Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating paralytic disorder caused by dysfunction and degeneration of motoneurons starting in adulthood. Recent studies using cell or animal models document that astrocytes expressing disease-causing mutations of human superoxide dismutase 1 (hSOD1) contribute to the pathogenesis of ALS by releasing a neurotoxic factor(s). Neither the mechanism by which this neurotoxic factor induces motoneuron death nor its cellular site of action has been elucidated. Here we show that acute exposure of primary wild-type spinal cord cultures to conditioned medium derived from astrocytes expressing mutant SOD1 (ACM-hSOD1G93A) increases persistent sodium inward currents (PCNa), repetitive firing, and intracellular calcium transients, leading to specific motoneuron death days later. In contrast to TTX, which paradoxically increased twofold the amplitude of calcium transients and killed motoneurons, reduction of hyperexcitability by other specific (mexiletine) and nonspecific (spermidine and riluzole) blockers of voltage-sensitive sodium (Nav) channels restored basal calcium transients and prevented motoneuron death induced by ACM-hSOD1G93A. These findings suggest that riluzole, the only FDA-approved drug with known benefits for ALS patients, acts by inhibiting hyperexcitability. Together, our data document that a critical element mediating the non-cell-autonomous toxicity of ACM-hSOD1G93A on motoneurons is increased excitability, an observation with direct implications for therapy of ALS.
Keywords: amyotrophic lateral sclerosis, motoneuron degeneration, sodium channel, hyperexcitability
amyotrophic lateral sclerosis (ALS) is a fatal, adult-onset paralytic disorder caused by degeneration of cranial and spinal motoneurons. Much of our understanding of this disease comes from studies of a subgroup of familial (FALS) cases that arises from mutations in the gene encoding for SOD1 (Beckman et al. 2001; Rosen et al. 1993; Valentine et al. 2005). Recent studies also raise the hypothesis that misfolded wild-type (WT) SOD1 is pathogenic in many individuals with sporadic ALS (Bosco et al. 2010; Haidet-Phillips et al. 2011). The precise mechanisms whereby mutant SOD1 or misfolded WT SOD1 is toxic to motoneurons are not defined. Also, the mechanisms of the beneficial actions of riluzole, the only FDA-approved ALS drug, have not yet been established (Bellingham 2011).
In vivo and in vitro studies using mutant human (h)SOD1 transgenic mice reveal many pathogenic changes in affected motoneurons, including hyperexcitability, disturbed calcium homeostasis, mitochondrial dysfunction, SOD1 aggregation, cytoskeletal disruption, activation of cell death signals, and oxidative stress (Bento-Abreu et al. 2010; Cleveland and Rothstein 2001; Pasinelli and Brown 2006; van Zundert et al. 2012). It is not clear yet whether these abnormalities are part of a primary or secondary event or the result of compensatory mechanisms.
To get insights into these issues, we previously investigated disturbances of physiological functions in motoneurons in acute slice preparations from the brain stem of neonatal hSOD1G93A mice [postnatal days (P)4–10], 2–3 mo prior to the appearance of motoneuron degeneration and clinical symptoms, and delineated functional alterations in motoneurons including increases in repetitive firing, synaptic transmission, and persistent sodium inward currents (PCNa) mediated by voltage-sensitive sodium (Nav) channels (van Zundert et al. 2008). To date, these are the earliest abnormalities documented for the hSOD1G93A mice (van Zundert et al. 2012). Our data, together with other findings on spinal and cortical neurons in cultures and slice preparations from mutant SOD-linked transgenic ALS mouse models, indicate that electrophysiological abnormalities in motoneurons are a very early event in ALS (Bories et al. 2007; Kuo et al. 2005; Pambo-Pambo et al. 2009; Quinlan et al. 2011), possibly because of disturbance of Nav channel activity causing an increment in PCNa (ElBasiouny et al. 2010; Kuo et al. 2005; Pieri et al. 2009; Quinlan et al. 2011; van Zundert et al. 2008, 2012).
Many elegant studies have shown that ALS is at least partially a non-cell-autonomous disease and that cells such as astrocytes expressing mutant hSOD1 contribute to the pathogenesis of ALS (reviewed in Ilieva et al. 2009). In in vitro models of ALS, for example, extensive death of primary spinal cord motoneurons and embryonic stem cell-derived motoneurons is induced when cells are cultured on astrocytes expressing hSOD1G93A or exposed to conditioned medium derived from astrocytes expressing mutant SOD1 (ACM-hSODG93A) (Di Giorgio et al. 2007; Nagai et al. 2007). However, the primary target of the toxic factor is not known. In the present study, using this ACM-hSODG93A in vitro model system, we have elucidated a critical role for hyperexcitability, mediated at least in part through increased Nav channel activity, in inducing motoneuron death.
MATERIALS AND METHODS
Animals.
Care and use of rodents was in accordance with National Institutes of Health guidelines and was approved by the Institutional Animal Care and Use Committees of Andres Bello University, the University of Concepción, and the University of Massachusetts. Hemizygous transgenic B6SJL mice carrying a high copy number of mutant hSOD1 (hSOD1G93A) or WT hSOD1 (hSOD1WT) were originally obtained from Jackson Laboratories (Bar Harbor, ME). Nontransgenic littermates (mSOD1WT) and transgenic mice overexpressing the gene for hSOD1WT were used as control subjects. Transgenes were identified by polymerase chain reaction (Rosen et al. 1993; van Zundert et al. 2008). The hSOD1G93A mice, but not the hSOD1WT mice, develop signs of neuromuscular deficits (tremor of the legs and loss of extension reflex of the hind paws) and have an average life span of 19–21 wk (Del Signore et al. 2009; Gurney et al. 1994; Scott et al. 2008).
Conditioned medium preparation.
ACM was prepared similarly as described previously (Nagai et al. 2007). Cultures of astrocytes were prepared from P1–2 WT mice and from transgenic mice expressing either hSOD1G93A or hSOD1WT. Cultures were maintained in DMEM (Hyclone SH30081.02) containing 10% FBS (Hyclone SH30071.03; lot ATC31648) and 1% penicillin-streptomycin (Invitrogen 15070-063) at 37°C and 5% CO2. Cultures reached confluence after ∼14 days and contained >95% glial fibrillary acidic protein (GFAP)-positive astrocytes. Residual microglial cells were removed by shaking in an orbital shaker (200 rpm in the incubator) for 6 h. Medium was then replaced by spinal culture medium (see below). After 7 days (21 DIV), ACM was collected, centrifuged (500 g for 10 min), and stored at −80°C. Before use, the ACM was supplemented with 4.5 mg/ml d-glucose (final concentration) and penicillin-streptomycin and filtered. We also added a chick hindlimb muscle extract (Sepulveda et al. 2010). For all experiments (including the motoneuron survival analysis, patch-clamp recordings, and calcium imaging), we used ACM that was diluted eightfold (12.5%); at this concentration ACM-hSOD1G93A strongly reduced motoneuron survival, whereas ACM-hSOD1WT was not toxic. The ACM was applied to ventral spinal cord cultures derived from rats because better-quality motoneurons can be obtained from these rodents relative to mice spinal cord cultures. The use of cocultures mixing different species (rat, mice, human) does not induce apparent side effects (see, e.g., Di Giorgio et al. 2007; Pehar et al. 2004; Nagai et al. 2007).
Primary neuronal cultures.
Pregnant Sprague-Dawley rats were deeply anesthetized with CO2, and primary spinal cultures were prepared from E14 pups (Sepulveda et al. 2010). Briefly, whole spinal cords were excised and placed into ice-cold HBSS (Invitrogen 14185-052) containing 50 μg/ml penicillin-streptomycin (Invitrogen 15070-063). The dorsal part of the spinal cord was removed with a small razor blade, and the ventral cord was minced and incubated with prewarmed HBSS containing 0.25% trypsin (Invitrogen 15090-046) in an incubator for 20 min at 37°C. After enzymatic treatment, the cells were transferred to a 15-ml tube containing neuronal growth medium [70% MEM (Invitrogen 11090-073), 25% Neurobasal media (Invitrogen 21103-049), 1% N-2 supplement (Invitrogen 17502-048), 1% l-glutamine (Invitrogen 25030-081), 1% penicillin-streptomycin (Invitrogen 15070-063), 2% horse serum (Hyclone SH30074.03; lot AQH24495), and 1 mM pyruvate (Sigma)]. Cells were precipitated, transferred to a new 15-ml tube containing 2 ml of growth medium, resuspended by mechanical agitation through fire-polished glass Pasteur pipettes of different tip diameters, and counted; 1 × 106 cells were plated on freshly prepared poly-l-lysine-coated six-well plates (1 mg/ml; mol wt 30,000–70,000; Sigma P2636) or 6-mm no. 00 coverslips (Menzel-Gläser) for calcium imaging experiments in growth medium; they were cultured for 7 days at 37°C under 5% CO2 and supplemented with 45 μg/ml E18 chick leg extract; medium was refreshed every 3 days. Riluzole (Sigma; 100 μM) was dissolved in distilled water (plus 10% Tween 20), while spermidine (Sigma), mexilitine (Sigma), and tetrodotoxin (TTX; Alomone) were dissolved in water and stored at 100 mg/ml at −20°C.
Cell labeling and counting.
Primary spinal cultures were fixed at 7 DIV with 4% paraformaldehyde and immunostained with an antibody against microtubule-associated protein 2 (MAP2, 1:400; Santa Cruz Biotechnology) to visualize all neurons (interneurons as well as motoneurons); immunostaining with the SMI-32 antibody (1:1,000; Sternberger Monoclonals) revealed the presence of unphosphorylated neurofilament-H, expressed specifically in motoneurons in spinal cord cultures (Nagai et al. 2007; Urushitani et al. 2006); previously we found that our WT primary spinal cultures typically contain at least 8–10% motoneurons until 12 DIV (Sepulveda et al. 2010). Fluorescent neurons were visualized with epifluorescent illumination on an Olympus IX81 microscope or on a Nikon C1 confocal microscope on which stacks of 0.50-μm optical sections were acquired through entire neurons. Labeling patterns were documented with a ×20 objective and a Q-Imaging Micropublisher 3.3 Real-Time Viewing camera; MAP2- and SMI-32-positive neurons were counted off-line within 20 randomly chosen fields, and the percentage of SMI-32-positive motoneurons within the total number of MAP2-positive cells was calculated. Each condition was replicated in at least three independent cultures and in duplicate.
Electrophysiology.
Whole cell patch-clamp recordings were taken from primary spinal neurons and analyzed as previously described (Sepulveda et al. 2010; van Zundert et al. 2008). For recording action potential (AP) firing and voltage-sensitive persistent sodium currents (PCNa) cells were maintained in a solution containing (in mM) 150 NaCl, 5.4 KCl, 2.0 CaCl2, 2.0 MgCl2, 10 HEPES (pH 7.4), and 10 glucose. After formation of a high-resistance seal and break-in (>800 MΩ), whole cell voltage signals were recorded with an Axopatch 200B amplifier (Molecular Devices). Pipette and whole cell capacitance and series resistance were compensated with amplifier circuitry. Signals were low-pass filtered (5 kHz) and digitized (5–40 kHz) on a PC with pCLAMP 9.2 software. The passive membrane properties were measured from those cells from which we recorded APs. The resting membrane potential was corrected for a calculated junction potential of 13.7 mV (JPCalc, Clampfit). For recording APs, a K gluconate-based internal solution was used containing (in mM) 17.5 KCl, 122.5 K gluconate, 9 NaCl, 1 MgCl2, 10 HEPES, 0.2 EGTA, 3 MgATP, and 0.3 GTP-Tris. APs were evoked by injection of rectangular depolarizing current pulses (10–40 pA for 300 ms). Cells were discarded if resting membrane potential was more than −40 mV or if a train of APs could not be generated in response to any depolarizing current steps. For recording PCNa, a Cs gluconate-based internal solution was used containing (in mM) 122.5 Cs gluconate, 17.5 CsCl, 8 NaCl, 10 HEPES, 0.2 EGTA, 2 MgATP, and 0.3 GTP-Na. In some experiments, we added CdCl2 (100 μM to the bath) to block voltage-gated calcium channels and TEA-Cl (5 mM to pipette and 10 mM to bath) and 4-aminopyridine (1 mM to the bath) to block potassium channels (Fleidervish et al. 2008). PCNa was recorded by applying a slow voltage ramp from −60 mV to +10 mV and back (14 mV/s) (Powers and Binder 2003). Cell-attached recordings were performed simultaneously with calcium imaging at 34°C, as previously described (Madrid et al. 2006), with 7- to 8-MΩ patch pipettes fabricated from GC150F-7.5 borosilicate glass capillaries (Harvard Apparatus) using Clampex 10.3.
Calcium imaging.
Calcium imaging studies were carried out under controlled temperature (34 ± 1°C) (Madrid et al. 2006) as previously described with some modification. Cultured spinal neurons were incubated with 5 μM fura-2 AM (Invitrogen) dissolved in standard extracellular solution and 0.02% Pluronic (Invitrogen) for 45 min at 37°C. Fluorescence measurements were made with a Nikon Ti inverted microscope fitted with a 12-bit cooled CCD camera (Orca C8484–03G02; Hamamatsu). Fura-2 was excited at 340 nm and 380 nm with a Polychrome V monochromator (Till Photonics), and the emitted fluorescence was filtered with a 510-nm long-pass filter; 340 to 380 nm ratios (at 1 Hz) were displayed online with HCImage software v.2.2.1 (Hamamatsu). Bath temperature was sampled simultaneously with intracellular calcium recordings with a BAT-12 microprobe thermometer, supplemented with an IT-18 T-thermocouple (Physitemp Instruments), using Clampex 10.3 Software (Molecular Devices). Coverslip pieces (6 mm in diameter, 55–80 μm in thickness; no. 00 from Menzel-Gläser) with cultured neurons were placed in a microchamber and continuously perfused (∼1 ml/min) with solutions warmed at 34 ± 1°C. The temperature was adjusted with a water-cooled computer-controlled Peltier device, with the outlet close to the imaging field and controlled by a feedback device.
Pharmacological treatments in culture.
Riluzole (Sigma) was dissolved in distilled water (plus 10% Tween 20) at 100 μM and added to cultures to final concentrations ranging from 25 nM to 500 nM. Spermidine (Sigma) was dissolved in water at 100 mg/ml and added to cultures to a final concentration of 10 μM. A 1 mM stock solution of TTX (Alomone) was prepared, citrate buffered, and added to cultures to final concentrations ranging from 1 to 500 nM. Mexiletine (Tocris) was dissolved in water to 100 mM and used at final concentrations ranging from 5 to 25 nM.
Data analysis.
ANOVA, followed by post hoc Tukey tests, was used to detect significant changes. Student's t-tests were used to compare the response of two populations to individual treatments. Nonparametric Kolmogorov-Smirnov test was used to test the significance of the cumulative distributions of the calcium transient amplitudes.
RESULTS
Chronic and short-term treatment of primary spinal cultures with ACM-hSOD1G93A triggers death of motoneurons.
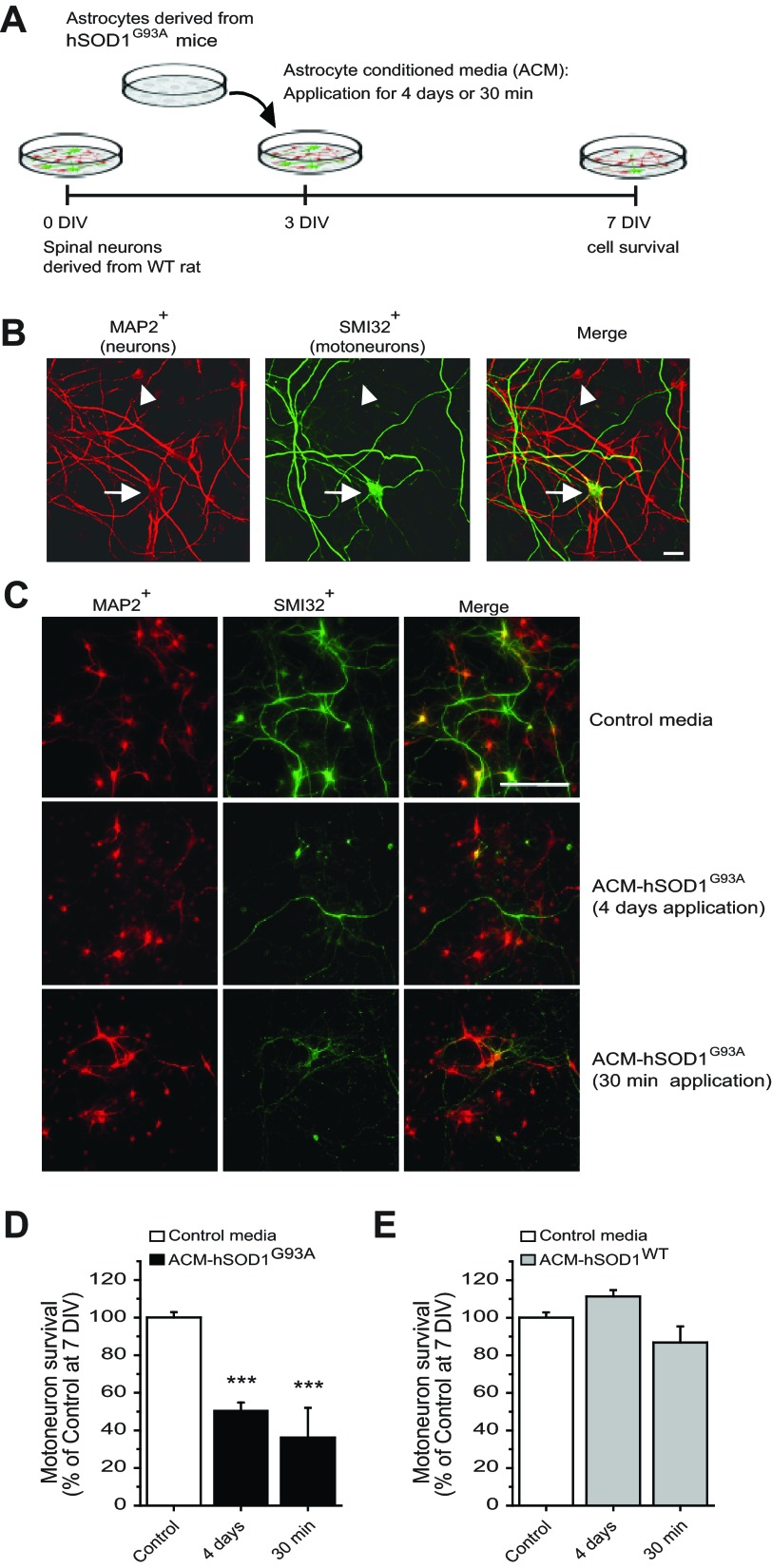
In vitro, chronic exposure of primary mouse spinal cord neurons to undiluted ACM from transgenic rodents expressing mutant hSOD1, including the G93A allele (ACM-hSOD1G93A), causes extensive (∼60%) and specific death of motoneurons (Nagai et al. 2007). The identity of the offending soluble toxic factors is not known. We have used this model to gain insight into the nature of the toxic soluble factors in ACM-hSOD1G93A and into its primary targets. ACM-hSOD1G93A prepared with astrocytes derived from hSOD1G93A mice was added at an eightfold dilution (see materials and methods) to WT primary rat spinal cultures at 3 DIV for either 4 days or 30 min; its effects on neuron survival were assessed at 7 DIV (Fig. 1A). To define the presence of both interneurons and motoneurons an antibody against MAP2 was used (Fig. 1, B and C). The SMI-32 antibody, which recognizes unphosphorylated neurofilament-H, was used to specifically identify motoneurons in spinal cord cultures (Fig. 1, B and C), as previously described (Nagai et al. 2007; Sepulveda et al. 2010; Urushitani et al. 2006).
Fig. 1.
Long-term and short-term treatment of primary spinal cultures with ACM-hSOD1G93A equally trigger cell death of motoneurons. A: flow diagram of experiment. Medium was conditioned for 7 days by astrocytes derived from transgenic mice overexpressing human (h)SOD1G93A (ACM-hSOD1G93A). Primary wild-type (WT) rat spinal cord cultures (3 DIV) were exposed to ACM-hSOD1G93A for 4 days or for 30 min; all were fixed at 7 DIV to assay cell survival with immunocytochemistry. B: fixed 7 DIV primary spinal cultures were double-labeled with anti-microtubule-associated protein 2 (MAP2) antibody (red) to visualize interneurons (arrowhead) and motoneurons (arrow) and with the SMI-32 antibody (green) to identify motoneurons (arrow). Scale bar, 25 μm. C: spinal cultures were treated with control medium for 4 days (top) or ACM-hSOD1G93A for either 4 days (middle) or 30 min (bottom), fixed at 7 DIV, and labeled with MAP2 and SMI-32. Note that a single, short-term 30-min exposure of spinal cord neurons to ACM-hSOD1G93A is as effective in triggering motoneuron cell death as chronic application. Interneurons are spared in both conditions. Scale bar, 200 μm. D: graph of the ratio of SMI-32+/MAP2+ neurons showing % of surviving motoneurons at 7 DIV after treatment with control medium or ACM-hSOD1G93A applied for 4 days or 30 min. E: % of surviving motoneurons at 7 DIV after 4-day or 30-min application of 3 DIV cultures of medium that was conditioned by astrocytes derived from transgenic mice overexpressing hSOD1WT (ACM-hSOD1WT). Values represent means ± SE from at least 3 independent experiments performed in duplicate, analyzed by 1-way ANOVA followed by a Tukey post hoc test. ***P < 0.001 relative to control medium at 7 DIV.
Consistent with previous studies (Nagai et al. 2007), chronic 4-day exposure (from 3 to 7 DIV) of spinal cultures to ACM-hSOD1G93A induced ∼50% motoneuron death (Fig. 1, C and D). In addition, we found that a 30-min exposure of 3 DIV spinal cultures to ACM-hSOD1G93A was sufficient to cause a similar extensive death of motoneurons analyzed at 7 DIV (Fig. 1, C and D). We also obtained control ACM from astrocytes harvested from transgenic mice that carry the nonpathological human WT SOD1 gene (ACM-hSOD1WT) or from nontransgenic mice (ACM-mSOD1WT). Neither of these media caused motoneuron death (Fig. 1D and data not shown). The finding that ACM-hSOD1WT was not toxic indicates that the factor inducing motoneuron death is specifically attributable to the hSOD1G93A mutation, rather than to the overexpression of the hSOD1 protein.
ACM-hSOD1G93A rapidly increases PCNa, neuronal excitability, and intracellular calcium dynamics.
We next sought to identify potential targets of the soluble toxic factors in the ACM-hSOD1G93A. We had previously shown in acute slice preparations from neonatal hSOD1G93A mice that mutant motoneurons displayed increases in repetitive firing, synaptic transmission, and PCNa (van Zundert et al. 2008). In light of these findings, we hypothesized that ACM-hSOD1G93A application enhances Nav channel permeability and thereby increases PCNa, excitability, and synaptic transmission, leading to sustained and toxic levels of sodium and calcium influxes that instigate motoneuron death. To get insights into this hypothesis, we first exposed 5–7 DIV spinal neurons to ACM-hSOD1G93A and analyzed functional changes by whole cell patch recordings and calcium imaging.
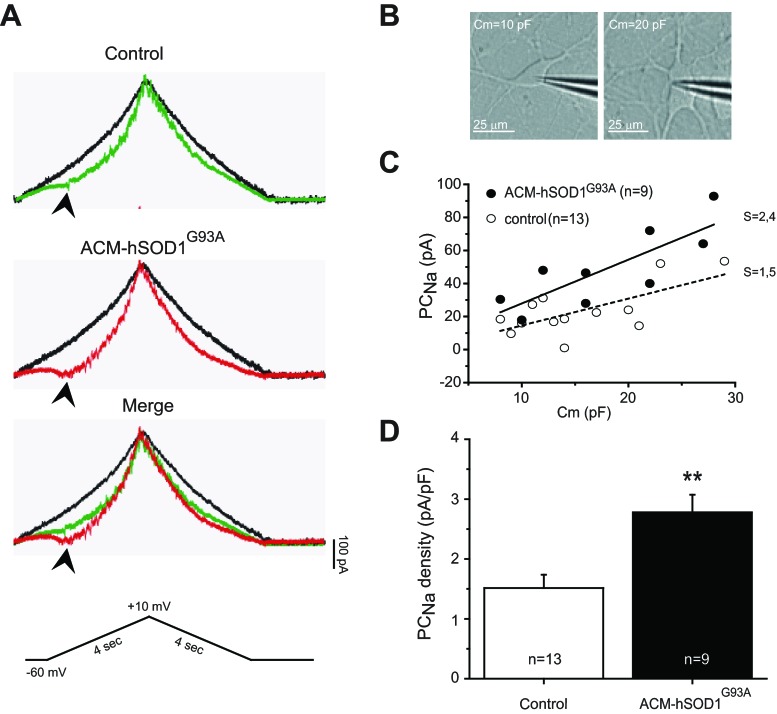
To analyze the effect of mutant SOD1 on the PCNa, we treated primary spinal cultures with ACM-hSOD1G93A for 30–90 min and selectively recorded this “noninactivating” current, independent of the transient sodium current (TNa), by applying a slow voltage ramp from −60 mV to +10 mV and back (14 mV/s) (Fig. 2A, bottom) as described previously (Kuo et al. 2005; Powers and Binder 2003; Theiss et al. 2007; van Zundert et al. 2008). Representative sample traces reveal that, compared with control, untreated cells (Fig. 2A), application of ACM-hSOD1G93A generated a marked inward current, which peaked at around −40 mV (Fig. 2A). Typically, much of the inward persistent current was eliminated by application of the Nav channel blocker TTX (1 μM) (Fig. 2A), indicating that the persistent inward current derives predominantly from Nav channels (PCNa) rather than voltage-sensitive calcium (Cav) channels in these young spinal cultures.
Fig. 2.
ACM-hSOD1G93A increases persistent sodium inward current (PCNa). A: representative persistent inward current traces generated by a slow triangular voltage-clamp command (14 mV/s) from a holding potential of −60 to +10 mV (4 s) and back (4 s) (bottom) in the absence (colored traces) or presence (black traces) of the voltage-sensitive sodium (Nav) channel blocker TTX (1 μM); recordings from a 6 DIV control spinal neuron (top) and a sister neuron incubated with ACM-hSOD1G93A for ≥30 min (middle). As can be observed from the merge (bottom), PCNa (arrowhead; TTX-sensitive inward current activated at voltages starting at around −40 mV) is significantly larger in neurons treated with ACM-hSOD1G93A (red traces) relative to the control cell (green traces). B: typical patched interneuron with cell membrane area [membrane capacitance (Cm)] of ∼10 pF (left) and typical patched motoneuron with Cm of ∼20 pF (right). C: PCNa of individual spinal cord neurons (5–7 DIV) were generated under control conditions (dashed line) or after application of ACM-hSOD1G93A for 30–90 min (solid line) by a slow voltage-clamp command (as in A) and plotted against its Cm. The best-fitted slopes (S) highlight that PCNa is dependent on the size of the neurons. D: averaged mean peak PCNa amplitude normalized to Cm (pA/pF) for 5–7 DIV control neurons and those incubated with ACM-hSOD1G93A. Values represent means ± SE from at least 9 neurons, analyzed by t-test. **P < 0.01 relative to control.
We next asked whether the ACM-hSOD1G93A-induced PCNa can be detected in all neurons (including interneurons) or is exclusive to motoneurons. As one approach to this problem we examined the relationship between the maximum PCNa (quantified by measuring the peak of the amplitude of the inward current after subtraction of the TTX-insensitive current) and membrane capacitance (Cm) as a surrogate marker for neuronal size. We observed that neurons with a Cm of >20 pF predominantly displayed a large soma (>20-μm diameter) and expressed five or more primary dendrites; these parameters are typical for motoneurons (Fig. 2B, right). In contrast, neurons with a Cm of <15 pF are likely to be interneurons, as they are smaller (<20-μm diameter) and typically contain three or four primary branches (Fig. 2B, left). The plots in Fig. 2C show that for control neurons as well as for neurons treated with ACM-hSOD1G93A, the magnitude of the sodium inward current is dependent on the size of the neuron: the larger the neuron, the larger the PCNa. After the inward current was normalized for the area of the cell membrane, the mean peak amplitude of the PCNa in primary spinal cultures treated with ACM-hSOD1G93A (2.8 ± 0.3 pA/pF) was twice the size of that for control neurons (1.5 ± 0.2 pA/pF; t-test, P < 0.01) (Fig. 2D).
To analyze the effect of mutant SOD1 on neuronal excitability, we performed current-clamp recordings to examine spiking frequency in response to depolarizing current steps. Remarkably, within minutes of ACM-hSOD1G93A application, the patched neuron started to fire APs at significantly higher rates (Fig. 3A). After 30 min of ACM-hSOD1G93A application, the average neuronal firing rate increased by up to 151 ± 17% (t-test, P < 0.05 relative to ACM-hSOD1WT at minute 30) (Fig. 3B). In contrast, application of ACM-hSOD1WT did not change AP firing rates during the same exposure (97 ± 11% relative to minute 0) (Fig. 3B). Analyses of both passive and active properties of spinal cord neurons before and after 30 min of bath application of ACM-hSOD1G93A did not reveal other significant differences (data not shown).
Fig. 3.
ACM-hSOD1G93A rapidly increases neuronal excitability. A: whole cell current-clamp recordings of control primary spinal neuron in the presence of ACM-hSOD1G93A. Action potentials (APs) are evoked by injection of rectangular depolarizing current pulses (bottom; 10, 20, and 40 pA for 300 ms). Representative membrane potential traces show that bath application of ACM-hSOD1G93A to 5–7 DIV primary spinal neurons rapidly increases the firing frequency (arrowheads). B: average change in AP firing frequency produced by 30-min application of ACM-hSOD1G93A or ACM-hSOD1WT. Values represent means ± SE from at least 5 neurons/time point, analyzed by t-test. *P < 0.05 compared with ACM-hSOD1WT at same time point.
We also performed some experiments to get insights into whether in addition to the non-cell-autonomous effects mediated by ACM-hSOD1G93A exogenous introduction of mutant SOD1 into neurons can induce cell-autonomous effects. For this, ventral spinal cord neurons (VSCNs, 4–5 DIV) were transfected with a CaPO4 transfection protocol (Sepulveda et al. 2010) with a plasmid encoding for either hSOD1G93A-GFP or hSOD1WT-GFP (Turner et al. 2005). Twenty-four hours after exogenous expression, voltage steps from −70 to −40 mV were applied to transfected neurons to generate Nav channel-dependent currents; because the transfection rate is very low (<5%) and VSCNs contain 90–92% interneurons and only 8–10% motoneurons, our analyses were performed on healthy-looking transfected interneurons. Note that electrophysiological recordings displayed in Figs. 2 and 3 also include predominantly interneurons. Because transfected neurons with hSOD1G93A-GFP (but hSOD1WT-GFP) did not display resting membrane potentials more negative than −40 mV, required to detect reliable APs, we were unable to determine whether the excitability of neurons expressing mutant SOD1 was increased. Next we applied voltage steps from −70 to −40 mV to generate Nav channel-dependent currents. We found that hSOD1G93A-GFP-transfected neurons displayed an approximately threefold increase in the maximum Nav channel-mediated current compared with hSOD1WT-GFP-expressing neurons [−3,906 ± 1,220 pA in hSOD1G93A-GFP (n = 3) vs. −1,101 ± 219 pA in hSOD1WT-GFP (n = 3); P < 0.001]. These results indicate that mutant SOD1G93A also mediates cell-autonomous effects through regulation of Nav channels.
Next we evaluated whether hSOD1G93A-GFP-transfected neurons displayed DNA damage (Hoechst and DAPI staining), membrane permeabilization (incorporation of rhodamine-conjugated dextran amine mini-Ruby), and caspase-3 activation (immunocytochemistry with a caspase-3 phosphorylation antibody). We exogenously introduced either hSOD1G93A-GFP or hSOD1WT-GFP into 4 DIV VSCNs and analyzed these parameters 1–4 days later. ACM-hSOD1G93A and H2O2 (as positive control) were also applied to the cultures. In contrast to H2O2 application, neither addition of ACM-hSOD1G93A nor expression of hSOD1G93A-GFP (or hSOD1WT-GFP) resulted in DNA damage, membrane permeabilization, or caspase-3 activation of interneurons (data not shown). These results are in agreement with our findings that ACM-hSOD1G93A application does not affect interneuron cell survival (Fig. 1) and indicate that although mutant SOD1G93A mediates cell-autonomous and non-cell-autonomous effects through regulation of Nav channels, the activation of these channels is insufficient to cause detectable pathology and cell death in interneurons.
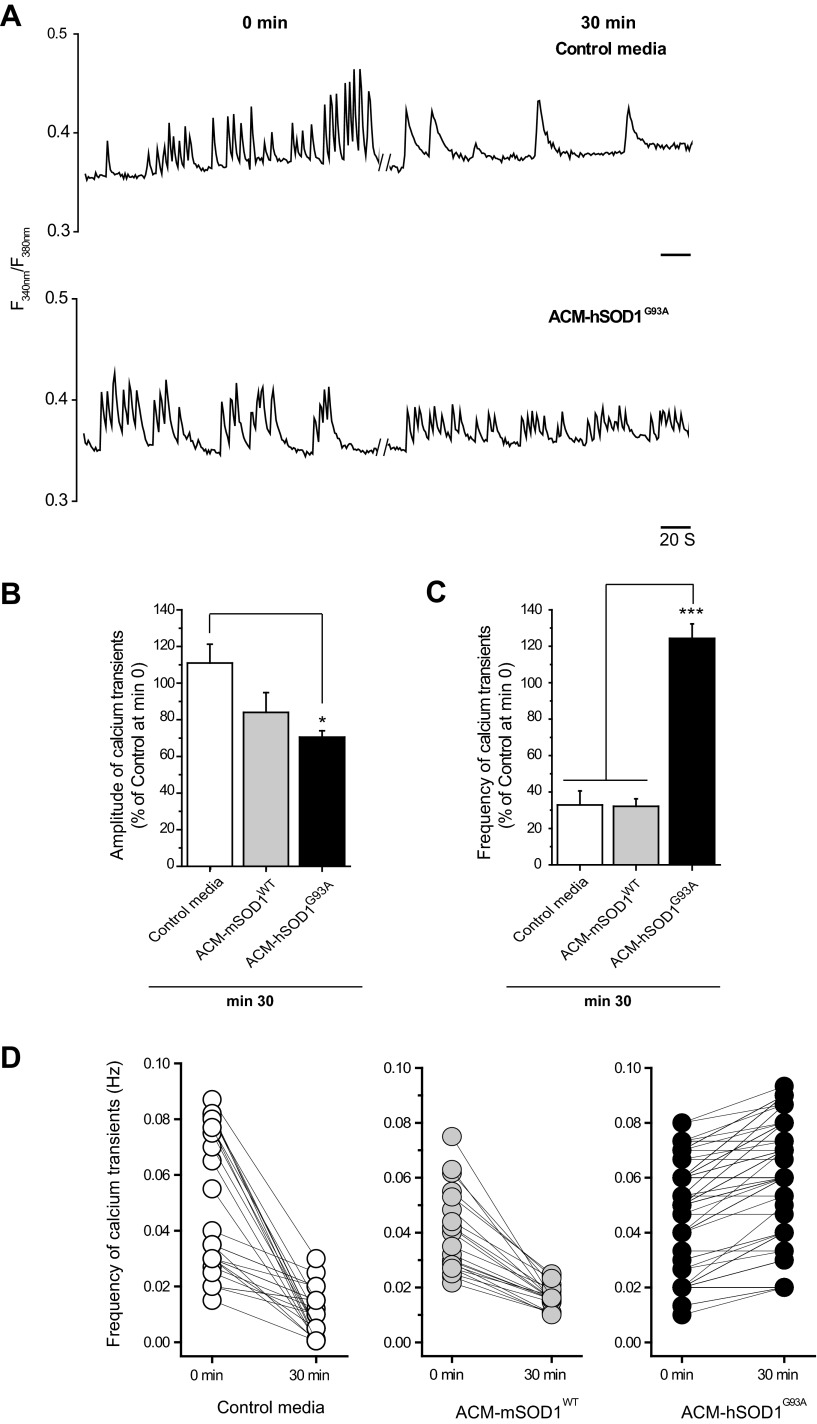
To determine whether hyperexcitability induced by ACM-hSOD1G93A also affects calcium homeostasis, we recorded intracellular calcium transients ([Ca2+]i) in spinal neurons in culture, using fura-2. In ∼43% of the neurons we observed spontaneous oscillations in [Ca2+]i as shown in the representative example traces in Fig. 4A. These calcium transients showed an averaged amplitude of 0.05 (ΔF340/F380) and a basal mean frequency of 0.06 Hz, often in bursts. The smallest value of ΔF340/F380 considered as a calcium transient was 0.01. Under control conditions or after administration of ACM-mSOD1WT the frequency of calcium transients declined until ∼30% of the initial value at minute 30 (Fig. 4, A and C). By contrast, 30-min application of ACM-hSOD1G93A markedly increased the frequency of the calcium transients (124 ± 8% of control at minute 0; ANOVA, P < 0.001 relative to control and ACM-hSOD1G93A at minute 30) (Fig. 4, A, C, and D). Short-term application of ACM-hSOD1G93A also significantly (P < 0.05) reduced the mean amplitude of the calcium transients (Fig. 4B).
Fig. 4.
ACM-hSOD1G93A rapidly increases the frequency of calcium transients in cultured spinal neurons. A: representative ratiometric intracellular calcium transient ([Ca2+]i) traces of 2 independent spinal neurons (5–7 DIV) measured before (minute 0, left) and 30 min after application of either control medium or ACM-hSOD1G93A (minute 30, right). Parallel lines correspond to 30-min incubation periods. B and C: mean fraction of calcium transient amplitudes (B) and frequencies (C) at 30 min after treatment with the different media, relative to control activity measured at minute 0. Values represent means ± SE from 23–45 neurons/condition, analyzed by 1-way ANOVA followed by a Tukey post hoc test. *P < 0.05 and ***P < 0.001 relative to control medium and ACM-mSOD1WT at minute 30. D: line series graphs of calcium transients of individual neurons at 0 and 30 min under control conditions (left) or after treatment with ACM-mSOD1WT (center) or ACM-hSOD1G93A (right).
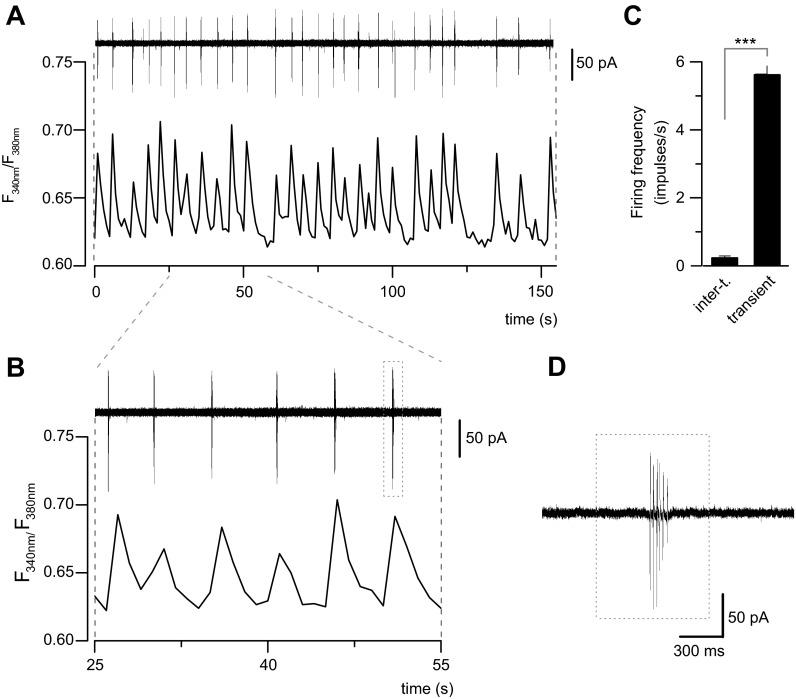
To study the correlation of calcium transients with the electrical excitability of cultured neurons under noninvasive conditions, we performed simultaneous recordings of [Ca2+]i and AP firing from the soma, using the cell-attached mode of the patch-clamp technique (Fig. 5). As we show in Fig. 5, A and B, spontaneous AP firing in these neurons appeared in bursts (see Fig. 5D), which are coincident with calcium transients (Fig. 5, A and B). The mean firing frequency during a calcium transient was 5.6 ± 0.3 impulses/s (Fig. 5C) quantified in 198 transients from four independent experiments. Intertransient periods were in parallel with an almost complete absence of AP firing (Fig. 5C). These results are consistent with the idea that calcium transients are directly related to spontaneous AP firing of cultured spinal cord neurons. Accordingly, the increase of the frequency of calcium transients induced by ACM-hSOD1G93A could be due to an increase in neuronal excitability.
Fig. 5.
Calcium transients in cultured spinal cord neurons correlate with AP firing. A: simultaneous recording of spontaneous AP firing in cell-attached mode (top) and calcium transients (bottom) in a spinal cord neuron. B: expanded timescale for the neuron in A. Note the coincidence of calcium transients with bursts of AP firing. C: bar graph showing the mean frequency of AP firing from 198 transients recorded in 4 cultured spinal cord neurons. Note that during intertransient (inter-t.) periods, AP firing is virtually absent. Values represent means ± SE. ***P < 0.001, t-test. D: action currents in an expanded timescale from the last burst in B (dotted box).
Effects of Nav channel blockers on functional alterations and motoneuron death induced by ACM-hSOD1G93A.
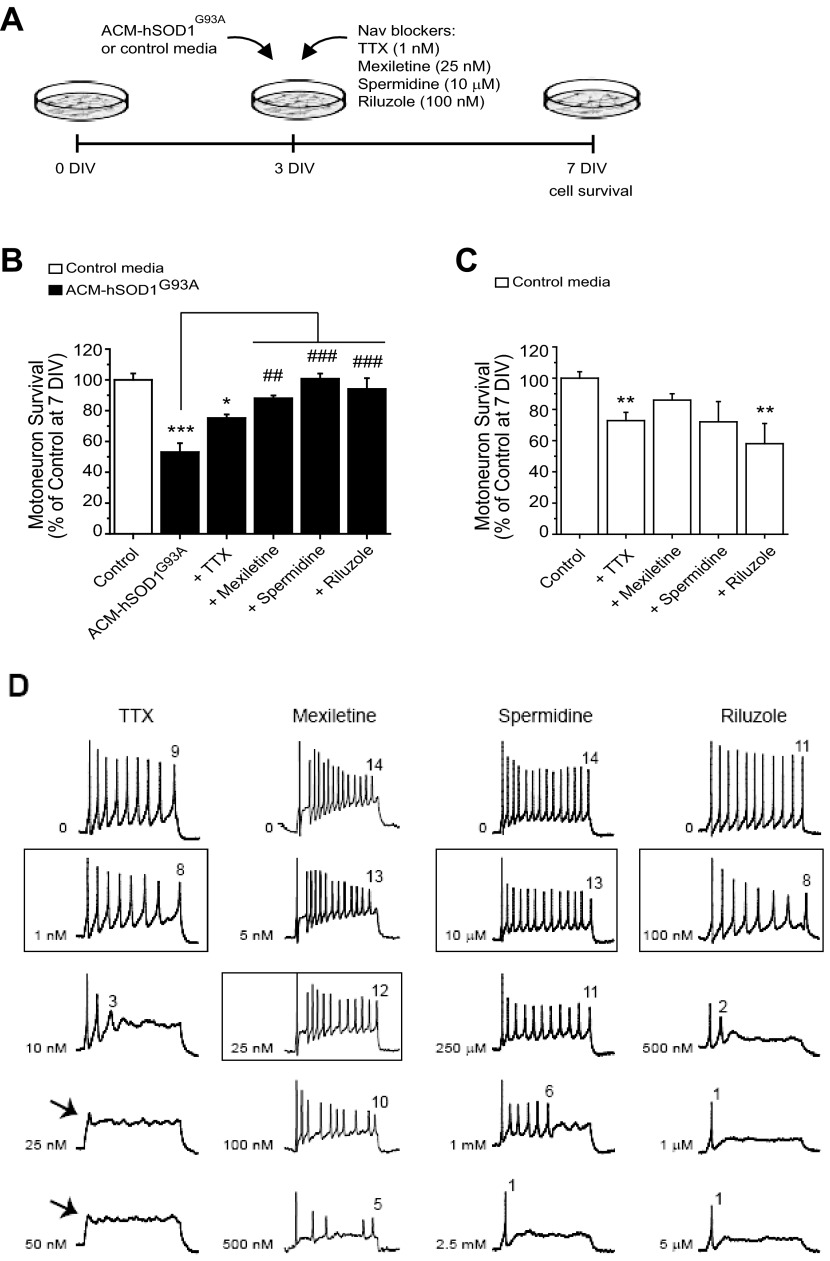
Because neuronal excitability regulates synaptic transmission and fluxes of sodium and calcium, we tested whether it would be possible to rescue motoneuron death induced by ACM-hSOD1G93A by pharmacological blockage of Nav channels to counterbalance the increased excitability evoked by this toxic medium. Toward that end, we tested TTX, mexiletine, spermidine, and riluzole, reagents well documented to block Nav channel permeability, thereby reducing PCNa and the frequency of APs (Bellingham 2011; Fleidervish et al. 2008; Kuo et al. 2005; Olschewiski et al. 2009; Theiss et al. 2007), as we also confirmed in the present study (see Fig. 6D for effects of drugs on excitability). The effects of multiple doses of each Nav channel blocker on the survival of motoneurons were assessed under control conditions and after coapplication with ACM-SOD1G93A (data not shown); for each drug the maximum effect in rescuing motoneuron survival is displayed in Fig. 6, A–C.
Fig. 6.
Nav channel blockers prevent ACM-hSOD1G93A-induced motoneuron death at doses that slightly reduce excitability. A: flow diagram of experiment. Medium (ACM-hSOD1G93A or control medium) was applied chronically at 3 DIV (as in Fig. 1) alone or together with the Nav blockers TTX (1 nM), mexiletine (25 nM), spermidine (10 μM), or riluzole (100 nM), and cell survival was assayed at 7 DIV. B: % of surviving motoneurons at 7 DIV treated with the different Nav blockers, relative to sister neurons treated with control medium (indicated with *) or to ACM-hSOD1G93A alone (indicated with #). Note that the drugs mexiletine, spermidine, and riluzole prevented motoneuron death induced by ACM-hSOD1G93A. TTX trends toward prevention but is unable to significantly reduce ACM-hSOD1G93A-mediated motoneuron death. C: effects of the same doses of Nav channel blockers, coapplied with control medium, on motoneuron survival. Note that the drugs did not improve survival of control neurons. Values represent means ± SE from at least 3 independent experiments, analyzed by 1-way ANOVA followed by a Tukey post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 relative to survival with control medium at 7 DIV; ##P < 0.01, ###P < 0.001 compared with survival with ACM-hSOD1G93A at 7 DIV. D: representative membrane potential traces (rectangular current pulse of 100 pA for 500 ms) of control 5–7 DIV primary spinal neurons treated with increasing concentrations of TTX, mexiletine, spermidine, or riluzole; the boxes highlight the doses used for the above motoneuron survival studies. Number stated above the last AP in each trace indicates the number of spikes in the train. Note that at the doses at which mexiletine, spermidine, and riluzole were able to prevent the ACM-hSOD1G93A-induced cell death, these compounds only eliminated a few spikes within the train of APs. At higher doses, spike suppression was strong and drugs killed motoneurons (data not shown). Also, while even high concentrations of mexiletine and spermidine do not suppress the initial firing, increasing doses of riluzole and TTX abruptly reduce the firing pattern and rate and ultimately abolish the first AP (arrows).
We first analyzed TTX, a classic neuronal Nav channel pore blocker that binds the “neurotoxin receptor at site 1” with IC50 values in the nanomolar range (reviewed in Catterall et al. 2005). We found that chronic application of TTX at a wide range of concentrations (1–500 nM) was unable to significantly prevent ACM-hSOD1G93A-induced motoneuron death; under the best circumstances, in the presence of ACM-SOD1G93A and 1 nM TTX motoneuron survival was only 75 ± 2% (Fig. 6B; P < 0.05 relative to control and P > 0.05 relative to ACM-SOD1G93A). Notably, however, we found that comparable administration of TTX to control spinal cord cultures also significantly killed motoneurons (Fig. 6C; P < 0.01 relative to control). In our view, this initially surprising result reflects a paradoxical effect of TTX on enhancing drastically the amplitude of calcium transients (see below), possibly by increasing neuronal excitability by homeostatic mechanisms (Desai et al. 1999; Fishbein and Segal 2007; Koch et al. 2010; Schonfeld-Dado et al. 2009). We next tested mexiletine, an orally active lidocaine analog, which is a local anesthetic and antiarrhythmic drug that targets the “local anesthetic receptor site” of Nav channels with strong use- or activity-dependent properties and with wide ranges of IC50 (Catterall et al. 2005; Olschewiski et al. 2009; Ragsdale et al. 1994). Importantly, we found that chronic coapplication of ACM-SOD1G93A with 25 nM mexiletine prevented motoneuron death (88 ± 2% survival; ANOVA, P < 0.01 relative to ACM-SOD1G93A and P > 0.05 relative to control) (Fig. 6B). We also observed that chronic application of this specific Nav channel blocker to control spinal cord cultures did not cause significant motoneuron cell death (Fig. 6C). The beneficial effects of mexiletine compared with TTX might be a consequence of the strong use-dependent properties of mexiletine. Thus antiarrhythmics such as mexiletine, unlike TTX, are very suitable for clinical use because they are able to curtail high-frequency firing of excitable neurons at concentrations that have little effect on basal activity. Consequently, as can be observed in the representative traces of Fig. 6D, although low doses of mexiletine (5 nM) reduce the number of spikes within the AP train, substantially increasing concentrations by 5 (25 nM) to 20 (100 nM) times causes only a gradual suppression in the neuronal activity. Even at very high doses of mexiletine (500 nM), APs are still generated and within a train pattern. This is very different from the influence of TTX (Fig. 6D), for which a 10-fold increase in concentration (from 1 to 10 nM) largely suppresses spiking and dissembles the train pattern. Slightly higher doses of TTX (≥25 nM) even suppress the initial firing altogether (Fig. 6D).
Next we analyzed the effects of the polyamine spermidine, which is known to affect the gating of various ion channels and serves as an endogenous, activity-dependent Nav channel blocker; its affinity is in the micromolar range but depends on the membrane potential (Fleidervish et al. 2008; Williams 1997). We found that 10 μM spermidine totally prevented motoneuron death induced by ACM-SOD1G93A (101 ± 3% survival; ANOVA, P < 0.001 relative to ACM-SOD1G93A and P > 0.05 relative to control) (Fig. 6B). In addition, large increases in spermidine concentrations (e.g., 25 times from 10 to 250 μM) eliminated only a few APs without altering spiking patterns until very high doses (1–2.5 mM) (Fig. 6D).
Finally, we tested riluzole, which over a large dose range (<1 μM to 1 mM) has multiple effects including inhibition of neurotransmitter release and potentiation of glutamate transporters (reviewed in Bellingham 2011). Riluzole also suppresses neuronal excitability at low concentrations from 100 nM to 5.0 μM by affecting Nav channels (Bellingham 2011; Kuo et al. 2005; Theiss et al. 2007). Interestingly, we found that chronic coapplication of ACM-SOD1G93A with 100 nM riluzole was able to completely prevent motoneuron death (94 ± 7% survival; ANOVA, P < 0.001 relative to ACM-SOD1G93A and P > 0.05 relative to control) (Fig. 6B). As with the other reagents, the dose of riluzole that prevented ACM-hSOD1G93A-induced motoneuron death caused only a slight reduction in spiking frequency (Fig. 6D). However, riluzole appears not to be an optimal therapeutic drug, as it causes death of control neurons (Fig. 6C), possibly because of the fact that this drug affects not only PCNa but also many other targets that influence neuronal activity, including calcium-dependent K+ currents, voltage-gated K+ currents, voltage-gated Ca2+ currents, etc. (Bellingham 2011). We also found that riluzole, at increasing concentrations, rapidly suppresses the spiking and train pattern, while it leaves the initial AP intact (Fig. 6D). Together, our data suggest that the favorable effects of mexiletine, spermidine, and riluzole on motoneuron survival are principally the result of counterbalancing the increases in neuronal excitability induced by ACM-hSODG93A.
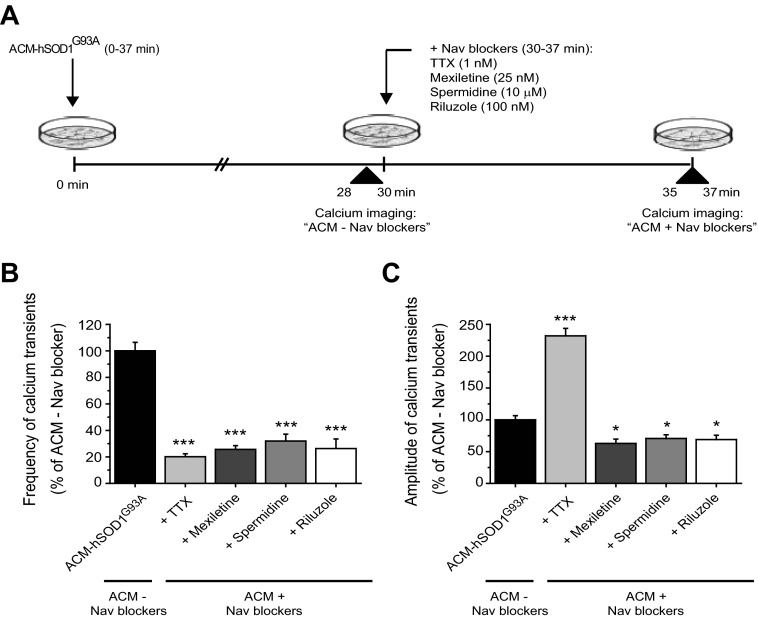
We next analyzed the effects of Nav channel blockers on the altered calcium homeostasis induced by ACM-hSOD1G93A (Fig. 7A), using the same concentrations that had been used to test cell survival. Application of mexiletine, spermidine, or riluzole to cultures incubated for the first 30 min with ACM-hSOD1G93A markedly decreased both the average frequency and the amplitude of the intracellular transients within minutes (Fig. 7, B and C). By contrast, application of TTX differentially affected the [Ca2+]i dynamics. Like the other Nav-blocking compounds, TTX significantly reduced the mean frequency of [Ca2+]i (Fig. 7B); however, it also strongly increased (by 225%) the mean amplitude of the remaining transients (Fig. 7C). As discussed below, this effect of TTX, which seems paradoxical by comparison with the effect of mexiletine, spermidine, and riluzole, may account for its toxicity to control neurons and its inability to prevent ACM-SOD1G93A-induced motoneuron death (see Fig. 6).
Fig. 7.
Acute application of Nav channel blockers has differential effects on calcium dynamics modified by ACM-hSOD1G93A. A: flow diagram of experiment. ACM-hSOD1G93A was applied on 5–7 DIV spinal cultures for 30 min (as in Fig. 3) to measure calcium transients (ACM − Nav blockers). Thereafter, Nav channel blockers TTX (1 nM), mexiletine (25 nM), spermidine (10 μM), and riluzole (100 nM) were applied to measure calcium transients of the same neurons 7 min later (ACM + Nav blockers); Nav channel blockers were used at the same concentrations as in Fig. 4. B and C: mean fraction of calcium transient frequencies (B) and amplitudes (C) after ACM-hSOD1G93A and drug treatments (ACM + Nav blockers) relative to ACM-hSOD1G93A alone (ACM − Nav blockers). Note that all Nav channel blockers markedly decreased mean frequency (to 20–30%). However, whereas mexiletine, spermidine, and riluzole significantly decreased the mean amplitude of the calcium transients (to 60%), TTX increases this parameter by ∼225%. Values represent means ± SE from 17–19 neurons/condition, analyzed by 1-way ANOVA followed by a Tukey post hoc test. *P < 0.05, ***P < 0.001 relative to 30-min ACM-hSOD1G93A.
DISCUSSION
Many studies report that ALS is at least partially a non-cell-autonomous disease and that astrocytes participate in the death of motoneurons (Cassina et al. 2008; Clement et al. 2003; Di Giorgio et al. 2007; Haidet-Phillips et al. 2011; Ilieva et al. 2009; Nagai et al. 2007; Yamanaka et al. 2008). In a unique established in vitro model of ALS, for example, extensive death of primary motoneurons is induced when spinal cultures are exposed to conditioned medium derived from astrocytes expressing SODG93A (ACM-hSODG93A) (Nagai et al. 2007). However, the primary target of the toxic factors is unknown. In the present study, we have used the in vitro system reported by Nagai et al. (2007) to demonstrate that even short-term (30 min) exposure of WT spinal cord neurons to ACM-hSOD1G93A suffices to trigger death of motoneurons. Moreover, acute exposure to ACM-hSOD1G93A rapidly increases levels of PCNa, excitability, and calcium transients of spinal neurons. Although the PCNa is only a small fraction (1–5%) of the total sodium current, this “noninactivating” component can have important effects on cell and network behavior. Because it can be activated close to the cell's resting potential, small increases in this current can enhance intrinsic excitability, alter spike initiation, amplify and prolong firing rate, and influence release of neurotransmitters (Crill et al. 1996; ElBasiouny et al. 2010; Goldin 2003; van Zundert et al. 2012). Persistent increases in this sodium current may thus lead to excessive influxes of sodium and calcium ions. Because motoneurons have a limited cytosolic calcium-buffering capacity, excessive uptake of calcium by mitochondria could be an initial step in a cascade of events that impair mitochondrial function and ultimately lead to motoneuron degeneration and death (Bento-Abreu et al. 2010; Lewinski and Keller 2005; van Zundert et al. 2012). Our finding that bigger VSCNs have a larger PCNa is likely an additional factor that can explain the selective vulnerability of motoneurons in this ALS model system. In light of the above data and previously reported experimental evidence obtained in neonatal hSOD1G93A mice and cultures (ElBasiouny et al. 2010; Kuo et al. 2005; Pieri et al. 2009; Quinlan et al. 2011; Schuster et al. 2011; van Zundert et al. 2008), it seems likely that an important mechanism by which mutant SOD1 induces motoneuron death is through the activation of PCNa, thereby increasing excitability, which in turn causes toxic levels of cytosolic calcium (van Zundert et al. 2012). This hypothesis is supported by the observation that exposure of spinal cultures to riluzole, mexiletine, and spermidine, three reagents that reduce PCNa and hyperexcitability, restored calcium homeostasis and prevented cell death induced by ACM-hSOD1G93A. On the basis of our findings, we support the view that induction of Nav channel-mediated hyperexcitability by mutant SOD1G93A is a central factor initiating motoneuron death in ALS.
Despite our findings with the Nav channel blockers mexiletine, riluzole, and spermidine, we were intrigued to observe that TTX (1 nM) was the only compound unable to effectively prevent motoneuron death when chronically coapplied with ACM-hSOD1G93A. The exact structural basis for how these different reagents block Nav channels is currently unknown; however, this should change rapidly with the recent establishment of the first crystal structure of a bacterial Nav channel obtained at 2.7-Å resolution (Payandeh et al. 2011). Of course, it is very plausible that the beneficial effects of riluzole and spermidine are completely independent of the suppression of Nav channels and involve other ion channels/receptors. For example, it is well known that riluzole can inhibit multiple voltage-gated calcium and potassium channels and potentiates the activity of glutamate transporters (Bellingham 2011). The polyamine spermidine also is able to block voltage-gated calcium and potassium channels and modulates several glutamate receptor types (Williams 1997). However, these additional targets of riluzole and spermidine do not explain why the specific Nav channel blocker mexiletine is able to prevent the ACM-hSOD1G93A-induced motoneuron death. Accordingly, in our view, it remains possible that the primary neuroprotective effect of mexiletine, spermidine, and riluzole in this in vitro system is mediated by the reduction in excessive Nav channel current.
If this is the case, the issue that arises is the apparent discrepancy between the effects of mexiletine and TTX: why does the latter Nav blocker not rescue motoneuron death induced by ACM-hSOD1G93A? Two considerations may explain this observation. First, although short-term coapplication of TTX (1 nM) with ACM-hSOD1G93A significantly decreased the frequency of calcium transients, paradoxically the amplitude of the remaining events was strongly increased. Second, many studies have shown that while TTX at high concentrations (1 μM) can suppress neuronal network activity, it induces neuronal death, presumably by induction of intrinsic and synaptic homeostatic plasticity processes. For example, in neuronal cultures TTX actually increased intrinsic excitability (Desai et al. 1999; Koch et al. 2010), enhanced AMPA receptor-mediated postsynaptic activity (Fishbein and Segal 2007), and reduced expression of GluR2, a subunit critical to block calcium entry through AMPA receptors (Schonfeld-Dado et al. 2009). These results suggest that events or pathways that are components of homeostatic plasticity, and compensate for the TTX-induced reduction in neuronal activity, can lead indirectly to injurious calcium influxes (Fishbein and Segal 2007; Schonfeld-Dado et al. 2009; Turrigiano 2011). It is plausible that in our culture system similar processes are induced by the chronic application of low doses of TTX, leading to specific death of vulnerable cells like motoneurons, which have a very limited calcium-buffering capacity. Notably, while 1 nM TTX killed untreated motoneurons, TTX doses of ≥500 nM were required to affect the survival of nonmotoneurons within the same culture (data not shown).
How do cytotoxic factors of the ACM-hSOD1G93A enhance the persistent sodium current of Nav channels? The amplitude of the PCNa is influenced by many factors, including the type of α-subunit underlying the currents (PCNa is greater for Nav1.1 and Nav1.6 compared with Nav1.2 and Nav1.3), the presence of Nav channel β-subunits, exposures to different reactive oxygen species, the activity of critical protein kinases (e.g., PKC), and levels of external potassium (Aman et al. 2009; Franceschetti et al. 2000; Goldin 2003; Hammarström and Gage 2000; Kassmann et al. 2008; Somjen and Muller 2000; van Zundert et al. 2012). In our experiments, the PCNa are not modified by 30 mM KCl (data not shown), suggesting that potassium fluxes are not the source of heightened Nav channel permeability. Additional experiments, beyond the scope of this work, will be required to define the specific factors within the ACM-hSOD1G93A that affect the PCNa.
As for understanding of the molecular and cellular basis of the astrocytic non-cell-autonomous toxicity, evidence exists showing that mutant SOD1-expressing astrocytes release factors such as glutamate, cytokines, chemokines, and death receptor-activated components that affect motoneuron survival (Aebischer et al. 2011; Hensley et al. 2006; Milanese et al. 2010; Pehar et al. 2004). Evaluation of the ACM generated by the laboratory of Przedborski (which is similar to our medium), however, discarded the idea that many of these molecules are likely to be involved in the toxicity of motoneuron death induced by mutant hSODG93A-expressing astrocytes (see Supplementary Fig. 5, Nagai et al. 2007). Precise identification of the neurotoxic factor(s) in the ACM-hSOD1G93A, however, is likely to be challenging; recent quantitative mass spectrometry studies, for example, reveal that 92 of 516 proteins in ACM-hSODG93A are enriched >1.5-fold relative to those in cell lysate (Greco et al. 2010). Furthermore, analyses of microarray gene expression showed that multiple genes involved in transcription, signaling, cell proliferation, extracellular matrix synthesis, response to stress, and steroid and lipid metabolism are expressed differentially in hSODG93A versus WT astrocytes (Vargas et al. 2008).
Together, the results reported here provide the first evidence indicating that the neurotoxicity of medium conditioned by astrocytes expressing ALS-linked mutated SODG93A is closely associated with its induction of motoneuron hyperexcitability. Our findings that mexiletine and spermidine (in addition to riluzole) completely reverse ACM-hSOD1G93A-induced motoneuron death in this ALS model system suggest that these agents are plausible candidate therapies for ALS patients. Finally, because patients with sporadic and familial ALS display similar pathology, have comparable clinical symptoms, and experience a modest but beneficial response to riluzole, it is conceivable that reduction of hyperexcitability by pharmacological agents will similarly prove beneficial in both forms of ALS.
GRANTS
This work was supported by ALS Therapy Alliance-CVS Pharmacy (B. van Zundert), Fondecyt 1101012 (B. van Zundert), Conicyt-Anillo ACT-1114 (B. van Zundert), Conicyt 24090204 (P. Izaurieta), Fondecyt 1100983 (R. Madrid), VRID-USACH (P. Rojas), and Conicyt-Anillo ACT-1113 (R. Madrid, P. Rojas). Additionally, R. H. Brown, Jr. is supported by National Institute of Neurological Disorders and Stroke (Grants 1R01 NS-050557 and RC2 NS-070-342), the ALS Therapy Alliance, Project ALS, the Angel Fund, the Pierre L. de Bourgknecht ALS Research Foundation, the Al-Athel ALS Research Foundation, and the ALS Family Charitable Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.F., P.I., F.R.M., D.G., F.R., R.H.B., R.M., and B.v.Z. conception and design of research; E.F., P.I., A.W., F.R.M., P.R., D.G., F.R., R.M., and B.v.Z. performed experiments; E.F., P.I., A.W., F.R.M., P.R., D.G., F.R., R.H.B., R.M., and B.v.Z. analyzed data; E.F., P.I., F.R.M., P.R., D.G., F.R., R.H.B., R.M., and B.v.Z. interpreted results of experiments; E.F., P.I., R.M., and B.v.Z. prepared figures; E.F., P.I., F.R.M., P.R., R.H.B., R.M., and B.v.Z. edited and revised manuscript; E.F., P.I., A.W., F.R.M., P.R., D.G., F.R., R.H.B., R.M., and B.v.Z. approved final version of manuscript; R.M. and B.v.Z. drafted manuscript.
ACKNOWLEDGMENTS
We thank M. Constantine-Paton, R. Horvitz, and F. Alvarez for helpful comments.
REFERENCES
- Aebischer J, Cassina P, Otsmane B, Moumen A, Seilhean D, Meininger V, Barbeito L, Pettmann B, Raoul C. IFNγ triggers a light-dependent selective death of motoneurons contributing to the non-cell autonomous effects of mutant SOD1. Cell Death Differ 18: 754–768, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman TK, Grieco-Calub TM, Chen C, Rusconi R, Slat EA, Isom LL, Raman IM. Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels. J Neurosci 29: 2027–2042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Estévez AG, Crow JP, Barbeito L. Superoxide dismutase and the death of motoneurons in ALS. Trends Neurosci 24: S15–S20, 2001 [DOI] [PubMed] [Google Scholar]
- Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther 17: 4–31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento-Abreu A, Van Damme P, Van Den Bosch L, Robberecht W. The neurobiology of amyotrophic lateral sclerosis. Eur J Neurosci 31: 2247–2265, 2010 [DOI] [PubMed] [Google Scholar]
- Bories C, Amendola J, Lamotte d'Incamps B, Durand J. Early electrophysiological abnormalities in lumbar motoneurons in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci 25: 451–459, 2007 [DOI] [PubMed] [Google Scholar]
- Bosco DA, Morfini G, Karabacak NM, Song Y, Gros-Louis F, Pasinelli P, Goolsby H, Fontaine BA, Lemay N, McKenna-Yasek D, Frosch MP, Agar JN, Julien JP, Brady ST, Brown RH. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci 13: 1396–1403, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de Leon A, Robinson KM, Mason RP, Beckman JS, Barbeito L, Radi R. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J Neurosci 28: 4115–4122, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57: 411–425, 2005 [DOI] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillée S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown RH, Julien JP, Goldstein LS, Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302: 113–117, 2003 [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2: 806–819, 2001 [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol 58: 349–362, 1996 [DOI] [PubMed] [Google Scholar]
- Del Signore SJ, Amante DJ, Kim J, Stack EC, Goodrich S, Cormier K, Smith K, Cudkowicz ME, Ferrante RJ. Combined riluzole and sodium phenylbutyrate therapy in transgenic amyotrophic lateral sclerosis mice. Amyotroph Lateral Scler 10: 85–94, 2009 [DOI] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci 2: 515–520, 1999 [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci 10: 608–614, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElBasiouny SM, Schuster JE, Heckman CJ. Persistent inward currents in spinal motoneurons: important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis. Clin Neurophysiol 121: 1669–1679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein I, Segal M. Miniature synaptic currents become neurotoxic to chronically silenced neurons. Cereb Cortex 17: 1292–1306, 2007 [DOI] [PubMed] [Google Scholar]
- Fleidervish IA, Libman L, Katz E, Gutnick MJ. Endogenous polyamines regulate cortical neuronal excitability by blocking voltage-gated Na+ channels. Proc Natl Acad Sci USA 105: 18994–18999, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschetti S, Taverna S, Sancini G, Panzica F, Lombardi R, Avanzini G. Protein kinase C-dependent modulation of Na+ currents increases the excitability of rat neocortical pyramidal neurons. J Physiol 528: 291–304, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AL. Mechanisms of sodium channel inactivation. Curr Opin Neurobiol 13: 284–290, 2003 [DOI] [PubMed] [Google Scholar]
- Greco TM, Seeholzer SH, Mak A, Spruce L, Ischiropoulos H. Quantitative mass spectrometry-based proteomics reveals the dynamic range of primary mouse astrocyte protein secretion. J Proteome Res 9: 2764–2774, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney M, Pu H, Chiu A, Dal Canto M, Polchow C, Alexander D, Caliendo J, Hentati A, Kwon Y, Deng H, Chen W, Zhai P, Sufit RL, Siddique T. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264: 1772–1775, 1994 [DOI] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, Rao M, Eagle A, Kammesheidt A, Christensen A, Mendell JR, Burghes AH, Kaspar BK. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol 29: 824–832, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström AK, Gage PW. Oxygen-sensing persistent sodium channels in rat hippocampus. J Physiol 529: 107–118, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Abdel-Moaty H, Hunter J, Mhatre M, Mou S, Nguyen K, Potapova T, Pye QN, Qi M, Rice H, Stewart C, Stroukoff K, West M. Primary glia expressing the G93A-SOD1 mutation present a neuroinflammatory phenotype and provide a cellular system for studies of glial inflammation. J Neuroinflammation 3: 2, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187: 761–772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassmann M, Hansel A, Leipold E, Birkenbeil J, Lu SQ, Hoshi T, Heinemann SH. Oxidation of multiple methionine residues impairs rapid sodium channel inactivation. Pflügers Arch 456: 1085–1095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H, Huh SE, Elsen FP, Carroll MS, Hodge RD, Bedogni F, Turner MS, Hevner RF, Ramirez JM. Prostaglandin E2-induced synaptic plasticity in neocortical networks of organotypic slice cultures. J Neurosci 30: 11678–11687, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JJ, Siddique T, Fu R, Heckman CJ. Increased persistent Na+ current and its effect on excitability in motoneurones cultured from mutant SOD1 mice. J Physiol 563: 843–854, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinski FV, Keller BU. Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS. Trends Neurosci 28: 494–500, 2005 [DOI] [PubMed] [Google Scholar]
- Madrid R, Donovan-Rodriguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci 26: 12512–12525, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanese M, Zappettini S, Jacchetti E, Bonifacino T, Cervetto C, Usai C, Bonanno G. In vitro activation of GAT1 transporters expressed in spinal cord gliosomes stimulates glutamate release that is abnormally elevated in the SOD1/G93A+ mouse model of amyotrophic lateral sclerosis. J Neurochem 113: 489–501, 2010 [DOI] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 10: 615–622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschewski A, Schnoebel-Ehehalt R, Li Y, Tang B, Bräu ME, Wolff M. Mexiletine and lidocaine suppress the excitability of dorsal horn neurons. Anesth Analg 109: 258–264, 2009 [DOI] [PubMed] [Google Scholar]
- Pambo-Pambo A, Durand J, Gueritaud JP. Early excitability changes in lumbar motoneurons of transgenic SOD1G85R and SOD1G93A-Low mice. J Neurophysiol 102: 3627–3642, 2009 [DOI] [PubMed] [Google Scholar]
- Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci 7: 710–723, 2006 [DOI] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature 475: 353–358, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehar M, Cassina P, Vargas MR, Castellanos R, Viera L, Beckman JS, Estévez AG, Barbeito L. Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem 89: 464–473, 2004 [DOI] [PubMed] [Google Scholar]
- Pieri M, Carunchio I, Curcio L, Mercuri NB, Zona C. Increased persistent sodium current determines cortical hyperexcitability in a genetic model of amyotrophic lateral sclerosis. Exp Neurol 215: 368–379, 2009 [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Persistent sodium and calcium currents in rat hypoglossal motoneurons. J Neurophysiol 89: 615–624, 2003 [DOI] [PubMed] [Google Scholar]
- Quinlan KA, Schuster JE, Fu R, Siddique T, Heckman CJ. Altered postnatal maturation of electrical properties in spinal motoneurons in a mouse model of amyotrophic lateral sclerosis. J Physiol 589: 2245–2260, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science 265: 1724–1728, 1994 [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362: 59–62, 1993 [DOI] [PubMed] [Google Scholar]
- Schonfeld-Dado E, Fishbein I, Segal M. Degeneration of cultured cortical neurons following prolonged inactivation: molecular mechanisms. J Neurochem 110: 1203–1213, 2009 [DOI] [PubMed] [Google Scholar]
- Schuster JE, Fu R, Siddique T, Heckman CJ. Effect of prolonged riluzole exposure on cultured motoneurons in a mouse model of ALS. J Neurophysiol 107: 484–492, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, Bostrom A, Theodoss J, Al-Nakhala BM, Vieira FG, Ramasubbu J, Heywood JA. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler 9: 4–15, 2008 [DOI] [PubMed] [Google Scholar]
- Sepulveda FJ, Bustos FJ, Inostroza E, Zuniga FA, Neve RL, Montecino M, van Zundert B. Differential roles of NMDA receptor subtypes NR2A and NR2B in dendritic branch development and requirement of RasGRF1. J Neurophysiol 103: 1758–1770, 2010 [DOI] [PubMed] [Google Scholar]
- Somjen GG, Müller M. Potassium-induced enhancement of persistent inward current in hippocampal neurons in isolation and in tissue slices. Brain Res 885: 102–110, 2000 [DOI] [PubMed] [Google Scholar]
- Theiss RD, Kuo JJ, Heckman CJ. Persistent inward currents in rat ventral horn neurons. J Physiol 580: 507–522, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Atkin JD, Farg MA, Zang DW, Rembach A, Lopes EC, Patch JD, Hill AF, Cheema SS. Impaired extracellular secretion of mutant superoxide dismutase 1 associates with neurotoxicity in familial amyotrophic lateral sclerosis. J Neurosci 25: 108–117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci 34: 89–103, 2011 [DOI] [PubMed] [Google Scholar]
- Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci 9: 108–118, 2006 [DOI] [PubMed] [Google Scholar]
- Valentine JS, Doucette PA, Potter SZ. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem 74: 563–593, 2005 [DOI] [PubMed] [Google Scholar]
- van Zundert B, Izaurieta P, Fritz E, Alvarez FJ. Early pathogenesis in the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J Cell Biochem 113: 3301–3312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert B, Peuscher MH, Hynynen M, Chen A, Neve RL, Brown RH, Constantine-Paton M, Bellingham MC. Neonatal neuronal circuitry shows hyperexcitable disturbance in a mouse model of the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J Neurosci 28: 10864–10874, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Pehar M, Díaz-Amarilla PJ, Beckman JS, Barbeito L. Transcriptional profile of primary astrocytes expressing ALS-linked mutant SOD1. J Neurosci Res 86: 3515–3525, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Modulation and block of ion channels: a new biology of polyamines. Cell Signal 9: 1–13, 1997 [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillée S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci 11: 251–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]