Abstract
TNF promotes skeletal muscle weakness, in part, by depressing specific force of muscle fibers. This is a rapid, receptor-mediated response, in which TNF stimulates cellular oxidant production, causing myofilament dysfunction. The oxidants appear to include nitric oxide (NO); otherwise, the redox mechanisms that underlie this response remain undefined. The current study tested the hypotheses that 1) TNF signals via neuronal-type NO synthase (nNOS) to depress specific force, and 2) muscle-derived reactive oxygen species (ROS) are essential co-mediators of this response. Mouse diaphragm fiber bundles were studied using live cell assays. TNF exposure increased general oxidant activity (P < 0.05; 2′,7′-dichlorodihydrofluorescein diacetate assay) and NO activity (P < 0.05; 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate assay) and depressed specific force across the full range of stimulus frequencies (1-300 Hz; P < 0.05). These responses were abolished by pretreatment with Nω-nitro-L-arginine methyl ester (L-NAME; a nonspecific inhibitor of NOS activity), confirming NO involvement. Genetic nNOS deficiency replicated L-NAME effects on TNF-treated muscle, diminishing NO activity (−80%; P < 0.05) and preventing the decrement in specific force (P < 0.05). Comparable protection was achieved by selective depletion of muscle-derived ROS. Pretreatment with either SOD (degrades superoxide anion) or catalase (degrades hydrogen peroxide) depressed oxidant activity in TNF-treated muscle and abolished the decrement in specific force. These findings indicate that TNF signals via nNOS to depress contractile function, a response that requires ROS and NO as obligate co-mediators.
Keywords: oxidative stress, inflammation, cytokines, diaphragm, weakness
tnf-α is a proinflammatory cytokine that promotes muscle weakness in a variety of chronic diseases (64). TNF depresses muscle force via two general mechanisms. Muscle atrophy is the mechanism recognized most widely. At the cellular level, prolonged TNF exposure activates pro-catabolic signaling and upregulates the ubiquitin-proteasome pathway to cause protein loss and reduce cell size (31, 36–39). The resulting decline in muscle mass inexorably leads to weakness.
The second mechanism, termed contractile dysfunction, is less well understood. TNF has the capacity to depress specific force of skeletal muscle, i.e., force/unit of the cross-sectional area, causing weakness, independent of muscle mass. Contractile dysfunction occurs at circulating TNF levels that do not cause muscle atrophy (35) and is stimulated via the TNF receptor subtype 1 (TNFR1) (21) and reflects loss of myofibrillar function (21, 49). Muscle-derived oxidants play a central role in this response. TNF/TNFR1 signaling increases general oxidant activity in the cytosol within minutes; antioxidant pretreatment buffers the rise in oxidant activity and preserves specific force, arguing for causality (21, 35). The oxidants that mediate TNF-induced dysfunction appear to include nitric oxide (NO) derivatives. Alloatti and associates (1) showed that brief exposure to TNF increases NO production and depresses specific force in extensor digitorum longus (EDL) muscle of guinea pig. Both responses were abolished by pharmacologic blockade of NO synthase (NOS), suggesting a role for NO. Although intriguing, these findings have not been confirmed independently.
Our current study addressed the redox mechanism by which TNF stimulates contractile dysfunction. Initial experiments assessed the biological robustness of NO involvement using a respiratory skeletal muscle (diaphragm) from a different species (mouse). We then tested two original hypotheses.
Hypothesis 1.
TNF signals via neuronal-type NOS (nNOS) to stimulate NO production and contractile dysfunction. The NOS isoform that mediates TNF/NO signaling is undefined. Skeletal muscle constitutively expresses all three isoforms: inducible, endothelial, and nNOS (26, 27, 44). These are independent gene products with distinct structures, intracellular locations, and mechanisms of regulation (55). The nNOS isoform transduces receptor-activated signaling cascades in other cell types (13, 51, 56) and localizes to the subsarcolemmal region of muscle fibers (7), placing nNOS in close physical proximity to the TNFR1 complex. We therefore assessed nNOS as a downstream component of TNF/TNFR1 signaling.
Hypothesis 2.
Reactive oxygen species (ROS) contribute to contractile dysfunction stimulated by TNF. Compared with NO and its derivatives, the ROS cascade may be generated by separate intracellular sources, is derived from a chemically distinct parent molecule, and can be regulated by independent mechanisms. Muscle-derived ROS are reported to function as second messengers in a variety of TNF-stimulated responses (30, 32, 39, 54); however, the role of ROS in contractile dysfunction has not been evaluated.
Hypotheses were tested by exposing diaphragm fiber bundles to recombinant TNF in vivo or in vitro. Live cell assays were used to measure changes in general oxidant activity, NO activity, and contractile function. The contributions of muscle-derived NO and ROS were evaluated using selective pharmacologic probes and genetic interventions. Our findings identify nNOS as the source of TNF-stimulated NO and demonstrate that muscle-derived ROS are obligate co-mediators of the subsequent contractile dysfunction.
METHODS
Animal care.
Experiments were performed using 6- to 8-wk-old male mice from two strains: wild-type (C57BL/6J; The Jackson Laboratory, Bar Harbor, ME) and nNOS or nNOS-deficient (nNOS−/−; B6.129S4-Nos1tm1Plh/J; The Jackson Laboratory) mice. Animals were maintained in a 12:12-h dark-light cycle and received water and food ad libitum. All procedures conformed to the guiding principles for use and care of laboratory animals of the American Physiological Society and were approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
Muscle preparations.
Each animal was anesthetized deeply by inhalation of isoflurane (Aerrane; Baxter Healthcare, Deerfield, IL) and killed by cervical dislocation, followed by exsanguination. The diaphragm muscle was excised quickly and placed in Krebs-Ringer solution (in mM: 137 NaCl, 5 KCl, 1 MgSO4, 1 NaH2PO4, 24 NaHCO3, and 2 CaCl2), equilibrated with 95% O2–5% CO2 (pH 7.3 at 37°C). For live-cell oxidant activity assays, the muscle was surgically separated into two hemidiaphragms. For measurement of contractile function, bundles of muscle fibers were surgically isolated with portions of the rib and central tendon attached.
Live-cell oxidant activity assays.
We used the fluorochrome probe 2′,7′-dichlorodihydrofluorescein diacetate (DCF; excitation/emission 492–495/517–527 nm; Molecular Probes, Eugene, OR) to measure cytosolic oxidant activity (42) and 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM; 495/515 nm; Molecular Probes) to measure cytosolic NO activity (29). Hemidiaphragms were pinned at near-optimal length (LO) in a bath containing oxygenated Krebs-Ringer solution at 37°C. Each muscle preparation was loaded with fluorochrome by incubation in DCF 20 μM or DAF-FM 5 μM for 20 min and then was incubated for 30 min in buffer alone (control) or TNF 500 ng/ml. In a subset of experiments, muscles were pretreated with SOD 1,000 U/ml (Sigma-Aldrich, St. Louis, MO), catalase 1 kU/ml (Sigma-Aldrich), Nω-nitro-L-arginine methyl ester 10 mM (L-NAME; Sigma-Aldrich), or Nω-nitro-D-arginine methyl ester 10 mM (D-NAME; Sigma-Aldrich) to assess the contributions of muscle-derived ROS or NO derivatives. Fluorescence emissions of the oxidized fluorochromes were measured from a 0.27-mm2 site on the muscle surface, and background emissions were measured from a muscle-free area of the incubation media using an epifluorescence microscope (Eclipse TE2000; Nikon Instruments, Melville, NY) and a charge-coupled device camera (CoolSNAP ES; Photometrics, Tuscon, AZ). Final values for fluorescence intensity were corrected for background emissions.
Molecular probe chemistry.
Experiments were performed in triplicate using a 24-well tissue-culture plate with a reaction volume of 1 ml. The sensitivities of DCF and DAF-FM to ROS and NO derivatives were tested using a cell-free assay. DCF diacetate 50 μM and DAF-FM diacetate 20 μM were preincubated with esterase 4 U/ml in Krebs buffer for 5 min to produce DCF and DAF-FM, respectively. Each fluorochrome was mixed with 1) TNF 15 ng/ml; 2) hydrogen peroxide (H2O2) 1 mM; 3) H2O2 plus catalase 1 kU/ml; 4) the NO donor, 3-(2-hydroxy-1-methyl-2-nitrosohydrazino)-N-methyl-1-propanamine (NOC-7; 100 μM; EMD4Biosciences, EMD Millipore, Billerica, MA); 5) NOC-7 plus hemoglobin 50 μM (NO scavenger; Sigma-Aldrich); or 6) Krebs-Ringer solution alone (control). After 10 min, fluorescence was measured using the microscope system described above.
Contractile studies.
Muscle fiber bundles were preconditioned by TNF exposure, prior to contractile measurements. For in vitro preconditioning, fiber bundles were incubated at 37°C with TNF 500 ng/ml 3-[2-hydroxy-1-(1-methyethyl)-2-nitrosohydrazino]-1-propanamine (NOC-5; 20 μM; EMD4Biosciences, EMD Millipore) as a source of exogenous NO derivative or buffer alone (control) before contractile measurements. For in vivo preconditioning, mice received intraperitoneal (ip) injections of TNF 100 μg/kg (Pierce Biotechnology, Thermo Fisher Scientific, Rockford, IL) or an equal volume of diluent prior to muscle excision. A subset of TNF-treated animals was pretreated with SOD 1 kU/μg, catalase 1 kU/μg, L-NAME 20 mg/kg, or D-NAME 20 mg/kg by ip injection prior to TNF administration.
Isometric contractile properties were measured in vitro. The fiber bundle was mounted by tying the rib to a glass rod and the central tendon to a force transducer (BG series 100 gm; Kulite Semiconductor Products, Leonia, NJ) using silk suture (4–0). The transducer was mounted on a micrometer used to adjust muscle length. The fiber bundle was positioned between platinum plate electrodes in a water-jacketed organ bath containing (+)-tubocurarine hydrochloride 0.025 mM (Sigma-Aldrich) in Krebs-Ringer solution, continuously gassed with 95% O2–5% CO2 at 37°C. Fiber-bundle length was adjusted to maximize twitch force (LO), stimulated using supramaxmimal voltage and 0.2-ms pulses (stimulator model S48; Grass Instrument Company, Quincy, MA).
Force-frequency characteristics were determined using stimulus frequencies of 1, 15, 30, 50, 80, 120, 150, and 250 Hz (500-ms train duration), delivered every 2 min and interspersed by maximal tetanic contractions (PO; 300 Hz). Transducer output was measured using a digital oscilloscope (model 546601B; Hewlett-Packard, Palo Alto, CA). After the force-frequency protocol, muscle length was measured using an electronic caliper (CD-6“ CS; Mitutoyo America, Aurora, IL). The fiber bundle was removed from the organ bath, excised from bone and connective tissue, blotted dry, and weighed. Fiber bundle weight and LO were used to calculate cross-sectional area (10). Specific force is expressed as N/cm2.
Western blot.
Tissue samples were homogenized in 2× protein loading buffer (120 mM Tris, pH 7.5, 200 mM DTT, 20% glycerol, 4% SDS, 0.002% bromphenol blue). Proteins were loaded and separated by 4–15% SDS-PAGE gel electrophoresis (Criterion; Bio-Rad Laboratories, Hercules, CA) at 200 V for 40 min. Total protein was assessed by staining using SimplyBlue (Invitrogen, Life Technologies, Carlsbad, CA). Proteins were wet transferred to polyvinyldifluoride membranes overnight at 100 mA. Membranes were blocked (Odyssey blocking buffer; LI-COR Biosciences, Lincoln, NE) for 1 h at room temperature and incubated with primary antibodies (anti-nNOS, ECM Biosciences, Versailles, KY; and mouse phospho-JNK and rabbit total JNK, Cell Signaling Technology, Danvers, MA) overnight in blocking buffer and an equal volume of PBS plus 0.1% Tween (PBST), followed by four, 5-min washes in PBST. Membranes were incubated with fluorescence-conjugated secondary antibodies (goat anti-mouse IRD 800; Rockland Immunochemicals, Gilbertsville, PA) in blocking buffer/PBST plus 0.01% SDS for 45 min, followed by four, 5-min washes. Membranes were dried, and blots were imaged using the Odyssey infared scanner (Odyssey infared system; LI-COR Biosciences) to quantify differences.
Statistical analysis.
All comparisons were performed using Prism 5.0b software (GraphPad Software, La Jolla, CA). Paired comparisons between DCF and DAF-FM data were evaluated using Student's paired t-tests. Differences between force-frequency curves were analyzed using two-way, repeated-measures ANOVA and a post hoc Turkey test. Differences were considered significant at the P < 0.05 level. Results are reported as means ± SE.
RESULTS
TNF effects on specific force and oxidant activity.
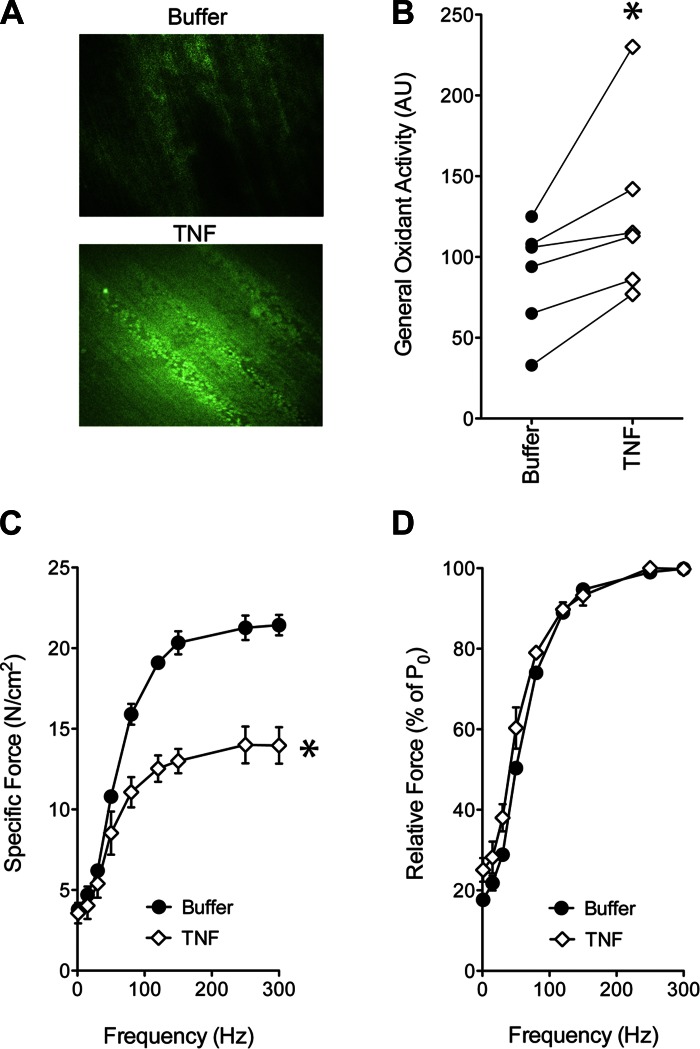
As illustrated in Fig. 1, TNF exposure increases general oxidant activity in the cytosolic compartment. This rise in oxidant activity is associated with decrements in specific force at all stimulus frequencies, such that relative force, as a percentage of maximal force, is relatively unaffected. These responses are abolished by antioxidant pretreatment (21, 35)—evidence that muscle-derived oxidants cause the fall in specific force stimulated by TNF.
Fig. 1.
Direct TNF exposure increases cytosolic oxidant activity and depresses specific force in diaphragm fiber bundles. A: representative fluorescence images depict hemidiaphragms loaded with 2′,7′-dichlorodihydrofluorescein diacetate (DCF) and incubated in buffer alone (top) or in TNF (bottom; 500 ng/ml, 30 min). B: general oxidant activity quantified from DCF fluorescence of hemidiaphragms, treated as in A. TNF increased emissions in 8 of 8 paired comparisons; *P < 0.05 (paired t-test), Buffer vs. TNF. C: the specific force-frequency relationship of murine diaphragm fiber bundles incubated with TNF (500 ng/ml, 30 min; open diamonds) was depressed across all experimental frequencies relative to control measured in buffer alone (closed circles); mean values shown ± SE; n = 3/group; *P < 0.05 (2-way ANOVA), Buffer vs. TNF. D: relative force as a percentage of maximal tetanic force (% of PO) was unaffected by TNF at all stimulus frequencies; means are shown ± SE.
Role of muscle-derived NO.
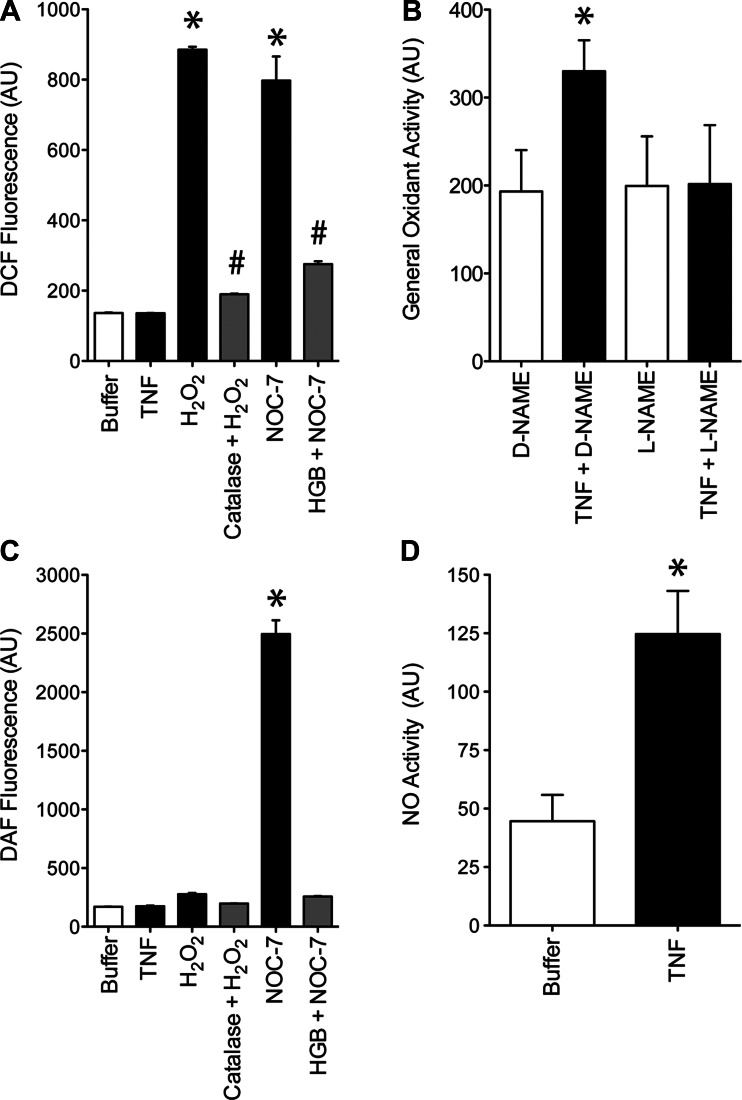
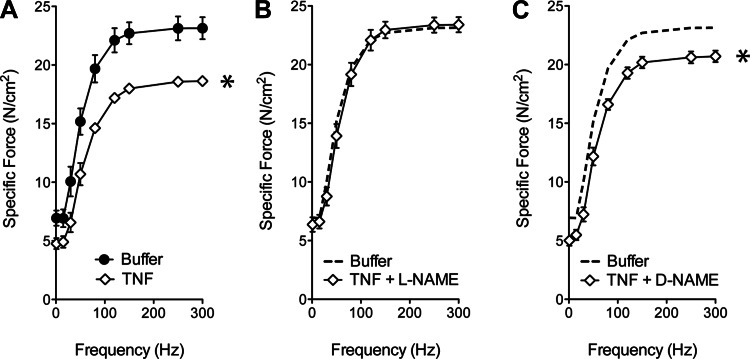
We monitor general oxidant activity using DCF, a fluorescence probe that detects both NO derivatives and ROS (Fig. 2A). To assess the contribution of NO, we tested TNF effects on general oxidant activity in muscle fibers pretreated with L-NAME (NOS inhibitor) or D-NAME (inactive enantiomer). As shown in Fig. 2B, TNF increased general oxidant activity in D-NAME-treated fibers, mimicking the response of muscle without drug treatment. L-NAME abolished this response—evidence that TNF increases general oxidant activity by stimulating NO production. NO involvement was tested further by use of DAF-FM, a fluorescence probe that preferentially detects NO derivatives (Fig. 2C); data from intact muscle fibers confirm that TNF increases cytosolic NO activity (Fig. 2D). The increased NO appears to depress contractile function. Decrements in specific force (Fig. 3A) are abolished by pretreating muscle with L-NAME (Fig. 3B) but not D-NAME (Fig. 3C). These findings closely resemble data from guinea pig EDL muscle (1) and confirm that NO mediates the contractile dysfunction stimulated by TNF.
Fig. 2.
TNF induces both reactive oxygen species (ROS) and nitric oxide (NO) in diaphragm fibers. A: reactivity of de-esterified DCF was measured by use of a cell-free system. Relative to buffer alone (Krebs), DCF emissions were unaffected by TNF (15 ng/ml). Hydrogen peroxide (H2O2; 1 mM) increased emissions, an effect abolished by catalase (1 kU/ml; H2O2 + catalase). The NO donor, 3-(2-hydroxy-1-methyl-2-nitrosohydrazino)-N-methyl-1-propanamine (NOC-7; 100 μM), increased emissions, an effect abolished by reduced hemoglobin (HGB; 50 μM; NOC-7 + HGB); n = 3/group; means shown ± SE; *P < 0.05, Krebs vs. NOC-7 or H2O2; #P < 0.05, H2O2 vs. H2O2 + catalase, NOC-7 vs. NOC-7 + HGB. B: muscles treated with TNF (500 ng/ml) plus Nω-nitro-D-arginine methyl ester [D-NAME; 10 uM; neuronal-type NO synthase (nNOS) inhibitor, inactive enantiomer] for 30 min increased DCF emissions, an effect that was abolished by treatment with Nω-nitro-L-arginine methyl ester (L-NAME; 10 uM; nNOS inhibitor, active enantiomer); mean values shown ± SE; n = 3/group; *P < 0.05 (paired t-test), TNF + D-NAME vs. all other groups. C: reactivity of de-esterified 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM) exposed under cell-free conditions to oxidants and redox interventions as in A. TNF, H2O2, and catalase had no effect on DAF-FM emissions relative to buffer alone. NOC-7 increased emissions, an effect abolished by reduced hemoglobin; n = 3/group; means ± SE; *P < 0.05, NOC-7 vs. NOC-7 + HGB. D: diaphragm treated with 500 ng/ml TNF (30 min) in vitro increased cytosolic NO activity, as measured by DAF-FM assay relative to buffer-treated muscles; n = 6/group; means ± SE; *P < 0.05 (paired t-test), Buffer vs. TNF.
Fig. 3.
Pharmacologic NOS inhibition protects contractile function of TNF-treated diaphragm in vivo. A: specific force of diaphragm fiber bundles from TNF-treated mice (100 μg/kg ip, 60 min; open diamonds) was depressed relative to data from buffer-treated control animals (closed circles); n = 3/group; means ± SE; *P < 0.05 (2-way ANOVA), TNF vs. Control. B: pharmacologic blockade of nNOS with L-NAME (20 mg/kg ip, 60 min) prevents the TNF-stimulated decrease in specific force compared with buffer-treated muscle (dotted line; reproduction of data in A for reference); n = 6/group. C: the inactive enantiomer D-NAME (20 mg/kg) did not alter the decrement of force in TNF-treated muscle (open diamonds) compared with buffer-treated muscle (dotted line; reproduction of data in A); n = 6/group; means ± SE; *P < 0.05 (2-way ANOVA), TNF + D-NAME vs. Buffer.
TNF signaling via nNOS.
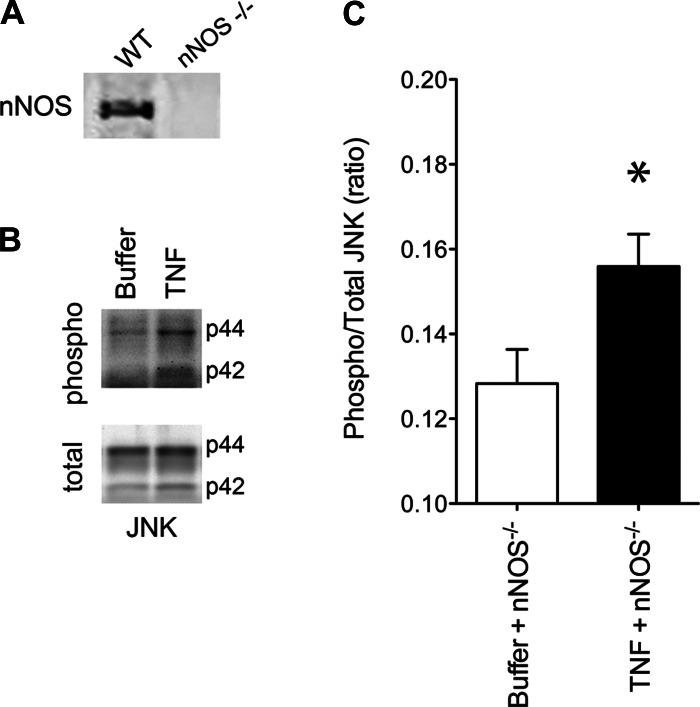
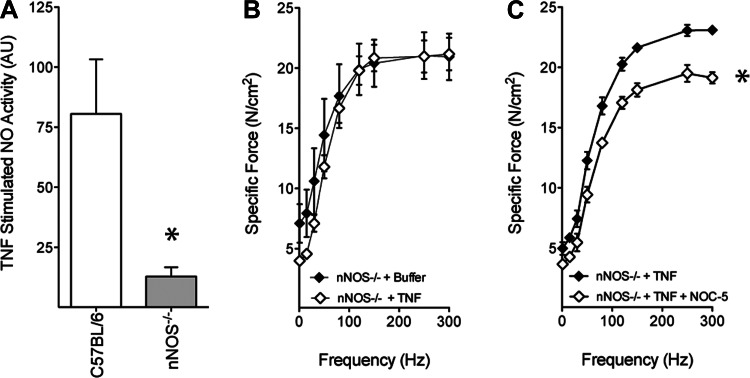
The NOS isoform responsible for TNF-stimulated NO production has not been defined. Based on its role in receptor-mediated signaling and subsarcolemmal localization, we evaluated nNOS as the NO source. Mice engineered for nNOS−/− were compared with control animals of similar genetic background. Muscle preparations were confirmed to be nNOS−/− by Western blot (Fig. 4A). The patency of TNF/TNFR1 signaling was assessed by measuring phosphorylation of JNK, an established response to TNFR1 activation (15, 20, 36). TNF consistently increased JNK phosphorylation (Fig. 4, B and C)—evidence that TNFR1 signaling remains intact in nNOS−/− muscle. Despite retaining TNFR1 function, NO signaling was disrupted profoundly by nNOS−/−. The NO activity stimulated by TNF was diminished by 84% in nNOS−/− muscle relative to genetically intact muscle (Fig. 5A). Contractile function was also protected. The specific force-frequency relationship of nNOS−/− muscle was not altered significantly by TNF exposure (Fig. 5B). This finding is not explained by NO insensitivity. Follow-up studies showed that direct exposure to NOC-5, an exogenous NO donor, depressed specific force of nNOS−/− muscle (Fig. 5C). This mirrors the contractile depression caused by NO donors in wild-type muscle (26, 48) and confirms that downstream targets of NO signaling are preserved. Overall, these data identify nNOS as the primary isoform by which TNF stimulates NO synthesis and show that nNOS is an essential component of the signaling pathway that causes contractile dysfunction.
Fig. 4.
TNF signaling remains intact after nNOS depletion in diaphragm muscle. A: Western blot illustrates constitutive expression of full-length nNOS (158 kDa) in diaphragm of wild-type (WT) mice and the absence of nNOS in diaphragm of nNOS-deficient (nNOS−/−) animals. B: Western blots illustrate the effects of TNF (in vitro; 500 ng/ml, 30 min) on JNK signaling in nNOS−/− muscle. C: quantification of B shows that TNF induces JNK phosphorylation in nNOS−/− muscle; data expressed as the ratio of phosphorylated:total JNK; means are shown ± SE; *P < 0.05 (paired t-test), TNF + nNOS−/− vs. Buffer + nNOS−/−.
Fig. 5.
nNOS-derived NO is essential for TNF-induced contractile dysfunction. A: TNF-stimulated (in vitro; 500 ng/ml, 30 min) NO activity, as measured by DAF-FM, is blunted in nNOS−/− muscle compared with muscle from strain-matched controls; n = 3/group; means ± SE; *P < 0.05 (paired t-test), C57BL/6 vs. nNOS−/−. B: TNF (100 μg/kg ip, 60 min) administration to nNOS−/− mice does not depress specific force of diaphragm fiber bundles; n = 3/group; means ± SE. C: reintroduction of NO {3-[2-hydroxy-1-(1-methyethyl)-2-nitrosohydrazino]-1-propanamine (NOC-5); 10 μM, 30 min} to TNF-treated nNOS−/− muscle in vitro recovers phenotypic depression of specific force; n = 3/group; means ± SE; *P < 0.05 (2-way ANOVA), nNOS−/− + TNF vs. nNOS−/− + TNF + NOC-5.
ROS as co-mediators.
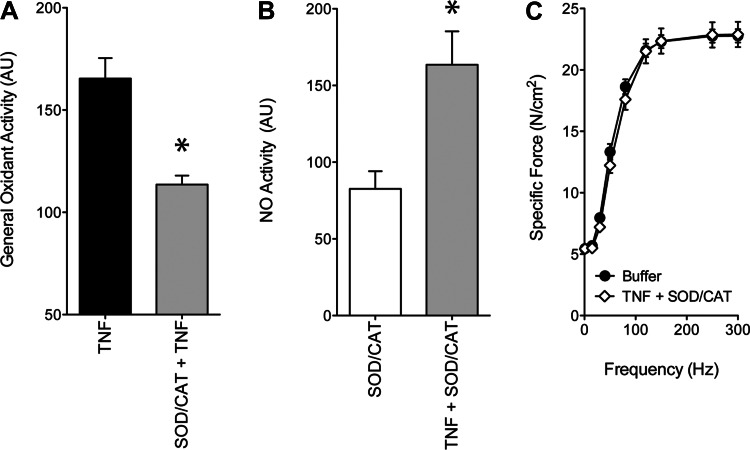
ROS are continually produced by skeletal muscle fibers, are major contributors to cytosolic oxidant activity under basal conditions, and are known to mediate TNF signaling. We tested the role of muscle-derived ROS in our system by use of exogenous catalase and SOD, anti-ROS enzymes that selectively deplete H2O2 and superoxide anions, respectively. In muscle exposed to TNF, catalase and SOD depressed general oxidant activity (Fig. 6A). This finding confirms that ROS are present in the cytosol of TNF-treated muscle and can be depleted experimentally by catalase and SOD. ROS depletion does not appear to disrupt TNF/NO signaling. NO activity is still stimulated by TNF, despite pretreatment with catalase and SOD (Fig. 6B). This rise in NO activity is essential for contractile dysfunction but does not appear to be sufficient. Muscle-derived ROS are also required. ROS depletion, by catalase and SOD, abolishes TNF effects on specific force (Fig. 6C), identifying ROS as obligatory co-mediators of contractile dysfunction.
Fig. 6.
Muscle-derived ROS co-mediates contractile dysfunction stimulated by TNF. A: mice were treated with TNF (100 μg/kg ip, 60 min) following pretreatment with buffer or SOD (1,000 U/μg ip, 60 min) and catalase (SOD/CAT; 1,000 U/μg ip, 60 min). Selective ROS depletion depressed general oxidant activity in fibers of TNF-treated animals, as measured by DCF fluorescence; n = 7/group; *P < 0.05 (paired t-test), TNF vs. SOD/CAT + TNF. B: NO activity in fibers treated in vitro with SOD/CAT (1,000 U/ml, 30 min) was increased by co-treatment in vitro with TNF (500 ng/ml, 30 min); n = 3/group; means ± SE; *P < 0.05 (paired t-test), SOD/CAT vs. SOD/CAT + TNF. C: pretreatment of mice with SOD/CAT (1,000 U/μg ip, 60 min; n = 6) prevented the decrement in specific force caused by TNF administration (100 μg/kg ip, 60 min; open diamonds) relative to control values (n = 3; closed circles); means ± SE.
DISCUSSION
TNF and contractile dysfunction.
TNF has a robust capacity to decrease the specific force of skeletal muscle. This response has been documented in respiratory muscle (21, 35, 61, 62) and limb muscle (1, 49, 67) of various mammalian species, although recent data suggest that individual muscles differ in their sensitivities to TNF (67). Contractile dysfunction can result from TNF overexpression in remote tissues (35, 67), systemic TNF administration in vivo (21, 62), or TNF incubation in vitro (1, 49, 61). Specific force is decreased within 1 h (21, 35) and at TNF plasma levels that are too low to cause muscle atrophy (35).
Our laboratory has a long-standing interest in the cellular mechanism of this response. Research using genetically engineered mice showed that contractile dysfunction is stimulated by TNF binding to the TNFR1 subtype (19, 21). The resulting decrement in specific force appears to be caused by myofilament dysfunction. In intact muscle fibers, TNF decreases specific force without depressing electrically stimulated calcium transients—results that suggested myofilament involvement (49). This interpretation was confirmed by studying permeabilized muscle fibers from TNF-treated animals; myofibrillar force is depressed, despite direct calcium activation (21).
The pathway that transduces the TNF/TNFR1 signal is less clear. Early reports implicated platelet-activating factor (1) or prostaglandin synthesis (61), but later research has focused on muscle-derived oxidants. TNF increases biochemical and genetic markers of oxidative stress (6, 8, 33) and stimulates intracellular oxidant activity (21, 33, 35)—changes that correlate with loss of force. More importantly, force can be preserved by pretreating muscle with nonspecific antioxidants (21, 35). These findings indicate that the rise in oxidant activity is required for contractile dysfunction but do not identify the redox cascade that is responsible.
NO signaling via nNOS.
A prior report by Alloatti and associates (1) indicated that TNF acts via muscle-derived NO to depress specific force. This finding is inconsistent with reports that TNF does not stimulate NO production by muscle cells (63) and that TNF opposes receptor-stimulated NO signaling (65). Therefore, we independently evaluated NO as a second messenger for TNF. Our data are entirely consistent with those of Alloatti et al. (1) and confirm their conclusion that TNF signals via NO to depress specific force. This concurrence was reached, despite qualitative differences between species (guinea pig vs. mouse) and muscle preparations (glycolytic limb muscle vs. aerobic respiratory muscle), which argues for biological robustness. The evidence that NO acts as a downstream messenger for TNF is consistent with the fact that direct NO exposure depresses specific force of skeletal muscle (26) by altering myofilament function (3).
Our current data identify nNOS as the source of TNF-stimulated NO. Skeletal muscle fibers constitutively express nNOS, which binds α1-syntrophin to associate with the dystrophin complex (7), localizing nNOS to the subsarcolemmal region. nNOS has a well-established role in transducing activation signals from membrane receptors, including κ-opioid receptors (13), N-methyl-D-aspartate receptors (56), and muscarinic M2 and M4 acetylcholine receptors (51). For G-protein coupled receptors, recent data show that nNOS complexes with RGSZ2, a regulator of G-protein signaling, and physically associates with membrane receptors to initiate redox signaling (51). The mechanism by which TNFR1 interacts with nNOS is less clear. To our knowledge, the current data are the first to show that nNOS is required for TNFR1 signaling in any cell type.
ROS as co-mediators.
We hypothesized that muscle-derived ROS contribute to TNF-induced dysfunction for several reasons. In our experience, ROS mimic the actions of TNF by increasing cytosolic oxidant activity (42) and depressing specific force of unfatigued muscle (47) via effects on myofilament function (2). Data from the current study clearly show that muscle-derived ROS are required for TNF-induced dysfunction. However, our data provide no evidence that TNF increases general ROS activity in the cytosol. It may be that ROS signaling occurs at low levels or in a compartmentalized process that protects the overall redox state of the cell (23). Alternatively, basal ROS levels may play a permissive role in TNFR1 signaling, for example, by enabling signal transduction via redox-sensitive proteins (17) or reacting with TNF-stimulated NO to generate peroxynitrite (40).
Clinical relevance.
Muscle weakness is a major complication of chronic inflammatory conditions, including chronic obstructive pulmonary disease (COPD), chronic heart failure, rheumatoid arthritis, and aging. Circulating TNF levels correlate inversely with muscle strength in these conditions (5, 9, 52, 66), suggesting a TNF-mediated process. Consistent with this thesis, chronic inflammatory conditions often increase biochemical markers of oxidative stress in muscle (4, 22, 41, 46, 60) and depress specific force (24, 34, 43, 57).
At present, there is no clinical standard of care nor a drug or nutritional strategy to preserve muscle function in chronic inflammatory conditions. Systemic TNF blockade could be beneficial, but anti-TNF therapy has significant side effects (14, 18, 59) and may increase mortality in some patient populations (11, 45). Alternatively, pharmacologic antioxidants could be used to target events downstream of TNF. For example, N-acetylcysteine (NAC) is a reduced thiol donor that opposes contractile dysfunction caused by TNF in animals (35) and improves quadriceps endurance in individuals with COPD (28). However, at clinical doses, NAC causes adverse reactions that can limit its use (16, 50). NO- and ROS-specific interventions are new approaches, suggested by our current findings. For example, several NOS inhibitors are available for experimental use in human subjects (12, 25, 53). Similarly, novel, small peptide molecules, which inhibit mitochondrial ROS production, are now being developed for clinical use (58). Our current data suggest that drugs in these two categories have the potential to alleviate weakness caused by TNF, which may be beneficial for individuals with chronic inflammatory disease.
Conclusion.
The signaling pathway by which TNF stimulates contractile dysfunction includes nNOS-derived NO and endogenous ROS as essential co-mediators. To our knowledge, obligate interdependence between these two redox cascades has not been demonstrated in skeletal muscle. This may simply reflect lack of scrutiny. Most studies of redox signaling, including reports from our laboratory, have addressed one cascade or the other but not both. In the future, it will be useful to test for NO/ROS interdependence in other aspects of muscle cell biology, e.g., glucose uptake, calcium regulation, and protein catabolism, which are redox sensitive.
GRANTS
Support for this project was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 5 R01-AR055974-04 (M. B. Reid) and the National Heart, Lung, and Blood Institute Grant 5 T32-HL086341-4 (S. A. Stasko).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.A.S., B.J.H., J.D.S., and M.B.R. conception and design of research; S.A.S., B.J.H., J.D.S., and J.S.M. performed experiments; S.A.S., B.J.H., J.D.S., and J.S.M. analyzed data; S.A.S., B.J.H., J.D.S., and M.B.R. interpreted results of experiments; S.A.S. and J.D.S. prepared figures; S.A.S., J.D.S., and M.B.R. drafted manuscript; S.A.S., J.D.S., J.S.M., and M.B.R. edited and revised manuscript; S.A.S., J.D.S., J.S.M., and M.B.R. approved final version of manuscript.
REFERENCES
- 1. Alloatti G, Penna C, Mariano F, Camussi G. Role of NO and PAF in the impairment of skeletal muscle contractility induced by TNF-alpha. Am J Physiol Regul Integr Comp Physiol 279: R2156–R2163, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509: 565–575, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of nitric oxide on single skeletal muscle fibres from the mouse. J Physiol 509: 577–586, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barreiro E, Peinado VI, Galdiz JB, Ferrer E, Marin-Corral J, Sanchez F, Gea J, Barbera JA. Cigarette smoke-induced oxidative stress: a role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am J Respir Crit Care Med 182: 477–488, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Beckham JC, Caldwell DS, Peterson BL, Pippen AM, Currie MS, Keefe FJ, Weinberg JB. Disease severity in rheumatoid arthritis: relationships of plasma tumor necrosis factor-alpha, soluble interleukin 2-receptor, soluble CD4/CD8 ratio, neopterin, and fibrin D-dimer to traditional severity and functional measures. J Clin Immunol 12: 353–361, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Bhatnagar S, Panguluri SK, Gupta SK, Dahiya S, Lundy RF, Kumar A. Tumor necrosis factor-alpha regulates distinct molecular pathways and gene networks in cultured skeletal muscle cells. PLoS One 5: e13262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84: 757–767, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J 15: 1753–1765, 1996 [PMC free article] [PubMed] [Google Scholar]
- 9. Cicoira M, Bolger AP, Doehner W, Rauchhaus M, Davos C, Sharma R, Al-Nasser FO, Coats AJ, Anker SD. High tumour necrosis factor-alpha levels are associated with exercise intolerance and neurohormonal activation in chronic heart failure patients. Cytokine 15: 80–86, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev 52: 129–197, 1972 [DOI] [PubMed] [Google Scholar]
- 11. Cottone M, Kohn A, Daperno M, Armuzzi A, Guidi L, D'Inca R, Bossa F, Angelucci E, Biancone L, Gionchetti P, Ardizzone S, Papi C, Fries W, Danese S, Riegler G, Cappello M, Castiglione F, Annese V, Orlando A. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol 9: 30–35, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cunha TM, Souza GR, Domingues AC, Carreira EU, Lotufo CM, Funez MI, Verri WA, Jr, Cunha FQ, Ferreira SH. Stimulation of peripheral kappa opioid receptors inhibits inflammatory hyperalgesia via activation of the PI3Kgamma/AKT/nNOS/NO signaling pathway. Mol Pain 8: 10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Simone C, Murri R, Maiorino A, Venier A, Caldarola G. Management of recurrent cutaneous abscesses during therapy with infliximab. Clin Ther 33: 1993–1996, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Eringa EC, Stehouwer CD, Walburg K, Clark AD, van Nieuw Amerongen GP, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-dependent vasoconstriction of skeletal muscle resistance arteries in the presence of tumor necrosis factor-alpha dependence on c-Jun N-terminal kinase. Arterioscler Thromb Vasc Biol 26: 274–280, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Ferreira LF, Campbell KS, Reid MB. N-acetylcysteine in handgrip exercise: plasma thiols and adverse reactions. Int J Sport Nutr Exerc Metab 21: 146–154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Fu B, Ustianowski AP, Watson KD, Lunt M, Symmons DP. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 mo of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 50: 124–131, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilliam LA, Moylan JS, Ferreira LF, Reid MB. TNF/TNFR1 signaling mediates doxorubicin-induced diaphragm weakness. Am J Physiol Lung Cell Mol Physiol 300: L225–L231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grounds MD, Radley HG, Gebski BL, Bogoyevitch MA, Shavlakadze T. Implications of cross-talk between tumour necrosis factor and insulin-like growth factor-1 signalling in skeletal muscle. Clin Exp Pharmacol Physiol 35: 846–851, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Hardin BJ, Campbell KS, Smith JD, Arbogast S, Smith J, Moylan JS, Reid MB. TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol 104: 694–699, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Jackson MJ, McArdle A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J Physiol 589: 2139–2145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45: 1–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jubrias SA, Odderson IR, Esselman PC, Conley KE. Decline in isokinetic force with age: muscle cross-sectional area and specific force. Pflugers Arch 434: 246–253, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Kellogg DL, Jr, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature 372: 546–548, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Kobzik L, Stringer B, Balligand JL, Reid MB, Stamler JS. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophys Res Commun 211: 375–381, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Koechlin C, Couillard A, Simar D, Cristol JP, Bellet H, Hayot M, Prefaut C. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am J Respir Crit Care Med 169: 1022–1027, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70: 2446–2453, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Kosmidou I, Vassilakopoulos T, Xagorari A, Zakynthinos S, Papapetropoulos A, Roussos C. Production of interleukin-6 by skeletal myotubes: role of reactive oxygen species. Am J Respir Cell Mol Biol 26: 587–593, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem 278: 2294–2303, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Lai CF, Shao JS, Behrmann A, Krchma K, Cheng SL, Towler DA. TNFR1-activated reactive oxidative species signals up-regulate osteogenic Msx2 programs in aortic myofibroblasts. Endocrinology 153: 3897–3910, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Langen RC, Schols AM, Kelders MC, Van Der Velden JL, Wouters EF, Janssen-Heininger YM. Tumor necrosis factor-alpha inhibits myogenesis through redox-dependent and -independent pathways. Am J Physiol Cell Physiol 283: C714–C721, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Levine S, Nguyen T, Kaiser LR, Rubinstein NA, Maislin G, Gregory C, Rome LC, Dudley GA, Sieck GC, Shrager JB. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med 168: 706–713, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Li X, Moody MR, Engel D, Walker S, Clubb FJ, Jr, Sivasubramanian N, Mann DL, Reid MB. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 102: 1690–1696, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 19: 362–370, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li YP, Lecker SH, Chen Y, Waddell ID, Goldberg AL, Reid MB. TNF-alpha increases ubiquitin-conjugating activity in skeletal muscle by up-regulating UbcH2/E220k. FASEB J 17: 1048–1057, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Li YP, Reid MB. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol 279: R1165–R1170, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J 12: 871–880, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Liaudet L, Vassalli G, Pacher P. Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front Biosci 14: 4809–4814, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, Mobius-Winkler S, Schubert A, Schuler G, Hambrecht R. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation 111: 1763–1770, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Murrant CL, Andrade FH, Reid MB. Exogenous reactive oxygen and nitric oxide alter intracellular oxidant status of skeletal muscle fibres. Acta Physiol Scand 166: 111–121, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Ottenheijm CA, Heunks LM, Sieck GC, Zhan WZ, Jansen SM, Degens H, de Boo T, Dekhuijzen PN. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 172: 200–205, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park CS, Park R, Krishna G. Constitutive expression and structural diversity of inducible isoform of nitric oxide synthase in human tissues. Life Sci 59: 219–225, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Pérez-Alvarez R, Díaz-Lagares C, García-Hernández F, Lopez-Roses L, Brito-Zerón P, Pérez-de-Lis M, Retamozo S, Bové A, Bosch X, Sanchez-Tapias JM., Forns X, Ramos-Casals M, BIOGEAS Study Group Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore) 90: 359–371, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Puente-Maestu L, Tejedor A, Lázaro A, de Miguel J, Alvarez-Sala L, González-Aragoneses F, Simón C, Agustí A. Site of mitochondrial reactive oxygen species production in skeletal muscle of COPD and its relationship with exercise oxidative stress. Am J Respir Cell Mol Biol 47: 358–362, 2012 [DOI] [PubMed] [Google Scholar]
- 47. Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol 75: 1081–1087, 1993 [DOI] [PubMed] [Google Scholar]
- 48. Reid MB, Kobzik L, Bredt DS, Stamler JS. Nitric oxide modulates excitation-contraction coupling in the diaphragm. Comp Biochem Physiol A Mol Integr Physiol 119: 211–218, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med 166: 479–484, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Reid MB, Stokic DS, Koch SM, Khawli FA, Leis AA. N-acetylcysteine inhibits muscle fatigue in humans. J Clin Invest 94: 2468–2474, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanchez-Blazquez P, Rodriguez-Munoz M, Bailon C, Garzon J. GPCRs promote the release of zinc ions mediated by nNOS/NO and the redox transducer RGSZ2 protein. Antioxid Redox Signal 17: 1163–1177, 2012 [DOI] [PubMed] [Google Scholar]
- 52. Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 64: 1183–1189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schrage WG, Wilkins BW, Johnson CP, Eisenach JH, Limberg JK, Dietz NM, Curry TB, Joyner MJ. Roles of nitric oxide synthase and cyclooxygenase in leg vasodilation and oxygen consumption during prolonged low-intensity exercise in untrained humans. J Appl Physiol 109: 768–777, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sriram S, Subramanian S, Sathiakumar D, Venkatesh R, Salerno MS, McFarlane CD, Kambadur R, Sharma M. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-kappaB. Aging Cell 10: 931–948, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 81: 209–237, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Szabadits E, Cserep C, Szonyi A, Fukazawa Y, Shigemoto R, Watanabe M, Itohara S, Freund TF, Nyiri G. NMDA receptors in hippocampal GABAergic synapses and their role in nitric oxide signaling. J Neurosci 31: 5893–5904, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Szentesi P, Bekedam MA, van Beek-Harmsen BJ, van der Laarse WJ, Zaremba R, Boonstra A, Visser FC, Stienen GJ. Depression of force production and ATPase activity in different types of human skeletal muscle fibers from patients with chronic heart failure. J Appl Physiol 99: 2189–2195, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Szeto HH, Schiller PW. Novel therapies targeting inner mitochondrial membrane–from discovery to clinical development. Pharm Res 28: 2669–2679, 2011 [DOI] [PubMed] [Google Scholar]
- 59. Tong D, Manolios N, Howe G, Spencer D. New onset sarcoid-like granulomatosis developing during anti-TNF therapy: an under-recognised complication. Intern Med J 42: 89–94, 2012 [DOI] [PubMed] [Google Scholar]
- 60. Vescovo G, Ravara B, Dalla Libera L. Skeletal muscle myofibrillar protein oxidation and exercise capacity in heart failure. Basic Res Cardiol 103: 285–290, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Wilcox P, Milliken C, Bressler B. High-dose tumor necrosis factor alpha produces an impairment of hamster diaphragm contractility. Attenuation with a prostaglandin inhibitor. Am J Respir Crit Care Med 153: 1611–1615, 1996 [DOI] [PubMed] [Google Scholar]
- 62. Wilcox PG, Wakai Y, Walley KR, Cooper DJ, Road J. Tumor necrosis factor alpha decreases in vivo diaphragm contractility in dogs. Am J Respir Crit Care Med 150: 1368–1373, 1994 [DOI] [PubMed] [Google Scholar]
- 63. Williams G, Brown T, Becker L, Prager M, Giroir BP. Cytokine-induced expression of nitric oxide synthase in C2C12 skeletal muscle myocytes. Am J Physiol Regul Integr Comp Physiol 267: R1020–R1025, 1994 [DOI] [PubMed] [Google Scholar]
- 64. Winkelman C. Inactivity and inflammation: selected cytokines as biologic mediators in muscle dysfunction during critical illness. AACN Clin Issues 15: 74–82, 2004 [DOI] [PubMed] [Google Scholar]
- 65. Xie J, Wang Y, Lippton H, Cai B, Nelson S, Kolls J, Summer WR, Greenberg SS. Tumor necrosis factor inhibits stimulated but not basal release of nitric oxide. Am Rev Respir Dis 148: 627–636, 1993 [DOI] [PubMed] [Google Scholar]
- 66. Yende S, Waterer GW, Tolley EA, Newman AB, Bauer DC, Taaffe DR, Jensen R, Crapo R, Rubin S, Nevitt M, Simonsick EM, Satterfield S, Harris T, Kritchevsky SB. Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax 61: 10–16, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zuo L, Nogueira L, Hogan MC. Effect of pulmonary TNF-alpha overexpression on mouse isolated skeletal muscle function. Am J Physiol Regul Integr Comp Physiol 301: R1025–R1031, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]