Abstract
The time course of the response and recovery after acute activity seen in exercise is not well understood. The goal of this work is to address how proteins of the thin filament (actin and its capping protein CapZ) are changed by 1 h of mechanical stimulation and return to baseline over time. Neonatal rat ventricular myocytes in culture were subjected to cyclic 10% strain at 1 Hz for 1 h to mimic increased mechanical loading during exercise. CapZ and actin dynamics were analyzed by fluorescence recovery after photobleaching (FRAP) using CapZβ1-GFP, actin-GFP, or actin-RFP. After cyclic strain, CapZ dynamics increased above resting controls and abated 2–3 h after cessation of the cyclic strain. Similarly, actin dynamics initially increased and abated 1.5–2 h after the end of stimulation. Neurohormonal hypertrophic stimulation by phenylephrine or norepinephrine treatments also elevated actin dynamics but required a much longer time of treatment (24–48 h) to be detectable. The actin capping mechanism was explored by use of expression of CapZβ1 with a COOH-terminal deletion (CapZβ1ΔC). Increased dynamics of actin seen with CapZβ1ΔC was similar to the response to cyclic strain. Thus it is possible that mechanical stimulation alters the dynamics for CapZ capping of the actin filament through the CapZβ1 COOH terminus, known as the β tentacle, thereby remodeling sarcomeres in cardiac myocytes. This adaptive mechanism, which is probably regulating thin-filament addition, declines a few hours after the end of a bout of exercise.
Keywords: actin assembly, exercise, cardiac hypertrophy, mechanotransduction, sarcomere, actin binding
the heart muscle remodels to the demands of exercise by regulation of the size and shape of the myocytes. To study mechanisms of cytoskeletal dynamics, myocytes can be cyclically strained to mimic exercise (31). Also, hypertrophy resulting from chronic demands can be mimicked by neurohormones such as norepinephrine (10). Hypertrophy requires addition of new sarcomeres controlled by the Z-disc-associated proteins for addition of actin molecules to thin filaments. Most research studies several days of cyclic strain or chronic neurohormonal treatment in neonatal myocytes, but less attention is given to the normal, briefer pattern of activity seen in physiological cyclic strain as in athletes. Here, a 1-h period of activity is applied to mimic the normal duration of activity and changes evaluated over several hours after the stimulation ends.
CapZ is a mushroom-like heterodimeric protein that “caps” the barbed end of an actin filament, slowing down actin filament assembly in vitro (7, 8). In striated muscle, the COOH terminal extended ends of the α- and β-subunits of CapZ are the major actin-binding sites and act like two “tentacles” that are critical for actin capping (16, 33). The flexibility of the β tentacle alters the mushroom structure, resulting in modified interactions with other proteins (9). Mutations or deletion of the β tentacle significantly affect capping properties (4, 17, 33). Also, the transgenic mouse with a β-tentacle mutation had impaired cardiac muscle structure (13). Thus the β tentacle is critical for CapZ-mediated actin filament assembly.
The actin cytoskeleton in mature muscle cells is a very stable structural component. Nonetheless, mature sarcomeric actin filaments in muscle cells incorporate G-actin within minutes, indicating their dynamic nature (11, 22). In recent studies, the dynamics of non-sarcomeric β-actin increased in adult cardiac myocytes under neurohormonal stimulation (3), but stimulation of α-sarcomeric actin has not been explored.
Pressure or volume overloading, such as aortic stenosis or increased peripheral resistance, initiates cardiac remodeling. When loads are encountered, cardiac myocytes integrate extracellular, intercellular, and intracellular forces into intracellular signals through the mechanotransduction pathways (21). However, destination for the signaling cascades and how filament assembly and sarcomere remodeling occur are less well understood. CapZ capping is dynamic, as assessed by fluorescence recovery after photobleach (FRAP) of CapZβ1-GFP (14). That study also found the dynamics of CapZ were elevated by neurohormonal stimulation such as phenylephrine (PE) and endothelin-1 (ET1) through a PKC- and PIP2-dependent pathway. Also, although calcium signaling plays an important role in mechanical stimulation of myocyte, CapZ is not modified by intracellular calcium (15).
The goal of these experiments using neonatal rat ventricular myocyte (NRVM) in culture is to find how CapZ regulates sarcomeric actin filament remodeling with cyclic strain and return to baseline after stimulation ends. The hypothesis is that 1 h of mechanical stimulation is sufficient to regulate actin dynamics by CapZ in NRVM, which return to the control level over time. First, the dynamics of CapZ in NRVM are analyzed after 1 h of cyclic mechanical strain, and the time course is tracked for the next 3 h. Second, the dependence of actin dynamics on CapZ is measured. Finally, a CapZ β tentacle mutant determines its role in both CapZ and actin dynamics.
MATERIALS AND METHODS
Cell culture.
Primary heart cultures were obtained from neonatal rats according to Institutional Animal Care and Use Committee and National Institutes of Health guidelines for the care and use of laboratory animals. Hearts were removed and cells were isolated from 1- to 2-day-old neonatal Sprague-Dawley rats with collagenase (Worthington), as previously described (6). The cells were re-suspended, filtered through a metal sieve to remove large material, and plated at high density (1,000 cells/mm2) in PC-1 medium (Biowhittaker/Cambrex) on fibronectin coated (25 μg/ml) FlexCell silicone membranes (200,000 cells/cm2). Cells were left undisturbed for 24 h in a 5% CO2 incubator. Unattached cells were removed by aspiration, and PC-1 media was replenished. Cells were left in incubator for another 24 h before the experiment.
Mechanical strain.
Seventy-two hours after cell isolation, with or without viral infection, cyclic mechanical strain was generated with a Flexcell Strain Unit (model FX-4000, Flexcell International, Hillsborough, NC). NRVMs were strained at 10% elongation biaxially at 1 Hz sinusoidally for 1 h in PC-1 medium. Strain magnitude, time, and waveform were user-assigned to the system that controls vacuum pressure to deliver calibrated strain values to available elastic substrates housed in the incubator.
Neurohormonal hypertrophic stimulation.
Control cells were either stimulated or left unstimulated before photobleaching. The neurohormonal treatment times chosen were sufficient to induce hypertrophy (14, 27). Phenylephrine (10 μM, Sigma-Aldrich) treatment was for 4 or 24 h before FRAP analysis. Norepinephrine (20 μM, Sigma-Aldrich) treatment was for 24 or 48 h before FRAP.
Adenovirus.
Forty-eight hours after cell isolation, recombinant adenoviruses used are CapZβ1-GFP and CapZβ1-GFP with the COOH-terminal deletion (CapZβ1ΔC-GFP). NRVMs were infected with CapZβ1 (MOI 20) or CapZβ1ΔC (MOI 20) for 60 min at 37°C diluted in PC-1 medium. Viral medium was then replaced with virus-free PC-1 medium for 24 h.
Actin-GFP and actin-RFP expression.
Forty-eight hours after cell isolation, actin-GFP and actin-RFP expression were induced by CellLight Reagents BacMam 2.0 actin-GFP or actin-RFP (Invitrogen). Two days after NRVM isolation, appropriate volume of CellLight Reagent (30 μl/1,000,000 cells) was used as modified from the manufacturer's instructions. Infected NRVMs are returned to the culture incubator for at least 16 h.
Immunostaining and confocal microscopy.
NRVMs were washed with PBS, fixed with 4% paraformaldehyde (Sigma Aldrich) for 10 min, placed in cold 70% ethanol, and stored at −20°C until immunostaining. Cells were rehydrated in PBS (Sigma Aldrich) for 10 min. After rehydration, cells were incubated on a shaker table in 1% BSA in PBS for 1 h at 25°C. Primary α-actinin (AbCam) antibody was diluted (1:250) in 1% BSA in PBS. The antibody was allowed to incubate on a shaker table at 4°C overnight. Cells were then rinsed in PBS at 25°C and blocked in 1% BSA in PBS for 1 h at 25°C. Secondary antibody (Molecular Probes) was diluted at a ratio of 1:500 in 1% BSA in PBS and incubated for 1 h at 25°C. Cells were washed in PBS. Anti-fade reagent with DAPI (Molecular Probes) was added, and cover slips were mounted on glass slides.
Analysis of fluorescence recovery after photobleaching.
Fluorescence recovery after photobleaching (FRAP) analysis was done as in previous publications (14, 18, 29). For FRAP of CapZβ1-GFP, up to 10 beating and well striated cells (as evidenced by CapZβ1-GFP) were randomly selected for each FRAP. FRAP images were obtained by a Zeiss LSM 710 confocal microscope. The intensity (Ifrap) of the region of interest (ROI; area = 3.75 × 3.75 μm) was observed both before (t0) and immediately after (t1) bleaching at full power. Data were collected every 2 min because of the slow dynamics of CapZ (24) with lower excitation power (1 to ∼3%) and 800- to ∼1,000-ms duration until the end of the 15 min. Images were analyzed using the Zeiss Imaging Browser. Plotted intensity values are given as a percentage of the difference between Ifrap (t0) and Ifrap (t1). Binding of CapZ to the actin filament has two binding states (30), so FRAP curves of CapZ were fit using nonlinear regression in OriginPro (OriginLab, Northampton, MA):
| (1) |
The average kinetic constant (Kfrap) for dynamics was calculated using the following formula:
| (2) |
For FRAP of actin-GFP and actin-RFP, data were collected every 10 s (actin-GFP) or 20 s (actin-RFP) because of the higher dynamics of the actin (20) until the end of the 8 min (28). Since actin binding has only one binding state (23), the equation for curve fitting using nonlinear regression in OriginPro was:
| (3) |
The average kinetic constant (Kfrap) was calculated using the following formula:
| (4) |
Statistics.
Data are presented as means ± SE. For FRAP assays, the sample number was defined as individual cells, of which one to five cells were analyzed per culture in at least three separate cultures analyzed per experimental condition. Statistical significance was determined for FRAP curves by Student's t-test. Significance was taken at P < 0.01 or P < 0.05 as indicated.
RESULTS
Effects of cyclic mechanical strain on sarcomeric CapZ dynamics.
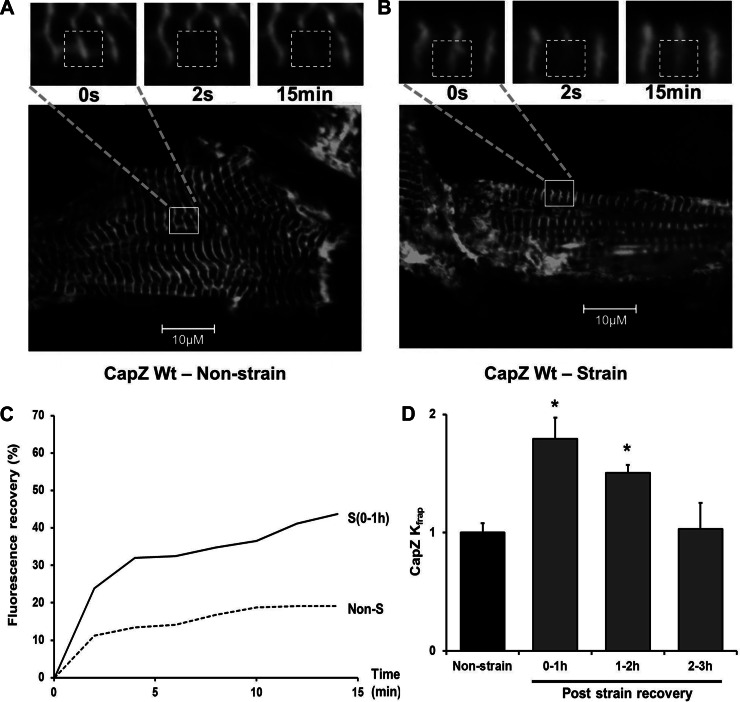
To test the potential of mechanical stimulation to induce thin-filament remodeling, the dynamics of the CapZ was measured by FRAP in NRVMs with or without 1 h of 1-Hz, 10% cyclic strain (Fig. 1, A and B). Within 1 h after cyclic strain, the FRAP profile was altered significantly (Fig. 1C). The kinetic constant (Kfrap) gained from the results of curve fitting (Eq. 2) was significantly increased by ∼80% within 1 h after cyclic strain (Fig. 1D and supplemental Table 1 available online at the Journal of Applied Physiology website) compared with nonstrained cells. The increased CapZβ1 dynamics abated after cessation of cyclic strain and returned to the control level by 2 h after the end of strain (Fig. 1D).
Fig. 1.
Time course of CapZβ1 dynamics after cyclic mechanical strain. Neonatal rat ventricular myocytes (NRVMs) infected by CapZβ1-GFP were either unstrained or subjected to 1-Hz cyclic 10% strain for 1 h. A and B: confocal images of CapZβ1-GFP with FRAP shown before bleach (0 s), after bleach (2 s), and 15 min later in cells in the following conditions: A, unstrained with resting spontaneous beating or B, 1 h after cyclic strain has ended. Region of interest (ROI) shown as dashed white boxes (3.75 μm × 3.75 μm). Inset: higher magnification images of area delineated by the solid white boxes in the lower magnification image. C: fluorescence recovery after photobleaching (FRAP) of CapZβ1-GFP as a percentage of prebleach intensity with unstrained control (dashed line, Non-S) and within 1 h after cyclic strain ended [solid line, S (0–1 h)]. D: Kfrap values for CapZβ1 normalized to unstrained cells; 0 to ∼1 h (n = 5), 1 to ∼2 h (n = 4), and 2 to ∼3 h (n = 4). Values are means ± SE. *Significant difference (P < 0.01).
Effects of cyclic mechanical strain on sarcomeric actin dynamics.
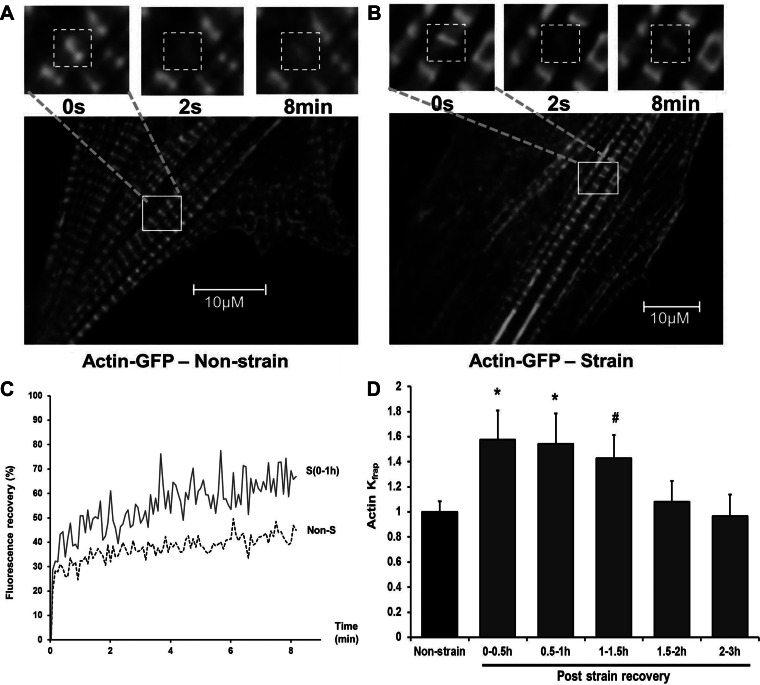
Because of the role of CapZ in the regulation of actin filament assembly, the dynamics of the sarcomeric actin may also be altered. NRVMs infected with actin-GFP fusion protein showed the striated pattern, permitting dynamics of sarcomeric actin to be tested by FRAP (Fig. 2, A and B). The profile of recovery of actin-GFP fluorescence is changed at the end of 1 h of cyclic strain (Fig. 2C). The kinetic constant (Kfrap) of actin-GFP gained from the results of curve-fitting (Eq. 4) is significantly increased by ∼50% within 1 h after cyclic strain (Fig. 2D and supplemental Table 2 available online at the Journal of Applied Physiology website). The elevated sarcomeric actin dynamics returned to the control level 1.5 to ∼2 h after the cessation of cyclic strain (Fig. 2D).
Fig. 2.
Time course for actin dynamics after cyclic mechanical strain. NRVMs infected by actin-GFP were either unstrained or subjected to 1-Hz cyclic 10% strain for 1 h. A and B: confocal images of actin-GFP with FRAP shown before bleach (0 s), after bleach (2 s), and 8 min later in cells in the following conditions: A, unstrained with resting spontaneous beating B, 1 h after cyclic strain has ended. Regions of interest (ROIs) are shown as dashed white boxes (3.75 × 3.75 μm). Inset: higher magnification images of area delineated by the solid white boxes in the lower-magnification image. C: FRAP of actin-GFP as a percentage of prebleach intensity with unstrained control (dashed line, Non-S) and within 1 h after cyclic strain ended [solid line, S (0–1h)]. D: Kfrap values for actin-GFP normalized to unstrained cells; 0 to ∼0.5 h (n = 6), 0.5 to ∼1 h (n = 5), 1 to ∼1.5 h (n = 4), 1.5 to ∼2 h (n = 8), and 2 to ∼3 h (n = 7) after strain were compared. Values are means ± SE. Significant difference: *P < 0.01; #P < 0.05.
Effects of neurohormonal hypertrophic stimulation on sarcomeric actin dynamics.
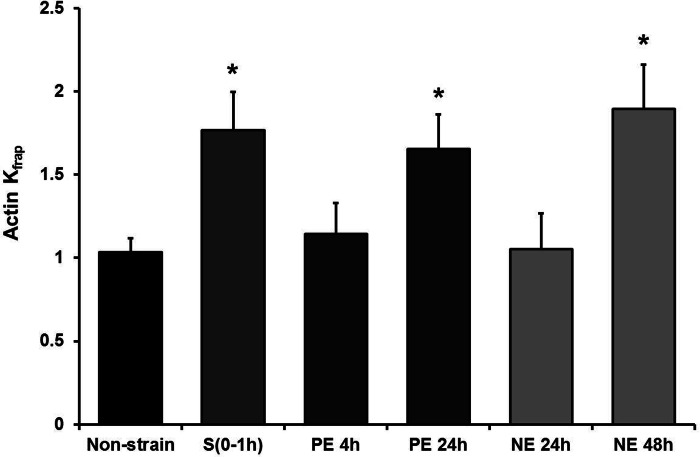
To test whether the sarcomeric actin dynamics are also elevated by neurohormonal stimulation as shown for CapZ (14), NRVMs were treated with PE (10 μM) or norepinephrine (NE; 20 μM), and FRAP of actin-GFP was measured (Fig. 3). FRAP results demonstrated that actin dynamics increased by ∼65% 24 h after PE treatment and by ∼90% 48 h after NE treatment. Importantly, the effective treatment time of 24 or 48 h for neurohormonal stimulation is much longer than the 1 h of cyclic strain.
Fig. 3.
Actin dynamics after neurohormonal stimulation. Kfrap values for actin-GFP FRAP in cells 4 h (n = 5) or 24 h (n = 4) after phenylephrine (PE) treatment and 24 h (n = 6) or 48 h (n = 4) after norepinephrine (NE) treatment, normalized to unstrained cells (Non-strain) or 0–1 h after strain. S(0–1h), 0–1 h after strain. Values are means ± SE. *Significant difference (P < 0.01).
Localization and dynamics of mutant CapZβ1-GFP fusion proteins in vitro.
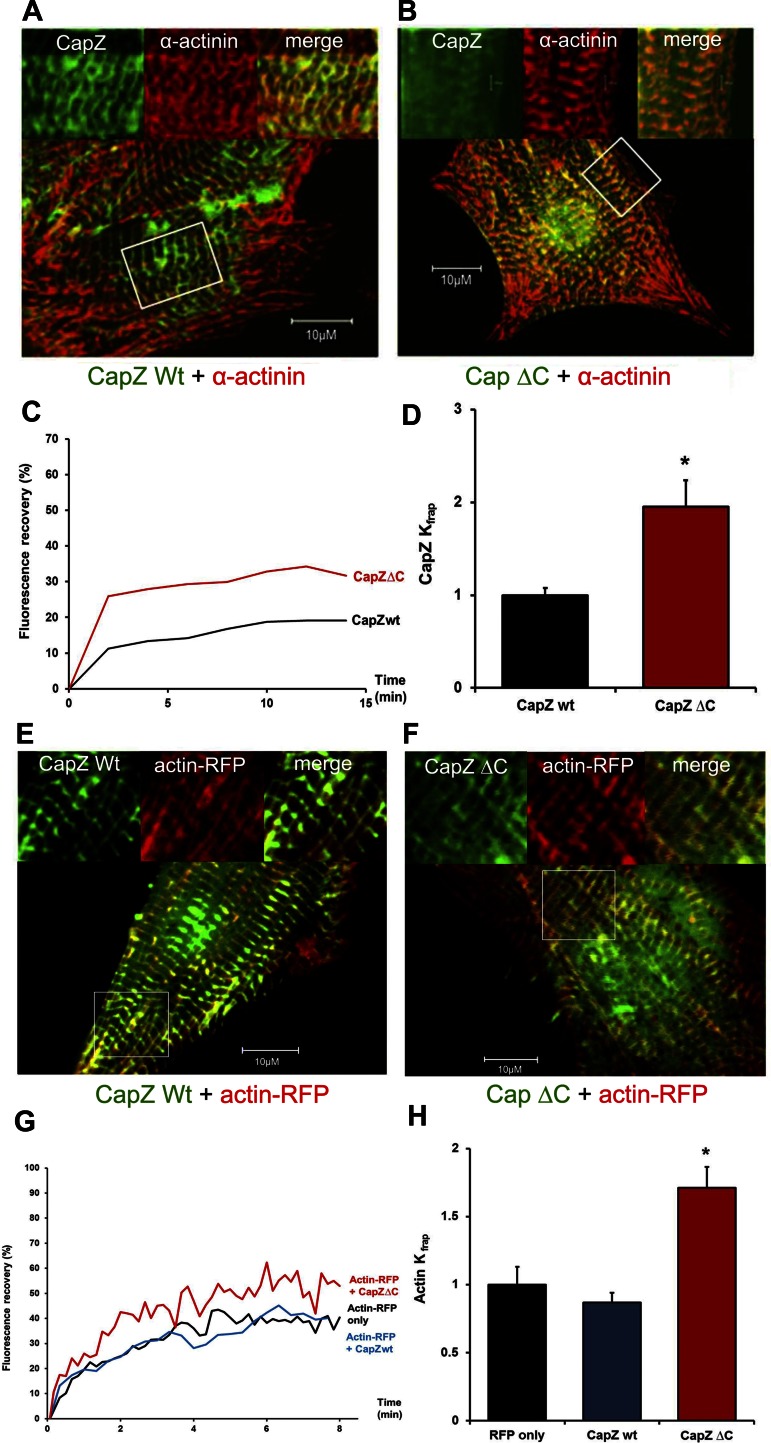
Since both CapZ and sarcomeric actin dynamics have similar time courses in response to cyclic strain and the previous studies are all from in vitro experiments, a possible interaction between CapZ and the actin filaments was addressed. It is known that the CapZβ1 mutant with its tentacle (COOH terminus) deleted (CapZβ1ΔC) is critical in CapZ-actin interaction (4). In our study, the CapZβ1ΔC-GFP showed a Z-disc localization (Fig. 4, A and B), but the recovery profile of CapZβ1ΔC-GFP in NRVMs showed a significant difference from wild-type CapZβ1-GFP (Fig. 4C). Results of curve-fitting by the two binding-state model (Eq. 2) demonstrated that CapZβ1ΔC had elevated Kfrap by ∼95%, confirming the importance of the β1 tentacle for CapZβ1 binding (Fig. 4D).
Fig. 4.
NRVMs expressing CapZβ1ΔC-GFP have higher CapZ and actin dynamics than wild-type CapZβ1-GFP. A and B: GFP and α-actinin antibodies show colocalization of both the wild-type and the mutant CapZβ1-GFP to the Z-disc. Boxed areas in whole cell are enlarged in insets. C: FRAP of wild-type CapZβ1-GFP (black line, CapZwt) or COOH-terminal deletion CapZ (red line, CapZΔC) as a percentage of prebleach intensity. D: Kfrap values normalized to unstrained cells have higher dynamics for CapZβ1ΔC-GFP (red bar, n = 4) compared with wild-type CapZβ1-GFP (black bar, n = 4). E and F: confocal images show localization of CapZ and its mutant at the Z-disc, and actin-RFP localization adjacent to Z-disc in the I-band. G: actin-RFP FRAP alone as a percentage of prebleach intensity in cells (black line, actin-RFP), or actin-RFP also expressing either CapZβ1-GFP (blue line, actin-RFP + CapZwt), or expressing the COOH-terminal deletion CapZ (red line, actin-RFP + CapZΔC). H: Kfrap values normalized to the cells expressing actin-RFP alone (n = 4) show a higher recovery rate of actin-RFP in the cells expressing CapZβ1ΔC-GFP (red bar, n = 5) than the cells expressing wild-type CapZβ1-GFP (blue bar, n = 5). Values are means ± SE. *Significant difference (P < 0.01).
Effects of CapZβ1 COOH-terminal tentacle on sarcomeric actin dynamics.
Alterations of the interaction between CapZ and actin filaments affect the rate of actin assembly in a polymerization assay (16). To test whether changes of CapZ binding by the CapZβ1 COOH terminus affects sarcomeric actin dynamics directly, NRVMs were infected with either wild-type CapZβ1-GFP or CapZβ1ΔC-GFP, followed by expression of actin-RFP. The cells expressing actin-RFP, CapZβ1-GFP + actin-RFP, or CapZβ1ΔC-GFP + actin-RFP (Fig. 4, E and F) were subjected to FRAP of actin-RFP. The recovery profile of actin-RFP in NRVMs showed no major difference between actin-RFP alone and CapZβ1-GFP + actin-RFP, but a significant difference in CapZβ1ΔC-GFP + actin-RFP was seen (Fig. 4G). Results of curve-fitting demonstrated that expression of CapZβ1ΔC-GFP significantly elevated dynamics of actin by ∼70% compared with cells expressing wild-type CapZβ1-GFP (Fig. 4H and supplemental Table 2 available online at the Journal of Applied Physiology website), suggesting that the β tentacle directly regulates actin dynamics.
DISCUSSION
In this study, three novel findings are reported. First, in the NRVM primary culture system, the dynamics of CapZ, one of the major regulators of actin-filament assembly, was found to be elevated by mechanical strain at the end of a brief period of stimulation but to abate over the next 2 h. Second, the dynamics of sarcomeric actin in NRVMs share a similar time course to CapZ for both the increase and abatement. Third, the β1-tentacle COOH terminus of CapZβ1 may play an important role in filament assembly since sarcomeric actin dynamics are significantly enhanced when the tentacle is deleted. Thus it seems possible that muscle hypertrophy stimulated by mechanical forces 1) begins to remodel the actin filament within an hour, 2) abates over 2 h after cessation of stimulation, and 3) involves the tentacle of the β1 subunit of CapZ.
Time course of the response of NRVM to mechanical stimulation.
Mechanotransduction rapidly reacts to stimulation through signaling and posttranslational modifications, such as phosphorylation of existing proteins, thereby initiating sarcomeric remodeling. Phosphorylation of focal adhesion kinase (FAK) peaks within 1 h after mechanical strain (25, 32) and returns to baseline levels 1–2 h after stimulation ends (2). The increased dynamics of both actin and CapZ after a brief period of cyclic strain are in the same time frame as for FAK. Thus the time course of CapZ and actin dynamics 2 h after stimulation ends suggests a reversal mechanism, such as dephosphorylation might occur. For example, protein phosphatase 1 (PP1), a well known phosphatase in NRVM, dephosphorylates PKC α in an hour (5) and possibly participates in the increase and abatement of CapZ dynamics. Also, the tensin homolog (PTEN) is a phosphatase that rapidly regulates the synthesis of phosphoinositide (PI), which itself is an important regulator of CapZ capping (24). Its role of dephosphorylating FAK in muscle cells (1, 12, 26) may suggest that it is a potential candidate for regulation of the active time course in response to mechanical stimulation.
Comparison of the effects of mechanical strain and neurohormonal hypertrophic stimulation.
Both mechanical strain and neurohormonal stimulation result in the increase of CapZ and sarcomeric actin dynamics, suggesting that both alter actin filament assembly during hypertrophy. PE and NE elicit hypertrophy and cardiac remodeling via small G proteins and share many signaling pathways with mechanical stimulation, such as Ras, Rac, Rho, and the downstream activation of MAPKs (19). Noticeably, although only 1 h of cyclic mechanical strain alters actin capping and actin dynamics, it takes days for neurohormonal stimulation to produce similar changes (1 day for PE, 2 days for NE).
Role of the β1 tentacle of CapZ in regulation of capping properties in NRVM.
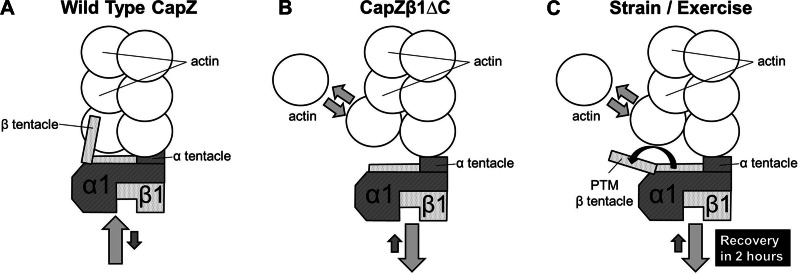
A model is proposed for the mechanism by which the interaction between CapZ and actin regulates actin assembly after a brief period of mechanical stimulation (Fig. 5). This model is based on results in NRVM of CapZ and actin dynamics after cyclic strain or with the CapZβ1 terminal deletion mutant. Under normal conditions when adaptation is not occurring, the two tentacles of CapZ tightly cap the barbed end of the actin filament, resulting in a low actin mobility and a low actin off rate (Fig. 5A). The deletion of the β tentacle significantly weakens the actin capping and increases CapZ dynamics, thus elevating the off rate of actin (Fig. 5B). After brief mechanical stimulation, the capping of CapZ is also decreased, and the off rate of actin is elevated (Fig. 5C). This is possibly due to posttranslational modifications of the β tentacle of CapZ that alter the actin off rate. The model suggests a relationship between CapZ capping and actin dynamics, but the experiments do not prove actin assembly directly in cultured cells. However, this is a likely mechanism, since biochemical methods in vitro show actin assembly when the capping is altered (17, 33).
Fig. 5.
Model of interactions between CapZ and sarcomeric actin. A: in unstrained conditions when NRVMs beat spontaneously but are not growing, the CapZ tightly caps the barbed end of the actin filament [on rate (light gray arrow) > off rate (dark gray arrow)]. B: the deletion of β tentacle significantly increases CapZ dynamics [off rate (light gray arrow) > on rate (dark gray arrow)], which weakens the binding of CapZ to the actin filament. The dynamics of the actin monomer on and off the actin filament is elevated (arrows with two directions). C: mechanical stimulation also significantly increases CapZ dynamics [off rate (light gray arrow) > on rate (dark gray arrow)], possibly because of structural change of the β tentacle (black arrow) through posttranslational modification. The β tentacle of CapZ is released from its actin-binding state, which leads to an elevated actin off rate (arrows with two directions).
The binding of CapZ to the actin filament, however, is not controlled only by the CapZβ tentacle. The CapZα tentacle is the target of CapZ inhibitors (30), but the regulatory role of the α tentacle in mechanical strain-induced sarcomeric actin remodeling has not yet been investigated. Undoubtedly, other partnering proteins, posttranslational modifications, and ions, such as calcium, are also involved in control of actin-filament assembly in response to different functional demands of the heart.
In conclusion, the interaction of CapZ and actin in the thin filament is altered after strain in NRVM in culture. A model is proposed to explain the possible role of the β tentacle of CapZ in NRVM after mechanical stimulation. One hour of cyclic strain is sufficient to increase the dynamics of sarcomeric actin through CapZβ1, and this process returns to baseline in only a few hours after cessation of the stimulation. Thus remodeling of actin thin filaments in cardiac myocytes lasts for only a few hours after a bout of exercise and may involve the COOH terminus (β tentacle) of CapZβ1. It is possible that cycles of activity and rest may be important for a healthy muscle, whereas the maintained stimulation may be a factor in the maladaptation that occurs in chronic cardiac disease.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-62426 (B. Russell) and American Heart Association Grant 12PRE12050371 (Y.-H. Lin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.H.L. and B.R. conception and design of research; Y.H.L. and J.L. performed experiments; Y.H.L. and E.R.S. analyzed data; Y.H.L., E.R.S., and B.R. interpreted results of experiments; Y.H.L. prepared figures; Y.H.L., J.L., and B.R. drafted manuscript; Y.H.L., J.L., and B.R. edited and revised manuscript; B.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Allen M. Samarel (Loyola University Chicago Stritch School of Medicine, Maywood, IL) for the gift of the CapZ DNA constructs. Dr. Ke Ma provided technical assistance with the FRAP experiments.
REFERENCES
- 1. Aikawa R, Nagai T, Kudoh S, Zou Y, Tanaka M, Tamura M, Akazawa H, Takano H, Nagai R, Komuro I. Integrins play a critical role in mechanical stress-induced p38 MAPK activation. Hypertension 39: 233–238, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Atherton PJ, Szewczyk NJ, Selby A, Rankin D, Hillier K, Smith K, Rennie MJ, Loughna PT. Cyclic stretch reduces myofibrillar protein synthesis despite increases in FAK and anabolic signalling in L6 cells. J Physiol 587: 3719–3727, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balasubramanian S, Mani SK, Kasiganesan H, Baicu CC, Kuppuswamy D. Hypertrophic stimulation increases beta-actin dynamics in adult feline cardiomyocytes. PLos One 5: e11470, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barron-Casella EA, Torres MA, Scherer SW, Heng HH, Tsui LC, Casella JF. Sequence analysis and chromosomal localization of human Cap Z. Conserved residues within the actin-binding domain may link Cap Z to gelsolin/severin and profilin protein families. J Biol Chem 270: 21472–21479, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, de Tombe PP. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res 101: 195–204, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Boateng SY, Hartman TJ, Ahluwalia N, Vidula H, Desai TA, Russell B. Inhibition of fibroblast proliferation in cardiac myocyte cultures by surface microtopography. Am J Physiol Cell Physiol 285: C171–C182, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Caldwell JE, Heiss SG, Mermall V, Cooper JA. Effects of CapZ, an actin capping protein of muscle, on the polymerization of actin. Biochemistry 28: 8506–8514, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Cooper JA, Pollard TD. Effect of capping protein on the kinetics of actin polymerization. Biochemistry 24: 793–799, 1985 [DOI] [PubMed] [Google Scholar]
- 9. Cooper JA, Sept D. New insights into mechanism and regulation of actin capping protein. Int Rev Cell Mol Biol 267: 183–206, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fischer JE, Horst WD, Kopin IJ. Norepinephrine metabolism in hypertrophied rat hearts. Nature 207: 951–953, 1965 [DOI] [PubMed] [Google Scholar]
- 11. Glacy SD. Pattern and time course of rhodamine-actin incorporation in cardiac myocytes. J Cell Biol 96: 1164–1167, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta A, Dey CS. PTEN and SHIP2 regulates PI3K/Akt pathway through focal adhesion kinase. Mol Cell Endocrinol 309: 55–62, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Hart MC, Cooper JA. Vertebrate isoforms of actin capping protein beta have distinct functions in vivo. J Cell Biol 147: 1287–1298, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hartman TJ, Martin JL, Solaro RJ, Samarel AM, Russell B. CapZ dynamics are altered by endothelin-1 and phenylephrine via PIP2- and PKC-dependent mechanisms. Am J Physiol Cell Physiol 296: C1034–C1039, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kilimann MW, Isenberg G. Actin filament capping protein from bovine brain. EMBO J 1: 889–894, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim K, Yamashita A, Wear MA, Maéda Y, Cooper JA. Capping protein binding to actin in yeast: biochemical mechanism and physiological relevance. J Cell Biol 164: 567–580, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim T, Cooper JA, Sept D. The interaction of capping protein with the barbed end of the actin filament. J Mol Biol 404: 794–802, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koshman YE, Kim T, Chu M, Engman SJ, Iyengar R, Robia SL, Samarel AM. FRNK inhibition of focal adhesion kinase-dependent signaling and migration in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 30: 2226–2233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li C, Hu Y, Mayr M, Xu Q. Cyclic strain stress-induced mitogen-activated protein kinase (MAPK) phosphatase 1 expression in vascular smooth muscle cells is regulated by Ras/Rac-MAPK pathways. J Biol Chem 274: 25273–25280, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Littlefield R, Almenar-Queralt A, Fowler VM. Actin dynamics at pointed ends regulates thin filament length in striated muscle. Nat Cell Biol 3: 544–551, 2001 [DOI] [PubMed] [Google Scholar]
- 21. McCain ML, Parker KK. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflügers Arch 462: 89–104, 2011 [DOI] [PubMed] [Google Scholar]
- 22. McKenna N, Meigs JB, Wang YL. Identical distribution of fluorescently labeled brain and muscle actins in living cardiac fibroblasts and myocytes. J Cell Biol 100: 292–296, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pollard TD, Mooseker MS. Direct measurement of actin polymerization rate constants by electron microscopy of actin filaments nucleated by isolated microvillus cores. J Cell Biol 88: 654–659, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schafer DA, Jennings PB, Cooper JA. Dynamics of capping protein and actin assembly in vitro: uncapping barbed ends by polyphosphoinositides. J Cell Biol 135: 169–179, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Senyo SE, Koshman YE, Russell B. Stimulus interval, rate and direction differentially regulate phosphorylation for mechanotransduction in neonatal cardiac myocytes. FEBS Lett 581: 4241–4247, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seqqat R, Guo X, Rafiq K, Kolpakov MA, Guo J, Koch WJ, Houser SR, Dell'italia LJ, Sabri A. Beta1-adrenergic receptors promote focal adhesion signaling downregulation and myocyte apoptosis in acute volume overload. J Mol Cell Cardiol 53: 240–249, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest 72: 732–738, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Skwarek-Maruszewska A, Hotulainen P, Mattila PK, Lappalainen P. Contractility-dependent actin dynamics in cardiomyocyte sarcomeres. J Cell Sci 122: 2119–2126, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Sprague BL, Pego RL, Stavreva DA, McNally JG. Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys J 86: 3473–3495, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takeda S, Minakata S, Koike R, Kawahata I, Narita A, Kitazawa M, Ota M, Yamakuni T, Maéda Y, Nitanai Y. Two distinct mechanisms for actin capping protein regulation-steric and allosteric inhibition. PLoS Biol 8: e1000416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Terracio L, Miller B, Borg TK. Effects of cyclic mechanical stimulation of the cellular components of the heart: in vitro. Cell Dev Biol 24: 53–58, 1988 [DOI] [PubMed] [Google Scholar]
- 32. Torsoni AS, Constancio SS, Nadruz W, Jr, Hanks SK, Franchini KG. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ Res 93: 140–147, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Wear MA, Yamashita A, Kim K, Maéda Y, Cooper JA. How capping protein binds the barbed end of the actin filament. Curr Biol 13: 1531–1537, 2003 [DOI] [PubMed] [Google Scholar]