Abstract
Effects of water deprivation on rhythmic bursting of sympathetic nerve activity (SNA) were investigated in anesthetized, bilaterally vagotomized, euhydrated (control) and 48-h water-deprived (WD) rats (n = 8/group). Control and WD rats had similar baseline values of mean arterial pressure, heart rate, end-tidal CO2, and central respiratory drive. Although integrated splanchnic SNA (sSNA) was greater in WD rats than controls (P < 0.01), analysis of respiratory rhythmic bursting of sSNA revealed that inspiratory rhythmic burst amplitude was actually smaller (P < 0.005) in WD rats (+68 ± 6%) than controls (+208 ± 20%), and amplitudes of the early expiratory (postinspiratory) trough and late expiratory burst of sSNA were not different between groups. Further analysis revealed that water deprivation had no effect on either the amplitude or periodicity of the cardiac rhythmic oscillation of sSNA. Collectively, these data indicate that the increase of sSNA produced by water deprivation is not attributable to either increased respiratory or cardiac rhythmic burst discharge. Thus the sympathetic network response to acute water deprivation appears to differ from that of chronic sympathoexcitation in neurogenic forms of arterial hypertension, where increased respiratory rhythmic bursting of SNA and baroreflex adaptations have been reported.
Keywords: sympathetic nerve activity, hypertension, paraventricular nucleus, rostral ventrolateral medulla, respiratory network
support of arterial pressure in water-deprived (WD) rats depends on the level of ongoing sympathetic nerve activity (SNA) (6, 7, 29, 37, 39, 43). Although water deprivation progressively unloads cardiopulmonary volume receptors, increases circulating hormones (vasopressin, oxytocin, angiotensin II), and raises body fluid osmolality (6, 40), data indicate that body fluid hyperosmolality is the principal driver of sympathetic network activity (6, 29). Hyperosmolality activates osmosensory neurons in the forebrain lamina terminalis (32–34, 40) and during water deprivation recruits a descending neural pathway to presympathetic neurons of the hypothalamic paraventricular nucleus (PVN). Water deprivation mainly activates glutamatergic PVN neurons (36) with axonal projections to downstream sympathetic regulatory targets, including reticulospinal neurons of the rostral ventrolateral medulla (RVLM) (4, 14) and sympathetic preganglionic neurons of the spinal intermediolateral cell column (5, 32, 36).
In mammals at rest, ongoing SNA to most end organs consists largely of discreet respiratory and cardiac rhythmic bursts of action potentials (2, 11, 12, 26). Respiratory rhythmic sympathetic burst discharge result from excitatory and inhibitory interactions among elements of the respiratory network (15, 22, 23) that are transmitted to the sympathetic network, mostly at the level of the RVLM. Cardiac rhythmic bursts of SNA result from pulse synchronous inhibitory entrainment of RVLM neurons by arterial baroreceptor inputs (11, 38).
In the present study, we tested the hypothesis that, during water deprivation, sympathetic network drive results in increased respiratory and cardiac rhythmic bursting of SNA. Support for this hypothesis stems from evidence summarized above that water deprivation activates a sympathetic regulatory pathway that targets vital elements of the respiratory network and the arterial baroreceptor reflex arc (18–20, 28). Further indirect support is derived from evidence that pontomedullary transection, which interrupts the neural pathway activated by water deprivation, disrupts both central respiratory modulation of SNA and arterial baroreflex function (3).
Here, the impact of water deprivation on rhythmic patterns of SNA was determined from averages of splanchnic SNA (sSNA) triggered by bursts of phrenic nerve activity (PNA) and ECG R-waves. We chose to record sSNA because its burst pattern is strongly modulated by the respiratory network (10, 13). Results indicate that baseline sSNA was increased in WD rats compared with controls, but, contrary to our hypothesis, inspiratory rhythmic sSNA burst amplitude was reduced, not increased, in WD rats, and activity during the expiratory phase of the respiratory cycle did not differ across groups. The cardiac rhythmic oscillation of sSNA was also similar in control and WD rats. Thus sympathetic network drive during water deprivation appears to mainly increase components of sSNA that are neither respiratory nor cardiac rhythmic.
METHODS
Animals.
All experimental and surgical procedures complied with guidelines set forth by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio. Adult male Sprague-Dawley rats (n = 22, 250–400 g) (Charles River Laboratories) were housed in a temperature-controlled room (22–23°C) with a 14:10-h light-dark cycle (lights on at 0700). Control rats had continuous access to food (Harlan Teklad LM-485, 0.3% NaCl) and tap water. WD rats had water but not food withheld for 48 h before initiating experimental protocols.
Animal preparation and surgery.
On experiment days, rats were anesthetized with a mixture of α-chloralose and urethane (80/800 mg/kg ip) (Sigma-Aldrich, St. Louis, MO). Catheters (PE-50 tubing) were inserted into the left femoral artery and both femoral veins to record arterial blood pressure and administer drugs, respectively. Vagus nerves were transected at the midcervical level, leaving aortic depressor nerves intact. A lead 1 ECG was recorded. After tracheal cannulation, rats were paralyzed with gallamine triethiodide (25 mg·kg−1·h−1 iv) and artificially ventilated with oxygen-enriched room air. End-tidal CO2 (ETCO2) was maintained between 5.0 and 5.5% by adjusting ventilation rate (80–100 breaths/min) and/or tidal volume (2–3 ml). Rats were placed in a stereotaxic device, and body temperature was maintained at 37 ± 1°C with a ventrally located water-circulating pad. Supplements of α-chloralose/urethane (10% of initial dose) were given as necessary to maintain adequate anesthesia, which was assessed by lack of a withdrawal reflex to noxious pinching of the hindpaw before paralysis and lack of a pressor response thereafter.
Phrenic and sympathetic nerve recording.
To record PNA, skin and muscle overlying the left scapula were incised and retracted laterally. The phrenic nerve was isolated near the brachial plexus and transected, and its proximal end placed on a bipolar silver wire electrode (A-M systems, 0.005-in. outer diameter). To record sSNA, the left greater splanchnic nerve was exposed through a retroperitoneal incision, isolated proximal to the adrenal gland, and placed on a bipolar stainless steel wire electrode (A-M systems, 0.005-in. outer diameter). To insulate recordings from body fluid, each nerve-electrode interface was covered with a silicon-based impression material (Super-Dent Light, Carlisle Laboratories). Signals were obtained through high-impedance probes connected to AC amplifiers that were equipped with half-amplitude frequency filters (band pass: 30–1,000 Hz) and a 60-Hz notch filter. Nerve signals were amplified (20,000–50,000×), full-wave rectified, RC integrated (time constant τ = 10 ms), and digitized at 1.5 kHz using a 1401plus analog-to-digital converter and Spike2 software (version 7.1, Cambridge Electronic Design). Noise in sSNA recordings was determined as a 3-min average of integrated voltage recorded 5 min after bolus injection of the ganglionic blocker hexamethonium (30 mg/kg iv).
Assessment of central respiratory drive.
Baseline central respiratory drive was determined and compared across groups of control and WD rats. Rats were ventilated with 100% O2 (ETCO2 = ∼5.5%) at baseline, and PNA burst amplitude and frequency were determined. Ventilation was then switched to 8% CO2 (balance O2), which increased PNA bursting to a stable maximum (∼10 min). PNA burst amplitudes at baseline and during hypercapnia were compared across groups.
Hematology.
After surgery, a 0.5-ml venous blood sample was drawn from each rat. Plasma protein concentration (Pprotein) was determined by refractometry (VWR International, Buffalo Grove, IL). Plasma osmolality (PosM) was measured from duplicate plasma samples using a freezing-point depression osmometer (model 3320, Advanced Instruments, Norwood, MA). Hematocrit (Hct) was determined from duplicate capillary tubes with a Lancer Hct tube reader (St. Louis, MO).
Data analysis.
Heart rate (HR) and respiratory rate were determined as the mean frequency of ECG R-wave and PNA burst synchronous events. Mean arterial pressure (MAP) was calculated as Pdiastolic + (Psystolic − Pdiastolic)/3, where Pdiastolic and Psystolic are diastolic and systolic blood pressure, respectively. Baseline values of HR, respiratory rate, and MAP were determined from 5-min segments of stable data.
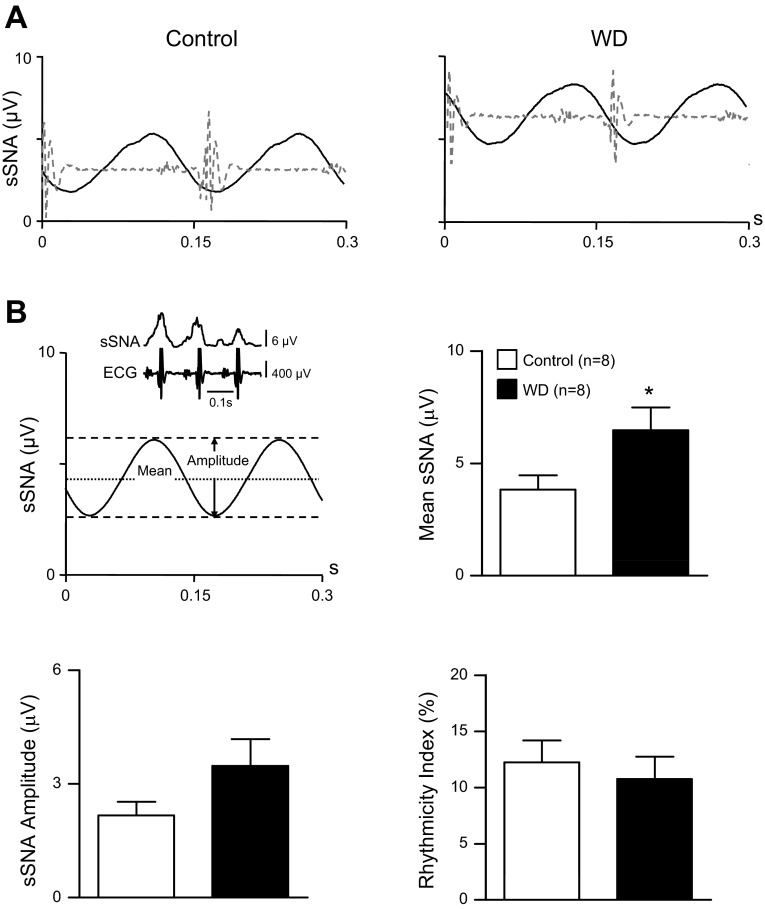
Averages of sSNA were constructed using the onset of 150 PNA bursts as trigger events (i.e., time zero) and consisted of a 0.3-s pretrigger period and a 1.6-s posttrigger period. The latter was equal to the average respiratory cycle duration (determined from the interval distribution of PNA burst events). Baseline sSNA was taken as the mean of the posttrigger voltage (see Fig. 2B, top left). Respiratory rhythmic sSNA oscillation amplitudes were quantified by subtracting the mean of the posttrigger voltage (i.e., baseline) from the voltage of each peak or trough appearing in the posttrigger period. Peak and trough amplitudes were expressed as a percent change from the mean value.
Fig. 2.
Effect of water deprivation on respiratory rhythmic sSNA. A: PNA burst-triggered averages of sSNA from a euhydrated control (left) and 48-h WD (right) rat. The timing of neural inspiration is indicated by PNA bursts (shaded dashed lines). B: features of respiratory modulated sSNA are shown in the top left. Examples in A show a prominent IP of sSNA occurred in the control and WD rat. Note that the amplitude was greater in the control than the WD rat. The IP was followed by an early expiratory (postinspiratory) trough (ET) and a small late expiratory peak (EP). Summary data (n = 8/group) in the top right reveal that the mean voltage of sSNA was significantly greater in WD rats (solid bar) compared with controls (open bar), whereas IP amplitude (bottom left) was significantly smaller in WD than control rats. Amplitudes of the early ET and late EP when present in control rats (see text for details) were similar across groups. Bottom right: rhythmicity index values. Note that respiratory modulation of sSNA across the entire respiratory cycle (Total) was significantly less in WD rats compared with controls. The IP was the major contributor to total respiratory modulation in control rats, but its role was significantly less in WD rats. Contributions of the early ET and late EP to overall respiratory modulation were similar across groups, with the late EP playing a minor role in overall respiratory modulation in both groups. Triggered averages of sSNA were constructed from 150 consecutive PNA bursts. Summary data are means ± SE. *P < 0.05 vs. control. †P < 0.0005 vs. control. Δ, Change.
To quantify cardiac rhythmic bursting of sSNA, triggered averages were constructed from ∼1,600 ECG R-waves concurrently recorded with segments of sSNA used for construction of PNA burst-triggered averages. Each R-wave-triggered sSNA average consisted of a 0.3-s posttrigger period (>2 cardiac cycles). Amplitude of the cardiac rhythmic sSNA oscillation was determined as the voltage difference between peaks and troughs (see Fig. 3B, top left).
Fig. 3.
Effect of water deprivation on cardiac rhythmic sSNA. A: representative ECG R-wave-triggered averages of sSNA from a control (left) and 48-h WD (right) rat. Data are from the same rats as in Fig. 2A. Timing of the cardiac cycle is indicated by superimposed ECG traces (shaded dashed lines). B: the top left shows characteristic features of cardiac rhythmic sSNA. Examples in A show that, whereas mean sSNA voltage was greater in the WD than the control rat, cardiac rhythmic sSNA oscillation amplitudes were similar. Summary data (n = 8/group) (top right) show that mean voltage from R-wave-triggered sSNA averages was significantly greater in WD rats (solid bar) compared with euhydrated controls (open bar). However, cardiac rhythmic sSNA oscillation amplitudes were not different across groups (bottom left). Cardiac rhythmicity index values (bottom right) indicate that overall cardiac modulation of sSNA was unchanged by water deprivation. Collectively, results likely reflect similar levels of MAP and HR in control and WD rats (see Table 1). Triggered averages of sSNA in A were constructed using ∼1,600 consecutive R-waves. Summary data are means ± SE. *P < 0.05 vs. control.
Respiratory and cardiac rhythmicity index calculations.
To quantify the overall level of respiratory rhythmicity present in each PNA burst-triggered sSNA average, the area under the curve (AUC) of the inspiratory peak (IP), expiratory (postinspiratory) trough (ET) and expiratory peak (EP) were summed and expressed as a ratio relative to the total AUC. Total AUC was determined as the product of the mean voltage and duration of the posttrigger period (∼1.6 s). Cardiac rhythmicity was calculated from R-wave-triggered sSNA averages by expressing the AUC of the signal oscillation around the mean as a ratio of the total AUC (mean voltage × cardiac cycle duration). All index values are expressed as a percent. Accordingly, a respiratory or cardiac rhythmicity index value of 100% indicates that all recorded sSNA was rhythmic with respect to the trigger event (PNA burst or ECG R-wave). Likewise, an index value of 0% indicates that no component of recorded sSNA was rhythmic with respect to the trigger.
Statistics.
Baseline sSNA MAP, HR, ETCO2, respiratory rate, Pprotein, PosM, and Hct were each compared across control and WD groups with unpaired Student's t-tests. Baseline sSNA as well as amplitudes and durations of respiratory and cardiac rhythmic sSNA bursts were compared across groups with Mann-Whitney U-tests. Statistical tests were performed using Prism software (version 5.0, GraphPad). In all cases, a critical value of P < 0.05 was considered statistically significant. Data in the text and Figs. 1–3 are expressed as means ± SE.
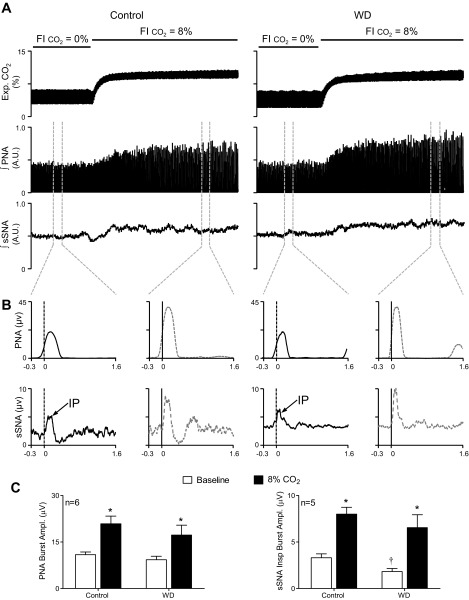
Fig. 1.
Effects of water deprivation on central respiratory drive and respiratory rhythmic splanchnic sympathetic nerve activity (sSNA) burst discharge at baseline and during hypercapnic ventilation. A: expired CO2, integrated phrenic nerve activity (PNA), and sSNA responses to ventilation with 8% fractional inspired CO2 (FiCO2) in a euhydrated control (left) and 48-h water-deprived (WD; right) rat. B: consistent with raw burst data in A, PNA burst-triggered averages of integrated PNA (top row) and integrated sSNA (bottom row) revealed that, compared with baseline (solid black lines), 8% FiCO2 (shaded dashed lines) in the control rat (left graphs) and the WD rat (right graphs) caused similar magnitude increases in the amplitude of the PNA burst and the inspiratory peak (IP) of sSNA. C: summary data from control and WD rats (n = 6/group) show that PNA burst amplitude (left) was similar at baseline (open bars) and was similarly and significantly increased by ventilation with 8% FiCO2 (solid bars). PNA burst-triggered averages of sSNA (right) in control and WD rats (n = 5/group) show that, although IP amplitude at baseline was significantly smaller (open bars) in WD rats, hypercapnic ventilation (solid bars) significantly increased IP amplitude in both groups. Collectively, these data indicate that central respiratory drive at baseline was similar in control and WD rats and that water deprivation did not change response sensitivity to CO2. Summary data are means ± SE. Triggered averages in B were constructed from onset of 40 PNA bursts. *P < 0.01 vs. baseline. †P < 0.05 vs. control. AU, arbitrary units.
RESULTS
Baseline sSNA, hemodynamic, and hematological values.
As expected (4, 5, 14, 33, 34), Table 1 shows that Pprotein, PosM, and Hct were significantly (P < 0.01) greater in 48-h WD rats than euhydrated controls (n = 8/group), indicating that WD rats were both hyperosmotic and hypovolemic. Despite this, baseline MAP and HR were similar across groups, which may be explained by a significantly (P < 0.05) greater level of baseline sSNA recorded in the WD group (Table 1). Our observation of elevated sympathetic outflow in anesthetized WD rats is consistent with a recent study in conscious rats (9).
Table 1.
Baseline hemodynamic, sympathetic nerve activity, and hematological values
| Group | n | MAP, mmHg | HR, beats/min | sSNA, μV | PosM, mosmol/kg | Pprotein, g/dl | Hct, % |
|---|---|---|---|---|---|---|---|
| Control | 8 | 98 ± 4 | 392 ± 11 | 3.8 ± 0.6 | 332 ± 4† | 4.6 ± 0.2 | 47 ± 1 |
| Water deprived | 8 | 96 ± 5 | 386 ± 12 | 6.5 ± 1.0* | 352 ± 3†* | 5.6 ± 0.1* | 55 ± 1* |
Values are means ± SE; n, no. of rats. MAP, mean arterial pressure; HR, heart rate; sSNA, splanchnic sympathetic nerve activity; PosM, plasma osmolality; Pprotein, Plasma protein concentration; Hct, hematocrit. Each variable was compared by one-way ANOVA.
Values are elevated by approximately 30 mosmol due to contribution of urethane-chloralose anesthesia.
P < 0.01 vs. control.
Effects of water deprivation on central respiratory drive.
To determine effects of water deprivation on respiratory rhythmic bursting of sSNA, experiments first quantified and compared the level of central respiratory drive across groups. Figure 1A shows examples of expiratory CO2, integrated PNA, and integrated sSNA recorded from a control (left) and WD (right) rat during ventilation with 100% O2 and after switching to hypercapnic ventilation with 8% CO2 to maximally increase central respiratory drive. Baseline MAP and responses to hypercapnia were similar across groups (data not shown). Figure 1B shows PNA burst-triggered averages of integrated PNA (top row) and integrated sSNA (bottom row) constructed from data shown in Fig. 1A. In both the control and WD rat, PNA burst amplitudes at baseline (100% O2) were ∼50% of their hypercapnic maxima. PNA burst-triggered averages of integrated sSNA constructed concurrently revealed that the IP amplitude increased to a similar degree in both the control and WD rat. Summary data in Fig. 1C indicate that PNA burst amplitudes (left, n = 6/group) at baseline (open bars) were similar between control and WD rats. Hypercapnic ventilation significantly (P < 0.01) increased PNA burst amplitude in both groups. Importantly, increases of PNA burst amplitude were not different between groups. Similarly, group data from PNA burst-triggered averages of sSNA (right, n = 5/group) indicate that, although IP amplitude at baseline was smaller on average in WD compared with control rats (P < 0.05), hypercapnic ventilation similarly and significantly (P < 0.01) increased IP amplitude in both groups. In other experiments, baseline values of ETCO2 in control (5.5 ± 0.1%) and WD (5.5 ± 0.1%) rats (n = 8/group) were identical, as were respiratory rate (i.e., PNA burst frequency) (control, 34 ± 1 beats/min; WD, 34 ± 2 beats/min), inspiratory time (control, 0.49 ± 0.01 s; WD, 0.52 ± 0.02 s), and expiratory time (control, 1.17 ± 0.08 s; WD, 1.06 ± 0.07 s). Collectively, data indicate that, under conditions of these experiments, central respiratory drive was similar among control and WD rats, and that CO2 sensitivity of PNA and sSNA was unaffected by water deprivation.
Effects of water deprivation on respiratory rhythmic sSNA.
Figure 2A shows a representative PNA burst-triggered sSNA average from a control (left) and WD (right) rat. Data were analyzed as shown in Fig. 2B (top left). Note that, in the control rat (Fig. 2A, left), a prominent IP was followed by an early ET and little evidence of a late EP. By contrast, mean voltage of sSNA across the entire respiratory cycle was greater in the WD rat (Fig. 2A, right), and a smaller amplitude IP was observed. Whereas the amplitudes of the ET were similar, the WD rat had a larger amplitude EP compared with the control rat. Group data in Fig. 2B (n = 8/group) indicate that the mean voltage of sSNA (top right) was significantly (P < 0.05) greater in the WD group than controls. Although the IP duration was similar in control (0.31 ± 0.02 s) and WD (0.37 ± 0.03 s) rats, Fig. 2B (bottom left) indicates that the IP amplitude relative to the mean was significantly (P < 0.001) smaller in the WD group (+68 ± 6%) compared with controls (+208 ± 20%). Water deprivation had no effect on either the amplitude or the duration of the ET. The late EP was observed in two of eight control rats and eight of eight WD rats. When present in control rats, however, the EP amplitude was similar to that of WD rats.
A comparison of overall respiratory modulation of sSNA across groups is shown in Fig. 2B, bottom right. Rhythmicity index values calculated over the entire respiratory cycle are shown left of the vertical dashed line (i.e., Total). Values indicate that the overall degree of respiratory modulation of sSNA was a significantly (P < 0.05) smaller percentage of the mean sSNA in WD rats (13.0 ± 1.3%) compared with controls (20.3 ± 2.9%). Data to the right of the vertical dashed line are rhythmicity index values for individual burst components of respiratory rhythmic sSNA. Consistent with WD rats having increased baseline sSNA and reduced IP amplitude, the IP rhythmicity index was significantly (P < 0.005) smaller in WD (4.3 ± 0.4%) compared with control (12.8 ± 1.4%) rats. Rhythmicity index values of the ET (WD, 5.7 ± 0.6%; control, 7.1 ± 1.1%) and EP (WD, 2.9 ± 0.3%, n = 8; control, 1.9 ± 0.1%, n = 2) were not different across groups, indicating that these components of respiratory modulated sSNA contributed similarly to the overall level of sSNA observed in control and WD rats.
Effects of water deprivation on cardiac rhythmic sSNA.
Figure 3A shows an R-wave-triggered average of sSNA from a control (left) and WD (right) rat. Data were analyzed as shown in Fig. 3B (top left). Note that the mean level of sSNA was greater in the WD rat than the control, but the amplitude of the cardiac rhythmic sSNA oscillation was similar. Group data in Fig. 3B (n = 8/group) reveal that the mean of sSNA was significantly (P < 0.05) greater in WD rats than controls (top right), which is consistent with data from PNA burst-triggered averages in Fig. 2B. Cardiac rhythmic sSNA oscillation amplitude was similar in WD (3.4 ± 0.6 μV) and control (2.2 ± 0.3 μV) rats (bottom left), which likely reflects similar baroreceptor reflex entrainment of sSNA, given similar values of resting MAP across groups (Table 1). Period length of the cardiac rhythmic oscillation of sSNA was similar across groups (control, 0.16 ± 0.01 s; WD, 0.16 ± 0.02 s), consistent with similar HR values. Cardiac rhythmicity index values are shown in Fig. 3B, bottom right. Values were similar across groups, indicating that the strength of cardiac modulation of sSNA was similar in WD (n = 8) and control (n = 8) rats.
DISCUSSION
Maintenance of arterial pressure during water deprivation depends on the level of ongoing SNA (4–6, 9, 27, 32–34, 39). Here, burst patterns of sSNA were quantified to determine which rhythmic components might contribute to cardiovascular homeostasis during water deprivation. Results indicate that rats deprived of water for 48 h had an overall increase of sSNA compared with euhydrated controls, but did not exhibit an accompanying increase of either respiratory or cardiac rhythmic burst discharge. Thus maintenance of cardiovascular homeostasis by heightened sympathetic network drive during water deprivation appears to depend on neural modulatory effects that selectively increase nonrespiratory and noncardiac rhythmic components of ongoing SNA. It should be noted that this conclusion is predicated on the assumption that the rhythmic patterning of sSNA reported here is representative of that which occurs among other sympathetic nerves during water deprivation. This remains to be demonstrated.
The increase of ongoing sSNA observed among WD rats is consistent with evidence of robust sympathetic network activation during dehydration (4, 6, 7, 9, 27, 29, 32–34, 36, 39). Sympathetic activation during water deprivation has a functionally significant impact on cardiovascular function. This conclusion is supported by Colombari et al. (9), who recently reported that MAP in conscious rats increases progressively during a 48-h period of water deprivation. Consistent with the present findings, these authors performed power analysis of systolic blood pressure variations and concluded that water deprivation significantly increased resting sympathetic discharge.
In the present study, we assessed the extent of overall respiratory modulation of sSNA. This was achieved by calculating a respiratory rhythmicity index from PNA burst-triggered averages of sSNA. Contrary to our hypothesis, this analysis revealed that water deprivation reduced, rather than increased, overall respiratory modulation of sSNA. Further analysis showed that water deprivation reduced the amplitude of the inspiratory burst of sSNA without affecting its duration and without significantly impacting the pattern of sSNA that occurred during expiratory phase of the respiratory cycle. Indeed, neither the early ET nor the late expiratory burst of sSNA differed across control and WD rats. A caveat to the latter conclusion is that a small but detectable EP of sSNA was consistently (8/8) observed in WD rats, but was seen infrequently (2/8) in euhydrated controls. This notwithstanding, the small amplitude of sSNA bursts that occurred more often in WD rats is not sufficient to account for an overall elevation of sSNA in this group, especially when considered together with the significant reduction of inspiratory rhythmic burst of sSNA in the WD group. Thus the overall decrease of respiratory rhythmicity of sSNA in WD rats appears to mostly reflect the observed decreased in the amplitude of inspiratory bursts.
It should be noted that the observed decrease of inspiratory bursting of sSNA in WD rats conflicts with a previous study (9), which reported that respiratory modulation of SNA was unchanged in WD rats. A possible explanation for this difference is that the earlier study was performed in conscious rats, while our rats were anesthetized with urethane-chloralose. Alternatively, we assessed respiratory modulation of SNA from PNA burst-triggered averages of directly recorded sSNA, whereas they used changes in the high-frequency component of the systolic blood pressure power spectrum to assess respiratory modulation. To our knowledge, the relative sensitivity of these two methods to detect changes in inspiratory vs. expiratory phase modulation of SNA has not been established.
It is important to emphasize that our observation of reduced respiratory rhythmicity of sSNA in WD rats could simply reflect a decrease of central respiratory drive compared with control rats. However, experiments using hypercapnic ventilation (see Fig. 1) indicate that this was not the case. Further support for this conclusion comes from data showing that baseline ETCO2, inspiratory time, and expiratory time, were all similar among control and WD rats. As noted above, small expiratory phase sSNA bursts were more frequently observed in WD rats, and admittedly this could reflect increased central respiratory drive. As discussed above, however, even with this being the case, greater expiratory phase sSNA is not sufficient to account for the observed increase of ongoing sSNA in WD rats.
Mechanisms underlying reduced inspiratory bursting of sSNA during water deprivation remain to be determined. One possibility is that presympathetic neurons of WD rats might have blunted responses to inspiratory rhythmic excitation from respiratory network inputs. Inspiratory bursts of SNA are dominantly driven by sympathoexcitatory neurons of the RVLM, which receive excitatory inputs from inspiratory pre-Bötzinger neurons (23). Whether or not individual RVLM neurons exhibit blunted inspiratory rhythmic discharge in WD rats has not been directly tested. Available evidence suggests that this might not be the case, since excitatory glutamatergic tonus and neuronal activity in RVLM are increased, not decreased, in WD rats (4, 32). Another argument against water deprivation-induced blunting of excitatory responses in RVLM stems from studies in salt-loaded rats, which have increased PosM akin to that produced by water deprivation. Salt-loaded rats have enhanced, not diminished, pressor and sympathoexcitatory responses to RVLM injection of l-glutamate (1, 17, 35). Additional studies are clearly needed to determine whether water deprivation produces a similar affect.
It is also possible that reduced inspiratory bursting of sSNA in WD rats could arise from an increase of convergent inspiratory rhythmic inhibition of sympathoexcitatory neurons. A possible source of such inhibition is GABAergic neurons in the caudal ventrolateral medulla, which have been recently reported to have increased activity during acute hypoxia (21). Whether these or other such neurons are activated during water deprivation remains untested.
A final consideration is that decreased inspiratory burst amplitude in WD rats could simply reflect the overall increase of sSNA that was observed during noninspiratory phases of respiratory cycle. This could simply have made the amplitude of the IP of sSNA appear smaller relative to baseline. Although our results do not entirely exclude this possibility, the smaller IP amplitude recorded at baseline in WD rats was clearly not due to a ceiling effect, as hypercapnic ventilation not only increased overall sSNA (Fig. 1A), but also increased IP amplitude similarly in control and WD rats (Fig. 1, B and C). Neural mechanisms that generate and regulate the level of nonrhythmic sSNA are not well described, but electrophysiological data indicate that neurons with tonic discharge are interspersed in the RVLM among neurons with respiratory rhythmic discharge (16, 24). Moreover, up to 50% of spinal sympathetic preganglionic neurons exhibit tonic firing that is neither respiratory nor cardiac rhythmic (16, 44). Even among respiratory rhythmic RVLM neurons, rhythmic discharges can be superimposed on a background of tonic (nonrhythmic) firing (12).
Sources of tonic drive to RVLM that are activated during water deprivation have not been identified, but one possibility is the hypothalamic PVN, where ∼25% of neurons projecting to RVLM have been documented to exhibit sympathetic-related discharge that is, nevertheless, tonically patterned (8). This possibility seems particularly intriguing, given literature evidence that PVN neuronal activity is required for maintenance of ongoing SNA and MAP in WD rats (14, 32–34, 36). It remains to be determined, however, if the subgroup of PVN-RVLM neurons with tonic sympathetic-related discharge is activated by water deprivation.
Previous reports indicate that baroreflex gain and its operating range are unaffected in conscious WD rabbits (42). This is consistent with the present study, which showed that water deprivation did not affect the amplitude of the cardiac rhythmic sSNA oscillation. The latter finding is not unexpected, given that baseline MAP and HR were similar in WD and control rats, suggesting that baroreceptor afferent input and baroreflex function overall were similar across groups.
The significance of the present findings lay in determining the extent to which specific rhythmic bursts of SNA contribute to regulation of cardiovascular function. Recent studies in rats made hypertensive by treatment with angiotensin II and a high-salt diet (41) or by exposure to chronic intermittent hypoxia (45, 46) have provided important insight in that both disease models exhibit exaggerated SNA bursting during expiration. These findings extend those of Simms et al. (30, 31) who provided key initial evidence to support the concept that respiratory modulation of SNA is an important factor in development of exaggerated neurogenic vasomotor tone in juvenile spontaneously hypertensive rats.
In summary, rats deprived of water for 48 h exhibited an increase of ongoing sSNA that could not be explained by an increase of either respiratory or cardiac rhythmic burst discharge. An overall increase of sSNA was observed in WD rats, despite having reduced inspiratory rhythmic burst amplitude, together with little or no change of either expiratory rhythmic or cardiac rhythmic activity. Over the past several years, studies have spawned a resurgence of interest in the role that respiratory rhythmic bursting of SNA might play in regulation of arterial blood pressure, particularly as a mechanism contributing to neurogenic forms of arterial hypertension (25, 30, 31, 41, 45, 46). Within this context, the present findings suggest that SNA that is not rhythmic with respect to either the respiratory or the cardiac cycle could be an additional important factor in cardiovascular regulation and control of arterial pressure. Additional studies are needed to further validate this concept and to determine the relative contributions of cardiac, respiratory, and nonrhythmic components of SNA to maintenance of homeostasis during acute physiological challenges (hemorrhage, hypoxia, cold stress, etc.), as well as chronic cardiovascular/metabolic diseases (hypertension, heart failure, renal disease, obesity, diabetes, etc.).
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants R01 HL-102310 and PO1 HL-088052 (G. M. Toney).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.W.H. and G.M.T. conception and design of research; W.W.H. and G.M.T. performed experiments; W.W.H. and G.M.T. analyzed data; W.W.H. and G.M.T. interpreted results of experiments; W.W.H. and G.M.T. prepared figures; W.W.H. and G.M.T. drafted manuscript; W.W.H. and G.M.T. edited and revised manuscript; W.W.H. and G.M.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Mary Ann Andrade, Alfredo S. Calderon, and Myrna Herrera-Rosales for excellent technical assistance.
REFERENCES
- 1. Adams JM, Bardgett ME, Stocker SD. Ventral lamina terminalis mediates enhanced cardiovascular responses of rostral ventrolateral medulla neurons during increased dietary salt. Hypertension 54: 308–314, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adrian ED, Bronk DW. Discharges in mammalian sympathetic nerves. J Physiol 74: 115–133, 1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baekey DM, Dick TE, Paton JF. Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp Physiol 93: 803–816, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Brooks VL, Freeman KL, Clow KA. Excitatory amino acids in rostral ventrolateral medulla support blood pressure during water deprivation in rats. Am J Physiol Heart Circ Physiol 286: H1642–H1648, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Brooks VL, Freeman KL, O'Donaughy TL. Acute and chronic increases in osmolality increase excitatory amino acid drive of the rostral ventrolateral medulla in rats. Am J Physiol Regul Integr Comp Physiol 287: R1359–R1368, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Brooks VL, Qi Y, O'Donaughy TL. Increased osmolality of conscious water-deprived rats supports arterial pressure and sympathetic activity via a brain action. Am J Physiol Regul Integr Comp Physiol 288: R1248–R1255, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Burnier M, Biollaz J, Brunner DB, Brunner HR. Blood pressure maintenance in awake dehydrated rats: renin, vasopressin, and sympathetic activity. Am J Physiol Heart Circ Physiol 245: H203–H209, 1983 [DOI] [PubMed] [Google Scholar]
- 8. Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience 118: 797–807, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Colombari DS, Colombari E, Freiria-Oliveira AH, Antunes VR, Yao ST, Hindmarch C, Ferguson AV, Fry M, Murphy D, Paton JF. Switching control of sympathetic activity from forebrain to hindbrain in chronic dehydration. J Physiol 589: 4457–4471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Czyzyk-Krzeska MF, Trzebski A. Respiratory-related discharge pattern of sympathetic nerve activity in the spontaneously hypertensive rat. J Physiol 426: 355–368, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Darnall RA, Guyenet P. Respiratory modulation of pre- and postganglionic lumbar vasomotor sympathetic neurons in the rat. Neurosci Lett 119: 148–152, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Dick TE, Hsieh YH, Morrison S, Coles SK, Prabhakar N. Entrainment pattern between sympathetic and phrenic nerve activities in the Sprague-Dawley rat: hypoxia-evoked sympathetic activity during expiration. Am J Physiol Regul Integr Comp Physiol 286: R1121–R1128, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Freeman KL, Brooks VL. AT(1) and glutamatergic receptors in paraventricular nucleus support blood pressure during water deprivation. Am J Physiol Regul Integr Comp Physiol 292: R1675–R1682, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Guyenet PG, Darnall RA, Riley TA. Rostral ventrolateral medulla and sympathorespiratory integration in rats. Am J Physiol Regul Integr Comp Physiol 259: R1063–R1074, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol Regul Integr Comp Physiol 256: R739–R750, 1989 [DOI] [PubMed] [Google Scholar]
- 17. Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 276: R1600–R1607, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Kc P, Balan KV, Tjoe SS, Martin RJ, Lamanna JC, Haxhiu MA, Dick TE. Increased vasopressin transmission from the paraventricular nucleus to the rostral medulla augments cardiorespiratory outflow in chronic intermittent hypoxia-conditioned rats. J Physiol 588: 725–740, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mack SO, Kc P, Wu M, Coleman BR, Tolentino-Silva FP, Haxhiu MA. Paraventricular oxytocin neurons are involved in neural modulation of breathing. J Appl Physiol 92: 826–834, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Mack SO, Wu M, Kc P, Haxhiu MA. Stimulation of the hypothalamic paraventricular nucleus modulates cardiorespiratory responses via oxytocinergic innervation of neurons in pre-Botzinger complex. J Appl Physiol 102: 189–199, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mandel DA, Schreihofer AM. Modulation of the sympathetic response to acute hypoxia by the caudal ventrolateral medulla in rats. J Physiol 587: 461–475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyawaki T, Goodchild AK, Pilowsky PM. Evidence for a tonic GABA-ergic inhibition of excitatory respiratory-related afferents to presympathetic neurons in the rostral ventrolateral medulla. Brain Res 924: 56–62, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Miyawaki T, Minson J, Arnolda L, Chalmers J, Llewellyn-Smith I, Pilowsky P. Role of excitatory amino acid receptors in cardiorespiratory coupling in ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 271: R1221–R1230, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Miyawaki T, Pilowsky P, Sun QJ, Minson J, Suzuki S, Arnolda L, Llewellyn-Smith I, Chalmers J. Central inspiration increases barosensitivity of neurons in rat rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 268: R909–R918, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Moraes DJ, Zoccal DB, Machado BH. Medullary respiratory network drives sympathetic overactivity and hypertension in rats submitted to chronic intermittent hypoxia. Hypertension 60: 1374–1380, 2012 [DOI] [PubMed] [Google Scholar]
- 26. Numao Y, Koshiya N, Gilbey MP, Spyer KM. Central respiratory drive-related activity in sympathetic nerves of the rat: the regional differences. Neurosci Lett 81: 279–284, 1987 [DOI] [PubMed] [Google Scholar]
- 27. O'Donaughy TL, Qi Y, Brooks VL. Central action of increased osmolality to support blood pressure in deoxycorticosterone acetate-salt rats. Hypertension 48: 658–663, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal-cord in the rat. J Comp Neurol 205: 260–272, 1982 [DOI] [PubMed] [Google Scholar]
- 29. Scrogin KE, Grygielko ET, Brooks VL. Osmolality: a physiological long-term regulator of lumbar sympathetic nerve activity and arterial pressure. Am J Physiol Regul Integr Comp Physiol 276: R1579–R1586, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Simms AE, Paton JF, Allen AM, Pickering AE. Is augmented central respiratory-sympathetic coupling involved in the generation of hypertension? Respir Physiol Neurobiol 174: 89–97, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol 587: 597–610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 287: R1172–R1183, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol 563: 249–263, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am J Physiol Regul Integr Comp Physiol 286: R719–R725, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Stocker SD, Madden CJ, Sved AF. Excess dietary salt intake alters the excitability of central sympathetic networks. Physiol Behav 100: 519–524, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol 494: 673–685, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stricker EM, Huang W, Sved AF. Early osmoregulatory signals in the control of water intake and neurohypophyseal hormone secretion. Physiol Behav 76: 415–421, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Sun MK, Guyenet PG. GABA-mediated baroreceptor inhibition of reticulospinal neurons. Am J Physiol Regul Integr Comp Physiol 249: R672–R680, 1985 [DOI] [PubMed] [Google Scholar]
- 39. Thornton RM, Proppe DW. Influence of vasoconstrictor systems on leg vasodilation during heating of dehydrated baboons. Am J Physiol Heart Circ Physiol 254: H11–H19, 1988 [DOI] [PubMed] [Google Scholar]
- 40. Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand 177: 43–55, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Toney GM, Pedrino GR, Fink GD, Osborn JW. Does enhanced respiratory-sympathetic coupling contribute to peripheral neural mechanisms of angiotensin II-salt hypertension? Exp Physiol 95: 587–594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trapani AJ, Undesser KP, Keeton TK, Bishop VS. Neurohumoral interactions in conscious dehydrated rabbit. Am J Physiol Regul Integr Comp Physiol 254: R338–R347, 1988 [DOI] [PubMed] [Google Scholar]
- 43. Veitenheimer BJ, Engeland WC, Guzman PA, Fink GD, Osborn JW. Effect of global and regional sympathetic blockade on arterial pressure during water deprivation in conscious rats. Am J Physiol Heart Circ Physiol 303: H1022–H1034, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou SY, Gilbey MP. Respiratory-related activity of lower thoracic and upper lumbar sympathetic preganglionic neurones in the rat. J Physiol 451: 631–642, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zoccal DB, Machado BH. Coupling between respiratory and sympathetic activities as a novel mechanism underpinning neurogenic hypertension. Curr Hypertens Rep 13: 229–236, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Zoccal DB, Paton JF, Machado BH. Do changes in the coupling between respiratory and sympathetic activities contribute to neurogenic hypertension? Clin Exp Pharmacol Physiol 36: 1188–1196, 2009 [DOI] [PubMed] [Google Scholar]