Abstract
Adaptive changes in glutamatergic signaling within the hypothalamic paraventricular nucleus (PVN) may play a role in the neurohumoral dysfunction underlying the hypertension induced by “slow-pressor” ANG II infusion. We hypothesized that these adaptive changes alter production of gp91phox NADPH oxidase (NOX)-derived reactive oxygen species (ROS) or nitric oxide (NO), resulting in enhanced glutamatergic signaling in the PVN. Electron microscopic immunolabeling showed colocalization of NOX2 and N-methyl-d-aspartate receptor (NMDAR) NR1 subunits in PVN dendrites, an effect enhanced (+48%, P < 0.05 vs. saline) in mice receiving ANG II (600 ng·kg−1·min−1 sc). Isolated PVN cells or spinally projecting PVN neurons from ANG II-infused mice had increased levels of ROS at baseline (+40 ± 5% and +57.6 ± 7.7%, P < 0.01 vs. saline) and after NMDA (+24 ± 7% and +17 ± 5.5%, P < 0.01 and P < 0.05 vs. saline). In contrast, ANG II infusion suppressed NO production in PVN cells at baseline (−29.1 ± 5.2%, P < 0.05 vs. saline) and after NMDA (−18.9 ± 2%, P < 0.01 vs. saline), an effect counteracted by NOX inhibition. In whole cell recording of unlabeled and spinally labeled PVN neurons in slices, NMDA induced a larger inward current in ANG II than in saline groups (+79 ± 24% and +82.9 ± 6.6%, P < 0.01 vs. saline), which was reversed by the ROS scavenger MnTBAP and the NO donor S-nitroso-N-acetylpenicillamine (P > 0.05 vs. control). These findings suggest that slow-pressor ANG II increases the association of NR1 with NOX2 in dendrites of PVN neurons, resulting in enhanced NOX-derived ROS and reduced NO during glutamatergic activity. The resulting enhancement of NMDAR activity may contribute to the neurohumoral dysfunction underlying the development of slow-pressor ANG II hypertension.
Keywords: NMDA receptors, NADPH oxidase, reactive oxygen species, nitric oxide, patch clamp, electron microscopy
the hypothalamic paraventricular nucleus (PVN) includes neurons containing neurotransmitters, such as glutamate (41, 48) and GABA (6), as well as neuromodulators like nitric oxide (NO) (3, 27, 47). The neurohumoral output from PVN neurons is crucial for coordinating neuroendocrine and autonomic responses, which are thought to play an important role in hypertension (8, 16, 21, 26, 28, 29, 32, 46). The blood pressure elevation induced by chronic administration of subpressor doses of ANG II depends on activation of ANG II type 1 receptors (AT1R) in the subfornical organ (SFO) (9, 50), a circumventricular structure that provides a major excitatory input to presympathetic neurons in the PVN (4, 15). These PVN neurons are also glutamatergic and project to sympathetic preganglionic neurons in the intermediolateral column of the spinal cord (41). Since glutamatergic neurotransmission and glutamate receptors in the PVN (5, 19) play a role in the modulation of presympathetic outputs (4, 31, 32), it is likely these important components of signaling within the SFO to PVN pathway are critical for ANG II-induced “slow-pressor” hypertension (15).
Oxidative stress induced by NADPH oxidase (NOX) plays a key role in the mechanisms of ANG II hypertension (33). Targeting NADPH oxidase to discrete subcellular compartments is an important mechanism for spatial and temporal restriction of reactive oxygen species (ROS) production (20, 25, 39, 43, 44, 49, 50). We have previously shown that a gp91phox-containing NOX2 is the major source of both N-methyl-d-aspartic acid (NMDA) and ANG II-induced free radicals production in cerebral cortex (18, 22) and selected brain stem nuclei (43). Therefore, ANG II-induced slow-pressor hypertension may mobilize NOX2 to sites near the NMDA receptor (NMDAR) in PVN neurons, resulting in enhanced ROS production in response to glutamate. Activation of ionotropic glutamate receptors, particularly NMDAR-mediated currents (13, 29, 42), enhances the production of both neuronal NO and ROS from NOX2 (7, 18, 23). These agents, either directly or through the formation of peroxynitrite (37), the reaction product of NO with superoxide, could mediate the adaptive changes in ion channel gating in PVN neurons, resulting in the neurohumoral alterations underlying the hypertension.
We used confocal microscopy and electron microscopic immunolabeling to determine whether coexpression of NOX2 and the essential NMDAR subunit NR1 in PVN neurons are affected by chronic systemic administration of a slow-pressor dose of ANG II. We also examined the effect of ANG II hypertension on NMDAR-mediated Ca2+ currents using patch-clamp recording in slices, as well as ROS and NO production in isolated PVN neurons. The results provide new evidence linking enhanced NMDAR-mediated currents, NOX2-dependent ROS production and decreased NO availability in PVN with ANG II slow-pressor hypertension.
MATERIALS AND METHODS
Animals.
Sixty-two male C57BL/6 mice (age: 8–12 wk; weight: 21–25 g) were obtained from an in-house colony. All procedures were approved by the Animal Care and Use Committee at Weill Cornell Medical College.
Materials.
ANG II, NMDA, LNNA, TTX, CNQX, MK801, MnTBAP, SNAP, pronase, and thermolysin were obtained from Sigma-Aldrich (St. Louis, MO, USA). The NOX2 peptide inhibitor gp-91ds-tat (39) was synthesized by Bio-Synthesis (Lewisville, TX). Dihydroethidium (DHE) and 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM) were purchased from Invitrogen (Carlsbad, CA).
Osmotic minipump implantation and blood pressure monitor.
As described elsewhere (9), wild-type mice were anesthetized intraperitoneally (ketamine + xylazine) and implanted subcutaneously with 14-day osmotic minipumps (ALZET; Durect, Cupertino, CA) loaded with saline or ANG II (600 ng·kg−1·min−1), a concentration widely used by us and others (9, 10, 12, 33, 50). A Hatteras MC-4000 tail-cuff blood pressure system (Cary, NC) was used to document the increase in systolic blood pressure produced by ANG II. In some saline and ANG II-infused mice, losartan (1 mg/kg; n = 3) or PD123319 (1 mg/kg; n = 3) (30) were administered intraperitoneally, at day 11 for three consecutive days before being killed at day 14.
Electron microscopy.
Naïve mice and mice receiving saline or ANG II for 14 days were anesthetized with pentobarbital sodium, and their brains were fixed by perfusion with heparin-saline (1,000 U/ml) followed consecutively by 3.8% acrolein (Polysciences) in 2% paraformaldehyde/0.1 mol/l phosphate buffer (PB), as described previously (13). A vibratome (Leica Microsystems, Wetzlar, Germany) was used to cut coronal sections of 40 μm through the PVN, according to the brain atlas of (17). The sections were then freeze-thawed and placed for 30 min in a solution of 1% sodium borohydride in 0.1 mol/l PB prior to incubation in a solution of primary antisera for 72 h at 4°C on an orbital shaker. The immunolabeling was reproduced using two different sets of affinity-purified primary antisera (1) 1:200 dilution of rabbit polyclonal anti-NMDAR NR1 antibody (no. AB1516, lot no. 0611044491; Chemicon, Temucula, CA) and a 1:25 dilution of mouse anti-gp91phox (NOX2) (no. 611415, lot no. 75531; BD Transduction Laboratories, San Jose, CA), and (2) 1:50 dilution of mouse anti-NMDAR NR1 monoclonal antibody (no. 556308, lot no. 17084; BD Pharmingen, San Diego, CA, USA) and 1:200 dilution of goat anti-gp91phox (no. sc-5827, lot no. A1311; Santa Cruz Biotechnology, Santa Cruz, CA). The antisera were visualized using an immunoperoxidase and immunogold silver labeling protocol that has previously been described in detail and used for electron microscopic analysis of PVN neurons (2, 13). Analysis of numbers of labeled profiles was done exclusively in tissue processed using immunogold for NR1 and immunoperoxidase for NOX2, but tissue processed using reverse markers was also qualitatively examined in the PVN of mice receiving saline or ANG II. For immune-peroxidase labeling, sections previously incubated with the primary antisera were placed for 30 min in a 1:400 dilution of biotinylated donkey anti-rabbit immunoglobulin, IgG (no. 711–065-152) for detection of rabbit anti-NR1 or 1:200 biotinylated donkey anti goat IgG (no. 705–165-147; Jackson Immunoresearch, West Grove, PA) for detection of goat anti-gp91phox. These incubations were followed by 30-min reaction in avidin-biotin complex (ABC) Vectastain ABC Elite kit (no. BA-2001, Vector Laboratories, Burlingame, CA). The reaction product was visualized by a 4–6-min incubation in 3,3′-diaminobenzadine (Sigma-Aldrich, St. Louis, MO) and hydrogen peroxide. For immunogold labeling, sections were next placed for 2 h in a 1:50 dilution of goat anti-mouse gp91phox or donkey anti-mouse NR1 ultrasmall immunogold (no. 25810; Electron Microscopy Sciences, Hatfield, PA) followed by a 6-min incubation in silver enhancement IntenSE M (no. RPN 491; GE Healthcare, Piscataway, NJ). The immunolabeled tissue was prepared for electron microscopy by processing in 2% osmium tetroxide for 1 h, followed by dehydration and embedment in Embed 812 resin. Ultrathin sections collected exclusively at the surface of the tissue were counterstained and analyzed for ultrastructural analysis using a Tecnai transmission electron microscope.
Confocal NMDAR NR1 immunostaining.
Mice receiving saline or ANG II for 14 days were anesthetized with pentobarbital sodium, and their brains were fixed by perfusion with heparin-saline (1,000 U/ml) followed by 4% paraformaldehyde/0.1 mol/l PB, as described previously (13). A vibratome (Leica Microsystems) was used to cut coronal sections of 40 μm through the PVN, according to the brain atlas of Franklin and Paxinos (17). Free-floating sections were processed for single-labeling of rabbit polyclonal anti-NMDA NR1 antibody (1:200 dilution; no. AB1516, lot no. 0611044491, Chemicon). Sections were washed and incubated with Cy5-conjugated anti-rabbit IgG (1:200 dilution). For quantification of Cy5-labeled NMDAR1 immunoreactivity, the PVN sections from saline and ANG II-recipient mice were processed under identical conditions and scanned at the confocal microscope (Zeiss LSM 510; Zeiss, Munich, Germany). Setting was kept constant across all samples within individual experiments. Fluorescence intensity was quantified using National Institutes of Health ImageJ software.
Real-time RT-PCR measurement of the mRNA of NMDAR NR1.
Quantification of NMDAR NR1 transcript levels was done by amplification of cDNA from total RNA with an iQ5 real-time PCR machine (19), using SYBR Green (quantabio) and primers specific for NR1 transcript: sense, 5′-CTGCTCTCTGCAACCTGACTT-3′ and anti-sense, 5′-TGCCACTAGGGCAGTGAAG-3 and β-actin mRNA. Primers used for β-actin are as follows: sense, 5′- CATCCTCTTCCTCCCTGGAGAAGA-3′; anti-sense, 5′-ACAGGATTCCATACCCAAGAAGGAAGG-3′. Standard amplification conditions were utilized according to the manufacturer's specifications (Bio-Rad iQ5; Bio-Rad, Hercules, CA). β-actin was used for relative quantitation by ΔCt method, expressed as 2−ΔCt.
Retrograde labeling of spinally projecting paraventricular neurons.
Mice receiving saline or ANG II for 10 days were anesthetized intraperitoneally (ketamine + xylazine), and their spinal cords were exposed at the T2-T4 level through dorsal laminectomy. Under a surgical microscope, the tip of a glass pipette filled with rhodamine-labeled fluorescent microsphere (FluoSpheres, 0.04 μm; Molecular Probes, Eugene, OR) was pressure-ejected (50 nl, Picospritzer III; Parker Hannifin, Fairfield, NJ) bilaterally into the intermediolateral nucleus region of the spinal cord, and the incision was sutured after the injection (29, 36).
ROS detection.
ROS levels in isolated PVN cells were assessed using DHE, as previously described (43). PVN cells were dissociated as described previously (13). In some studies, rhodamine-labeled spinally projecting PVN neurons were first identified using FITC filter. All isolated cells were incubated with DHE (1 μmol/l) for 30 min. The fluorescence intensity was measured using a Nikon diaphot 300 inverted microscope (Nikon, Japan) equipped with a charge-coupled device (CCD) camera (Princeton Instruments, Trenton, NJ) and HE bromide filter (45). Time-resolved fluorescence was measured every 30 s with an exposure time of 100 ms using image analysis software (IPLab; Scanalytics, Fairfax, VA). For baseline measurement, ROS-dependent fluorescence was expressed as relative fluorescence units (RFU). The increase in ROS signal induced by NMDA was expressed as the ratio of Ft/Fo, where Fo is the baseline fluorescence before application of NMDA, and Ft is fluorescence in the same cell after application of NMDA (18, 43). Recordings were started after stable baseline fluorescence readings were achieved. In some experiments, cells were pretreated with the NMDA receptor antagonist MK801 (10 μmol/l), or the NOX2 peptide inhibitor gp-91ds-tat (1 μmol/l). Time control experiments in which NMDA was not perfused were performed in parallel to assure the stability of the preparation.
NO detection.
Isolated PVN cells were incubated with the NO-sensitive fluorescent dye DAF-FM (5 μmol/l; Molecular Probes) for 30 min and then rinsed for 30 min. DAF-FM imaging was performed using an inverted fluorescence microscope (Nikon) equipped with a CCD camera (Princeton Instruments) and FITC filter (45). Time-resolved fluorescence was measured every 30 s with an exposure time of 200 ms using image analysis software (IPLab, Scanalytics). For baseline measurement, NO-dependent fluorescence was expressed as RFU. Changes in NO signal induced by NMDA was expressed as the ratio Ft/Fo, where Fo is the baseline fluorescence before application of NMDA, and Ft is fluorescence in the same cell after application of NMDA (13). In some experiments, PVN cells were pretreated with the nonselective nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine (LNNA) (100 μmol/l), or the NOX2 peptide inhibitor gp-91ds-tat (1 μmol/l).
Patch recordings of NMDAR-mediated currents of PVN neurons in brain slices. Saline- and ANG II-infused mice were anesthetized with 2% isoflurane, and their brains were removed and immersed into ice-cold sucrose-artificial cerebrospinal fluid (s-aCSF) composed of (in mmol/l): 26 NaHCO3, 1 NaH2PO4, 3 KCl; 5 MgSO4, 0.5 CaCl2, 10 glucose; 248 sucrose; 95% O2 and 5% CO2, at pH 7.3. Coronal slices (200-μm thickness) containing the PVN were cut using Leica VT1000s vibratome (Leica Microsystems) and stored in a custom-designed chamber containing lactic acid (l)-aCSF with 95% O2 and 5% CO2 at 32°C for 1 h. The l-aCSF was composed of (in mmol/l): 124 NaCl, 26 NaHCO3, 5 KCl, 1 NaH2PO4, 2 MgSO4, 2 CaCl2, 10 glucose, 4.5 lactic acid, at pH 7.4. PVN slices were then transferred to a glass-bottom recording chamber and continuously perfused with gassed l-aCSF at 2 ml/min. The PVN region was identified using lateral ventricle, fornix, and optic chiasm as landmarks. Rhodamine-labeled spinal PVN neurons in brain slices were briefly identified under an E600 epifluorescence microscope (Nikon, Tokyo, Japan) with a combination of FITC filter and differential interference contrast optics. Neurons located in the medial one-third of the PVN area between the third ventricle and the fornix were patched for whole cell recording (27) using Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Because current flow through NMDAR channels is largely blocked by Mg2+ ions at resting membrane potentials (34), to elicit NMDA receptor-mediated ROS formation and inward currents maximally, before the recording, cells were superfused with a modified Mg2+-free l-aCSF (in mmol/l: 121 NaCl, 5 KCl, 1.8 CaCl2, 0.01 glycine, 1 Na-pyruvate, 20 glucose, 26 NaHCO3, 1 NaH2PO4, 4.5 lactic acid, 95% O2 and 5% CO2, at pH 7.4), and then NMDA (10–300 μmol/l)-containing buffers. To block voltage-gated Na+ channels and non-NMDA receptor-mediated cation channels, 1 μmol/l TTX and 5 μmol/l CNQX were added to the Mg2+-free l-aCSF buffer. The holding potential was at −60 mV and NMDA (30 μmol/l)-containing Mg2+-free extracellular solution was perfused toward the patched neuron for 30 s (13, 42). Patch-pipette tip resistances were 5–8 MΩ as filled with an intracellular solution (in mmol/l): 130 K-gluconate; 10 NaCl, 1.6 MgCl2, 1 EGTA, 10 HEPES, 2 Mg-ATP, adjusted to pH 7.3. After formation of a GigaΩ seal, the electrode capacitance was nullified. After breaking the patch membrane, the cell membrane capacitance (Cm) was read directly from Membrane Test of Window pClamp 8.2 (Axon Instruments, Union City, CA) and was used as an indicator of cell size. Cell membrane capacitance and series resistance were monitored throughout the recording, with series resistance generally compensated >80%. Signals were low-pass filtered at 2 kHz and acquired at a sampling rate of 5–10 kHz.
Data analysis.
Data are expressed as means ± SE. Two-group comparisons were analyzed by paired or unpaired t-test, or ANOVA two-tailed t-test. Multiple comparisons were evaluated by ANOVA followed by Tukey's post hoc test with paired test. Differences in the number of dendrites coexpressing NR1 and NOX2 in the EM study were statistically evaluated by χ2-test. For all tests, differences were considered statistically significant at P < 0.05. For dose-response relationships between ROS/NO formation and NMDA, data were collected when a stable change induced by each dose of NMDA was reached.
RESULTS
Slow-pressor ANG II infusion increases systolic arterial pressure.
Infusion of saline for 14 days did not alter systolic arterial pressure (118 ± 3 mmHg at day 0 and 120 ± 3 mmHg at day 14; P > 0.05, n = 14). However, ANG II infusion (600 ng·kg−1·min−1 sc) increased systolic arterial pressure from 117 ± 2 mmHg at day 0 to 140 ± 4 mmHg at day 14 (P < 0.01; n = 15).
ANG II increases colocalization of NOX2 and NR1 in dendrites of PVN neurons.
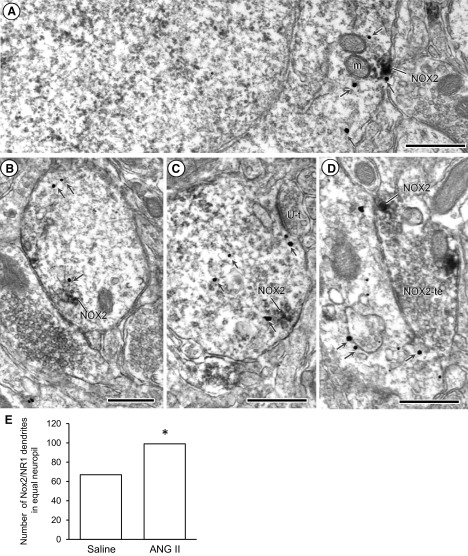
NOX2 immunoperoxidase was localized to cytoplasmic endomembranes that were often found near mitochondria and/or plasmalemmal surfaces in the PVN neuropil (Fig. 1A). NOX2-labeled somata, as well as dendrites (Fig. 1, B and C) also contained NR1 immunogold, which was affiliated with cytoplasmic endomembranes and the cell surface. Occasionally NOX2 immunoperoxidase labeling was detected in aggregates along nonsynaptic portions of the plasma membrane in axon terminals that were presynaptic to dendrites containing NR1-immunogold (Fig. 1D). A similar subcellular distribution was seen in PVN tissue processed using immunogold for NOX2 and immunoperoxidase for NR1 detection. In this tissue, there were no differences in the plasmalemmal or cytoplasmic density of NOX2 in dendrites in mice receiving ANG II compared with saline (P > 0.05). However, ANG II-recipient mice showed significantly more dually labeled NOX2/NR1 dendritic profiles (+48%, P < 0.05) than saline-recipient mice in an equal area of PVN neuropil (Fig. 1E). While NOX2 and NR1 were also often seen in proximity to each other in neuronal soma, as well as in glia, these associations were not quantitatively examined.
Fig. 1.
Electron microscopy images of the somatodendritic distribution of NADPH oxidase (NOX2; immunoperoxidase) and N-methyl-d-aspartate receptor (NMDAR) NR1 immunogold (arrows) in the paraventricular nucleus (PVN). A: NOX2 labeling is aggregated along endomembranes near a mitochondrion and the plasmalemmal surface of a neuronal soma that also shows cytoplasmic immunogold labeling for NMDA NR1. B and C: dendritic profiles show colocalization of NOX2 and NMDA NR1. D: dendrite-expressing NR1 receives a synaptic contact from an axon terminal that contains NOX2 immunoreactivity. Scale bar = 500 nm. m, mitochondria; U-t, unlabeled terminals. E: ANG II infusion increases the number of dendritic profiles dually labeled for NOX2 and NR1 in equal areas of PVN neuropil. Forty-five micrographs per animal were taken at a magnification of ×19,000. Analysis was done in a tissue area of 977 μm2 from three mice with the total area of 2,931 μm2. *P < 0.05 using χ2-square test.
ANG II does not alter NR1 expression in the PVN.
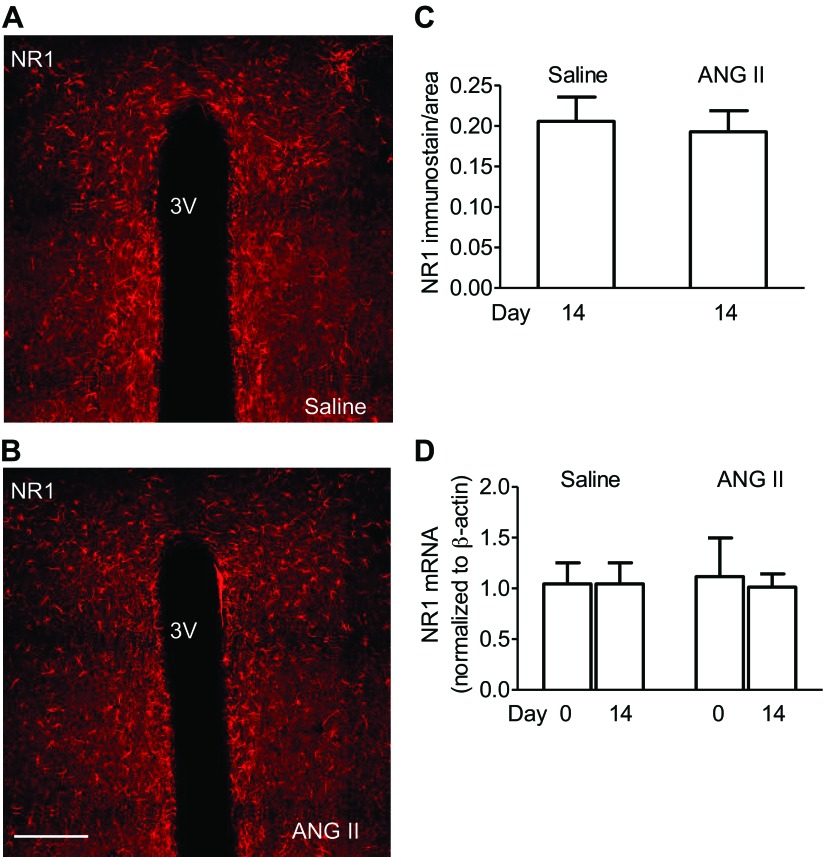
Representative confocal microscopic images show the NR1 immunoreactivity in the PVN cells from saline (Fig. 2A) and ANG-II (Fig. 2B) recipient mice. Quantitative confocal microscopy analysis demonstrated that NR1 immunoreactivity in the PVN did not differ between saline and ANG II-recipient mice (P > 0.05; 5 or 6 slices/group from 3 mice each) (Fig. 2C). In addition, no difference in NR1 mRNA levels, assessed by real-time PCR in PVN, were observed following ANG II infusion (P > 0.05 vs. saline; n = 4 mice/group) (Fig. 2D).
Fig. 2.
ANG II infusion does not alter NMDAR NR1 subunit expression in the PVN. A and B: representative confocal microscopic images of NR1 immunoreactivity in the PVN from saline and ANG-II recipient mice are shown. Scale bar = 250 μm. 3V, the third ventricle. C: histograms summarize quantitative analysis of NR1 immunolabeling in PVN sections from saline and ANG II-recipient mice. D: histograms summarize NR1 mRNA levels detected by real-time PCR in PVN tissue punched from saline and ANG II-recipient mice. Unpaired t-test or ANOVA and Tukey's paired test was used.
ANG II enhances baseline ROS and NMDAR-induced ROS production in PVN cells.
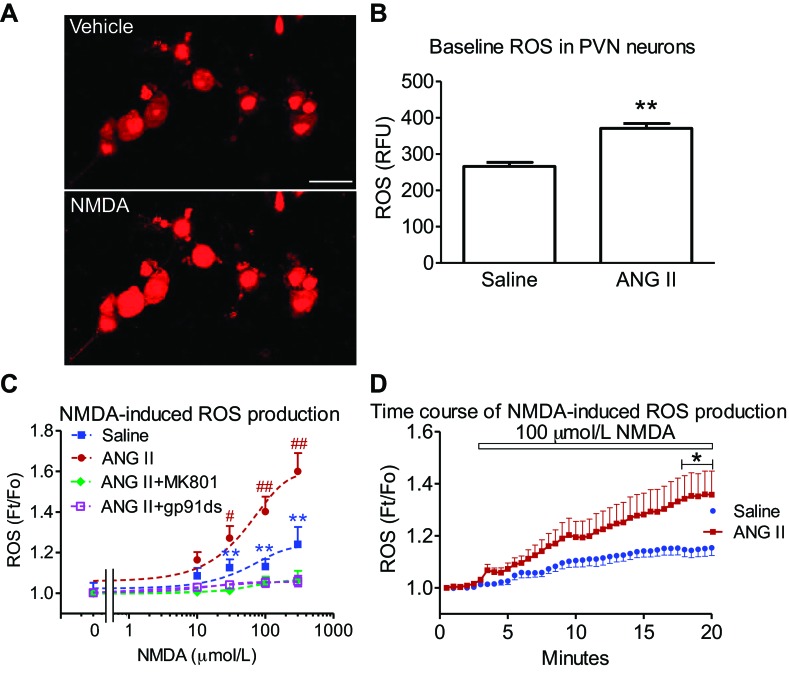
Isolated PVN neurons from ANG II-infused mice displayed higher-baseline ROS-dependent fluorescence than those infused with saline (P < 0.01 vs. saline; n = 21–25 cells/group, from three mice each) (Fig. 3B). NMDA increased ROS production in PVN cells of saline-infused mice in a dose-dependent manner (30–300 μmol/l NMDA vs. vehicle; P < 0.01; n = 14–15/group, from two mice each) (Fig. 3C). In PVN cells from ANG II-infused mice, NMDA (30–300 μM)-induced ROS productions were enhanced (P < 0.05 and P < 0.01 vs. saline; n = 14–21/group, from two mice each) (Fig. 3C). The ROS increase was gradual and reached a plateau between 15 and 20 min (P < 0.05 vs. saline, n = 9/group) (Fig. 3D). The increased ROS was blocked by pretreatment with the NMDA receptor antagonist MK801 (10 μmol/l, P < 0.01 vs. ANG II; n = 9, from two mice each) or the NADPH-oxidase peptide inhibitor gp-91ds-tat (1 μmol/l; P < 0.01 vs. ANG II; n = 8, from two mice each) (Fig. 3C). These data indicate that chronic ANG II-infusion enhances NMDAR-evoked, NADPH-oxidase-dependent ROS production in PVN neurons.
Fig. 3.
ANG II infusion enhances baseline and NMDA-mediated reactive oxygen species (ROS) production in PVN cells. A: representative DHE images of ROS-dependent fluorescence induced by NMDA (100 μmol/l) in PVN cells from ANG II-recipient mice. Scale bar = 20 μm. B: baseline ROS production in PVN cells from saline and ANG II-recipient mice is shown. **P < 0.01 vs. saline. C: NMDA increased ROS dose-dependently in isolated PVN cells of saline-infused mice, an effect abolished by MK 801 (10 μmol/l) or gp-91ds-tat (1 μmol/l). NMDA-mediated ROS production is enhanced in PVN cells of mice receiving ANG II infusion. **P < 0.01 vs. vehicle; #P < 0.05 vs. vehicle or saline-infusion; ##P < 0.01 vs. vehicle or saline-infusion using ANOVA and Tukey's test. D: effect of NMDA on the time course of ROS-dependent fluorescence assessed by DHE in PVN cells from saline and ANG II-infused mice. *P < 0.05 using unpaired t-test.
ANG II enhances baseline ROS and NMDAR-induced ROS production in spinally projecting PVN neurons.
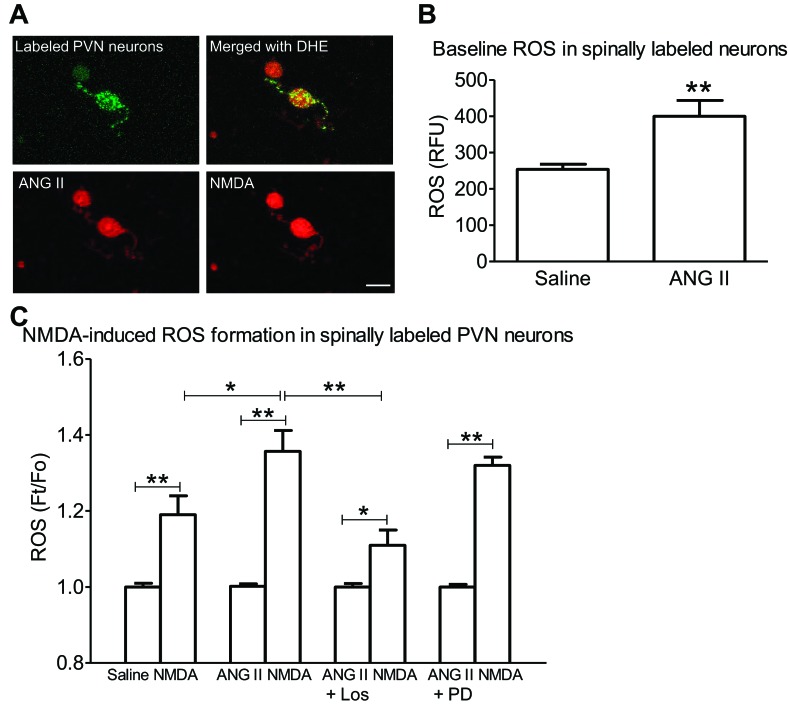
To identify spinally projecting PVN neurons, rhodamine-labeled FluoSphere were injected into the thoracic spinal cord. Isolated PVN neurons labeled with FluoSphere were identified with fluorescence illumination (Fig. 4A). These spinally labeled PVN neurons from ANG II-infused mice also exhibited higher baseline ROS than controls receiving saline (P < 0.01 vs. saline; n = 7/group, from 3 mice each). Application of NMDA (100 μM) induced ROS production in spinally labeled PVN neurons, an effect that was greater in ANG II-treated mice than in controls (NMDA vs. saline, P < 0.05; n = 7/group, from 3 mice each) (Fig. 4C). The enhancement was attenuated by in vivo treatment with the AT1R antagonist losartan (P < 0.01 vs. ANG II alone, n = 7–10/group, from three mice each), but not the AT2R antagonist PD123319 (P > 0.05 vs. ANG II alone, n = 7 or 8/group, from 3 mice each) (Fig. 4C).
Fig. 4.
ANG II enhances NMDA-induced ROS production in spinally projecting PVN neurons. A: microinjection of rhodamine-labeled microspheres into the thoracic spinal cord leads to retrograde labeling (green) of PVN neurons from an ANG II-infused mouse (top). NMDA increases ROS production in these neurons (bottom). Scale bar = 20 μm. B: baseline ROS in PVN cells is increased in ANG II-infused mice. **P < 0.01 vs. saline, unpaired t-test. C: NMDA-induced ROS production is enhanced in spinally labeled PVN neurons of ANG II-infused mice. The enhancement was attenuated in ANG II-infused mice treated with losartan (Los) (1 mg/kg ip), but not PD123319 (PD) (1 mg/kg ip). *P < 0.05. **P < 0.01 vs. saline, ANOVA and Tukey's paired test.
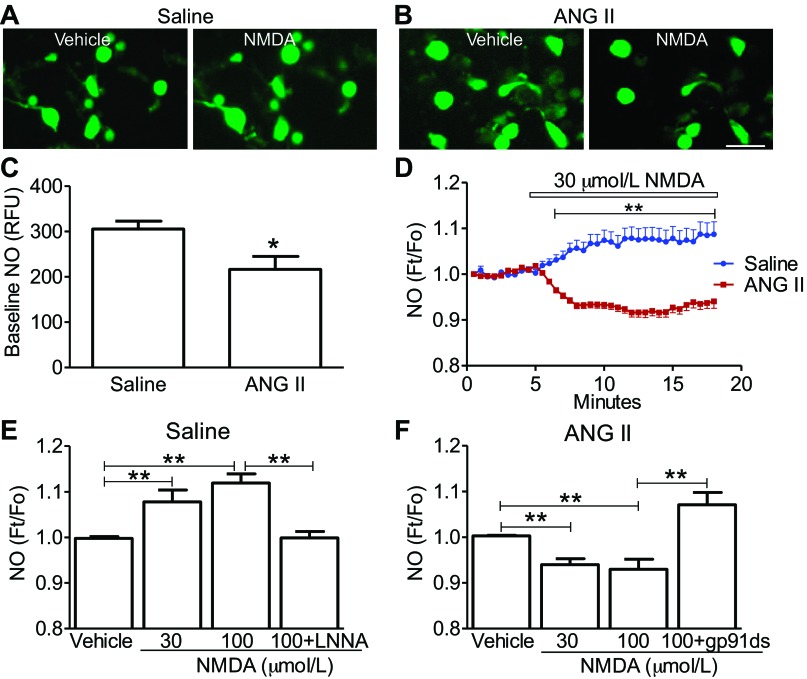
ANG II suppresses the ability of NMDAR to increase NO in PVN cells.
Modulation of NO levels and glutamatergic signaling can affect the neurohumoral output of PVN neurons (27, 40). Therefore, we examined whether ANG II-infusion alters NMDA-induced NO production in isolated PVN cells. Cells from the ANG II group displayed a lower baseline NO than the saline group (P < 0.05 vs. saline; n = 17 or 18/group, from 2 mice each) (Fig. 5C). An example of the time course of immediate changes in NO induced by NMDA in mice receiving saline or ANG II is shown in Fig. 5D. NMDA-increased NO signal in the saline-treated group (P < 0.01 for 30 μmol/l and 100 μmol/l vs. vehicle; n = 8–10/group, from two mice each), which was prevented by the coapplication with the NOS inhibitor l-NNA (P > 0.01 vs. from vehicle; n = 8 or 9/group, from two mice each) (Fig. 5E) (13). In the ANG II-treated group, however, NMDA evoked a decrease in the NO signal (P < 0.01 vs. vehicle, n = 9–10/group, from two mice each), an effect that was reversed by the NADPH-oxidase peptide inhibitor gp-91ds-tat (P < 0.01 vs. 100 μmol/l NMDA; n = 9–10/group, from two mice each) (Fig. 5F). These data indicate that ANG II infusion suppresses the ability of NMDAR to increase NO and that the effect depends on NADPH-oxidase-derived ROS.
Fig. 5.
ANG II infusion attenuates baseline nitric oxide (NO) and NMDA-induced NO production in dissociated PVN cells. A: representative images of 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM) fluorescence in PVN neurons of saline-recipient mouse are shown. NMDA increased NO-dependent fluorescence. Scale bar in A and B = 20 μm. B: representative images of DAF-FM fluorescence in PVN neurons from an ANG II-recipient mouse are shown. NMDA reduced NO-dependent fluorescence in PVN cells of ANG II-recipient mice. C: PVN cells from ANG II-infused mice have reduced NO production compared with PVN cells of mice receiving saline; *P < 0.05, using unpaired t-test. D: effect of NMDA on the time course of NO-dependent fluorescence in isolated PVN cells from saline and ANG II-treated mice; **P < 0.01 using an unpaired t-test. E: NMDA increases NO-dependent fluorescence in PVN cells of saline-infused mice, an effect prevented by the nitric oxide synthase inhibitor LNNA (100 μmol/l). **P < 0.01, using ANOVA and Tukey's test. F: NMDA reduces NO-dependent fluorescence in PVN cells of ANG II-treated mice. The suppression of NO formation is reversed by coapplied NOX2 peptide inhibitor gp-91ds-tat (1 μmol/l). **P < 0.01, using ANOVA and Tukey's test.
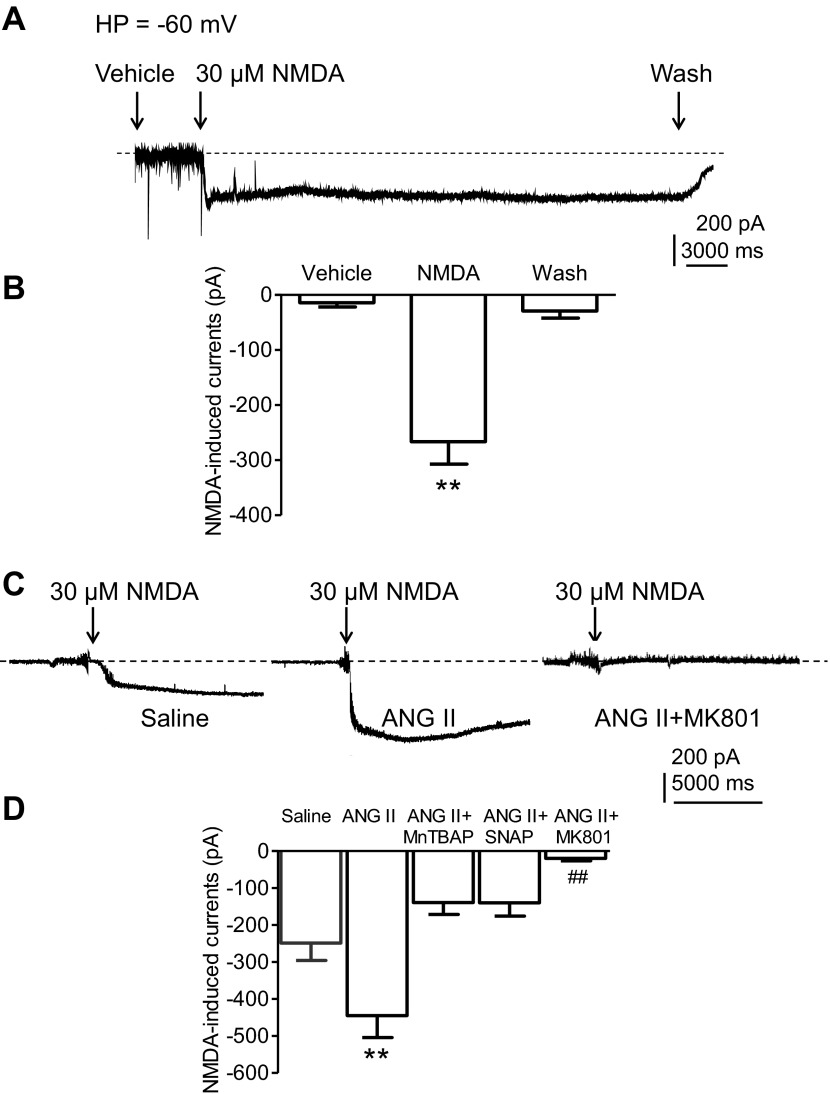
ANG II enhances NMDAR-mediated currents in PVN neurons.
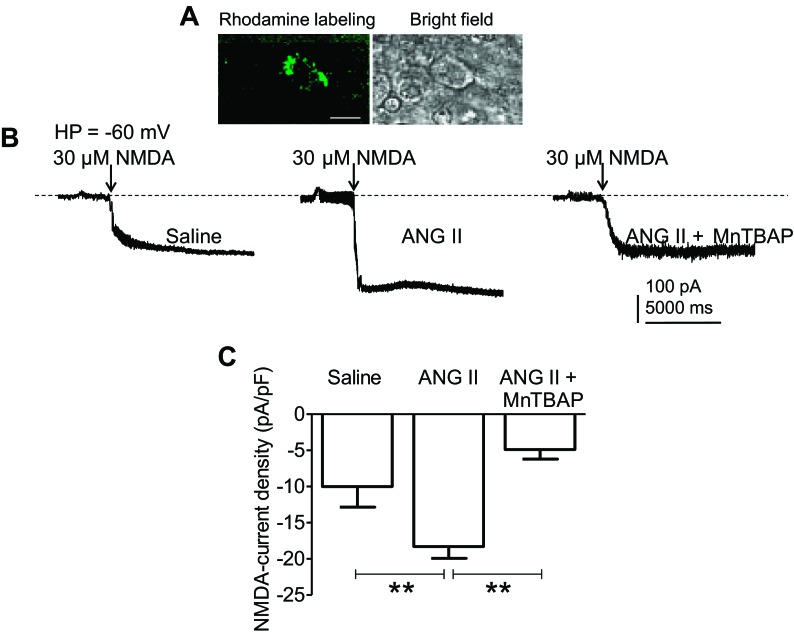
Whole cell patch-clamp recordings were performed on neurons located in the medial one-third of the PVN area between the third ventricle and the fornix, where NR1 is abundant (Fig. 2, A and B) and most spinally projecting neurons are located (27). In saline-infused mice, NMDA (30 μmol/l) reversibly induced an inward current (n = 8, from three mice), an effect reversed by washout of NMDA and blocked by MK801 (13). Such current was significantly larger in PVN neurons from ANG II-recipient mice (n = 13, from four mice) than saline-recipient mice (P < 0.01 vs. saline, n = 10, from three mice) (Fig. 6, C and D). The enhancement was blocked by the ROS scavenger MnTBAP (P < 0.01 vs. ANG II, n = 6, from two mice), the NO donor SNAP (P < 0.05 vs. ANG II, n = 7, from three mice) or MK801 (P < 0.01 vs. ANG II, n = 12, from three mice) (Fig. 6C). Spinally projecting PVN neurons labeled from thoracic cord injection of rhodamine-labeled microspheres were isolated and membrane capacitance (Cm) was measured using the whole cell configuration. No significant differences in Cm were observed in PVN neurons from saline (saline: 25.6 ± 2.1 pF; n = 4, from two mice) and ANG II-infused groups (ANG II: 27.4 ± 2.5 pF; n = 4, from two mice; P > 0.05), or MnTBAP-treated neurons from the ANG II group (24.2 ± 1.2 pF; n = 5, from two mice; P > 0.05). NMDA treatment increased current density more in neurons from the ANG II than in the saline group (P < 0.01 vs. saline; n = 5, from two mice), an effect that was prevented by MnTBAP (100 μmol/l, P < 0.01 vs. ANG II, n = 5, from three mice) (Fig. 7). These results indicate that ROS are required for enhancement of the inward current induced by NMDAR activation in PVN neurons of ANG II-infused mice.
Fig. 6.
ANG II infusion enhances NMDA-receptor-mediated currents in PVN neurons. A: representative whole cell recordings of the current triggered by NMDA (30 μmol/l) in one PVN neuron in brain slices of a saline-infused mouse are shown. The current is reversed by wash with the control buffer. B: histograms illustrate the amplitudes of NMDA-currents in PVN neurons from saline-infused mice. **P < 0.01 vs. vehicle and wash using ANOVA and Tukey's test. C: whole cell recordings show currents triggered by NMDA (30 μmol/l) in three PVN neurons in brain slices from mice treated with saline infusion (left), ANG II infusion (middle), and ANG II infusion in brain slices treated with MK801 (right) (10 μmol/l), respectively. D: histograms illustrate that an enhancement of NMDA currents in PVN neurons in brain slice is subjected to ANG II infusion. This enhancement is suppressed by MnTBAP (100 μmol/l) or SNAP (30 μmol/l). NMDA currents are blocked by MK801 (10 μmol/l) **P < 0.01 vs. saline, MnTBAP, SNAP, or MK801. ##P < 0.01 vs. saline, ANG II MnTBAP, or SNAP using ANOVA and Tukey's test.
Fig. 7.
ANG II infusion enhances NMDA receptor-mediated currents in spinally projecting PVN neurons. A: retrograde labeling of spinally projecting PVN neurons following microinjection of rhodamine-labeled microsphere into the thoracic spinal cord. Scale bar = 20 μm. B: whole cell patch-clamp recording on spinally labeled PVN neurons showing an enhancement of NMDA-induced currents in ANG II-infused mice (left and middle), an effect prevented by MnTBAP (100 μmol/l) (right). HP = −60 mV. C: enhancement of NMDA current density (pA/pF) in spinally labeled PVN neurons of ANG II-infused mice. This enhancement is suppressed by MnTBAP. **P < 0.01 vs. saline or MnTBAP, using ANOVA, and Tukey's test.
DISCUSSION
There are several novel findings in this study. First, we demonstrated that ANG II-induced slow pressor hypertension is accompanied by an increase in dendritic colocalization of NMDA NR1 subunits and NOX2 in PVN neurons. Second, we showed that ANG II hypertension is evoked in mice having an increase in NOX2-dependent ROS production and reduced NO in PVN. Third, the increase in ROS production is associated with enhanced glutamatergic NMDAR-mediated currents in PVN neurons. Collectively, these observations support the hypothesis that the hypertension induced by administration of subpresssor doses of ANG II is linked to glutamatergic-induced production of ROS, leading to reduced NO signal and facilitation of glutamatergic signaling in PVN neurons. These adaptive changes in PVN may contribute to the neurohumoral dysfunction underlying slow-pressor ANG II hypertension.
ANG II alters the subcellular distribution of NR1 and NOX2 leading to increased colocalization in PVN dendrites.
The NOX2 immunolabeling along endomembranes and plasma membrane in many somatodendritic profiles, as well as in a few axon terminals in the PVN, is consistent with the subcellular localization of NOX2 previously described in brain stem autonomic nuclei (44). However, a new finding is that ANG II increases the number of dendritic profiles dually labeled for NOX2 and NR1. This phenomenon is likely to reflect NOX2 trafficking to membrane sites of NMDA receptor activation, since there was no observed increase in mRNA expression. Dendritic mobilization of NOX2 in PVN dendrites of mice showing ANG II-induced slow pressor hypertension is consistent with our recent demonstration of NOX p47phox subunit trafficking in PVN dendrites in this hypertension model (12). These observations, collectively, suggest that ANG II slow-pressor hypertension leads to a compartmental redistribution of NMDA receptors and associated signaling molecules in PVN neurons, which may be critical for the enhanced ROS production and glutamate currents observed in these cells.
ANG II increases ROS production and reduces NO levels in PVN.
Another finding of this study is that ANG II increases baseline ROS production and enhances the ROS induced by NMDAR activation. The increase in NMDA-triggered ROS production is consistent with our ultrastructural data, indicating that NOX2 and the NMDA receptor subunit NR1 are more frequently colocalized in dendritic compartments in this model of hypertension. In contrast to ROS, baseline NO and NMDA-induced NO increases were reduced in the PVN of mice receiving ANG II infusion. ROS and NO are highly reactive mediators that are coregulated by complex mechanisms highly dependent on the microdomains in which they are produced (7, 18). In neocortical neurons, NO triggers ROS production by activating NOX2 via cGMP and PKG (18). On the other hand, in other models, ROS have been shown to reduce NO availability by reacting to form peroxynitrite (37, 45). The present results suggest that NMDA receptor activation increases both ROS and NO in the PVN of saline-infused mice. However, with ANG II infusion, the increase in ROS produced by NMDA receptor activation is enhanced and is associated with reduced cellular NO levels, suggesting that the excess of ROS acts to suppress NO (45). In support of this hypothesis, inhibition of ROS production with a NOX peptide inhibitor restores NO production in PVN cells. Furthermore, a NO donor is able to attenuate NMDA currents in PVN neurons. ANG II-derived ROS could suppress NO either by formation of peroxynitrite or by inhibiting neuronal NOS enzymatic activity (11, 35, 40). Irrespective of the mechanisms involved, the present data provide new evidence that ANG II administration alters the balance between NO and ROS in PVN, which, in turn, has with profound effects on local glutamatergic signaling.
ANG II enhances NMDA currents in PVN neurons.
We also found that ANG II increases the amplitude of NMDA currents in PVN neurons, an effect not associated with increased expression of the NR1 subunit of NMDAR. These findings raise the possibility that in PVN, not only AMPA receptors (19, 26), but also NMDAR may contribute to the heightened presympathetic activity and hypertension produced by systemic ANG II (29, 31). Indeed, the involvement of NMDAR in PVN is consistent with evidence showing that microinjection of glutamate or NMDA into the PVN increases arterial blood pressure, sympathetic nerve activity, and plasma norepinephrine concentration in rats (31). We found that bath-applied NMDA induced a noninactivating inward current, which was enhanced in neurons of ANG II-infused mice and was blocked by NMDA receptor antagonist MK801. It remains unclear, however, whether synaptic and/or extrasynaptic NMDA receptors are responsible for this current. Similar noninactivating inward currents have been reported in hypothalamic magnocellular neurons, which were mediated by activation of extrasynaptic NMDA receptors (38). Therefore, further studies will have to address the synaptic or extrasynaptic source of these currents in our preparation.
What are the mechanisms by which ANG II infusion increases NMDA currents in PVN? ROS are well known to modulate NMDA currents (23, 24), which may explain why NMDA triggered larger ionic currents in ANG II-infused PVN neurons. Furthermore, local ROS production in dendritic segments may regulate the NMDAR activity by inactivating protein tyrosine phosphatase or by associations with lipid rafts and endosomes that are involved in NMDA receptor trafficking (14). NO could act to suppress NMDA currents by nitrosylating its redox modulatory site (1). Therefore, both increased ROS and reduced NO may participate in the upregulation of synaptic NMDA currents induced by ANG II. Taken together, our data suggest that increased ROS production, reduced NO, and enhanced NMDAR currents are critical steps in the increase in the output of presympathetic PVN neurons, which serve the neurohumoral dysfunction mediating ANG II slow-pressor hypertension.
Perspectives and Significance
We studied the role of NMDAR in the regulation of ROS and NO homeostasis in PVN neurons in the slow pressor ANG II hypertension model in mice. We found that NMDAR NR1 and NOX2 subunits are colocalized at postsynaptic dendrites of the PVN, especially those from ANG II hypertensive mice. NMDAR-mediated ROS production was significantly enhanced, but NMDAR-induced NO formation was attenuated in PVN neurons from ANG II-recipient mice. These effects on ROS and NO have an impact on NMDAR-mediated inward currents in PVN neurons, because both ROS and NO counterbalance NMDAR-mediated currents enhanced by ANG II infusion. Similar enhancements of NMDAR-mediated ROS and currents were also observed in spinally projecting PVN neurons isolated from ANG II-recipient mice. These results suggest that NMDAR-mediated NO production may offset the deleterious effects of NMDAR-mediated ROS in the PVN, possibly, by suppressing NMDAR-mediated currents. Our findings unveil a previously unrecognized mechanism underlying the interaction between ROS and NO in the PVN during hypertension. Such an interaction may be relevant to clinical conditions like hypertension and cardiac failure, in which the balance between central NO and ROS is altered (20, 27, 40, 46). However, the functional significance of the interaction between NMDAR-mediated NO and ROS in the regulation of sympathetic outflow and blood pressure remains to be defined. Further studies using in vitro and in vivo approaches will be required to address these issues.
GRANTS
Support was provided for this article by National Institute of Health Grant HL-096571.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.W., R.L.D., C.I., and V.M.P. conception and design of research; G.W., C.G.C., J.C., G.F., J.M.-L., T.A.M., M.R.G., and J.A. performed experiments; G.W., C.G.C., J.C., G.F., M.R.G., J.A., C.I., and V.M.P. analyzed data; G.W., C.G.C., J.A., R.L.D., C.I., and V.M.P. interpreted results of experiments; G.W., C.G.C., J.A., C.I., and V.M.P. prepared figures; G.W., C.I., and V.M.P. drafted manuscript; G.W., C.G.C., R.L.D., C.I., and V.M.P. edited and revised manuscript; G.W., C.I., and V.M.P. approved final version of manuscript.
REFERENCES
- 1. Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci 25: 510–517, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Aicher SA, Punnoose A, Goldberg A. mu-Opioid receptors often colocalize with the substance P receptor (NK1) in the trigeminal dorsal horn. J Neurosci 20: 4345–4354, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bains JS, Ferguson AV. Nitric oxide regulates NMDA-driven GABAergic inputs to type I neurones of the rat paraventricular nucleus. J Physiol 499: 733–746, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol Regul Integr Comp Physiol 268: R625–R633, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol 77: 3396–3400, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Bowers G, Cullinan WE, Herman JP. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J Neurosci 18: 5938–5947, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci 12: 857–863, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burmeister MA, Young CN, Braga VA, Butler SD, Sharma RV, Davisson RL. In vivo bioluminescence imaging reveals redox-regulated activator protein-1 activation in paraventricular nucleus of mice with renovascular hypertension. Hypertension 57: 289–297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao X, Peterson JR, Wang G, Anrather J, Young CN, Guruju MR, Burmeister MA, Iadecola C, Davisson RL. Angiotensin II-dependent hypertension requires cyclooxygenase 1-derived prostaglandin E2 and EP1 receptor signaling in the subfornical organ of the brain. Hypertension 59: 869–876, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, Pickel VM, Davisson RL, Iadecola C. Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin II hypertension. J Neurosci 32: 4878–4886, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng WH, Lu PJ, Ho WY, Tung CS, Cheng PW, Hsiao M, Tseng CJ. Angiotensin II inhibits neuronal nitric oxide synthase activation through the ERK1/2-RSK signaling pathway to modulate central control of blood pressure. Circ Res 106: 788–795, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Coleman CG, Wang G, Faraco G, Marques Lopes J, Waters EM, Milner TA, Iadecola C, Pickel VM. Membrane trafficking of NADPH oxidase p47phox in paraventricular hypothalamic neurons parallels local free radical production in angiotensin II slow-pressor hypertension. J Neurosci 33: 4308–4316, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coleman CG, Wang G, Park L, Anrather J, Delagrammatikas GJ, Chan J, Zhou J, Iadecola C, Pickel VM. Chronic intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus. J Neurosci 30: 12103–12112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delint-Ramirez I, Fernandez E, Bayes A, Kicsi E, Komiyama NH, Grant SG. In vivo composition of NMDA receptor signaling complexes differs between membrane subdomains and is modulated by PSD-95 and PSD-93. J Neurosci 30: 8162–8170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology 89: 370–376, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus—a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets 12: 717–727, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1997 [Google Scholar]
- 18. Girouard H, Wang G, Gallo EF, Anrather J, Zhou P, Pickel VM, Iadecola C. NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci 29: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herman JP, Eyigor O, Ziegler DR, Jennes L. Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat. J Comp Neurol 422: 352–362, 2000 [PubMed] [Google Scholar]
- 20. Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-κB activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res 82: 503–512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol Regul Integr Comp Physiol 256: R1325–R1330, 1989 [DOI] [PubMed] [Google Scholar]
- 22. Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res 95: 1019–1026, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem 94: 299–306, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol 80: 452–457, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal 11: 2607–2619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li DP, Byan HS, Pan HL. Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci 32: 372–380, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li DP, Chen SR, Pan HL. Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic GABA release. J Neurophysiol 88: 2664–2674, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Li DP, Pan HL. Increased group I metabotropic glutamate receptor activity in paraventricular nucleus supports elevated sympathetic vasomotor tone in hypertension. Am J Physiol Regul Integr Comp Physiol 299: R552–R561, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol 586: 1637–1647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li H, Gao Y, Grobe JL, Raizada MK, Katovich MJ, Sumners C. Potentiation of the antihypertensive action of losartan by peripheral overexpression of the ANG II type 2 receptor. Am J Physiol Heart Circ Physiol 292: H727–H735, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 291: H2847–H2856, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Llewellyn T, Zheng H, Liu X, Xu B, Patel KP. Median preoptic nucleus and subfornical organ drive renal sympathetic nerve activity via a glutamatergic mechanism within the paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 302: R424–R432, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lob HE, Schultz D, Marvar PJ, Davisson RL, Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension 61: 382–387, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309: 261–263, 1984 [DOI] [PubMed] [Google Scholar]
- 35. Melaragno MG, Fink GD. Role of ANG II in hypertension produced by chronic inhibition of nitric oxide synthase in conscious rats. Am J Physiol Heart Circ Physiol 271: H806–H811, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Milner TA, Thompson LI, Wang G, Kievits JA, Martin E, Zhou P, McEwen BS, Pfaff DW, Waters EM. Distribution of estrogen receptor beta containing cells in the brains of bacterial artificial chromosome transgenic mice. Brain Res 1351: 74–96, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J 31: 2741–2748, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Astrocytes modulate a postsynaptic NMDA-GABAA-receptor crosstalk in hypothalamic neurosecretory neurons. J Neurosci 33: 631–640, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ Res 89: 408–414, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Sharma NM, Zheng H, Mehta PP, Li YF, Patel KP. Decreased nNOS in the PVN leads to increased sympathoexcitation in chronic heart failure: role for CAPON and ANG II. Cardiovasc Res 92: 348–357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol 494: 673–685, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suh YH, Terashima A, Petralia RS, Wenthold RJ, Isaac JT, Roche KW, Roche PA. A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat Neurosci 13: 338–343, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension 48: 482–489, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci 24: 5516–5524, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang G, Coleman CG, Glass MJ, Zhou P, Yu Q, Park L, Anrather J, Pickel VM, Iadecola C. Angiotensin II type 2 receptor-coupled nitric oxide production modulates free radical availability and voltage-gated Ca2+ currents in NTS neurons. Am J Physiol Regul Integr Comp Physiol 302: R1076–R1083, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xue B, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK. PVN adenovirus-siRNA injections silencing either NOX2 or NOX4 attenuate aldosterone/NaCl-induced hypertension in mice. Am J Physiol Heart Circ Physiol 302: H733–H741, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension 58: 966–973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol 448: 217–229, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res 95: 532–539, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004 [DOI] [PubMed] [Google Scholar]