Abstract
Behavioral deficits in visuomotor planning and control exhibited by children with developmental coordination disorder (DCD) have been extensively reported. Although these functional impairments are thought to result from “atypical brain development,” very few studies to date have identified potential neurological mechanisms. To address this knowledge gap, electroencephalography (EEG) was recorded from 6- to 12-yr-old children with and without DCD (n = 14 and 20, respectively) during the performance of a visuomotor drawing task. With respect to motor performance, typically developing (TD) children exhibited age-related improvements in key aspects of motor planning and control. Although some children with DCD performed outside this TD landscape (i.e., age-related changes within the TD group), the group developmental trajectory of the children with DCD was similar to that of the TD children. Despite overall similarities in performance, engagement of cortical resources in the children with DCD was markedly different from that in their TD counterparts. While the patterns of activation are stable in TD children across the age range, the young children with DCD exhibited less engagement of motor cortical brain areas and the older children with DCD exhibited greater engagement of motor cortical brain areas than their TD peers. These results suggest that older children with DCD may employ a compensatory strategy in which increased engagement of relevant motor resources allows these children to perform comparably to their TD peers. Moreover, the magnitude of activation was related to several kinematic measures, particularly in children with DCD, suggesting that greater engagement in motor resources may underlie better behavioral performance.
Keywords: EEG, development, spectral power, atypical development
approximately six percent of school-aged children are diagnosed with developmental coordination disorder (DCD). This motor learning disorder is characterized by marked impairment in the performance of activities of daily living requiring movement coordination and interferes with the child's academic achievement (American Psychiatric Association 2004). In particular, children with DCD exhibit marked deficits in movement planning (Smyth et al. 1997) and adaptive visuomotor behavior (Kagerer et al. 2004, 2006; King et al. 2011a, 2011b) in reaching and drawing tasks. Although the motor performance of children with DCD has been extensively studied, the neurophysiological mechanisms underlying these functional deficits are not well known. Kaplan and colleagues (Kaplan et al. 1998) have suggested that DCD may be due to “atypical brain development.” However, the relationship between the movement planning deficits exhibited by children with DCD and differences in cortical activation patterns in relevant brain structures is unclear. To address this knowledge gap, the present study not only characterized the cortical dynamics underlying motor planning and control in children with DCD by electroencephalography (EEG) but also investigated the relationship between cortical dynamics and movement kinematics in children with and without DCD.
The temporal sensitivity of EEG makes it an ideal tool to study preparatory and ongoing cortical processes during the performance of motor tasks. In particular, movement-related cortical potentials (MRCPs) obtained from scalp locations overlying the supplementary motor area, premotor cortex, and primary motor cortex have revealed age-related differences in typically developing (TD) children and adults (Chiarenza 1990; Chiarenza et al. 1983; Pangelinan et al. 2011; Warren and Karrer 1984). Specifically, whereas adults exhibit larger negative-going waveforms in these relevant motor planning and control areas prior to and immediately after movement onset, this pattern of activity is attenuated or absent in children.
A complementary approach to time-domain analyses (i.e., MRCPs) is an examination of changes in the frequency content of the EEG between rest conditions and during the performance of a movement task. Many studies have reported task-related spectral power changes (desynchrony) in the alpha and beta frequency bands in adults during the preparation of motor tasks (Gerloff et al. 1998; Manganotti et al. 1998; Neuper and Pfurtscheller 2001; Pfurtscheller 1989; Pfurtscheller and Berghold 1989). More recently, these analyses have been applied to developmental data and have led to two important findings. First, young children lack the characteristic task-related changes (decreases) in alpha power reflecting a relative lack of movement preparation compared with older children and adults (Bender et al. 2005). Second, young children exhibit a relative increase in frontal cortical areas compared with motor cortical areas during motor planning, which may reflect the greater effort or attention needed for young children to plan and control arm movements (Pangelinan et al. 2011). As the motor performance of children with DCD is often more similar to that of much younger TD children, it follows that children with DCD may also exhibit a lack of alpha desynchrony over motor cortical brain areas while exhibiting a relative increase in frontal desynchrony during motor planning.
The present study examined movement kinematics as well as time- and frequency-domain analyses of EEG during the performance of a center-out drawing task to determine whether children with DCD exhibit a different developmental trajectory in behavioral and cortical dynamics compared with TD children. We also examined whether on average children with DCD differ from TD children after any age-related differences are accounted for. It was expected that children with DCD would exhibit EEG patterns that reflect a relative lack of engagement of relevant cortical motor resources and greater cortical activation from compensatory frontal brain regions. The results from this study provide insights into the neural mechanisms underlying visuomotor performance in children with DCD.
METHODS
Participants.
Children were recruited from the local university area. Children with DCD were referred to our study by local elementary school resource teachers, physical and occupational therapists, and/or parent support groups for children with developmental disabilities. All of the children in the DCD group were receiving educational support either via an individualized education plan (IEP) or the US Rehabilitation Act of 1973 (Section 504). These supports included untimed testing, a scribe for note taking, assistive technology (portable word processors), and/or modified home/classwork assignments. Table 1 provides details for the two groups. Fourteen children with DCD (8 girls, 6 boys; age range: 6.1–12.3 yr) and 20 TD children (10 girls, 10 boys; age range: 6.0–12.6 yr) were included in this study. Three additional 6- to 7-year olds with DCD were recruited for the study but were unable to complete the task and are not included in the analysis. The Institutional Review Board at the University of Maryland College Park approved all procedures. Prior to participation, parents and children provided informed consent and assent, respectively. For their participation, the children received a modest monetary compensation and a choice of an age-appropriate prize.
Table 1.
Group demographics and MABC2 performance details
| Group | Number of Participants, boys/girls | Mean Age, yr | Median MABC2 Total Percentile | Median MABC2 Manual Dexterity Percentile |
|---|---|---|---|---|
| DCD | 6/8 | 10.1 (2.0) | 5 (0.1–9) | 5 (0.01–9) |
| TD | 10/10 | 9.0 (2.1) | 63 (25–95) | 50 (25–99) |
Values in parentheses are SD (age) or range (MABC2 percentiles). DCD, developmental coordination disorder; TD, typically developing.
Inclusion criteria.
Parents completed a pediatric health questionnaire to provide details about their child's overall development. This questionnaire also inquired about the diagnosis of any general medical conditions and developmental learning disabilities (i.e., attention deficit hyperactivity disorder, autism spectrum disorder, speech/language difficulties, and academic problems). This questionnaire also asked whether motor coordination difficulties interfered with common activities of daily living, including tying shoes, buttoning clothing, taking notes in class, completing tests or school assignments, and participating in playground activities. The children completed a 10-item handedness test (Fagard and Corroyer 2003) to ensure right-hand dominance and the Movement Assessment Battery for Children, Second Edition (MABC2; Henderson and Sugden 2007) to characterize their motor skill ability in the areas of manual dexterity, ball skills, and balance.
TD children were eligible for inclusion on the basis of the following criteria: no history of neurological deficits, no head injuries/concussions, no learning or developmental disabilities, right-handedness, and performance on the MABC2 ≥25th percentile.
Inclusion criteria for the children with DCD were no history of neurological deficits, no head injuries/concussions, no diagnosis of pervasive developmental disabilities (e.g., autism spectrum disorder), no diagnosis of a medical condition that would impact movement (e.g., cerebral palsy), and right-handedness. In addition, on the basis of the parent questionnaire and the performance on the MABC, all children with DCD met the DSM-IV criteria for DCD: A: marked impairments in activities requiring motor coordination (MABC2 Manual Dexterity ≤9th percentile, median MABC 5th percentile; MABC2 Total Score ≤ 9th percentile, median MABC 5th percentile);1 B: motor coordination interferes with academic achievement or activities of daily living; and C: the disturbance is not due to a general medical condition2 as per parent report. Table 1 provides summary percentile scores (median and range) on the MABC2 for the two groups.
Experimental apparatus and procedures.
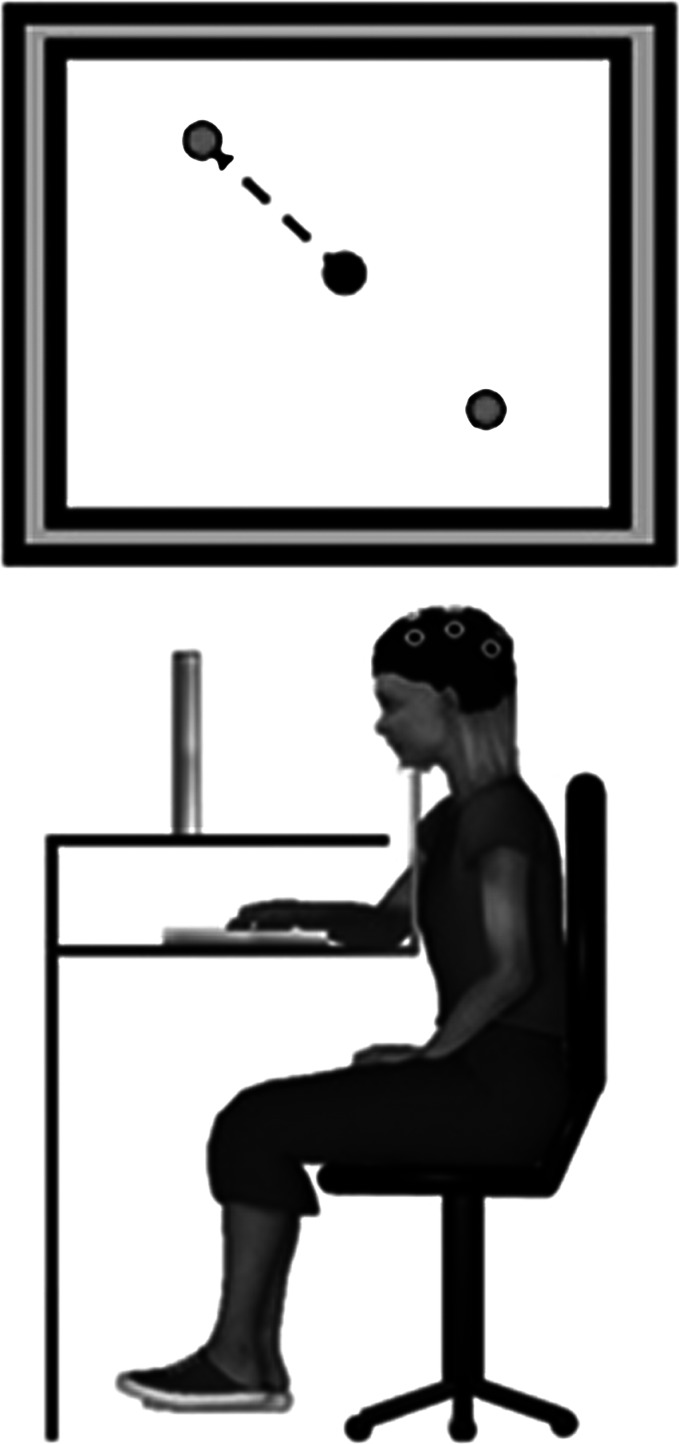
The data collection procedures were similar to previous studies in our lab (Contreras-Vidal and Kerick 2004; Pangelinan et al. 2011). The experimental setup is depicted in Fig. 1. Participants were seated at a table facing a computer monitor (21 in.) with the center of the screen positioned at eye level. A chin rest was used to stabilize and maintain the participant's head position, and the heights of the chair and chin rest were adjusted for each participant. Vision of the hand/arm was occluded via a wooden platform upon which the computer screen was positioned; a digitizing tablet (12 in. × 12 in.; WACOM In-Tuos, Vancouver, BC, Canada) was placed underneath.
Fig. 1.
Experiment setup. Top: the monitor displayed the center circle and the two peripheral targets. Bottom: the participants were seated at a desk with their head stabilized with a chin rest. The participants made self-selected and self-initiated center-out drawing movements with a digitized pen on a digitizing tablet for each of 60 trials.
Custom programs using OASIS software (Kikosoft, Nijmegen, The Netherlands) were used for stimulus presentation and tablet data acquisition. The participants made line-drawing/aiming movements in the horizontal plane with a computerized pen and a digitizing tablet. The sampling rate of the digitizing tablet was 200 Hz. The monitor provided real-time visual feedback of pen movement. The OASIS program also generated event markers that were synchronized with the EEG data collection indicating the beginning of a trial, target appearance, movement onset, target acquisition, and the end of a trial.
Continuous EEG was acquired at a sampling rate of 512 Hz from 11 surface tin electrodes housed within a stretchable Lycra cap (Electro Cap International, Eaton, OH) with Neuroscan Scan software (version 4.3, Herndon, VA). These electrode sites are consistent with the International 10/20 system and included the following regions: frontal (F3, Fz, F4), central (C3, Cz, C4), parietal (P3, Pz, P4), and occipital (O1, O2). Eye movement artifacts were recorded from electrodes placed superior and inferior to the left eye and on the lateral canthi of the left and right eyes. Average mastoids served as the common reference, and FPz served as the common ground. All channel impedances were maintained at or below 10 kΩ. However, acceptable impedances (below 10 kΩ) for the occipital sites (O1 and O2) were difficult to obtain for some of the participants because of interference caused by hair displacement. These sites were not included in the final analysis. Continuous EEG signals were amplified (20,000×) and digitally filtered (0.01 Hz and 100 Hz) with Grass (12A5) Neurodata Acquisition Amplifiers (Grass Technology, Astro-Med, West Warwick, RI). Prior to the drawing task, 2 min of eyes-open and eyes-closed resting EEG were recorded as baseline EEG measures.
The participants completed 12 practice trials to become familiar with the digital pen, tablet, and computer display. For some of the young children, an additional 12 trials were provided if the participant did not demonstrate an understanding of the task after the first practice set. Figure 1, top, depicts the behavioral task as presented on the computer monitor. The participants began a trial by moving the digital pen into a central home position indicated by a circle (0.5-cm diameter) presented on the computer monitor in the center of the workspace. Upon entering the home position, two target circles (each 0.5 cm in diameter) were presented 5 cm from the home position and located at 135° and 315° with respect to the home position. The participants were instructed to select one of the two targets and “plan or think how they will move quickly and accurately from the home position and stop in the target circle.” The participants had to remain motionless in the start position for 2 s. The purpose of this hold period was to provide the participants with sufficient time for target selection and movement planning, and to allow ample time for electrophysiological data acquisition during this phase of the task. There was no external cue to move after the 2-s hold period; however, if the participants left the home position too soon (<2 s), the targets would disappear and the trial would restart. After the hold period, the participants made one fast and straight movement with the digitizing pen from the home position to the target. The participants were able to see the pen trace displayed on the computer screen in real time. Once the pen reached the target position, the targets and pen trace disappeared and the participant returned the pen to the home position to begin the next trial. Between trials, the experimenter periodically reminded the participants to move “as quickly and as straight as possible.” The participants were free to choose the location of the target for each trial but were instructed to move to each of the targets equally across the 60 trials. On average both groups of children met this requirement (mean DCD: 30.0/30.0, mean TD: 31.1/28.9).
Data analysis.
Behavioral data analyses were consistent with previously reported studies conducted in our lab (King et al. 2011a, 2011b; Pangelinan et al. 2011) and conducted with programs written in MATLAB version 7.10 (MathWorks, Natick, MA). The time series of x and y positions for each trial were filtered with an 8th-order dual-pass Butterworth filter (cutoff frequency 10 Hz). Automated algorithms were used to mark the x/y position and time of movement onset and offset. Each trial was visually inspected and manually remarked if the onset/offset were incorrect. The following behavioral variables were computed from the movement trajectories: peak velocity (cm/s), movement time (MT, s), movement length (ML, cm), normalized jerk (NJ, unitless), root mean squared error (RMSE), and variability of initial direction error (VIDE, °). Peak velocity was the maximum velocity between the onset and offset. MT was the total time between movement onset and offset. ML was the total distance of the movement trajectory. NJ was calculated as the rate of change of the acceleration (j) normalized by MT and ML:
| (1) |
RMSE was computed as the average deviation between an ideal vector between the movement onset to offset (xa and ya) and the actual movement trajectory. N represents the number of sampled points in the trajectory:
| (2) |
Initial directional error (IDE) was calculated as the angular deviation between actual movement trajectory 80 ms after movement onset (initial movement direction prior to visual feedback correction) and an ideal straight vector from the onset to target. VIDE was assessed as the standard deviation of the IDE scores for each subject across all movements.
EEGLAB version 9.0.3 (Delorme and Makeig 2004) was used to re-reference data to average mastoids and apply filters. The following filters were applied to the data. For the time-domain analyses a 10-Hz low-pass filter with a 24 dB/octave roll-off was used. All subsequent analyses were conducted with customized programs written in MATLAB 7.10. Data were epoched/segmented into 1,000-ms windows beginning 500 ms prior to and 500 ms after movement onset. Data were baseline corrected with a 125-ms time window prior to the start of the epoch (725 ms prior to movement onset). These data were visually inspected for excessive movement and ocular artifacts. For the time-domain analyses or MRCPs, the 60 trials were averaged in time for each electrode site. The mean MRCP amplitude was computed for the period prior to (−500 ms to onset) and after (onset to 500 ms) movement onset for each trial for each electrode. For the spectral analysis, fast Fourier transforms (FFTs) were applied to data from the behavioral task as well as 1,000-ms epochs from the resting (eyes open) baseline condition. Power spectra were segmented into the alpha (8–12 Hz) and beta (13–30 Hz) bands. These bands were selected for their relevance to motor tasks (Gerloff et al. 1998). Spectral data were then log-transformed to meet the requirements for the statistical analysis (homogeneity of residuals). Task-related spectral power (TRSpec) was computed for alpha and beta frequency bands as
| (3) |
Statistical analysis.
SAS 9.1 software (SAS Institute, Cary, NC) was used to separately examine the group developmental trajectories and mean group differences after accounting for age. First, to examine the group differences in the developmental trajectories for each of the dependent measures, the following age-based regression model was employed:
| (4) |
where Y = dependent measure, β0, β1 = estimated fixed effects for the TD group (intercept and slope), γ0, γ1 = adjustments to the β parameters for the DCD group, C = 0 for the TD group and 1 for the DCD group, and e = residuals. The β0 parameter represents the intercept term for the TD group. The γ0 parameter is the adjustment to the TD intercept term; the sum of β0 and γ0 is equal to the intercept for the DCD group. Intercept terms were excluded from the discussion of the results because the value of these parameter estimates (i.e., when age = 0 yr) does not provide any meaningful conclusions. The β1 parameter represents the age-related changes (i.e., slope) for the TD children. The γ1 parameter is an adjustment to the TD slope parameter for the DCD group; the sum of β1 and γ1 is equal to the age-related changes for the DCD group. This statistical approach was employed by King and colleagues (King et al. 2012) to examine developmental trajectories in force control in children with and without DCD. Note that for the EEG dependent measures, regression models were created for each electrode of interest.
Second, to examine mean group differences after accounting for the effect of age, each dependent measure (behavioral and EEG) was analyzed by analysis of covariance (ANCOVA) with age as the covariate.
For the analysis of movement-related cortical potentials, the following electrodes were examined before and after movement onset: Fz, C3, Cz, and C4. These electrode sites were selected for their relevance to motor planning and were found to be sensitive to age-related differences in children in our previous work (Pangelinan et al. 2011). For TRSpec, separate analyses were examined corresponding to the frontal, central, and parietal regions for each of the two frequency bands of interest (alpha and beta).
Pearson's correlations between all behavioral and EEG measures were used to determine brain-behavior relationships across all children. For all statistical analyses, the level of significance was set to P < 0.05.
RESULTS
Movement kinematics.
Table 2 provides the beta coefficients, standard error, and significance level for the slope parameters in the regression analyses used to assess differences in the developmental trajectories for the performance measures.
Table 2.
Regression coefficients and standard error for kinematic analysis
| Behavioral DV | TD Slope (β1) | DCD Slope (β1 + γ1) | Difference in Slopes |
|---|---|---|---|
| MT | −0.11 (0.03)‡ | −0.01 (0.03) | 0.10 (0.04)* |
| ML | −0.14 (0.07)* | −0.10 (0.8) | 0.05 (0.10) |
| VIDE | −1.60 (0.32)‡ | −0.86 (0.38)* | 0.74 (0.50) |
| NJ | −41.92 (20.15)* | −45.16 (24.80)* | −6.11 (30.56) |
| PV | 1.05 (0.71) | −1.22 (0.95) | −1.84 (1.10) |
| RMSE | −0.02 (0.25) | −0.01 (0.02) | 0.01 (0.03) |
Values in parentheses are SE. DV, dependent variable; MT, movement time; ML, movement length; VIDE, variability of initial directional error; NJ, normalized jerk; PV, peak velocity; RMSE, root mean squared error.
P < 0.05,
P < 0.001.
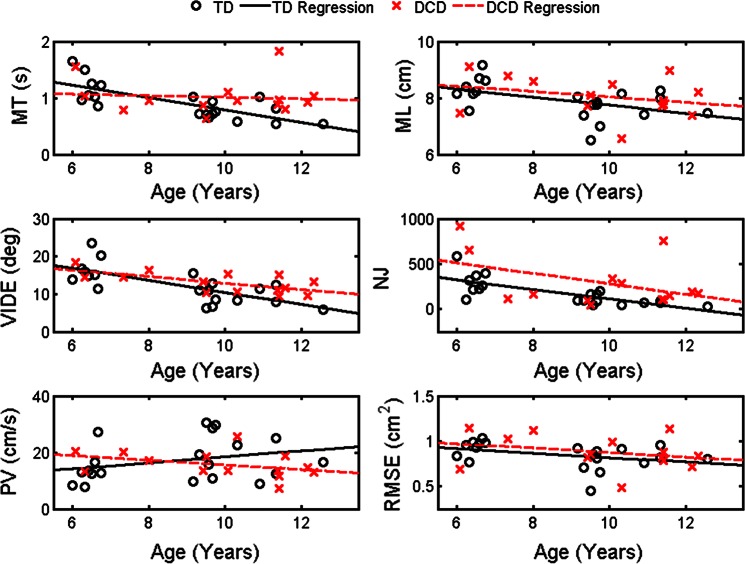
The regression analysis for each behavioral dependent measure revealed that the age-based regression slope for TD children was significant for MT, VIDE, ML, and NJ (P < 0.05 for all; Fig. 2). Similarly, the age-based regression slope for the DCD children was also significant for VIDE and NJ (P < 0.05 for both) but not for ML or MT. The slope coefficients for RMSE and peak velocity were not significant for either group. Moreover, no group differences were revealed for the slope and intercept terms for any dependent measure.
Fig. 2.
Movement kinematics with respect to age and group: movement time (MT; top left), movement length (ML; top right), variability of initial directional error (VIDE; middle left), normalized jerk (NJ; middle right), peak velocity (PV; bottom left), and root mean squared error (RMSE; bottom right). Regression for children with developmental coordination disorder (DCD) is indicated by the dashed red line; regression for typically developing (TD) children is indicated by the solid black line.
To assess mean group differences after accounting for the effects of age, each behavioral variable was assessed by ANCOVA with age as the covariate. After accounting for age, a mean group difference was evident for NJ (P < 0.05). The DCD group exhibited greater adjusted NJ (estimate: 326.4, standard error: 48.1) than the TD group (estimate: 158.1, standard error: 40.0). No additional mean group differences were revealed for the other behavioral variables (P > 0.05).
Taken together, in contrast to our hypotheses, these behavioral results suggest that the behavioral developmental trajectories of the two groups did not differ for any measure. Moreover, only after the effect of age was accounted for was a group mean difference found for one of the six behavioral dependent measures. Thus the behavioral performance was, by and large, similar across the two groups.
Movement-related cortical potentials.
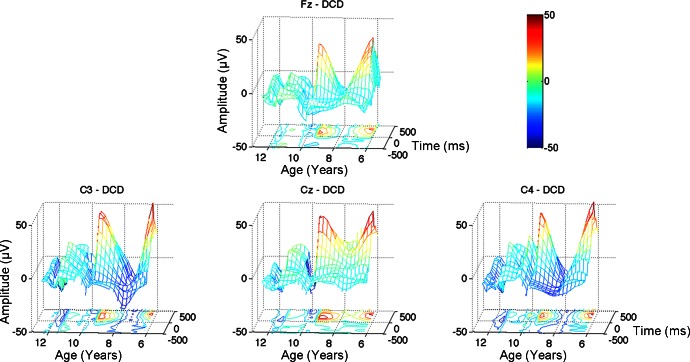
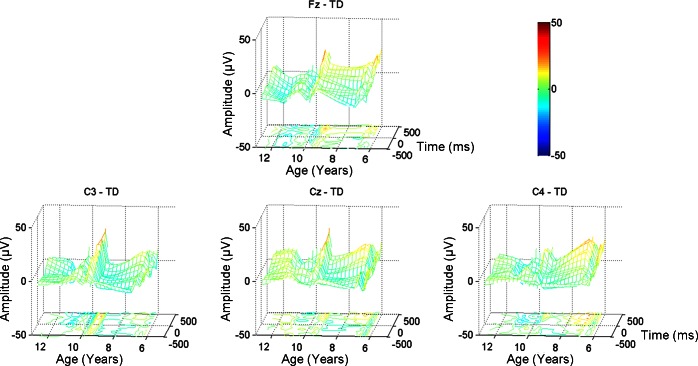
Figures 3 and 4 depict the time-averaged MRCPs for children with DCD and TD children, respectively. To highlight the developmental differences within each group, each individual's MRCP waveforms are incorporated into a mesh grid with age plotted along the x-axis, time (−500 ms to +500 ms with respect to movement onset) along the y-axis, and MRCP magnitude along the z-axis. Below the mesh grid is a contour map to highlight peaks (positive and negative) in the grid. The characteristic MRCP waveform consists of an increasingly negative amplitude leading to and immediately following movement onset (depicted as cool colors in Figs. 3 and 4). Several children in the DCD group, particularly the young children, exhibit very positive MRCP amplitudes after movement onset (i.e., warm colors in Figs. 3 and 4).
Fig. 3.
Movement related cortical potentials (MRCPs): children with DCD. Top: Fz. Bottom: C3 (left), Cz (center), and C4 (right). Each individual's MRCP waveforms are incorporated into the mesh grid. Age is plotted along the x-axis; time (−500 ms to +500 ms with respect to movement onset) is plotted along the y-axis; and MRCP magnitude is plotted along the z-axis. A contour map is plotted below the grid to highlight peaks (positive and negative) in the waveform grid. The color bar indicates the magnitude of the MRCP waveforms.
Fig. 4.
MRCPs: TD children. Top: Fz. Bottom: C3 (left), Cz (center), and C4 (right). Each individual's MRCP waveforms are incorporated into the mesh grid. Age is plotted along the x-axis; time (−500 ms to +500 ms with respect to movement onset) is plotted along the y-axis; and MRCP magnitude is plotted along the z-axis. A contour map is plotted below the grid to highlight peaks (positive and negative) in the waveform grid. The color bar indicates the magnitude of the MRCP waveforms.
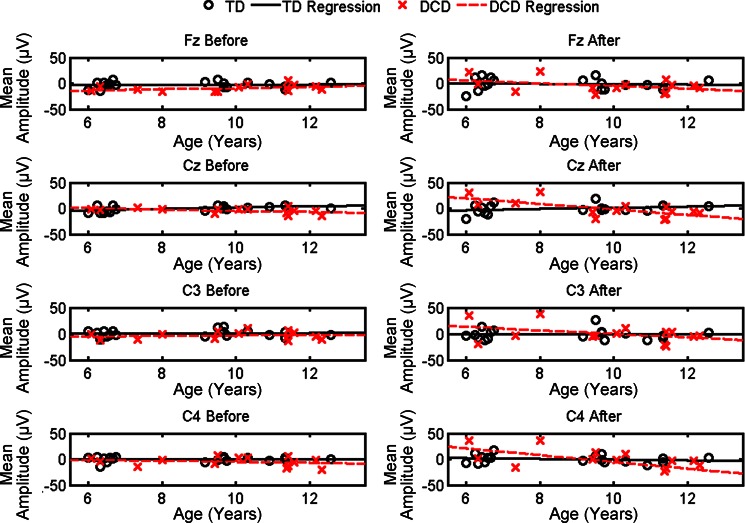
Analyses were conducted on mean MRCP amplitude before (−500 ms to movement onset) and after (onset to +500 ms) movement onset for Fz, Cz, C3, and C4. Regression analyses were used to assess the developmental trajectories of brain activation across groups. The slope coefficients, standard errors, and significance level for the regression analysis are provided in Table 3. Figure 5 depicts the significant age-related changes evident in the mean MRCP amplitude by group.
Table 3.
Regression coefficients and standard error for MRCP analysis
| MRCP DV | TD Slope (β1) | DCD Slope (β1 + γ1) | Difference in Slopes |
|---|---|---|---|
| Fz before | 0.12 (0.70) | 1.14 (0.84) | 1.02 (1.10) |
| Fz after | −0.40 (1.37) | −2.65 (1.61) | −2.25 (2.12) |
| Cz before | 1.38 (0.57) | −1.21 (0.67) | −2.59 (0.88)† |
| Cz after | 1.25 (1.16) | −5.34 (1.537)‡ | −6.58 (1.80)† |
| C3 before | 0.23 (0.81) | −0.42 (0.96) | 0.19 (1.26) |
| C3 after | −0.01 (1.52) | −3.31 (1.80)† | −3.30 (2.35)* |
| C4 before | 0.08 (0.76) | −0.93 (0.90) | −1.00 (1.18) |
| C4 after | −0.73 (1.83) | −6.60 (2.16)† | −5.86 (2.83)* |
Values in parentheses are SE. MRCP, movement-related cortical potential.
P < 0.05,
P < 0.01,
P < 0.001.
Fig. 5.
Mean MRCP amplitudes with respect to age and group: Fz (1st row), Cz (2nd row), C3 (3rd row), and C4 (4th row) averaged over the 500 ms before movement onset (left) and after movement onset (right). Regression for children with DCD is indicated by the dashed red line; regression for TD children is indicated by the solid black line.
This analysis revealed significant age-related changes (slope coefficients) for the children with DCD for Cz and C4 after onset (P < 0.001 and P < 0.01, respectively). The regression slopes were not significant for the TD children (P > 0.05). Interestingly, significant group differences in the regression slope were found for Cz before onset (P < 0.01), Cz after onset (P < 0.01), and C4 after onset (P < 0.05). These results suggest a different developmental trajectory for the children with DCD compared with TD children. Specifically, compared with their TD counterparts, the young children with DCD exhibit less engagement (greater positivity) for these motor cortical areas, whereas the older children with DCD exhibit greater engagement (greater negativity).
After accounting for age, significant mean group differences were revealed for Fz before onset [F(30) = 14.78, P < 0.001] and Cz before onset [F(30) = 4.51, P < 0.05]. No additional mean group differences were found for the other MRCP dependent measures (P > 0.05). These data suggest that after age-related differences were accounted for the children with DCD exhibited greater mean activation (greater negative amplitudes) than their TD peers. These results are consistent with our hypothesis that overall the children with DCD would exhibit greater activation compared with the TD cohort.
Task-related spectral power for alpha and beta.
No significant mean differences were found for any of the TRSpec measures (frontal, central, and parietal) for either frequency band (alpha and beta). Moreover, the regression analysis failed to reveal significant age-related changes for either group for the TRSpec measures (P > 0.05).
Correlations between EEG and kinematic measures.
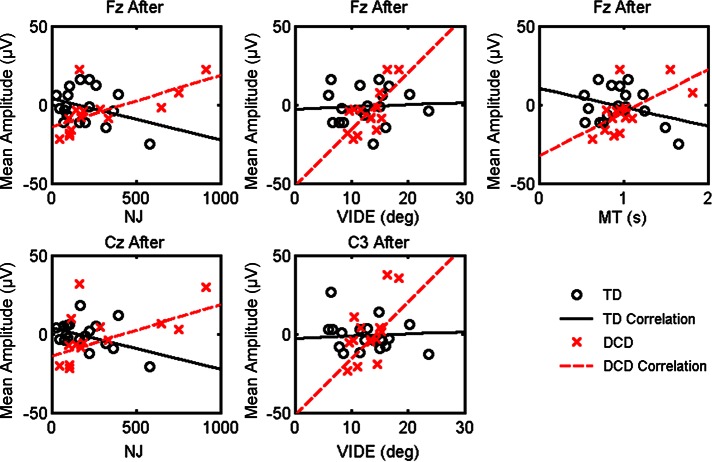
Figure 6 depicts the significant correlations between the MRCP components and the kinematic dependent measures for the TD children and children with DCD. Pearson's correlation revealed significant positive relationships for the children with DCD, but no significant relationships were revealed for the TD children. For the children with DCD, the MRCP amplitude for Fz after movement onset was correlated with NJ (r = 0.65, P < 0.01), VIDE (r = 0.71, P < 0.001), and MT (r = 0.62, P < 0.05). The MRCP amplitude for Cz after movement onset was correlated with NJ (r = 0.56, P < 0.05). MRCP amplitude at site C3 after movement onset was positively related to VIDE (r = 0.64, P < 0.05). These results suggest that greater mean negativity in the MRCP waveforms following movement onset is related to decreased variability in directional planning and greater smoothness in children with DCD.
Fig. 6.
Significant correlations: dependent measures NJ, VIDE, and MT. These dependent measures are depicted with respect to MRCP amplitude after movement onset for Fz (top), Cz (bottom left), and C3 (bottom center). The linear fit for children with DCD is indicated by the dashed red line, and the linear fit for the TD children is indicated by the solid black line.
DISCUSSION
This study is the first to examine differences in EEG cortical dynamics and movement kinematics in the context of a visuomotor task in children with and without DCD. The kinematic performance of TD children suggests age-related improvements in several aspects of motor planning and control (MT, ML, smoothness, and directional variability). The children with DCD exhibited age-related improvements limited to movement smoothness and variability of directional planning. Interestingly, although the performance of some children with DCD fell outside the TD landscape (i.e., age-related changes within the TD group), the developmental trajectory of the children with DCD was similar to that of the TD children. After accounting for age, group differences were only revealed for movement smoothness. Despite overall similarities in motor performance, the engagement of cortical resources in the children with DCD is markedly different from their TD counterparts. The activation patterns of the TD children are stable across the age range (no age-related differences), which suggests that even young TD children engage motor cortical resources during this task. In contrast to the TD children, the young children with DCD exhibit greater positivity in movement-related brain regions, whereas older children with DCD exhibit greater negativity, particularly after the initiation of movement. These results suggest that the older children with DCD may employ a compensatory strategy in which greater engagement of relevant motor cortical resources allows for motor performance equivalent to that of their TD peers. In addition, this study revealed that greater engagement in movement-related brain areas (i.e., MRCP negativity) is related to better kinematic performance (less variability, greater movement smoothness, and faster movements) in the children with DCD. Taken together, the results from this study provide insights into the differences in cortical dynamics in children with and without DCD and how the cortical dynamics relate to behavioral performance in these children.
It is worthwhile to note that no systematic differences were found for any behavioral or EEG measures across trials. Therefore, the activation patterns and performance of the two groups were consistent and stable during the task. Therefore, any group differences, or lack thereof, reported here are not likely transient.
The performance of the children with DCD on this goal-directed drawing task was not different from that of their TD counterparts in terms of the developmental trajectory of behavioral improvements across age. These results confirm other studies that found that the performance of children with DCD does not differ from that of TD children for simple discrete drawing or aiming movements (Hyde and Wilson 2011; Smits-Engelsman et al. 2003; Wilmut and Wann 2008). It is also possible that the age-related improvements in the DCD group reported for the planning and control measures may be due, in part, to the fact that the older children with DCD moved more slowly than their TD peers. This may reflect that the older children with DCD sacrificed speed for accuracy. In contrast, the TD children show age-related improvements in both speed (MT) and accuracy (ML), suggesting that older TD children are able to plan and control their movement well without compromising speed and accuracy.
Although the developmental trajectories do not appear to differ for the behavioral measures, after age was accounted for, the children with DCD exhibited less smooth movements (greater NJ) compared with the TD children, suggesting that kinematic control was difficult for children with DCD even for this simple task. It is likely that for more complex tasks, such as movements requiring greater involvement of additional joints or body segments or movements in which task conditions change (e.g., stop-signal or double-step tasks), the behavioral performance of children with DCD will degrade compared with that of TD children for all kinematic measures.
Divergent trajectories in brain activation patterns.
To accomplish levels of behavioral performance similar to those of the TD children, the older children with DCD engage cortical motor resources to a greater extent than their TD counterparts. This seeming lack of efficient cortical activation confirms a previous study using fMRI, which also found greater activation in children with DCD compared with control subjects for a set of visuospatial brain regions (Zwicker et al. 2010). The authors of this previous study attributed this increase in visuospatial brain activation to a dependence on vision to guide motor performance. In the context of the present study, we did not find global differences in brain activation. Rather, differences in activation were constrained to brain regions involved in motor planning and control. It is likely that this discrepancy between the present study and the previous study is due to the methodology used to assess cortical activation (EEG vs. MRI) and the nature of the two tasks (discrete drawing vs. maze tracing). The time-sensitive nature of EEG allows us to track real-time changes in cortical activation linked directly to the task planning and performance. Thus the results from the present study suggest that older children with DCD continue to engage motor cortical areas to support online control of the movement (i.e., after the initiation of a ballistic movement), whereas TD children do not require enhanced engagement of motor areas to perform the task effectively. If the present study employed a task that required continuous monitoring of performance online or if the task was dynamic (e.g., task constraints changed during performance), it is possible that additional brain regions might be implicated.
Previous studies using PET to measure glucose metabolism in adults have reported that movement preparation is associated with greater metabolism in movement-related cortical (sensorimotor, premotor, supplementary motor) and subcortical (basal ganglia, thalamus, and cerebellum) regions (Deiber et al. 1996; Jahanshahi et al. 1995). Similar findings have been also been reported in adults with fMRI, such that activation of the supplementary motor, cingulate motor, and primary motor areas was exhibited during the preparation of self-initiated movements (Ball et al. 1999; Cunnington et al. 2002, 2003). Moreover, greater movement complexity has been found to engage the supplementary and cingulate motor areas to a greater extent than less complex movements in adults (Deiber et al. 1999). Interestingly, a study by Toma and colleagues (Toma et al. 2002) used fMRI activity to constrain a source analysis of movement-related EEG and reported that the likely generators of the negative-going EEG waveform are the sensorimotor and supplementary motor areas. Taken together, these studies suggest that greater activation of these brain regions could underlie the greater task-related negativity exhibited by the older children with DCD, while attenuated activation of these brain regions could underlie the lack of task-related negativity exhibited by young children with DCD.
With respect to the findings from the TD children, our previous work examining age-related differences in MRCP waveforms across children and adults also reported differences between the behavioral performance of young children and older children despite no difference in the MRCP amplitudes (Pangelinan et al. 2011). These findings differ from previous studies examining developmental differences in MRCP waveforms during the performance of simple motor responses (Bender et al. 2005; Chiarenza 1990; Chiarenza et al. 1983; Warren and Karrer 1984); young children did not exhibit negative-going waveforms in those studies. However, it appears that the goal-directed, self-selected, self-initiated nature of the drawing task employed presently, and in our previous work, elicits consistent negative MRCP amplitudes even for young TD children. Interestingly, the young children with DCD exhibit positive-amplitude MRCP waveforms, suggesting that these children do not engage appropriate brain regions, particularly after movement onset (i.e., during online motor control).
Brain-behavior relationships.
Our previous study (Pangelinan et al. 2011) reported that the magnitude of MRCPs was related to the quality of motor performance in TD children and adults. The present study replicates and extends the previous findings, reporting a relationship between task-related negativity in relevant brain regions and improved kinematic performance in children with and without DCD. It was also revealed that engagement of motor-related cortical regions (midline frontal, midline central, and left central regions) after movement onset was related to faster movement times, smoother trajectories, and/or reduced directional variability. This finding substantiates the claim that an increased engagement of motor planning and control brain regions would directly relate to better online performance. These results were particularly striking for children with DCD.
The activation patterns and behavioral performance of the older TD children support a neural efficiency hypothesis in which those with greater motor (or cognitive) skill demonstrate a relative refinement in the activation across the cortex. This work has been supported by previous research in our lab investigating cortical processes of highly skilled versus novice athletes (Hatfield et al. 2004; Kerick et al. 2004). The relative attenuation of brain activity demonstrated by the older TD children, compared with the older children with DCD, may reflect automatization and skill in performing the visuomotor task. With increased practice on this task or handwriting-specific training the children with DCD may improve their behavioral performance and exhibit a similar reduction in motor cortical brain activation. Indeed, practice and learning effects have been found in the brain activity and behavior of adults for a similar task in which the pen trace is rotated abruptly (visuomotor adaptation paradigm) and participants must adapt movements for this new visuomotor environment (Contreas-Vidal and Kerick 2004; Gentili et al. 2011). Thus evaluating the cortical dynamics of children with DCD before and after behavioral training may provide an additional metric for skill acquisition even after behavioral performance no longer reveals significant improvement.
Future directions.
Future studies are necessary to confirm the results presented here. In particular, it would be worthwhile to determine whether children with DCD are still able to maintain performance equivalent to that of TD children if the task complexity increases. We would hypothesize that increased activation of cortical resources may not be sufficient to maintain behavioral performance and that children with DCD may begin to recruit additional neural resources to complete the tasks.
As mentioned above, it would also be worthwhile to compare the efficacy of different behavioral training programs using both the behavioral outcomes as well as brain dynamics. Currently, many different behavioral interventions are used to help children with DCD with fine motor and handwriting difficulties. Even with behavioral therapy, many children with DCD, particularly those with severe perceptual-motor difficulties, do not resolve their motor difficulties across childhood and adolescence (Cantell et al. 2003). Therefore, it is imperative that the behavioral interventions used are evaluated at the level of both brain and behavior to determine whether that therapy should be continued or whether alternative treatments are necessary. It is likely that the cortical dynamics will provide important insights into efficacy of behavioral treatments even once the behavioral outcomes have plateaued. Not only will this brain-based approach to therapy be useful for matching individual children with movement difficulties with interventions, but it will also provide valuable evidence regarding the persistence/resolution of DCD.
GRANTS
Support for this research was provided by the University of Maryland Kinesiology Graduate Research Initiative Project (GRIP) and the Ruth L. Kirschstein National Research Service Award Predoctoral Fellowship-Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) F31 HD-061210 awarded to M. M. Pangelinan. Additional support was provided by Eunice Kennedy Shriver NICHD R01 HD-42527 awarded to J. E. Clark.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.M.P., B.D.H., and J.E.C. conception and design of research; M.M.P. performed experiments; M.M.P. analyzed data; M.M.P., B.D.H., and J.E.C. interpreted results of experiments; M.M.P. prepared figures; M.M.P. drafted manuscript; M.M.P., B.D.H., and J.E.C. edited and revised manuscript; M.M.P., B.D.H., and J.E.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the children and their parents for their participation in this study. We thank Kelly Klein, Leah Bush, and Lauren Weiss for their assistance with data collection. A special thanks to Bradley R. King for his thoughtful review of the manuscript.
Footnotes
We acknowledge that the suggested research diagnostic criterion for DCD is a MABC2 Total Score ≤5th percentile. MABC2 total scores between the 5th and 15th percentiles are considered “at risk” for movement difficulties. We accepted children with total scores up to the 9th percentile if the child's manual dexterity scored at or below the 5th percentile, providing support that the child exhibits marked impairments in visuomotor abilities relevant to the behavioral task assessed.
None of the children in the present study presented with intellectual disabilities, so criterion D was not applicable to the diagnosis of DCD.
REFERENCES
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Arlington, VA: Am. Psychiatric Assoc., 2004 [Google Scholar]
- Ball T, Schreiber A, Feigel B, Wagner M, Lucking CH, Kristeva-Feige R. The role of higher-order motor areas in voluntary movement as revealed by high-resolution EEG and fMRI. Neuroimage 10: 682–694, 1999 [DOI] [PubMed] [Google Scholar]
- Bender S, Weisbrod M, Bornfleth H, Resch F, Oelkers-Ax R. How do children prepare to react? Imaging maturation of motor preparation and stimulus anticipation by late contingent negative variation. Neuroimage 27: 737–752, 2005 [DOI] [PubMed] [Google Scholar]
- Cantell M. Two distinct pathways for developmental coordination disorder: persistence and resolution. Hum Mov Sci 22: 413–431, 2003 [DOI] [PubMed] [Google Scholar]
- Chiarenza GA. Motor-perceptual function in children with developmental reading disorders: neuropsychophysiological analysis. J Learn Disabil 23: 375–385, 1990 [DOI] [PubMed] [Google Scholar]
- Chiarenza GA, Papakostopoulos D, Giordana F, Guareschi-Cazzullo A. Movement-related brain macropotentials during skilled performances. A developmental study. Electroencephalogr Clin Neurophysiol 56: 373–383, 1983 [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Kerick SE. Independent component analysis of dynamic brain responses during visuomotor adaptation. Neuroimage 21: 936–945, 2004 [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage 15: 373–385, 2002 [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and readiness for voluntary movement: a high-field event-related fMRI study of the Bereitschafts-BOLD response. Neuroimage 20: 404–412, 2003 [DOI] [PubMed] [Google Scholar]
- Deiber MP, Ibanez V, Sadato N, Hallett M. Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J Neurophysiol 75: 233–247, 1996 [DOI] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M. Mesial motor area in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol 81: 3065–3077, 1999 [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004 [DOI] [PubMed] [Google Scholar]
- Fagard J, Corroyer D. The association between developmental coordination disorder and other developmental traits. Dev Psychobiol 43: 44–56, 2003 [DOI] [PubMed] [Google Scholar]
- Gentili RJ, Bradberry TJ, Oh H, Hatfield BD, Contreras-Vidal JL. Cerebral cortical dynamics during visuomotor transformation: adaptation to a cognitive-motor executive challenge. Psychophysiology 48: 813–824, 2011 [DOI] [PubMed] [Google Scholar]
- Gerloff C, Richard J, Hadley J, Schulman AE, Honda M, Hallett M. Functional coupling and regional activation of human cortical motor areas during simple, internally paced and externally paced finger movements. Brain 121: 1513–1531, 1998 [DOI] [PubMed] [Google Scholar]
- Hatfield BD, Haufler AJ, Hung T, Spalding TW. Electroencephalographic studies of skilled psychomotor performance. J Clin Neurophysiol 21: 144–156, 2004 [DOI] [PubMed] [Google Scholar]
- Henderson SE, Sugden DA, Barnett AL. Movement Assessment Battery for Children (2nd ed.) (Movement ABC-2) London, UK: Pearson Assessment, 2007 [Google Scholar]
- Hyde C, Wilson PH. Dissecting online control in Developmental Coordination Disorder: a kinematic analysis of double-step reaching. Brain Cogn 75: 232–241, 2011 [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain 118: 913–933, 1995 [DOI] [PubMed] [Google Scholar]
- Kagerer FA, Bo J, Contreras-Vidal JL, Clark JE. Visuomotor adaptation in children with developmental coordination disorder. Motor Control 8: 450–460, 2004 [DOI] [PubMed] [Google Scholar]
- Kagerer FA, Contreras-Vidal JL, Bo J, Clark JE. Abrupt, but not gradual visuomotor distortion facilitates adaptation in children with developmental coordination disorder. Hum Mov Sci 25: 622–633, 2006 [DOI] [PubMed] [Google Scholar]
- Kaplan BJ, Wilson B, Dewey D, Crawford SG. DCD may not be a discrete disorder. Hum Mov Sci 17: 471–490, 1998 [Google Scholar]
- Kerick SE, Douglass LW, Hatfield BD. Cerebral cortical adaptations associated with visuomotor practice. Med Sci Sport Exerc 36: 118–129, 2004 [DOI] [PubMed] [Google Scholar]
- King BR, Clark JE, Oliveira MA. Developmental delay in finger torque control in children with developmental coordination disorder. Dev Med Child Neurol 54: 932–937, 2012 [DOI] [PubMed] [Google Scholar]
- King BR, Harring JR, Oliveira MA, Clark JE. Statistically characterizing intra- and inter-individual variability in children with developmental coordination disorder. Res Dev Disabil 32: 1388–1398, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, Kagerer FA, Harring JR, Contreras-Vidal JL, Clark JE. Multisensory adaptation of spatial-to-motor transformations in children with developmental coordination disorder. Exp Brain Res 212: 257–265, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P, Gerloff C, Toro C, Katsuta H, Sadato N, Zhuang P, Leocani L, Hallett M. Task-related coherence and task-related spectral power changes during sequential finger movements. Electroencephalogr Clin Neurophysiol 109: 50–62, 1998 [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol 43: 41–58, 2001 [DOI] [PubMed] [Google Scholar]
- Pangelinan MM, Kagerer FA, Momen B, Hatfield BD, Clark JE. Electrocortical dynamics reflect age-related differences in movement kinematics among children and adults. Cereb Cortex 21: 737–747, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G. Functional topography during sensorimotor activation studied with event-related desynchronization mapping. J Clin Neurophysiol 6: 75–84, 1989 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Berghold A. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr Clin Neurophysiol 72: 250–258, 1989 [DOI] [PubMed] [Google Scholar]
- Smits-Engelsman B. Fine motor deficiencies in children with developmental coordination disorder and learning disabilities: an underlying open-loop control deficit. Hum Mov Sci 22: 495–513, 2003 [DOI] [PubMed] [Google Scholar]
- Smyth MM, Mason UC. Planning and execution of action in children with and without developmental coordination disorder. J Child Psychol Psychiatry 38: 1023–1037, 1997 [DOI] [PubMed] [Google Scholar]
- Toma K, Matsuoka T, Mima T, Waldvogel D, Koshy B, Hanakawa T, Shill H, Hallett M. Generators of movement-related cortical potentials: fMRI-constrained EEG dipole source analysis. Neuroimage 17: 161–173, 2002 [DOI] [PubMed] [Google Scholar]
- Warren C, Karrer R. Movement-related potentials in children. Ann NY Acad Sci 425: 489–495, 1984 [DOI] [PubMed] [Google Scholar]
- Wilmut K, Wann J. The use of predictive information is impaired in the actions of children and young adults with Developmental Coordination Disorder. Exp Brain Res 191: 403–418, 2008 [DOI] [PubMed] [Google Scholar]
- Zwicker JG, Missiuna C, Harris SR, Boyd LA. Brain activation of children with developmental coordination disorder is different than peers. Pediatrics 126: e678–e686, 2010 [DOI] [PubMed] [Google Scholar]