Abstract
During sleep, changes in brain rhythms and neuromodulator levels in cortex modify the properties of individual neurons and the network as a whole. In principle, network-level interactions during sleep can be studied by observing covariation in spontaneous activity between neurons. Spontaneous activity, however, reflects only a portion of the effective functional connectivity that is activated by external and internal inputs (e.g., sensory stimulation, motor behavior, and mental activity), and it has been shown that neural responses are less correlated during external sensory stimulation than during spontaneous activity. Here, we took advantage of the unique property that the auditory cortex continues to respond to sounds during sleep and used external acoustic stimuli to activate cortical networks for studying neural interactions during sleep. We found that during slow-wave sleep (SWS), local (neuron-neuron) correlations are not reduced by acoustic stimulation remaining higher than in wakefulness and rapid eye movement sleep and remaining similar to spontaneous activity correlations. This high level of correlations during SWS complements previous work finding elevated global (local field potential-local field potential) correlations during sleep. Contrary to the prediction that slow oscillations in SWS would increase neural correlations during spontaneous activity, we found little change in neural correlations outside of periods of acoustic stimulation. Rather, these findings suggest that functional connections recruited in sound processing are modified during SWS and that slow rhythms, which in general are suppressed by sensory stimulation, are not the sole mechanism leading to elevated network correlations during sleep.
Keywords: auditory cortex, sleep, primate, sensory, hearing, electrophysiology
the brain undergoes a number of state changes throughout the course of the day ranging from fully engaged by one particular event (attention) to virtually unconscious of all external events (sleep). The effects of these behavioral states on neural responses are often studied by measuring mean firing rates of neurons. For example, attention has been shown to increase neural responses to stimuli (Moran and Desimone 1985; Motter 1994). Mean activity levels, however, are only a first-order approximation of underlying network changes and often give little insight into changes in the computational capacity of the network. For example, recent attention studies have found that changes in neural correlations or joint neural firing strongly impact the coding of the stimulus at the population level despite moderate changes in firing rates (Cohen and Maunsell 2009; Mitchell et al. 2009).
How neural correlations change in cortex during sleep is not well-understood because few studies have conducted simultaneous recordings from well-isolated single neurons during natural sleep. In auditory cortex, the mean population response to sounds is similar during wakefulness, slow-wave sleep (SWS), and rapid eye movement sleep (REM; Edeline et al. 2001; Issa and Wang 2008; Pena et al. 1999). However, neurons are modulated heterogeneously (some neurons exhibit increased responses, whereas others exhibit decreased responses; Edeline et al. 2001; Issa and Wang 2008), and excitatory and inhibitory strength are weakened during SWS, suggesting that other aspects of network operation can change without modifying overall activity (Issa and Wang 2011). Importantly, slow oscillations in SWS can increase neural synchronization by coordinately modulating neural activity up and down at long time scales (Steriade et al. 1993, 2001). This phenomenon, however, has been demonstrated in local field potentials (LFPs) and operates at long temporal scales (seconds) and large spatial scales (millimeters; Destexhe et al. 1999). In particular, little is known about how short-time-scale local neural interactions such as those involved in rapid sensory processing change beyond what would be predicted by slow, global mechanisms during SWS.
In the present study, we recorded simultaneously from multiple neurons in primate auditory cortex and measured local neural correlations during natural sleep. Using acoustic stimulation, we studied the natural operation of the cortical network when engaged in sensory processing for comparison with spontaneous activity measured in previous work. We show that SWS has distinct effects on local functional connectivity during sound stimulation despite minimal changes in firing rate.
MATERIALS AND METHODS
Subjects and surgery.
Neural data were collected from 5 hemispheres of 4 common marmosets (Callithrix jacchus; 2 males, 2 females) used in our 2 previous studies (Issa and Wang 2008, 2011). Two stainless steel headposts were implanted for head fixation (Lu et al. 2001), and animals were adapted to sleeping in a primate restraint chair while sounds were playing (Issa and Wang 2008). All experimental procedures were approved by the Johns Hopkins University Animal Care and Use Committee.
Physiological recordings.
Animals slept for 5–10 sleep cycles (6–8 h), and EEG and video monitoring were used to determine sleep state (Issa and Wang 2008). During a sleep cycle, animals would pass through one stable period of SWS (∼10–20 min) followed by a shorter period of REM (∼5–10 min). Transitions into SWS from light sleep were gradual and were marked by an increase in the amplitude of 1- to 3-Hz oscillations and a decrease in power at higher frequencies. Transitions into REM from SWS were more acute and included an increase in power at high (>10 Hz) frequencies and a noticeable decrease in muscle tone as the marmoset tail would drop and uncurl.
Extracellular activity was measured using 2- to 5-MΩ tungsten microelectrodes (A-M Systems). Spike waveforms were sorted online using a template-matching algorithm (Worgotter et al. 1986). Units were not recorded unless well-isolated and stable so that they could be reliably tested across behavioral states. After isolating one unit and waiting for further settling of the electrode, we would encounter a second unit in 10–20% of cases resulting in a paired recording. Only stable paired recordings that could subsequently be tested in all three sleep states (wakefulness, SWS, and REM) were included in analysis. Cases where a second unit was only present for one or two sleep states were not included. If waveforms from two units were difficult to sort or overlapping, the electrode was moved until one unit, the other, or both were clearly discernible. Mean signal-to-noise ratio (SNR) of units in paired recordings was 23 dB (peak-to-peak amplitude/standard deviation = 14x). Our methods of paired recordings closely followed Zohary et al. (1994), who used a similar algorithm and criteria for spike sorting. Despite the overall recording quality, we found that ∼10% of spikes were misclassified on average [area under receiver operating characteristic (ROC) of pairwise waveform distances between neurons vs. within a given neuron]. This number pools over variation over the course of a recording (up to 2 h) and is thus an upper limit on classification error. Although poor spike assignment can artificially boost the absolute magnitude of correlations (Ecker et al. 2010), we were primarily interested in the relative correlation levels across states and not their absolute magnitudes. As a result, repeating analyses using only pairs of units with low misclassification error did not change the main findings.
Neural activity was recorded in primary auditory cortex (A1) and lateral belt (LB; see Issa and Wang 2008). Data from A1 and LB were pooled together in analysis since modulation of neural responses during sleep is similar in A1 and LB (Issa and Wang 2008), and only a small sample of neuron pairs was recorded in LB (n = 12 out of 72 pairs used in main analysis). LFPs (band-pass filtered, 1–300 Hz) were only collected in 2 hemispheres in 2 animals (1 male, 1 female). Spiking activity and LFPs were measured on the same electrode raising the concern that low-frequency energy from spike waveforms could leak into the high-frequency range of the LFP, creating spurious unit-LFP correlations. We addressed this concern in three ways. First and foremost, all of our conclusions were based on relative comparisons across the three behavioral states (awake, SWS, and REM) and not on absolute values. Given that spiking levels are similar across these three states (Issa and Wang 2008), spike-LFP cross talk is not expected to introduce differences across behavioral states. Second, we limited our analysis to power below 120 Hz, which was well below the low-pass filter cutoff (300 Hz). Third, as an extra precaution, we only used units with <20-dB SNR in our analyses as we found that high SNR units (>22 dB) created correlations in the upper LFP frequencies (120–300 Hz).
Stimuli.
Stimuli consisted of tones, noise, sinusoidal amplitude-modulated tones, click trains, and marmoset vocalizations. Stimuli were chosen to optimize the response of the first neuron encountered in a pair as detailed in our previous work (see Issa and Wang 2008). In the 10–20% of recordings where a second neuron was isolated, we would continue using the same stimuli optimized for the first neuron under the assumption that nearby neurons tend to be driven by similar stimuli. An average of seven stimuli were tested per pair in all three behavioral states, and neurons were well-driven on average (mean per unit = 6 SE above baseline). Stimuli were delivered in free field in a soundproof acoustic isolation chamber from a speaker located 0.9 m directly in front of the animal.
Analysis.
An adaptive windowing algorithm was used for event detection (Issa and Wang 2008; Legendy and Salcman 1985). Event windows from both units in a pair were concatenated, and driven firing rates were computed over this larger window (driven activity: median analysis window = 435 ms) and spontaneous rate-subtracted. Spontaneous firing rates were estimated using the prestimulus period (spontaneous activity: median analysis window = 500 ms). For LFPs, we computed the power spectral density using the Welch moving-average method (100- or 128-point Hamming window, 50% overlap of segments) across the complete trial (spontaneous and driven periods, median analysis window = 700 ms) to ensure more accurate estimates of power in low frequencies; similar results were obtained using only the spontaneous (median analysis window = 200 ms) or the first 200 ms of the driven window to compute LFP power. Spectral power was computed in seven frequency bands: δ = 0.7–4.2 Hz, θ = 4.2–7.5 Hz, α = 7.5–12 Hz, β = 12–20 Hz, γ = 20–50 Hz, high-γ (hγ) = 40–120 Hz, ultra-hγ = 120–300 Hz. Spectrum estimates were normalized by the sum of the power in all frequencies and rescaled by the original power of the raw signal yielding units of V2/Hz. In the present study, correlations were computed between neural firing rates and LFP power (see our previous study for spike-field coherence measures during sleep; Issa and Wang 2011).
Correlations between two simultaneously recorded units or between units and LFP power were computed using a normalized correlation measure (Bair et al. 2001):
where ri are the firing rates (or LFP power) on the ith trial that are mean-subtracted and normalized by the standard deviation across all trials to obtain z-score zi. It is important to note that simple increases in mean firing rate or in overall LFP power (e.g., during SWS) would not increase correlations since z-scored rates/power are mean-corrected and variance-normalized. Neuron-neuron or neuron-LFP pairs were only included in correlation analysis if tested in all three behavioral states such that all comparisons (awake vs. SWS and awake vs. REM) are based on an equal number of recorded pairs tested with identical stimuli. It is important to note, however, that the pool of neurons used in neuron-neuron analyses was only partially overlapping with the neurons used in neuron-LFP analyses (21 of 72 total pairs) because LFPs were only recorded in monkeys 3 and 4 (whereas paired recordings were also made in monkeys 1 and 2) and LFP analyses excluded neurons with large waveforms (which was typical of paired recordings where isolation was particularly strong). Restricting neuron-LFP analyses to only those neurons also present in neuron-neuron analyses resulted in similar trends as the larger neuron-LFP pool. Correlations were computed for all stimuli tested regardless of auditory drive. Unless otherwise noted, we report the mean correlation for each pair by averaging across stimuli. Repeating our analyses using a subset of stimuli that passed an auditory drive criterion (response of both units in a pair >6 SE above baseline; n = 45 pairs of 72 recorded) did not change results. Our approach was similar to a previous study that also collapsed correlations across all stimuli (Zohary et al. 1994) because of limited variation in correlation measures across stimulus type or drive (Gutnisky and Dragoi 2008; Kohn and Smith 2005). Correlations were computed over large windows (hundreds of milliseconds) since we saw little evidence of synchrony at finer time scales (tens of milliseconds) suggesting a low likelihood of recording two directly connected neurons. We did not test longer time-scale correlations since our data were not collected continuously but in a trial-by-trial fashion precluding long (seconds or more) time windows. Mean differences were tested for significance using the Wilcoxon rank-sum test. Linear correlations were computed using the Pearson correlation coefficient, and values were Fisher z-transformed before statistical testing of significance. Percentage gain measures comparing two behavioral states were computed using %Gain = 100·(state 1 − state 2)/max(|state 1|, |state 2|). In all figures, error bars represent ±1 SE.
RESULTS
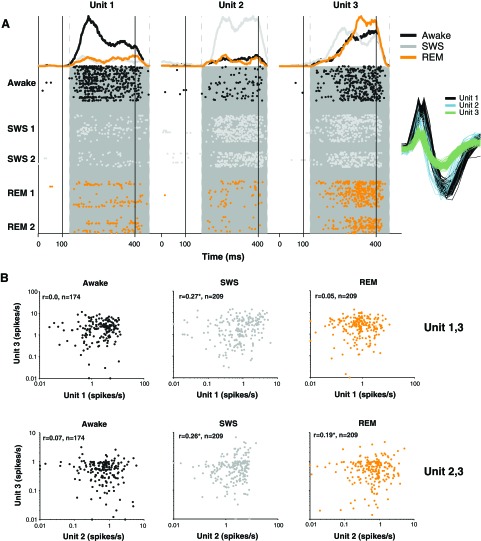
An example recording of three simultaneous single units is shown in Fig. 1. Although recorded on the same electrode and thus within close proximity of each other, these three units showed widely different responses during sleep (Awake-SWS-REM, unit 1: high-low-low, unit 2: low-high-low, unit 3: high-high-high; Fig. 1A) consistent with the heterogeneity of firing rate modulation observed in cortex during sleep (Edeline et al. 2001; Issa and Wang 2008). By recording simultaneously from neighboring neurons, we were able to measure noise correlations in each behavioral state. In the example in Fig. 1, correlations in trial-by-trial firing rates were not significant during wakefulness in both pairs tested (unit 1,3 and unit 2,3; Fig. 1B, left) but were highly significant in SWS (Fig. 1B, middle). This increase during SWS occurred despite the fact that unit 1 firing rate decreased in SWS, whereas unit 2 firing rate increased in SWS (Fig. 1A). In other words, trial-by-trial noise covariance increased in SWS even though firing rates either increased or decreased in sleep.
Fig. 1.
Example units recorded simultaneously. A: 3 well-isolated single units (signal-to-noise ratio: unit 1 = 38 dB, unit 2 = 29 dB, unit 3 = 28 dB; see inset for spike waveforms taken from the end of the recording) were recorded simultaneously for an episode of sleep [slow-wave sleep (SWS) 1 and rapid eye movement sleep (REM) 1] followed by wakefulness and a 2nd episode of sleep (SWS 2 and REM 2; 94 min total). These 3 units showed 3 different patterns of modulation during sleep. Unit 1 was strongly driven by a sinusoidal amplitude-modulated (sAM) tone [carrier frequency = 8.7 kHz, modulation frequency = 128 Hz, 30-dB sound pressure level (SPL)] during wakefulness, but its response was strongly attenuated in SWS (gain = −73%) and REM (gain = −69%). On the other hand, unit 2 responded most strongly in SWS and had a weak response in wakefulness (gain in SWS = 83%; sAM tone: carrier frequency = 8.7 kHz, modulation frequency = 1 Hz, 30-dB SPL). Finally, unit 3 gave a consistent response across all states (gain in SWS = 15%, gain in REM = 18%; sAM tone: carrier frequency = 8.7 kHz, modulation frequency = 128 Hz, 30-dB SPL). Stimulus onset on each trial was at 100 ms, and stimulus offset was at 400 ms (vertical black lines). Gray boxes denote analysis window for computing firing rates (see materials and methods). B: joint firing rates for pairs of units driven by the same stimulus (*significance at the P < 0.01 level). Units 1 and 3 were both driven by a 128-Hz sAM tone. During wakefulness, firing rates on individual trials were not correlated (r = 0.004, P = 0.96; top left). However, during SWS, units 1 and 2 showed covariation in trial responses (r = 0.27, P < 0.01; top middle). Covariation became minimal in REM (r = −0.05, P = 0.47; top right). Similarly, units 2 and 3 (both driven by a 1-Hz sAM tone) showed strong trial-by-trial covariation in firing rate in SWS (r = 0.26, P < 0.01; bottom left) but not during wakefulness (r = 0.07, P = 0.38; bottom middle). Note that the increase in correlation of units 1 and 2 with unit 3 occurred despite firing rates changing in opposite directions (unit 1 decreased whereas unit 2 increased firing rate in SWS).
Increased correlations in neural firing during SWS compared with wakefulness and REM were observed across the population of recorded pairs [median awake = 0.08 ± 0.02, SWS = 0.20 ± 0.02, REM = 0.10 ± 0.02, P(awake vs. SWS) = 0.002, P(awake vs. REM) = 0.99, n = 72; Fig. 2A]. A similar trend for increased correlation in SWS compared with wakefulness was seen even in the best isolated unit pairs [median awake = 0.05 ± 0.04, SWS = 0.23 ± 0.04, P(awake vs. SWS) = 0.01, n = 10, <6% spike sort errors compared with 11% errors in whole sample]. The difference in correlations in SWS compared with wakefulness and REM could not be accounted for by differences in spike sorting as misclassification error rates were similar across all three states [median awake = 9.2 ± 0.8%, SWS = 8.5 ± 0.8%, REM = 7.2 ± 0.8%, P(awake vs. SWS) = 0.93, P(awake vs. REM) = 0.22, n = 80 units]. We observed an increase in unit-unit correlations during SWS even for pairs where spike misclassification errors, and hence spurious correlations, decreased in SWS (change in correlation when spike misclassification errors in SWS compared with wakefulness increased = 0.05 ± 0.03 vs. decreased = 0.05 ± 0.04, P = 0.94, nincreased = 32, ndecreased = 47). An increase in correlations during SWS was also observed for stimuli that weakly drove responses and was robust to different levels of stimulus drive [median awake = −0.02 ± 0.07, SWS = 0.15 ± 0.06, P(awake vs. SWS) = 0.47, n = 20, response in both units ≤0.33 SE above mean during wakefulness, which is the lower quartile of acoustic drive compared with 4.7 SE drive across all stimuli].
Fig. 2.
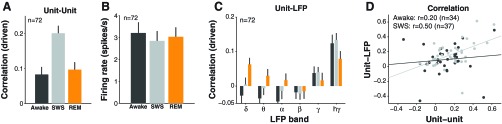
Local and global correlations during acoustic stimulation in sleep. A: local neural correlations increased in SWS relative to wakefulness and REM during sound playing. B: firing rates were similar between wakefulness, SWS, and REM during acoustic stimulation. C: neural firing was weakly negatively correlated with low-frequency local field potential (LFP) power (δ = 0.7–4.2 Hz, θ = 4.2–7.5 Hz, α = 7.5–12 Hz, β = 12–20 Hz) and positively correlated with high-frequency LFP power [γ = 20–50 Hz, high-γ (hγ) = 40–120 Hz], and across behavioral states, the magnitude of unit-LFP correlations was similar. D: global (unit-LFP-hγ) measures of neural correlation became strongly predictive of local (unit-unit) measures of correlation in SWS (lines correspond to least-squares linear fits).
Unlike noise correlations, overall driven firing rates were similar in all three states [median awake = 3.21 ± 0.49,SWS = 2.85 ± 0.45, REM = 3.04 ± 0.43, P(awake vs. SWS) = 0.61, P(awake vs. REM) = 0.63, n = 72; Fig. 2B]. Firing rates only changed by 2% in SWS compared with wakefulness (median = 1.8%, P = 0.70, t-test, n = 72), but firing rate correlations increased by 26% (median = 26.5%, P = 0.0001, t-test, n = 72). This increase in correlation occurred even when firing rates for both neurons in a pair decreased during SWS compared with wakefulness (change in correlation: both units decreased firing rate = 0.04 ± 0.10 vs. increased firing rate = 0.03 ± 0.03, P = 0.45, nboth down = 13, nboth up = 20). Finally, in our previous work, we had found differential effects of SWS on firing rates across sound level (Issa and Wang 2011), but when we separated SWS effects on neural correlations for quiet and loud sound levels in the present data, we found that correlations generally increased in SWS regardless of the sound level tested {change in correlation: quiet [0- to 20-dB sound pressure level (SPL)] = 0.06 ± 0.05 vs. loud [70- to 90-dB SPL] = 0.10 ± 0.04, P = 0.79, nquiet = 68, nloud = 64 stimuli}.
Neuron-neuron correlations were based on measurement of units recorded simultaneously on the same electrode and hence within close proximity of each other (∼100 μm). To test more global correlations, we used the LFP as a proxy for activity at larger spatial scales. In particular, LFP power in the γ-band is thought to represent the collective activities of local groups of neurons in a 1- to 2-mm diameter region (Mitzdorf 1985) as evidenced by its correlation with both neural activity (Kayser et al. 2007; Kreiman et al. 2006; Liu and Newsome 2006; Ray and Maunsell 2011) and local blood oxygenation signals used in fMRI (Logothetis et al. 2001). When we tested neural correlations with more global LFP-hγ activity (40–120 Hz), we found that correlations were higher in SWS compared with wakefulness and REM, although this difference did not reach significance in the comparison of SWS and wakefulness [median awake = 0.12 ± 0.03, SWS = 0.13 ± 0.02, REM = 0.08 ± 0.02, P(awake vs. SWS) = 0.20, P(awake vs. REM) = 0.01, n = 72; Fig. 2C]. In addition, we found that unit-unit measures became strongly predictive of unit-LFP-hγ measures in SWS (r = 0.50, P = 0.002, n = 37), suggesting that local and global behavior become more consistent than in wakefulness and REM (wakefulness: r = 0.20, P = 0.53, n = 34; REM: r = −0.13, P = 0.23, n = 33; Fig. 2D). In the lower LFP frequency bands, correlations with neural activity were small in overall magnitude and were highest in REM (Fig. 2C).
Unlike during acoustic stimulation, unit-unit correlations during spontaneous activity did not increase in SWS compared with wakefulness and REM [median awake = 0.16 ± 0.03, SWS = 0.19 ± 0.02, REM = 0.14 ± 0.03, P(awake vs. SWS) = 0.79, P(awake vs. REM) = 0.47, n = 65; Fig. 3A]. This result may seem surprising because slow oscillations are expected to coordinate spontaneous activity during SWS (Steriade et al. 1993); however, we have previously shown that during acoustic stimulation slow oscillations play a more limited role in modulating neural firing in primary auditory cortex (Issa and Wang 2011). Second, we observed that unit-unit correlations in spontaneous activity were somewhat predictive of unit-unit correlations during acoustic stimulation (wakefulness: r = 0.43, P < 0.01, n = 122; SWS: r = 0.39, P < 0.01, n = 136; Fig. 3B). One possibility is that the slope of the relationship between spontaneous and driven activity correlations changes between states without the absolute magnitude of spontaneous correlations changing between states, but we found a similar slope between states (Fig. 3B). We emphasize, however, that these measures of firing rate covariation were qualitatively different; spontaneous activity correlations were 50% higher on average than those during acoustic stimulation (wakefulness: median spontaneous = 0.15 ± 0.02 vs. driven = 0.10 ± 0.02, P = 0.02, n = 122; Fig. 3C), consistent with previous work (Jermakowicz et al. 2009; Smith and Kohn 2008). This difference could not be explained by a firing rate effect; at matched firing rates, spontaneous activity correlations were consistently higher than correlations during acoustic stimulation (Fig. 3D), and correlation strength did not depend on the mean firing rate in a pair (mean firing in a pair vs. unit-unit correlation, r = −0.001, P = 0.96, n = 2,029 stimuli). Correlations were higher when both neurons in a pair jointly had high firing rates (upper vs. lower quartile of firing rates in population: median correlation = 0.18 ± 0.04 vs. −0.08 ± 0.02, P = 0.06, nupper = 94, nlower = 351; upper vs. lower quartile of stimuli within each pair: median correlation = 0.27 ± 0.04 vs. 0.11 ± 0.04, P = 0.36, n = 47, nupper = 91, nlower = 90), but this increasing trend with joint firing rate would predict higher, not lower, correlations during acoustic drive compared with spontaneous activity.
Fig. 3.
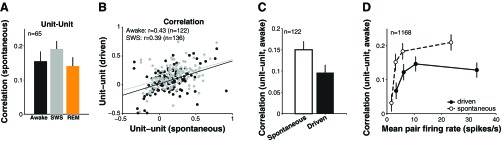
Neural correlations during spontaneous activity in sleep. A: local neural correlations did not change in SWS relative to wakefulness and REM during spontaneous activity. B: neural correlations measured during spontaneous activity were predictive of correlation patterns during driven activity (lines correspond to least-squares linear fits). C: trial-by-trial covariation in recorded pairs was 50% stronger during spontaneous activity as during acoustic drive in wakefulness. D: correlations were higher in spontaneous activity than in stimulus driven activity (not spontaneous rate-subtracted) even for matched mean firing rates in a pair.
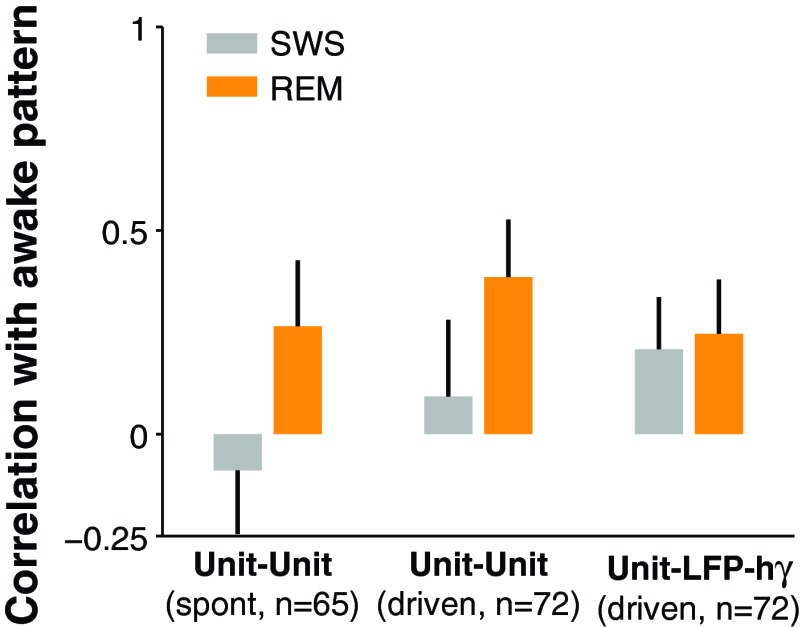
Finally, we asked whether the pattern of correlations (i.e., which pairs of neurons were strongly or weakly correlated) changed during sleep. We found that correlations patterns in SWS were dissimilar from those in wakefulness (spontaneous: r = −0.09, P = 0.48; driven: r = 0.11, P = 0.34, n = 72), whereas REM correlation patterns retained weak but significant similarity to awake patterns in both spontaneous and driven periods (spontaneous: r = 0.27, P = 0.03; driven: r = 0.40, P = 0.0004, n = 65; Fig. 4). Furthermore, at the unit-LFP-hγ level, correlation patterns were weakly preserved across states (wakefulness vs. SWS: r = 0.21, P = 0.08; wakefulness vs. REM: r = 0.21, P = 0.08; Fig. 4, right bars). In sum, the pattern (Fig. 4) and the magnitude (Fig. 2) of unit-unit and unit-LFP-hγ correlations were modified in SWS compared with wakefulness.
Fig. 4.
Patterns of correlation during sleep. The pattern of correlations in SWS (which pairs were strongly or weakly correlated) was not similar to the pattern in wakefulness, whereas REM correlation patterns had stronger similarity to awake patterns. This trend was present in the local (unit-unit) but not global (unit-LFP-hγ) measures used for computing correlation. spont, Spontaneous.
DISCUSSION
We have shown that neural correlations during acoustic stimulation are higher in SWS than during wakefulness and REM. Correlations increased in local (unit-unit) measures but not as strongly in global (unit-LFP) measures, which might reflect correlated activity at different spatial scales. Importantly, the strongest effects of SWS were observed during evoked activity, not spontaneous activity. Previous work had not studied single neuron correlations using sensory stimulation during sleep, instead focusing on unit-LFP or unit-EEG correlations (Destexhe et al. 1999) or correlations during spontaneous neural firing (Wilson and McNaughton 1994). Here, we used acoustic stimulation to drive strong responses in the network during sleep. It remains an open question whether using similar methods in other cortical sensory areas (e.g., somatosensory cortex) would reveal similar changes in neural correlation during SWS. However, our results are broadly consistent with an increasing body of literature showing that neural correlations increase in unaroused states (i.e., sleep or quiet waking), whereas neural responses become decorrelated in aroused states (e.g., attention or active exploration; Cohen and Maunsell 2009; Destexhe et al. 1999; Poulet and Petersen 2008) or during stimulation of reward centers (Goard and Dan 2009). An important exception may be REM, which as an unaroused state showed similar correlation patterns to wakefulness and tends to share other neural response properties with aroused behavioral states (Issa and Wang 2011).
How the network transitions from a default mode of correlation to less-correlated activity during attention or sensory stimulation remains an open question. In primary visual cortex, activation of local acetylcholine receptors leads to decorrelated network states (Goard and Dan 2009), and in studies of attention, increased activity in inhibitory neurons may be involved in decorrelating responses (Mitchell et al. 2007). Another possible coordinating mechanism are global oscillations, which become stronger during SWS (Destexhe et al. 1999; for review, see Kohn et al. 2009). Contrary to the idea of slow oscillations, which have been shown to increase LFP-LFP coherence in spontaneous activity during SWS (Destexhe et al. 1999) and which would have increased correlations in general (Steriade et al. 1993), a novel finding of this study is that correlated activity increased specifically during acoustic stimulation, suggesting that shared local inputs recruited during acoustic drive may be important additional factors. This does not rule out an effect of slow oscillations in our work. We have previously observed a clear influence of slow oscillations on spontaneous neural firing as measured by spike-field coherence, but this pattern was strongly disrupted during acoustically driven activity (Issa and Wang 2011; see their Fig. 7). It is plausible that the influence of slow oscillations is reduced by external stimulation, which is thought to disrupt these oscillations (Nauhaus et al. 2009).
Besides global mechanisms such as slow oscillations, our previous work suggested that local inputs also change during SWS. Specifically, response suppression decreased during SWS at high sound levels, whereas response excitation decreased at low sound levels, leading to small overall average change in firing rates (Issa and Wang 2011). Given that excitatory and inhibitory inputs may both be changing in SWS, it is difficult to speculate how noise correlations would be modified in sleep. We attempted to isolate the role of suppressive inputs in modifying noise correlations by examining different sound levels or by examining pairs for which responses were less suppressed in SWS, but we found no clear evidence that weaker suppression in SWS uncovered stronger correlations. In fact, both excitation and inhibition are likely to contribute common inputs. For example, divisive normalization mechanisms typically involve common inhibitory input such that weaker inhibition would decrease correlations in SWS as opposed to the increase we observed (Cohen and Maunsell 2009). Further knowledge about the underlying circuit will be required to determine the mechanisms leading to different levels of noise correlation across behavioral states such as attention and sleep.
Correlations between neurons are more difficult to measure than mean firing rates and tend to be small in magnitude. However, they can provide insight into network architecture and functional connectivity. For example, even weak pairwise correlations can imply strongly correlated global states (Schneidman et al. 2006) and have been used to account for higher-order correlations in the retina (Schneidman et al. 2006; Shlens et al. 2006). For larger networks of neurons such as in cortex, theoretical work suggests that pairwise correlations will tend to have almost no predictive power as network size increases (Roudi et al. 2009), and a recent empirical study has shown the importance of higher-order correlations beyond second order in accounting for simultaneously recorded neural populations in primary visual cortex (Koster et al. 2013). At a more functional level, correlations among neurons can limit sensory coding capacity if neural tuning curves are highly overlapping, which is unlikely, or if suboptimal readout schemes such as simple averaging are used (Abbott and Dayan 1999; Zohary et al. 1994). Neural correlations may be a proxy for the functional independence of inputs and the effectiveness of sensory processing. Under this view, SWS sits at one end of a continuous spectrum of arousal level and sensory processing with attention at the other end reflecting peak arousal and performance and reduced correlations (Cohen and Maunsell 2009). Correlated activity, however, is also thought to be an indicator of coordinated replay of waking experience during sleep (Wilson and McNaughton 1994). This points to an alternative interpretation that increases in correlation during deep sleep, although limiting auditory processing of external events, may reflect a novel state of the network conducive to processing internal events.
GRANTS
This research was supported by National Institute on Deafness and Other Communication Disorders Grant DC-03180 (X. Wang) and a Whitaker Fellowship (E. B. Issa).
DISCLOSURES
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
E.B.I. and X.W. conception and design of research; E.B.I. performed experiments; E.B.I. analyzed data; E.B.I. and X.W. interpreted results of experiments; E.B.I. prepared figures; E.B.I. drafted manuscript; E.B.I. and X.W. edited and revised manuscript; E.B.I. and X.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank A. Pistorio, E. Bartlett, D. Bendor, P. Crum, and Y. Zhou for help with animal care.
Present address of E. B. Issa: McGovern Institute for Brain Research, Dept. of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139.
REFERENCES
- Abbott LF, Dayan P. The effect of correlated variability on the accuracy of a population code. Neural Comput 11: 91–101, 1999 [DOI] [PubMed] [Google Scholar]
- Bair W, Zohary E, Newsome WT. Correlated firing in macaque visual area MT: time scales and relationship to behavior. J Neurosci 21: 1676–1697, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 12: 1594–1600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci 19: 4595–4608, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker AS, Berens P, Keliris GA, Bethge M, Logothetis NK, Tolias AS. Decorrelated neuronal firing in cortical microcircuits. Science 327: 584–587, 2010 [DOI] [PubMed] [Google Scholar]
- Edeline JM, Dutrieux G, Manunta Y, Hennevin E. Diversity of receptive field changes in auditory cortex during natural sleep. Eur J Neurosci 14: 1865–1880, 2001 [DOI] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci 12: 1444–1449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnisky DA, Dragoi V. Adaptive coding of visual information in neural populations. Nature 452: 220–224, 2008 [DOI] [PubMed] [Google Scholar]
- Issa EB, Wang X. Altered neural responses to sounds in primate primary auditory cortex during slow-wave sleep. J Neurosci 31: 2965–2973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa EB, Wang X. Sensory responses during sleep in primate primary and secondary auditory cortex. J Neurosci 28: 14467–14480, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermakowicz WJ, Chen X, Khaytin I, Bonds AB, Casagrande VA. Relationship between spontaneous and evoked spike-time correlations in primate visual cortex. J Neurophysiol 101: 2279–2289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Petkov CI, Logothetis NK. Tuning to sound frequency in auditory field potentials. J Neurophysiol 98: 1806–1809, 2007 [DOI] [PubMed] [Google Scholar]
- Kohn A, Smith MA. Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. J Neurosci 25: 3661–3673, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A, Zandvakili A, Smith MA. Correlations and brain states: from electrophysiology to functional imaging. Curr Opin Neurobiol 19: 434–438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster U, Sohl-Dickstein J, Gray CM, Olshausen BA. Higher order correlations within cortical layers dominate functional connectivity in microcolumns. arXiv:1301.0050 [q-bio.NC], 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiman G, Hung CP, Kraskov A, Quiroga RQ, Poggio T, DiCarlo JJ. Object selectivity of local field potentials and spikes in the macaque inferior temporal cortex. Neuron 49: 433–445, 2006 [DOI] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol 53: 926–939, 1985 [DOI] [PubMed] [Google Scholar]
- Liu J, Newsome WT. Local field potential in cortical area MT: stimulus tuning and behavioral correlations. J Neurosci 26: 7779–7790, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001 [DOI] [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nat Neurosci 4: 1131–1138, 2001 [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55: 131–141, 2007 [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 63: 879–888, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev 65: 37–100, 1985 [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science 229: 782–784, 1985 [DOI] [PubMed] [Google Scholar]
- Motter BC. Neural correlates of attentive selection for color or luminance in extrastriate area V4. J Neurosci 14: 2178–2189, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauhaus I, Busse L, Carandini M, Ringach DL. Stimulus contrast modulates functional connectivity in visual cortex. Nat Neurosci 12: 70–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena JL, Perez-Perera L, Bouvier M, Velluti RA. Sleep and wakefulness modulation of the neuronal firing in the auditory cortex of the guinea pig. Brain Res 816: 463–470, 1999 [DOI] [PubMed] [Google Scholar]
- Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454: 881–885, 2008 [DOI] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol 9: e1000610, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudi Y, Nirenberg S, Latham PE. Pairwise maximum entropy models for studying large biological systems: when they can work and when they can't. PLoS Comput Biol 5: e1000380, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneidman E, Berry MJ, Segev R, Bialek W. Weak pairwise correlations imply strongly correlated network states in a neural population. Nature 440: 1007–1012, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlens J, Field GD, Gauthier JL, Grivich MI, Petrusca D, Sher A, Litke AM, Chichilnisky EJ. The structure of multi-neuron firing patterns in primate retina. J Neurosci 26: 8254–8266, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Kohn A. Spatial and temporal scales of neuronal correlation in primary visual cortex. J Neurosci 28: 12591–12603, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13: 3252–3265, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85: 1969–1985, 2001 [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science 265: 676–679, 1994 [DOI] [PubMed] [Google Scholar]
- Worgotter F, Daunicht WJ, Eckmiller R. An on-line spike form discriminator for extracellular recordings based on an analog correlation technique. J Neurosci Methods 17: 141–151, 1986 [DOI] [PubMed] [Google Scholar]
- Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature 370: 140–143, 1994 [DOI] [PubMed] [Google Scholar]