Abstract
Bacterial pneumonia is one of the leading causes of disease-related morbidity and mortality in the world, in part because the diagnostic tools for pneumonia are slow and ineffective. To improve the diagnosis success rates and treatment outcomes for bacterial lung infections, we are exploring the use of secondary electrospray ionization-mass spectrometry (SESI-MS) breath analysis as a rapid, noninvasive method for determining the etiology of lung infections in situ. Using a murine lung infection model, we demonstrate that SESI-MS breathprints can be used to distinguish mice that are infected with one of seven lung pathogens: Haemophilus influenzae, Klebsiella pneumoniae, Legionella pneumophila, Moraxella catarrhalis, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae, representing the primary causes of bacterial pneumonia worldwide. After applying principal components analysis, we observed that with the first three principal components (primarily comprised of data from 14 peaks), all infections were separable via SESI-MS breathprinting (P < 0.0001). Therefore, we have shown the potential of this SESI-MS approach for rapidly detecting and identifying acute bacterial lung infections in situ via breath analysis.
Keywords: bacteria, breath analysis, lung infection, SESI-MS, VOC
lower respiratory infections, including both community and hospital acquired infections (HAIs), are the leading burden of disease in the world, and the third leading cause of mortality (29). In the United States, ventilator-associated pneumonia is responsible for ∼15% of all HAIs, and 36% of HAI-related deaths (22), at an estimated annual cost of $0.78–1.50 billion in this country alone (41). The high morbidity and mortality of pneumonia is due in part to the lack of effective diagnostics. The typical culture-based methods for identifying pneumonia etiologies are slow, requiring days for pathogen identification, with a success rate at ∼20% (16). Even with the most sophisticated and thorough molecular-based analyses at in-patient care facilities, nearly half of pneumonia etiologies cannot be identified (14, 16, 49). Treatment choices, such as the administration of antibiotics, should be based on the pathogen causing the pneumonia, but until more accurate and faster diagnostics are developed, treatment decisions will continue to be partly speculative (2).
Molecular diagnostics (e.g., genomic and protein-based methods) are vast improvements over traditional culture-based methods; however, these protocols still rely on recovering pathogen material from the infection site (25). This presents a significant obstacle for diagnosing lower respiratory infections, particularly in young children, because they do not reliably produce sputum for clinical analysis (10, 40). Therefore, rapid, noninvasive methods for determining the etiology of lung infections in situ could significantly improve diagnosis success rates and treatment outcomes for lower respiratory infections. Breath-based diagnostics eliminate the need for sputum production and are under development. For instance, electronic nose sensors have been used to monitor the expired breath gases of ventilated patients and can detect the presence of pneumonia and respiratory tract infections (17, 18). In addition, others have demonstrated that breath may be used to diagnose the cause of lung infections because Pseudomonas aeruginosa, Mycobacterium tuberculosis, and Aspergillus fumigates infections can be differentiated from uninfected controls using breath analyses (8, 15, 31, 32, 37, 40, 43). However, all of these studies have focused on the presence vs. absence of a single pathogen, or in the case of electronic nose sensors, on the presence vs. absence of disease without etiological data.
The ultimate goal in developing a new diagnostic tool is to identify unknown causes of disease, and diagnosing the etiologies of lung infections directly from breath will require a robust and unique breathprint for each infectious species. We have previously explored the utility of secondary electrospray ionization-mass spectrometry (SESI-MS) for differentiating two common opportunistic lung pathogens in situ in a murine infection model (51). SESI-MS is a technique that can rapidly characterize volatile mixtures, separating the components by their mass-to-charge (m/z) ratio, yielding a mass spectral fingerprint of the mixtures (3, 26). We have found that the SESI-MS fingerprints of breath, also known as breathprints, of mice infected with Staphylococcus aureus and P. aeruginosa are unique and reproducible, and that the breath can be used to differentiate strains of P. aeruginosa in situ (51). In the experiments described herein, we aim to further prove the utility of SESI-MS breathprints for diagnosis by analyzing the breath of mice with lung infections caused by one of seven different bacterial lung pathogens: Haemophilus influenzae, Klebsiella pneumoniae, Legionella pneumophila, Moraxella catarrhalis, P. aeruginosa, S. aureus, and Streptococcus pneumoniae, representing the primary causes of bacterial pneumonia worldwide (14, 20, 34, 36, 49). We observe that in addition to markers that may be used to distinguish infected lungs from healthy controls, the SESI-MS breathprints from all seven lung infections are unique, moving the concept of breath-based diagnostics another step closer to practical application.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study were H. influenzae ATCC 51907, P. aeruginosa PAO1-UW, S. aureus RN450 (courtesy of Prof. G. L. Archer, Virginia Commonwealth University, Richmond, VA), L. pneumophila ATCC 33152, S. pneumoniae ATCC 6301, M. catarrhalis ATCC 43628, and K. pneumoniae ATCC 13883. Before the bacteria were inoculated into the mice airways, strains were incubated aerobically in tryptic soy broth (16 h, 37°C; final cell counts >109 CFU/ml). After breath collection, the lungs were harvested and homogenized in 1 ml PBS, and lung bacterial cell counts were obtained by plating.
Mice and microbial airway exposure protocols.
Six- to 8-wk-old male C57BL/6J mice were purchased from The Jackson Laboratories (Bar Harbor, ME). The protocols for animal infection and respiratory physiology measurements were approved by the Institutional Animal Care and Use Committee, in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines. All mice were housed in the Association for Assessment and Accreditation of Laboratory Animal Care–accredited animal facility at the University of Vermont (Burlington, VT). Overnight cultures of bacteria were measured for optical density, centrifuged at 13,000 g for 1 min, washed twice with PBS, and resuspended in 40 μl PBS to give the desired concentration of bacteria (listed in Table 1). Mice were briefly anesthetized (isoflurane by inhalation) and infected by oropharyngeal aspiration as described previously (1, 48). Additional mice were exposed to 40 μl PBS as a negative control. Six mice per group were exposed and tests were conducted over several days to ensure data reproducibility.
Table 1.
Infection doses and end-point bacterial cell counts, six mice per group were tested in this study
| Bacteria | Infection dose (CFU/lung) | Infection time (h) | Lung harvest bacterial counts (CFU/lung) | SE |
|---|---|---|---|---|
| Moraxella catarrhalis | 1.0 × 108 | 3 | 2.4 × 106 | 7.0 × 104 |
| Klebsiella pneumoniae | 1.0 × 107 | 24 | 7.8 × 104 | 2.2 × 103 |
| Pseudomonas aeruginosa | 1.0 × 107 | 24 | 3.5 × 105 | 5.6 × 103 |
| Staphylococcus aureus | 1.0 × 108 | 24 | 1.6 × 106 | 1.1 × 105 |
| Streptococcus pneumoniae | 5.0 × 106 | 24 | 2.0 × 105 | 1.1 × 104 |
| Haemophilus influenzae | 1.0 × 108 | 48 | 5.4 × 105 | 4.6 × 103 |
| Legionella pneumophila | 2.5 × 106 | 48 | 1.7 × 104 | 2.0 × 102 |
Mice ventilation and breath sample collection.
At 3 h, 24 h, or 48 h post infection (Table 1), the mice were anesthetized with pentobarbital and their tracheas were cannulated. The mice were placed on the ventilator and paralyzed with intraperitoneal pancuronium bromide (0.5 mg/kg), and an electrocardiogram was used to monitor heart rate to ensure proper anesthesia. Breath coming out of the ventilator was collected in 5-liter Tedlar bags (SKC, Eighty Four, PA) at 180 breaths/min with a positive end-expiratory pressure of 3 cmH2O for 40–60 min.
Bronchoalveolar lavage fluid: hematology and lung damage assays.
After breath collection, 1 ml of cold PBS with 5% fetal bovine serum (FBS) was instilled into the lungs and the bronchoalveolar lavage fluid (BALF) was collected through the cannula installed previously for ventilation. BALF cells were pelleted and immediately resuspended in the same solution (PBS + 5% FBS). Total cells were counted using an ADVIA cell counter (Bayer, Terrytown, NY). Then, BALF cells were fixed onto glass slides (2 × 104 cells/slide), stained with Hema-3 (Biochemical Sciences, Swedesboro, NJ), and the leukocytes were counted (300/slide) and categorized as macrophages, eosinophils, polymorphonuclear neutrophils (PMNs), or lymphocytes on the basis of characteristic morphology and staining.
In vivo lung tissue damage was determined by measuring lactose dehydrogenase activity (LDH) in BALF samples using the CytoTox 96 NonRadioactive Cytotoxicity Assay (Promega, Madison, WI), according to the manufacturer's instructions.
Secondary electrospray ionization–mass spectrometry (SESI-MS) and breath sampling.
SESI-MS breath analysis was performed in positive-ion mode within 1 h of breath collection, as previously described (27, 50) on a modified SCIEX API 3000 mass spectrometer (Concord, ON, Canada; for a detailed schematic of the SESI-MS system, see Ref. 3). The breath sample was introduced into the reaction chamber for 30 s at a flow rate of 3 liters/min, and supplemented with 2 liters/min CO2 (99.99%) at ambient temperature. Formic acid [0.1% (v/v)] was used as the electrospray solution, delivered at a flow rate of 5 nl/s through a nonconductive silica capillary (40 μm i.d.). The operation voltage was ∼3.5 kV, and the declustering, focusing, and entrance potentials for the mass spectrometer were set to 5 V, 350 V, and 2 V, respectively. Spectra were collected for 30 s as an accumulation of 10 scans. The system was flushed with CO2 between samples until the spectrum returned to background levels.
Data analysis and statistics.
Analyst 1.4.2 software (Applied Biosystems, Foster City, CA) was used for spectra collection and raw data processing. The mass spectra shown are the average spectra of all sample replicates in each group. Full scan spectra are blank-subtracted (the blank spectrum is humidified room air collected using the same procedure as for mice breath) and normalized to the peak of greatest intensity. MS/MS fragmentation spectra of some high-abundance peaks in the breath sample were also collected and built into a small database using NIST MS search V 2.0 software (National Institute of Standards and Technology, Gaithersburg, MD). The spectral pattern comparison algorithms of the NIST V 2.0 software were used to assess the similarities between the fragmentation patterns, as previously described (9, 28, 42). For this study, peaks from different breath samples that have fragmentation pattern match scores of 700 or greater (≥70% match) are considered identical peaks (i.e., identical variables) for subsequent principal component analysis, described below.
The statistical significance of total leukocytes, PMN counts from BALF, and LDH activity between infection and control groups were determined by a t-test (3-h infection) or one-way ANOVA (24- and 48-h infections) using JMP version 10 (SAS Institute, Cary, NC). To meet the assumption of normal distribution for the ANOVA and t-test, total leukocytes and PMN counts were log-transformed before the analysis. SAS version 9.2 (SAS Institute) and JMP version 10 were used to conduct spectral principal component analysis (PCA) on absolute intensity spectra, and to determine the statistical significance of observed PCA score differences. Peaks between 20 and 200 m/z and signal-to-noise ratios greater than 2 were used as variables for PCA, while all experimental replicates were used as observations.
RESULTS
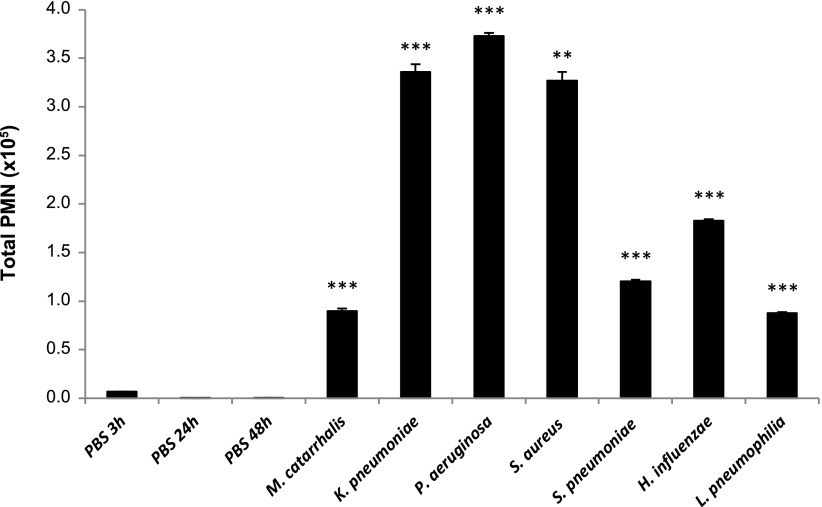
We employed a murine lung infection model using seven different bacteria, establishing a 3-h infection with M. catarrhalis; 24-h infections with K. pneumoniae, P. aeruginosa, S. aureus, and S. pneumoniae; and 48-h infections with H. influenzae and L. pneumophila (Table 1). Bacterial cell counts from lung homogenates indicate that bacteria are present in the lungs at the time of breath collection. The data also show that there is a clearing of bacteria from the initial lung inoculum, as is expected for these doses and infection times (4, 11, 13, 19, 24). To confirm the establishment of infection, the BALF leukocyte cell count, PMN total count, and LDH activity were measured. We observed that the BALF leukocytes were significantly increased in the infection groups vs. controls in most cases (data not shown), which is consistent with previous mouse infection models for each pathogen in this study (5, 21, 23, 38, 39, 47, 48). PMN infiltration is one of the most important steps during the innate immune response against bacterial infections (35), and we observed that the total PMN count was significantly different from that of the control groups in all instances (Fig. 1), with P < 0.001 (t-test or one-way ANOVA). Further evidence of infection can be ascertained by the presence of lung damage, measured by extracellular LDH activity in BALF (45). We report here that LDH levels were higher in the BALF of all infected mice compared with uninfected controls (P < 0.05), except for S. pneumoniae. Taken together, the leukocyte cell counts, total PMN counts, and LDH activity indicate that infections were established for all bacteria.
Fig. 1.
Total number of polymorphonuclear neutrophils (PMNs) in bronchoalveolar lavage fluid (BALF). Statistical significance determined by t-test (3-h infection) or one-way ANOVA (24- and 48-h infections); ***P < 0.0001, **P < 0.001 compared with the corresponding PBS-treated mice (control) as per Table 1. Values represent mean ± SE of all replicates in each group.
The utility of SESI-MS breathprinting relies on high intergroup differences between breathprints from different infections, coupled with high intragroup reproducibility. We calculated the average Spearman correlation coefficients between biological replicate breathprints within a single group to assess the reproducibility of SESI-MS. For six out of seven infection groups and all three PBS control groups, the reproducibility of the breathprints is high, ranging from 0.81 to 0.94 (standard deviation ≤0.09). The exception is M. catarrhalis (0.64 ± 0.14), possibly because of its quick clearance rate (typically less than 4 h) coupled with the short time scale (3 h) between infecting inoculation and breath measurement (4, 12).
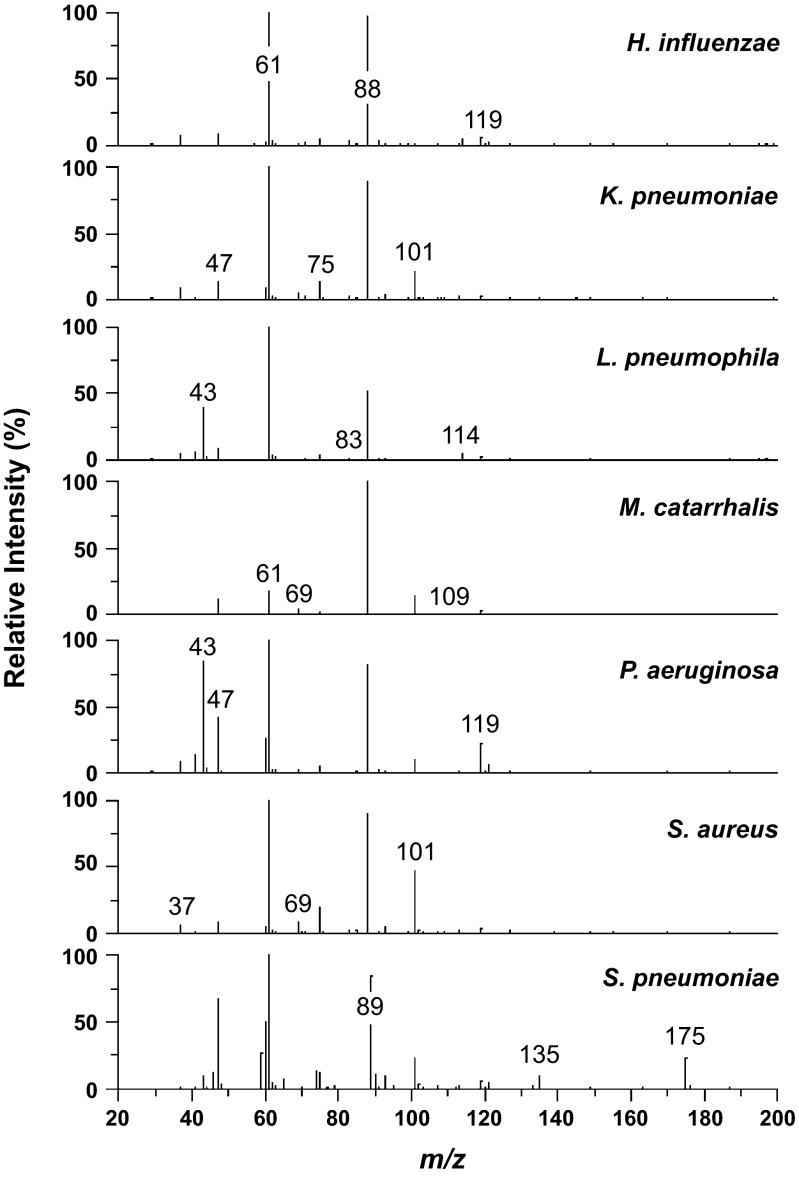
The SESI-MS breathprints for infected mice show unique patterns for each bacterium (Fig. 2). To compare and contrast the details of the breathprint patterns from the seven bacterial species involved in this study, we list the peaks from each breathprint in Table 2. Comparing the presence and absence of peaks across these seven infection groups (Table 2) and the uninfected controls (data not shown), we find that M. catarrhalis has two unique peaks (m/z = 54 and 92), K. pneumoniae has two unique peaks (m/z = 145 and 183), S. aureus has one (m/z = 81), and S. pneumoniae has three (m/z = 46, 59, and 74), contributing distinguishing markers in the breathprints for these infections. Beyond the unique peaks for individual species, the intensities of the common peaks in the spectra also carry information, as observed by the patterns generated in Table 2. For example, peak m/z = 61 can be measured from all seven bacterial infections, with intensities varying by an order of magnitude (106 to 107 cps), whereas peaks m/z = 41 and 119 vary by two orders of magnitude. The patterns of peak intensities across the breathprint mass range also confer unique information for bacterial identification.
Fig. 2.
SESI-MS breathprints from mice with H. influenzae, K. pneumoniae, L. pneumophila, M. catarrhalis, P. aeruginosa, S. aureus, or S. pneumoniae lung infections. The spectra shown here are representative spectra (average of six replicates from each group).
Table 2.
Absolute intensities of breathprint peaks from mice infected with one of seven lung pathogens
| Peaks | MC | KP | SA | PA | SP | HI | LP | Peaks | MC | KP | SA | PA | SP | HI | LP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 27† | ++ | ++ | 108 | +++ | ++ | ||||||||||
| 29 | ++ | +++ | ++ | +++ | +++ | +++ | 109 | ++ | +++ | +++ | ++ | ++ | |||
| 33 | ++ | ++ | + | 110 | + | ++ | |||||||||
| 37* | ++++ | +++ | +++ | +++ | ++++ | +++ | 111 | ++ | ++ | ||||||
| 39 | + | ++ | 112 | + | ++ | +++ | |||||||||
| 41* | ++ | +++ | +++ | ++++ | +++ | ++ | ++++ | 113* | +++ | +++ | +++ | +++ | +++ | ||
| 42* | ++ | ++ | 114 | +++ | +++ | +++ | |||||||||
| 43* | ++++ | ++++ | ++++ | 119† | +++ | +++ | +++ | ++++ | ++++ | +++ | +++ | ||||
| 44* | +++ | +++ | +++ | 120 | ++ | +++ | +++ | +++ | |||||||
| 46 | ++++ | 121 | +++ | ++++ | +++ | ||||||||||
| 47 | ++++ | ++++ | +++ | ++++ | +++++ | ++++ | ++++ | 123 | ++ | ||||||
| 48 | ++ | ++ | +++ | +++ | ++ | 125 | ++ | ++ | ++ | ||||||
| 54 | ++ | 127* | ++ | +++ | +++ | +++ | ++ | +++ | +++ | ||||||
| 59 | ++++ | 129 | ++ | ||||||||||||
| 60 | ++++ | +++ | ++++ | +++++ | +++ | 133 | ++ | +++ | |||||||
| 61 | ++++ | +++++ | +++++ | ++++ | +++++ | +++++ | +++++ | 135 | +++ | ++++ | |||||
| 62 | ++ | +++ | +++ | +++ | ++++ | +++ | +++ | 136 | ++ | ++ | ++ | ||||
| 63 | +++ | +++ | +++ | +++ | +++ | +++ | 139 | +++ | +++ | ++ | +++ | ++ | |||
| 65 | + | ++ | ++++ | 141 | ++ | ||||||||||
| 69 | +++ | +++ | +++ | +++ | +++ | ++ | 143 | +++ | ++ | +++ | |||||
| 70 | ++ | ++ | +++ | ++ | +++ | 145 | +++ | ||||||||
| 71 | +++ | ++ | +++ | ++ | 147 | + | ++ | ||||||||
| 74 | ++++ | 149 | ++ | +++ | +++ | +++ | +++ | +++ | ++ | ||||||
| 75† | +++ | ++++ | ++++ | +++ | ++++ | +++ | +++ | 150 | + | ++ | ++ | ||||
| 76 | ++ | +++ | +++ | + | ++ | 151 | ++ | ||||||||
| 77 | ++ | ++ | ++ | ++ | +++ | ++ | 152 | + | |||||||
| 79 | +++ | 153 | ++ | ||||||||||||
| 81 | ++ | 155 | ++ | ++ | ++ | ++ | ++ | ||||||||
| 83 | +++ | +++ | ++ | +++ | +++ | 157 | ++ | ++ | |||||||
| 84 | ++ | + | ++ | 159 | +++ | ++ | ++ | ||||||||
| 85 | + | +++ | +++ | +++ | +++ | ++ | 161 | ++ | |||||||
| 88* | +++++ | +++++ | ++++ | ++++ | +++++ | ++++ | 163 | +++ | +++ | ||||||
| 89* | +++++ | 165 | ++ | ||||||||||||
| 90 | ++++ | 167 | ++ | ++ | ++ | ||||||||||
| 91 | ++ | +++ | +++ | +++ | +++ | +++ | +++ | 170 | +++ | ++ | +++ | +++ | ++ | ||
| 92 | ++ | 175 | ++++ | ||||||||||||
| 93† | ++ | +++ | +++ | +++ | ++++ | +++ | +++ | 176 | +++ | ||||||
| 95 | +++ | 177 | ++ | ++ | ++ | ++ | |||||||||
| 97 | ++ | +++ | 183 | ++ | |||||||||||
| 99* | ++ | +++ | +++ | ++ | +++ | ++ | 185 | ++ | ++ | ++ | |||||
| 100 | ++ | ++ | 187 | +++ | ++ | +++ | +++ | +++ | +++ | ||||||
| 101 | ++++ | ++++ | ++++ | ++++ | ++++ | +++ | 191 | ++ | ++ | ||||||
| 102 | +++ | +++ | ++++ | 195 | ++ | ++ | ++ | ||||||||
| 103 | +++ | +++ | ++ | +++ | ++ | 197 | ++ | ++ | +++ | ++ | |||||
| 107 | +++ | +++ | ++ | +++ | +++ | 199 | +++ | +++ |
MC, M. catarrhalis; KP, K. pneumoniae; SA, S. aureus; PA, P. aeruginosa; SP, S. pneumoniae; HI, H. influenzae; LP, L. pneumophila. Order of peak intensity (cps): +103; ++104; +++105; ++++106; +++++107. Peaks that have loadings with an absolute value >0.7 on principal component 1 (*) and 2 (†).
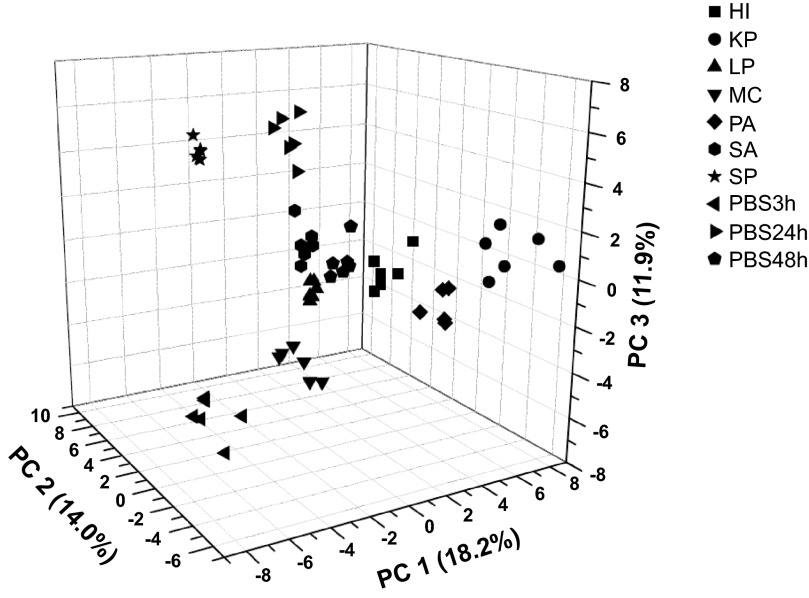
To determine the statistical difference between groups, we performed principal components analysis with the absolute intensities of the breathprint peaks (Fig. 3). Using the first three principal components (PC), accounting for 44.1% of the total variance, all infections were separable via their SESI-MS breathprints (P < 0.0001). In addition, all of the infection breathprints are separated from uninfected controls using the first three PCs. Examining the PC loadings for each individual peak, where the closer the loading value is to 1, the higher the peak-to-PC influence, we found that 10 peaks have strong contributions (absolute PC loadings >0.7) to PC1, four peaks have strong contribution to PC2 (Table 2).
Fig. 3.
Principal components analysis of spectral breathprints from mice with lung infections caused by H. influenzae (HI), K. pneumoniae (KP), L. pneumophila (LP), M. catarrhalis (MC), P. aeruginosa (PA), S. aureus (SA), S. pneumoniae (SP), and three PBS control groups. Six mice per group were tested in this study.
DISCUSSION
MS/MS fragmentation of breath volatiles, which can be used for compound identification and peak verification, is a capability that is afforded by SESI-MS, unlike similar mass spectrometry methods such as selected ion flow tube–mass spectrometry (SIFT-MS) and proton transfer reaction–mass spectrometry (PTR-MS) (6, 7, 46). We conducted more than 200 MS/MS product ion scans to obtain peak fragmentation data on the most abundant peaks from each breath sample, then we used NIST 08 MS software to compare the fragmentation patterns between biological replicates and between bacterial groups. We confirmed that all peaks with the same m/z have similar fragmentation patterns (match score >700), and therefore should be recognized as the same compound or group of compounds. Comparing our SESI-MS data to previously published breath analyses, seven peaks listed in Table 2 (peaks m/z = 101, 103, 107, 121, 129, 143, and 157), which are observed in the breath of the infected mice in our study, could be tentatively assigned as compounds that have been identified by Peters and colleagues (30, 44), whereas standards tests will be needed before these identifications can be confirmed. The studies by Peters et al. examine inflammation markers (i.e., no infection involved), and therefore, these seven peaks in the breath of infected mice may be markers that are host-derived, rather than pathogen metabolites. We hypothesize that portions of the distinguishing patterns in the SESI-MS breathprints for each pathogen are also host-derived, with the immune system mounting bacterium-specific responses to some infections. We are presently conducting experiments to parse apart the bacterium and host contributions to SESI-MS breathprints.
Transitioning SESI-MS breathprinting from a mouse model to diagnosing human lung infections is an admittedly large step that we aim to take in the near future. The biggest hurdle will be accommodating the high interindividual variability that exists in the human breath volatilome (33). In developing breath-based diagnostics, it will be necessary to address the influences that genetic, environmental, and behavioral factors have over breath volatiles, which will require many more than six subjects per infection group as was used in these mouse experiments. However, the breathprints of the seven different infections were observed to be highly unique and reproducible, even with the small group size used in this study (six mice per group; P < 0.0001), demonstrating the incredible amount of information contained in each breathprint and suggesting that it will be possible to overcome the variability we expect to encounter in human breathprints. In addition, it has been shown that highly specific and sensitive breath tests for human lung infections can be developed when multiple breath volatiles are used for diagnosis (37). Because SESI-MS breathprints measure the relative abundances of many breath volatiles simultaneously, we feel that it holds promise for diagnosing human bacterial lung infections in the future.
To the best of our knowledge, this is the first study to compare and contrast the breath volatile biomarkers from lung infections caused by H. influenzae, K. pneumoniae, L. pneumophila, M. catarrhalis, P. aeruginosa, S. aureus, and S. pneumoniae. We have demonstrated that SESI-MS breathprinting can be used to distinguish all seven bacterial infections in situ (P < 0.0001), providing evidence that SESI-MS can be a powerful tool for the detection and identification of bacterial lung infections using breath analysis.
GRANTS
Support for this study was provided by National Center for Research Resources Grant 5P20RR021905-07, National Institute of General Medical Sciences Grant 8 P20 GM103496-07, National Aeronautical and Space Administration EPSCoR Grant NNH09ZNE002C, and by a postdoctoral fellowship from the Cystic Fibrosis Foundation to H.D. Bean.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.Z., H.D.B., and J.E.H. conception and design of research; J.Z., H.D.B., and J.J.-D. performed experiments; J.Z. and J.J.-D. analyzed data; J.Z., H.D.B., and J.J.-D. interpreted results of experiments; J.Z. prepared figures; J.Z. and J.J.-D. drafted manuscript; J.Z., H.D.B., J.J.-D., and J.E.H. edited and revised manuscript; J.Z., H.D.B., J.J.-D., and J.E.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Lennart Lundblad, Nirav Dapthary, and Minara Aliyeva from the Vermont Lung Center ventilation facility for their help. We thank Jenna Allard for her technical assistance for the mice work; Dr. Fiona Baird for her contribution to experimental design; and Pierre Galea, Jackson Sengle, and Frederick Naumann for their assistance with breath collection.
REFERENCES
- 1. Allard JB, Rinaldi L, Wargo MJ, Allen G, Akira S, Uematsu S, Poynter ME, Hogan DA, Rincon M, Whittaker LA. Th2 allergic immune response to inhaled fungal antigens is modulated by TLR-4-independent bacterial products. Eur J Immunol 39: 776–788, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anevlavis S, Bouros D. Community acquired bacterial pneumonia. Expert Opin Pharmacother 11: 361–374, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Bean HD, Zhu J, Hill JE. Characterizing bacterial volatiles using secondary electrospray ionization mass spectrometry (SESI-MS). J Vis Exp 52 (Online). http://www.jove.com/details.php?id=2664 [accessed Nov. 1, 2012]. [DOI] [PMC free article] [PubMed]
- 4. Becker PD, Bertot GM, Souss D, Ebensen T, Guzman CA, Grinstein S. Intranasal vaccination with recombinant outer membrane protein CD and adamantylamide dipeptide as the mucosal adjuvant enhances pulmonary clearance of Moraxella catarrhalis in an experimental murine model. Infect Immun 75: 1778–1784, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broug-Holub E, Toews GB, Van Iwaarden JF, Strieter RM, Kunkel L, Paine R, 3rd, Standiford TJ. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun 65: 1139–1146, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bunge M, Araghipour N, Mikoviny T, Dunkl J, Schnitzhofer R, Hansel A, Schinner F, Wisthaler A, Margesin R, Mark TD. On-line monitoring of microbial volatile metabolites by proton transfer reaction-mass spectrometry. Appl Environ Microbiol 74: 2179–2186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carroll W, Lenney W, Wang TS, Ŝpanêl P, Alcock A, Smith D. Detection of volatile compounds emitted by Pseudomonas aeruginosa using selected ion flow tube mass spectrometry. Pediatr Pulmonol 39: 452–456, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Chambers ST, Syhre M, Murdoch DR, McCartin F, Epton MJ. Detection of 2-pentylfuran in the breath of patients with Aspergillus fumigatus. Med Mycol 47: 468–476, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Cristoni S, Molin L, Lai A, Bernardi LR, Pucciarelli S, Agostini M, Bedin C, Nitti D, Seraglia R, Repetto O, Dibari VF, Orlandi R, Sinues PM, Traldi P. Maldi-MS-NIST library approach for colorectal cancer diagnosis. Rapid Commun Mass Spectrom 23: 2839–2845, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Esposito S, Principi N. Unsolved problems in the approach to pediatric community-acquired pneumonia. Curr Opin Infect Dis 25: 286–291, 2012 [DOI] [PubMed] [Google Scholar]
- 11. Essilfie AT, Simpson JL, Horvat JC, Preston JA, Dunkley ML, Foster PS, Gibson PG, Hansbro PM. Haemophilus influenzae infection drives IL-17-mediated neutrophilic allergic airways disease. PLoS Pathog 7: e1002244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forsgren A, Brant M, Riesbeck K. Immunization with the truncated adhesin Moraxella catarrhalis immunoglobulin D-binding protein (MID764–913) is protective against M. catarrhalis in a mouse model of pulmonary clearance. J Infect Dis 190: 352–355, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Frutuoso MS, Hori JI, Pereira MS, Junior DS, Sonego F, Kobayashi KS, Flavell RA, Cunha FQ, Zamboni DS. The pattern recognition receptors NOD1 and NOD2 account for neutrophil recruitment to the lungs of mice infected with Legionella pneumophila. Microbes Infect 12: 819–827, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Gentile A, Bardach A, Ciapponi A, Garcia-Marti S, Aruj P, Glujovsky D, Calcagno JI, Mazzoni A, Colindres RE. Epidemiology of community-acquired pneumonia in children of Latin America and the Caribbean: a systematic review and meta-analysis. Int J Infect Dis 16: e5-e15, 2012 [DOI] [PubMed] [Google Scholar]
- 15. Gilchrist FJ, Razavi C, Webb AK, Jones AM, Ŝpanêl P, Smith D, Lenney W. An investigation of suitable bag materials for the collection and storage of breath samples containing hydrogen cyanide. J Breath Res 6: 036004, 2012 [DOI] [PubMed] [Google Scholar]
- 16. Herrero FS, Olivas JB. Microbiology and risk factors for community-acquired pneumonia. Semin Respir Crit Care Med 33: 220–231, 2012 [DOI] [PubMed] [Google Scholar]
- 17. Hockstein NG, Thaler ER, Torigian D, Miller WT, Deffenderfer O, Hanson CW. Diagnosis of pneumonia with an electronic nose: correlation of vapor signature with chest computed tomography scan findings. Laryngoscope 114: 1701–1705, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Hockstein NG, Thaler ER, Yuanqing L, Lee DD, Hanson CW. Correlation of pneumonia score with electronic nose signature: a prospective study. Ann Otol Rhinol Laryngol 114: 504–508, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Hoogendijk AJ, Diks SH, Peppelenbosch MP, Van Der Poll T, Wieland CW. Kinase activity profiling of Gram-negative pneumonia. Mol Med 17: 741–747, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunter JD. Ventilator associated pneumonia. Postgrad Med J 82: 172–178, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kapetanovic R, Jouvion G, Fitting C, Parlato M, Blanchet C, Huerre M, Cavaillon JM, Adib-Conquy M. Contribution of NOD2 to lung inflammation during Staphylococcus aureus-induced pneumonia. Microbes Infect 12: 759–767, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Klevens RM, Edwards JR, Richards CL, Jr, Horan TC, Gaynes RP, Pollock DA, Cardo DM. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 122: 160–166, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kyd JM, Cripps AW, Murphy TF. Outer-membrane antigen expression by Moraxella (Branhamella) catarrhalis influences pulmonary clearance. J Med Microb 47: 159–168, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Li GQ, Bai XG, Li CR, Yang XY, Hu XX, Yuan M, Zhang WX, Lou RH, Guo HY, Jiang JD, You XF. In vivo antibacterial activity of chinfloxacin, a new fluoroquinolone antibiotic. J Antimicrob Chemother 67: 955–961, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Lung ML, Codina G. Molecular diagnosis in HAP/VAP. Curr Opin Crit Care 18: 487–494, 2012 [DOI] [PubMed] [Google Scholar]
- 26. Martinez-Lozano P, de la Mora J. Electrospray ionization of volatiles in breath. Int J Mass Spectrom 265: 68–72, 2007 [Google Scholar]
- 27. Martínez-Lozano P, de la Mora J. On-line detection of human skin vapors. J Am Soc Mass Spectrom 20: 1060–1063, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Martinez-Lozano P, Zingaro L, Finiguerra A, Cristoni S. Secondary electrospray ionization-mass spectrometry: breath study on a control group. J Breath Res 5: 2011 [DOI] [PubMed] [Google Scholar]
- 29. Mathers C, Boerma T, Fat DM. The global burden of disease: 2004 update. Geneva, Switzerland: World Health Organization, 2008 [Google Scholar]
- 30. Neuhaus S, Seifert L, Vautz W, Nolte J, Bufe A, Peters M. Comparison of metabolites in exhaled breath and bronchoalveolar lavage fluid samples in a mouse model of asthma. J Appl Physiol 111: 1088–1095, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Phillips M, Basa-Dalay V, Bothamley G, Cataneo RN, Lam PK, Natividad MP, Schmitt P, Wai J. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis 90: 145–151, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Phillips M, Cataneo RN, Condos R, Erickson GA, Greenberg J, La Bombardi V, Munawar MI, Tietje O. Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis 87: 44–52, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Phillips M, Herrera J, Krishnan S, Zain M, Greenberg J, Cataneo RN. Variation in volatile organic compounds in the breath of normal humans. J Chromatogr B Biomed Sci Appl 729: 75–88, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Prayle A, Atkinson M, Smyth A. Pneumonia in the developed world. Paediatr Respir Rev 12: 60–69, 2011 [DOI] [PubMed] [Google Scholar]
- 35. Razavi HM. Pulmonary neutrophil infiltration in murine sepsis: role of inducible nitric oxide synthase. Am J Respir Crit Care Med 170: 227–233, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Rello J. Bench-to-bedside review: therapeutic options and issues in the management of ventilator-associated bacterial pneumonia. Crit Care 9: 259–265, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robroeks CM, van Berkel JJ, Dallinga JW, Jobsis Q, Zimmermann LJ, Hendriks HJ, Wouters MF, van der Grinten CP, van de Kant KD, van Schooten FJ, Dompeling E. Metabolomics of volatile organic compounds in cystic fibrosis patients and controls. Pediatr Res 68: 75–80, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Rosseau S, Hocke A, Mollenkopf H, Schmeck B, Suttorp N, Kaufmann SH, Zerrahn J. Comparative transcriptional profiling of the lung reveals shared and distinct features of Streptococcus pneumoniae and influenza A virus infection. Immunology 120: 380–391, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roy CR, Fontana MF, Banga S, Barry KC, Shen X, Tan Y, Luo ZQ, Vance RE. Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila. PLoS Pathog 7: e1001289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scott-Thomas A, Syhre M, Pattemore P, Epton M, Laing R, Pearson J, Chambers S. 2-Aminoacetophenone as a potential breath biomarker for Pseudomonas aeruginosa in the cystic fibrosis lung. BMC Pulm Med 10: 56, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scott RD., II The direct medical costs of healthcare-associated infections in U. S. hospitals and the benefits of prevention. Atlanta: Centers for Disease Control and Prevention, 2009 [Google Scholar]
- 42. Sinues PM, Alonso-Salces RM, Zingaro L, Finiguerra A, Holland MV, Guillou C, Cristoni S. Mass spectrometry fingerprinting coupled to National Institute of Standards and Technology Mass Spectral search algorithm for pattern recognition. Anal Chim Acta 755: 28–36, 2012 [DOI] [PubMed] [Google Scholar]
- 43. Syhre M, Manning L, Phuanukoonnon S, Harino P, Chambers ST. The scent of Mycobacterium tuberculosis—part II breath. Tuberculosis 89: 263–266, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Vautz W, Nolte J, Bufe A, Baumbach JI, Peters M. Analyses of mouse breath with ion mobility spectrometry: a feasibility study. J Appl Physiol 108: 697–704, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Vijeyaratnam GS, Corrin B. Pulmonary histiocytosis simulating desquamative interstitial pneumonia in rats receiving oral iprindole. J Pathol 108: 105–113, 1972 [DOI] [PubMed] [Google Scholar]
- 46. Wang TS, Smith D, Ŝpanêl P. Selected ion flow tube, SIFT, studies of the reactions of H3O+, NO+ and O2+ with compounds released by Pseudomonas and related bacteria. Int J Mass Spectrom 233: 245–251, 2004 [Google Scholar]
- 47. Wang X, Moser C, Louboutin JP, Lysenko ES, Weiner DJ, Weiser JN, Wilson JM. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J Immunol 168: 810–815, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Wargo MJ, Gross MJ, Rajamani S, Allard JL, Lundblad LK, Allen GB, Vasil ML, Leclair LW, Hogan DA. Hemolytic phospholipase C inhibition protects lung function during Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 184: 345–354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 67: 71–79, 2012 [DOI] [PubMed] [Google Scholar]
- 50. Zhu J, Bean HD, Kuo YM, Hill JE. Fast detection of volatile organic compounds from bacterial cultures by secondary electrospray ionization-mass spectrometry. J Clin Microbiol 48: 4426–4431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu J, Bean HD, Wargo MJ, Leclair LW, Hill JE. Detecting bacterial lung infections: in vivo evaluation of in vitro volatile fingerprints. J Breath Res 7: 016003, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]