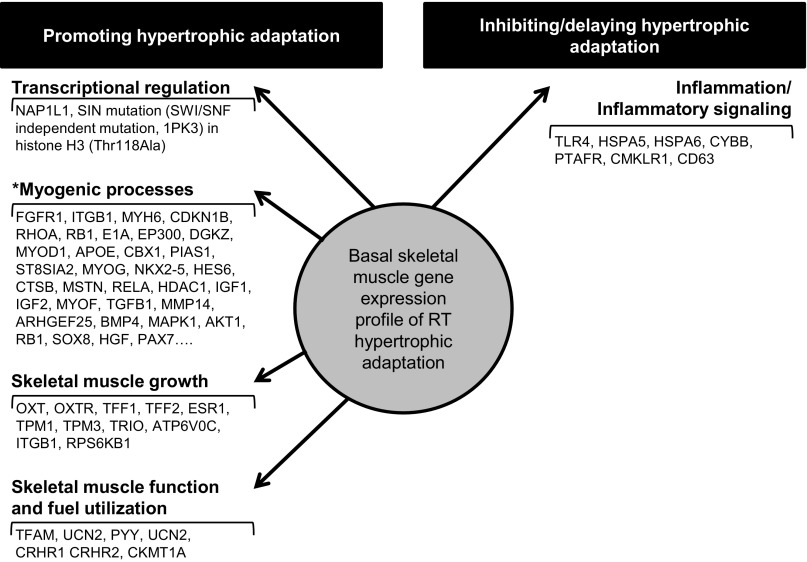
Functional categorization with IPA determined Gene Expression as the top differentially regulated molecular and cellular functional category among those that distinguished Xtr from Non and Mod. Within the broad functional categories, IPA defined subfunctions that allowed us to further interpret the differentially expressed genes. Within Gene Expression, the top subfunctions distinguishing Xtr from Non (P = 2.00E-15) and Mod (P = 7.65E-06) were Expression of RNA and Activation of DNA Endogenous Promoter, respectively. After manually annotating and understanding the biological role of the genes classified by IPA within these functional categories, we renamed this functional grouping of genes as Transcriptional Regulation (Fig. 1). Related to transcriptional regulation, NAP1L1 was 17.4- and 7.3-fold higher in Xtr and Mod (vs. Non), respectively, making it the most highly upregulated gene in the responders. A SIN mutation (SWI/SNF independent mutation, 1PK3) in histone H3 (Thr118Ala) was the second highest differentially expressed gene (16.4- and 4.4-fold higher) in Xtr and Mod (vs. Non), respectively. Lastly, histone H3 (and its variants) displayed stepwise greater expression from Non to Mod to Xtr, with 8.6-fold greater expression in Xtr compared with Non.
Fig. 1.
Functional annotation of the basal skeletal muscle gene expression profile associated with resistance training (RT)-induced myofiber hypertrophy. Functions and subfunctions were determined with Ingenuity Pathways Analysis (IPA) and available gene ontology. *Additional differentially expressed genes are found in Supplemental Table S1.
Skeletal muscle development and function.
Skeletal muscle development and function (SMDF) was a top five functional category for the Xtr vs. Mod and the Mod vs. Non comparisons and was also an overrepresented functional category in Xtr vs. Non (P < 0.05). Subfunctions of SMDF (defined by IPA and gene ontology) enhanced biological interpretation of the differentially expressed genes (Supplemental Table S1).1 Broadly, we identified three functional categories (i.e., myogenic processes, skeletal muscle growth, and skeletal muscle function and fuel utilization) that summarized genes within SMDF.
Under myogenic processes we further identified subcategories of biological relevance. Related to myogenesis, one gene, paxillin (PXN), had stepwise higher mRNA levels from Non to Mod to Xtr, which was validated by qPCR in Xtr vs. Non (1.5-fold greater expression, P = 0.03). Three genes were expressed at levels unique to Xtr vs. Non and Mod [V-rel reticuloendotheliosis viral oncogene homolog A (RELA), CREB binding protein (CREBBP), and mitogen-activated protein kinase 14 (MAPK14)]. On the other hand, two genes classified under myogenesis had levels of gene expression unique to Non [fibroblast growth factor receptor 1 (FGFR1) and integrin, beta 1 (ITGB1)]. Classified under Satellite Cell Activation and Function, we found stepwise higher expression from Non to Mod to Xtr for sex-determining region Y-box 8 (SOX8) and hepatocyte growth factor (HGF). The gene expression of paired box 7 (PAX7), a biomarker of satellite cells, was higher in Xtr compared with the other two clusters. While not specifically categorized under SMDF, Quantity of Muscle Cells was a subfunction for differentially expressed genes in the Xtr vs. Non and Mod. Interestingly, in the Xtr vs. Mod comparison, IPA predicted that the function quantity of muscle cells was higher in the Xtr (z-score = 2.089). Several genes classified under cell cycle progression and differentiation were uniquely differentially expressed in Xtr, including lower expression of cyclin-dependent kinase inhibitor 1B (p27Kip); Ras homolog gene family, member A (RHOA); E1A binding protein p300 (EP300); chromobox homolog 1 (CBX1); recombination signal binding protein for immunoglobulin kappa J region (RBPJ); and protein inhibitor of activated STAT 1 (PIAS1); and higher expression of diacylglycerol kinase zeta (DGKZ); MyoD, apolipoprotein E (APOE); ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 2 (ST8SIA2); myogenin; and NK2 transcription factor related locus 5 (NKX2–5) in the Xtr vs. Non and Mod. Stepwise higher expression from Non to Mod to Xtr was noted for hairy and enhancer of split family (HES6). There were also two genes uniquely differentially expressed in Non higher levels of cathepsin B (CTSB) and lower levels of insulin-like growth factor 2 (IGF2) in the Non vs. Xtr and Mod. It is also noteworthy that the expression of myostatin (MSTN) was 3.15-fold greater in the Xtr vs. Non. There was a stepwise increase in expression from Non to Xtr for two genes classified under morphology of muscle cells and myofibers, including tropomyosin 1 (TPM1) and tropomyosin 3 (TPM3). Additionally, there was higher expression, specific to the Non, for ATPase, H+ transporting, lysosomal 16 kDa, V0 subunit c (ATP6V0C), but lower expression of myosin heavy chain 6 (MYH6). The majority of the genes classified under muscle development had expression specific to the Xtr (10/21), or there was a stepwise increase in expression (4/21). There was a stepwise increase in expression from Non to Mod to Xtr for several genes involved with skeletal muscle growth, including oxytocin (OXT), trefoil factor 1 (TFF1), and estrogen receptor 1 (ESR1). Additionally, OXT receptor (OXTR) and trefoil factor 2 (TFF2) were upregulated in the Xtr vs. Non and Mod. Genes related to the negative regulation of skeletal muscle function and fuel utilization were primarily lower in the Xtr vs. Non [tribbles homolog 3 (TRIB3), AE binding protein 1 (AEBP1), and CD36], while genes that could positively influence skeletal muscle function and fuel utilization were upregulated in the Xtr [peptide tyrosine tyrosine (PYY), transcription factor A, mitochondrial (TFAM), urocortin 2 (UCN2), creatine kinase, mitochondrial 1A (CKMT1A), corticotropin releasing hormone receptor 1 and 2 (CRHR1 and CRHR2, respectively)].
After minimizing the IPA network analysis to include only genes that had a ≥2.0-fold difference in expression between the Xtr vs. Non, we identified two networks that show the interconnectivity of the differentially expressed genes related to not only SMDF, but also to Transcriptional Regulation (Figs. 2 and 3). Incorporating genes related to myogenesis and muscle growth (e.g., PXN, ESR1, GREB1) and transcriptional regulation (e.g., NAP1L1), IPA network 3 (score = 35, Fig. 2) clustered 24 of the differentially expressed genes between Xtr vs. Non that had ≥2.0-fold expression difference. While only 24 genes with ≥2.0-fold expression difference were used to determine the network score, other genes that were differentially expressed but with <2.0-fold expression difference between Xtr vs. Non were also incorporated. The top functions, as indicated by IPA for this network include Cell Death and Survival, Tissue Morphology, and Cell Cycle. IPA also clustered 21 differentially expressed genes with a ≥2.0-fold expression difference between Xtr and Non (score = 29, network 7, Fig. 3) that were related to skeletal muscle development and function (e.g., UCN2, FOXC2, FGF, ICMT). Similar to network 3, differentially expressed genes with a ≥2.0-fold expression difference between Xtr vs. Non were also incorporated. The top functions, as indicated by IPA for this network, include Organ Morphology, Skeletal Muscular System Development and Function, and Embryonic Development.
Fig. 2.
IPA network 3 (score = 35) clustered 24 (of 35 in the network) of the differentially expressed genes between Xtr vs. Non using genes with ≥2.0-fold expression difference. Genes with ≥2.0-fold expression difference indicated with fold change value. Remaining genes had <2.0-fold expression difference between Xtr vs. Non. Red nodes indicate genes with greater expression in the Xtr vs. Non; green nodes indicate genes with reduced expression in the Xtr vs. Non; white nodes indicate genes that are not differentially expressed, but related to this network. *Transcript represented by multiple probe-sets. Mod, modest responder; Non, nonresponder; Xtr, extreme responder.
Fig. 3.
IPA network 7 (score = 29) clustered 21 (of 35 in the network) of the differentially expressed genes between Xtr vs. Non using genes with ≥2.0-fold expression difference. See Fig. 2 for description of nodes.
Inflammation and inflammatory signaling.
Many of the differentially expressed genes classified by IPA under Infectious Disease were related to inflammatory signaling, acute immune response, and mitochondrial dysfunction and thus were recategorized as inflammation and inflammatory signaling. In addition to Infectious Disease being a top biological function in the comparison between the Xtr vs. Non, within this functional category were two of the top five subcategories (Infection by Virus and Infection of Cells) with the greatest significance (i.e., the smallest P values) and all five of the subfunctions containing the lowest z-score. For the Xtr vs. Non, IPA predicted a decrease in functions related to Infection of cells and by infectious agents (e.g., cells, virus, HIV, Retroviridae, tumor cell lines, HIV-1, cervical cancer cell lines, embryonic cell lines, kidney cell lines, epithelial cell lines) based on the z-scores. Among the genes classified within these functional categories were genes with lower expression in the Xtr vs. Non, including Toll-like receptor 4 (TLR4), two of the heat shock proteins 70 kDa isoforms (HSPA5 and HSPA6), cytochrome b-245 beta polypeptide (CYBB), platelet activating factor receptor (PTAFR), chemokines-like receptor 1 (CMKLR1), and CD63.
Protein level follow-up.
Several differentially expressed genes [HES6, annexin A2, myogenin, NF-κB p50/105, p27, cyclin D1, MyoD, ESR1, acetylated histone H3 (K36), α-tubulin] were selected for follow-up protein analysis at baseline and 24 h after the first full RE bout. In resting muscle prior to any RE, the amount of acetylated histone H3 (K36) protein was higher in the responder clusters (Mod vs. Non, P = 0.003; Xtr vs. Non, P = 0.06). Main time effects for p27 (P < 0.001) and α-tubulin (P < 0.001) were driven by the responder clusters: Mod and Xtr each decreased p27 and increased α-tubulin protein content in response to a single RE bout, while no changes were seen in Non (Fig. 4). For the remaining proteins analyzed, no cluster differences or RE responses were detected (data not shown).
Fig. 4.
Protein levels by response cluster (Non, Mod, Xtr) in the basal, pretraining state and 24 h after the first full bout of RT for p27 (A), α-tubulin (B), and acetylated histone H3 (K36) (C). Representative immunoblots are shown in D. †Different from baseline; *different from Non at baseline. P < 0.05.
DISCUSSION
The present study is the first to identify differences in the resting, pretraining skeletal muscle transcriptome among clusters of human subjects found to have widely disparate myofiber hypertrophy responses to RT. The molecular profile, particularly of the Xtr and Non, demonstrated a large number of genes with mRNA levels unique to each response cluster. The interconnectivity of differentially expressed genes related to skeletal muscle growth potential (i.e., Xtr and Non, Figs. 2 and 3) highlights not only the gene relationships, but also the functional mechanisms that likely underlie skeletal muscle hypertrophy. As suggested by functional categories representing differentially expressed genes among the three clusters, the skeletal muscle of sedentary persons appeared primed for growth in Xtr while perhaps inhibited from hypertrophy in Non.
Transcriptional regulation.
Increased mRNA translation (i.e., protein synthesis) is required for myofiber hypertrophy; therefore, translational regulation via putative signaling pathways (e.g., mTOR/p70S6K) has been widely studied in models of muscle hypertrophy. An important, but commonly overlooked aspect of protein synthesis is transcriptional regulation, as sufficient amounts of rRNA and mRNA are required for ribosomal biogenesis and mRNA translation. We have previously demonstrated increased total skeletal muscle RNA following 16 wk of RT in all responder groups, but only Xtr increased total muscle RNA (26%) 24 h after the first bout of RE (24). While it is unknown how altered transcriptional regulation contributes to the responder phenotype, a more robust expression of rRNA (∼85% of total RNA) following RE would likely promote protein synthesis via enhanced translational capacity. Several candidate transcripts identified in these analyses may be important for the differential regulation of gene expression among responder clusters. NAP1L1 is a member of the nucleosome assembly protein 1-like family that associates with chromatin to preferentially incorporate acetylated core histones, relax the chromatin structure, and facilitate transcription factor binding to DNA (2). NAP1L1 at higher transcript levels have been found in Emery-Dreifuss muscular dystrophy patients' skeletal muscle, where it presumably tries to remove acetylated histones from MyoD target promoters to facilitate myogenesis (3). Additionally, the SIN mutation (SWI/SNF independent mutation, 1PK3) in histone H3 (Thr118Ala) was differentially expressed among responder clusters. Histone SIN mutations result in nucleosome destabilization (33), which weakens the barrier to transcription and allows more efficient traversal by RNA polymerase II (21). Although this histone H3 SIN mutation enhances nucleosome traversal, it also decreases nucleosome survival upon traversal, which may, in part, explain the robust levels of NAP1L1 in Xtr and Mod, as NAP1L1 mediates nucleosome formation (34). Additionally, there was the stepwise increase in expression of histone H3 (and its variants). Acetylated histone H3 (K36) was chosen for follow-up immunoblotting, as this acetylation site may enhance transcriptional activity and has been found to be increased in skeletal muscle following exercise (31). Interestingly, we found that, although RE did not change acetylated H3 (K36) protein levels, both Mod and Xtr had higher (≈31%) acetylated H3 (K36) protein than Non at baseline, suggesting that those who achieve proficient RT-induced myofiber hypertrophy may have altered chromatin structure that may confer an advantage toward RT-induced hypertrophic gene transcription. Overall the findings support the idea that basal expression of genes involved in transcriptional regulation likely plays an important role in determining one's capacity for RT-induced myofiber hypertrophy.
Myogenic processes.
Satellite cells (i.e., skeletal muscle progenitor cells) play an obligatory role in skeletal muscle repair and regeneration subsequent to injury and may also contribute to RT-induced hypertrophy (35). The transcript profile of Xtr supports the importance of satellite cell-mediated myonuclear addition for muscle growth. Higher mRNA levels of satellite cell markers PAX7 and SOX8 in Xtr (vs. Non and Mod) corroborates our previous report of a greater number of satellite cells in the Xtr (vs. Non and Mod) at baseline and following 16 wk of RT (35). A number of factors could be driving satellite cell differences in the Xtr, such as the growth factor HGF, a putative activator of satellite cells (1, 32), which was upregulated in Xtr (vs. Non and Mod). Additionally, protein level follow-up of the cell cycle inhibitor p27 indicates that only Mod and Xtr decreased p27 levels (14 and 16%, respectively) following acute RE. These observations suggest a readiness of the Xtr muscle to activate resident muscle progenitor cells when necessary.
Activated and proliferating muscle progenitor cells withdraw from the cell cycle and commit to the skeletal muscle lineage (i.e., differentiation) with the assistance of several factors, [e.g., myogenic regulatory factors (MRFs)]. Our results indicate that two MRFs (MyoD and myogenin) were differentially expressed in the Xtr. Previously, we did not detect statistically significant differences in these MRFs at baseline (5). A potential reason for the disparate results between the prior and current work is the high likelihood that the gene probes were different between the two gene expression platforms (microarray vs. PCR). There were several differentially expressed auxiliary molecules to the MRFs that are involved with myogenic differentiation. Cyclins D1 and D2 (cyclin D1 and CCND2, respectively) were downregulated in Xtr compared with Non, which may facilitate withdrawal from the cell cycle and commitment to the muscle cell lineage. However, at the protein level we found no cluster differences in cyclin D1. We also observed greater gene expression specific to the Xtr (vs. Non and Mod) for several genes that play a role in differentiation including APOE, DGKZ, MSTN, and HES6. Kang et al. (22) reported reduced muscle wound healing in APOE knockout mice that was associated with decreased myogenin levels. DGKZ is a negative regulator of cell cycle progression (14) such that decreased expression of DGKZ (via siRNA) impairs muscle cell differentiation (15). MSTN is generally thought of as an inhibitor of muscle growth as it suppresses cell proliferation and thus attenuates cell number. However, in the current study, higher expression of MSTN in the Xtr vs. Non could suggest a role in myoblast differentiation (19, 19a). We have shown previously that lower myostatin levels are not essential for skeletal muscle hypertrophy (25) and that myostatin mRNA levels in human muscle actually correlate positively with muscle mass (23). Lastly, baseline gene expression of HES6 was 9.2- and 4.2-fold lower in Non vs. Xtr and Mod, respectively. HES6 is a basic helix-loop-helix transcription factor, downstream of MyoD that promotes normal myogenic differentiation (18, 29). These findings suggest a unique basal gene expression profile among Non that may lead to differential myogenic responses to exercise compared with subjects who proficiently achieve RT-induced myofiber hypertrophy (Mod and Xtr).
Skeletal muscle growth.
The skeletal muscle molecular profile of Xtr indicated greater signal intensities in the Xtr for ESR1, OXT, OXTR, and several factors involved with the estrogen signaling pathway (e.g., TFF1 and NCOR1), which may reveal a novel mechanism for RT-induced myofiber hypertrophy. Estrogen has been shown to stimulate satellite cell activation and proliferation (13), and the estrogen receptor is necessary for postinjury satellite cell activation and recruitment (12). OXTR was identified as a “driver” of anabolic steroid-induced increases in bovine skeletal muscle mass; however, downstream signaling events from OXTR and OXT, which would initiate gains in skeletal muscle mass, could not be elucidated from that study (9). We were unable to measure circulating OXT or estrogen levels in the present study. Whether baseline levels of these hormones are important for muscle mass is difficult to interpret because there were no baseline differences in myofiber cross-sectional area (CSA) among the three response clusters (5). Along similar lines, the transcription of SOX8, a transcription factor that binds the promoter region of GREB1 (i.e., a “classic estrogen-induced gene”), may also be important for increased hypertrophic capacity. It is important to note that the expression of SOX8 coincides with ESR1 and OXT expression in each response cluster, which supports the hypothesis of De Jager et al. (9).
Skeletal muscle function and fuel utilization.
Our data suggest that persons who did not respond to 16 wk of RT (i.e., Non) may have had a functional and metabolic disadvantage. For example, the Non had lower expression of UCN2 and its receptor, CRHR2, which have previously been shown to increase skeletal muscle mass and force and increase myofiber CSA in nonatrophying mouse muscle and minimize the loss of mass, force, and CSA in denervated mouse muscle (20). UCN2 is a novel regulator of fuel utilization (glucose homeostasis and metabolic functions) through negative regulation of glucose uptake (8) and has been shown to increase the contractile force of skeletal muscle (20). Additionally, the Non had lower levels (vs. Xtr and Mod) of mRNA for TFAM, a marker of mitochondrial biogenesis. While not directly implicated in RT-induced myofiber hypertrophy, mitochondrial biogenesis has been deemed important for skeletal muscle regeneration (10, 42). In fact, MyoD and myogenin expression correspond with the expression of TFAM and other biomarkers of mitochondrial biogenesis (42). On the other hand, Xtr may have been at a metabolic advantage, having 7.9- and 3.3-fold higher levels of mitochondrial creatine kinase 1A than Non and Mod, respectively.
Inflammation and inflammatory signaling.
Expression differences between Xtr and Non suggest Non could have heightened localized inflammation that may interfere with the ability for skeletal muscle to respond to a hypertrophic stimulus. Several of the differentially expressed genes that were upregulated in Non are associated with NF-κB signaling, a pathway known to be linked with heightened inflammation and skeletal muscle atrophy (28). Genomic signatures that suggest heightened inflammation and inflammatory and metabolic dysfunction have been demonstrated in other populations, which might also have an impaired growth capacity (36, 39).
Nonresponders or delayed responders?.
The unique transcript profile noted in those individuals classified as Non may be at least partially responsible for the failed hypertrophy adaptation, suggesting that these individuals were at a disadvantage prior to RT compared with the Mod and Xtr. On the other hand, it is possible that Non are actually capable of experiencing RT-induced myofiber growth comparable with Mod or Xtr but, to achieve this, may require an alternative prescription and/or extended training duration. The protein level results for α-tubulin, which increased only in Mod and Xtr after the first exposure to a full RE bout and could be considered a surrogate marker of RE-induced cytoskeletal protein synthesis, strongly suggest the muscles of Non were less sensitive to the prescribed dose of RE. We speculate that individuals clustered as Non may require a greater stimulus or longer duration of the same stimulus to remodel/hypertrophy their myofibers. Future research is necessary to determine whether unique translational patterns following unaccustomed resistance exercise play a role in the failed hypertrophy among Non.
Conclusions
These results indicate that unique transcript profiles do exist among the response clusters prior to RT-induced hypertrophy. It is important to emphasize that these differences are observed in the untrained state, suggesting that the muscles of each of the response clusters were at different states of preparedness to respond to RT. The molecular profiles suggest responders benefited from enhanced transcriptional regulation, myogenic and satellite cell function, and muscle growth signaling, while individuals who did not experience RT-induced myofiber hypertrophy expressed a profile indicative of enhanced inflammatory signaling. An attractive future direction would be the exploration of various exercise prescriptions (i.e., doses) to identify an optimal strategy that would induce myofiber hypertrophy in individuals with a pretraining transcript profile similar to those we clustered in this study as nonresponders.
GRANTS
5R01AG017896 (M. M. Bamman), VA Merit Review (M. M. Bamman), 5T32 DK-062710-01A1 (A. Thalacker-Mercer), and CTSA 5UL1RR-025777.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.T.-M., X.C., J.C., and M.M.B. conception and design of research; A.T.-M., M.S., J.C., S.T.W., and M.M.B. performed experiments; A.T.-M., M.S., X.C., and M.M.B. analyzed data; A.T.-M., M.S., S.T.W., and M.M.B. interpreted results of experiments; A.T.-M., M.S., and M.M.B. prepared figures; A.T.-M., M.S., and M.M.B. drafted manuscript; A.T.-M., M.S., X.C., and M.M.B. edited and revised manuscript; A.T.-M., M.S., X.C., J.C., S.T.W., and M.M.B. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We sincerely thank the participants for their efforts and S. C. Tuggle for administering the resistance training program and all associated testing.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol 165: 307–312, 1995. [DOI] [PubMed] [Google Scholar]
- 2. Attia M, Forster A, Rachez C, Freemont P, Avner P, Rogner UC. Interaction between nucleosome assembly protein 1-like family members. J Mol Biol 407: 647–660, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Bakay M, Wang Z, Melcon G, Schiltz L, Xuan J, Zhao P, Sartorelli V, Seo J, Pegoraro E, Angelini C, Shneiderman B, Escolar D, Chen YW, Winokur ST, Pachman LM, Fan C, Mandler R, Nevo Y, Gordon E, Zhu Y, Dong Y, Wang Y, Hoffman EP. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration. Brain 129: 996–1013, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen MC. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol 84: 157–163, 1998. [DOI] [PubMed] [Google Scholar]
- 5. Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol 102: 2232–2239, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol 97: 1329–1337, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Brown AB, McCartney N, Sale DG. Positive adaptations to weight-lifting training in the elderly. J Appl Physiol 69: 1725–1733, 1990. [DOI] [PubMed] [Google Scholar]
- 8. Chen A, Brar B, Choi CS, Rousso D, Vaughan J, Kuperman Y, Kim SN, Donaldson C, Smith SM, Jamieson P, Li C, Nagy TR, Shulman GI, Lee KF, Vale W. Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle. Proc Natl Acad Sci USA 103: 16580–16585, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Jager N, Hudson NJ, Reverter A, Wang YH, Nagaraj SH, Cafe LM, Greenwood PL, Barnard RT, Kongsuwan KP, Dalrymple BP. Chronic exposure to anabolic steroids induces the muscle expression of oxytocin and a more than fiftyfold increase in circulating oxytocin in cattle. Physiol Genomics 43: 467–478, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Duguez S, Feasson L, Denis C, Freyssenet D. Mitochondrial biogenesis during skeletal muscle regeneration. Am J Physiol Endocrinol Metab 282: E802–E809, 2002. [DOI] [PubMed] [Google Scholar]
- 11. Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucl Acids Res 30: 207–210, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Enns DL, Iqbal S, Tiidus PM. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol (Oxf) 194: 81–93, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol 104: 347–353, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Evangelisti C, Astolfi A, Gaboardi GC, Tazzari P, Pession A, Goto K, Martelli AM. TIS21/BTG2/PC3 and cyclin D1 are key determinants of nuclear diacylglycerol kinase-zeta-dependent cell cycle arrest. Cell Signal 21: 801–809, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Evangelisti C, Riccio M, Faenza I, Zini N, Hozumi Y, Goto K, Cocco L, Martelli AM. Subnuclear localization and differentiation-dependent increased expression of DGK-zeta in C2C12 mouse myoblasts. J Cell Physiol 209: 370–378, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 263: 3029–3034, 1990. [PubMed] [Google Scholar]
- 17. Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol 64: 1038–1044, 1988. [DOI] [PubMed] [Google Scholar]
- 18. Gao X, Chandra T, Gratton MO, Quelo I, Prud'homme J, Stifani S, St-Arnaud R. HES6 acts as a transcriptional repressor in myoblasts and can induce the myogenic differentiation program. J Cell Biol 154: 1161–1171, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garikipati DK, Rodgers BD. Myostatin stimulates myosatellite cell differentiation in a novel model system: evidence for gene subfunctionalization. Am J Physiol Regul Integr Comp Physiol 302: R1059–R1066, 2012. [DOI] [PubMed] [Google Scholar]
- 19a. Garikipati D, Rodgers BD. Myostatin inhibits myosatellite cell proliferation and consequently activates differentiation: evidence for endocrine-regulated transcript processing. J Endocrinol 215: 177–187, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Hinkle RT, Donnelly E, Cody DB, Bauer MB, Sheldon RJ, Isfort RJ. Corticotropin releasing factor 2 receptor agonists reduce the denervation-induced loss of rat skeletal muscle mass and force and increase non-atrophying skeletal muscle mass and force. J Muscle Res Cell Motil 25: 539–547, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Hsieh FK, Fisher M, Ujvari A, Studitsky VM, Luse DS. Histone Sin mutations promote nucleosome traversal and histone displacement by RNA polymerase II. EMBO Rep 11: 705–710, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang J, Albadawi H, Patel VI, Abbruzzese TA, Yoo JH, Austen WG, Jr, Watkins MT. Apolipoprotein E−/− mice have delayed skeletal muscle healing after hind limb ischemia-reperfusion. J Vasc Surg 48: 701–708, 2008. [DOI] [PubMed] [Google Scholar]
- 23. Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288: E1110–E1119, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Kim JS, Petrella JK, Cross JM, Bamman MM. Load-mediated downregulation of myostatin mRNA is not sufficient to promote myofiber hypertrophy in humans: a cluster analysis. J Appl Physiol 103: 1488–1495, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Kim JS, Petrella JK, Cross JM, Bamman MM. Load-mediated downregulation of myostatin mRNA is not sufficient to promote myofiber hypertrophy in humans: a cluster analysis. J Appl Physiol 103: 1488–1495, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Kosek DJ, Bamman MM. Modulation of the dystrophin-associated protein complex in response to resistance training in young and older men. J Appl Physiol 104: 1476–1484, 2008. [DOI] [PubMed] [Google Scholar]
- 27. Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 101: 531–544, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med 86: 1113–1126, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malone CM, Domaschenz R, Amagase Y, Dunham I, Murai K, Jones PH. Hes6 is required for actin cytoskeletal organization in differentiating C2C12 myoblasts. Exp Cell Res 317: 1590–1602, 2011. [DOI] [PubMed] [Google Scholar]
- 30. Mayhew DL, Hornberger TA, Lincoln HC, Bamman MM. Eukaryotic initiation factor 2B epsilon induces cap-dependent translation and skeletal muscle hypertrophy. J Physiol 589: 3023–3037, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGee SL, Fairlie E, Garnham AP, Hargreaves M. Exercise-induced histone modifications in human skeletal muscle. J Physiol 587: 5951–5958, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller KJ, Thaloor D, Matteson S, Pavlath GK. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am J Physiol Cell Physiol 278: C174–C181, 2000. [DOI] [PubMed] [Google Scholar]
- 33. Muthurajan UM, Bao Y, Forsberg LJ, Edayathumangalam RS, Dyer PN, White CL, Luger K. Crystal structures of histone Sin mutant nucleosomes reveal altered protein-DNA interactions. EMBO J 23: 260–271, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okuwaki M, Kato K, Nagata K. Functional characterization of human nucleosome assembly protein 1-like proteins as histone chaperones. Genes Cells 15: 13–27, 2010. [DOI] [PubMed] [Google Scholar]
- 35. Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104: 1736–1742, 2008. [DOI] [PubMed] [Google Scholar]
- 36. Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, Li J, Guo W, Andrade FH. A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum Mol Genet 11: 263–272, 2002. [DOI] [PubMed] [Google Scholar]
- 37. Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol 112: 1625–1636, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thalacker-Mercer AE, Dell'Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics 40: 141–149, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thalacker-Mercer AE, Petrella JK, Bamman MM. Does habitual dietary intake influence myofiber hypertrophy in response to resistance training? A cluster analysis. Appl Physiol Nutr Metab 34: 632–639, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Timmons JA, Knudsen S, Rankinen T, Koch LG, Sarzynski M, Jensen T, Keller P, Scheele C, Vollaard NB, Nielsen S, Akerström T, MacDougald OA, Jansson E, Greenhaff PL, Tarnopolsky MA, van Loon LJ, Pedersen BK, Sundberg CJ, Wahlestedt C, Britton SL, Bouchard C. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J Appl Physiol 108: 1487–1496, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wagatsuma A, Kotake N, Yamada S. Muscle regeneration occurs to coincide with mitochondrial biogenesis. Mol Cell Biochem 349: 139–147, 2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.