Abstract
Taste processing in the rostral nucleus of the solitary tract (rNST) is subject to modulatory influences including opioid peptides. Behavioral pharmacological studies suggest an influence of μ-opioid receptors in rNST, but the underlying mechanism is unknown. To determine the cellular site of action, we tested the effects of the μ-opioid receptor agonist DAMGO in vitro. Whole cell patch-clamp recordings were made in brain stem slices from GAD67-GFP knockin mice expressing enhanced green fluorescent protein (EGFP) under the control of the endogenous promoter for GAD67, a synthetic enzyme for GABA. Neuron counts showed that ∼36% of rNST neurons express GABA. We recorded monosynaptic solitary tract (ST)-evoked currents (jitter ≤ 300 μs) in both GAD67-EGFP-positive (GAD67+) and GAD67-EGFP-negative (GAD67−) neurons with equal frequency (25/31; 22/28), but the inputs to the GAD67+ neurons had significantly smaller paired-pulse ratios compared with GAD67− neurons. DAMGO (0.3 μM) significantly suppressed ST-evoked currents in both cell types (mean suppression = 46 ± 3.3% SE), significantly increased the paired-pulse ratio of these currents, and reduced the frequency of spontaneous miniature excitatory postsynaptic currents but did not diminish their amplitude, indicating a presynaptic site of action. Under inhibitory amino acid receptor blockade, DAMGO was significantly more suppressive in GAD67+ neurons (59% reduction) compared with GAD67− neurons (35% reduction), while the reverse was true in normal artificial cerebrospinal fluid (GAD67+: 35% reduction; GAD67−: 57% reduction). These findings suggest that DAMGO suppresses activity in rNST neurons predominantly via a presynaptic mechanism, and that this effect may interact significantly with tonic or evoked inhibitory activity.
Keywords: taste, GAD67-GFP knockin mouse, disinhibition, GABA
the rostral nucleus of the solitary tract (rNST) is the site of the first central synapse in the afferent taste pathway, receiving input from the 7th, 9th, and 10th cranial nerves (Lundy and Norgren 2004). Although it serves as a distribution node for both local and ascending gustatory pathways, it also constitutes a complex intrinsic processing network subject to modulatory influences from forebrain gustatory and homeostatic regions (Bradley 2007; Lundy and Norgren 2004; Smith and Travers 2008). Opioids are one among several classes of neuromodulators that have been demonstrated to be present and/or affect taste responses in the rNST (Davis and Smith 1997; King et al. 1993; Li et al. 2003; Travers and Travers 2010), and opioid receptors, including the μ- and δ-subtypes, are located in the rostral, gustatory, as well as caudal visceral regions of the nucleus (Huang et al. 2000; Li et al. 2003; Mansour et al. 1994; Monteillet-Agius et al. 1998; Moriwaki et al. 1996). Indeed, depending on the precise location, intra-NST infusions of opioids affect not only gustatory but also visceral and ingestive functions (Herman et al. 2009, 2010; Kinzeler and Travers 2011; Kotz et al. 1997).
In the caudal nucleus of the solitary tract (cNST) and associated dorsal motor nucleus of the vagus, μ-opioid receptors (MORs) act at multiple loci, exerting not only presynaptic inhibitory effects on primary afferent fibers but also postsynaptic inhibitory effects on intrinsic neurons (Appleyard et al. 2005; Poole et al. 2007; Rhim et al. 1993; Rhim and Miller 1994). In addition, MOR activation in this region has a powerful effect on vago-vagal reflexes controlling gastric function and tone. Interestingly, a recent study (Herman et al. 2012) demonstrated gastric effects due to interactions between the manipulation of cNST μ-opioid and GABAergic receptors consistent with effects on cNST neurons observed in vitro (Herman et al. 2010).
In the rNST, microinjection of the mixed μ/δ ligand met-enkephalin suppresses taste-evoked responses (Li et al. 2003), and in vitro studies suggest that an underlying basis for this effect is δ-opioid receptor-mediated postsynaptic inhibition of (excitatory) parabrachial nucleus (PBN)-projection neurons (Zhu et al. 2009). However, immunohistochemical studies imply that MORs are also located on primary gustatory afferents (Li et al. 2003). Moreover, infusion of the MOR agonist DAMGO into this substrate results in complex changes in oromotor reflex behaviors that include abbreviating the duration of lick bouts and augmenting the oral rejection response, effects compatible with underlying inhibitory and disinhibitory processes, respectively (Kinzeler and Travers 2011). Thus it would appear that in addition to postsynaptic influences of δ-receptors on excitatory projection neurons, opioid mechanisms in the rNST include MOR-mediated effects exerted through other mechanisms or cell types, similar to their effects in the caudal region of the nucleus.
To begin to explore the cellular basis of μ-opioid influence in rNST, we examined the effect of DAMGO on solitary tract (ST)-evoked responses in an in vitro slice preparation in a strain of transgenic mice that express enhanced green fluorescent protein (EGFP) under the control of the endogenous promoter for GAD67, a synthetic enzyme for GABA (Tamamaki et al. 2003). We determined that DAMGO had a primarily presynaptic suppressive effect on ST-evoked input to both GAD67-EGFP-positive (GAD67+) and GAD67-EGFP-negative (GAD67−) rNST neurons. Recording from this model also enabled us to characterize synaptic properties of these identified neurons, and we found that monosynaptic input to GAD67+ neurons was characterized by a significantly smaller paired-pulse ratio (PPR) than input to GAD67− neurons.
METHODS
Animal model.
We used a transgenic mouse expressing EGFP under the control of the endogenous promoter for GAD67, a synthetic enzyme for GABA. In contrast to the commercially available “GIN” mouse, which only expresses GFP in a particular subset of rNST GABAergic neurons (Travers et al. 2007; Wang and Bradley 2010a), this GAD67-GFP knockin strain has been shown to produce EGFP in virtually all cells expressing GAD67 (Brown et al. 2008; Kaneko et al. 2008; Ono et al. 2005; Tamamaki et al. 2003), allowing for a more complete identification of GABAergic neurons in the NTS. Because of reports that monosynaptic inputs to GABAergic neurons in the rNST may diminish during development in rat (Wang et al. 2012) but evidence for presynaptic inhibition of monosynaptic vagal inputs to cNST GABAergic neurons in mature as well as young mice (Glatzer et al. 2007), we included both mouse pups (P14–18) and adult mice (6–11 wk) in our study. Initially we tested mouse pup brain slices in artificial cerebrospinal fluid (ACSF) and sometimes observed both inhibitory and excitatory inputs after afferent stimulation that precluded measurement of jitter essential to an interpretation of monosynaptic input. We therefore recorded from a population of rNST neurons while suppressing inhibitory currents with a bath application of a gabazine-strychnine (GS) cocktail. This latter protocol was maintained in the adult group. All experimental protocols were approved by the Ohio State University Institutional Animal Care and Use Committee in accordance with guidelines from the National Institutes of Health.
Slice preparation.
Acute slices were prepared for electrophysiological recording after anesthetization with urethane (33% urethane, 10 ml/kg), decapitation, and rapid removal and cooling of the brain. In in vitro experiments urethane has been shown to wash out in considerably less time than our hour-long incubation step prior to recording, and thus it was unlikely to have had any influence on our neurons at the time of recording (Hara and Harris 2002; Sceniak and Maciver 2006). The blocked brain stem was glued to a ceramic block with cyanoacrylate glue, and coronal brain stem slices 250–300 μm thick were cut with a sapphire blade on a vibratome (model 1000, Vibratome, St. Louis, MO) in an ice-cold carboxygenated cutting solution containing (in mM) 110 choline, 25 NaHCO3, 3 KCl, 7 MgSO4, 1.5 NaH2PO4, 10 d-glucose, and 0.5 CaCl2. Slices containing the NST rostral to where it moves laterally away from the fourth ventricle were identified and incubated in a carboxygenated ACSF containing (in mM) 124 NaCl, 25 NaHCO3, 3 KCl, 1 MgSO4, 1.5 NaH2PO4, 10 d-glucose, and 1.5 CaCl2 at 32°C for 1 h prior to recording.
Electrophysiological recording and stimulation of solitary tract.
Slices were transferred to a recording chamber and perfused with 36°C ACSF at a rate of 1–2 ml/min. The NST and ST were visualized with a Nikon E600FN microscope, and a bipolar stimulating electrode made of twisted insulated wire was placed on the ST under visual control. Stimulation of the ST consisted of two current pulses 0.15 ms in duration separated by 33 ms and delivered at 0.3 Hz. The interstimulus interval (ISI) of 33 ms was chosen to minimize summation between the responses to the first and second stimuli while also allowing the first stimulus to influence the probability of release in response to the second stimulus. It also falls within the range of ISIs used by other investigators studying this brain region (Appleyard et al. 2005; Zhu et al. 2009). Threshold was defined as the current intensity that evoked a response 50% of the time, and the experimental stimulus intensity was set at 20 μA above threshold to ensure a reliable response. In some cases, the presence of an additional, higher-threshold evoked response precluded the use of current values 20 μA above threshold, and in these cases the highest current that did not activate the additional input was used. Stimulus intensities ranged from 5 to 140 μA.
After placement of the stimulating electrode, cells in the rNST were visualized with IR-DIC optics and epifluorescent illumination to identify cells that were either GAD67+ or GAD67−. Approximately equal numbers of GAD67+ and GAD67− neurons were targeted throughout the course of the study. Once a neuron was identified, a whole cell patch-clamp recording was made with a 4- to 6-MΩ pulled glass pipette filled with an intracellular solution containing (in mM) 130 K-gluconate, 10 EGTA, 10 HEPES, 1 CaCl2, 1 MgCl2, and 2 ATP, at pH 7.2–7.3 and osmolality 290–295 mosmol/kgH2O. An initial seal of >1 GΩ and a membrane resistance of >100 mΩ were inclusion criteria for seal and cell viability. Recordings were made with an A-M Systems model 2400 amplifier and recorded with pCLAMP software (Molecular Devices, Sunnyvale, CA).
Recording protocols.
Cells were initially held in current clamp and injected with 1-s current steps sufficient to elicit action potentials (typically 0.05–0.1 nA). Their response to ST stimulation was assessed in voltage clamp at −70 mV to record evoked excitatory postsynaptic currents (EPSCs). Cells that did not display a positive action potential overshoot or a repeatable, time-locked response to ST stimulation were excluded from further study; such exclusions were very rare. In a separate group of cells, we measured the effect of DAMGO on miniature EPSCs in the presence of 1 μM tetrodotoxin (TTX), 2 mM CaCl2, 5 μM gabazine, and 5 μM strychnine. These cells were held in voltage clamp at −70 mV and were included in the study if their baseline rate of miniature EPSCs exceeded 0.5 Hz.
Drug conditions, tracers, and immunohistochemistry.
DAMGO was bath applied to each cell at 0.3 μM for 5.5 min, followed by a washout period in normal ACSF. This concentration fell in the middle of the range of concentrations reported in the literature (Herman et al. 2012; Poole et al. 2007; Rhim et al. 1993; Rhim and Miller 1994) and was ideal for our purposes because in most cases it caused considerable but incomplete suppression of the evoked response, which allowed us to measure a PPR throughout the protocol. One of three baseline drug conditions was used: normal ACSF as described above, normal ACSF containing 5 μM gabazine and 5 μM strychnine, or normal ACSF containing gabazine, strychnine, and 1 μM CTAP, a MOR antagonist (Glatzer et al. 2007; Glatzer and Smith 2005). Gabazine, strychnine, and CTAP, when used, were applied to the cell for a minimum of 15 min prior to the collection of baseline data and were maintained throughout application of DAMGO and washout.
Four groups of data were collected with 1) mouse pups in ACSF (n = 16 neurons), 2) mouse pups in GS (n = 23 neurons), 3) adult mice in GS (n = 12 neurons), and 4) adult mice in GS-CTAP (n = 5 neurons). In addition, TTX-resistant miniature EPSCs were recorded in several rNST neurons in order to provide additional information about the mechanism (pre- or postsynaptic) of DAMGO suppression.
In most instances, the location of the electrode was determined from images taken at the time of recording. In a few cases (n = 4), cells were filled with 0.5% biocytin. After recording, slices were fixed in 4% paraformaldehyde and processed with a streptavidin-conjugated Alexa Fluor to visualize the biocytin-filled neuron and processes and immunostained for the P2X2 receptor (P2X2r), a putative marker for gustatory afferents (Finger et al. 2005) in the rostral part of the nucleus. Briefly, slices were fixed for 24–48 h, followed by rinses in phosphate-buffered saline (PBS) and blocking in 5% donkey serum in PBS-0.3% Triton X-100 for 1 h, and incubated with the primary rabbit anti-P2X2 antibody (Alomone Labs, 1:10,000) at 4°C for 48 h. After PBS rinses, the tissue was incubated overnight at 4°C with the secondary antibody (fluorescent donkey anti-rabbit IgG, 1:500 dilution; Invitrogen) and then rinsed again with PBS prior to imaging.
To determine the proportion of rNST neurons that express GAD67, sections from three adult GAD67-GFP knockin mice were prepared and immunostained with the neuronal marker NeuN (Mullen et al. 1992). Mice were deeply anesthetized and perfused with saline followed by 4% paraformaldehyde, and the brain was removed and cryoprotected in 20% sucrose phosphate buffer (PB). Brain stem sections (30 μm) were cut on a freezing microtome, and those from the NST were rinsed in PBS, with nonspecific binding sites blocked in 5% donkey serum, and then incubated overnight at room temperature in a primary antiserum against NeuN (Millipore, MAB377, 1:1,000 in PB-0.2% Triton X-100), rinsed again in PBS, and then incubated in a fluorescently tagged (Alexa Fluor 546) anti-mouse secondary antibody (1:500; Invitrogen) overnight before final rinses in PBS and dilute PB. A section representing the middle of the rNST along its rostro-caudal axis was selected from each of the three cases, and photomicrographs were taken with a laser scanning confocal microscope (Olympus Fluoview 1000) with appropriate lasers and filter settings for viewing the native EGFP (argon 2/488 nm, 480–495 nm) and Alexa Fluor 546 (HeNe1, 543 nm, 550–660). EGFP and NeuN neurons were counted by viewing individual z-levels from confocal stacks taken at 2-μm intervals with a ×20 lens (NA = 0.85; 0.621 μm/pixel). Cells positive for NeuN immunofluorescence and for native EGFP were counted separately and the ratio reported. The neuron densities were calculated from the number of neurons and the area within the rNST borders.
Data collection and analysis.
ST-evoked amplitude data were collected in 2.2-min increments (40 sweeps each) during baseline (immediately preceding DAMGO application), DAMGO (beginning 3.3 min after the onset of DAMGO application), and washout (beginning 6.6 min after the return to baseline solution) conditions, and measurements from individual sweeps were averaged within these periods. Although there were typically 40 sweeps in each condition, some data were collected with multiple stimulus intensities and in those cases only 10 sweeps were available from each threshold-defined input (with the time periods remaining the same). Measurements were taken with Clampfit software (Molecular Devices) and analyzed with Systat.
The responses to ST stimulation were measured as the maximum amplitude of the evoked current relative to baseline, and the PPR was calculated as the ratio of the amplitude of the second response to the first. In cases where an accurate PPR could not be measured during DAMGO application (usually because of near-complete suppression or an increase in the failure rate), the cells were excluded from the PPR analysis. The latency was measured as the time from the peak of the stimulus artifact to the onset of the response, and jitter was calculated as the standard deviation of the latency measurements (Doyle and Andresen 2001). Data were analyzed with t-tests or ANOVA as appropriate. Significance was defined as P < 0.05. Where arithmetic means are reported, we provide the SE. The PPR analysis was done separately for cells with polysynaptic input (defined as jitter > 300 μs) because of the potential for a polysynaptic pathway to complicate the measurement of a change in the PPR.
Miniature EPSCs were identified with Mini Analysis software, and their rates and amplitudes were compared across the baseline, DAMGO, and washout conditions. These conditions were defined as 2-min segments immediately before DAMGO application (minutes 3–5), 3 min after the onset of DAMGO application (minutes 8–10), and 7 min after the beginning of washout (minutes 17–19).
RESULTS
A significant proportion of rNST neurons are GAD67+.
To estimate the proportion of rNST neurons that are GABAergic, we stained for the neuronal marker NeuN in tissue from three GAD67-EGFP knockin mice. Overall, GAD67+ neurons accounted for 35.6 ± 4.8% of rNST neurons. The average density was 2,400 ± 540 cells/mm2 for GABAergic neurons and 6,700 ± 660 cells/mm2 for NeuN-labeled neurons. There were no obvious differences in the distribution of GAD67+ neurons across rNST subdivisions (Fig. 1). We took advantage of the broad distribution of GAD67-EGFP expression to record from both GAD67+ and GAD67− neurons throughout the nucleus (Table 1).
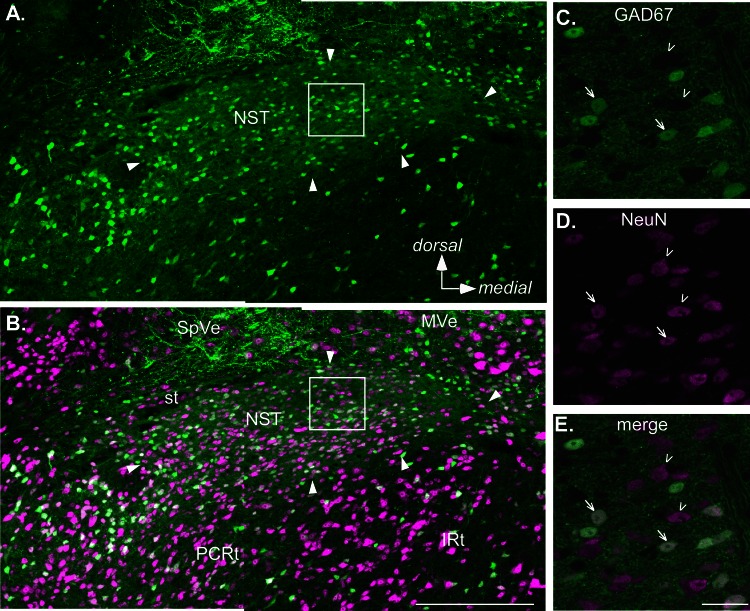
Fig. 1.
Confocal images of a section from a level of the rostral nucleus of the solitary tract (rNST) in an adult GAD67+ mouse approximately midway between the rostral pole of the nucleus and where the NST abuts the IVth ventricle. Sections were also immunostained for the neuronal marker NeuN. A and B: low-power maximum-intensity projections showing the distribution of GAD67+, enhanced green fluorescent protein (EGFP)-stained neurons and fibers (green, A) and EGFP expression merged with NeuN staining (magenta, B). Arrowheads indicate the borders of the nucleus. Note, however, that the most medial pole contains sparse somal label for either marker and corresponds with a region occupied by preganglionic parasympathetic neurons that constitute the rostral pole of the dorsal motor nucleus of the vagus. Aside from this region, GAD67+ neurons are distributed throughout the nucleus; likewise, the nucleus is characterized by profuse EGFP staining of the neuropil. C–E. higher-magnification images at a single z-plane. Arrows indicate examples of double-stained neurons; arrowheads indicate neurons stained only with NeuN. Scale bars, 250 μm (A and B), 25 μm (C–E). IRt, intermediate subdivision of the medullary reticular formation; MVe, medial vestibular nucleus; PCRt, parvocellular reticular formation; SpVe, spinal vestibular nucleus; st, solitary tract.
Table 1.
Numbers of responses from GAD67+ and GAD67− neurons in rNST
| RC | V | RC/V | RL | Unknown | Total | |
|---|---|---|---|---|---|---|
| GAD67− | 16 (14) | 4 (3) | 5 (1) | 3 (2) | 0 | 28 (22) |
| GAD67+ | 8 (7)* | 9 (6) | 7 (5) | 4 (4)† | 3 (3) | 31 (25) |
Values are numbers of responses from GAD67+ and GAD67− neurons recorded in the different rostral nucleus of the solitary tract (rNST) subdivisions: rostral central (RC), ventral (V), and rostral lateral (RL). A number of neurons were recorded in an area that could not clearly be defined as either RC or V (RC/V) or in an unknown location. In most cases, a given response represents a single neuron, but in 3 cases 2 distinct responses could be observed. Numbers in parentheses indicate the subset of neurons in a given subdivision with monosynaptic responses, as defined by jitters ≤ 300 μs. Monosynaptic responses could be recorded throughout the nucleus.
One of these neurons was between RC and RL;
one of these neurons was between RL and V.
A majority of both GAD67+ and GAD67− neurons receive monosynaptic ST input.
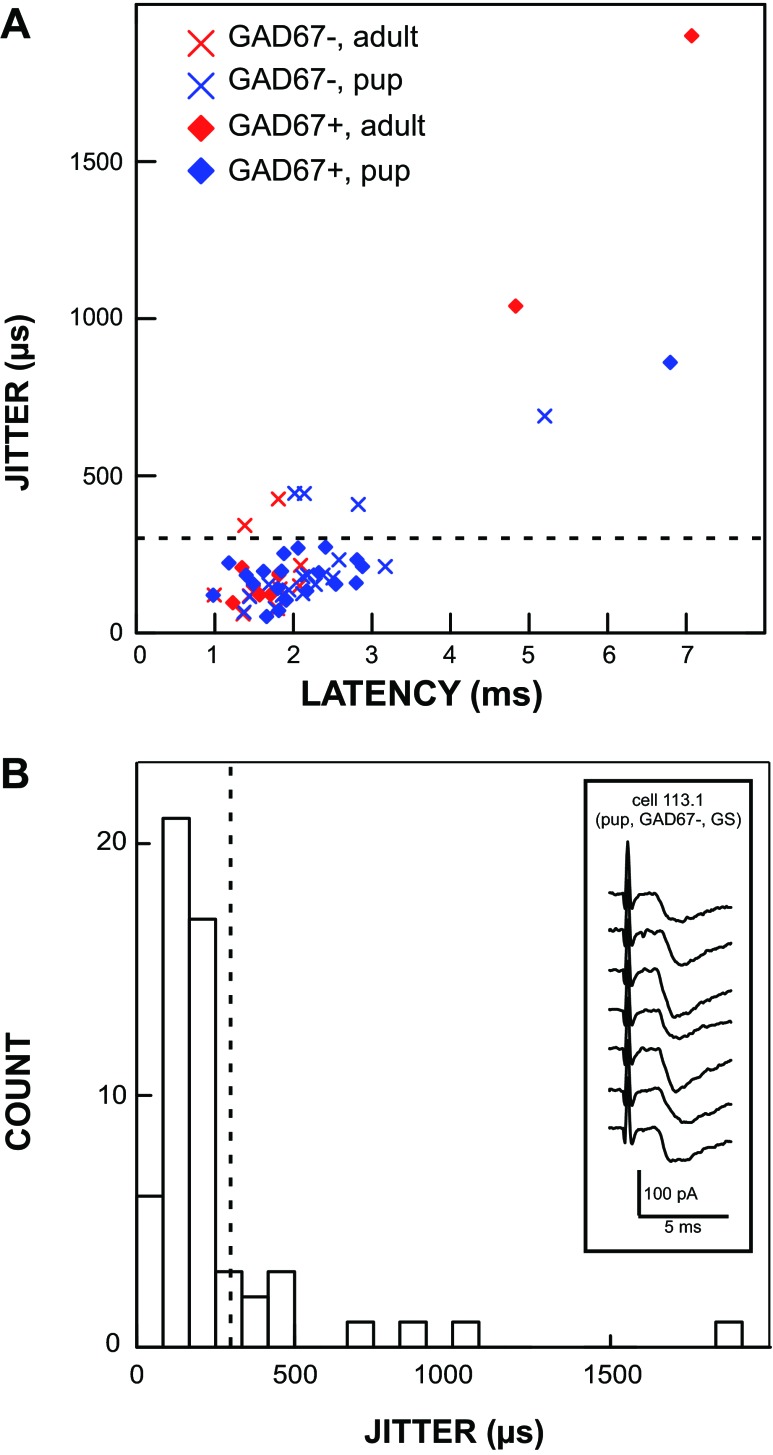
Across the entire population, responses in GAD67+ and GAD67− neurons had, respectively, average latencies of 2.41 ± 1.49 ms and 2.11 ± 0.79 ms (P = 0.34) and average jitter values of 290 ± 381 μs and 261 ± 239 μs (P = 0.74). Although a jitter of <200 μs has often been used as a criterion for monosynaptic input to cNST (Doyle and Andresen 2001), in the present study we used a value of 300 μs (Fig. 2) because a recent study demonstrated that rNST neurons with anatomically defined direct ST inputs can have jitters that reach this value (Wang and Bradley 2010b). With a jitter criterion of <300 μs, most ST-evoked responses in rNST neurons were monosynaptic in both adult (78%) and pup (80%) populations. Likewise, most ST-evoked responses were monosynaptic in both GAD67+ (81%) and GAD67− (79%) neurons. It is important to note that since we made an effort to isolate the lowest threshold response from each cell, our recordings were likely biased toward monosynaptic inputs because polysynaptic inputs would have to overcome the threshold of an interneuron to be detectable.
Fig. 2.
Many cells, including GAD67+ neurons, received monosynaptic afferent input from the solitary tract (ST), and this innervation persisted into adulthood. A plot of latency vs. jitter (A) shows that most responses elicited by ST stimulation had a jitter value < 300 μs. A histogram of jitter values (B) shows the cutoff (dashed line) that we used to define mono- vs. polysynaptic innervation at 300 μs. Inset in B shows an example of raw data from a single neuron with an average latency of 1.69 ms and a jitter of 150 μs. GS, gabazine-strychnine.
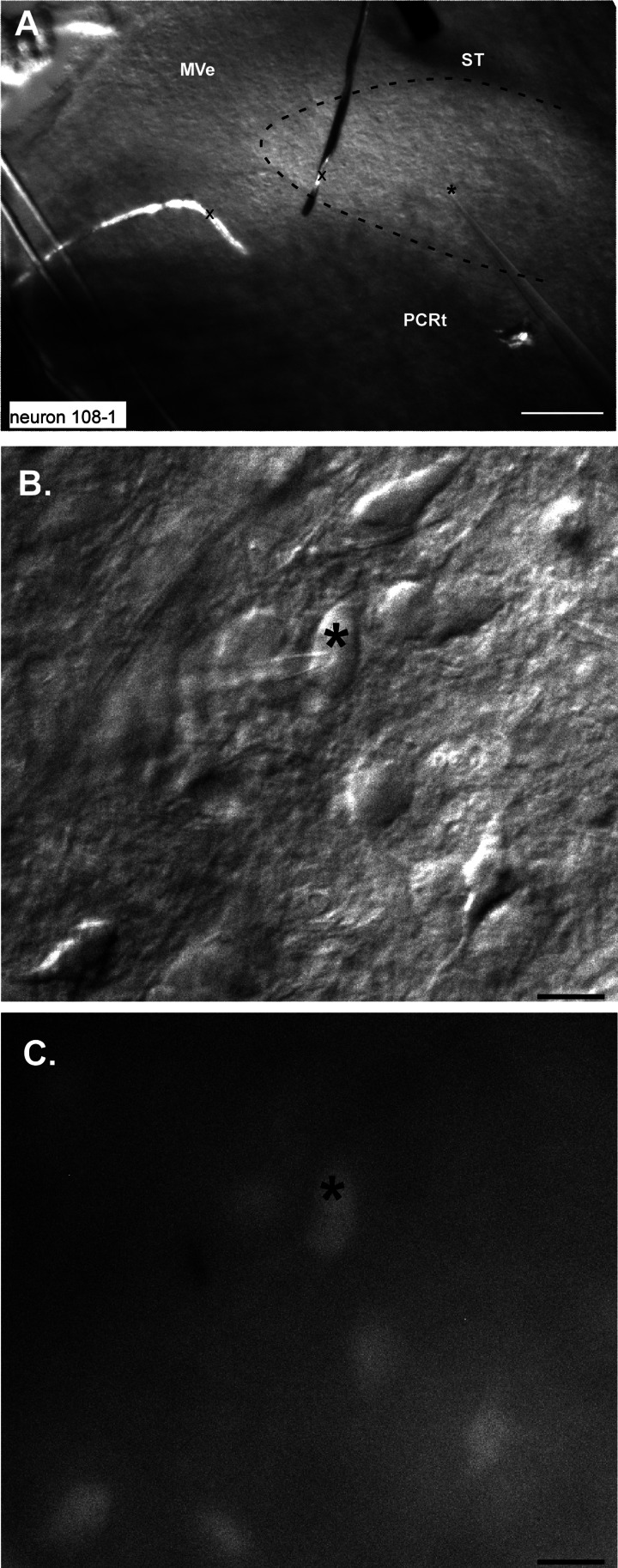
A small subset of biocytin-filled neurons (n = 4) were recovered and the sections immunostained for P2X2r. All of these neurons received monosynaptic inputs (defined as jitter < 300 μs), and three of four were located in the P2X2r terminal field, presumably corresponding to the central terminations of gustatory afferents (Finger et al. 2005) and to the rostral central (Whitehead 1988) subnucleus, whereas the remaining neuron was ventral to the P2X2r staining. In fact, we recorded a number of low-jitter, presumably monosynaptic responses in a similar ventral location, corresponding to the position of the ventral subdivision (Table 1; Fig. 3).
Fig. 3.
Photomicrographs of a ventrally located site where a GAD67+ neuron was recorded. A: low-magnification DIC image showing the stimulating electrode on the solitary tract (ST) and the recording electrode in the ventral 1/3 of the nucleus; the position of the tip is indicated by *. The approximate outlines of the rNST are indicated by the dashed line. Pieces of debris (x) cut across the medial part of rNST and medial vestibular nucleus (MeV). PCRt, parvocellular reticular formation. B: high-magnification DIC image showing the pipette on the recorded neuron. C: high-magnification fluorescent image showing that the cell is GAD67+. Scale bars, 250 μm (A), 25 μm (B and C).
DAMGO suppresses ST-evoked input to rNST neurons.
Across the population (n = 54), DAMGO had a generally suppressive effect on ST-evoked responses save for one response that increased by 53% over baseline. The suppression was reversible in many individual cells and across the population (Fig. 4, A and B). For the remaining 53 responses the mean suppression was 46 ± 3.6%, and this effect was nearly identical in pups and adults (45% and 48%, respectively). Nevertheless, there was considerable heterogeneity in effect size, ranging from nearly total suppression to virtually none (Fig. 4C).
Fig. 4.
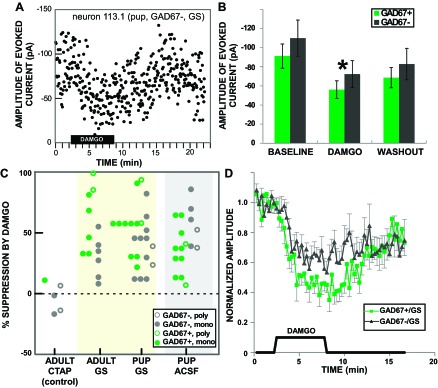
DAMGO suppressed the response of rNST neurons to ST-evoked input. A: effect of DAMGO on the amplitude of a ST-evoked current in a single GAD67− neuron under gabazine-strychnine (GS) blockade in a pup. ST-evoked responses were measured at 3.33-s intervals. B: mean ± SE amplitudes of rNST responses in GAD67+ and GAD67− neurons across the population before (baseline), during (DAMGO), and after (washout) DAMGO application (n = 53; the 1 response facilitated by DAMGO was omitted). ANOVA indicated an overall effect of DAMGO (*P < 0.0005) but no effect of GAD67-EGFP status or DAMGO × GAD67-EGFP interaction; Bonferroni-adjusted post hoc comparisons revealed significant differences between all 3 conditions: baseline, DAMGO, and washout (all P < 0.005). C: dot density plot showing the degree to which individual responses were suppressed by DAMGO (response increment omitted). Responses are grouped by animal age and bath solution: artificial cerebral spinal fluid (ACSF), gabazine and strychnine (GS), and gabazine and strychnine with the μ-opioid receptor (MOR) antagonist CTAP (CTAP). Symbols indicate monosynaptic vs. polysynaptic responses and GAD67-EGFP status. D: effect of DAMGO shown for normalized responses over time for GAD67+ (n = 15) and GAD67− (n = 15) neurons recorded when gabazine and strychnine were included in the bath. Individual measurements are for the moving average of 5 adjacent time points. When inhibitory amino acid receptors were blocked, DAMGO had a significantly larger effect on ST-evoked responses in GAD67+ than in GAD67− neurons, although responses in both cell types were affected.
In the pooled data (Fig. 4B), suppression by DAMGO did not vary between GAD67+ and GAD67− neurons (P = 0.188). Closer scrutiny, however, suggested that GAD67+ and GAD67− neurons were differentially sensitive to DAMGO depending on whether they were under inhibitory blockade. It was possible to make this comparison directly with the data collected in pups, since DAMGO was applied in both ACSF and GS baseline conditions. When the slice was bathed in ACSF GAD67− neurons were significantly more suppressed (57.3%) compared with GAD67+ neurons (34.6%), but when the slice was bathed in GS GAD67+ neurons showed more suppression (55.8%) compared with GAD67− neurons (38.5%) (ANOVA, DAMGO × inhibitory blockade, P = 0.006). The greater suppression in GAD67+ neurons compared with GAD67− neurons was consistent in the adult experiments, which were all performed in the presence of GS [suppression in GAD67+ 63.5% vs. GAD67− 29.6% (P = 0.003)]. Figure 4D shows the difference between DAMGO's effects on GAD67+ and GAD67− neurons as a function of time for the GS group (pups + adults).
DAMGO has a presynaptic site of action.
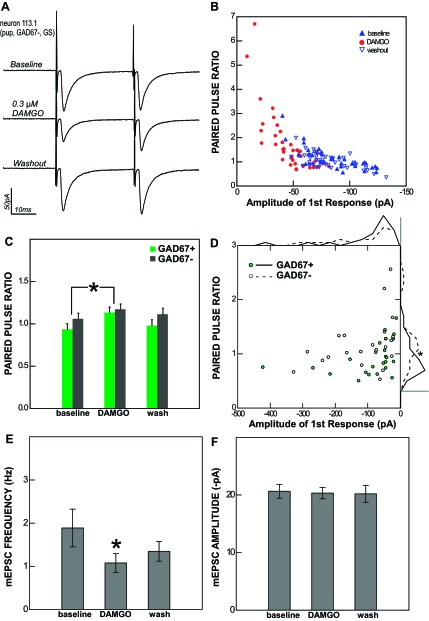
The PPR is calculated as the second response to stimulation divided by the first and reflects the use- and time-dependent short-term plasticity of transmitter release from the presynaptic terminal (Zucker and Regehr 2002). Many rNST neurons displayed significant trial-to-trial variability in their response to ST stimulation, and this variability allowed us to visualize the relationship between the amplitude of the first response and the PPR within single cells, as shown in the example in Fig. 5B. When the first response was smaller, the PPR tended to be larger, possibly because of the effects of residual calcium in the terminal coupled with a smaller degree of vesicle depletion (Debanne et al. 1996). Conversely, when the first response was larger, the PPR tended to be smaller. This relationship yielded a characteristic amplitude vs. PPR plot, and in the presence of DAMGO the points shifted so that as the amplitude of the first pulse decreased the PPR increased.
Fig. 5.
The effect of DAMGO on rNST neurons is presynaptic. A: the response of an rNST neuron to ST stimulation (averages of 40 sweeps in voltage clamp) before, during, and after the application of 0.3 μM DAMGO. B: single data points showing the relationship between the amplitude of the 1st response to ST stimulation and the paired-pulse ratio (PPR) for the same cell in each condition (baseline, DAMGO, and washout). C: across the population, the average PPR increases during DAMGO application for both GAD67+ and GAD67− neurons. *P < 0.001. D: scatterplot of average baseline amplitude and PPR values for individual monosynaptic ST-evoked responses (n = 44) showing an inverse relationship between the 1st response amplitude and PPR for both GAD67+ and GAD67− neurons. The PPRs of monosynaptic responses to ST stimulation were significantly smaller in GAD67+ neurons than in GAD67− neurons. This effect may be seen most clearly in the frequency polygons (binned in 15 equal segments) displayed outside the graph area; scale bars correspond to a frequency of 10 neurons. E: the frequency of miniature excitatory postsynaptic current (mEPSCs; tested in 7 neurons, 2 GAD67+ and 5 GAD67−) was significantly reduced by DAMGO. *P < 0.014. F: the average amplitude of mEPSCs was unaffected by DAMGO. Taken together, these results indicate a presynaptic site of action.
This kind of change in the PPR accompanying a change in the response amplitude typically indicates a presynaptic site of action, while an unchanged PPR is indicative of a postsynaptic effect (Zucker and Regehr 2002). For monosynaptic responses (n = 44) we observed a significant increase in the PPR in conjunction with DAMGO suppression (mean increase = 0.166, P < 0.001). This was true for both GAD67+ (mean increase = 0.195, P = 0.004) and GAD67− (mean increase = 0.127, P = 0.035) neurons (Fig. 5C). There were no differences between adults and pups or between ACSF and GS bathing solutions in the increase of the PPR under DAMGO.
For the population of monosynaptic responses, the magnitude of the PPR change was positively correlated with the magnitude of DAMGO suppression (Pearson's r = 0.396, P = 0.005). In other words, the more the first response was suppressed by DAMGO, the greater the relative increase in the second response. Polysynaptic responses (n = 10) were analyzed separately because of their potential to complicate the measurement of PPR changes. Although polysynaptic responses did show an increase in the PPR accompanying suppression by DAMGO, the change was not statistically significant (P = 0.096).
Monosynaptic ST-evoked responses in GAD67+ neurons had a mean PPR (0.92) that was significantly less than that of GAD67− neurons (1.19) (P < 0.03). That is, with a 33-ms ISI, ST input to GAD67+ neurons displayed short-term depression compared with short-term facilitation for GAD67− neurons. This was the case despite a tendency for GAD67− neurons to have nominally larger ST-evoked responses (P = 0.36), which would if anything be likely to give rise to a smaller PPR (Fig. 5D).
To confirm the presynaptic site of action, we recorded miniature EPSCs in seven rNST neurons from adult animals (2 GAD67+ and 5 GAD67−) during the application of DAMGO. The average baseline frequency was 1.89 ± 1.06 Hz, and the average amplitude was 20.6 ± 2.9 pA. DAMGO decreased the average frequency to 1.08 ± 0.53 Hz (Fig. 5E; P = 0.014) but did not significantly alter the average amplitude (20.3 ± 2.4 pA; Fig. 5F; P = 0.54). This is consistent with a presynaptic mechanism of action, since the frequency of release was altered while the postsynaptic response to a quantum of neurotransmitter remained unchanged.
DAMGO directly hyperpolarizes some rNST neurons.
We observed infrequent (n = 5) postsynaptic effects of DAMGO in rNST. In all five cases, the holding current (at Vhold = −70 mV) became more positive (−22.6 ± 7.1 vs. −8.5 ± 18.5 pA) and the membrane resistance was reduced (994 ± 509 vs. 612 ± 343 MΩ) with the application of DAMGO. These effects were reversible with washout and are consistent with the direct activation of an inhibitory conductance. Aside from these five cells, membrane resistance in all cells remained on average within 22% of baseline levels throughout DAMGO application.
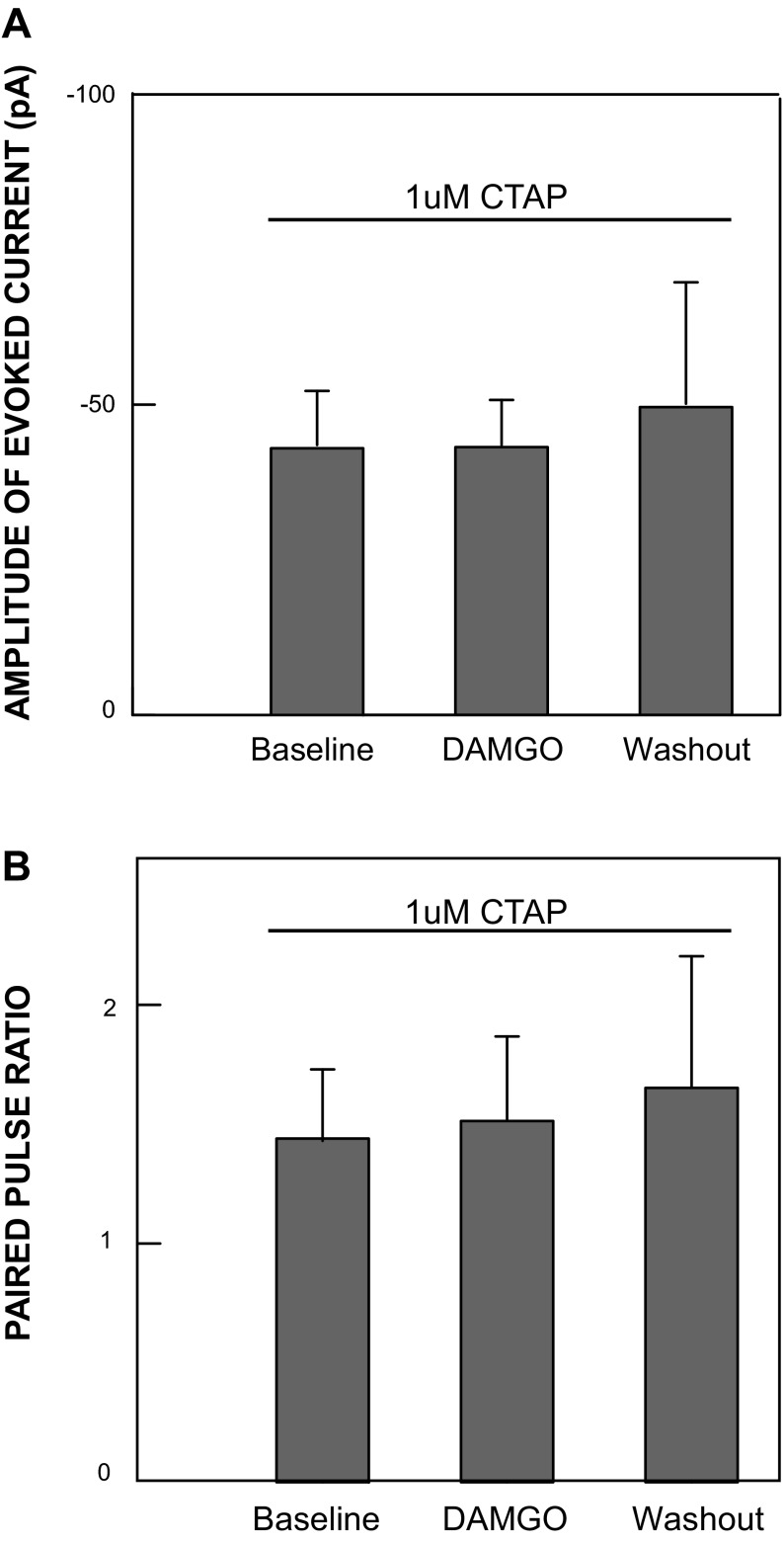
All DAMGO responses were abolished by the μ-opioid antagonist CTAP.
We tested the activity of DAMGO in the presence of the MOR antagonist CTAP in neurons from adult animals (4 GAD67− and 1 GAD67+). No DAMGO effects on amplitude (P = 0.95) or PPR (P = 0.4) were observed in any of the CTAP control cells (Fig. 4C; Fig. 6), indicating that the responses to DAMGO were due to specific activation of MORs.
Fig. 6.
All DAMGO effects are abolished in the presence of the μ-opioid antagonist CTAP: mean amplitudes of evoked responses (A) and PPRs (B) from 5 neurons recorded under MOR receptor blockade accomplished by adding 1 μM CTAP to a bathing solution containing normal ACSF with gabazine (5 μM) and strychnine (5 μM). Under these conditions, no significant change in the amplitude of the evoked response or PPR was observed when 0.3 μM DAMGO was added, indicating that DAMGO effects in the absence of CTAP blockade were specifically mediated by MORs.
DISCUSSION
The results of the present study demonstrate that the MOR agonist DAMGO has a suppressive effect on ST-evoked rNST responses that acts primarily through a presynaptic mechanism on both GAD67+ and GAD67− neurons. Although both types of neurons received substantial monosynaptic input, we observed that they differed in their response to a paired-pulse stimulation protocol. At the stimulus frequency tested, ST input to GAD67+ neurons had a smaller PPR than input to GAD67− neurons, reflecting a tendency for inputs to GAD67+ neurons to exhibit more short-term depression while inputs to GAD67− neurons exhibited more short-term facilitation.
The GAD67-EGFP knockin mouse.
The original report of the knockin mouse model used in this study compared immunohistochemistry for GAD67 and GABA to EGFP expression in neocortical neurons and reported nearly complete overlap of EGFP with both GABA itself and the synthetic enzyme (Tamamaki et al. 2003). Thus it would appear that this mouse is a more effective model for comparing properties of GABAergic with non-GABAergic neurons than some other commercially available strains. For example, the “GIN” mouse expresses EGFP mainly in somatostatin GABAergic neurons in the neocortex (Oliva et al. 2000), and in the rNST EGFP expression is confined to just half of the GABAergic cells (Wang and Bradley 2010a) and is particularly abundant in the ventral subnucleus (Travers et al. 2007; Wang and Bradley 2010a). This preferential distribution contrasts sharply with the homogeneous pattern observed in the GAD67-EGFP knockin mouse in the present investigation (Fig. 1). Nevertheless, coexpression of GABA and EGFP has not yet been evaluated in NST, and it remains possible that there is a subpopulation of GABAergic neurons in the GAD67-GFP knockin that are not evident. For example, in the olfactory bulb, although some GABAergic neurons express both GAD67 and GAD65, some only express one of the two isoforms (Parrish-Aungst et al. 2007).
The proportion of rNST neurons expressing GAD67.
Previous estimates of the proportion of rNST neurons that are GABAergic have been based on GABA immunoreactivity in the chorda tympani (CT) terminal field of rats (18%; Lasiter and Kachele 1988) or on counts of GFP-expressing neurons in the ventral subdivision in the “GIN” mouse (24%; Wang and Bradley 2010a). We report a proportion of ∼36% throughout the nucleus in the GAD67-EGFP knockin mouse, which is notably higher than previous studies. This perhaps reflects the strength of using a genetic model to label the GABAergic neurons but could also reflect differences between rats and mice or between the subdivisions of the rNST.
ST stimulation evokes monosynaptic responses in GABAergic rNST neurons.
We recorded from an approximately equal number of GAD67+ and GAD67− neurons in rNST and observed no differences between the latency and jitter of these two groups. This was true in the mouse pups both in normal extracellular ACSF and when we added gabazine and strychnine to remove any confounding effect of evoked inhibition. The same experiment in adults verified that the lack of a difference in latency and jitter was not dependent on development since the latency and jitter measurements of all four populations (GAD67+/− in adult/pup) overlapped extensively (Fig. 2). These results are similar to those from the cNST, where 50–70% of GABAergic neurons have been shown to be monosynaptically innervated by the ST (Bailey et al. 2008; Glatzer et al. 2007). However, our report of a high proportion of rNST GAD67+ neurons with monosynaptic input appears at odds with a recent anatomical report (Wang et al. 2012) that showed that only a small fraction of identified CT afferent fibers make synaptic contact with GABAergic neurons in the adult rat. It is important to note that we report the percentage of GABAergic neurons receiving monosynaptic ST input, while Wang et al. report the percentage of CT terminals that synapse onto GABAergic neurons. These are not necessarily mutually exclusive observations; it is possible, for example, that non-GABAergic neurons receive much denser innervation from the ST, which would reconcile the observations. In addition, there are no available anatomical data on the relationship between NST GABAergic cells and glossopharyngeal afferents, another population of fibers we undoubtedly stimulated. Moreover, the difference between the studies could reflect a species difference in innervation pattern.
Monosynaptic input to inhibitory rNST neurons implies a mechanism of feedforward or lateral inhibition in the nucleus at the level of the primary afferent synapse. Although the function of this inhibition is unknown, results from in vivo recording studies suggest that GABAergic inhibition in the rNST may sharpen chemosensitive tuning profiles (Smith and Li 1998). One can imagine multiple configurations of afferent inputs and first-order neurons that could underlie such a function, but more information about the distribution and convergence patterns of individual afferents will be required in order to define a more precise role for these monosynaptic inputs to inhibitory rNST neurons in taste processing.
Short-term synaptic plasticity.
The PPRs that we report at an ISI of 33 ms are considerably higher than those that have been previously reported in the rNST, even at similar ISIs (Wang and Bradley 2010b; Zhu et al. 2009). Since the PPR can be significantly affected by experimental conditions that are typically not held constant between studies, most notably stimulus frequency (Zucker and Regehr 2002) and temperature (Klyachko and Stevens 2006), it is difficult to make a direct comparison between different studies. Nevertheless, we frequently observed facilitatory responses, which have not been reported in the rNST in vitro but are consistent with an in vivo report showing that a subpopulation of rNST neurons responds with significant facilitation to paired-pulse stimulation of the glossopharyngeal nerve (Hallock and Di Lorenzo 2006). Under the experimental conditions of the present study (33-ms ISI at 36°C), GAD67− neurons received inputs characterized by larger (facilitatory) PPRs compared with inputs received by GAD67+ (GABAergic) neurons.
That these GAD67−, perhaps glutamatergic, neurons have a “facilitatory bias” could imply a more linear transformation of the sensory input compared with the depressive response in GAD67+ neurons (MacLeod 2011). Future work characterizing the frequency dependence of the PPR in identified neurons as well as their projection status might shed light on the function of this difference in short-term synaptic plasticity.
DAMGO (presynaptically) suppresses ST-evoked responses in the rNST.
In light of the observations that DAMGO reduced the PPR of evoked responses and the frequency, but not the amplitude, of miniature EPSCs it seems likely that DAMGO acts mainly presynaptically to suppress primary afferent input by reducing release probability. MORs have not been demonstrated specifically on identified gustatory afferents, but there is nevertheless strong support for an anatomical basis for this effect. MORs exist in the petrosal as well as the nodose and jugular ganglia (Ding et al. 1998), although their expression has not been studied in the geniculate. In addition, several published reports show photomicrographs of MOR staining in incoming ST afferents at the level of the rNST (Li et al. 2003; Monteillet-Agius et al. 1998; Moriwaki et al. 1996), and electron microscopic studies report the receptor on axon terminals in the rostral part of the nucleus (Huang et al. 2000).
In the present study, presynaptic inhibition mediated by MORs was observed in young and adult animals and in both GAD67+ and GAD67− neurons. Presynaptic inhibition mediated by MORs occurs for other select types of primary afferents: nociceptive Aδ and C fiber projections to the spinal cord (see, e.g., Heinke et al. 2011) as well as vagal visceral inputs to diverse neuron types in the cNST including GABAergic (Glatzer et al. 2007; Glatzer and Smith 2005), A2 catecholaminergic (Cui et al. 2012), and proopiomelanocortin (POMC) (Appleyard et al. 2005) neurons. MOR-mediated presynaptic inhibition also occurs for intrinsic inhibitory and excitatory synaptic transmission in the cNST (Glatzer and Smith 2005).
Although presynaptic inhibition was evident for both GAD67+ and GAD67− cells, the magnitude of suppression was a function of both GAD67-EGFP status and the presence of inhibitory blockade. Presynaptic inhibition was stronger for GAD67− neurons in ACSF but stronger for GAD67+ neurons when GABAA and glycine receptors were blocked. These results imply an interaction between the effects of μ-opioid and inhibitory amino acid receptors in rNST. Similar interactions are common in other parts of the CNS, such as the rostral ventromedial medulla (Pedersen et al. 2011) and ventral tegmental area (Johnson and North 1992). The fact that inhibitory blockade preferentially increases the impact of DAMGO on GAD67+ neurons hints that inhibition in the rNST may affect GABAergic and non-GABAergic neurons differently, and that differences could be systematically related to DAMGO sensitivity. Additional evidence, including recordings from the same cells before and after inhibitory amino acid receptor blockade, would be useful in verifying this hypothesis.
DAMGO also appears to directly inhibit a small proportion of rNST neurons by increasing a hyperpolarizing conductance, consistent with reports of postsynaptic effects of MOR activation in cNST (Poole et al. 2007; Rhim et al. 1993). We saw this effect infrequently, however, and when we did it was typically small. It is conceivable that calcium chelation by EGTA in our recording pipettes could have prevented or reduced any calcium-dependent postsynaptic effects of DAMGO, but this seems unlikely in light of the postsynaptic DAMGO effects seen by other groups using the same concentration of intracellular EGTA (Poole et al. 2007; Rhim et al. 1993). It is also possible that if we took steps to maximize the effect of a small inhibitory conductance change we would observe an enhanced postsynaptic response more frequently. At any rate, MOR-mediated inhibition in the rNST appears similar to that observed in the cNST, where activation of MORs presynaptically suppresses afferent input to many second-order neurons (Appleyard et al. 2005; Cui et al. 2012; Glatzer et al. 2007; Poole et al. 2007) but also has a postsynaptic inhibitory influence on a more restricted population (Poole et al. 2007).
Functional significance of MOR modulation in rNST.
Opioids modulate a wide range of visceral and autonomic processes in the brain stem. In the rNST, varicose fibers containing endogenous ligands for MORs are plentiful, including the enkephalins, which activate δ-opioid receptors as well as MORs (Raynor et al. 1994), and the endomorphins, which are specific MOR agonists (Zadina et al. 1997). The source of these fibers is not certain, but likely origins include the cNST and hypothalamus, regions compatible with a role for MOR modulation related to visceral state. Enkephalinergic neurons are distributed in a wide variety of CNS regions (see, e.g., Fallon and Leslie 1986), and such cells often project locally (Fields 2004; Llewellyn-Smith et al. 2005; Poulin et al. 2006; Schneider and Walker 2007). Moreover, enkephalinergic neurons are found in the caudal as well as the rostral NST (Davis and Kream 1993; Fallon and Leslie 1986; Murakami et al. 1987; Stornetta et al. 2001), but endomorphinergic soma are located only in the cNST at the level of area postrema and the hypothalamus near and in the dorsomedial and arcuate nuclei (Martin-Schild et al. 1999; Pierce and Wessendorf 2000).
Notably, activation of MORs in several forebrain regions such as the nucleus accumbens strongly increases food intake, particularly of palatable substances (see, e.g., Pecina and Berridge 2000). In the cNST, MOR activation suppresses ST input to A2 (Cui et al. 2012) and POMC (Appleyard et al. 2005) neurons, effects also compatible with an orexigenic influence since these neuronal populations have been implicated in satiety (Rinaman 2011). Although early reports suggested that rNST DAMGO infusions also increased food intake (Kotz et al. 1997), these studies used rather large infusions and the feeding effect was delayed, suggesting that the orexigenic effect may have arisen from ligand spread that suppressed satiety signals in cNST (Kinzeler and Travers 2011). Indeed, although smaller DAMGO infusions in rNST profoundly impacted the sensory-motor response to intraoral infusion of taste stimuli, they did not prolong the response to sucrose, which would have been consistent with a MOR-induced increase in feeding. Rather, effects were generally suppressive in that both the rate of intraoral ingestive and rejection responses and the bout duration of ororhythmic responses to QHCl and water were reduced. The reduction of ST-evoked responses observed in the present study, particularly in GAD67− neurons, forms a likely basis for producing shorter bout durations since bout duration is a positive function of stimulus concentration (Davis 1973; Grill and Norgren 1978) and consequently the magnitude of the evoked rNST response (see, e.g., Ganchrow and Erickson 1970). Indeed, in vivo infusions of met-enkephalin into the rNST suppress taste-evoked activity in rNST neurons (Li et al. 2003), although this inhibition probably arises from a combination of postsynaptic inhibition by δ-opioid receptors (Zhu et al. 2009) as well as the MOR-mediated inhibition observed in the present study.
Paradoxically, in the behavioral studies DAMGO infusions also changed a neutral licking response to water to an oral rejection “gape” response typically associated with infusions of bitter stimuli. This effect of DAMGO may reflect a disinhibitory mechanism. We have previously demonstrated that infusions of the GABAA antagonist bicuculline into the reticular formation and overlying rNST produced large-amplitude gapes in response to taste stimuli that normally elicited small-amplitude mouth openings; i.e., gapes are produced by suppressing GABAergic activity (disinhibition) (Chen and Travers 2003). A disinhibitory interaction between MOR and GABAergic neurons in cNST has also been postulated to account for increased excitability in vago-vagal reflexes (Herman et al. 2009). Thus we propose that MOR suppression of ST-evoked responses in GABAergic rNST neurons could, in principle, mediate the large-amplitude gape responses to water stimulation observed after DAMGO infusions into the rNST (Kinzeler and Travers 2011) by a similar mechanism of MOR-mediated disinhibition.
Overall, activation of MORs in the rNST has behavioral effects that could arise from both inhibitory and disinhibitory mechanisms, consistent with our findings that both GAD67+ and GAD67− neurons receive DAMGO-sensitive monosynaptic input from the ST. The fact that MORs modulate incoming orosensory signals to GABAergic as well as to putative excitatory rNST neurons underscores the complexity of processing in this first-order gustatory relay.
GRANTS
This work was supported by National Institutes of Health grants DC-00416, DC-00417, and DE 014320 and Grants-in-Aid for Scientific Research 22300105 and 23115503 (Y. Yanagawa) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.J.B., S.P.T., and J.B.T. conception and design of research; A.J.B. performed experiments; A.J.B., S.P.T., and J.B.T. analyzed data; A.J.B., S.P.T., and J.B.T. interpreted results of experiments; A.J.B., S.P.T., and J.B.T. prepared figures; A.J.B., S.P.T., and J.B.T. drafted manuscript; A.J.B., Y.Y., S.P.T., and J.B.T. edited and revised manuscript; A.J.B., Y.Y., S.P.T., and J.B.T. approved final version of manuscript.
REFERENCES
- Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci 25: 3578–3585, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS). J Neurophysiol 99: 1712–1722, 2008 [DOI] [PubMed] [Google Scholar]
- Bradley BE. The Role of the Nucleus of the Solitary Tract in Gustatory Processing. Boca Raton, FL: CRC, 2007 [PubMed] [Google Scholar]
- Brown RE, McKenna JT, Winston S, Basheer R, Yanagawa Y, Thakkar MM, McCarley RW. Characterization of GABAergic neurons in rapid-eye-movement sleep controlling regions of the brainstem reticular formation in GAD67-green fluorescent protein knock-in mice. Eur J Neurosci 27: 352–363, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Travers JB. Inactivation of amino acid receptors in medullary reticular formation modulates and suppresses ingestion and rejection responses in the awake rat. Am J Physiol Regul Integr Comp Physiol 285: R68–R83, 2003 [DOI] [PubMed] [Google Scholar]
- Cui RJ, Roberts BL, Zhao H, Andresen MC, Appleyard SM. Opioids inhibit visceral afferent activation of catecholamine neurons in the solitary tract nucleus. Neuroscience 222: 181–190, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ, Kream RM. Distribution of tachykinin- and opioid-expressing neurons in the hamster solitary nucleus: an immuno- and in situ hybridization histochemical study. Brain Res 616: 6–16, 1993 [DOI] [PubMed] [Google Scholar]
- Davis BJ, Smith DV. Substance P modulates taste responses in the nucleus of the solitary tract of the hamster. Neuroreport 8: 1723–1727, 1997 [DOI] [PubMed] [Google Scholar]
- Davis JD. The effectiveness of some sugars in stimulating licking behavior in the rat. Physiol Behav 11: 39–45, 1973 [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol 491: 163–176, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Li JL, Lu BZ, Wang D, Zhang ML, Li JS. Co-localization of mu-opioid receptor-like immunoreactivity with substance P-LI, calcitonin gene-related peptide-LI and nitric oxide synthase-LI in vagal and glossopharyngeal afferent neurons of the rat. Brain Res 792: 149–153, 1998 [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J Neurophysiol 85: 2213–2223, 2001 [DOI] [PubMed] [Google Scholar]
- Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol 249: 293–336, 1986 [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci 5: 565–575, 2004 [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310: 1495–1499, 2005 [DOI] [PubMed] [Google Scholar]
- Ganchrow JR, Erickson RP. Neural correlates of gustatory intensity and quality. J Neurophysiol 33: 768–783, 1970 [DOI] [PubMed] [Google Scholar]
- Glatzer NR, Derbenev AV, Banfield BW, Smith BN. Endomorphin-1 modulates intrinsic inhibition in the dorsal vagal complex. J Neurophysiol 98: 1591–1599, 2007 [DOI] [PubMed] [Google Scholar]
- Glatzer NR, Smith BN. Modulation of synaptic transmission in the rat nucleus of the solitary tract by endomorphin-1. J Neurophysiol 93: 2530–2540, 2005 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143: 263–279, 1978 [DOI] [PubMed] [Google Scholar]
- Hallock RM, Di Lorenzo PM. Effects of electrical stimulation of the glossopharyngeal nerve on cells in the nucleus of the solitary tract of the rat. Brain Res 1113: 163–173, 2006 [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg 94: 313–318, 2002 [DOI] [PubMed] [Google Scholar]
- Heinke B, Gingl E, Sandkuhler J. Multiple targets of mu-opioid receptor-mediated presynaptic inhibition at primary afferent Adelta- and C-fibers. J Neurosci 31: 1313–1322, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Alayan A, Sahibzada N, Bayer B, Verbalis J, Dretchen KL, Gillis RA. Mu-opioid receptor stimulation in the medial subnucleus of the tractus solitarius inhibits gastric tone and motility by reducing local GABA activity. Am J Physiol Gastrointest Liver Physiol 299: G494–G506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Cruz MT, Sahibzada N, Verbalis J, Gillis RA. GABA signaling in the nucleus tractus solitarius sets the level of activity in dorsal motor nucleus of the vagus cholinergic neurons in the vagovagal circuit. Am J Physiol Gastrointest Liver Physiol 296: G101–G111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Gillis RA, Vicini S, Dretchen KL, Sahibzada N. Tonic GABAA receptor conductance in medial subnucleus of the tractus solitarius neurons is inhibited by activation of mu-opioid receptors. J Neurophysiol 107: 1022–1031, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wang H, Pickel VM. Rostrocaudal variation in targeting of N-methyl-d-aspartate and mu-opioid receptors in the rat medial nucleus of the solitary tract. J Comp Neurol 421: 400–411, 2000 [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12: 483–488, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Tamamaki N, Owada H, Kakizaki T, Kume N, Totsuka M, Yamamoto T, Yawo H, Yagi T, Obata K, Yanagawa Y. Noradrenergic excitation of a subpopulation of GABAergic cells in the basolateral amygdala via both activation of nonselective cationic conductance and suppression of resting K+ conductance: a study using glutamate decarboxylase 67-green fluorescent protein knock-in mice. Neuroscience 157: 781–797, 2008 [DOI] [PubMed] [Google Scholar]
- King MS, Wang L, Bradley RM. Substance P excites neurons in the gustatory zone of the rat nucleus tractus solitarius. Brain Res 619: 120–130, 1993 [DOI] [PubMed] [Google Scholar]
- Kinzeler NR, Travers SP. Mu-opioid modulation in the rostral solitary nucleus and reticular formation alters taste reactivity: evidence for a suppressive effect on consummatory behavior. Am J Physiol Regul Integr Comp Physiol 301: R690–R700, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyachko VA, Stevens CF. Temperature-dependent shift of balance among the components of short-term plasticity in hippocampal synapses. J Neurosci 26: 6945–6957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz CM, Billington CJ, Levine AS. Opioids in the nucleus of the solitary tract are involved in feeding in the rat. Am J Physiol Regul Integr Comp Physiol 272: R1028–R1032, 1997 [DOI] [PubMed] [Google Scholar]
- Lasiter PS, Kachele DL. Organization of GABA and GABA-transaminase containing neurons in the gustatory zone of the nucleus of the solitary tract. Brain Res Bull 21: 623–636, 1988 [DOI] [PubMed] [Google Scholar]
- Li CS, Davis BJ, Smith DV. Opioid modulation of taste responses in the nucleus of the solitary tract. Brain Res 965: 21–34, 2003 [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Dicarlo SE, Collins HL, Keast JR. Enkephalin-immunoreactive interneurons extensively innervate sympathetic preganglionic neurons regulating the pelvic viscera. J Comp Neurol 488: 278–289, 2005 [DOI] [PubMed] [Google Scholar]
- Lundy RF, Norgren R. Gustatory System. San Diego, CA: Elsevier Academic, 2004 [Google Scholar]
- MacLeod KM. Short-term synaptic plasticity and intensity coding. Hear Res 279: 13–21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol 350: 412–438, 1994 [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol 405: 450–471, 1999 [PubMed] [Google Scholar]
- Monteillet-Agius G, Fein J, Anton B, Evans CJ. ORL-1 and mu opioid receptor antisera label different fibers in areas involved in pain processing. J Comp Neurol 399: 373–383, 1998 [DOI] [PubMed] [Google Scholar]
- Moriwaki A, Wang JB, Svingos A, van Bockstaele E, Cheng P, Pickel V, Uhl GR. Mu opiate receptor immunoreactivity in rat central nervous system. Neurochem Res 21: 1315–1331, 1996 [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development 116: 201–211, 1992 [DOI] [PubMed] [Google Scholar]
- Murakami S, Okamura H, Yanaihara C, Yanaihara N, Ibata Y. Immunocytochemical distribution of met-enkephalin-Arg6-Gly7-Leu8 in the rat lower brainstem. J Comp Neurol 261: 193–208, 1987 [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci 20: 3354–3368, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Yanagawa Y, Koyano K. GABAergic neurons in inferior colliculus of the GAD67-GFP knock-in mouse: electrophysiological and morphological properties. Neurosci Res 51: 475–492, 2005 [DOI] [PubMed] [Google Scholar]
- Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J Comp Neurol 501: 825–836, 2007 [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res 863: 71–86, 2000 [DOI] [PubMed] [Google Scholar]
- Pedersen NP, Vaughan CW, Christie MJ. Opioid receptor modulation of GABAergic and serotonergic spinally projecting neurons of the rostral ventromedial medulla in mice. J Neurophysiol 106: 731–740, 2011 [DOI] [PubMed] [Google Scholar]
- Pierce TL, Wessendorf MW. Immunocytochemical mapping of endomorphin-2-immunoreactivity in rat brain. J Chem Neuroanat 18: 181–207, 2000 [DOI] [PubMed] [Google Scholar]
- Poole SL, Deuchars J, Lewis DI, Deuchars SA. Subdivision-specific responses of neurons in the nucleus of the tractus solitarius to activation of mu-opioid receptors in the rat. J Neurophysiol 98: 3060–3071, 2007 [DOI] [PubMed] [Google Scholar]
- Poulin JF, Chevalier B, Laforest S, Drolet G. Enkephalinergic afferents of the centromedial amygdala in the rat. J Comp Neurol 496: 859–876, 2006 [DOI] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol 45: 330–334, 1994 [PubMed] [Google Scholar]
- Rhim H, Glaum SR, Miller RJ. Selective opioid agonists modulate afferent transmission in the rat nucleus tractus solitarius. J Pharmacol Exp Ther 264: 795–800, 1993 [PubMed] [Google Scholar]
- Rhim H, Miller RJ. Opioid receptors modulate diverse types of calcium channels in the nucleus tractus solitarius of the rat. J Neurosci 14: 7608–7615, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol 300: R222–R235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sceniak MP, Maciver MB. Cellular actions of urethane on rat visual cortical neurons in vitro. J Neurophysiol 95: 3865–3874, 2006 [DOI] [PubMed] [Google Scholar]
- Schneider SP, Walker TM. Morphology and electrophysiological properties of hamster spinal dorsal horn neurons that express VGLUT2 and enkephalin. J Comp Neurol 501: 790–809, 2007 [DOI] [PubMed] [Google Scholar]
- Smith DV, Li CS. Tonic GABAergic inhibition of taste-responsive neurons in the nucleus of the solitary tract. Chem Senses 23: 159–169, 1998 [DOI] [PubMed] [Google Scholar]
- Smith DV, Travers SP. Central neural processing of taste information. In: The Senses: A Comprehensive Reference, edited by Basbaum AI, Kaneko A, Shepherd GM, Westheimer G. San Diego: Academic, 2008, p. 289–327 [Google Scholar]
- Stornetta RL, Schreihofer AM, Pelaez NM, Sevigny CP, Guyenet PG. Preproenkephalin mRNA is expressed by C1 and non-C1 barosensitive bulbospinal neurons in the rostral ventrolateral medulla of the rat. J Comp Neurol 435: 111–126, 2001 [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol 467: 60–79, 2003 [DOI] [PubMed] [Google Scholar]
- Travers JB, Herman K, Yoo J, Travers SP. Taste reactivity and Fos expression in GAD1-EGFP transgenic mice. Chem Senses 32: 129–137, 2007 [DOI] [PubMed] [Google Scholar]
- Travers JB, Travers SP. Microcircuitry of the rostral nucleus of the solitary tract. In: Handbook of Brain Microcircuits, edited by Shepherd GM, Grillner S. Oxford, UK: Oxford Univ. Press, 2010 [Google Scholar]
- Wang M, Bradley RM. Properties of GABAergic neurons in the rostral solitary tract nucleus in mice. J Neurophysiol 103: 3205–3218, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Bradley RM. Synaptic characteristics of rostral nucleus of the solitary tract neurons with input from the chorda tympani and glossopharyngeal nerves. Brain Res 1328: 71–78, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Corson J, Hill D, Erisir A. Postnatal development of chorda tympani axons in the rat nucleus of the solitary tract. J Comp Neurol 520: 3217–3235, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead MC. Neuronal architecture of the nucleus of the solitary tract in the hamster. J Comp Neurol 276: 547–572, 1988 [DOI] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature 386: 499–502, 1997 [DOI] [PubMed] [Google Scholar]
- Zhu M, Cho YK, Li CS. Activation of delta-opioid receptors reduces excitatory input to putative gustatory cells within the nucleus of the solitary tract. J Neurophysiol 101: 258–268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405, 2002 [DOI] [PubMed] [Google Scholar]