Abstract
Inhalation of tumor necrosis factor-alpha (TNF-α), a proinflammatory cytokine, induces airway hyperresponsiveness, and the underlying mechanism is not fully understood. Hypersensitivity of vagal bronchopulmonary C-fiber afferents is known to contribute to the airway hyperresponsiveness during an airway inflammatory reaction. Because activation of these afferents can elicit pulmonary chemoreflexes, this study was designed to determine if a pretreatment with TNF-α induced airway inflammation and enhanced the pulmonary chemoreflex sensitivity in anesthetized mice; and if so, whether the effect was mediated through activation of either or both of the TNF receptors, p55 and p75. Our results showed that TNF-α instilled into the lung caused an increased sensitivity of pulmonary chemoreflex responses to various chemical stimulants of the vagal bronchopulmonary C-fiber afferents. The increased sensitivity was found 24 h later, persisted at 48 h, and then gradually declined after several days. The TNF-α-induced airway hypersensitivity was accompanied by airway inflammation as shown by a striking elevation of the levels of eosinophils and neutrophils, several potent bronchoactive inflammatory mediators, and proinflammatory cytokines in the bronchoalveolar lavage fluid. Furthermore, the increase in pulmonary chemoreflex response caused by TNF-α was partially abrogated in both p55-null and p75-null mice, but completely abolished in p55/p75-null mice. In conclusion, TNF-α pretreatment induced airway inflammation and a sustained elevation of pulmonary chemoreflex sensitivity, which was mediated through an activation of both types of TNF receptors.
Keywords: airway inflammation, airway reflex, C-fibers, transient receptor potential vanilloid type 1, asthma
an important role of tumor necrosis factor-alpha (TNF-α), a proinflammatory cytokine, in the pathogenesis of allergic asthma has been widely recognized (4, 20, 42). TNF-α was detected in bronchoalveolar lavage fluid (BALF), sputum, exhaled breath condensate, and serum of asthmatic patients during asthmatic attack or following antigen inhalation challenge (25, 36). TNF-α is co-released with certain inflammatory mediators such as histamine and tryptase from a number of inflammatory cells in the airways (e.g., macrophages, mast cells, etc.) and can induce diverse and potent inflammatory responses by enhancing the release of various proinflammatory/chemotactic mediators and facilitating the infiltration of neutrophils and eosinophils into the airways (6, 28, 42). Indeed, inhalation of TNF-α can induce airway hyperresponsiveness accompanied by airway inflammation in healthy humans (43). A recent study has further demonstrated that prolonged (24–48 h) incubation of isolated rat pulmonary nodose and jugular sensory neurons with TNF-α induced a pronounced potentiating effect on the sensitivity of these sensory neurons to a number of chemical activators, particularly those activating the transient receptor potential vanilloid type 1 (TRPV1) receptor (21). However, the effect of TNF-α on the sensitivity of these TRPV1-expressing pulmonary sensory nerves in intact animals is not known.
TRPV1 is predominantly expressed in nonmyelinated (C-) sensory fibers. The C-fiber afferents innervating the respiratory tract play an important role in regulating cardiorespiratory functions under both normal and abnormal physiological conditions (12, 31). Furthermore, hypersensitivity of vagal bronchopulmonary C-fibers is known to contribute to the airway hyperresponsiveness during airway inflammatory reaction (24). Because activation of these C-fiber afferents can elicit pulmonary chemoreflexes in intact animals (12, 31), we hypothesized that, if a pretreatment with TNF-α enhances the sensitivity of these C-fiber afferents, it should augment the pulmonary chemoreflex responses elicited by chemical stimulants. Therefore, this study was designed to determine: 1) if a pretreatment with TNF-α elevated the sensitivities of pulmonary chemoreflex responses to both TRPV1 and non-TRPV1 activators in anesthetized mice; 2) if so, whether the increased sensitivity was associated with airway inflammatory reaction induced by the TNF-α pretreatment; and 3) the role of TNF receptors (TNFRs) in the potentiating effects of TNF-α on pulmonary chemoreflex responses.
MATERIALS AND METHODS
The procedures described below were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health, and were also approved by the University of Kentucky Institutional Animal Care and Use Committee.
Animal preparations.
The experiments were carried out in four groups of young adult mice (3–4 mo old) as follows: 1) wild-type (WT; B6129SF2/J); 2) TNF-α receptor double homozygous mutant mice (129S-Tnfrsf1atm1ImxTnfrsf1btm1Imx/J) in which both types of TNFRs, TNFR1 (p55) and TNFR2 (p75), were mutated (p55/p75-null); 3) p55-null mice (C57BL/6-Tnfrsf1atm1Imx/J); and 4) p75-null mice (129S2-Tnfrsf1btm1Mwm/J). All mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
During the experiment, mice were anesthetized by intraperitoneal injection of α-chloralose (0.07 mg/g) and urethane (1 mg/g); the supplemental doses of α-chloralose and urethane (∼1/10 of the initial doses) were injected intravenously to maintain abolition of pain reflexes elicited by paw pinch. The trachea was cannulated, and mice were breathing spontaneously via a tracheal cannula. The jugular vein and femoral artery were cannulated for administering chemicals and for measuring blood pressure (ABP), respectively. Body temperature was maintained at ∼36°C by a heating pad. At the end of the experiment, the animals were euthanized by intravenous injection of KCl.
Intratracheal instillation of TNF-α or vehicle.
Mice were anesthetized with inhalation of 2% isoflurane via a nose cone connected to a vaporizer (Protech international model 61020-WBB, Boerne, TX). A small (∼0.5 cm) midline incision was made on the ventral neck skin to expose the trachea. Under sterile conditions, TNF-α solution (10 μg/ml; 0.03 ml) or its vehicle (PBS, 0.03 ml) was instilled into the trachea via a needle (28-gauge). After the incision was closed, the mice were allowed to recover from anesthesia, and experiments were carried out at different time points (1–2 or 6–7 days) after the instillation.
Pulmonary chemoreflex experiment.
Respiratory flow was measured with a heated mouse pneumotachograph (dead space: 40 μl; airflow resistance: 0.018 cmH2O·ml−1·s−1; linear flow range: 0–3 ml/s; designed and manufactured by the University of Kentucky Center for Manufacturing) connected to the tracheal cannula and a differential pressure transducer (Validyne MP45-14, Northridge, CA). Tidal volume (Vt), expiratory duration (Te), respiratory frequency (f), and minute ventilation (V̇e) were analyzed on a breath-by-breath basis by an online computer (Biocybernetics TS-100, Taipei, Taiwan). ABP and heart rate (HR) were measured by a pressure transducer (Statham P23AC, Hato Rey, Puerto Rico) and also analyzed by the online computer. The sampling rate was set at 1,000 Hz in this study. Results analyzed by computer were routinely compared with those by hand calculation for accuracy.
Pulmonary chemoreflex responses were elicited by bolus intravenous injections of capsaicin (Cap; a TRPV1 activator; 0.25–1.00 μg/kg), phenylbiguanide (PBG; a 5-HT3 activator; 30–120 μg/kg), or allyl isothiocyanate (AITC; a TRPA1 activator; 0.3–0.9 mg/kg).
Analyses of airway inflammatory cells and mediators.
One day after intratracheal instillation of TNF-α or vehicle, mice were anesthetized by intraperitoneal injection of α-chloralose and urethane as described earlier, and BALF was obtained by injecting a total of 1.1 ml of PBS (lavage twice consecutively at 0.6 ml and 0.5 ml) via the trachea cannula. The collected BALF was centrifuged at 1,500 rpm, 4°C for 10 min. The pelleted cells were treated with 0.24 ml Tris-buffered ammonium chloride solution (pH 7.2) to lyse red blood cells. The remaining cells were washed with 1.2 ml PBS supplemented with Hank's buffer solution with 20% fetal bovine serum (FBS). Total cell counts were determined by using a hemocytometer. Differential leukocyte counts were carried out using cytospin and standard morphologic criteria. The cytospin procedure was performed by University of Kentucky Clinical Laboratory. A minimum of 500 leukocytes were counted by two different individuals, and the data were then averaged.
To compare the inflammatory mediators and cytokines released in the BALF, supernatants were transferred to other tubes for ELISA of inflammatory mediators and cytokines including leukotriene (LT) B4, LTC4/D4/E4, histamine, prostaglandin E2 (PGE2), thromboxane B2 (TXB2), pentraxin 3 (PTX3), interleukin (IL)-1β and IL-13. The assay of the same chemical in the BALF collected from different treatment groups was performed at the same time to avoid any possible experimental error and variability. These inflammatory mediators and cytokines were selected for measurements based on the previous reports indicating their possible changes in response to the TNF-α treatment (7, 37, 38, 42).
Experimental protocols.
Four study series were performed. For Study 1, two matching groups of WT mice were pretreated with intratracheal instillation of PBS and TNF-α, respectively, as described above. Chemoreflex responses were tested and compared between these two groups 24 h later; the protocol was designed based on our preliminary observations. In Study 2, to determine the long-term effect of the TNF-α treatment, chemoreflex responses were tested and compared between three matching groups of WT mice receiving different protocols: 1) 1–2 days after PBS; 2) 1–2 days after TNF-α; 3) 6–7 days after TNF-α; PBS and TNF-α were administered (intratracheal instillation) in an identical manner. In Study 3, to determine whether TNF-α pretreatment induced airway inflammation, BALF was obtained for measurements of inflammatory cells and mediators from five groups of mice 24 h after intratracheal instillation of either PBS or TNF-α as follows: 1) naïve group (WT mice receiving no surgery or treatment); 2) Veh group [WT mice pretreated with vehicle (PBS, 0.03 ml)]; 3) and 4) TNF-α 1 day group and 7 day group, respectively [WT mice 1 and 7 days after pretreatment with TNF-α (10 μg/ml, 0.03 ml), respectively]; and 5) p55/p75-null + TNF-α group [p55/p75-null mice pretreated with TNF-α (10 μg/ml, 0.03 ml)]. In Study 4, to investigate the role of TNFRs, pulmonary chemoreflex responses to Cap, PBG, and AITC were compared between WT mice and p55/p75-null mice 24 h after pretreatment with TNF-α. In Study 5, the relative roles of TNFR1 and TNFR2 in the TNF-α-induced hypersensitivity were further determined by comparing the reflex responses to Cap and PBG between p55-null and p75-null mice.
Statistical analysis.
Data were reported as means ± SE and analyzed using one-way and two-way repeated-measures ANOVA, unless mentioned otherwise. Pairwise comparisons for ANOVA were made with a post hoc analysis (Fisher's least significant difference). A value of P < 0.05 was considered significant.
When the apneic responses were compared between the four groups of mice (WT, p55-null, p75-null, and p55/p75-null), a linear mixed model with the between-subjects factor (group) and within-subjects factors (dose of Cap or PBG) was constructed. The covariance structure of the repeated measurements within the same subject was unstructured since the compound symmetry assumption made by a conventional two-way ANOVA procedure was not supported by the data. A post hoc comparison of means was based on the interaction between the factors group and dose.
Chemical agents.
TNF-α was purchased from Prospec Bio (Rehovot, Israel). ELISA kits for LTC4/D4/E4, PGE2, LTB4, TXB2 and histamine were purchased from Cayman chemical company (Ann Arbor, MI), PTX3 and IL1-β from R&D systems (Minneapolis, MN), and IL-13 from eBioscience (San Diego, CA). Isoflurane was purchased from Bulter animal health supply (Dublin, OH). Hank's buffer solution and FBS were purchased from Gibco (Grand Island, NY). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
RESULTS
Study 1: pulmonary chemoreflex responses potentiated by TNF-α pretreatment.
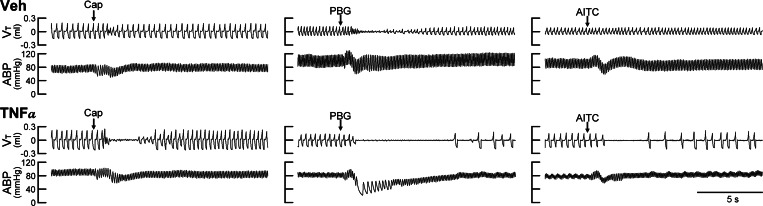
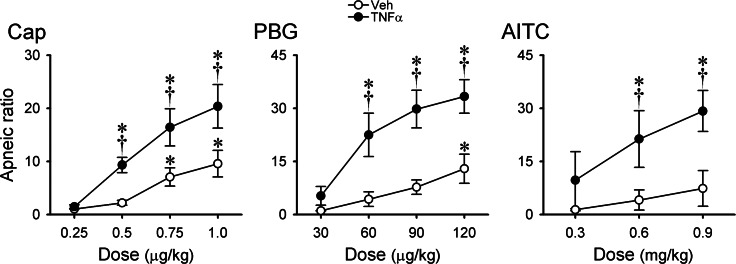
We found no significant difference in any of the baseline respiratory and cardiovascular variables (Vt, f, V̇e, ABP, and HR) between the two groups of mice 1 day after they received pretreatments with vehicle (Veh) and TNF-α (P > 0.05, n = 6; Table 1). However, TNF-α pretreatment significantly potentiated the pulmonary chemoreflex responses; representative examples are shown in Fig. 1. Bolus (intravenous) injection of a low dose of Cap (0.5 μg/kg) in a mouse pretreated with vehicle caused only mild cardiorespiratory depression (Fig. 1), whereas the same Cap challenge caused distinct pulmonary chemoreflex responses, characterized by a long apnea accompanied by abrupt and transient decrease in HR and ABP, in a mouse pretreated with TNF-α (Fig. 1). When the apneic response was standardized by calculating the apneic ratio as apneic duration/20-breath average of base-line Te, the group data showed that the apneic responses to Cap injections were dose dependent in the Veh group (Fig. 2). In comparison, the same Cap challenges elicited significantly larger apneic responses to Cap in the TNF-α-treated group in the entire dose range, except at the lowest dose of 0.25 μg/kg. Furthermore, a potentiating effect of the TNF-α pretreatment with similar pattern and magnitude was also found in the pulmonary chemoreflex responses to intravenous injections of PBG and AITC, as shown in Figs. 1 and 2.
Table 1.
Comparison of baseline respiratory and cardiovascular variables after TNF-α and vehicle pretreatments in anesthetized mice
| Frequency (bpm) | Tidal Volume (ml) | Ventilation (ml/min) | Blood Pressure (mmHg) | Heart Rate (bpm) | |
|---|---|---|---|---|---|
| Veh | 160.1 ± 6.4 | 0.115 ± 0.012 | 18.13 ± 1.72 | 87.70 ± 9.49 | 491.7 ± 22.0 |
| TNF-α | 158.6 ± 7.4 | 0.098 ± 0.006 | 15.35 ± 1.49 | 85.76 ± 6.20 | 473.9 ± 31.4 |
| P | 0.884 | 0.224 | 0.252 | 0.868 | 0.651 |
Values are means ± SE; n = 6. Tumor necrosis factor-alpha (TNF-α) group: intratracheal instillation of TNF-α (10 μg/ml, 0.03 ml); vehicle (Veh) group: intratracheal instillation of vehicle (PBS, 0.03 ml).
Fig. 1.
Experimental records illustrating the effect of tumor necrosis factor-alpha (TNF-α) pretreatment on pulmonary chemoreflexes elicited by capsaicin (Cap), phenylbiguanide (PBG), and allyl isothiocyanate (AITC) in anesthetized spontaneously breathing mice. Treatments were made 24 h before the experiments; Veh: intratracheal instillation of vehicle (PBS, 0.03 ml); TNF-α: intratracheal instillation of TNF-α (10 μg/ml, 0.03 ml). Intravenous bolus (0.05 ml volume) injections of Cap (0.5 μg/kg), PBG (60 μg/kg), and AITC (0.6 mg/kg) were made at the arrows. Body weights in the Veh group (top) from left to right were 35.3, 30.1, and 30.1 g, respectively; TNF-α group (bottom) were 34.5, 27.0, and 27.0 g, respectively. Vt, tidal volume; ABP, arterial blood pressure.
Fig. 2.
Dose-related apneic responses to Cap, PBG, and AITC in anesthetized, spontaneously breathing mice after pretreatments with TNF-α and its vehicle. Treatments were made 24 h before the experiments; Veh: intratracheal instillation of vehicle (PBS, 0.03 ml); TNF-α: intratracheal instillation of TNF-α (10 μg/ml, 0.03 ml). Apneic ratio was calculated as (apneic duration/20-breath average of baseline expiratory duration). Responses to Cap, PBG, and AITC were tested in different groups of mice; n = 6 in each group (except in the Veh group response to AITC, n = 5). Data are means ± SE. *Significantly different from lowest dose in each group (P < 0.05); †significantly different from corresponding data in the Veh group (P < 0.05).
Study 2: chronic effects of TNF-α pretreatment on pulmonary chemoreflex.
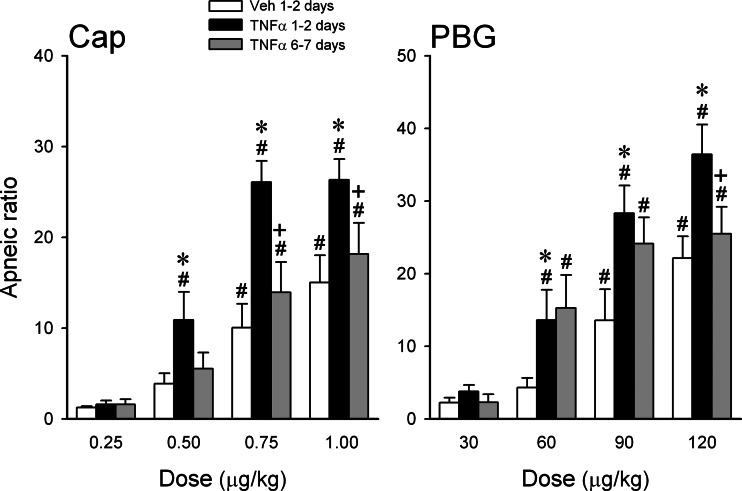
In our pilot experiments, we found that the potentiating effect of TNF-α on pulmonary chemoreflex was present in anesthetized mice when tested within 10–100 min after the intratracheal instillation. However, the effect was not as pronounced as 24 h later, and was further masked by an irregular and unstable baseline breathing pattern, presumably resulting from the acute effects of intratracheal instillation. In addition, in a small number of mice (n = 3), we also found that the potentiating effect of TNF-α pretreatment was still clearly present after 3–5 days, but began to decline. Hence this study series was intended to determine if the effect of TNF-α persisted 6–7 days after the instillation. Our results showed that the apneic reflex responses to Cap and PBG clearly increased when tested on either 1 or 2 days after the TNF-α pretreatment; because there was no statistically significant difference (P > 0.1; paired t-test) in the responses between 1 day (n = 3) and 2 days (n = 3), these data were pooled (Fig. 3). The enhanced apneic response declined significantly toward the baseline at 6–7 days after the instillation, which were no longer different from that in the Veh group (Fig. 3, left; n = 6). The augmented apneic response to PBG also showed similar but less consistent recovery at 6–7 days; it declined significantly at the high dose (120 μg/kg), but was not significantly different from that at 1–2 days at the medium (60 μg/kg) and low doses (30 μg/kg) of PBG (Fig. 3, right; n = 6).
Fig. 3.
Chronic effects of TNF-α pretreatment on the apneic responses to Cap and PBG. Measurements were made in 3 different groups of mice, n = 6 in each group: 1) Veh: 1–2 days after intratracheal instillation of vehicle (PBS, 0.03 ml); 2) and 3): 1–2 days and 6–7 days after intratracheal instillation of TNF-α (10 μg/ml, 0.03 ml), respectively. Data are means ± SE. *Significantly different from the corresponding response in mice pretreated with vehicle; +significantly different from the corresponding response in mice at 1–2 days after TNF-α pretreatment; #significantly different from the lowest dose in the same group.
Study 3: airway inflammation induced by TNF-α pretreatment.
Differential cell count in the BALF showed that the total number of leukocyte cells was significantly higher in the WT mice (n = 5) 1 day after a pretreatment with TNF-α than that in the other four groups as follows: naïve group (P < 0.05, n = 5); Veh group (P < 0.05, n = 5); TNF-α (7-day) group (P < 0.05, n = 6); and p55/p75-null + TNF-α group (P < 0.05, n = 5). No significant difference was found between these four other groups (Table 2). The percentages of eosinophils and neutrophils in the TNF-α (1-day) group were >15-fold higher than that in naïve, Veh, TNF-α (7-day), and p55/p75-null + TNF-α groups (Table 2), and again, no significant difference was found in either eosinophils or neutrophils between these four other groups (Table 2). Although the percentages of lymphocytes and monocytes were lower in the TNF-α (1-day) group, the total numbers of these cells were higher than the other four groups.
Table 2.
Effect of TNF-α pretreatment on leukocyte counts in bronchoalveolar lavage fluid
| Group | Total Cells (× 105) | Eosinophils (%) | Neutrophils (%) | Basophils (%) | Lymphocytes (%) | Monocytes (%) |
|---|---|---|---|---|---|---|
| Naïve (n = 5) | 4.55 ± 1.30 | 0.98 ± 0.14 | 0.98 ± 0.27 | 0.17 ± 0.12 | 17.14 ± 2.86 | 80.74 ± 2.54 |
| Veh (n = 5) | 3.37 ± 1.44 | 1.21 ± 0.27 | 1.16 ± 0.20 | 0.17 ± 0.11 | 16.38 ± 1.56 | 81.08 ± 1.42 |
| TNF-α 1 day (n = 5) | 10.35 ± 3.24* | 15.99 ± 1.11* | 34.27 ± 2.55* | 0.35 ± 0.06 | 9.91 ± 0.54* | 39.47 ± 3.64* |
| TNF-α 7 days (n = 6) | 3.79 ± 1.13 | 0.38 ± 0.03 | 0.44 ± 0.05 | 0.13 ± 0.05 | 5.95 ± 1.35* | 93.10 ± 1.35* |
| p55/p75-null + TNF-α (n = 5) | 4.24 ± 0.61 | 0.30 ± 0.06 | 0.58 ± 0.11 | 0.16 ± 0.07 | 7.79 ± 1.78* | 91.17 ± 1.82* |
Values are means ± SE. Naïve group: wild-type (WT) mice receiving no surgery or treatment; Veh group: WT mice pretreated with vehicle (PBS); TNF-α 1 and 7 days groups: WT mice 1 and 7 days after pretreatment with TNF-α, respectively; p55/p75-null + TNF-α group: p55/p75-null mice pretreated with TNF-α. Both vehicle and TNF-α were delivered by intratracheal instillation. Bronchoalveolar lavage fluid was collected at 24 h after pretreatment with TNF-α or vehicle (except the TNF-α 7 days group).
Significantly different from corresponding data in the naïve group (P < 0.05)
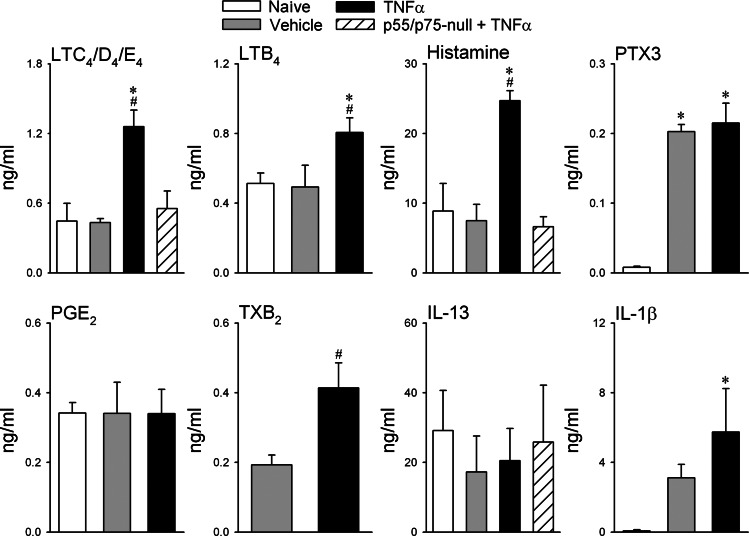
ELISA results showed that TNF-α pretreatment significantly elevated the levels of LTB4, LTC4/D4/E4, histamine, TXB2, and IL-1β in the BALF of WT mice, compared with that in the naïve, Veh, and p55/p75-null groups, and there was no significant difference in these inflammatory mediators and cytokine between these three other groups (Fig. 4; notice that TXB2 was not measured in naïve animals). In contrast, there was no difference in the level of PGE2 and IL-13 in BALF among all the groups. The levels of PTX3 were distinctly higher in both TNF-α and Veh groups than that in the naïve group, but there was no difference between TNF-α and Veh groups.
Fig. 4.
Effect of TNF-α pretreatment on inflammatory mediators in bronchoalveolar lavage fluid (BALF). Naïve group: wild-type (WT) mice receiving no surgery or treatment; Veh group: WT mice pretreated with vehicle (PBS); TNF-α group: WT mice pretreated with TNF-α; p55/p75-null + TNF-α group: p55/p75-null mice pretreated with TNF-α. Both vehicle and TNF-α were delivered by intratracheal instillation. BALF was collected 24 h after pretreatment of TNF-α or vehicle. LTB4 and LTC4/D4/E4: leukotrienes B4 and C4/D4/E4, respectively; PTX3: pentraxin 3; PGE2: prostaglandin E2; TXB2, thromboxane B2; IL-13 and IL-1β: interleukins 13 and 1β, respectively. Each data point represents means ± SE of the measurements made in 5 mice; the total number of mice used in these groups varied: n = 15 in both Veh and TNF-α groups, n = 10 in naïve group, and n = 5 in p55/p75-null + TNF-α group. Thus, some tests (e.g., LTB4, IL-1β) were only performed in 2 or 3 groups of mice. *Significantly different from naïve group (P < 0.05); #significantly different from Veh group (P < 0.05).
Study 4: the role of TNF-α receptors in the potentiating effect of TNF-α.
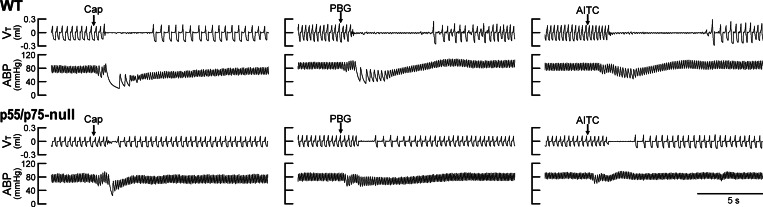
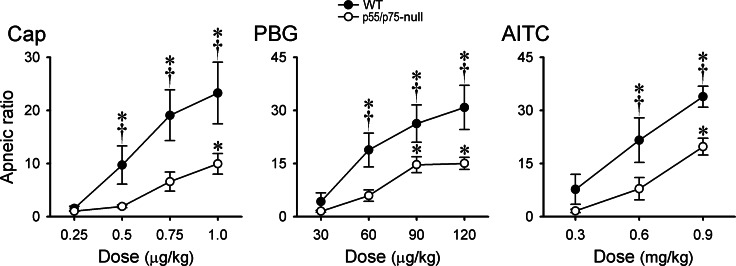
We found no significant difference in any of the baseline respiratory and cardiovascular variables between WT mice and p55/p75-null mice 1 day after TNF-α pretreatment (P > 0.05, n = 6 each; Table 3). However, after the TNF-α pretreatment, the pulmonary chemoreflex responses to Cap, PBG, and AITC were clearly stronger in WT mice than that in the p55/p75-null mice, as shown by the representative examples in Fig. 5. Group data further showed that apneic responses to these chemical activators of bronchopulmonary C-fibers were significantly higher in the WT mice than that in the p55/p75-null mice 24 h after both groups received the TNF-α pretreatment except the lowest doses (Fig. 6). In fact, the cardiorespiratory responses in p55/p75-null mice pretreated with TNF-α were similar to that in the WT mice pretreated with vehicle (PBS) shown in Figs. 1 and 2.
Table 3.
Comparison of baseline respiratory and cardiovascular variables in anesthetized wild-type and p55/p75-null mice
| Group | Frequency (bpm) | Tidal Volume (ml) | Ventilation (ml/min) | Blood Pressure (mmHg) | Heart Rate (bpm) |
|---|---|---|---|---|---|
| Wild type | 154.3 ± 6.8 | 0.108 ± 0.005 | 16.58 ± 1.36 | 81.07 ± 7.75 | 537.0 ± 39.3 |
| p55/p75-null | 161.1 ± 10.4 | 0.100 ± 0.010 | 15.83 ± 0.99 | 75.26 ± 4.05 | 504.5 ± 38.1 |
| P | 0.594 | 0.470 | 0.667 | 0.522 | 0.566 |
Values are means ± SE; n = 6. Both groups of mice were pretreated with intratracheal instillation of TNF-α (10 μg/ml, 0.03 ml) 24 h before measurements.
Fig. 5.
Experimental records illustrating the effect of TNF-α pretreatment on pulmonary chemoreflexes elicited by Cap, PBG, and AITC in anesthetized WT and p55/p75-null mice. TNF-α (10 μg/ml, 0.03 ml) was administered by intratracheal instillation 24 h before the experiments. Intravenous bolus (0.05 ml volume) injections of Cap (0.5 μg/kg), PBG (60 μg/kg), and AITC (0.6 mg/kg) were made at arrows. Body weights in WT mice (top) from left to right were 32.1, 34.5, and 34.5 g, respectively; p55/p75-null mice were 28.3, 26.6, and 26.6 g, respectively.
Fig. 6.
Effects of TNF-α pretreatment on the apneic responses to Cap, PBG, and AITC in anesthetized WT and p55/p75-null mice. TNF-α (10 μg/ml, 0.03 ml) was administered by intratracheal instillation 24 h before the experiments. Data are means ± SE (n = 6). *Significantly different from lowest dose in each group (P < 0.05); †significantly different from the corresponding data in p55/p75- null mice (P < 0.05).
Study 5: the relative roles of TNFR1 and TNFR2 in the potentiating effect of TNF-α.
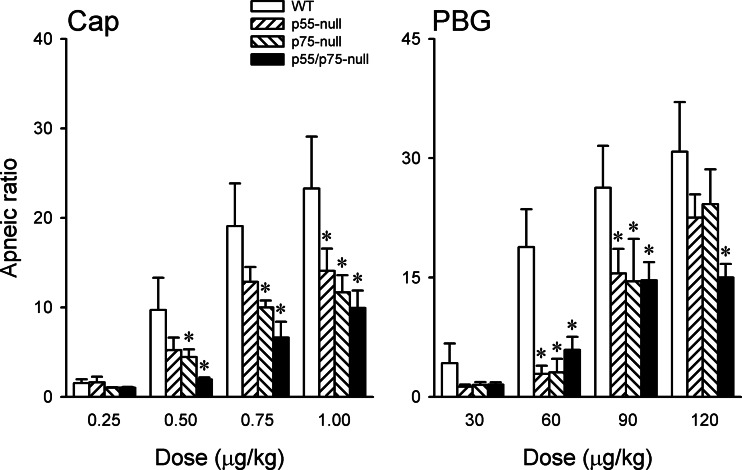
After the TNF-α pretreatment, the increases in apneic responses to 0.5, 0.75, and 1.0 μg/kg Cap in p75-null mice were all significantly smaller than that in the WT mice, but only the response to 1.0 μg/kg was smaller in p55-null mice than that in WT mice (Fig. 7). The TNF-α-induced increases in apneic responses to PBG were significantly attenuated in both p55-null and p75-null mice, compared with that in WT mice, except at the highest dose (120 μg/kg) (Fig. 7). Furthermore, there was no significant difference between p55-null and p75-null mice in the responses of apneic ratio, HR, and ABP to either Cap or PBG.
Fig. 7.
Relative roles of TNFR1 and TNFR2 in the effects of TNF-α pretreatment on the apneic responses to Cap and PBG. TNF-α (10 μg/ml, 0.03 ml) was administered by intratracheal instillation 24 h before the experiments. Measurements were made in 4 different groups of mice: WT, p55-null (TNFR1 deletion), p75-null (TNFR2 deletion), and p55/p75-null; n = 6 in each group. Data from WT and p55/p75 groups were the same as those presented in Fig. 6. Data are means ± SE (n = 6). *Significantly different from WT group; since the interaction hypothesis is detected with reduced statistical power, P < 0.1 was considered significant in the post hoc analysis.
DISCUSSION
Results of the present study showed that TNF-α instilled into the lung caused an increased sensitivity of pulmonary chemoreflex responses to chemical stimulants of vagal bronchopulmonary C-fiber afferents, including both TRPV1 (Cap) and non-TRPV1 activators (PBG and AITC). This sensitizing effect was found 24 h after the TNF-α treatment, persisted at 48 h, and then gradually declined after several days. The TNF-α-induced hypersensitivity was associated with airway inflammation as shown by striking elevation of the levels of inflammatory cells (neutrophils and eosinophils), autacoids (LTs, histamine), and proinflammatory cytokines (IL-1β) in the BALF. Furthermore, both the increase in pulmonary chemoreflex sensitivity and airway inflammation were completely abrogated in the p55/p75-null mice that were pretreated with TNF-α.
It is well recognized that TNF-α plays an important role in the pathogenesis of chronic inflammatory airway diseases such as asthma. TNF-α is stored in granules and released from mast cells, macrophages, neutrophils, dendritic cells, and other inflammatory cells via the immunoglobulin E-dependent mechanism(s) (4, 16, 20, 42). The proinflammatory effects of TNF-α are mediated through the activation of TNFR1 and TNFR2 (41). Both these TNFRs are present on a wide variety of cell types and are involved in a wide range of immunological responses and inflammatory reactions, but they have distinct molecular masses (55 kDa and 75 kDa), immunoreactivity, and activation mechanisms (8, 41). Binding of TNF-α to these receptors activates certain intracellular signaling pathways that lead to a wide array of cellular responses; for example, activation of TNFR1 can activate nuclear factor-kappa B pathway and mitogen-activated protein kinase pathways, including the extracellular signal-regulated kinase (ERK) (1–3, 15). Activated ERK is then translocated to the nucleus and can alter the gene expression, which has been implicated in the TNF-α-induced overexpression of TRPV1 in nociceptive neurons (19, 44). In addition, activation of TNFRs can also exert a chemotactic action, upregulate the leukocyte-endothelial cell adhesive molecules E-selectin and vascular cell adhesion molecule-1, enhance the production of Th2 cytokines, and cause infiltration and degranulation of these inflammatory cells in the airways (6, 21, 26, 42, 43). Some of the autacoids released from degranulation of these inflammatory cells may in turn generate a secondary sensitizing effect on bronchopulmonary C-fiber endings and augment the pulmonary chemoreflex responses (31, 32). Whether these signaling pathways and mechanisms are involved in the sensitizing effect of TNF-α on C-fiber afferents observed in this study remains to be determined. Our results showed that the TNF-α-induced increase in pulmonary chemoreflex sensitivity was partially abolished in both p55-null and p75-null mice, and was completely abrogated in p55/p75 mice (Figs. 6 and 7). Furthermore, there was no significant difference in the apneic response to either Cap or PBG between p55-null and p75-null mice, indicating that both types of TNFRs were involved in the sensitizing effects of TNF-α.
To investigate a possible involvement of inflammatory mediators, we selected several mediators for measurements in this study because previous reports indicated their possible releases in the airways resulting from the action of TNF-α (7, 37, 38, 42). Our ELISA data showed distinct increases in LTB4, LTC4/D4/E4, histamine, and TXB2 in the BALF samples obtained from TNF-α-treated animals, compared with that in the naïve (untreated and non-operated) and vehicle-treated animals (Fig. 4). The cysteinyl LTs, LTC4/D4/E4, also known as the slow-reacting substances of anaphylaxis, are produced in cells expressing LTC4 synthase such as eosinophils and mast cells. These LTs are potent bronchoconstrictors and major contributors to the pathophysiology of asthma, and can induce symptoms of bronchoconstriction, increased vascular permeability, and mucus secretion, etc. (5, 27). Furthermore, it has been reported that these endogenously released lipoxygenase products can activate the TRPV1 receptor expressed in the nociceptors in peripheral tissues (22, 24, 40). LTB4 is produced and released from neutrophils and other leukocytes, is a potent chemoattractant for other inflammatory cells, and can induce the formation of reactive oxygen species by these cells (10, 18). Histamine is mainly produced, stored, and released from the granules of mast cells and basophils upon immunological and chemical actions, and can cause significant bronchoconstrictive and other cardiovascular effects (14). In addition, histamine has been shown to enhance the baseline activity and excitability of bronchopulmonary C-fiber afferents (30, 32). TXB2 is a stable and inactive metabolite of TXA2; the latter is a potent prostanoid produced mainly by platelets and known to cause bronchoconstriction and vasoconstriction. TXA2 has also been shown to activate pulmonary afferents and elicit chemoreflex responses (39). TNF-α instillation into the lung also induced a marked increase in the production of IL-1β in the BALF. IL-1β is produced by macrophages and other inflammatory cells, and has been shown to play a prominent role in the development of airway inflammation and hyperresponsiveness in bronchial asthma (11). A stimulatory effect of IL-1β on the nociceptors in the lung, including pulmonary C-fiber and Aδ afferents, has been reported (17, 46).
PTX3 is a member of the pentraxin superfamily of proteins that are involved in acute immunological response to tissue injury (34). PTX3 is known to be rapidly produced and released by neutrophils and other phagocytes in response to inflammatory signals generated by cytokines such as TNF-α (35). Thus we reasoned that the intratracheal instillation of TNF-α should cause an increase in PTX3 in the BALF. However, we were surprised that no difference in the level of PTX3 was found between the BALF obtained from TNF-α- and vehicle-treated mice, and both PTX3 levels were distinctly higher than that obtained from naïve animals (without surgery or any treatment; Fig. 4). One possible explanation is that the PTX3 released in response to the surgical wound and needle puncture on the trachea during the intratracheal instillation is far greater than the tissue inflammation/injury caused specifically by the TNF-α challenge in this study.
A previous study in our laboratory reported that prolonged incubation (24–48 h) of isolated pulmonary sensory neurons with TNF-α only enhanced their sensitivity to TRPV1 and TRPA1 activators, and not to non-TRP activators of these neurons (21). However, in the present study TNF-α pretreatment augmented the sensitivity of reflex responses not only to Cap, but also to PBG (Figs. 1 and 2); PBG, a selective activator of 5-HT3 receptor, is not known to activate any of the TRP channels. We believe that the discrepancy between these two studies is related to the fact that TNF-α administered in vivo induced leukocyte infiltration and endogenous release of mediators in the lungs in these animals (Table 2 and Fig. 4). As described earlier, some of these inflammatory mediators can enhance the sensitivity of bronchopulmonary C-fiber afferents and pulmonary chemoreflex responses to chemical stimulants such as PBG (12, 21, 31, 32), though we did not attempt to identify any specific mediators that are primarily responsible in this study. Indeed, the potentiated pulmonary chemoreflex responses induced by the TNF-α treatment were absent in p55/p75-null mice (Figs. 4–6) when the accompanying airway inflammatory reactions were abrogated in these mice. Furthermore, the finding that the chemoreflex hypersensitivity subsided when the inflammatory reaction recovered 7 days after the TNF pretreatment in WT mice (Table 2 and Fig. 3) seems to support this hypothesis. However, these new findings in the present study do not rule out the possibility suggested by Hu and co-workers (21) that TNF-α alone can induce a sensitizing effect and/or overexpression of the TRPV receptors in the airway C-fiber sensory neurons.
Morphological and neurophysiological evidence clearly show that vagal C-fiber afferents innervate all levels of the respiratory tract, from trachea to alveoli, in various mammalian species including humans (12, 31, 32, 45), and they represent the majority of the sensory nerves arising from the lung structures (23). It is well recognized that the afferent activity generated by these C-fiber endings plays an important role in eliciting the pulmonary defense reflexes against the assaults by various chemical irritants in the respiratory tract (12, 31). When these afferent endings are activated, action potentials conducted through the vagus nerves to the commissural subnucleus of the nucleus tractus solitarius elicit the pulmonary chemoreflex response as illustrated in this study (12, 31). Other responses include airway irritation, urge to cough, and dyspnea (9, 29). In addition, intense and/or sustained stimulation of these afferents can cause airway smooth muscle contraction, mucous hypersecretion, vasodilation, and extravasation of macromolecules in the tracheobronchial tree (12, 32). These airway responses are known to be mediated through both the cholinergic reflex pathways and the local “axonal reflex”; the latter involves the release of several bioactive tachykinins and calcitonin gene-related peptides from these sensory endings (13, 33). Although we did not measure these other responses in this study, it seems logical to postulate that when the excitability of C-fiber afferents is enhanced by endogenous TNF-α, these airway and vascular responses to a given stimulus will be amplified.
In conclusion, results of this study showed that intratracheal instillation of TNF-α induced an inflammatory reaction as evidenced by the infiltration of inflammatory cells and releases of potent bronchoactive autacoids in the airways. These effects were accompanied by a pronounced and sustained elevation of pulmonary chemoreflex responses, which was mediated through an activation of both types of TNFRs. Taken together, these results suggest that the hypersensitivity of bronchopulmonary C-fiber afferents may contribute, at least in part, to the airway hyperresponsiveness associated with the increasing level of TNF-α found in airway inflammatory diseases.
GRANTS
This study was supported in part by NIH Grant HL-96914 and Department of Defense DMRDP/ARATD award administered by the U.S. Army Medical Research & Materiel Command (USAMRMC) Telemedicine & Advanced Technology Research Center (TATRC) under Contract Number W81XWH-10-2-0189.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.-L.L., Y.-J.L., and M.J.G. performed experiments; R.-L.L., R.K., Y.-J.L., M.J.G., and L.-Y.L. analyzed data; R.-L.L., R.K., and L.-Y.L. interpreted results of experiments; R.-L.L. and L.-Y.L. prepared figures; R.-L.L. and L.-Y.L. drafted manuscript; R.-L.L., Y.-J.L., and L.-Y.L. approved final version of manuscript; L.-Y.L. conception and design of research; L.-Y.L. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Chayse Martin, Michelle Lim, Reyno Tapia, and Robert Morton for their technical assistance and performing the differential cell counts.
REFERENCES
- 1. Barbin G, Roisin MP, Zalc B. Tumor necrosis factor alpha activates the phosphorylation of ERK, SAPK/JNK, and P38 kinase in primary cultures of neurons. Neurochem Res 26: 107–112, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 11: 372–377, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102: 12248–12252, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med 354: 697–708, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bisgaard H. Pathophysiology of the cysteinyl leukotrienes and effects of leukotriene receptor antagonists in asthma. Allergy 56, Suppl 66: 7–11, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol 10: 471–480, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Brightling C, Berry M, Amrani Y. Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol 121: 5–12, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brockhaus M, Schoenfeld HJ, Schlaeger EJ, Hunziker W, Lesslauer W, Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci USA 87: 3127–3131, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burki NK, Lee LY. Mechanisms of dyspnea. Chest 138: 1196–1201, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cho KJ, Seo JM, Kim JH. Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol Cell 32: 1–5, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chung KF, Barnes PJ. Cytokines in asthma. Thorax 54: 825–857, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984 [DOI] [PubMed] [Google Scholar]
- 13. De Swert KO, Joos GF. Extending the understanding of sensory neuropeptides. Eur J Pharmacol 533: 171–181, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Dunford PJ, Holgate ST. The role of histamine in asthma. Adv Exp Med Biol 709: 53–66, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Furukawa K, Mattson MP. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem 70: 1876–1886, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Gosset P, Tsicopoulos A, Wallaert B, Joseph M, Capron A, Tonnel AB. Tumor necrosis factor alpha and interleukin-6 production by human mononuclear phagocytes from allergic asthmatics after IgE-dependent stimulation. Am Rev Respir Dis 146: 768–774, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Gu Q, Lee LY. Pulmonary chemoreflex sensitivity is enhanced by intratracheal instillation of interleukin-1β in anesthetized rats. Am J Respir Crit Care Med 169: 2004 [Google Scholar]
- 18. Henderson WR., Jr Role of leukotrienes in asthma. Ann Allergy 72: 272–278, 1994 [PubMed] [Google Scholar]
- 19. Hensellek S, Brell P, Schaible HG, Brauer R, Segond von Banchet G. The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci 36: 381–391, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, Beckett P, Al Ali M, Chauhan A, Wilson SJ, Reynolds A, Davies DE, Holgate ST. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax 60: 1012–1018, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu Y, Gu Q, Lin RL, Kryscio R, Lee LY. Calcium transient evoked by TRPV1 activators is enhanced by tumor necrosis factor-α in rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 299: L483–L492, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA 97: 6155–6160, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst 5: 165–176, 1982 [DOI] [PubMed] [Google Scholar]
- 24. Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta 1772: 915–927, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Keatings VM, O'Connor BJ, Wright LG, Huston DP, Corrigan CJ, Barnes PJ. Late response to allergen is associated with increased concentrations of tumor necrosis factor-alpha and IL-5 in induced sputum. J Allergy Clin Immunol 99: 693–698, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Kips JC, Tavernier J, Pauwels RA. Tumor necrosis factor causes bronchial hyperresponsiveness in rats. Am Rev Respir Dis 145: 332–336, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Kumar A, Busse W. Eosinophils in asthma. In: Inflammatory Mechanisms in Asthma, edited by Holgate S, Busse W. New York: Marcel Dekker, 1998, p. 247 [Google Scholar]
- 28. Lassalle P, Gosset P, Delneste Y, Tsicopoulos A, Capron A, Joseph M, Tonnel AB. Modulation of adhesion molecule expression on endothelial cells during the late asthmatic reaction: role of macrophage-derived tumour necrosis factor-alpha. Clin Exp Immunol 94: 105–110, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee LY. Respiratory sensations evoked by activation of bronchopulmonary C-fibers. Respir Physiol Neurobiol 167: 26–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee LY, Morton RF. Histamine enhances vagal pulmonary C-fiber responses to capsaicin and lung inflation. Respir Physiol 93: 83–96, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Lee LY, Undem BJ. Brochopulmonary vagal sensory nerves. In: Advances in Vagal Afferent Neurobiology, edited by Undem BJ, Weinreich Boca Raton, FL: CRC Press, 2005, p. 279–313 [Google Scholar]
- 33. Lundberg JM, Saria A. Polypeptide-containing neurons in airway smooth muscle. Annu Rev Physiol 49: 557–572, 1987 [DOI] [PubMed] [Google Scholar]
- 34. Manfredi AA, Rovere-Querini P, Bottazzi B, Garlanda C, Mantovani A. Pentraxins, humoral innate immunity and tissue injury. Curr Opin Immunol 20: 538–544, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Mantovani A, Garlanda C, Bottazzi B. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine 21, Suppl 2: S43–S47, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Matsunaga K, Yanagisawa S, Ichikawa T, Ueshima K, Akamatsu K, Hirano T, Nakanishi M, Yamagata T, Minakata Y, Ichinose M. Airway cytokine expression measured by means of protein array in exhaled breath condensate: correlation with physiologic properties in asthmatic patients. J Allergy Clin Immunol 118: 84–90, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, Galli SJ. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol 120: 48–55, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Oyoshi MK, Bryce P, Goya S, Pichavant M, Umetsu DT, Oettgen HC, Tsitsikov EN. TNF receptor-associated factor 1 expressed in resident lung cells is required for the development of allergic lung inflammation. J Immunol 180: 1878–1885, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Shams H, Scheid P. Effects of thromboxane on respiration and pulmonary circulation in the cat: role of vagus nerve. J Appl Physiol 68: 2042–2046, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci USA 99: 10150–10155, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today 13: 151–153, 1992 [DOI] [PubMed] [Google Scholar]
- 42. Thomas PS. Tumour necrosis factor-alpha: the role of this multifunctional cytokine in asthma. Immunol Cell Biol 79: 132–140, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med 152: 76–80, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Utreras E, Futatsugi A, Rudrabhatla P, Keller J, Iadarola MJ, Pant HC, Kulkarni AB. Tumor necrosis factor-alpha regulates cyclin-dependent kinase 5 activity during pain signaling through transcriptional activation of p35. J Biol Chem 284: 2275–2284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, Priestley JV. Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience 141: 1533–1543, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Yu J, Lin S, Zhang J, Otmishi P, Guardiola JJ. Airway nociceptors activated by pro-inflammatory cytokines. Respir Physiol Neurobiol 156: 116–119, 2007 [DOI] [PubMed] [Google Scholar]