Abstract
Oral ingestion of sodium bicarbonate (bicarbonate loading) has acute ergogenic effects on short-duration, high-intensity exercise. Because sodium bicarbonate is 27% sodium, ergogenic doses (i.e. 300 mg·kg−1) result in sodium intakes well above the Dietary Reference Intakes upper limit of 2300 mg/day. Therefore, it is conceivable that bicarbonate loading could have hypertensive effects. Therefore, we performed a double-blind cross-over trial to evaluate the hypothesis that bicarbonate loading increases resting and exercise blood pressure (BP). A secondary hypothesis was that bicarbonate loading causes gastrointestinal distress. Eleven endurance-trained men and women (exercise frequency, 4.6±0.4 sessions/wk; duration, 65±6 min/session) underwent testing on two occasions in random sequence: once after bicarbonate loading (300 mg·kg−1) and once after placebo ingestion. BP and heart rate (HR) were measured before bicarbonate or placebo consumption, 30 minutes after consumption, during 20 min of steady state submaximal cycling exercise, and during recovery. Bicarbonate loading did not affect systolic BP during rest, exercise, or recovery (p=0.38 for main treatment effect). However, it resulted in modestly higher diastolic BP (main treatment effect, +3.3±1.1 mmHg, p=0.01) and higher HR (main treatment effect, +10.1±2.4 bpm, p=0.002). Global ratings of gastrointestinal distress severity (0–10 scale) were greater after bicarbonate ingestion (5.1±0.5 vs. 0.5±0.2, p<0.0001). Furthermore, 10 of the 11 subjects (91%) experienced diarrhea, 64% experience bloating and thirst, and 45% experienced nausea after bicarbonate loading. In conclusion, although a single, ergogenic dose of sodium bicarbonate does not appear to have acute, clinically important effects on resting or exercise BP, it does cause substantial GI distress.
Keywords: exercise, diarrhea, blood pressure, sodium, sodium bicarbonate, human
1. INTRODUCTION
A large body of evidence indicates that oral ingestion of sodium bicarbonate (bicarbonate loading) has ergogenic effects on subsequent short-duration, high-intensity exercise (for reviews and meta-analyses, see [1–3]). For example, it increases running time to exhaustion, increases maximal-effort swimming velocity, and results in greater power output during cycling [4–7]. Based on a meta-analysis of 59 studies, bicarbonate loading increases high-intensity, short-duration exercise power output by 1.7% [2]. These effects result from increases in serum and extracellular bicarbonate levels, which increase acid buffering capacity and attenuate the development of metabolic acidosis during intense exercise [4–9]. Although the prevalence of bicarbonate loading and dosing methods among athletes has not been studied, the most commonly used ergogenic dose of sodium bicarbonate in scientific studies is 300 mg·kg−1 [2]. Because sodium bicarbonate is 27% sodium (i.e. 23 g of the 84 g molar mass of sodium bicarbonate is sodium [10]), this dose provides quantities of sodium that greatly exceed the Dietary Reference Intakes Tolerable Upper Intake Level of 2300 mg per day [11]. For example, the ergogenic dose for a 70 kg athlete contains ~5700 mg sodium.
Changes in dietary sodium are known to alter blood pressure (BP) [12–15]. Although the acute blood pressure effects of a single oral sodium load in humans has not been studied, a 10–20 min infusion of 800–2500 mg sodium in solution increases mean and systolic BP by ~10 mmHg [16–18]. Additionally, BP increases by ~6 mmHg systolic and ~3–4 mmHg diastolic after increasing dietary sodium by 2300 mg/d for 4 weeks [19]. Therefore, it is conceivable that the large doses of sodium ingested during bicarbonate loading could have adverse effects on BP. Such an effect might be especially concerning if it persists during exercise, when BP increases in proportion to exercise intensity, and might increase the risk of adverse events, such as hemorrhagic stroke [20].
We hypothesized that acute oral bicarbonate loading, which has been shown in many studies to have beneficial effects on exercise performance [1–3], may have adverse effects on health. More specifically, the primary research objective was to evaluate, in humans, if a single, 300 mg·kg−1 oral dose of sodium bicarbonate increases resting and exercise BP, as compared to BP measured after placebo ingestion. Furthermore, because some studies have reported that bicarbonate loading causes gastrointestinal (GI) distress [21–23], a secondary objective was to determine if bicarbonate loading is associated with the development of symptoms of GI distress. The rationale for performing this study was to identify potentially adverse effects of a well-known nutrition practice that is used for enhancing exercise performance in humans.
2. METHODS AND MATERIALS
a. Participants
Exercise trained men and women were recruited from the Saint Louis metropolitan area. Screening consisted of questionnaires to evaluate medical history, medication use, and exercise and diet histories. Body weight was measured on a calibrated balance beam scale (Detecto, Webb City, MO) with the participant dressed in light clothing (e.g. shorts and t-shirt) and no shoes. Height was measured with a wall-mounted stadiometer (Health-o-meter, Sunbeam Products, Inc., Boca Raton, FL). Height and weight were used to calculate body mass index (BMI, kg/m2). In accordance with published standards [24], waist circumference was measured at the narrowest bilateral aspect of the abdomen and hip circumference was measured at the maximal posterior protuberance of the buttocks. Screening BP was measured according to published criteria [25], as described below. Volunteers were excluded if they: (a) were not in the age range of 18–55 yr, (b) were not exercise trained (defined as performing vigorous endurance exercise at least 30 min/d, 3 times per week, for the past 3 months), (c) had major chronic disease or conditions in which exercise or sodium bicarbonate supplementation was contraindicated, (d) were taking anti-hypertensive medications, or (e) were classified as “moderate” or “high” risk for medical complications during exercise according to American College of Sports Medicine guidelines [26]. The Saint Louis University Institutional Review Board approved the study protocol and all participants provided informed written consent to participate in the study.
b. Study Design and Randomization
The study consisted of a double-blind cross-over trial in which study participants underwent testing on two occasions: once after bicarbonate loading (300 mg·kg−1) and once after placebo ingestion. The intervention sequence was randomized, such that half of the participants received the bicarbonate treatment first, and the others received placebo first. The two trials were separated by 1 to 2 weeks. The bicarbonate and placebo solutions were prepared and administered to the participants by study personnel who were not involved in data collection. Investigator and subject blinding was maintained until all data collection was completed.
c. Bicarbonate Loading and Blinding
Sodium bicarbonate and placebo solutions were administered to the participants in opaque bottles to avoid visual differentiation. The sodium bicarbonate solution consisted of 300 mg·kg−1 sodium bicarbonate mixed into 592 mL of water, which is similar to acute loading protocols used in previous studies [22,27–30]. The placebo consisted of 582 mL of sodium-free carbonated water (La Croix, Sundance Beverage Co, Fort Lauderdale, FL) with 10 mL of non-alcoholic bitters, which together provided a similar taste and effervescent quality to the bicarbonate solution. The participants were encouraged to consume the beverages in ≤5 minutes. To determine the efficacy of blinding, and after all other aspects of study participation were complete, the participants were asked if they knew which drink (bicarbonate or placebo) they received in each of the 2 trials.
d. Study Trials
During each trial, the participants reported to the laboratory after a 4-hour fast. BP and heart rate (HR) were measured multiple times as follows: (a) after arriving at the laboratory and resting (seated) for 5 minutes, (b) 30 minutes after consuming the experimental drink (i.e. sodium bicarbonate solution or placebo), (c) after 10 and 20 minutes of steady-state exercise on a mechanically-braked cycle ergometer (Monark 827e, Monark Exercise AB, Vansbro, Sweden), and (d) after 2 minutes of passive recovery from exercise with the subject seated in a chair. On average (±SE), steady state exercise started 41± 1 min after drink consumption; therefore, the exercise BP measures were taken at approximately 50 and 60 minutes after dosing. The timing for our protocol was based on research which demonstrates that changes in plasma pH and bicarbonate concentrations are near maximal (~70%) 30 minutes after a 300 mg·kg−1 oral bicarbonate load at maximum change 60 min after loading [27,31].
During the first of the two trials for each subject, cycling consisted of an initial stepwise increase in work rate until 60% heart rate reserve (HRR) was attained [where 60% HRR = 0.60 × (maximal HR − resting HR) + resting HR]; then, an additional 20 minutes of steady state exercise was performed at the work rate that initially elicited 60% of HRR. For the incremental portion of the exercise protocol, resistance on the cycle ergometer was set at 1.0 kg and was then increased by 0.5 kg every 3 minutes until target HR was attained. On average (±SE), 60% of HRR was attained 6.8±0.5 min after initiating exercise. Pedal rate was initially self-selected but was required to remain constant throughout the exercise protocol. Pedal rate and resistance were continuously monitored by the technician to ensure that they remained constant during the 20 minutes of steady state exercise. During the second of the two trials for each subject, the work rate and pedal rate from trial 1 was replicated and was not dependent on HR response. No warm-up exercise was performed in either trial.
e. Dietary and Exercise Control
To avoid possible confounding effects of variations in dietary factors (e.g. sodium or fluid intake) and exercise on study outcomes, participants kept a diary of all foods and beverages consumed and all exercise performed during the 24 hours prior to the first trial and were instructed to follow the same diet and exercise routine during the 24 hours prior to their second trial. Participants were also advised to refrain from using nutritional supplements and over-the-counter medications for 1 week before each study trial.
f. Blood Pressure, Heart Rate, and Rate Pressure Product Measurements
All BP and HR measures were performed by a technician who was formally trained in these methods and certified by the American College of Sports Medicine as a Health/Fitness Specialist. BPs were measured using auscultation and a mercury sphygmomanometer according to JNC 7 guidelines [25]. In brief, resting BP was initially measured twice in each arm after 5 minutes of seated rest; thereafter, the arm with the higher BP was used for all subsequent BP measurements. If duplicate measures were discrepant by >5 mmHg, additional measures were made. Duplicate measures at each assessment time point were averaged [25]. During exercise, BP was measured after 10 and 20 minutes of steady-state exercise.
Resting HRs were measured by manually palpating the radial artery for a 60 seconds. Exercise HRs were monitored with a wristwatch-type HR monitor (Polar RS200, Polar Electro Oy, Kempele, Finland) at the same time intervals as described for exercise BPs.
Mean BP was calculated as 2/3rds diastolic BP + 1/3rd systolic BP. Rate pressure product was calculated as the product of HR and systolic BP and was used as an index of myocardial oxygen consumption (or myocardial work) [32].
g. Cardiorespiratory Fitness
Predicted maximal oxygen uptake (VO2max) was measured using standard methods [26] and used as an index of cardiorespiratory fitness. In brief, a regression equation was generated for each subject based on the relationship between resting and submaximal exercise HR and predicted oxygen uptake (VO2). Resting HR was measured as described above and resting VO2 was assumed to be 3.5 mL/kg/min. Exercise HR was measured during the submaximal exercise which was performed during the placebo exercise trial to avoid any possible effects of bicarbonate on HR. Exercise VO2 was predicted based on a standard equation for predicting VO2 from cycle ergometry work rates [26]. The resulting regression equation was used to predict VO2 at age-predicted maximal HR, where age-predicted maximal HR = 208−0.7*age in years [33] and this value was considered VO2max.
h. Gastrointestinal Discomfort
The participants were advised to inform the investigators about any symptoms of gastrointestinal (GI) distress or discomfort experienced at any time during the study trial (such as nausea, vomiting, diarrhea, bloating, cramping, lightheadedness, and thirst). Thus, the nature of the symptom monitoring was open-ended and did not involve specific questions at set time intervals. At the end of the trail, participants were asked to use a verbal numeric rating scale to provide a global rating of the severity of GI distress when it was at its worst during the trial (0–10 scale, with zero reflecting no GI distress at all and 10 being the most severe GI distress imaginable). Verbal numeric rating scales are common and valid for measuring patient-assessed global pain [34,35] and have also been validated for use in evaluating global symptom severity in patients with irritable bowel syndrome [36].
i. Sample Size Determination
Sample size was calculated a priori based on a standard deviation for duplicate BP measures of 10 mmHg, a desired statistical power of 0.80, an alpha error rate of 0.05 for a two-tailed test, and a clinically relevant blood pressure effect size of 10 mmHg. Results indicated that 10 subjects would be needed. Eleven subjects were enrolled to account for possible drop out; however, no subjects dropped out.
j. Statistical Analyses
Baseline values (before dosing) for quantitative outcomes were compared by using paired t-tests. For quantitative outcomes measured after dosing, two-factor (treatment × time) repeated measures ANCOVAs were used in which the dependent variable was the outcome, as measured after dosing, and the covariate was the baseline value of the outcome (as measured before dosing). GI symptom frequencies were compared by using single-sided non-parametric Cohen’s Kappa Coefficients, which account for the repeated measures nature of the data. Significance was accepted at the p≤0.05 level. All values are reported as means ± standard error of the mean. Statistical analyses were performed with SAS (Enterprise Guide, version 4.3, SAS Institute Inc., Cary, NC).
3. RESULTS
a. Subject Characteristics
Eleven volunteers met the inclusion criteria for the study and were enrolled; all completed both trials and provided valid data. Subject characteristics are provided in Table 1. Although 18–55 year-old individuals were eligible, all enrolled participants were 22–28 years of age. Similar numbers of men and women participated. Some of the men had BMI values >25.0 kg/m2, suggesting that they were overweight. Although body composition was not measured, none of these individuals had waist circumferences that would suggest overweight or obesity (i.e. all were <102 cm [37]) and based on our subjective observations, BMI was high in these individuals because of high muscle mass, not adiposity. Strength training histories were not obtained.
Table 1.
Subject characteristics.
| Age (yrs) | 24.6 ± 0.5 |
| Sex | |
| Men | 5 (45%) |
| Women | 6 (55%) |
| Height (cm) | |
| Men | 182.9 ± 4.5 |
| Women | 167.4 ± 4.3 |
| Weight (kg) | |
| Men | 90.4 ± 5.7 |
| Women | 58.7 ± 2.2 |
| BMI (kg/m2) | |
| Men | 26.9 ± 0.5 |
| Women | 21.0 ± 0.4 |
| Waist circumference, cm | |
| Men | 89.2 ± 3.0 |
| Women | 70.9 ± 2.3 |
| Waist-to-Hip Ratio | |
| Men | 0.83 ± 0.03 |
| Women | 0.73 ± 0.02 |
| Resting Blood Pressure, mmHg | |
| Systolic | 117 ± 2 |
| Diastolic | 75 ± 2 |
| Predicted VO2max, mL/kg/min | |
| Men | 48.5 ± 6.0 |
| Women | 36.2 ± 1.0 |
| Exercise frequency, days/wk | 4.6 ± 0.4 |
| Exercise duration, min/session | 65 ± 6 |
| Exercise intensity (subjective exertion during endurance exercise) | |
| Light | 1 (9%) |
| Moderate | 10 (91%) |
| Hard | 7 (64%) |
Data are means ± SE or frequencies reported as counts and percentages. VO2max, maximal oxygen uptake (predicted based on resting and submaximal exercise). The total sample size was 11.
Based on normative data for cardiorespiratory fitness (i.e. VO2max) in 20–29 year old men and women [26], men in our study were at the 71st percentile and women were at the 67th percentile, indicating that the participants were moderately exercise trained. Most study participants (n=10, 91%) reported that they did not avoid salt or sodium in their diet; one subject reported intentionally avoiding dietary sodium for health purposes.
b. Bicarbonate Dosing
The average dose of sodium bicarbonate that was administered was 22.0 ± 1.7 g (3.6% in solution) with a range of 15.4–31.6 g. This provided 5857 ± 454 mg sodium (range 4109–8437 mg).
c. Blood Pressure, Heart Rate, and Rate Pressure Product
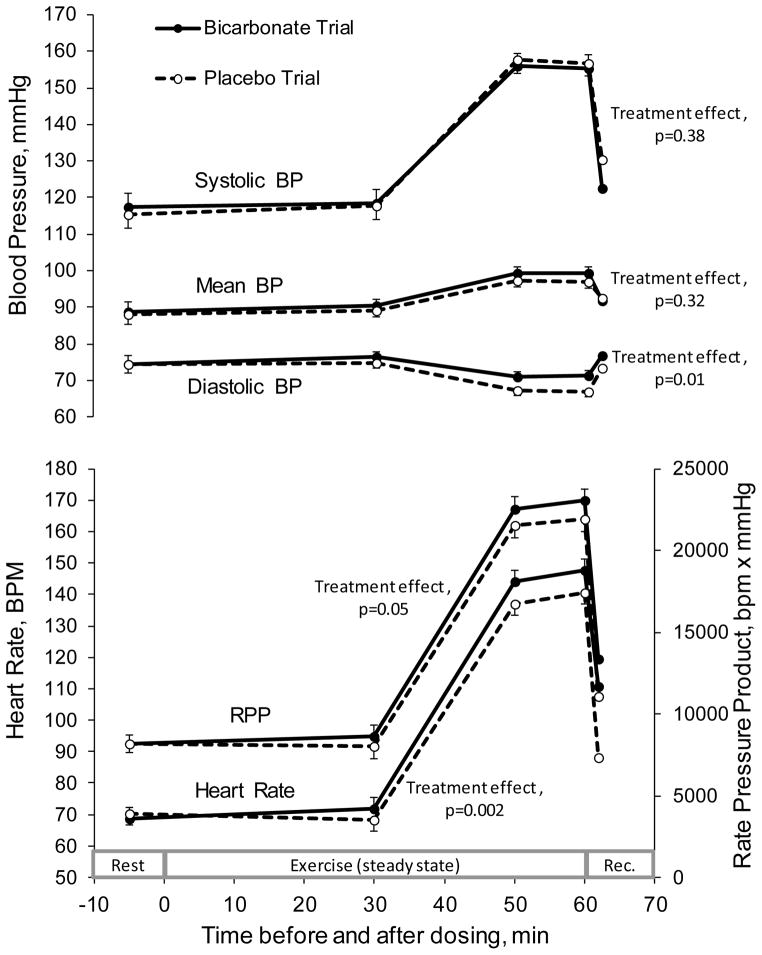
At baseline (before bicarbonate or placebo ingestion) no differences were observed between the bicarbonate and placebo trials for resting BP (systolic, diastolic, or mean), HR, or rate pressure product (Figure 1). As expected, exercise resulted in increases (all p<0.0001 for main effect of time) in systolic and mean BPs, a decrease in diastolic BP, and increases in HR and rate pressure product; all measures returned toward baseline during recovery from exercise (Figure 1). This pattern of change from rest to exercise and recovery did not differ between the bicarbonate and placebo trials for any of the outcomes (p-values for treatment by time interaction were as follows: systolic BP, p=0.76; mean BP, p=0.87; diastolic BP, p=0.80; HR, p=0.08; and rate pressure product, p=0.80). Because none of the interactions effects were significant, no time point-specific paired comparisons were performed. However, tests for main treatment effects indicate that bicarbonate loading resulted in higher diastolic BP (3.3±1.1 mmHg, p=0.01), HR (10±2 bpm, p=0.002), and rate pressure product (1254±575 bpm x mmHg, p=0.05). No significant main treatment effects were observed for systolic and mean BP (Figure 1).
Figure 1.
Hemodynamic responses to oral ingestion of sodium bicarbonate (300 mg/kg) and placebo during rest and steady-state constant workload exercise.
Data (means±SE) were analyzed by using two-factor (treatment × time) repeated measures ANCOVAs, in which the dependent variable was the outcome, as measured after dosing, and the covariate was the baseline value of the outcome, as measured before dosing. Initial analyses evaluated the interaction between treatment and time; only when the interaction term was not significant (as was the case for all outcomes) were the main effects evaluated. P-values shown for treatment effects reflect differences in outcome measures when all values for the bicarbonate trial are compared in aggregate to the values in the placebo trial. Eleven subjects completed both the bicarbonate and placebo trials.
d. Gastrointestinal Distress
At baseline, before bicarbonate and placebo administration, none of the study participants reported symptoms of GI distress. In response to bicarbonate loading, all 11 subjects (100%) reported symptoms of GI distress, with diarrhea, thirst and bloating being the most common complaints (Table 2). In the bicarbonate trial, all subjects reported having at least two of the symptoms shown in Table 2, with one subject who reported having 5 of the 9 different symptoms, two who reported having 4 symptoms, six who reported having 3 symptoms, and one who reported 2 symptoms. In contrast, the only complaints about GI discomfort in the placebo trial were thirst by one subject and hunger by another subject. During the bicarbonate trial, the average rating of global GI distress severity was 5.1±0.5 (0–10 rating scale). This was 10-fold greater (P< 0.0001) than the average rating of 0.5±0.2 during the placebo trial.
Table 2.
Gastrointestinal symptom frequencies during each of the two study trials.
| Symptom | Bicarbonate Trial | Placebo Trial | P-value |
|---|---|---|---|
| Nausea | 5 (45%) | 0 (0%) | 0.006* |
| Vomiting | 1 (9%) | 0 (0%) | 0.153 |
| Diarrhea | 10 (91%) | 0 (0%) | <0.001* |
| Bloating | 7 (64%) | 0 (0%) | <0.001* |
| Cramping | 2 (18%) | 0 (0%) | 0.069 |
| Heartburn | 1 (9%) | 0 (0%) | 0.153 |
| Lightheadedness | 2 (18%) | 0 (0%) | 0.069 |
| Thirst | 7 (64%) | 1 (9%) | 0.004* |
| Hunger | 0 (0%) | 1 (9%) | 0.153 |
Data are counts with percentages in parentheses. Participants were advised to inform the investigators about any symptoms of gastrointestinal (GI) distress or discomfort experienced at any time during the study trial. The sampled included all 11 study participants, all of whom completed both trials. P-values are from one-sided Cohen’s kappa coefficients.
Designates a significant difference in symptom frequency between trials based on Bonferroni-corrected p-values of ≤0.006 (i.e. 0.05 ÷ 9 comparisons).
4. DISCUSSION
The principal finding of this study is that bicarbonate loading, with a dose of sodium bicarbonate that is commonly used for exercise performance enhancement (i.e. 300 mg·kg−1), modestly increases diastolic blood pressure (~3–4 mmHg, p=0.01) during rest, exercise, and recovery from exercise in healthy young adults. While a long-term increase of this magnitude would be expected to increase the risk of death due to stroke and other vascular diseases by ~30–40% [38], it is not likely to be problematic with isolated occurrences of bicarbonate loading because the effects are short-lived. Furthermore, while an increase in systolic pressure might be expected to acutely increase the risk of hemorrhagic stroke [20], especially during exercise when systolic pressures are high, diastolic pressure, which decreases modestly during exercise, would not increase peak forces on the vasculature and therefore wouldn’t be expected to increase the risk of vascular rupture. Therefore, form a clinical perspective, it doesn’t seem likely that the small transient increases in diastolic blood pressure that results from bicarbonate loading would be associated with health risks. In this context, we reject the research hypothesis and conclude that bicarbonate loading does not have clinically relevant adverse effects on BP.
Research has demonstrated that an 800–2500 mg infusion of sodium [16–18] and a daily increase in dietary sodium of 2300 mg/d [19] increase blood pressure by ~6–10 mmHg systolic and ~3–4 mmHg diastolic. Therefore, it is not clear why a sodium load of nearly 6000 mg in our study did not affect systolic BP at all and did not have larger effects on diastolic BP; however, several factors warrant consideration. First, our study sample consisted of young men and women who, by virtue of being young, were not likely to be salt-sensitive (salt sensitivity is often defined as a ≥5 mmHg increases in BP with a ~3500–7000 mg/d increase in dietary sodium [39,40], although testing protocols and definitions vary). The prevalence of salt-sensitivity in young adults is ~13% [41]. Future studies should target populations in which salt-sensitivity is more prevalent such as middle- to older-aged individuals (≥45–50 yr, ~20–40% prevalence [41,42]) or African Americans (~20–50% prevalence [40,43]). It is also possible that sodium from sources other than sodium chloride does not affect BP. Some research has demonstrated that moderate sodium doses (~3200 mg/d) from sodium bicarbonate [44] and large sodium doses (~5520 mg/d, similar to that used in the present study) from sodium citrate [45] do not adversely affect BP, while equimolar amounts of sodium chloride do increase BP (~5–16 mmHg). Other research, however, has demonstrated that sodium bicarbonate (5750 mg/d sodium) does increase BP (mean arterial pressure increases ~5 mmHg), albeit to a lesser extent than equimolar doses of sodium chloride (~11 mmHg) [46]. It is also conceivable that athletes and exercise trained individuals, who frequently have large sodium losses in the form of sweat, may be able to tolerate large doses of sodium without adverse effects on BP.
Another possible explanation for the negligible changes in BP is that the sufficient time was not allowed for the full effect of the sodium load to develop, as resting BP was measured 30 min after dosing and exercise measures were taken at 50 and 60 min after dosing. To our knowledge, the time course of BP changes after a single, large oral sodium load has not been studied. However, a 3% sodium chloride infusion (~800 – 2500 mg sodium in solution) increases BP by ~10 mmHg within 20 minutes [16–18]. Because intestinal sodium absorption is rapid (~6900 mg/h) [47], rapid effects on BP might also be expected after an oral sodium load.
An unexpected finding was a higher HR after bicarbonate loading, which was evident during rest, exercise (at the same work rate in the two trials), and recovery. An explanation for this phenomenon is not possible based on data from the present study. However, it is conceivable that the marginally higher diastolic BP might have been involved through its effects on vascular conductance and cardiac hemodynamics [48]. Alternatively, it is possible that the GI effects of bicarbonate loading were associated with alterations in autonomic nervous system activity, which would have their own effects on HR and cardiovascular function [49]. Nonetheless, the finding of a higher HR during steady-state exercise (and the resulting rise in rate pressure product, as an index of myocardial oxygen consumption [32]) raises the possibility that bicarbonate loading may have negative effects on aerobic exercise performance, although proper studies on this issue are needed.
A secondary but important finding of this study was the ubiquitous and severe gastrointestinal distress that resulted from bicarbonate loading. While the reason for this is not clear, ingestion of hypertonic solutions, such as the sodium bicarbonate solution provided in the study (~880 mOsm/L), causes an intraluminal osmotic load and water shift from plasma (~300 mOsm/L) and extracellular fluid to the intestinal lumen [50]. This would be expected to contribute to osmotic diarrhea (gastric dumping) and/or related gastrointestinal distress, and might contribute to systemic dehydration (this might explain the lack of increase in BP and the high prevalence of thirst). Accordingly, all participants reported multiple forms of GI distress after bicarbonate loading. These findings are consistent with those from other studies, in which GI distress was prevalent (63–100%) and/or severe (~6 on 10-point visual analog scale) [21,23,51] but not with others in which GI distress was less problematic [5,22,52].
Studies have not evaluated the prevalence and frequency of bicarbonate loading by athletes in the “real world.” Therefore, it is somewhat difficult to know the full ramifications of the findings from our study. For example, if athletes use bicarbonate loading to enhance daily training sessions and these practices continue for years, it is conceivable that the modest BP effects observed in the present study might have long-term health consequences [38]. Furthermore, studies have not characterized the bicarbonate loading methods that are used by athletes; therefore, it is difficult to know if our loading protocol is similar to “real world” practices. It is possible that athletes are using loading protocols that circumvent GI distress problems. In this context, it is noteworthy that laboratory research has identified some bicarbonate loading strategies that may reduce GI distress problems including co-ingestion of the bicarbonate with a large volume of fluid (14 mL/kg body mass, ~ 1 liter for a 70 kg athlete), using a long ingestion period (60 min), and taking the bicarbonate in capsule form, rather than solution [22]. It is also likely that smaller bicarbonate doses result in less GI distress than large doses; however, based on a recent meta-analysis, this would also likely result in a smaller ergogenic effect [2].
A limitation of the present study was that the subject blinding was not effective, as all participants were able to distinguish the bicarbonate solution from placebo. While a lack of blinding is a major concern for studies in which exercise performance is the outcome (as this depends heavily on motivation) [53], this could not explain the BP results because the results were essentially negative. Furthermore, it is unlikely that the lack of blinding caused the GI distress that we reported. Therefore, in the context of the present study, the lack of effective blinding was not problematic. However, it would be prudent for future studies to utilize a different strategy for blinding (e.g. use of sodium bicarbonate capsules and matching placebo capsules). Another limitation is that we evaluated BP during steady-state endurance exercise, not the high-intensity, short duration types of exercise that bicarbonate loading benefits. The rationale for this was that BP measurements taken during sprinting or other types of high-intensity exercise are very difficult to obtain and would be of questionable validity. However, because bicarbonate loading did not affect systolic pressure and diastolic pressure was only minimally affected, it does not seem likely that BPs during higher intensity exercise would be of concern. Perhaps future studies could use intra-arterial catheters to acquire blood pressure measurements during high-intensity sprint-type exercise.
In conclusion, although the recommended dose of sodium bicarbonate that is used for exercise performance enhancement (i.e. 300 mg·kg−1) contains a large amount of sodium, results from the present study suggest that it does not acutely increase resting or exercise systolic blood pressure and has only minor effects on diastolic pressure, which are likely negligible from a safety perspective. Therefore, it does not appear as though bicarbonate loading acutely increases the risk for medical complications associated with resting or exercise hypertension, at least in young healthy men and women. Future studies are perhaps needed to evaluate blood pressure responses to frequent bicarbonate loading (as might be used for regular training sessions) and for longer term loading protocols. Results from this study also confirm other recent studies by showing that bicarbonate loading has adverse effects on the gastrointestinal system, as evidenced by diarrhea and other symptoms of GI distress, which might increase the risk of medical complications for athletes and negate the performance enhancing effects.
Acknowledgments
We are grateful to the study participants for their cooperation. This research was supported by the Department of Nutrition and Dietetics at Saint Louis University.
ABBREVIATIONS
- BP
blood pressure
- HR
heart rate
- HRR
heart rate reserve
- GI
gastrointestinal
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McNaughton LR, Siegler J, Midgley A. Ergogenic effects of sodium bicarbonate. Curr Sports Med Rep. 2008;7:230–236. doi: 10.1249/JSR.0b013e31817ef530. [DOI] [PubMed] [Google Scholar]
- 2.Carr AJ, Hopkins WG, Gore CJ. Effects of acute alkalosis and acidosis on performance: a meta-analysis. Sports Med. 2011;41:801–814. doi: 10.2165/11591440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Peart DJ, Siegler JC, Vince RV. Practical recommendations for coaches and athletes: a meta-analysis of sodium bicarbonate use for athletic performance. J Strength Cond Res. 2012;26:1975–1983. doi: 10.1519/JSC.0b013e3182576f3d. [DOI] [PubMed] [Google Scholar]
- 4.Bishop D, Claudius B. Effects of induced metabolic alkalosis on prolonged intermittent-sprint performance. Med Sci Sports Exerc. 2005;37:759–767. doi: 10.1249/01.mss.0000161803.44656.3c. [DOI] [PubMed] [Google Scholar]
- 5.Van Montfoort MC, Van Dieren L, Hopkins WG, Shearman JP. Effects of ingestion of bicarbonate, citrate, lactate, and chloride on sprint running. Med Sci Sports Exerc. 2004;36:1239–1243. doi: 10.1249/01.mss.0000132378.73975.25. [DOI] [PubMed] [Google Scholar]
- 6.Price M, Moss P, Rance S. Effects of sodium bicarbonate ingestion on prolonged intermittent exercise. Med Sci Sports Exerc. 2003;35:1303–1308. doi: 10.1249/01.MSS.0000079067.46555.3C. [DOI] [PubMed] [Google Scholar]
- 7.Lindh AM, Peyrebrune MC, Ingham SA, Bailey DM, Folland JP. Sodium bicarbonate improves swimming performance. Int J Sports Med. 2008;29:519–523. doi: 10.1055/s-2007-989228. [DOI] [PubMed] [Google Scholar]
- 8.Bishop D, Edge J, Davis C, Goodman C. Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability. Med Sci Sports Exerc. 2004;36:807–813. doi: 10.1249/01.mss.0000126392.20025.17. [DOI] [PubMed] [Google Scholar]
- 9.Douroudos II, Fatouros IG, Gourgoulis V, Jamurtas AZ, Tsitsios T, Hatzinikolaou A, Margonis K, Mavromatidis K, Taxildaris K. Dose-related effects of prolonged NaHCO3 ingestion during high-intensity exercise. Med Sci Sports Exerc. 2006;38:1746–1753. doi: 10.1249/01.mss.0000230210.60957.67. [DOI] [PubMed] [Google Scholar]
- 10.PubChem. [Accessed March 26, 2013]; http://pubchem.ncbi.nlm.nih.gov/
- 11.Food and Nutrition Board. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington D.C: The National Academies Press; 2005. [Google Scholar]
- 12.Frisoli TM, Schmieder RE, Grodzicki T, Messerli FH. Salt and hypertension: is salt dietary reduction worth the effort? Am J Med. 2012;125:433–439. doi: 10.1016/j.amjmed.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 14.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, III, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 15.Obarzanek E, Proschan MA, Vollmer WM, Moore TJ, Sacks FM, Appel LJ, Svetkey LP, Most-Windhauser MM, Cutler JA. Individual blood pressure responses to changes in salt intake: results from the DASH-Sodium trial. Hypertension. 2003;42:459–467. doi: 10.1161/01.HYP.0000091267.39066.72. [DOI] [PubMed] [Google Scholar]
- 16.Charkoudian N, Eisenach JH, Joyner MJ, Roberts SK, Wick DE. Interactions of plasma osmolality with arterial and central venous pressures in control of sympathetic activity and heart rate in humans. Am J Physiol Heart Circ Physiol. 2005;289:H2456–H2460. doi: 10.1152/ajpheart.00601.2005. [DOI] [PubMed] [Google Scholar]
- 17.Farquhar WB, Paul EE, Prettyman AV, Stillabower ME. Blood pressure and hemodynamic responses to an acute sodium load in humans. J Appl Physiol. 2005;99:1545–1551. doi: 10.1152/japplphysiol.00262.2005. [DOI] [PubMed] [Google Scholar]
- 18.Farquhar WB, Wenner MM, Delaney EP, Prettyman AV, Stillabower ME. Sympathetic neural responses to increased osmolality in humans. Am J Physiol Heart Circ Physiol. 2006;291:H2181–H2186. doi: 10.1152/ajpheart.00191.2006. [DOI] [PubMed] [Google Scholar]
- 19.Melander O, von Wowern F, Frandsen E, Burri P, Willsteen G, Aurell M, Hulthen UL. Moderate salt restriction effectively lowers blood pressure and degree of salt sensitivity is related to baseline concentration of renin and N-terminal atrial natriuretic peptide in plasma. J Hypertens. 2007;25:619–627. doi: 10.1097/HJH.0b013e328013cd50. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA American Heart Association Stroke C, Council on Cardiovascular N, Council on E, Prevention, Council for High Blood Pressure R, Council on Peripheral Vascular D, Interdisciplinary Council on Quality of C, Outcomes R. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 21.Carr AJ, Gore CJ, Dawson B. Induced alkalosis and caffeine supplementation: effects on 2,000-m rowing performance. Int J Sport Nutr Exerc Metab. 2011;21:357–364. doi: 10.1123/ijsnem.21.5.357. [DOI] [PubMed] [Google Scholar]
- 22.Carr AJ, Slater GJ, Gore CJ, Dawson B, Burke LM. Effect of sodium bicarbonate on [HCO3-], pH, and gastrointestinal symptoms. Int J Sport Nutr Exerc Metab. 2011;21:189–194. doi: 10.1123/ijsnem.21.3.189. [DOI] [PubMed] [Google Scholar]
- 23.Cameron SL, McLay-Cooke RT, Brown RC, Gray AR, Fairbairn KA. Increased blood pH but not performance with sodium bicarbonate supplementation in elite rugby union players. Int J Sport Nutr Exerc Metab. 2010;20:307–321. doi: 10.1123/ijsnem.20.4.307. [DOI] [PubMed] [Google Scholar]
- 24.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign: Human Kinetics Publishers, Inc; 1988. [Google Scholar]
- 25.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 26.American College of Sports M. ACSM’s guidelines for exercise testing and prescription. Baltimore: Williams & Wilkins; 2006. [Google Scholar]
- 27.Siegler JC, Midgley AW, Polman RC, Lever R. Effects of various sodium bicarbonate loading protocols on the time-dependent extracellular buffering profile. J Strength Cond Res. 2010;24:2551–2557. doi: 10.1519/JSC.0b013e3181aeb154. [DOI] [PubMed] [Google Scholar]
- 28.McNaughton LR. Bicarbonate ingestion: effects of dosage on 60 s cycle ergometry. J Sports Sci. 1992;10:415–423. doi: 10.1080/02640419208729940. [DOI] [PubMed] [Google Scholar]
- 29.Siegler JC, Gleadall-Siddall DO. Sodium bicarbonate ingestion and repeated swim sprint performance. J Strength Cond Res. 2010;24:3105–3111. doi: 10.1519/JSC.0b013e3181f55eb1. [DOI] [PubMed] [Google Scholar]
- 30.Gaitanos GC, Nevill ME, Brooks S, Williams C. Repeated bouts of sprint running after induced alkalosis. J Sports Sci. 1991;9:355–370. doi: 10.1080/02640419108729896. [DOI] [PubMed] [Google Scholar]
- 31.Renfree A. The time course for changes in plasma [h+] after sodium bicarbonate ingestion. Int J Sports Physiol Perform. 2007;2:323–326. doi: 10.1123/ijspp.2.3.323. [DOI] [PubMed] [Google Scholar]
- 32.Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57:549–556. doi: 10.1161/01.cir.57.3.549. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 34.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Immpact Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine (Phila Pa 1976) 2000;25:3140–3151. doi: 10.1097/00007632-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel B, Strickland A, Naliboff BD, Mayer EA, Chang L. Predictors of patient-assessed illness severity in irritable bowel syndrome. Am J Gastroenterol. 2008;103:2536–2543. doi: 10.1111/j.1572-0241.2008.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 38.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert K, Nian H, Yu C, Luther JM, Brown NJ. Fenofibrate lowers blood pressure in salt-sensitive but not salt-resistant hypertension. J Hypertens. 2013;31:820–829. doi: 10.1097/HJH.0b013e32835e8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson DK, Bayer L, Krishnamoorthy JS, Ampey-Thornhill G, Nicholson SC, Sica DA. The prevalence of salt sensitivity in an African-American adolescent population. Ethn Dis. 1999;9:350–358. [PubMed] [Google Scholar]
- 41.Overlack A, Ruppert M, Kolloch R, Gobel B, Kraft K, Diehl J, Schmitt W, Stumpe KO. Divergent hemodynamic and hormonal responses to varying salt intake in normotensive subjects. Hypertension. 1993;22:331–338. doi: 10.1161/01.hyp.22.3.331. [DOI] [PubMed] [Google Scholar]
- 42.Overlack A, Ruppert M, Kolloch R, Kraft K, Stumpe KO. Age is a major determinant of the divergent blood pressure responses to varying salt intake in essential hypertension. Am J Hypertens. 1995;8:829–836. doi: 10.1016/0895-7061(95)00213-9. [DOI] [PubMed] [Google Scholar]
- 43.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 44.Luft FC, Zemel MB, Sowers JA, Fineberg NS, Weinberger MH. Sodium bicarbonate and sodium chloride: effects on blood pressure and electrolyte homeostasis in normal and hypertensive man. J Hypertens. 1990;8:663–670. doi: 10.1097/00004872-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Kurtz TW, Al Bander HA, Morris RC., Jr “Salt-sensitive” essential hypertension in men. Is the sodium ion alone important? N Engl J Med. 1987;317:1043–1048. doi: 10.1056/NEJM198710223171702. [DOI] [PubMed] [Google Scholar]
- 46.Schmidlin O, Forman A, Sebastian A, Morris RC., Jr Sodium-selective salt sensitivity: its occurrence in blacks. Hypertension. 2007;50:1085–1092. doi: 10.1161/HYPERTENSIONAHA.107.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Visscher MB, Varco RH, Carr CW, Dean RB, Erickson D. Sodium ion movement between the intestinal lumen and blood. Am J Physiol. 1944;141:488–505. [Google Scholar]
- 48.Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. Central Circulatory Adjustments to Dynamic Exercise; pp. 162–203. [Google Scholar]
- 49.O’Leary DS, Potts JT. The Cardiovascular System: Design and Control. In: Tipton CM, Sawka MN, Tate CA, Terjung RL, editors. ACSM’s Advanced Exercise Physiology. 1. Baltimore: Lippincott Williams & Wilkins; 2006. pp. 314–325. [Google Scholar]
- 50.Gisolfi CV, Summers RW, Schedl HP, Bleiler TL, Oppliger RA. Human intestinal water absorption: direct vs. indirect measurements. Am J Physiol. 1990;258:G216–G222. doi: 10.1152/ajpgi.1990.258.2.G216. [DOI] [PubMed] [Google Scholar]
- 51.Siegler JC, Marshall PW, Bray J, Towlson C. Sodium bicarbonate supplementation and ingestion timing: does it matter? J Strength Cond Res. 2012;26:1953–1958. doi: 10.1519/JSC.0b013e3182392960. [DOI] [PubMed] [Google Scholar]
- 52.McNaughton L, Backx K, Palmer G, Strange N. Effects of chronic bicarbonate ingestion on the performance of high-intensity work. Eur J Appl Physiol Occup Physiol. 1999;80:333–336. doi: 10.1007/s004210050600. [DOI] [PubMed] [Google Scholar]
- 53.McClung M, Collins D. “Because I know it will!”: placebo effects of an ergogenic aid on athletic performance. J Sport Exerc Psychol. 2007;29:382–394. doi: 10.1123/jsep.29.3.382. [DOI] [PubMed] [Google Scholar]