Abstract
Exercise, in the form of moderate daily treadmill training following nerve transection and repair leads to enhanced axon regeneration, but its effect on functional recovery is less well known. Female rats were exercised by walking continuously, at a slow speed (10 m/min), for 1 h/day on a level treadmill, beginning 3 days after unilateral transection and surgical repair of the sciatic nerve, and conducted 5 days/wk for 2 wk. In Trained rats, both direct muscle responses to tibial nerve stimulation and H reflexes in soleus reappeared earlier and increased in amplitude more rapidly over time than in Untrained rats. The efficacy of the restored H reflex was greater in Trained rats than in Untrained controls. The reinnervated tibialis anterior and soleus were coactivated during treadmill locomotion in Untrained rats. In Trained animals, the pattern of activation of soleus, but not tibialis anterior, was not significantly different from that found in Intact rats. The overall length of the hindlimb during level and up- and downslope locomotion was conserved after nerve injury in both groups. This conservation was achieved by changes in limb orientation. Limb length was conserved effectively in all rats during downslope walking but only in Trained rats during level and upslope walking. Moderate daily exercise applied immediately after sciatic nerve transection is sufficient to promote axon regeneration, to restore muscle reflexes, and to improve the ability of rats to cope with different biomechanical demands of slope walking.
Keywords: exercise, axon regeneration, peripheral nerve
traumatic peripheral nerve injuries occur commonly, and even though axons in peripheral nerves are capable of considerable regeneration, only about 10% of these patients ever recover full function (Frostick et al. 1998; Scholz et al. 2009). A common reason given for the poor functional outcomes is the slow process of axon regeneration (Gordon 2009). Thus one potential target for therapeutic intervention in treating peripheral nerve injuries might be to enhance the process of axon regeneration. Indeed, a number of rehabilitative approaches have been advocated to promote functional recovery following injury to peripheral nerves (reviewed in Udina et al. 2011). In studies from our laboratory in mice (Sabatier et al. 2008), exercise in the form of moderate daily treadmill training results in a marked enhancement of axon regeneration in cut nerves. Others have reported a variety of different training paradigms in different injury models (crush or transection) in rats that result in enhanced axon regeneration (Asensio-Pinilla et al. 2009; Ilha et al. 2008; Seo et al. 2006; Udina et al. 2011). We have used a combination of transgenic and knockout mice to show that the enhancement of axon regeneration produced by this moderate exercise is the result of an autocrine neurotrophin stimulation of regeneration of injured axons (Wilhelm et al. 2012).
Whether the enhancement of axon regeneration produced by exercise leads to improved functional recovery is less clear. Using nerve conduction studies, Navarro and colleagues (Asensio-Pinilla et al. 2009) studied the effects of treatment of rats with 4 wk of twice daily exercise after sciatic nerve transection and repair. For both the tibialis anterior (TA) and plantar foot muscles, training produced only very modest increases in the amplitude of direct muscle (M) responses to stimulation of the sciatic nerve above the transection until 2 mo following injury, when the responses were significantly larger. A similar effect was observed using passive cycling of the limbs (Udina et al. 2011). Only when treadmill exercise was combined with brief electrical stimulation was the magnitude of this functional outcome measure greater at earlier survival times (Asensio-Pinilla et al. 2009).
Interpretations of the effectiveness of any treatment in improving functional recovery following peripheral nerve injury also should include behavioral analyses. The repertoire of such assays applied to laboratory rodents has been reviewed recently (Brushart 2011; Wood et al. 2011). A complication of the use of many of these assays is the behavioral compensation made in response to the initial lesions. We have argued that analysis of slope walking is a behavioral assay in which those compensations are less complicating (Sabatier et al. 2011b). The biomechanical demands of walking up and down slopes are quite different (Carlson-Kuhta et al. 1998; Maas et al. 2009), and intact animals and humans adapt to those demands by altering muscle activation patterns and overall limb movements (Lay et al. 2006; Sabatier et al. 2011b). Our laboratory has shown that, in Untrained rats, recovering from sciatic nerve injuries, their ability to change either the pattern or intensity of activation of reinnervated muscles or the movements of the affected hindlimb when walking on different slopes is lost (Hamilton et al. 2011; Sabatier et al. 2011a; Sabatier et al. 2011b). Thus we propose to evaluate the efficacy of exercise as a treatment following nerve injury by measuring the extent of restoration of the ability of rats to adapt to walking on different slopes. The overall goal of this paper is to report the results of nerve conduction studies and studies of slope walking on the effects of exercise on functional recovery following sciatic nerve injury in rats. A preliminary report of these findings has been made (Boeltz et al. 2010).
MATERIALS AND METHODS
General methods.
All methods used were approved by the institutional animal care and use committee of Emory University. Experiments were conducted using 12 female rats (body weight ∼250 g). Females were chosen because they autotomize (chew toes) less following peripheral nerve injuries than male rats. Our original experiments were conducted using eight Sprague-Dawley rats, but, because several of these rats did autotomize and had to be removed from the study prematurely, we added four Lewis rats to the study. None of these rats had to be euthanized prematurely. Thus data from the longest survival times studied include recordings made in smaller numbers of animals, as indicated throughout the text. Originally, six rats were assigned to a Trained group and six to an Untrained group. Two Lewis rats were assigned to each group. Pretransection measurements from both groups were pooled to form a third, Intact group for some analyses. All rats in the Untrained and Trained groups tolerated the nerve repair surgery and were studied throughout the postinjury period. At the conclusion of the study period, all experimental animals were euthanized using an intraperitoneal injection of 100 mg/kg of pentobarbital. Some of the data from some (n = 4) of the Sprague-Dawley rats in the Untrained group have been reported previously (Sabatier et al. 2011b). They are included here for comparative purposes.
Recording hardware was implanted into ketamine-xylazine anesthetized animals for chronic use. All implanted electrodes were constructed as described previously (English et al. 2007a). Bipolar fine-wire EMG electrodes were implanted into the soleus (SOL) and TA muscles to record electrical potentials produced by these muscles. The wires were secured in place with fine (6–0 nylon) sutures to minimize their movement while the muscle is contracting. Wire electrodes were implanted into the muscle mass, in an anatomically consistent location in each muscle, where, based on preliminary experiments, the atrophy of the muscles following denervation would be unlikely to result in the exposure of deinsulated tips of the wires outside of the muscle. Wire locations were visualized postmortem and judged to be in place in all animals studied. A bipolar stimulating cuff electrode, made of Silastic tubing and the same fine wire (Stein et al. 1977), was implanted around the exposed tibial nerve below its branching from the sciatic nerve. The design of these cuffs is for them to fit rather loosely on the nerve so that any swelling of the nerve after implantation did not induce a strangulation of the tissues. At postmortem, there was no visible effect of the cuff on the nerve in any of the rats studied, except for an anticipated increase in connective tissue surrounding the cuff. All wires, including those attached to the stimulating cuff, were led subcutaneously to a small plug (Plastics One, Roanoke, VA, part no. MS363), ∼1 cm in diameter, mounted on the head of the animal with stainless steel bone screws implanted into the skull and secured with dental acrylic.
Before transecting the sciatic nerve, the tibial nerve was stimulated through the cuff, and evoked EMG activity was recorded from SOL and TA in the anesthetized animals. The sciatic nerve was then cut just above its branching into tibial, common fibular, and sural trunks and proximal to the implanted cuff, using sharp scissors, and the proximal and distal stumps of the cut nerve were aligned and secured in place using fibrin glue. The glue was manufactured at the time of use from equal parts of thrombin and a 1:1 mixture of fibrinogen and fibronectin (MacGillivray 2003). Once the sciatic nerve was repaired and the fibrin glue was cured (1–2 min) to form a clot about the repaired nerve, the surgical site was closed in layers with appropriate sutures.
Exercise.
Treadmill training began on the third day following nerve repair. Trained rats walked on a custom treadmill on a level surface at 10 m/min for 1 h, 5 days/wk. This is a very slow speed, and rats, even those with sciatic nerve injuries, manage this training paradigm without any adverse effects. Animals were selected for inclusion into this study, if they are able to run on the treadmill in presurgical screenings, at a speed of up to 20 m/min without the need for reinforcement. To avoid any complications of preinjury exercise, rats were not exposed to the treadmill for at least 2 wk before nerve repair surgery. Rats assigned to the Untrained group were not exposed to the treadmill during the study period, except during locomotion studies done at 2, 4, and 10 wk after nerve transection and repair, when the rats walked on the treadmill for a total of 10 min over a period of at least 30 min.
Nerve conduction studies.
Muscle reinnervation was assessed by stimulating the tibial nerve just below the nerve transection and recording EMG activity from muscles (SOL and TA) innervated ∼15 mm below the nerve transection. We chose to stimulate below the injury site primarily so that we could stimulate only axons that had regenerated, not all axons. Stimulation of the tibial nerve, rather than the entire sciatic nerve, also enabled us to avoid any volume conducted EMG artifact that might result from activating the large adjacent hamstring muscles. In Intact rats, tibial nerve stimulation resulted in two compound muscle action potentials in SOL: a direct muscle response, also known as the M response, and the H reflex (Fig. 1A, Intact). The latter reflects the synaptic activation of motoneurons in response to stimulation of afferent axons in the tibial nerve. Rats were free to move about their cages during the nerve conduction studies, but mostly they did not move and curled up in a corner of the cage. We did not control for the animal's movements, but instead opted to control for resting EMG activity levels before the delivery of each stimulus. Ongoing EMG activity in the SOL was monitored, and when such activity was maintained for 10 s within a user-defined voltage window, a very short (0.1 ms) constant-voltage stimulus pulse was delivered to the tibial nerve via the implanted nerve cuff electrode, under computer control, and the EMG activity in SOL and TA was recorded. Stimuli were delivered no more frequently than once every 3 s, as that has been shown to produce consistent responses without muscle fatigue (English et al. 2007a). At each recording session, ramps of successively increasing stimulus voltages, ranging from subthreshold to supramaximal, were applied to generate a full H-reflex recruitment curve. Maximum M responses (MMax) and the maximum H reflexes (HMax) were recorded separately. Final responses for each animal at each recording session were the result of averages of multiple stimulus presentations, corrected for background EMG activity, as our laboratory has described previously (English et al. 2007a). Recordings were made at multiple times over a period of 10–15 wk following sciatic nerve transection and repair.
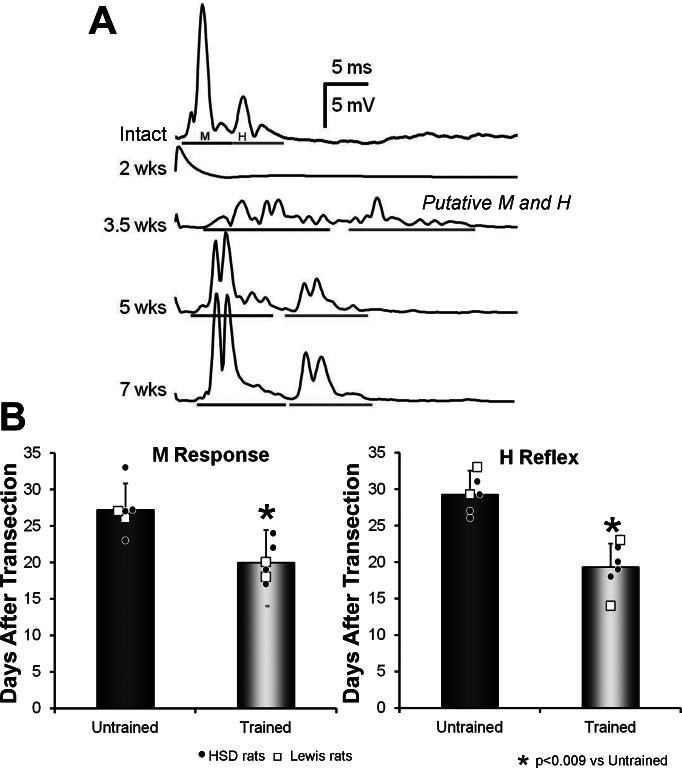
Fig. 1.
Effects of exercise on restoration of evoked EMG responses. A: two evoked EMG potentials were found in the soleus (SOL) muscle in response to stimulation of the tibial (Tib) nerve: a direct muscle (M) response and, at a slightly longer latency, an H reflex. The different traces show these potentials recorded prior to (Intact) and at different times after transection and repair of the sciatic nerve. All were recorded from a single, Untrained rat, and each trace is an average of the responses to 100 stimulus presentations. Gray bars below each trace indicate the time windows chosen for the M responses and H reflexes at the different recovery times. Note that only a stimulus artifact, and no evoked responses, were found at the earliest postinjury time point (2 wk). At the time of earliest recording of restored activity (3.5 wk), multiple small potentials were recorded. Dividing them into M responses and H reflexes was somewhat arbitrary, so that these potentials are referred to as putative M responses and putative H reflexes. B: mean (±SD) values for the first day after nerve transection and repair that M responses (left) and H reflexes (right) could be evoked are shown for Untrained rats and rats that had been treadmill trained (TT) for 2 wk. Small symbols in each bar in these graphs represent the values obtained from individual rats. Different symbols are shown for Sprague-Dawley (HSD) and Lewis rats. *P < 0.009 vs. Untrained.
We measured the latency from stimulus application to the initiation of the M response and the H reflex, as well as the average rectified amplitudes in time windows adjusted to be appropriate to these responses in each muscle (see below). Before nerve transection, a baseline recording of the M response and H reflex was determined in each rat. These measurements were repeated on the third day following nerve transection and repair, when treadmill training began in the Trained group. After the 2-wk treadmill training program ended, recordings of evoked EMG activity were made 5 days/wk until the first H reflex could be recorded. Recordings of evoked EMG activity were made at weekly intervals thereafter. The amplitudes of MMax responses and HMax reflexes were expressed as a percentage of the corresponding peak amplitudes obtained in pretransection recordings. Average intensities were computed for rats in the Untrained and Trained groups during weekly intervals after nerve transection and repair, and the data (posttransection time vs. amplitude) were fit with a least squares linear regression method. Significance of differences in slopes between groups was made using multiple linear regression analysis (Statistica, StatSoft).
Locomotor EMG activity.
In Intact rats, SOL and TA are reciprocally activated during locomotion (Sabatier et al. 2011b; Thota et al. 2005). However, following transection and repair of the sciatic nerve in Untrained rats, coactivation of the TA and SOL is common (Gramsbergen et al. 2000; Sabatier et al. 2011b). Activity in these muscles during treadmill locomotion was recorded from implanted EMG electrodes at 4 and 10 wk after nerve transection and repair in Trained rats and was compared both to data from Untrained rats at similar times after nerve repair and to data obtained from rats prior to nerve transection (Intact rats). Details of the recording conditions are given in our laboratory's previous publications (Sabatier et al. 2011b; Sabatier et al. 2012). Muscle activity and video records were synchronized to an LED pulse in the video field whose timing was recorded with the EMG activity.
Factor Analysis with Principal Components was used as a classification scheme to investigate whether the timing of EMG activity in SOL and TA during treadmill locomotion was different in Intact, Untrained, and Trained rats. From single-frame analysis of video records, the timing of paw contact and paw off during step cycles in which rats walked at constant speed on the treadmill was determined and used to extract the EMG activity during individual step cycles. The extracted EMG activity in SOL and TA during these selected step cycles was rectified, low-pass filtered at 10 Hz, and normalized to 100 samples, representing percentiles of the step cycle, using a cubic spline interpolation function. Activity was then averaged within slopes over several step cycles in the same experiment and subjected to Principal Components Analysis (PCA) (Statistica, StatSoft). The results of PCA are a set of new correlated variables, or principal components. Since, in our data set, the first three principal components accounted for more than 60% of the variance in the data, and because no significant differences were found in factor loadings (see below) for factors 4–6, we restricted our analysis to the first three principal components. For each of these three factors, we determined factor loadings, the correlations between the principal components, and the original data set. For each of the three treatment groups defined above, the pattern of locomotor EMG activity in SOL and TA at different times after denervation and on different slopes was characterized by the factor loadings for the first three principal components associated with that activity. Means of these three factor loadings for different animals at the different times and speeds/slopes were determined, and significance of differences in their means was evaluated using ANOVA, with post hoc paired [Fisher least significant difference (LSD)] testing, where appropriate. Any two vectors that differed significantly (P < 0.05) in one or more factor loading were considered to be derived from different patterns of locomotor EMG activity.
Kinematics.
Following recorded locomotion sessions, videos of the selected step cycles were subjected to single-frame analysis. Three previously marked reflective spots located over the greater trochanter, lateral malleolus, and fifth metatarsophalangeal (MP) joint were assigned Cartesian coordinates. A position vector between the markers at the greater trochanter and the fifth MP joint was determined from each frame. The magnitude of this vector was defined as the length of the entire extensible hindlimb. The direction of the vector is the angle of the entire limb to the treadmill belt. This vector was used as a global measure of limb movement. In a recent study, the measurement of the position of the hip joint during treadmill locomotion using X-ray video recordings was compared with that obtained with markers placed as described above (Bauman and Chang 2010). These authors described a significant difference between the methods and attributed the differences to skin movements over the pelvis during walking. Acknowledging the importance of these observations, we want to make very clear that, when we refer to measurements of limb length in this paper, we mean the distance between the applied skin markers, and not necessarily the actual distance between the hip and MP joints. We are very likely not really measuring limb length accurately, but regard our measure of limb length as a reasonable facsimile. We determined three parametric measures, each, for limb length and limb angle (minimum, maximum, and average) in selected step cycles in the different treatment groups. Significance of differences in these measures between Intact, Untrained, and Trained rats was determined using ANOVA with appropriate post hoc paired testing, as described above.
Terminal experiments.
The extent of misdirection of regenerating axons was evaluated in terminal acute physiology experiments. Under ketamine-xylazine anesthesia, the sciatic nerve was exposed at least 1 cm above the transection site. The epineurium was incised with sharp irridectomy scissors, and the tibial and common fibular fascicles were separated, as described by Evans et al. (1991). A stimulating cuff electrode was placed around the exposed nerves, one at a time, and used to stimulate muscle fibers innervated by axons coursing in that branch. Evoked activity in SOL and TA muscles was recorded using patch-type EMG electrodes (Loeb and Gans 1986) placed on the surfaces of the muscles. Within the defined M-response windows, mean rectified and integrated EMG activity was measured for each muscle in response to stimulating each of the nerves. The proportion of the total activity evoked in reinnervated muscles produced by tibial or common fibular nerve stimulation was determined and compared with that recorded from contralateral, intact muscles using an unpaired t-test (α = 0.05).
RESULTS
Effects of exercise on restoration of evoked SOL EMG activity.
Tibial nerve stimulation evoked an M response and an H reflex in the SOL muscle of Intact rats (Fig. 1A, Intact). Both evoked potentials disappear in denervated muscles (Fig. 1A, 2 wk), but later reappear as muscle fibers are reinnervated. They are first observed at a longer latency than found in intact SOL muscles, probably a reflection of the small size of the regenerating axons at that time and their diminished axonal conduction velocity, as has been observed by others (Foehring et al. 1986). In addition, these early restored potentials are found, not as a single compound action potential, but as a temporally dispersed series of small potentials (Fig. 1A, 3.5 wk). Over time, the latencies to the two evoked potentials shorten, and the distinction between M responses and H reflexes becomes more clear (Fig. 1A, 5 wk and 7 wk). For this reason, we considered the earliest potentials as putative M responses and putative H reflexes. The distinction between the two early responses in each muscle thus is somewhat arbitrary (English et al. 2007a).
In Trained rats, the putative M response appeared significantly (unpaired t-test, P < 0.002) earlier (on average, 20.00 days vs. 27.80 days) than in Untrained rats (Fig. 1B). Following transection and repair of the rat sciatic nerve, a putative SOL H reflex is found to appear slightly later than the earliest observation of a restored M response in Untrained rats (English et al. 2007a). As noted above for the reappearance of the M response, the putative SOL H reflex is first seen significantly earlier (unpaired t-test, P < 0.009) in Trained rats compared with Untrained rats (on average, 20.00 days vs. 29.25 days) (Fig. 1B).
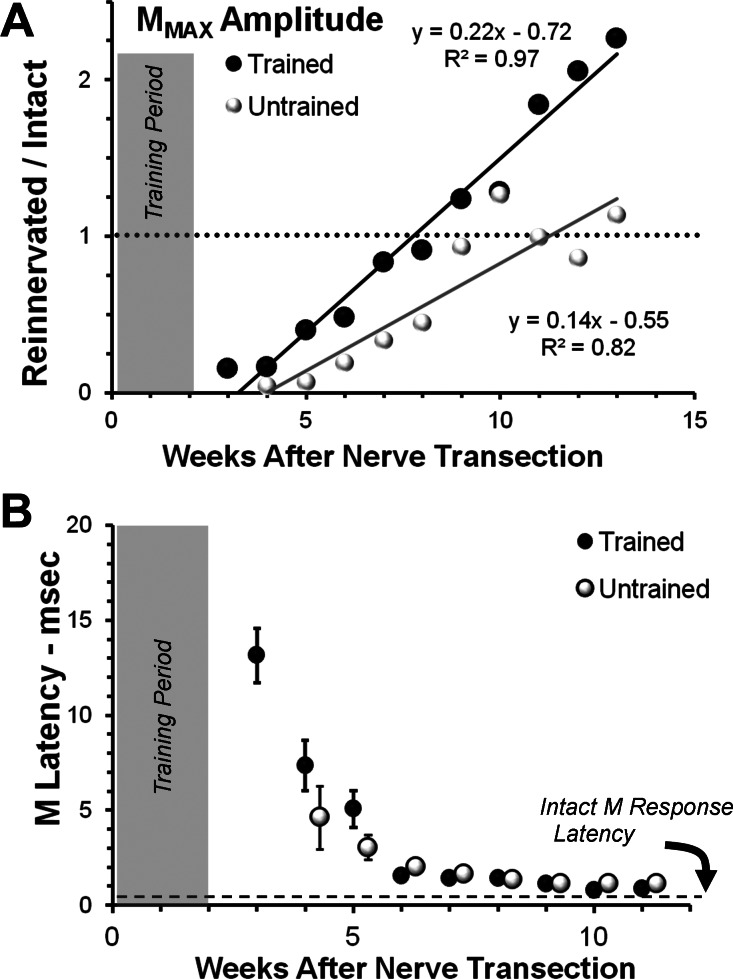
The amplitude of the largest restored MMax increased linearly over time in both Trained and Untrained animals (Fig. 2A). These data were subjected to multiple linear regression analysis, using survival time, treatment group (Trained = 1 or Untrained = 0), and their product as independent variables and M-response amplitude as the single dependent variable. Correlations of the data to the regression lines were very high, accounting for 82 and 97% of the variance in the respective data sets (Fig. 2A). Regression coefficients were significantly different from zero for all three independent variables, including the product of survival time and treatment (P < 0.00009). Two inferences are drawn from this analysis. The slopes of the individual regression lines for the two treatment groups both are significantly different from zero, and the slopes of these lines for Trained and Untrained rats are significantly different from one another. Based on the magnitude of these differences in slopes of the regression lines in the two groups, we conclude that, over the entire period of study, the amplitude of the evoked M response increased faster with time in Trained rats than in Untrained rats. When the data are scaled as a function of the M-response amplitude found prior to nerve transection, M responses that were significantly greater than 1.0 (greater than pretransection, Fig. 2A, horizontal dashed line) were found in Trained rats only.
Fig. 2.
Time course of recovery of the M response in Trained and Untrained rats. A: the mean amplitude of the maximal M response (MMax; average rectified activity in the M time window) is shown at different times after sciatic nerve transection and repair for Trained rats (black symbols) and Untrained rats (white symbols). All values are expressed as a proportion of the MMax amplitude recorded from that rat prior to nerve transection. The horizontal dotted line is at 1.0, indicating the amplitude of this pretransection response. Data from Trained and Untrained groups were fitted with linear least squares regression lines. Equations for the lines and correlation coefficients are shown next to each line. The shaded area at the left of the graph indicates the time course of treadmill training in the Trained animals. B: the mean latency from stimulus onset to the start of the M response is shown at different times after sciatic nerve transection and repair for Trained rats and Untrained rats. The horizontal dashed line represent the M-response latency found in Intact rats (0.5 ms). Error bars represent the SE of the mean for each time point. No error bars are visible at times longer than 5 wk, because the SE of the mean is smaller than the symbols used. Data from Untrained animals were offset slightly to the right of data points from Trained animals to avoid overlap.
In both Untrained and Trained rats, the initial restored M response was found at a longer latency than found in Intact rats. This latency decreased rather rapidly over time in both groups until it was no longer different from that found in Intact rats, but not until ∼10 wk postoperative in both groups (Fig. 2B). The interanimal variability in mean M-response latency was quite large at first, and we believe that this is accounted for by the difficulty in producing accurate measurements of latency when very small potentials are encountered. This large variability was found only at the first two measurement times. By 6 wk after nerve repair surgery, it was very small. At all times studied, data from the two Lewis rats in each group in this series were in the same range as the data from the four Sprague-Dawley rats in each group.
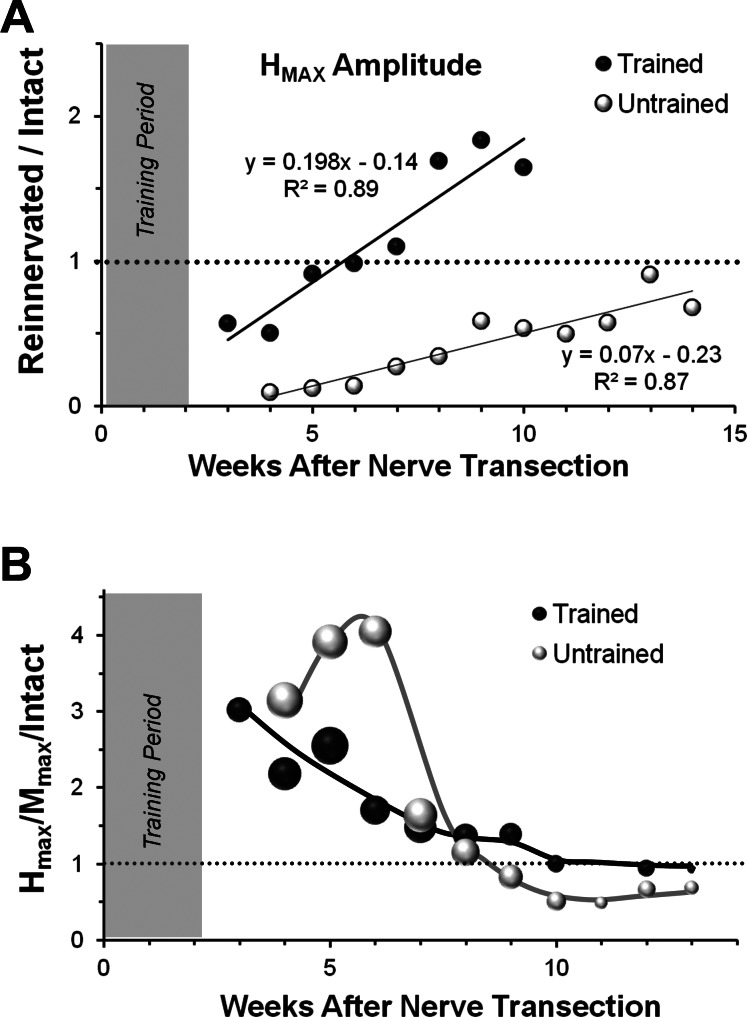
The amplitude of the restored SOL H reflex also increased linearly over time in both Untrained and Trained rats (Fig. 3A). Using multiple linear regression analysis described above, the slopes of the lines fitted to the data for both Untrained and Trained rats were found to be significantly different from zero, and that of the Trained group is greater than the slope of the line fitted to the data from the Untrained group. Over the entire period of study, the amplitude of the SOL H reflex increased faster in Trained rats than in Untrained rats. Based on the same multiple linear regression analysis, the slopes of the lines for the M responses and H reflexes were compared within each treatment group. Slopes of regression lines were significantly different for Untrained rats, but not Trained rats, suggesting that the ability of afferent axon stimulation to produce an EMG response was greater in Trained rats.
Fig. 3.
Time course of recovery of the H reflex in TT and Untrained rats. A: the mean amplitude of the maximal H reflex (HMax; average rectified activity in the H time window) is shown at different times after sciatic nerve transection and repair for Trained rats (black symbols) and Untrained controls (white symbols). All values are expressed as a proportion of the HMax amplitude recorded from that rat prior to nerve transection. The horizontal dotted line is at 1.0, indicating the amplitude of this pretransection response. Data from Trained and Untrained controls were fitted with linear least squares regression lines. Equations for the lines and correlation coefficients are shown next to each line. The shaded area at the left of the graph indicates the time course of treadmill training in the Trained animals. B: the same data are reexpressed as a proportion of the MMax amplitude at the different times studied. All values of this HMAX-to-MMax ratio are, in turn, expressed as a proportion of the same ratio determined from that rat prior to nerve transection (HMAX/MMax/Intact). The horizontal dotted line is at 1.0, indicating the amplitude of this pretransection ratio. The sizes of the symbols used are proportional to the SD for each time point. The lines for each group are not fitted to any function, but are included only to emphasize differences between Untrained and Trained groups. The shaded area at the left of the graph indicates the time course of treadmill training in the Trained animals.
A way of comparing the efficacy of the restored H reflex in reinnervated muscles to that found in Intact rats is to compute the ratio of the HMax amplitude to the MMax amplitude (Navarro et al. 2007). The time course of recovery of this HMax-to-MMax ratio (HMax/MMax) for Trained and Untrained rats following sciatic nerve transection and repair is shown in Fig. 3B. Values of HMax/MMax are expressed as a proportion of the HMax/MMax measured prior to transection (HMax/MMax/Intact). In both Trained and Untrained rats, the HMax/MMax at the earliest times of recovery is nearly three times greater than found prior to transection. In Untrained rats, this large response was maintained or increased slightly over a 3-wk period and then declined rapidly until a stable ratio was established at a level significantly lower than pretransection levels (Fig. 2B, and English et al. 2007a). In Trained rats, the initial response declined more gradually and stabilized at approximately 9 wk after nerve repair surgery. The HMax/MMax over the time period from 10 to 13 wk after nerve transection and repair was significantly larger in Trained rats than found in Untrained rats (ANOVA: F1,6 = 5.987, P < 0.038). In Trained rats, the mean HMax/MMax over this time period, when scaled to the same ratio prior to nerve transection and repair (1.02, SD 0.24), is not significantly different from 1.0. Over the same time period, the ratio for Untrained rats (0.52, SD 0.20) remains significantly less than 1.0. Thus one effect of exercise is a restoration of the efficacy of this spinal reflex to its prelesion magnitude.
Effects of exercise on the specificity of muscle reinnervation.
After injury to nerves as large as the sciatic nerve, many regenerating motor axons are directed into terminal nerve branches that lead either to the same muscle targets they innervated prior to injury or to anatomical synergists. Other motor axons are misdirected to inappropriate nerve branches, such as those supplying anatomical antagonists, and yet other regenerating motor axons are known to branch to both appropriate and inappropriate nerve branches (English 2005b; English et al. 2009). We evaluated the extent to which exercise might contribute to these two different forms of axon misdirection in two sets of parallel experiments.
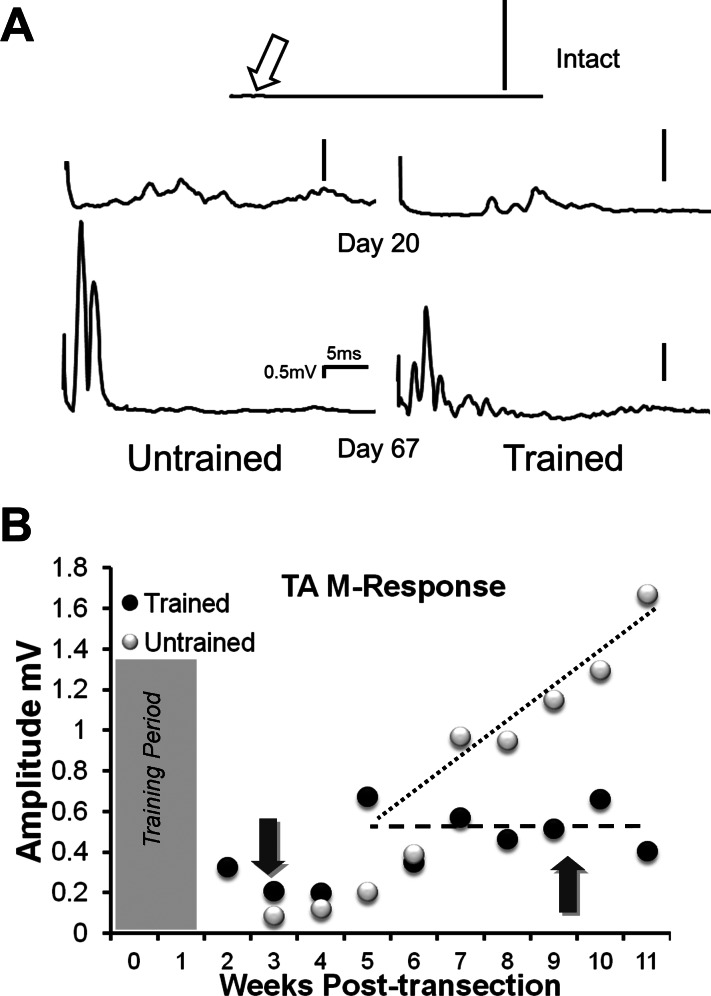
The two main terminal branches of the sciatic nerve, the common fibular and tibial nerves, supply muscles that are anatomical antagonists. To study the effect of exercise on the extent of branching of regenerating motor axons into both of these branches, we conducted axon reflex experiments. In all animals studied, we found evidence of this branching: a significant EMG response to tibial nerve stimulation was found in the reinnervated TA muscle (Fig. 4A). We measured the amplitudes of these potentials at different times after sciatic nerve transection in Untrained and Trained rats. Stimulation of the tibial nerve in Intact rats resulted in a very small potential (<0.005 mV in amplitude) that could be recorded from EMG electrodes in the TA muscle and identified only when using signal averaging (Fig. 4A, Intact, open arrow). We assume that this potential represents unavoidable EMG cross talk (English and Weeks 1989), and that this very small potential represents the quite laudable extent of fidelity of our recording system. Because it is so small, this data point is not included in Fig. 4B. In both groups of animals, slightly larger potentials were recorded in TA over the first 3–5 wk after nerve injury (Fig. 4A, Day 20). Over the next 6–7 wk, the amplitude of these potentials increased in a linear manner (slope = 0.16, R2 = 0.713, P < 0.008) in the Untrained rats (Fig. 4B, white symbols), and by the end of the study period, substantial potentials could be recorded from the TA muscle in response to stimulation of the tibial nerve (Fig. 4A, Untrained, Day 67). In Trained rats, a clear difference was found. The amplitude of the potentials did not increase over the last 6–7 wk of the study period (slope = −0.0023, R2 = 0.024, P = 0.908) (Fig. 4B, black symbols), and, at the end of the study period, significantly smaller potentials were recorded in Trained vs. Untrained rats (unpaired t-test, P < 0.007) (Fig. 4A, Trained, Day 67). To the extent that the linear increase in amplitude found in Untrained rats might reflect the addition or retention/maturation of regenerated TA motor units that are shared with targets of the tibial nerve, no such effect was found in Trained rats. Thus exercise has a positive effect on this form of axon misdirection.
Fig. 4.
The time course of the amplitude of tibialis anterior (TA) muscle responses to electrical stimulation of the Tib nerve distal to transection of the sciatic nerve is shown. A: examples of evoked EMG traces (axon reflexes) recorded from TA in an Intact rat and in an Untrained (left) and a Trained (right) rat at an early innervation time, 20 days after nerve transection, and a later time, 67 days after nerve transection. Each trace represents the average of 100 stimulus presentations. The open arrow in the top trace points to a very small potential that probably represents unavoidable EMG cross talk in the Intact rats. Vertical scale bar in each trace is 0.5 mV. B: the mean amplitude of the MMax (average rectified activity in the M time window) is shown at different times after sciatic nerve transection and repair for Trained (black symbols) and Untrained rats (white symbols). Different dashed lines do not represent regression lines, but are included only to emphasize differences between Untrained and Trained groups. The dark arrows point to the time at which the traces in A were obtained. The shaded area at the left of the graph indicates the time course of treadmill training in the Trained animals.
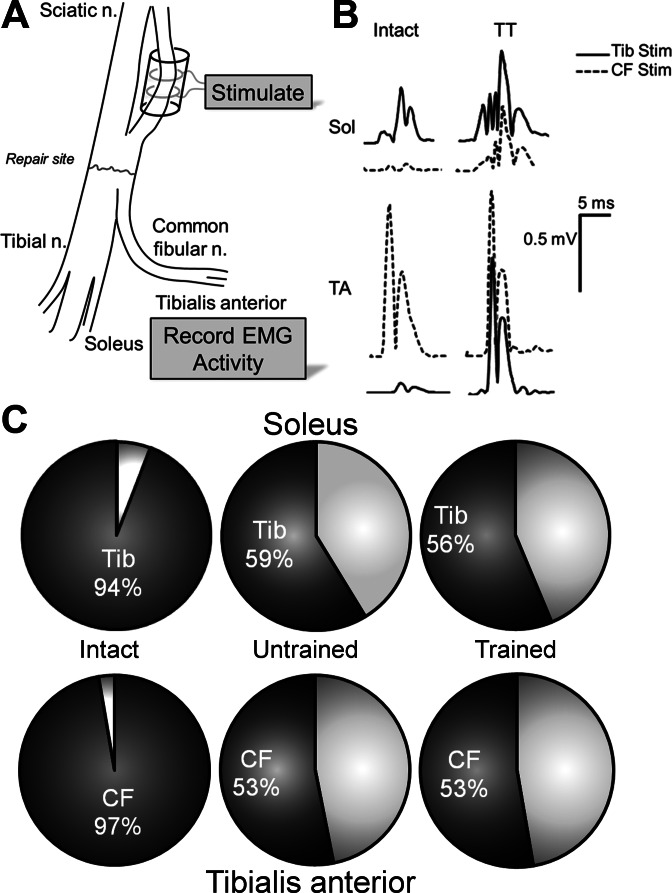
To evaluate a second form of regenerating motor axon misdirection, the routing of motor axons that had coursed in one sciatic nerve branch prior to injury and then were found in the other branch after regeneration, we stimulated fascicles of the sciatic nerve that formed the tibial and common fibular nerves well proximal to the lesion site, using the method described by Evans et al. (1991) and monitored activity evoked in SOL and TA in Trained and Untrained rats. Data from these experiments were compared with similar data from the contralateral intact TA and SOL muscles, where little or no such misdirection of axons would be expected. Results are shown in Fig. 5.
Fig. 5.
Specificity of muscle reinnervation in TT and Untrained rats. A: as part of a terminal experiment, at least 10 wk following sciatic nerve transection and repair, the Tib and common fibular (CF) fascicles of the sciatic nerve were dissected apart above the surgical repair site. A cuff electrode was placed about each of the dissected nerves (shown about CF branch in this diagram) and used to stimulate axons of motoneurons coursing in that branch. B: representative potentials recorded from patch electrodes on the SOL and TA muscles were made in response to stimulation of the Tib and CF fascicles of the sciatic nerve proximal to the nerve transection. Traces are shown for the Intact side and the reinnervated side (TT) of a rat 15 wk after transection and repair of the sciatic nerve and 2 wk of posttransection exercise. C: proportions of MMax in the SOL (top row) and TA (bottom row) muscles produced by stimulating the Tib and CF nerves are shown. In each pie chart, the proportion of the response produced by stimulation of the anatomically appropriate nerve is shown by dark shading. That produced by stimulation of the anatomically inappropriate nerve is shown by lighter shading.
If the sciatic nerve is intact, the evoked response produced by tibial nerve stimulation in SOL and by common fibular stimulation in TA was dominant. This is the response that would be anticipated. Small potentials, constituting 3–4% of the total M-response amplitude, were recorded in TA from tibial nerve stimulation and in SOL from common fibular stimulation (Fig. 5B, Intact). These were considered to be the result of either incomplete isolation of the nerve fascicle during dissection or unavoidable spread of stimulus current from the stimulated fascicle in these acute experiments. In contrast, significant potentials could be recorded in both the reinnervated TA and SOL muscles from stimulating either the tibial or the common fibular nerve (Fig. 5B, TT). Slightly more than one-half of the total M response found in the reinnervated SOL muscle was produced by axons originating in the tibial branch, and slightly more than one-half of the M response in the reinnervated TA muscle was produced by axons originating in the common fibular nerve (Fig. 5C). Slightly less than one-half of muscle reinnervation is by axons originating in functionally inappropriate nerves. In both Trained and Untrained rats, these proportions are not significantly different from each other, but are significantly different from those found after stimulating the same branches of intact nerves. Thus, as our laboratory has asserted previously for mice (English et al. 2009), treadmill training does not affect the extent of misdirection and aberrant reinnervation of muscle targets.
Effects of exercise on locomotor EMG activity.
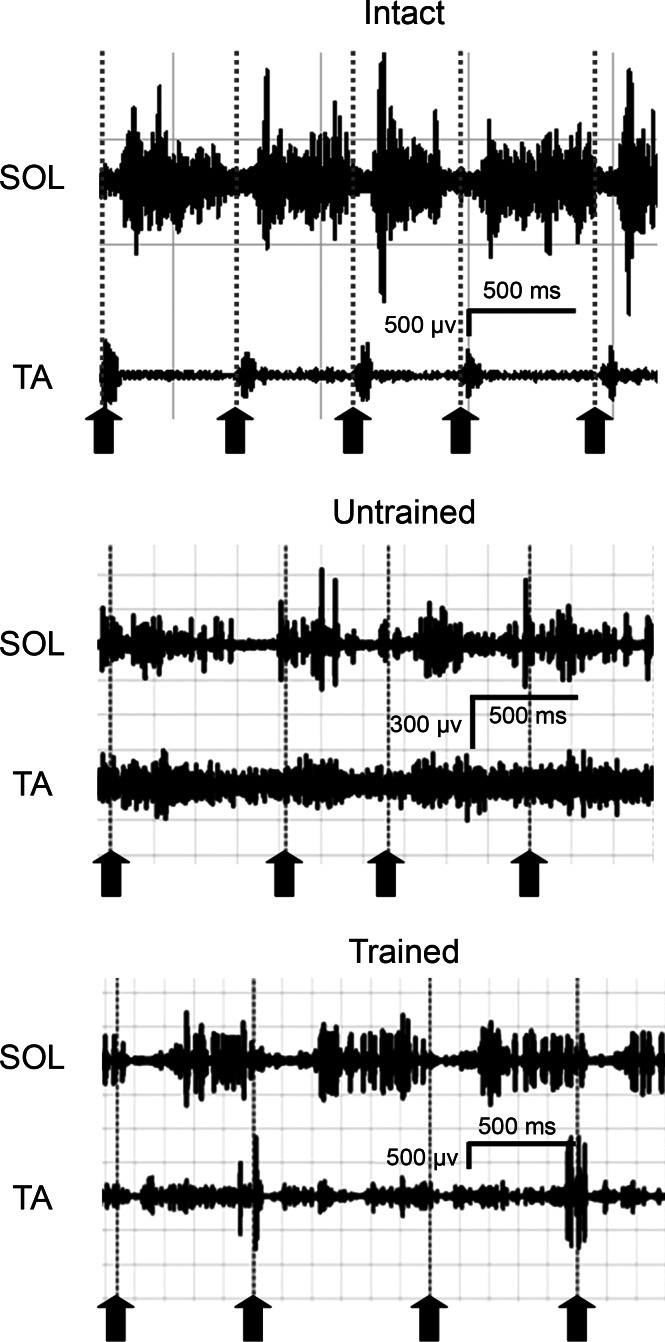
The timing of activation of the TA and SOL muscles during treadmill locomotion on three different slopes was compared among three different treatment groups: Intact, Untrained, and Trained. Representative raw locomotor EMG traces obtained are shown in Fig. 6. In both of the panels with recordings from reinnervated muscles, the amplitude of SOL activity is smaller, relative to those found in TA, than found in Intact rats. Note also that the strict reciprocal activation of TA and SOL found in Intact rats is not present in these panels.
Fig. 6.
Locomotor EMG activity in SOL and TA muscles during treadmill locomotion. Representative samples of activity recorded while walking on a level treadmill at 11 m/min are shown for an Intact rat (top) and for rats 10 wk after transection and repair of the sciatic nerve and Untrained (middle) or Trained rat (bottom). Activity was synchronized with video recordings. Vertical dotted lines and upward pointing arrows are placed at the onset of the swing phase of the step cycle, when the paw is removed from the treadmill belt.
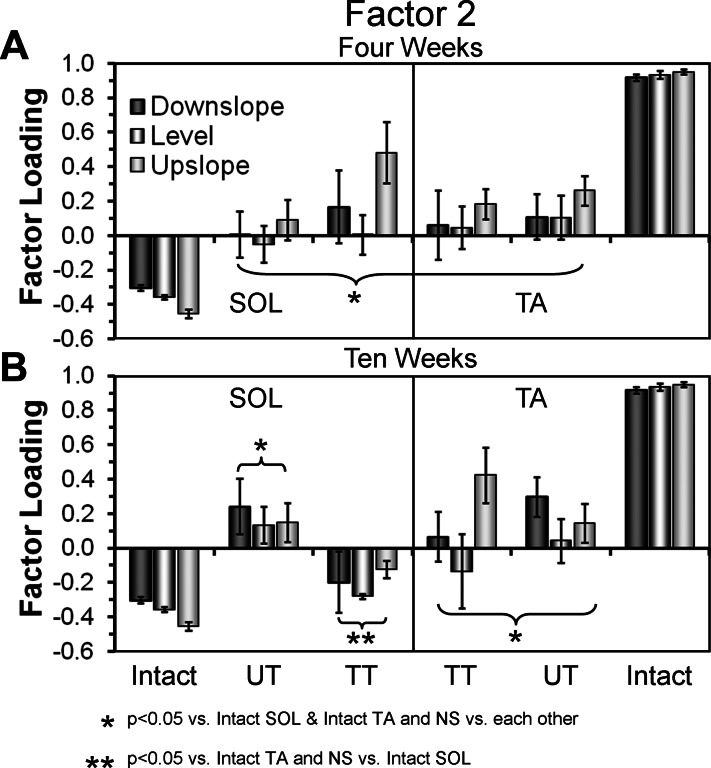
We used factor analysis with principal components (PCA) to evaluate the significance of any changes in activation pattern of these two muscles following muscle reinnervation in Trained and Untrained rats. Significant differences between groups were found for all three slopes studied at both survival times. For downslope walking, significant differences were found for factor 1 (F9,36 = 3.549, P < 0.003063) and factor 2 (F9,36 = 8.106, P < 0.000002). For upslope walking, significant differences were found for factor 2 (F9,36 = 16.717, P < 2 × 10−10) and factor 3 (F9,36 = 3.666, P < 0.002). For level walking, significant differences were found for factor 2 (F9,36 = 9.520, P < 0.0000004). Mean factor loadings for factor 2, where significant differences were found for all three slopes, are shown for the 4- and 10-wk survival time groups in Fig. 7.
Fig. 7.
Results of factor analysis using principal components (PCA) applied to locomotor EMG activity in the SOL and TA muscles. Activity during multiple individual step cycles was averaged for both muscles during level, upslope, and downslope walking at 11 m/min. Mean values (+SE) of factor loadings for factor 2, which accounts for 20% of the variance in the data set, are shown from activity recorded at 4 wk (A) and 10 wk (B) after sciatic nerve transection and repair. Bars representing data from downslope, level, and upslope treadmill locomotion are shown in different shadings. Data from the SOL muscle are shown to the left of each panel. Similar findings from the TA muscle are shown to the right in each panel. Data from Intact, Untreated (UT), and TT rats are shown as groups. The data points from Intact rats are the same in both A and B. *P < 0.05 vs. Intact SOL and Intact TA, and nonsignificant (NS) vs. each other. **P < 0.05 vs. Intact TA, and NS vs. Intact SOL.
In Intact rats, significant differences in mean factor loadings were found between SOL and TA for all three slopes studied (LSD, average P < 0.003) (Fig. 7, A and B, Intact). Our laboratory has argued previously that this very large difference is a reflection of the strict reciprocal activation of TA and SOL found during locomotion in Intact rats (Sabatier et al. 2011b; Thota et al. 2005). In Untrained rats, all of the factor loadings are significantly different from those of both TA and SOL in Intact rats, at both times studied and at all slopes (LSD, average P < 0.01) (Fig. 7, A and B, UT), consistent with the use of unique patterns of activation during locomotion; one that is different from that of either SOL or TA in Intact rats. In addition, no significant differences in factor loadings between TA and SOL among the Untrained rats were found at any slope (LSD, average P = 0.809). We interpret this finding as a reflection of the coactivation of these functional antagonists observed during locomotion in these animals, both here and by others (Gramsbergen et al. 2000). No significant differences were found between the 4-and 10-wk times examined.
Factor loadings for TA in the Trained group also were significantly different from those for both TA and SOL of Intact animals at both times studied (LSD, average P < 0.004) (Fig. 7, A and B, TT), as were the factor loadings for SOL at the 4-wk survival time (average P < 0.03). In contrast, by 10 wk after sciatic nerve transection, all factor loadings (for all PCs) for SOL in the Trained group were not significantly different from those for SOL in the Intact rats at all slopes studied (LSD, average P = 0.486). We interpret this finding to mean that moderate exercise applied immediately following sciatic nerve injury has an effect on the timing of activation of the reinnervated SOL during locomotion, at all three slopes studied, at 10 wk, but not 4 wk, after injury.
Effects of exercise on hindlimb movements during treadmill locomotion.
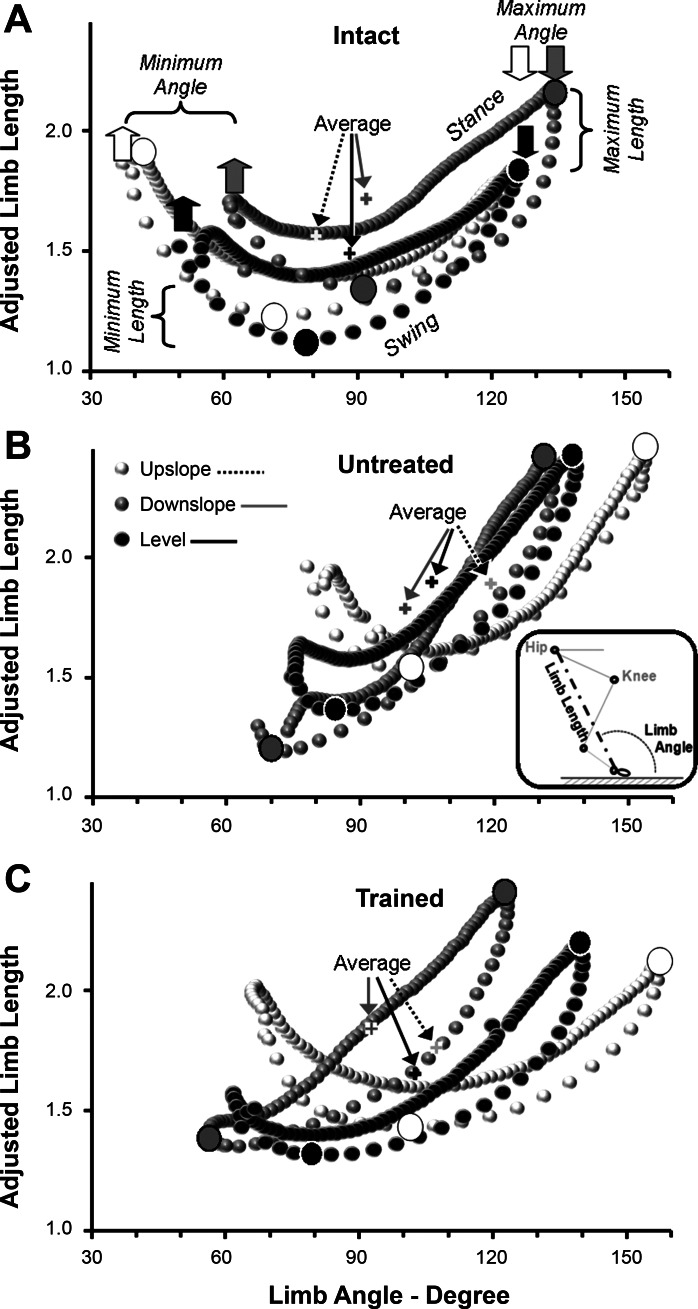
We used a global measure of hindlimb movements (Chang et al. 2009) to study the effects of exercise on hindlimb movements following sciatic nerve transection and repair. Marked points on the skin over the greater trochanter and the fifth MP joint were used to define a limb length position vector. The magnitude of this vector was calculated as the distance between these two skin markers and is termed limb length. The direction of this vector reflects the orientation of the limb to the treadmill belt and is termed limb angle (Fig. 8B, inset).
Fig. 8.
Whole hindlimb kinematics during treadmill locomotion on different slopes. From two-dimensional analysis of video records, a global limb vector of the hindlimb was calculated. The magnitude of this vector is limb length, the distance between skin markers placed over the greater trochanter and the fifth metatarsophalangeal joint, and its direction is limb angle, the orientation of the limb to the treadmill belt (see inset in B). For analysis, limb length was scaled to femur length in each rat and is reported as Adjusted Limb Length. In each panel, changes in limb angle during the step cycle are plotted against adjusted limb length. Data from Intact rats are shown in A. In B and C, data are from UT rats and from TT rats, respectively, 10 wk after transection and surgical repair of the sciatic nerve. Data from downslope, level, and upslope walking at 11 m/min are shown by symbols of different shading. Each of the resulting graphs in each panel forms an irregular shape. Data points in each shape represent the average of several rats (see Table 1). Paw contact, the end of the swing phase, is found at the extreme right of each shape (down arrows) and corresponds to the time of maximal Limb Angle. Paw off, the end of the stance phase, is found to the extreme left of each shape (up arrows) and corresponds to the time of minimum Limb Angle. Data points in the swing phase are found along the bottoms of each shape, and data points from the stance phase are along the top of each shape. In A, the timing of maximum and minimum Adjusted Limb Lengths are indicated by larger filled circles. Average values of limb angle and adjusted limb lengths are indicated by arrows in each panel.
Changes in these two components of the global limb vector during the step cycle in Intact, Untrained, and Trained rats are shown in Fig. 8. In each panel, data points from level, upslope, and downslope walking are indicated by different shading. Some of the data from Intact and Untrained rats have been presented in a slightly different form in previous papers from our laboratory (Sabatier et al. 2011a, 2011b). They form controls against which the data from Trained rats can be compared. In each panel, the magnitude of the global limb vector is displayed as adjusted limb length, the ratio of limb length to femur length, so that animals of different sizes could be compared at different survival times. For each of the two components of the global limb vector, three measures were chosen for parametric analysis. Average, minimum, and maximum values of limb angle and adjusted limb length over the entire step cycle were determined and are indicated by horizontal and vertical lines in Fig. 8A. All of these measurements were subjected to a one-way ANOVA. For each parameter, if a significant result was obtained from the omnibus test, paired, post hoc tests (LSD) were conducted. These data are summarized in Table 1. Mean values that are significantly different (P < 0.05) from Intact rats are indicated by asterisks.
Table 1.
Limb kinematic parameters during treadmill locomotion
| Time, wk | n | Average Angle, ° | Minimum Angle, ° | Maximum Angle, ° | Average Length | Minimum Adjusted Length | Maximum Adjusted Length | |
|---|---|---|---|---|---|---|---|---|
| Intact | ||||||||
| Downslope | 7 | 91.78 ± 6.83 | 56.68 ± 8.74 | 126.88 ± 5.92 | 1.72 ± 0.12 | 1.24 ± 0.17 | 2.20 ± 0.12 | |
| Level | 7 | 88.04 ± 3.09 | 51.52 ± 3.67 | 125.82 ± 4.82 | 1.49 ± 0.18 | 1.11 ± 0.15 | 1.85 ± 0.21 | |
| Upslope | 7 | 80.58 ± 4.31 | 37.23 ± 1.65 | 123.93 ± 7.84 | 1.57 ± 0.14 | 1.18 ± 0.13 | 1.81 ± 0.13 | |
| Untrained | ||||||||
| Downslope | 2 | 6 | 100.87 ± 9.35 | 68.27 ± 14.12 | 133.49 ± 4.96 | 1.75 ± 0.35 | 1.20 ± 0.30 | 2.31 ± 0.43 |
| 4 | 6 | 100.88 ± 12.62* | 63.67 ± 13.22 | 138.08 ± 14.44* | 1.79 ± 0.39 | 1.19 ± 0.23 | 2.39 ± 0.59 | |
| 10 | 3 | 99.94 ± 12.34 | 66.01 ± 17.79 | 133.86 ± 6.89 | 1.79 ± 0.21 | 1.18 ± 0.07 | 2.40 ± 0.37 | |
| Level | 2 | 6 | 107.98 ± 11.22* | 69.32 ± 13.52* | 135.48 ± 7.88* | 1.70 ± 0.19 | 1.52 ± 0.34 | 2.18 ± 0.15* |
| 4 | 6 | 106.05 ± 7.35* | 67.07 ± 6.73* | 140.14 ± 7.32* | 1.73 ± 0.45 | 1.53 ± 0.39 | 2.17 ± 0.46 | |
| 10 | 4 | 105.90 ± 8.91* | 73.93 ± 6.75* | 138.95 ± 10.68* | 1.90 ± 0.17* | 1.55 ± 0.25 | 2.46 ± 0.32* | |
| Upslope | 2 | 6 | 110.46 ± 14.92* | 69.19 ± 17.38* | 151.72 ± 14.88* | 1.65 ± 0.33 | 1.17 ± 0.29 | 2.06 ± 0.26* |
| 4 | 6 | 109.68 ± 7.26* | 63.99 ± 8.91* | 155.37 ± 7.87* | 1.83 ± 0.34 | 1.32 ± 0.31 | 2.34 ± 0.39* | |
| 10 | 3 | 118.94 ± 11.68* | 77.77 ± 13.66* | 154.22 ± 3.90* | 1.99 ± 0.22* | 1.48 ± 0.32 | 2.50 ± 0.14* | |
| Trained | ||||||||
| Downslope | 2 | 5 | 94.05 ± 6.76 | 66.62 ± 7.37 | 121.47 ± 8.20† | 1.66 ± 0.12 | 1.25 ± 0.15 | 2.08 ± 0.14 |
| 4 | 4 | 91.85 ± 5.29 | 60.34 ± 14.41 | 123.51 ± 9.26† | 1.85 ± 0.44 | 1.35 ± 0.33 | 2.34 ± 0.56 | |
| 10 | 4 | 89.76 ± 4.23 | 63.51 ± 12.05 | 128.51 ± 6.58 | 1.44 ± 0.54 | 0.97 ± 0.46 | 1.90 ± 0.61 | |
| Level | 2 | 5 | 101.80 ± 9.19* | 68.71 ± 12.88* | 134.89 ± 8.80 | 1.62 ± 0.17* | 1.27 ± 0.14 | 1.96 ± 0.21 |
| 4 | 4 | 100.40 ± 7.66* | 65.21 ± 10.46* | 135.59 ± 6.12* | 1.78 ± 0.45 | 1.33 ± 0.41 | 2.23 ± 0.50 | |
| 10 | 4 | 100.50 ± 8.91* | 58.93 ± 6.01 | 142.08 ± 11.77* | 1.74 ± 0.30 | 1.27 ± 0.35 | 2.20 ± 0.42 | |
| Upslope | 2 | 5 | 115.91 ± 6.54* | 75.55 ± 5.29* | 156.28 ± 9.70* | 1.51 ± 0.06* | 1.15 ± 0.06 | 1.87 ± 0.10 |
| 4 | 4 | 108.00 ± 4.40* | 65.11 ± 6.04* | 150.89 ± 12.32* | 1.62 ± 0.27 | 1.25 ± 0.17 | 1.89 ± 0.47 | |
| 10 | 4 | 111.06 ± 5.26* | 64.90 ± 5.70* | 157.22 ± 4.86* | 1.79 ± 0.21 | 1.05 ± 0.45 | 1.80 ± 0.45 |
Values are means ± SD; n, no. of rats. Length measurements are adjusted limb lengths. See text for details.
P < 0.05 vs. Intact.
P < 0.05 vs. Untrained.
The most striking outcome of this analysis is that global limb length is conserved by the recovering animals, as Chang et al. (2009) have suggested for cats and our laboratory has proposed for rats (Sabatier et al. 2011b). Among the 54 paired comparisons of adjusted limb length (three survival times × three parametric measures × three slopes × two treatment groups) made in the recovering rats, only nine significant (P < 0.05) differences (17%) were encountered. Most of these differences were for maximum adjusted limb length in Untreated rats. In contrast, significant differences in mean limb angle were found in 38 of the 54 comparisons made (70%). In all of these instances, mean limb angle measures were significantly greater than those found in Intact rats. The implication of this observation is that, in both Trained and Untrained rats, a strategy for conserving limb length during locomotion by increasing limb angle is adopted.
A second outcome of this analysis is related to the ability of the rats to conserve limb length when walking on different slopes. When Intact rats walk with the treadmill belt inclined downward, the swing phase is prolonged, and the maximum adjusted limb length is significantly greater than found during level walking, as the limb is extended to contact the treadmill belt (Fig. 8A, Maximum Length). This increased limb length persists throughout the step cycle. During upslope walking, maximum length occurs at the end of the stance phase when the angle of the limb is much reduced, as contact with the belt is prolonged (Fig. 8A, Minimum Angle). These adaptive changes to slope walking in Intact rats are not apparent in Untreated rats. There is barely any detectable difference in limb angle or limb length at different slopes (Fig. 8B), despite the very different biomechanical requirements for slope walking. The pattern of limb movement during walking on all three slopes resembles that described above for downslope walking in the Intact rats. As our laboratory has argued previously (Sabatier et al. 2011b), the adaptive strategy of conserving limb length by increasing limb angle is very effective during downslope walking. Of the 18 paired comparisons made for downslope walking, only two (maximum angle and average angle at 4 wk in the Untrained group) were significant (Table 1).
In marked contrast, slope-related adaptive changes in limb movements were observed in Trained rats (Fig. 8C). The same success in conserving both limb angle and adjusted limb length as found in Untreated rats was found during downslope walking. In Trained rats, maximal limb length was not significantly different from Intact rats during both level and upslope walking, and, at least by the 10-wk posttransection survival time, this conservation was achieved by smaller increases in average and minimum limb angle than found in Untrained rats (Table 1). Thus 2 wk of exercise applied immediately following sciatic nerve injury are sufficient to enable these animals to adapt the movements of their hindlimbs to the differing mechanical demands of walking on different slopes.
DISCUSSION
Moderate exercise in the form of treadmill training has been shown to enhance axon regeneration following peripheral nerve injury in mice (English et al. 2009; Sabatier et al. 2008) and rats (Asensio-Pinilla et al. 2009; Ilha et al. 2008; Seo et al. 2006; Udina et al. 2011), but its full effects on functional recovery are not known. We hypothesized that moderate exercise in the form of daily treadmill training during the first 2 wk following transection and repair of the rat sciatic nerve would lead to improved functional recovery. The main finding of this study was that 2 wk of daily treadmill training following sciatic nerve injury in rats results in improvements in at least four aspects of functional recovery compared with Untrained rats receiving the same injury.
Exercise results in enhanced functional muscle reinnervation.
The reappearance of an M response and an H reflex in SOL to tibial nerve stimulation in awake behaving animals was used to measure the timing of functional muscle reinnervation. In Trained rats, both evoked potentials reappeared significantly earlier than in Untrained rats. The simplest explanation for this effect is that exercise promoted regenerating axon elongation, as has been shown anatomically in mice (English et al. 2009; Sabatier et al. 2008). The amplitude of both of these potentials increased linearly in all rats during the 10- to 15-wk study period. This finding also is consistent with our laboratory's previous anatomical observations in exercised mice (English 2005a) and following brief electrical stimulation in rats (Al-Majed et al. 2000). Elongation of regenerating axons (Al-Majed et al. 2000) and muscle fiber reinnervation (English et al. 2007b), both are temporally staggered over several weeks following nerve transection and repair. The linear increase in amplitudes of these responses to tibial nerve stimulation is likely the result of some combination of the reinnervation of the SOL muscle by progressively more regenerating motor axons and the enlargement of reinnervated muscle fibers. Because nearly one-half of reinnervated SOL muscle fibers stop expressing slow myosin heavy chain isoforms and begin to express fast isoforms following sciatic nerve injury (Mendler et al. 2008), we also cannot rule out a contribution of this fiber phenotype transformation to the increase in evoked EMG amplitudes.
However, the slopes of the lines fitted to the data from Trained rats were significantly greater than the corresponding slopes for Untrained rats, suggesting that, at any given time during recovery, the regeneration process was more mature in the Trained animals. These findings are comparable to the results of nerve conduction studies reported by Navarro and colleagues (Asensio-Pinilla et al. 2009; Udina et al. 2011) who found that 4 wk of application of an exercise protocol that was even less intense than the one used here resulted in larger M responses at 60 days after injury. Coupled with the results presented above, that a similar enhancement of evoked EMG amplitudes is found as long as 100 days after injury, we suggest that the effects of moderate exercise after peripheral nerve injury influence the regeneration process for a considerable time after the exercise has ended.
By 11 wk after sciatic nerve transection and repair, the amplitude of both the SOL M response and the H reflex in Trained rats is much larger than that found in Untrained rats and also nearly twice that found in the same muscle prior to sciatic nerve transection. Others have reported more modest relative activity (Asensio-Pinilla et al. 2009; Udina et al. 2011). We can only speculate as to why such a large increase in amplitudes was found in the Trained rats. Despite finding in our postmortem examinations that the EMG wires were in the same locations in the muscles as where we had implanted them prior to nerve transection, it seems likely that the geometric relationship between our implanted EMG electrodes and the surrounding muscle fibers may change as the fibers first atrophy with denervation and then recover somewhat after reinnervation. In both Untreated and Treated rats, such a change might mean that the number of muscle fibers in close proximity to the moderately high spatial resolution EMG electrodes used in the present study might be greater than encountered in muscles of Intact rats. The compound muscle action potentials recorded in fully reinnervated muscles, either directly (M response) or via the H reflex, then might be expected to be larger than those recorded from activating the same number of motor units in the muscles in Intact rats. We believe that the larger-than-pretransection potentials recorded only in Trained rats reflects the faster and more extensive reinnervation of SOL muscle fibers found with moderate exercise. The restoration of evoked potential amplitudes to pretransection levels found in Untrained rats thus might be interpreted as evidence of incomplete reinnervation.
Exercise restores H reflex efficacy in reinnervated muscles.
The ratio of the amplitude of the largest H reflex recorded to that of the largest M response in the same experiment is considered a reflection of the efficacy of the reflex, a measure of the portion of the total available SOL motoneuron pool that is recruited into activity by the electrical stimulation of peripheral afferent axons (Navarro et al., 2007). Because it is a comparison of the amplitudes of two potentials, it is assumed to be independent of the amount of muscle reinnervation. This HMax/MMax differed markedly in Trained and Untrained rats. In both Trained and Untrained rats, this ratio is initially larger than found in Intact rats, as has been observed by others (Asensio-Pinilla et al. 2009; Navarro et al. 2007; Udina et al. 2011). In Untrained rats, this very large response persists for 3–4 wk and then decreases, at first rapidly and then more gradually over a 4-wk period, until it reaches a stable ratio significantly below that of Intact rats (English et al. 2007a). Thus, by the end of the study period, we would conclude that relatively fewer SOL motor units are recruited into the H reflex in Untrained rats than found in Intact rats. In Trained rats, the initial HMax/MMax decreases gradually until it stabilizes at a value similar to that noted in Intact rats, indicating that a similar proportion of the available motoneuron pool is recruited into activity by peripheral afferent stimulation in Trained and Intact rats.
Peripheral nerve transection results in a withdrawal of the synaptic inputs onto the somata and proximal dendrites of motoneurons, a phenomenon known as synaptic stripping (Blinzinger and Kreutzberg 1968). Although many of these withdrawn inputs are later restored, most of those originating from primary sensory neurons, the afferent axons contributing to the H reflex, are not (Alvarez et al. 2010, 2011), and this permanent loss of inputs may explain the loss of the stretch reflex in self-reinnervated muscles (Cope et al. 1994). We have found a similar withdrawal of synaptic terminals in Untrained mice following sciatic nerve transections, but no evidence for stripped inputs on axotomized motoneurons in mice after moderate daily exercise (English et al. 2011). It is tempting to speculate that this effect of exercise on the structure of circuitry in the central nervous system might underlie the restoration of the full HMax/MMax in our Trained rats, but more data would be needed to support such a conclusion.
Exercise has a modest effect on locomotor activation patterns of reinnervated muscles.
In Untrained rats, the pattern of activation of the SOL and TA muscles during treadmill locomotion shifts from strict reciprocity (Sabatier et al. 2011b; Thota et al. 2005) to coactivation (Sabatier et al. 2011b) following recovery from sciatic nerve injury. A similar outcome was noted when Trained rats were studied at early reinnervation times, but by 10 wk after nerve repair, the pattern of activity of the reinnervated SOL was not significantly different from that of the SOL muscle of Intact rats during locomotion on all three slopes. Because the timing of SOL activity has been shown to differ between level and upslope walking in Intact rats (Sabatier et al. 2011b), this alteration in the timing of activity of the reinnervated SOL at all three slopes must reflect at least a modest restoration of the ability of the rat to adapt the pattern of reinnervated muscle activity to the different mechanical demands of slope walking.
Such a change in the timing of activity could be related to the specificity with which regenerating axons reinnervated functionally appropriate vs. inappropriate muscle targets. Our laboratory has shown here and elsewhere (English et al. 2009) that exercise has little effect on one form of misdirection of regenerating motor axons, the precision with which those axons reinnervate peripheral targets. However, we also found that a second form of axon misdirection, the branching of regenerating axons to reinnervate both the TA muscle and muscle targets of the tibial nerve was greatly reduced in Trained relative to Untrained rats. The extent of such dual innervation, as noted in the results of tibial nerve axon reflex experiments (Fig. 4), was similar in the two experimental groups until about 6 wk after sciatic nerve transection and repair. After that time, the extent of branched innervation increased in the Untrained rats, but not in Trained rats. Whether the lesser amount of branched muscle reinnervation found in Trained rats, and by implication a greater ability to activate the TA and SOL muscles independently, is the source of the restored pattern of SOL activation during locomotion is not clear at this time. The contribution of branched muscle reinnervation could be evaluated by study of the activation patterns of individual reinnervated motor units.
A similar effect of training was not found in the reinnervated TA muscle: the locomotor activity pattern of the reinnervated TA muscle at both times studied and on all slopes was not significantly different in Trained and Untrained rats. Thus SOL and TA are coactivated during locomotion in the Trained rats, but the pattern of coactivation is different from the pattern of coactivation found in Untrained rats at 10 wk, or even that in Trained rats at earlier survival times. The effect of the exercise is a reduction in the amount of SOL activity during the swing phase, but cocontraction with TA during the stance phase (see Fig. 6, bottom). The fact that nearly one-half of the reinnervation of both the SOL and TA muscles is from functionally inappropriate motoneurons (see Fig. 5) could account for the coactivation of these muscles during walking on all slopes in the Untrained rats, provided the outputs of the spinal circuits that regulate the timing of activation of the motoneurons reinnervating these muscles during locomotion was not changed. Because the timing of SOL locomotor activity was changed in the Trained rats at 10 wk, but not 4 wk after injury, and the misdirection of regenerating axons was the same as found in Untrained rats, we suggest that the outputs of the spinal circuitry that regulates the timing of activation of the motoneurons reinnervating the SOL muscle during locomotion must have been changed by the exercise. In particular, we would speculate that the activation of motoneurons that had previously innervated TA, but had regenerated to reinnervate SOL, must have been changed in the Trained rats, and that the change occurred sometime between 4 and 10 wk after nerve transection. Testing of this speculative hypothesis awaits further study.
Our laboratory has postulated (Sabatier et al. 2011b) that the coactivation of the functional antagonistic TA and SOL muscles found in Untrained rats might be a part of an adaptive strategy to stiffen the ankle joint during both the stance and swing phases of the step cycle. In this context, we consider that the improved SOL reinnervation found following only 2 wk of moderate daily exercise might not be sufficient to counteract such a strategy following a nerve injury that affects both TA and SOL. In mice, we found that a similar training paradigm to the one employed in this study resulted in more profound effects on axon regeneration in the tibial branch of the sciatic nerve than the common fibular branch, but, if the amplitude of the exercise was increased by training on an upslope inclined treadmill, then the effect on axon regeneration in the two nerve branches was more balanced (Sabatier et al. 2010). A future study should investigate the effect of this upslope training on locomotor EMG activity.
Exercise improves hindlimb movement patterns during locomotion on different slopes.
Locomotion on different slopes requires different limb movement strategies to cope with the different biomechanical demands (Carlson-Kuhta et al. 1998; Maas et al. 2009). Previous observations in Untrained rats (Sabatier et al. 2011b) and in cats (Chang et al. 2009) have been used to argue that hindlimb length is a conserved variable in these strategies. Variability in limb length measurements, both between animals and between different steps in the same animals, is very much smaller than any other measure of limb or joint kinematics (Sabatier et al. 2011b). The range of limb lengths encountered during locomotion at all three slope conditions is conserved by changes in the angle of orientation of the limb, especially during level and upslope walking. One goal of this conservation of limb length is proposed to help maintain the coordination of movements of the different limbs (Chen et al. 2011).
Sciatic nerve transection causes paralysis of the muscles that act about the ankle and foot. Walking is accomplished by activation of innervated proximal musculature and little use of the ankle and foot (Bain et al. 1989). The foot lies flat on the treadmill belt during stance, but changes in hip and knee movements result in an increase in limb angle and an attempt to maintain the overall length of the hindlimb to match the pattern found in Intact rats (and, presumably, the contralateral hindlimb). This new walking pattern is retained following muscle reinnervation, where it is reinforced by coactivation of functional antagonists (SOL and TA) about the ankle. This compensatory strategy was not changed by our exercise protocol. All measures of limb angle were increased significantly in both Untrained and Trained rats at all times studied. What was changed by exercise was the effectiveness of this strategy to cope with the different mechanical demands of locomotion on slopes. Limb length was conserved successfully by all rats during downslope walking, but conservation of limb length by changing the orientation of the limb was successful during level and upslope locomotion only in the Trained rats.
The exercise protocol used does enhance functional recovery from peripheral nerve injury by enhancing axon regeneration, by facilitating a faster than normal recovery time of reflexes, and by restoring the efficacy of the H reflex. Although it does not result in a return to full functional recovery, at least as measured during locomotion, the enhancements of muscle and reflex function undoubtedly contribute to a restored ability of the animals to adapt to the different biomechanical demands of slope walking. We conclude that this restored ability represents a modest, but significant, improvement over the Untrained group. We feel that exercise as a therapy for peripheral nerve injury has significant potential for translation to clinical use, but more study is needed. It is possible that the brief period of very moderate exercise used in the present study, while sufficient to promote nerve regeneration and modest functional recovery, is not optimal to promote full functional recovery. Different paradigms could have a more pronounced effect on functional recovery. However, because different exercise protocols, including passive limb movements, have resulted in improvements in axon regeneration after peripheral nerve injury (reviewed in Udina et al. 2011), one also cannot rule out that the effects we have observed are the result of the increased environmental enrichment associated with exercise, as has been suggested by others (Gomez-Pinilla et al. 2011). Identification of the critical features of exercise that promote axon regeneration and improved functional recovery will have to be a priority, if our findings are to be translated to clinical use.
GRANTS
This work was supported by grant HD032571 from the US Public Health Service.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.B., M.I., K.M., J.N., K.P., S.J.R., E.W., and A.W.E. performed experiments; T.B., M.I., K.M., J.N., K.P., S.J.R., E.W., and A.W.E. analyzed data; T.B., M.I., K.M., J.N., K.P., S.J.R., E.W., and A.W.E. interpreted results of experiments; T.B., M.I., K.M., J.N., K.P., S.J.R., E.W., and A.W.E. prepared figures; T.B., M.I., K.M., J.N., K.P., S.J.R., E.W., and A.W.E. drafted manuscript; T.B., M.I., K.M., J.N., K.P., S.J.R., E.W., and A.W.E. edited and revised manuscript; T.B., M.I., K.M., J.N., K.P., S.J.R., E.W., and A.W.E. approved final version of manuscript; J.N., S.J.R., and A.W.E. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Dr. Michael Kutner for advice and help with all of the statistical methods used. Dr. Manning Sabatier read and commented on earlier versions of this paper, and his help is greatly appreciated.
REFERENCES
- Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci 20: 2602–2608, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Bullinger KL, Titus HE, Nardelli P, Cope TC. Permanent reorganization of Ia afferent synapses on motoneurons after peripheral nerve injury. Ann NY Acad Sci 1198: 231–241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol 106: 2450–2470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol 219: 258–265, 2009 [DOI] [PubMed] [Google Scholar]
- Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 83: 129–138, 1989 [DOI] [PubMed] [Google Scholar]
- Bauman JM, Chang YH. High-speed X-ray video demonstrates significant skin movement errors with standard optical kinematics during rat locomotion. J Neurosci Methods 186: 18–24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat 85: 145–157, 1968 [DOI] [PubMed] [Google Scholar]
- Boeltz T, Ireland M, Mathis K, Nicolini J, Poplavski K, Rose SJ, Wilson E, Sabatier MJ, English AW. Treadmill training and functional recovery after peripheral nerve injury. 2010 Neuroscience Meeting San Diego, CA: Society for Neuroscience, 2010 [Google Scholar]
- Brushart TM. Nerve Repair. New York: Oxford University Press, 2011, p. 463 [Google Scholar]
- Carlson-Kuhta P, Trank TV, Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol 79: 1687–1701, 1998 [DOI] [PubMed] [Google Scholar]
- Chang YH, Auyang AG, Scholz JP, Nichols TR. Whole limb kinematics are preferentially conserved over individual joint kinematics after peripheral nerve injury. J Exp Biol 212: 3511–3521, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen L, Wang Y, Wolpaw JR, Chen XY. Operant conditioning of rat soleus H-reflex oppositely affects another H-reflex and changes locomotor kinematics. J Neurosci 31: 11370–11375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope TC, Bonasera SJ, Nichols TR. Reinnervated muscles fail to produce stretch reflexes. J Neurophysiol 71: 817–820, 1994 [DOI] [PubMed] [Google Scholar]
- English AW, Weeks OI. Electromyographic cross-talk within a compartmentalized muscle of the cat. J Physiol 416: 327–336, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol 490: 427–441, 2005a [DOI] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol 490: 427–441, 2005b [DOI] [PubMed] [Google Scholar]
- English AW, Chen Y, Carp JS, Wolpaw JR, Chen XY. Recovery of electromyographic activity after transection and surgical repair of the rat sciatic nerve. J Neurophysiol 97: 1127–1134, 2007a [DOI] [PubMed] [Google Scholar]
- English AW, Gupta M, Schwartz GA. Anatomical reformation of neuromuscular synapses after nerve transection is accelerated by treadmill exercise. Program No. 511.2, 2007 Neuroscience Meeting [Google Scholar]
- English AW, Cucoranu D, Mulligan A, Sabatier M. Treadmill training enhances axon regeneration in injured mouse peripheral nerves without increased loss of topographic specificity. J Comp Neurol 517: 245–255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Wilhelm JC, Sabatier MJ. Enhancing recovery from peripheral nerve injury using treadmill training. Ann Anat 193: 354–361, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PJ, Bain JR, Mackinnon SE, Makino AP, Hunter DA. Selective reinnervation: a comparison of recovery following microsuture and conduit nerve repair. Brain Res 559: 315–321, 1991 [DOI] [PubMed] [Google Scholar]
- Foehring RC, Sypert GW, Munson JB. Properties of self-reinnervated motor units of medial gastrocnemius of cat. II. Axotomized motoneurons and time course of recovery. J Neurophysiol 55: 947–965, 1986 [DOI] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery 18: 397–405, 1998 [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Agoncillo T, Frostig R. The influence of naturalistic experience on plasticity markers in somatosensory cortex and hippocampus: effects of whisker use. Brain Res 1388: 39–47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus 26: E3, 2009 [DOI] [PubMed] [Google Scholar]
- Gramsbergen A, Ijkema-Paassen J, Meek MF. Sciatic nerve transection in the adult rat: abnormal EMG patterns during locomotion by aberrant innervation of hindleg muscles. Exp Neurol 161: 183–193, 2000 [DOI] [PubMed] [Google Scholar]
- Hamilton SK, Hinkle ML, Kaufman MR, Nicolini J, Rambo L, Rexwinkle AR, Rose SR, Sabatier MJ, Backus D, English AW. Misdirection of regenerating axons and functional recovery following sciatic nerve injury in rats. J Comp Neurol 259: 21–33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilha J, Araujo RT, Malysz T, Hermel EE, Rigon P, Xavier LL, Achaval M. Endurance and resistance exercise training programs elicit specific effects on sciatic nerve regeneration after experimental traumatic lesion in rats. Neurorehabil Neural Repair 22: 355–366, 2008 [DOI] [PubMed] [Google Scholar]
- Lay AN, Hass CJ, Gregor RJ. The effects of sloped surfaces on locomotion: a kinematic and kinetic analysis. J Biomech 39: 1621–1628, 2006 [DOI] [PubMed] [Google Scholar]
- Loeb GE, Gans C. Electromyography for Experimentalists. Chicago, IL: University of Chicago Press, 1986 [Google Scholar]
- Maas H, Gregor RJ, Hodson-Tole EF, Farrell BJ, Prilutsky BI. Distinct muscle fascicle length changes in feline medial gastrocnemius and soleus muscles during slope walking. J Appl Physiol 106: 1169–1180, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray TE. Fibrin sealants and glues. J Card Surg 18: 480–485, 2003 [DOI] [PubMed] [Google Scholar]
- Mendler L, Pinter S, Kiricsi M, Baka Z, Dux L. Regeneration of reinnervated rat soleus muscle is accompanied by fiber transition toward a faster phenotype. J Histochem Cytochem 56: 111–123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 82: 163–201, 2007 [DOI] [PubMed] [Google Scholar]
- Sabatier M, Redmon N, Schwartz G, English A. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol 211: 489–493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier M, Ngoc To B, Nicolini J, English AW. Effect of axon misdirection on recovery of EMG activity and kinematics after peripheral nerve injury. Cells Tissues Organs 193: 298–309, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier MJ, Kaufman M, English AW. Effects of upslope treadmill exercise on axon regeneration in peripheral nerves. Abstr Amer Cong Sports Medicine 2010 [Google Scholar]
- Sabatier MJ, To BN, Nicolini J, English AW. Effect of slope and sciatic nerve injury on ankle muscle recruitment and hindlimb kinematics during walking in the rat. J Exp Biol 214: 1007–1016, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier MJ, To BN, Rose S, Nicolini J, English AW. Chondroitinase ABC reduces time to muscle reinnervation and improves functional recovery after sciatic nerve transection in rats. J Neurophysiol 107: 747–757, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz T, Krichevsky A, Sumarto A, Jaffurs D, Wirth GA, Paydar K, Evans GR. Peripheral nerve injuries: an international survey of current treatments and future perspectives. J Reconstr Microsurg 25: 339–344, 2009 [DOI] [PubMed] [Google Scholar]
- Seo TB, Han IS, Yoon JH, Hong KE, Yoon SJ, Namgung U. Involvement of Cdc2 in axonal regeneration enhanced by exercise training in rats. Med Sci Sports Exerc 38: 1267–1276, 2006 [DOI] [PubMed] [Google Scholar]
- Stein RB, Nichols TR, Jhamandas J, Davis L, Charles D. Stable long-term recordings from cat peripheral nerves. Brain Res 128: 21–38, 1977 [DOI] [PubMed] [Google Scholar]
- Thota AK, Watson SC, Knapp E, Thompson B, Jung R. Neuromechanical control of locomotion in the rat. J Neurotrauma 22: 442–465, 2005 [DOI] [PubMed] [Google Scholar]
- Udina E, Puigdemasa A, Navarro X. Passive and active exercise improve regeneration and muscle reinnervation after peripheral nerve injury in the rat. Muscle Nerve 43: 500–509, 2011 [DOI] [PubMed] [Google Scholar]
- Wilhelm JC, Cucoranu D, Xu M, Chmielewski S, Holmes T, Lau KS, Bassell GJ, English AW. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J Neurosci 32: 5002–5009, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MD, Kemp SW, Weber C, Borschel GH, Gordon T. Outcome measures of peripheral nerve regeneration. Ann Anat 193: 321–333, 2011 [DOI] [PubMed] [Google Scholar]