Abstract
Heat stress is associated with increased fatigue perception and decrements in function for individuals with multiple sclerosis (MS). Similarly, healthy individuals experience decrements in exercise performance during hyperthermia. Alterations in central nervous system (CNS) function during hyperthermia include reduced voluntary activation of muscle and increased effort perception. The purpose of this investigation was to test the hypothesis that passive heat exposure in MS patients will produce increased subjective fatigue and impairments in physiological measures of central conduction and cortical excitability compared with healthy individuals. Eleven healthy individuals and 11 MS patients completed a series of transcranial magnetic stimulation studies to examine central conduction and cortical excitability under thermoneutral and heat-stressed (HS) conditions at rest and after a fatiguing thumb abduction task. Passive heat stress resulted in significantly greater fatigue perception and impairments in force production in MS patients. Central motor conduction time was significantly shorter during HS in controls; however, in MS patients normal increases in conduction velocity with increased temperature were not observed centrally. MS patients also exhibited decreased cortical excitability during HS, evidenced by significant increases in resting motor threshold, decreased MEP amplitude, and decreased recruitment curve slope. Both groups exhibited postexercise depression of MEP amplitude, but the magnitude of these decrements was amplified in MS patients during HS. Taken together, these results suggest that CNS pathology in MS patients played a substantial role in reducing cortical excitability during HS.
Keywords: passive hyperthermia, recruitment curve, transcranial magnetic stimulation
high internal temperature, ranging from 38.6 to 40.3°C, is associated with voluntary exhaustion during aerobic exercise, despite variations in baseline core temperature, heat storage rates, and final skin temperature (4, 10). Similarly, hyperthermia has been shown to produce motor fatigue, evidenced by decreased voluntary activation during sustained maximal voluntary contractions (MVC) (16, 34). Cardiovascular strain during thermal stress contributes to the development of fatigue; however, evidence also suggests that hyperthermia alters central nervous system (CNS) functions, resulting in alterations in voluntary activation of muscle, as well as changes in perception of effort (18, 24, 33). Little is known about the effects of moderate core temperature increases, in the range between 0.5 to 1.0°C, on CNS function in healthy individuals, although some evidence suggests that decrements in function may begin to appear when core temperatures exceed normal levels (18, 24, 33).
Individuals with multiple sclerosis (MS), a demyelinating disease of the CNS, commonly experience fatigue that is exacerbated by relatively small (0.5°C) increases in core temperature (11). It has been hypothesized that heat-related fatigue in MS is a form of central fatigue, produced by conduction slowing and/or block in areas of demyelination in the corticospinal system (11, 29). It would therefore be expected that thermal stress would produce increases in fatigue perception and exaggerated decrements in task performance in MS patients compared with healthy individuals. However, experimental support for this hypothesis is derived from studies of demyelinated peripheral nerve (11, 22).
Transcranial magnetic stimulation (TMS) is a noninvasive technique that has been used to study conduction properties of the corticospinal tract and excitability of the motor cortex, as well as CNS changes in response to thermal stress. Comparing motor-evoked potential amplitude (MEPamp) before and after fatiguing exercise is one method used to examine central fatigue (5). Postexercise depression (PED), a generalized decrease of MEPamp following fatiguing exercise, has been demonstrated in healthy individuals (1, 2, 13, 28) and MS patients (14, 21) but has not been examined with respect to thermal stress. Cortical excitability can be examined through analysis of the recruitment curve, in which MEPamp are assessed relative to increases in stimulation intensity. The slope of this recruitment curve is a function of cortical excitability (24). Additionally, assessment of changes in the cortical silent period (SP), the interruption of ongoing EMG activity induced by motor cortex stimulation, may indicate altered inhibitory activity to corticospinal neurons (31). Therefore, the primary aim of the present investigation was to test the hypothesis that passive heat exposure in MS patients will lead to increased subjective fatigue and impairments in physiological measures of central conduction and cortical excitability compared with healthy individuals.
METHODS
Subjects
Eleven healthy individuals and 11 individuals with definite relapsing-remitting MS (all between 18 and 55 yr of age) were studied (Table 1). All MS patients demonstrated stable disease condition for the 3 mo preceding the study and had no spasticity or hyperreflexia in the upper extremities. Each MS patient was matched to a control for gender, age (within 5 yr), body mass index (BMI, within 3 units), and physical activity (frequency ±1 day/wk and duration ±30 min/session). Physical activity was assessed by interview. All subjects provided written informed consent to participate in the protocol that was approved by the University of Utah Institutional Review Board. MS patients were examined according to the outline of the Minimal Record of Disability for Multiple Sclerosis (30), and patients with an Expanded Disability Status Scale score between one and six, with self-reported fatigue exacerbated by thermal stress participated. The Fatigue Impact Scale (8) was used to assess the overall effect of subjective fatigue, as well as effects on cognitive, physical, and social functions.
Table 1.
Subject characteristics
| Control | MS | |
|---|---|---|
| n | 11 (7 F) | 11 (7 F) |
| Age, yr | 37.4 ± 9.6 | 38.9 ± 6.9 |
| BMI, kg/m2 | 23.5 ± 3.6 | 27.1 ± 4.9 |
| Physical activity frequency, days/wk | 3.6 ± 2.3 | 3.8 ± 3.0 |
| Physical activity duration, min | 42 ± 18.6* | 24 ± 17.8 |
| FIS | ||
| Total | 10 ± 9.2* | 61 ± 39.1 |
| Cognitive | 3.6 ± 3.3* | 15.5 ± 12.4 |
| Physical | 2.0 ± 2.3* | 17.3 ± 7.5 |
| Social | 4.3 ± 4.2* | 27.7 ± 21.2 |
| Years since MS diagnosis | NA | 5.6 ± 2.0 |
| EDSS | NA | 1.9 (1.0 −3.0) |
Values are means ± SD; n, no. of subjects. MS, multiple sclerosis; F, females; BMI, body mass index; FIS, Fatigue Impact Scale; EDSS, Expanded Disability Status Scale.
Significant difference from MS, P < 0.05. NA, not available.
Instrumentation
Heart rate was obtained from an electrocardiogram (Biopac System, Santa Barbara, CA). Internal temperature was indexed from an ingestible capsule telemetry system (HTI Technologies, Palmetto, FL). The telemetry pill correlates well with other methods of internal temperature measurement (20). Mean skin temperature was measured via the weighted average of four thermocouples (chest, back, thigh, and calf) taped to the skin.
Measures of Central Conduction and Cortical Excitability
Nerve conduction.
Median nerve conduction from elbow to wrist was assessed. Muscle responses were recorded from the abductor pollicus brevis (APB) with 1-cm silver disc electrodes in a belly-tendon montage. EMG responses were displayed using a Synergy (Viasys) EMG system. Sweep speed was set at 5–10 ms/div and amplification set at 5,000 for compound muscle action potential (CMAP) and 500–1,000 for motor-evoked potentials, filtered at a band pass of 10–2,000 Hz. Peak-to-peak CMAP amplitude of APB resulting from supramaximal stimulation at the wrist was determined, as well as distal motor latency. Median nerve conduction velocity was calculated from stimulation at the wrist and elbow. Minimum F-wave latency from 20 trials was recorded for subsequent central motor conduction time (CMCT) calculations.
TMS.
A Magstim 2002 (Wales, UK) magnetic stimulator and a 70-mm double coil were used for magnetic stimulation. Subjects were tested in a semireclined supine position and were right hand dominant, except for one left-handed control. For each subject, an estimate for the intrinsic hand muscle area in the motor cortex was marked on a snug-fitting swim cap 5 cm lateral to the vertex. Stimuli were centered over this point and in the surrounding region to determine the site at which TMS evoked a maximal motor response in the right APB muscle. With the magnet over this “hot spot,” an outline of the stimulator was traced on the cap to mark the proper position for subsequent TMS studies. Resting motor threshold (RMT) was determined by gradually increasing stimulus intensity, beginning at 50% maximal stimulator output, until a detectable MEP (50 μV) was elicited in 4–6 out of 10 stimuli (3). Baseline MEPs were recorded at 120% RMT from the resting right APB muscle. Right APB muscle activity was monitored to ensure EMG silence during the trials. Fifteen MEPs, recorded at 10-s intervals, were obtained for analysis of CMCT and peak-to-peak MEPamp. CMCT was calculated as follows (26):
where MEPlat is shortest MEP latency of 15 trials recorded from the APB, DML is distal motor latency determined from supramaximal median nerve stimulation at the wrist, and FW is minimum F-wave latency from 20 trials.
Recruitment curves.
MEP recruitment curves were determined from stimulus intensities of 100, 110, 120, and 130% of RMT, as described by Chen et al. in 1998 (3). At each stimulus intensity, 12–15 responses were recorded at 15-s intervals and were subsequently expressed relative to the peak baseline CMAP amplitude obtained during the corresponding thermal condition. Averaged values were used to plot recruitment curves for MS patients and controls during heat stress (HS) and thermoneutral (TN) conditions. Recruitment curve slope was determined in each subject during HS and TN conditions from the steepness of the regression line through the four data points (25).
Cortical SP.
Stimulation intensity was increased to 1.5 times RMT and delivered during antigravity preactivation of the right APB muscle, in which subjects were asked to fully abduct the thumb for each of the SP recordings (12). This level of activation was estimated to be ∼15–20% of MVC. Fifteen recordings, obtained at 10-s intervals, were averaged for determination of SP duration.
Motor fatigue task.
The motor fatigue task, consisting of 3 min of sustained, maximal-effort thumb abduction exercise, followed the baseline TMS studies. Thumb abduction force was measured using a fixed platform containing a force transducer positioned over the thumb and hand, and the forearm and hand were supported in a fixed supinated position. Force data were acquired at 20 Hz and stored to a DataPac data acquisition system for later analysis of peak force and elapsed time to reach 50% force reduction (T50%).
PED.
Immediately following the motor fatigue task, TMS was applied at 120% RMT at 1, 4, 6, 8, and 10 min postexercise. Sets of eight MEPs, which were later averaged, were obtained at each of these times at an interstimulus time interval of 15 s.
Protocol
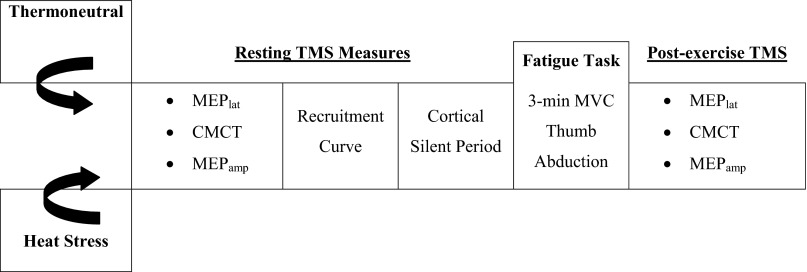
Measures of central conduction and cortical excitability were performed during TN and mild whole body HS trials in randomized order and separated by at least 1 wk. A protocol schematic is illustrated in Fig. 1. Upon arrival to the laboratory, each study volunteer swallowed the telemetry pill and was instrumented for heart rate and skin temperature measurements. Next, the individual was dressed in a tube-lined perfusion suit that permitted the control of body temperature by changing the temperature of water circulating through the suit (Med-Eng, Ottawa, Canada). The perfusion suit covered the entire body with the exception of the head, hands, and feet. On average, suit perfusion began 15 min after ingestion of the telemetry pill. During the HS condition, 46°C water was perfused through the suit until internal temperature increased ∼0.6°C (range: 0.5–0.8°C) over 45–60 min. Measures of central conduction and cortical excitability were performed while maintaining internal temperature constant. Following the HS trial, body temperature was normalized to baseline by perfusing cool water through the tube-lined suit. During the TN condition, 34°C water was perfused for a standard time period of 60 min to approximate the time required to increase core temperature during the HS trial. Following the standard time period, measures of central conduction and cortical excitability were performed while core temperature was maintained at resting levels.
Fig. 1.
Schematic of protocol. All tests were performed during thermoneutral and heat-stressed conditions. TMS, transcranial magnetic stimulation; MEPlat, motor-evoked potential latency; CMCT, central motor conduction time; MEPamp, motor-evoked potential amplitude; MVC, maximal voluntary contraction.
Heart rate, core temperature, skin temperatures (chest, back, thigh, and calf), and blood pressure were continuously monitored during each trial. A nine-point thermal sensation (0 = very cold to 8 = very hot) and a five-point thermal discomfort (1 = comfortable to 5 = intolerable) were used to determine the thermal comfort at 10-min intervals throughout each trial (6). In addition, subjective ratings of fatigue were recorded using a 0–100 visual analog scale. MS patients were also monitored, after the HS trial, until body temperature returned to baseline levels and any signs and symptoms had resolved.
Data Analysis
Group differences in physical characteristics and underlying fatigue levels were identified using unpaired t-tests. Thermal, cardiovascular, and subjective responses as well as baseline electrophysiological responses were examined with 2 (group) × 2 (treatment) repeated-measures (RM) ANOVA.
Pre- and postfatigue task MEPlat and CMCT were initially analyzed using 2 (group) × 2 (treatment) × 2 (time) RM ANOVA. Examination of within-group treatment and time effects was assessed with separate 2 × 2 RM ANOVAs for controls and MS patients. Treatment and group differences in peak force and the T50% were assessed using 2 × 2 RM ANOVA.
Serial MEPs obtained for recruitment curve analysis and PED were analyzed with 2 (group) × 2 (treatment) × 4 or 6 (time) RM ANOVAs that determined main effects and interactions. To specifically examine within-group treatment and time effects, separate RM ANOVAs were used. Planned contrasts were built into the model to examine time and interaction (treatment by time) effects relative to baseline values. Significance was set at P < 0.05. All data are presented as means ± SD.
RESULTS
Subject Characteristics
Although each MS patient was matched to a healthy control for age (within 5 yr), BMI (within 3 units), and physical activity (frequency within 1 day/wk; duration within 30 min/session), the MS group on average had higher BMI and participated in significantly less physical activity than controls (Table 1). Additionally, underlying fatigue levels were significantly higher in MS patients compared with controls (Table 1). In general, MS patients represented an active and mild to moderately impaired subgroup of MS patients.
Thermal, Cardiovascular, and Subjective Responses
The HS condition produced significant mean increases in core temperature (0.6°C) and in skin temperature (3.3 and 3.7°C for healthy controls and MS patients) (P < 0.001, Table 2). These responses were paralleled by significant HS treatment effects for thermal sensation (hotter) and thermal comfort (more uncomfortable) in both groups (P < 0.001). Self-reported fatigue increased during HS in both healthy controls and MS patients (P < 0.001), although MS patients reported significantly greater increases in fatigue (0.015). For both groups, heart rate was significantly higher and mean arterial pressure (MAP) was significantly lower during HS (P < 0.001). MAP was significantly higher in MS patients than controls during both treatments (P < 0.05).
Table 2.
Thermal, cardiovascular, and subjective responses during HS and TN conditions
| Control |
MS |
RM ANOVA |
||||
|---|---|---|---|---|---|---|
| HS | TN | HS | TN | Group P value | Treatment P value | |
| Tcore, °C | 37.6 ± 0.25† | 37.0 ± 0.37 | 37.6 ± 0.21† | 37.0 ± 0.35 | 0.850 | <0.001 |
| Mean Tskin, °C | 37.0 ± 0.46† | 33.8 ± 0.91 | 37.3 ± 0.64† | 33.7 ± 1.41 | 0.767 | <0.001 |
| Thermal sensation | 6.9 ± 0.85† | 3.7 ± 0.80* | 7.3 ± 0.61† | 4.5 ± 0.67 | 0.025 | <0.001 |
| Thermal comfort | 3.2 ± 1.01† | 1.1 ± 0.31 | 3.9 ± 1.05† | 1.3 ± 0.45 | 0.107 | <0.001 |
| Fatigue (0–100) | 21 ± 19.5*† | 10 ± 11.0 | 48 ± 27.2† | 18 ± 16.9 | 0.029 | <0.001 |
| Heart rate, beats/min | 83 ± 12.6† | 65 ± 8.3 | 88 ± 13.5† | 71 ± 8.9 | 0.238 | <0.001 |
| MAP, mmHg | 79 ± 6.0*† | 82 ± 9.7* | 88 ± 5.4† | 90 ± 8.6 | 0.007 | 0.070 |
Values are means ± SD. RM, repeated measures; HS, heat stress; TN, thermoneutral; Tcore, core temperature; mean Tskin, mean skin temperature; MAP, mean arterial pressure.
Significant difference from MS for corresponding condition, P < 0.05.
Significant within-group difference from TN condition, determined by post hoc analysis.
Force
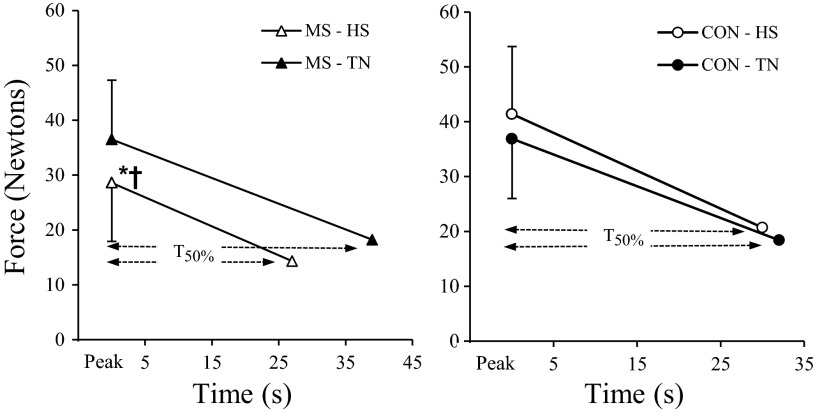
A significant overall treatment by group interaction was observed for peak force [F(1,17) = 11.73, P = 0.003], which was due to divergent peak force results during HS, with controls showing a small increase [12%, F(1,8) = 3.44, P = 0.101] and MS patients demonstrating a significant decrease of 22% [F(1,9) = 8.90, P = 0.015] (see Fig. 2). For T50%, a significant overall treatment effect was observed [F(1,17) = 5.15, P = 0.037]. Post hoc analyses indicated that T50% was not affected by the thermal condition in controls [F(1,8) = 0.52, P = 0.523], but, in MS patients, HS significantly reduced T50% compared with TN [F(1,9) = 5.43, P = 0.045, Fig. 2]. The rate of force decline was the same for HS and TN conditions in MS patients due to treatment differences in peak force.
Fig. 2.
Mean (+SD) peak force measurements and elapsed time to reach 50% force reduction (T50%) for multiple sclerosis (MS) patients and healthy controls (CON) during thermoneutral (TN) and heat stress (HS) conditions. *Significantly lower peak force during HS condition, P < 0.05. †Significantly shorter T50% during HS condition, P < 0.05.
Electrophysiological Measures
Resting measures.
There were no significant overall group differences for any resting electrophysiological measure (Table 3). However, significant overall treatment effects were seen for CMAP latency [F(1,20) = 15.23, P = 0.001], F-wave latency [F(1,20) = 10.44, P = 0.004], median nerve conduction velocity [F(1,20) = 15.02, P = 0.001], and RMT [F(1,20) = 13.21, P = 0.002]. Post hoc analyses indicated that HS resulted in significantly shorter CMAP latency (P = 0.005) and faster median nerve conduction velocity (P = 0.003) in controls. The HS condition resulted in decreased F-wave latency and increased RMT in both groups but reached statistical significance only in MS patients (P = 0.046 and 0.094 for F-wave latency and 0.013 and 0.068 for RMT in MS patients and controls, respectively). No group or treatment differences were observed for cortical SP duration.
Table 3.
Resting electrophysiological responses to HS and TN conditions
| Control |
MS |
RM ANOVA |
||||
|---|---|---|---|---|---|---|
| HS | TN | HS | TN | Group P value | Treatment P value | |
| CMAP amplitude, mV | 13.1 ± 3.7 | 14.7 ± 4.2 | 12.4 ± 3.2 | 11.8 ± 3.5 | 0.241 | 0.492 |
| CMAP latency, ms | 3.22 ± 0.49* | 3.81 ± 0.61 | 3.23 ± 0.75 | 3.61 ± 0.51 | 0.589 | 0.004 |
| Min F latency, ms | 26.38 ± 1.56 | 26.96 ± 1.61 | 25.63 ± 1.74* | 27.08 ± 1.57 | 0.711 | 0.008 |
| Median NCV, m/s | 61.4 ± 6.3* | 57.6 ± 4.8 | 58.5 ± 6.4 | 57.1 ± 5.9 | 0.495 | 0.001 |
| SP Duration, ms | 182 ± 13.8 | 182 ± 11.6 | 188 ± 12.9 | 189 ± 17.6 | 0.262 | 0.907 |
| RMT, % | 61.0 ± 6.0 | 60.2 ± 6.6 | 63.1 ± 8.0* | 61.5 ± 7.7 | 0.583 | 0.002 |
Values are means ± SD. Overall RM ANOVA results illustrate Group effects (across HS and TN conditions) and Treatment effects (across groups). Post hoc within-group differences between conditions are indicated. CMAP, compound muscle action potential; Min F, minimum F-wave latency of 20 trials; NCV, nerve conduction velocity; SP, cortical silent period; RMT, resting motor threshold.
Significant within-group difference from TN condition, determined by post hoc analysis.
Pre-post motor fatigue task measures.
The overall RM ANOVA indicated no significant group differences for MEPlat [F(1,20) = 0.88, P = 0.357] or CMCT [F(1,20) = 0.06, P = 0.812]. Separate analyses to examine treatment and time effects were performed for MS patients and controls. Significant within-group treatment effects for MEPlat were seen in MS patients [F(1,10) = 9.33, P = 0.012] and controls [F(1,10) = 12.99, P = 0.005], with shorter latencies recorded during HS. For CMCT the treatment effect, decreased latency during HS, was significant in controls [F(1,10) = 11.46, P = 0.007] but not MS patients [F(1,10) = 0.74, P = 0.410, Table 4]. There were no significant time (pre- to postmotor fatigue task) effects for MEPlat or CMCT in controls [F(1,10) < 0.06, P > 0.807, Table 4]. For MS patients, CMCT and MEPlat increased after the fatigue task; however, neither reached statistical significance [F(1,10) = 3.66, P = 0.085 and F(1,10) = 4.88, P = 0.052 for CMCT and MEPlat, respectively].
Table 4.
Pre- and postfatigue task MEP latencies and central motor conduction times
| Control |
MS |
|||||
|---|---|---|---|---|---|---|
| HS | TN | Tx P value | HS | TN | Tx P value | |
| MEPlat, ms | ||||||
| Preexercise | 22.27 ± 1.01* | 23.91 ± 1.81 | 0.005 | 22.36 ± 1.63* | 23.49 ± 1.14 | 0.012 |
| Postexercise | 22.45 ± 1.13* | 23.91 ± 2.02 | 22.73 ± 1.49* | 23.88 ± 1.28 | ||
| Time P value | 0.762 | 0.052 | ||||
| CMCT, ms | ||||||
| Preexercise | 8.07 ± 1.00* | 8.94 ± 1.33 | 0.007 | 8.47 ± 1.44 | 8.73 ± 0.93 | 0.410 |
| Postexercise | 8.15 ± 1.00* | 8.54 ± 1.11 | 8.78 ± 1.42 | 9.00 ± 1.25 | ||
| Time P value | 0.475 | 0.085 | ||||
Values are means ± SD. MEPlat, motor-evoked potential latency elicited by transcranial magnetic stimulation; CMCT, central motor conduction time. Tx column indicates within-group treatment effect across both time points; Time row indicates within-group pre- to postexercise effects across both treatments determined from RM ANOVA.
Significant within-group difference from TN condition at given time point, determined by post hoc analysis.
Recruitment curve.
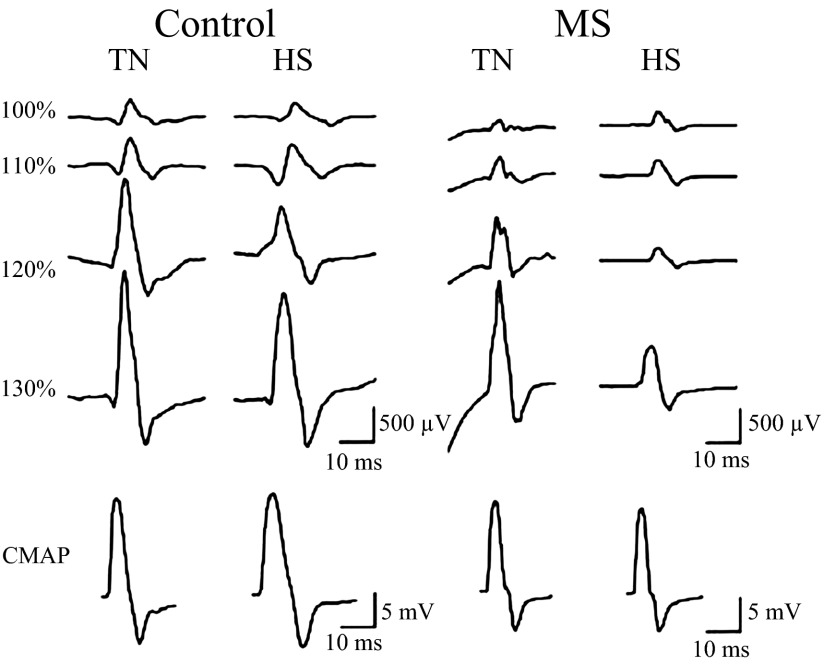
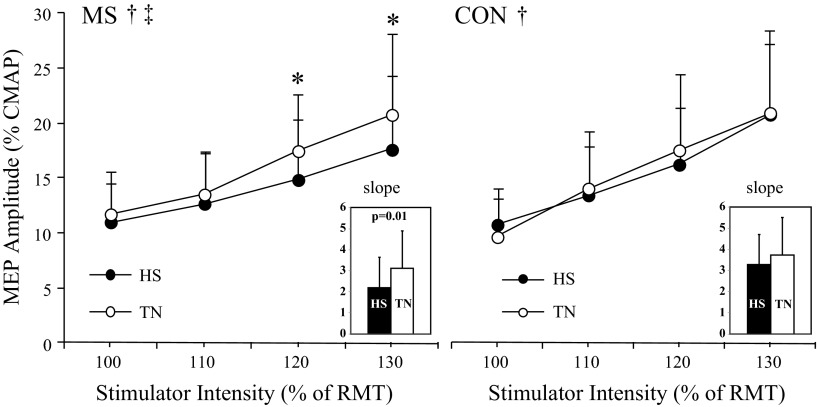
Representative MEP and CMAP traces are shown for a typical control and MS patient during HS and TN conditions, illustrating stimulation intensity and treatment effects (Fig. 3). The overall 4 (intensity) by 2 (treatment) by 2 (group) RM ANOVA revealed significant intensity [F(1.7, 34.8) = 60.1, P < 0.001] and intensity by treatment [F(3,59) = 2.94, P = 0.041] effects, indicating increased MEPamp (expressed relative to CMAP amplitude obtained during the same thermal condition) at stimulus intensities above RMT during both TN and HS conditions, with larger increases seen during the TN condition (Fig. 4). Separate RM analyses for MS patients and controls showed significant intensity effects in both groups [F(1.6, 16) > 25.5, P < 0.001]. Significant treatment and intensity by treatment effects were observed only in MS patients [F(1,10) = 4.95, P = 0.050 and F(2.9, 28.5) = 4.24, P = 0.015, respectively]. Post hoc contrasts included in the RM model indicated that in MS patients during HS, MEPamp were significantly lower at 120 and 130% of RMT [F(1,10) > 6.18, P < 0.032]. In controls, treatment and intensity by treatment effects were not statistically significant [F(1,10) < 1.10, P > 0.360; Fig. 4].
Fig. 3.
Representative motor-evoked potentials (MEPs) for a healthy control and MS patient during TN and HS conditions at 100, 110, 120, and 130% resting motor threshold (RMT). The row on the bottom shows corresponding baseline compound muscle action potential (CMAP) traces (different scale indicated). In this example, stimulus artifacts were evident in the MS patient during the TN condition. In general, these effects were not specific to any group or treatment and resolved before the MEP.
Fig. 4.
Recruitment curves during TN and HS conditions for MS patients (left) and healthy controls (right) are depicted. MEP amplitudes are expressed relative to CMAP amplitude. Recruitment curve slopes are indicated in the inset. *Significant within-group intensity by treatment effect, P < 0.05. †Significant within-group treatment (TN vs. HS) effect, P < 0.05. ‡Significant within-group intensity by treatment interaction, P < 0.05.
Recruitment curve slope was determined from the steepness of the regression line through individual response curves during HS and TN conditions. RM ANOVA of recruitment curve slope indicated a significant treatment effect [F(1,20) = 4.52, P = 0.046] but no group differences or treatment by group interaction [F(1,20) < 1.48, P > 0.239]. In MS patients, recruitment curve slope was significantly reduced during HS compared with TN [t(10) = 3.13, P = 0.011; Fig. 4, inset].
PED.
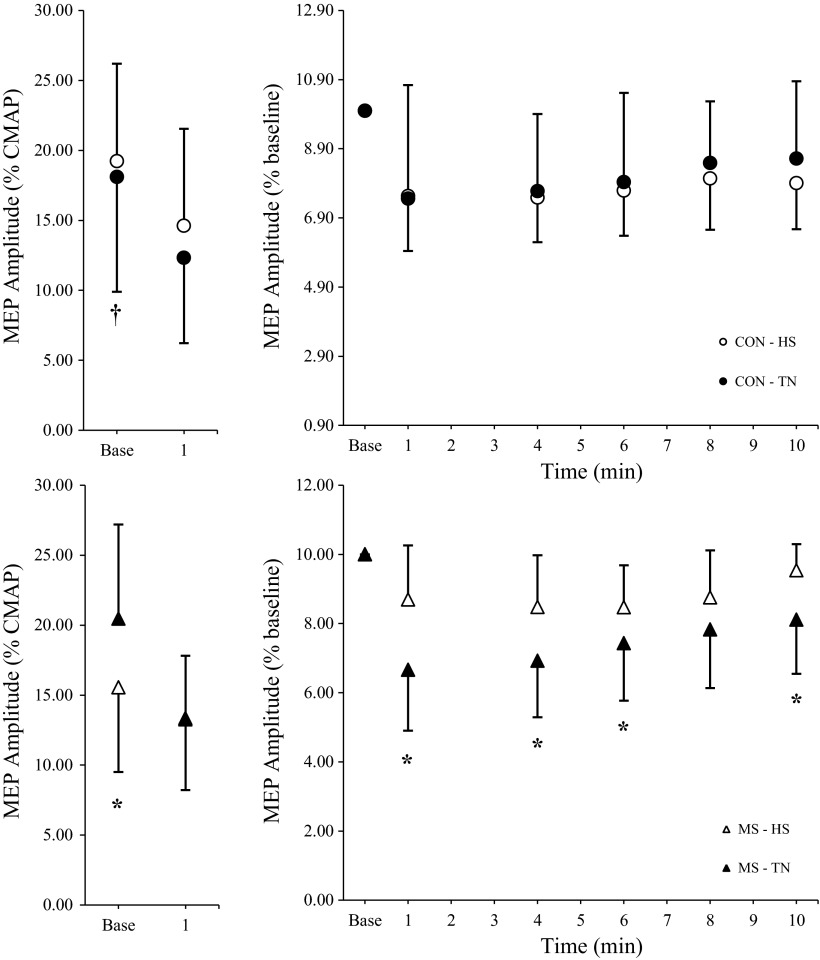
In MS patients, the HS condition resulted in significantly lower baseline MEPamp (expressed relative to CMAP amplitude) compared with the TN condition (P < 0.05) and compared with controls during both treatments (P < 0.05; Fig. 5, left). HS had no significant effect on baseline MEPamp in healthy controls.
Fig. 5.
Baseline and 1-min postfatigue task group differences in MEP amplitudes (MEPamp, left) for healthy controls (top) and MS patients (bottom). Postexercise changes in MEPamp relative to baseline (right) indicate no treatment (HS vs. TN) differences for controls (top) and significantly smaller relative changes in MS patients during HS compared with TN (bottom). *Significant within-group treatment effect, P < 0.05. †Significant between-group difference across treatments, P < 0.05.
Recovery MEPamp were recorded at 1, 4, 6, 8, and 10 min after the fatigue task and are expressed relative to preexercise levels (Fig. 5, right). In controls, RM ANOVA indicated a significant time effect [F(3.1, 30.6) = 6.14, P = 0.002] but no treatment effect (HS vs. TN) [F(1, 30.6) = 1.77, P = 0.213] or treatment by time interaction [F(3.3, 32.1) = 0.38, P = 0.781] for postexercise MEPamp. Contrasts included in the ANOVA model showed that MEPamp were significantly lower relative to baseline at all recovery time points in controls (P < 0.002). For the MS group, significant time [F(5,50) = 16.13, P < 0.001], treatment [F(1,50) = 4.95, P = 0.050], and treatment by time interaction [F(2.7, 27.2) = 3.84, P = 0.024] effects were observed. Contrasts derived from the treatment by time interaction illustrate significantly different treatment responses in MS patients at 1, 4, 6, and 10 min postexercise [F(1,10) > 4.99, P < 0.049], with smaller relative decreases in postexercise MEPamp during HS compared with TN (Fig. 5, right). These smaller relative postexercise decreases in the MS group during HS reflect baseline differences in MEPamp (Fig. 5, left).
DISCUSSION
To our knowledge, this is the first study to directly assess the effect of passive heat exposure on central conduction and cortical excitability in MS patients. The passive HS employed in the study produced significant perceptual changes, including increased subjective fatigue in both MS patients and healthy controls. Fatigue ratings during HS were significantly higher in MS patients than controls. It is important to note that fatigue perception increased significantly at rest, well before the motor fatigue task, suggesting that the effects of thermal stress were responsible. This is consistent with reports of heat-sensitive MS patients who experience greater fatigue when exposed to warm environments.
In addition to increased fatigue perception, reductions in peak force and faster decline in force were observed in MS patients during HS and are likely due to CNS lesions that are affected by heat, which could lead to recruitment of fewer motor units (conduction block) (11). In controls, there were no heat-related differences in peak force or force decline, which is in contrast to Nybo and Nielsen's work showing that, in healthy individuals, sustained maximal voluntary force declined significantly faster during hyperthermia (19). There are several possible explanations for this discrepancy. One possibility is that the degree of HS in the present study was not sufficient to appreciably decrease central drive in healthy individuals. In the study by Nybo and Nielsen, the degree of hyperthermia was substantially higher, core temperature of 40°C, compared with the moderate level in the present study. In addition, hyperthermia was achieved actively, altering peripheral metabolic characteristics that could further influence peak force. Still, other studies (16, 33) have demonstrated progressive decreases in MVC and voluntary activation with increasing core temperatures in the range of the present study. It is possible that mechanisms of task failure for the small hand muscles used in the present study differ from those of the knee extensors and plantar flexors of those studies (7, 16).
In healthy controls, resting MEPlat and CMCT were significantly shorter during the HS condition, reflecting increased peripheral and central nerve conduction velocity that is consistent with previous work that showed linear increases in conduction velocity of healthy nerve fibers with increasing temperature in the range of 28–40°C (22). In contrast, HS resulted in increased peripheral conduction velocity in MS patients, but no change in CMCT. In demyelinated neurons, studies have shown that increased temperature reduces the conduction safety factor and can produce conduction slowing and/or conduction block (11, 22, 29). For MS patients, the apparent inability of central pathways to increase conduction velocity during conditions of increased core temperature may reflect altered conduction characteristics due to demyelination in the CNS. Interestingly, the additional demands of sustained motor activity appear to amplify these effects in MS patients, as evidenced by trends toward increased MEPlat (P = 0.052) and CMCT (P = 0.085) immediately following the motor fatigue task. Similarly, our previous work showed temporary functional declines in motor performance following combined arm/leg cycling exercise even when core temperature increases were prevented (36). This suggests that both sustained and intermittent motor activity can alter central conduction characteristics and ultimately impair task performance in MS patients.
Recruitment curve characteristics paralleled fatigue perception and decreases in force production in that cortical excitability, indicated by recruitment curve slope, decreased in MS patients during HS. Proposed mechanisms to explain the effect of HS on cortical excitability include alterations in output from thermoregulatory centers in the hypothalamus or other structures, including the motor cortex (19, 23, 34). In the context of the present study, these explanations are somewhat problematic because the HS condition did not reduce cortical excitability in healthy controls (Fig. 4). The small range of stimulus intensities used to determine recruitment curves used in the present study as described by Chen et al. (3) may have resulted in an overall underestimation of slope due to missing responses evoked at higher stimulation intensities. Although this explanation could account for the lack of a thermal effect in controls, it is also possible that the degree of HS was not sufficient to produce central fatigue in controls. This is consistent with the controls' force characteristics, which also suggest that the degree of HS in the present study did not increase fatigue. Nonetheless, a more extensive recruitment curve that included higher stimulus intensities would have clarified this issue.
The significant reduction in recruitment curve slope in MS patients during HS was expected and corresponds to reports of increased symptomatology with increased temperature (11). It is reasonable to assume that demyelinated pathways in the CNS respond to increased temperature similarly to demyelinated peripheral nerve, with small increases in temperature capable of blocking of action potentials (11). The observed increase in RMT in MS patients during HS supports this concept.
Changes in cortical SP duration reflect spinal and cortical inhibitory mechanisms (9). Taylor et al. observed SP prolongation during sustained MVC of elbow flexors attributed to net decreases in cortical output, most likely due to intracortical inhibition (ICI) (31, 32). In the present study, SP duration was not affected by HS in either group. Using paired pulse TMS, Liepert and colleagues observed decreased postexercise ICI in MS patients with fatigue but no changes in cortical SP and suggested that SP duration was dependent on inhibitory mechanisms independent from those involved with ICI (14). HS and/or fatiguing exercise may influence different inhibitory circuits than those assessed using cortical SP.
Both MS patients and healthy controls demonstrated PED of evoked potentials. This has been documented in previous studies and is thought to indicate decreased corticospinal excitability (13, 14, 21). The intracortical mechanism for PED may be analogous to the spinal mechanisms of postactivation depression of the H-reflex, including possible depletion of the available neurotransmitter pool or increased presynaptic inhibition (27). Postexercise changes in cortical excitability may also be influenced by peripheral signaling from group III/IV afferents that inhibit motor areas (18). Temperature-sensitive TRP receptors are present on these afferent fibers and interact with metabolite-detecting ASIC and P2X receptors and thus could enhance inhibitory afferent signaling (15).
No treatment effects were evident for postexercise MEP responses in healthy controls, but in MS patients HS produced blunted MEP amplitudes at baseline and at all postfatigue task time points. As mentioned earlier, HS in combination with sustained contractions results in even greater decrements in central activation (4, 17, 19). It would be useful to examine measures of cortical excitability at a range of temperatures produced both passively and during active conditions to examine the additional effect of peripheral signaling from muscle afferents on central responses.
To summarize, mild passive HS resulted in greater fatigue perception and impairments in force production in MS patients and corresponding evidence for cortical degeneration or dysfunction, including significantly increased RMT, decreased MEPamp, and decreased recruitment curve slope (35). PED of MEPamp was evident in controls and MS patients; however, the combination of HS and sustained exercise magnified these decrements in MS patients. Taken together, these results suggest that CNS pathology in MS patients played a substantial role in reducing cortical excitability. Central conduction (CMCT) was significantly shorter during HS in healthy controls; however, in MS patients, normal increases in conduction velocity with increased temperature were not observed centrally. Both groups exhibited increased peripheral nerve conduction velocity, supporting the concept that differences between MS patients and controls were of central origin.
GRANTS
This project was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R15-AR-050435-01A2.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.T.W. conception and design of research; A.T.W., T.A.V., J.V., and S.L.D. performed experiments; A.T.W., T.A.V., and J.V. analyzed data; A.T.W. and S.L.D. interpreted results of experiments; A.T.W. and S.L.D. prepared figures; A.T.W. drafted manuscript; A.T.W., T.A.V., J.V., and S.L.D. edited and revised manuscript; A.T.W., T.A.V., J.V., and S.L.D. approved final version of manuscript.
REFERENCES
- 1. Brasil-Neto JP, Cohen LG, Hallett M. Central fatigue as revealed by postexercise decrement of motor evoked potentials. Muscle Nerve 17: 713–719, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Brasil-Neto JP, Pascual-Leone A, Valls-Sole J, Cammarota A, Cohen LG, Hallett M. Postexercise depression of motor evoked potentials: a measure of central nervous system fatigue. Exp Brain Res 93: 181–184, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol 80: 2870–2881, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Cheung SS, Sleivert GG. Multiple triggers for hyperthermic fatigue and exhaustion. Exerc Sport Sci Rev 32: 100–106, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Curra A, Modugno N, Inghilleri M, Manfredi M, Hallett M, Berardelli A. Transcranial magnetic stimulation techniques in clinical investigation. Neurology 59: 1851–1859, 2002 [DOI] [PubMed] [Google Scholar]
- 6. DuBois AB, Harb ZF, Fox SH. Thermal discomfort of respiratory protective devices. Am Ind Hyg Assoc J 51: 550–554, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol 586: 11–23, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci 21: 9–14, 1994 [PubMed] [Google Scholar]
- 9. Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol 81: 257–262, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol 86: 1032–1039, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Guthrie TC, Nelson DA. Influence of temperature changes on multiple sclerosis: critical review of mechanisms and research potential. J Neurol Sci 129: 1–8, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Hoppner J, Kunesch E, Buchmann J, Hess A, Grossmann A, Benecke R. Demyelination and axonal degeneration in corpus callosum assessed by analysis of transcallosally mediated inhibition in multiple sclerosis. Clin Neurophysiol 110: 748–756, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Liepert J, Kotterba S, Tegenthoff M, Malin JP. Central fatigue assessed by transcranial magnetic stimulation. Muscle Nerve 19: 1429–1434, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Liepert J, Mingers D, Heesen C, Bäumer T, Weiller C. Motor cortex excitability and fatigue in multiple sclerosis: a transcranial magnetic stimulation study. Mult Scler 11: 316–321, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morrison S, Sleivert GG, Cheung SS. Passive hyperthermia reduces voluntary activation and isometric force production. Eur J Appl Physiol 91: 729–736, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Morrison SA, Sleivert GG, Neary JP, Cheung SS. Prefrontal cortex oxygenation is preserved and does not contribute to impaired neuromuscular activation during passive hyperthermia. Appl Physiol Nutr Metab 34: 66–74, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Nybo L. Brain temperature and exercise performance. Exp Physiol 97: 333–339, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol 91: 1055–1060, 2001 [DOI] [PubMed] [Google Scholar]
- 20. O'Brien C, Hoyt RW, Buller MJ, Castellani JW, Young AJ. Telemetry pill measurement of core temperature in humans during active heating and cooling. Med Sci Sports Exerc 30: 468–472, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Petajan JH, White AT. Motor-evoked potentials in response to fatiguing grip exercise in multiple sclerosis patients. Clin Neurophysiol 111: 2188–2195, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Rasminsky M. The effects of temperature on conduction in demyelinated single nerve fibers. Arch Neurol 28: 287–292, 1973 [DOI] [PubMed] [Google Scholar]
- 23. Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol 105: 340–344, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Roelands B, Meeusen R. Alterations in central fatigue by pharmacological manipulations of neurotransmitters in normal and high ambient temperature. Sports Med 40: 229–246, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Rosenkranz K, Williamon A, Rothwell JC. Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J Neurosci 27: 5200–5206, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rossini P, Caramia M, Zarola F. Central motor tract propagation in man: studies with non-invasive, unifocal, scalp stimulation. Brain Res 66: 88–100, 1987 [DOI] [PubMed] [Google Scholar]
- 27. Samii A, Lopez-Devine J, Wasserman EM, Dalakas MC, Clark K, Grafman J, Hallett M. Normal postexercise facilitation and depression of motor evoked potentials in postpolio patients. Muscle Nerve 21: 948–950, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Samii A, Wassermann EM, Hallett M. Post-exercise depression of motor evoked potentials as a function of exercise duration. Electroencephalogr Clin Neurophysiol 105: 352–356, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Schauf CL, Davis FA. Impulse conduction in multiple sclerosis: a theoretical basis for modification by temperature and pharmacological agents. J Neurol Neurosurg Psychiatry 37: 152–161, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Society NMS. Minimal Record of Disability for Multiple Sclerosis. New York, NY: National Multiple Sclerosis Society, 1985 [Google Scholar]
- 31. Taylor JL, Allen GM, Butler JE, Gandevia SC. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol 89: 305–313, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol 490: 519–528, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas MM, Cheung SS, Elder GC, Sleivert GG. Voluntary muscle activation is impaired by core temperature rather than local muscle temperature. J Appl Physiol 100: 1361–1369, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Todd G, Butler JE, Taylor JL, Gandevia SC. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol 563: 621–631, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vucic S, Burke T, Lenton K, Ramanathan S, Gomes L, Yannikas C, Kiernan MC. Cortical dysfunction underlies disability in multiple sclerosis. Mult Scler 18: 425–432, 2012 [DOI] [PubMed] [Google Scholar]
- 36. White AT, Wilson TE, Davis SL, Petajan JH. Effect of precooling on physical performance in multiple sclerosis. Mult Scler 6: 176–180, 2000 [DOI] [PubMed] [Google Scholar]