Abstract
The kidneys maintain extracellular K+ homeostasis by altering K+ excretion to match K+ intake. Because this can occur without changes in plasma K+ concentrations ([K+]), how the kidneys sense K+ intake is unclear. We tested the hypothesis that the pituitary plays a critical role in signaling K+ intake to the kidneys. If this hypothesis is true, hypophysectomy would impair kidney responses to altered K+ intake. Hypophysectomized (Hypox) and sham-operated control rats (n = 8 each) were compared for their abilities to adjust K+ excretion during a transition from normal to reduced (to one-third of normal) K+ intake, followed by a reversal to normal K+ intake. Food was provided only at night, and renal K+ excretion was determined both for absorptive (night or feeding) and postabsorptive (day or nonfeeding) periods. In normal rats, both absorptive and postabsorptive renal K+ excretion were changed in parallel to the changes in K+ intake, indicating a rapid adaptation of normal kidneys to altered K+ intake. In Hypox rats, whereas absorptive renal K+ excretion was changed in response to changes in K+ intake, postabsorptive K+ excretion was not responsive (P < 0.001), indicating impaired renal responses to altered K+ intake. In addition, Hypox rats, compared with control rats, showed K+ intolerance (increases in plasma [K+]) upon feeding (i.e., K+ intake) at night or following an intravenous K+ infusion (P < 0.01), indicating an impairment of acute renal responses to K+ intake. These data support that the pituitary plays a key role in the signaling of K+ intake to the kidneys (and kidney responses to altered K+ intake).
Keywords: potassium excretion, potassium sensing, potassium tolerance, brain, gut factor
k+ homeostasis is critical for normal cardiovascular and neuromuscular function (2, 14). Extracellular K+ homeostasis is maintained by renal and extrarenal mechanisms. The kidneys have a remarkable capacity to regulate K+ excretion to match K+ intake (13, 32); K+ excretion is increased with increased K+ intake and is dramatically suppressed during K+ deprivation. This function of the kidney is critical for chronic K+ balance and has been well recognized for several decades (8, 15). In addition, extrarenal tissues, such as liver and skeletal muscle, provide K+ buffering capacity by moving K+ into and out of extracellular fluid, which is critically important in the acute regulation of extracellular K+ after a meal (2, 19). Our previous studies (5–7) demonstrated that extrarenal tissues also respond to altered K+ intake: insulin-stimulated cellular K+ uptake is profoundly suppressed during K+ deprivation. Thus, both renal K+ excretion and extrarenal cellular K+ uptake are suppressed during K+ deprivation, indicating concerted mechanisms for extracellular K+ conservation when K+ intake is reduced. We found a significant correlation between the degrees of change in renal and extrarenal K+ handling with decreased K+ intake (6), suggesting a common signal or factor for K+ regulation by the kidneys and by other tissues. Importantly, these changes occurred without changes in plasma levels of K+ ([K+]) or aldosterone (5, 6), the classic regulators of renal K+ excretion, when K+ intake was reduced to one-third of normal. These observations suggest that there may be a previously unidentified factor(s) that regulates renal and/or extrarenal K+ handling in response to altered K+ intake.
How do the kidneys respond to altered K+ intake when there are no changes in plasma K+ or aldosterone level? One possibility is that dietary K+ intake is sensed by the gut, and the gut releases a signaling peptide or hormone to increase renal K+ excretion. To test this possibility, we previously examined a few candidate peptides but failed to identify such a gut peptide (23). Alternatively, the gut may send the signal of K+ intake to the brain, and the brain may regulate renal K+ excretion. There is substantial evidence that the brain is involved in the regulation of K+ excretion, and some of this regulation is mediated by pituitary peptides (25). An excellent example is the regulation of kidney function by the brain following acute uninephrectomy. Uninephrectomy in anesthetized rats causes approximately twofold increases in Na+ and K+ excretion in the remaining kidney. A series of studies by Humphreys and colleagues (11, 17, 18, 29, 30) established that these effects are mediated by γ-melanocyte-stimulating hormone (γ-MSH) secreted by the pituitary after uninephrectomy and that an afferent neural pathway from the kidney plays an important role. We propose that the brain monitors dietary K+ intake (input) and makes adjustments in renal K+ excretion (output) to maintain K+ balance; the sensing of K+ intake in the gut may be transmitted to the hypothalamus via an afferent neural pathway, as is the signal from the kidney, and the hypothalamus may regulate renal K+ excretion via pituitary hormones (25, 20, 21). If this hypothesis is true, hypophysectomy would impair the signaling of K+ intake to the kidneys, and, as a result, the kidneys would lose their ability to adjust K+ excretion in response to altered K+ intake. In addition, with impaired signaling of K+ intake, the kidneys of hypophysectomized (Hypox) animals would be in K+ conservation states, resulting in reduced K+ excretion and/or a risk for hyperkalemia with sudden increases in K+ intake. The present study was designed to test whether all of these expectations are met.
Responses of the kidneys to altered K+ intake have often been studied with very large increases in K+ intake or total K+ deprivation. These conditions are associated with significant changes in plasma K+ and aldosterone concentrations, which would result in secondary changes and thereby confound the interpretation of the findings. As mentioned above, we demonstrated that a modest decrease in K+ intake (i.e., to one-third of normal) in rats was accompanied by downregulation of renal K+ excretion without changes in plasma K+ or aldosterone levels (5, 6). Such experimental conditions offered an excellent opportunity to study kidney responses to altered K+ intake independent of plasma K+ or aldosterone. In the present study, Hypox and sham-operated control rats were compared for their abilities to adjust K+ excretion during a transition from normal to reduced (to one-third of normal) K+ intake, followed by a reversal to normal K+ intake. In addition, plasma [K+] and K+ excretion profiles were compared between control and Hypox rats during feeding at nights or an intravenous K+ infusion.
METHODS
Animals.
Hypox and sham-operated Wistar rats (weighing 250–300 g) were obtained from Charles River Laboratories (Wilmington, MA). Animals were individually housed under controlled temperature (22 ± 2°C) and lighting (12-h light, 6 AM–6 PM; 12-h dark, 6 PM–6 AM) with free access to water. Food was given only at night (6 PM–8 AM; see below). Hypox rats ate less than normal rats and were provided 5% dextrose water at night, that is, during feeding. This increased water intake and urine flow in Hypox rats. During the day (without feeding and without dextrose in water), water intake, plasma osmolality, and plasma sodium and potassium concentrations were all normal in hypophysectomized rats, consistent with previous studies showing that a portion of pituitary stalk remains after hypophysectomy to secrete vasopressin and maintain normal water and electrolyte balances. Hypox rats were studied 2–4 wk after hypophysectomy. Hypophysectomy was validated by a weight screen; rats are expected to exhibit no weight gain (vs. 20–30 g per week for sham controls) after a successful hypophysectomy. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Southern California.
Feeding with different K+ diets.
Hypox and sham control rats underwent three sequential periods of different K+ intake, 3 days of normal, 3 days of reduced (to one-third of normal), and the final 2 days of normal K+ intake. The animals were fed only at night to allow measurement of both absorptive (during feeding) and postabsorptive (during nonfeeding) urinary K+ excretion (see below). During the initial period of normal K+ intake, the control animals were fed ad libitum with diets prepared from K+-deficient powdered rat chow (TD 88239; Harlan Teklad, Madison, WI), supplemented with 1% K+. The diet was gelled by dissolving 30 g agarose by heating in 500 ml of deionized water and adding to 500 g of powdered diet (22). Gelled diets were cut in small blocks and stored at −4°C until use. Hypox rats consumed significantly less diet, and, to match K+ intake between control and Hypox rats, the K+ content of the diet for Hypox rats was increased to 2%, and additional K+ was provided in drinking water. During the period of reduced K+ intake, all of the K+ contents in the diets and drinking water (Hypox rats) were reduced to one-third of those during the initial control (i.e., normal K+ intake) period. Finally, during the final period of normal K+ intake, K+ contents in the diets and drinking water were restored to those during the initial control period.
Measurement of absorptive and postabsorptive K+ excretion.
To study whether altered K+ intake has differential effects on absorptive vs. postabsorptive K+ excretion, the animals were fed only at night; diets were provided at 6 PM and removed at 8 AM on the following day. Urinary K+ excretion was determined every day for absorptive (6 PM–8 AM) and postabsorptive (10 AM–6 PM) periods, the latter of which began 2 h after food removal. The animal cages had a wire floor, and a mesh screen was placed underneath the wire floor to separate feces from urine passed and avoid the contamination of urine by fecal K+ (16).
Catheterization.
In some experiments, Hypox and sham control rats were studied for plasma [K+] responses to feeding at night or an intravenous K+ infusion. For these experiments, tail-artery and/or tail-vein catheters were placed for blood sampling and/or K+ infusion, respectively. Tail-vein catheters were placed the day before the experiment, and tail-artery catheters were placed on the morning of the experiment (∼7 AM), as previously described (6, 16). To protect tail blood-vessel catheters during the experiments, the animals were placed in individual cages with tail restraints and adapted to the conditions for at least 4 days before the experiment. The animals were free to move about and were allowed unrestricted access to food (when provided) and water.
Measurement of K+ tolerance.
The effects of intravenous K+ infusion on plasma [K+] and renal K+ excretion were studied in Hypox and sham control rats. The experiments were performed in the postabsorptive state, starting at ∼1 PM. K+ was infused as 300 mM KCl (16) for 2 h at a constant rate of 100 mg·kg−1·h−1. The K+ infusion occurred via a tail vein. Blood samples were collected for determination of plasma [K+] at variable intervals during 2-h preinfusion, 2-h K+ infusion, and 3-h washout periods. Urinary K+ excretion was also determined during these periods by collecting urine every hour throughout the experiment using specially designed cages. The animal cages had a wire floor but were open at the bottom and were placed on an aluminum tray. The tray was replaced every hour to collect urine. To avoid the contamination of urine passed by fecal K+, a mesh screen was placed underneath the wire floor to separate feces from urine. To obtain a constant flow of urine, animals were infused with a constant volume (4 ml/h) of fluid (saline or saline + KCl during the K+ infusion period) throughout the experiment. This volume infusion did not appear to affect K+ excretion (16). In some experiments in Hypox rats, corticosterone was intravenously infused at a rate of 15–20 μg/h for 24 h prior to the K+ infusion experiment to restore plasma corticosterone levels. Animals were killed by an overdose of pentobarbital sodium at the end of experiment.
Assays and calculations.
Plasma and urine K+ and Na+ levels were determined by flame photometry using a Radiometer FLM 3 flame photometer, as previously reported (6, 16). Plasma corticosterone levels were analyzed using an ELISA kit from AssayPro (St. Charles, MO). In the K+ tolerance experiments, the basal rate of urinary K+ excretion was calculated by averaging the values determined hourly during the 2-h preinfusion period. Increases in urinary K+ excretion (ΔUKV) were then calculated as the sum of urinary K+ excretions during the 2-h K+ infusion and the 3-h washout periods minus the 5-h equivalent of basal K+ excretion (i.e., basal K+ excretion × 5 h). Basal plasma [K+] was calculated as the average of the preinfusion and final-hour plasma [K+]. Total area under the plasma [K+] curve (tAUCK) was calculated using the trapezoidal method during the 2-h K+ infusion and the 3-h washout periods. Area under the [K+] curve above basal (ΔAUCK) was then calculated by subtracting the area under the baseline (i.e., basal plasma [K+] × 5 h) from tAUCK. The ratio of ΔUKV to ΔAUCK then indicated the efficiency of the kidneys to increase K+ excretion in response to increases in plasma [K+] (16).
Statistical analysis.
Data are expressed as means ± SE. The significance of differences in mean values between groups was assessed by Student's t-test. Differences were considered significant at P < 0.05.
RESULTS
K+ intake.
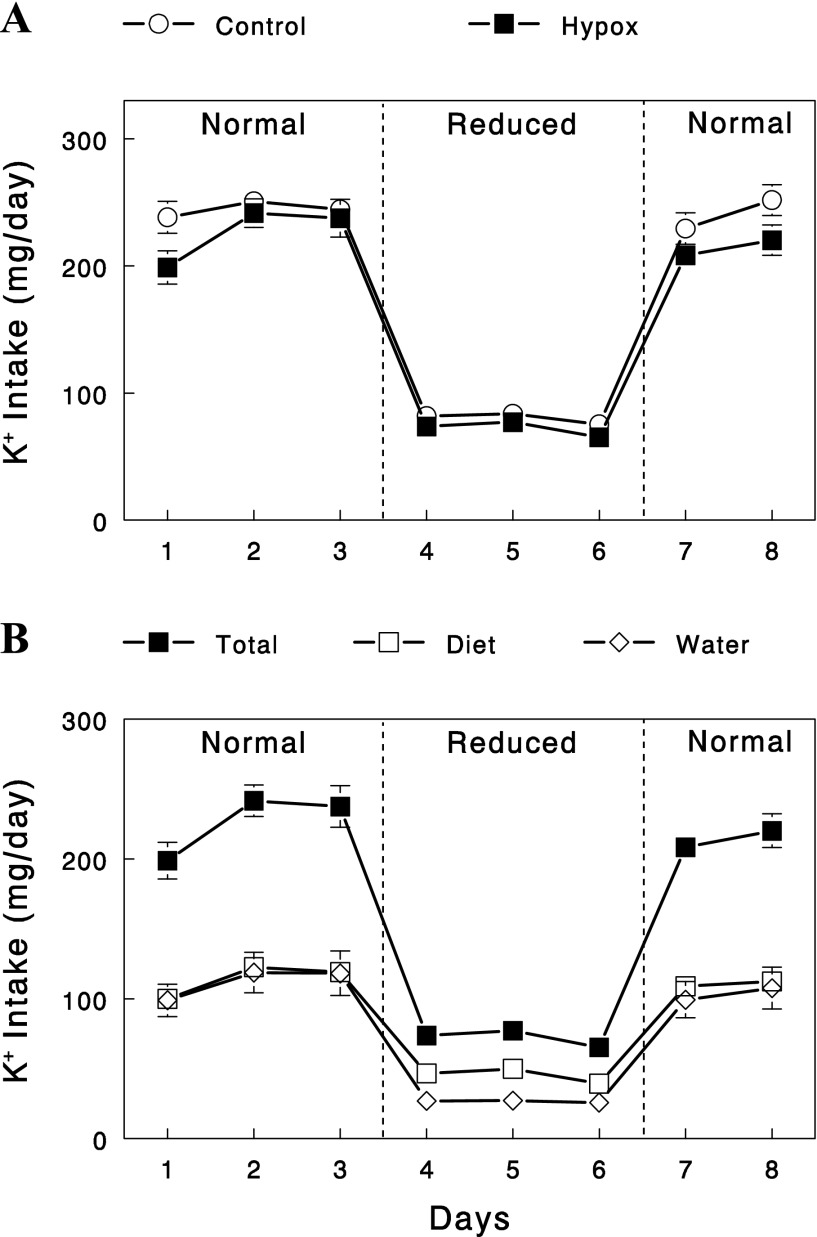
Control and Hypox rats underwent three sequential periods of different K+ intake, 3 days of normal, 3 days of reduced, and the final 2 days of normal K+ intake (Fig. 1). During the initial period of normal K+ intake, the control animals were fed ad libitum with a diet containing 1% K+. The amount of diet consumed was about ∼24.5 g per day, resulting in K+ intake of about ∼245 mg/day. Hypox rats consumed significantly less food (∼5.7 g/day). To match K+ intake between control and Hypox rats, the K+ content in their diet for Hypox rats was increased to 2%, and additional K+ was provided in drinking water. Figure 1 shows that K+ intake was matched between control and Hypox rats, and in Hypox rats, K+ intake occurred equally via diet and drinking water. During the period of reduced K+ intake, all of the K+ contents in diets and drinking water (Hypox rats) were reduced to one-third of those during the initial control period. Finally, during the final period of normal K+ intake, K+ contents in diets and drinking water were restored to those during the initial control period. K+ intake, estimated on the basis of diet and water consumption, showed three distinct periods of normal, reduced (to one-third of normal), and normal K+ intake as designed, and this pattern was well matched between control and Hypox rats (Fig. 1A).
Fig. 1.
A: changes in daily K+ intake in sham-operated (control) and hypophysectomized (Hypox) rats during the sequential periods of normal, reduced, and normal K+ intake. Food was available only at night (6 PM–8 AM). B: changes in daily K+ intake via diet vs. water in Hypox rats. Food and K+-containing water were available only at night. Data are means ± SE for eight experiments for each group.
K+ excretion during feeding at night (absorptive K+ excretion).
Absorptive K+ excretion was determined every day during feeding at night (6 PM–8 AM). In control animals, absorptive K+ excretion was decreased when K+ intake was reduced and was restored when K+ intake was normalized (Fig. 2). The changes in K+ excretion were parallel to the changes in K+ intake, and the adaptation of the kidney to altered K+ intake was very rapid, completed within a day of changes in K+ intake, consistent with previous studies (12, 27, 28). A similar pattern of changes in absorptive K+ excretion was observed in Hypox rats. Although K+ excretion was slightly higher during the initial control period, the percent changes in K+ excretion in responses to altered K+ intake were identical between control and Hypox rats (Fig. 2B).
Fig. 2.
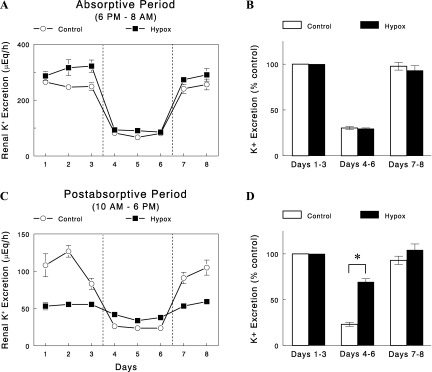
A and B: changes in absorptive (6 PM–8 AM) renal K+ excretion in control and Hypox rats. C and D: changes in postabsorptive (10 AM–6 PM) renal K+ excretion in control (○ and open bars) and Hypox (■ and closed bars) rats. Data are expressed as means ± SE for eight experiments for each group. *P < 0.001 vs. control.
K+ excretion during daytime (postabsorptive K+ excretion).
K+ excretion was also determined during postabsorptive or nonfeeding periods during daytime (10 AM–6 PM). In control animals, postabsorptive K+ excretion, like absorptive K+ excretion, was altered as K+ intake was changed (Figs. 2C). The changes in postabsorptive K+ excretion were parallel to the changes in K+ intake. Thus, both absorptive and postabsorptive K+ excretion were altered in parallel to the changes in K+ intake. In Hypox rats, two distinct differences were noted. First, postabsorptive K+ excretion was substantially lower (by ∼50%, P < 0.01) during the periods of normal K+ intake (but not during the period of reduced K+ intake). Second, the changes in K+ excretion in response to reduced K+ intake were substantially smaller; postabsorptive K+ excretion was decreased by 77% in control rats, in parallel to the decrease in K+ intake, but decreased only by 31% in Hypox rats (Fig. 2D, P < 0.001 vs. control). Thus, when measured during postabsorptive periods, kidney responses to altered K+ intake were substantially impaired in Hypox rats. This was in contrast to the apparently normal responses of absorptive K+ excretion, measured at night during feeding, to altered K+ intake.
Plasma [K+] during feeding at night.
We next examined whether the apparently normal responses of K+ excretion in Hypox rats at nights were associated with any changes in plasma [K+]. Plasma [K+] was measured in control and Hypox rats before, during, and after a feeding period at night (Fig. 3A). Plasma [K+] was comparable between the groups in the postabsorptive state (i.e., during daytime). Feeding, which was started at 6 PM, only slightly increased plasma [K+] in control rats; average plasma [K+] during the first 4 h of feeding was 4.6 meq/l, only 0.2 meq/l higher than that prior to feeding (4.4 meq/l; Fig. 3B). In contrast, in Hypox rats, plasma [K+] increased substantially within an hour of feeding and remained elevated throughout the feeding period; average plasma [K+] during the first 4 h of feeding was 5.6 meq/l, significantly higher than that prior to feeding (4.6 meq/l; P = 0.01). Thus, the increases in plasma [K+] were five-fold larger in Hypox rats than in control rats during feeding at night (P < 0.01), indicating impaired K+ homeostasis in Hypox rats.
Fig. 3.
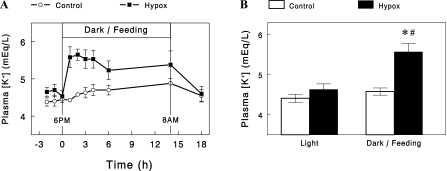
A: time courses of changes in plasma [K+] during feeding at night in control and Hypox rats. B: average plasma [K+] before (light) and during feeding (night; first 4 h) in control and Hypox rats. Data are expressed as means ± SE for four experiments for each group. *P < 0.01 vs. control; #P = 0.01 vs. light.
Na+ excretion.
Na+ excretion during absorptive periods was higher in control than in Hypox rats (Fig. 4A, P < 0.001), as expected from the lower food (and thus Na+) intake in Hypox rats. In contrast, postabsorptive Na+ excretion was similar between the two groups (Fig. 4B), suggesting that impaired postabsorptive K+ excretion in these rats (with normal K+ intake) was not due to altered renal Na+ transport or excretion. Na+ excretion (both absorptive and postabsorptive) was fairly constant throughout the three periods of different dietary K+ intake in both control and Hypox rats. Thus, changing K+ intake did not produce any appreciable alteration in Na+ excretion, suggesting independent homeostatic control for Na+ and K+.
Fig. 4.
Changes in absorptive (A: 6 PM–8 AM) and postabsorptive (B: 10 AM–6 PM) renal Na+ excretion in control and Hypox rats. Data are expressed as means ± SE for eight experiments for each group.
Impaired K+ tolerance in Hypox rats.
As discussed in the introduction, Hypox animals may be in K+ conservation states with impaired signaling of K+ intake. To test this idea, plasma [K+] and renal K+ excretion was measured in control and Hypox rats before and during an intravenous K+ infusion (100 mg·kg−1·h−1) in postabsorptive (nonfeeding) states. Prior to the K+ infusion, plasma [K+] was similar between the groups, but renal K+ excretion and clearance were lower in Hypox than in control rats (Fig. 5, A–C). During the 2-h K+ infusion, plasma [K+] increased in both groups, but the increases were substantially larger in Hypox rats. Renal efficiency for K+ excretion in response to increases in plasma [K+], calculated as increases in renal K+ excretion normalized to increases in plasma [K+] (see methods), was substantially lower in Hypox rats during K+ infusion (P < 0.01). Thus, reduced renal efficiency for K+ excretion caused K+ intolerance (i.e., increases plasma [K+]) in Hypox rats. Interestingly, these changes in K+ intolerance were similar to those in normal rats maintained on one-third of normal K+ intake for 3 days (Fig. 5D). Thus, the absence of pituitary function in Hypox rats produced phenotypes similar to those in normal rats, in which K+ intake (and its signaling) was reduced (see discussion).
Fig. 5.
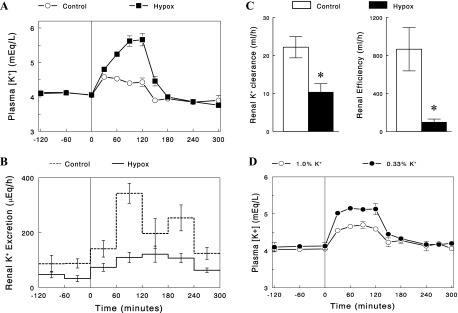
Changes in plasma [K+] (A) and renal K+ excretion (B), as well as renal K+ clearance (C) before and during an intravenous K+ infusion (100 mg·kg−1·h−1) in control and Hypox rats. C: renal K+ clearance (left) was calculated as UKV/[K+] in the basal state (prior to K+ infusion), and renal efficiency for K+ excretion (the ratio of ΔUKV to ΔAUCK; right) during K+ infusion was calculated as described in methods (open bars for control and solid bars for Hypox rats). D: changes in plasma [K+] before and during an intravenous K+ infusion (100 mg·kg−1·h−1) in normal rats maintained on a normal 1% K+ diet or a low (0.33%) K+ diet for 3 days. Data are expressed as means ± SE (n = 4 or 5). *P < 0.01 vs. control.
Effects of corticosterone treatment on K+ tolerance in Hypox rats.
Plasma corticosterone levels were low in Hypox rats, compared with control rats (20 ± 3 vs. 173 ± 20 ng/ml). Because glucocorticoids are known to affect renal K+ excretion (31, 33), we next tested whether restoration of corticosterone levels reverses reduced renal K+ excretion and/or K+ intolerance in Hypox rats. Corticosterone was intravenously infused at a rate of 15–20 μg/h for 24 h prior to the intravenous K+ infusion. The corticosterone infusion increased plasma corticosterone to levels (220 ± 46 ng/ml) statistically not different from the control levels (P > 0.05), which had no significant effects on plasma [K+] or renal K+ excretion in the postabsorptive state or during the K+ infusion (Fig. 6; P > 0.05).
Fig. 6.
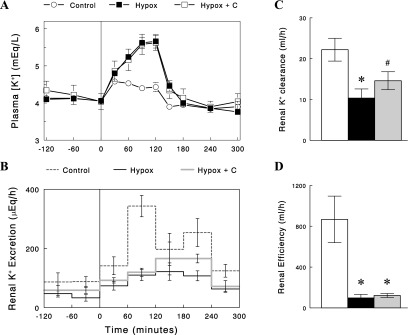
Changes in plasma [K+] (A) and renal K+ excretion (B) before and during an intravenous K+ infusion (100 mg·kg−1·h−1) in Hypox rats treated with corticosterone (C) for 24 h (Hypox + C). The data in control and Hypox rats (untreated) are the same as those used in Fig. 5. Renal K+ clearance (C) was calculated as UKV/[K+] in the basal state (prior to K+ infusion), and renal efficiency (D) for K+ excretion (the ratio of ΔUKV to ΔAUCK) during K+ infusion was calculated as described in methods (open bars for control, solid bars for Hypox rats, and gray bars for Hypox rats treated with corticosterone). Data are expressed as means ± SE; n = 4 or 5. *P < 0.01 vs. control. #P < 0.05 vs. control (one-tailed t-test).
DISCUSSION
The goal of the present study was to test for specific impairments in K+ homeostasis predicted to occur in Hypox rats on the basis of our hypothesis that the pituitary is directly involved in renal responses to altered K+ intake. As discussed in the introduction, the predicted impairments were a loss of renal ability to adjust K+ excretion in response to altered K+ intake, and a risk for hyperkalemia with sudden increases in K+ intake or an intravenous K+ load. The present data demonstrate that all of these expectations were met, supporting the hypothesis.
When maintained on normal K+ intake (at night), postabsorptive renal K+ excretion was significantly lower in Hypox than in control rats (Fig. 2), indicating impaired renal function for K+ excretion. In contrast, K+ excretion at night (i.e., during feeding) in Hypox rats was similar to that of control rats. This inconsistency may be explained by larger increases in plasma [K+] in Hypox rats during feeding at night (Fig. 3). Impaired renal function for K+ excretion might be compensated by the effect of high plasma [K+] to increase K+ excretion (8, 9). Thus, the apparently normal K+ excretion in Hypox rats at night occurred at the expense of elevated plasma [K+]. Because of the compensatory effects of plasma [K+], K+ excretion at night (i.e., during K+ intake) may be dictated by K+ intake, relatively independent of modest impairment of renal function, resulting in changes in absorptive K+ excretion parallel to changes in K+ intake in Hypox rats. In contrast, during postabsorptive periods, when K+ intake was absent and plasma [K+] was not elevated, renal K+ excretion might faithfully represent renal efficiency for K+ excretion. Therefore, renal adaptation to altered K+ intake may be better studied with postabsorptive than absorptive K+ excretion, and the lack of changes in postabsorptive K+ excretion with altered K+ intake in Hypox rats may indicate an inability of these rats to appropriately adapt to altered K+ intake.
The present study demonstrates that plasma [K+] was normal in Hypox rats in postabsorptive states, but renal K+ excretion and renal K+ clearance were markedly reduced. In addition, K+ tolerance was profoundly reduced in Hypox rats, resulting in larger increases in plasma [K+] following an intravenous K+ load, compared with those in control rats (Fig. 5); Some of these defects have been previously reported in Hypox animals or human subjects with decreased pituitary function (1, 33) and have been attributed to hormonal [e.g., growth hormone (10)] or other changes [e.g., decreased GFR (4)] associated with pathological conditions. Importantly, we found that these defects in Hypox rats could be reproduced in normal rats maintained on reduced K+ intake (and thus reduced signaling of K+ intake to the kidneys) for 3 days. These data suggest the intriguing possibility that the defects in Hypox rats may be due to impairment of signaling of K+ intake to the kidneys, consistent with the hypothesis that the pituitary is involved in this signaling. Thus, without being informed of dietary K+ intake, Hypox rats may be in K+ conservation states all the time (even with normal or increased K+ intake), resulting in K+ intolerance and a lack of responsiveness to K+ intake.
It is interesting to note that Hypox rats had normal postabsorptive plasma [K+] with normal K+ intake, although their renal excretion of K+ was impaired. We believe that this is due to reduced K+ release from skeletal muscle. Thus, while the output (i.e., renal excretion) was reduced, the input from skeletal muscle to the extracellular fluid was also reduced, and plasma [K+] could be maintained to be normal. Reduced K+ release from skeletal muscle during the postabsorptive periods may be explained by reduced K+ uptake (and thus, K+ storage) by skeletal muscle during the feeding periods, due to the absence of gut factor effects (see below) in Hypox rats, which is consistent with the finding that K+ intake was slightly less (Fig. 1A), but K+ excretion was slightly greater in Hypox than in control rats (Fig. 2A). It remains to be studied whether there is a mechanism coordinating the changes in K+ release from muscle and renal K+ excretion to maintain normal plasma [K+].
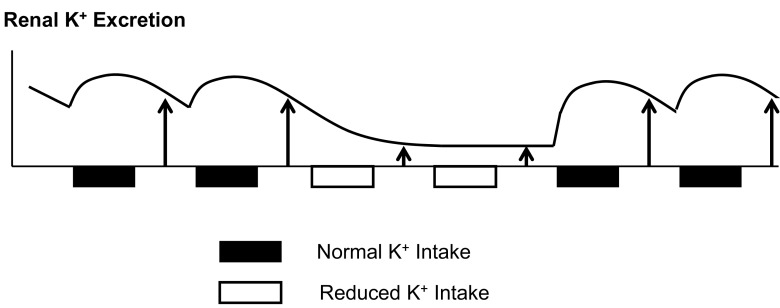
Rabinowitz and colleagues (3, 24, 26) suggested that meal-induced kaliuresis cannot be fully accounted for by increases in plasma K+ and aldosterone concentrations. They proposed a kaliuretic reflex arising from K+ sensing in the splanchnic bed that increases renal K+ excretion during dietary K+ intake. This idea was supported by Morita and colleagues (20, 21), who demonstrated that intraportal KCl infusion in anesthetized rats increased hepatic afferent nerve activity and urinary K+ excretion in the absence of increases in plasma [K+]. Consistent with these studies, we recently reported evidence for the existence of a mechanism activated by gut sensing of K+ intake (or “gut factor”) that enhances renal K+ excretion (16, 23). On the basis of this concept, we suggest that renal K+ excretion is upregulated because of gut factor effects during normal K+ intake but may be reduced during K+ deprivation (or reduced K+ intake) due to a lack of (or reduced) gut factor effects (Fig. 7). The exact nature of the gut factor has not been revealed, but the present data provide important clues to the mode of the gut factor. When overnight K+ intake was increased, K+ excretion increased not only during feeding (i.e., K+ intake) periods but also during subsequent nonfeeding (i.e., postabsorptive) periods (Fig. 2). These data suggest that gut factor effects may persist for a substantial period after feeding. On the other hand, when K+ intake was reduced, postabsorptive K+ excretion on the following day was reduced, suggesting that gut factor effects from the earlier feeding (with normal K+ intake) did not last for more than 24 h. These data are compatible with the possibility that a humoral factor is released from the gut or the pituitary upon gut sensing of dietary K+ intake and acts on kidneys to increase K+ excretion for a substantial period up to a day in rats. As discussed in the introduction, our previous study tested for a few candidate gut and pituitary peptides, such as GLP-1, GIP, guanylin, uroguanylin, α-MSH, γ-MSH, and arginine vasopressin, and found that these peptides are not affected by K+ intake. However, the pituitary (the gut) secretes many other peptides (or substances) whose function is still unknown, and there is the possibility that some of them or as yet unidentified humoral substances may be involved in K+ homeostasis. Identifying such a peptide or substance would be a breakthrough in our understanding of K+ homeostasis but may require a systematic (e.g., peptidomic) approach requiring tremendous effort. The present study provides a justification for such a study.
Fig. 7.
A schematic diagram illustrating hypothetical changes in renal K+ excretion in response to dietary K+ intake. The squares represent feeding at night with a normal K+ (solid squares) or a K+-free diet (open squares). The vertical arrows (heights) represent renal K+ excretion determined during nonfeeding or postabsorptive states. It is assumed that a gut factor is activated in response to normal K+ intake to increase renal K+ excretion, and this effect lasts for a day. Without normal K+ intake, renal K+ excretion is reduced to conserve extracellular K+.
It is interesting to note that impaired postabsorptive K+ excretion was observed in Hypox rats when K+ intake was normal but not when K+ intake was reduced (Fig. 2C). In fact, postabsorptive K+ excretion was slightly higher in Hypox than in control rats when K+ intake was reduced to one-third of normal (Fig. 2C). These data suggest that the defects in the kidneys of Hypox rats may not be due to general suppression of renal K+ excretion (e.g., due to decreases in GFR). Rather, the defects may arise from impaired sensing of K+ intake and subsequent control, which render Hypox rats in K+ conservation states all the time, as discussed above. Thus, whereas K+ excretion was enhanced in normal rats with dietary K+ intake (due to gut factor effects), such control might not occur in Hypox rats, causing notable differences in K+ excretion between normal and Hypox rats with increased K+ intake. Similar observations were made with intravenous K+ tolerance, whereas it was enhanced with increased K+ intake in normal rats (Fig. 5D), such effects were not seen in Hypox rats (data not shown), causing larger differences with increased K+ intake. A lack of gut factor effects also explains larger increases in plasma [K+] during feeding in Hypox rats (Fig. 3), representing a risk of hyperkalemia with sudden increases in K+ intake in Hypox animals or human subjects with impaired pituitary function.
The pituitary secretes many hormones with important functions, including but not limited to growth hormone (GH), ACTH, thyroid-stimulating hormone, and sex hormone-stimulating hormones. The absence of the pituitary in Hypox rats is expected to cause low levels of GH, corticosterone, thyroid hormones, and sex hormones, causing drastic changes in many body systems, possibly including those involved in K+ homeostasis. Therefore, the present study cannot exclude the possibility that hypophysectomy impairs K+ homeostasis by altering systems not directly involved in the signaling of K+ intake to the kidneys. Glucocorticoids have been suggested to play a role in the regulation of renal K+ excretion, likely by altering sodium reabsorption (31). In the present study, a restoration of normal plasma corticosterone levels by an intravenous corticosterone infusion for 24 h did not normalize impaired K+ homeostasis in Hypox rats. It remains to be studied whether the impairments of K+ homeostasis seen in Hypox rats can be normalized by an acute or a prolonged restoration of other hormonal changes in these rats.
Perspectives and Significance
The present study demonstrates impaired renal response to altered K+ intake in Hypox rats under several different experimental settings. These data support a gut-brain-kidney axis in the signaling of K+ intake to the kidneys, although we cannot exclude the possibility that hypophysectomy impairs K+ homeostasis by altering systems not directly involved in the signaling. Future studies are warranted to directly test whether the pituitary is indeed involved in the signaling of K+ intake to the kidneys and, if so, to identify a pituitary hormone(s) whose secretion is affected by K+ intake to regulate renal K+ excretion.
GRANTS
This study was supported by National Institutes of Health DK-080233 (to J. H. Youn.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.T.O., J.K., and J.H.Y. conception and design of research; Y.T.O. performed experiments; Y.T.O. and J.H.Y. analyzed data; Y.T.O., J.K., and J.H.Y. interpreted results of experiments; Y.T.O. and J.H.Y. prepared figures; Y.T.O., J.K., and J.H.Y. edited and revised manuscript; Y.T.O., J.K., and J.H.Y. approved final version of manuscript; J.H.Y. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Larry Rabinowitz for his critical reading of the manuscript and providing helpful comments.
REFERENCES
- 1. Banks RO. Natriuresis and kaliuresis in saline-expanded, long-term hypophysectomized rats. Proc Soc Exp Biol Med 172: 502–507, 1983 [DOI] [PubMed] [Google Scholar]
- 2. Bia MJ, DeFronzo RA. Extrarenal potassium homeostasis. Am J Physiol Renal Fluid Electrolyte Physiol 240: F257–F268, 1981 [DOI] [PubMed] [Google Scholar]
- 3. Calo L, Borsatti A, Favaro S, Rabinowitz L. Kaliuresis in normal subjects following oral potassium citrate intake without increased plasma potassium concentration. Nephron 69: 253–258, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Caverzasio J, Faundez R, Fleisch H, Bonjour JP. Tubular adaptation to Pi restriction in hypophysectomized rats. Pflügers Arch 392: 17–21, 1981 [DOI] [PubMed] [Google Scholar]
- 5. Chen P, Guzman JP, Leong PK, Yang LE, Perianayagam A, Babilonia E, Ho JS, Youn JH, Wang WH, McDonough AA. Modest dietary K+ restriction provokes insulin resistance of cellular K+ uptake and phosphorylation of renal outer medulla K+ channel without fall in plasma K+ concentration. Am J Physiol Cell Physiol 290: C1355–C1363, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Choi CS, Lee FN, McDonough AA, Youn JH. Independent regulation of in vivo insulin action on glucose versus K+ uptake by dietary fat and K+ content. Diabetes 51: 915–920, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Choi CS, Thompson CB, Leong PK, McDonough AA, Youn JH. Short-term K+ deprivation provokes insulin resistance of cellular K+ uptake revealed with the K+ clamp. Am J Physiol Renal Physiol 280: F95–F102, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Gennari FJ, Segal AS. Hyperkalemia: An adaptive response in chronic renal insufficiency. Kidney Int 62: 1–9, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Giebisch G, Sullivan LP, Whittembury G. Relationship between tubular net sodium reabsorption and peritubular potassium uptake in the perfused Necturus kidney. J Physiol 230: 51–74, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirschberg R, Kopple JD. Effects of growth hormone and IGF-I on renal function. Kidney Int Suppl 27: S20–S26, 1989 [PubMed] [Google Scholar]
- 11. Humphreys MH, Lin SY, Wiedemann E. Renal nerves and the natriuresis following unilateral renal exclusion in the rat. Kidney Int 39: 63–70, 1991 [DOI] [PubMed] [Google Scholar]
- 12. Jackson CA. Rapid renal potassium adaptation in rats. Am J Physiol Renal Fluid Electrolyte Physiol 263: F1098–F1104, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Jamison RL. Potassium recycling. Kidney Int 31: 695–703, 1987 [DOI] [PubMed] [Google Scholar]
- 14. Kurtzman NA, Gonzalez J, DeFronzo R, Giebisch G. A patient with hyperkalemia and metabolic acidosis. Am J Kidney Dis 15: 333–356, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Laroche-Joubert N, Doucet A. Collecting duct adaptation to potassium depletion. Semin Nephrol 19: 390–398, 1999 [PubMed] [Google Scholar]
- 16. Lee FN, Oh G, McDonough AA, Youn JH. Evidence for gut factor in K+ homeostasis. Am J Physiol Renal Physiol 293: F541–F547, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Lin SY, Chaves C, Wiedemann E, Humphreys MH. A gamma-melanocyte stimulating hormone-like peptide causes reflex natriuresis after acute unilateral nephrectomy. Hypertension 10: 619–627, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Lin SY, Wiedemann E, Humphreys MH. Role of the pituitary in reflex natriuresis following acute unilateral nephrectomy. Am J Physiol Renal Fluid Electrolyte Physiol 249: F282–F290, 1985 [DOI] [PubMed] [Google Scholar]
- 19. McDonough AA, Thompson CB, Youn JH. Skeletal muscle regulates extracellular potassium. Am J Physiol Renal Physiol 282: F967–F974, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Morita H, Fujiki N, Hagiike M, Yamaguchi O, Lee K. Functional evidence for involvement of bumetanide-sensitive Na+K+2Cl− cotransport in the hepatoportal Na+ receptor of the Sprague-Dawley rat. Neurosci Lett 264: 65–68, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Morita H, Fujiki N, Miyahara T, Lee K, Tanaka K. Hepatoportal bumetanide-sensitive K+-sensor mechanism controls urinary K+ excretion. Am J Physiol Regul Integr Comp Physiol 278: R1134–R1139, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Nguyen MT, Yang LE, Fletcher NK, Lee DH, Kocinsky H, Bachmann S, Delpire E, McDonough AA. Effects of K+-deficient diets with and without NaCl supplementation on Na+, K+, and H2O transporters' abundance along the nephron. Am J Physiol Renal Physiol 303: F92–F104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oh KS, Oh YT, Kim SW, Kita T, Kang I, Youn JH. Gut sensing of dietary K+ intake increases renal K+excretion. Am J Physiol Regul Integr Comp Physiol 301: R421–R429, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rabinowitz L. Aldosterone and potassium homeostasis. Kidney Int 49: 1738–1742, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Rabinowitz L, Aizman RI. The central nervous system in potassium homeostasis. Front Neuroendocrinol 14: 1–26, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Rabinowitz L, Sarason RL, Yamauchi H. Effects of KCl infusion on potassium excretion in sheep. Am J Physiol Renal Fluid Electrolyte Physiol 249: F263–F271, 1985 [DOI] [PubMed] [Google Scholar]
- 27. Rabinowitz L, Sarason RL, Yamauchi H, Yamanaka KK, Tzendzalian PA. Time course of adaptation to altered K intake in rats and sheep. Am J Physiol Renal Fluid Electrolyte Physiol 247: F607–F617, 1984 [DOI] [PubMed] [Google Scholar]
- 28. Rabinowitz L, Wydner CJ, Smith KM, Yamauchi H. Diurnal potassium excretory cycles in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 250: F930–F941, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Ribstein J, Humphreys MH. Endogenous opioids and electrolyte excretion after contralateral renal exclusion. Am J Physiol Renal Fluid Electrolyte Physiol 244: F392–F398, 1983 [DOI] [PubMed] [Google Scholar]
- 30. Ribstein J, Humphreys MH. Renal nerves and cation excretion after acute reduction in functioning renal mass in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 246: F260–F265, 1984 [DOI] [PubMed] [Google Scholar]
- 31. Stanton B, Giebisch G, Klein-Robbenhaar G, Wade J, DeFronzo RA. Effects of adrenalectomy and chronic adrenal corticosteroid replacement on potassium transport in rat kidney. J Clin Invest 75: 1317–1326, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stokes JB. Potassium intoxication: pathogenesis and treatment. In: The Regulation of Potassium Balance, edited by Seldin DW, Giebisch G. New York, NY: Raven, p. 157–174, 1989 [Google Scholar]
- 33. van Buren M, Rabelink TJ, Koppeschaar HP, Koomans HA. Role of glucocorticoid in excretion of an acute potassium load in patients with Addison's disease and panhypopituitarism. Kidney Int 44: 1130–1138, 1993 [DOI] [PubMed] [Google Scholar]