Abstract
Background:
To establish a ultra-pressure liquid chromatography (UPLC) method for the determination of 5 phenolic acids including neochlorogenic acid, chlorogenic acid, caffeic acid, cryptochlorogenic acid, and cynarin in fried Fructus xanthii from different production sites and its dispensing granule.
Materials and Methods:
An Acquity BEH C18 chromatographic column (100 mm × 2.1 mm,1.7 μm) was used to perform the determination, which was maintained at 40°C throughout the analysis. Mobile phase was composed of methanol and water containing 0.1% phosphoric acid (v/v) with flow rate at 0.4 mL/min under gradient elution, and detection wavelength was set to 325 nm for monitoring the separation.
Results:
Neochlorogenic acid, chlorogenic acid, caffeic acid, cryptochlorogenic acid, and cynarin have shown good linearity (r2≥0.9997) within 0.959–239.75, 0.9408–235.2, 0.1638–40.95, 0.6744–67.44, and 0.47–117.5 μg/mL, and their average recoveries were 100.09%, 99.98%, 101.74%, 99.83%, and 99.63%, respectively.
Conclusion:
The UPLC method established in this study was rapid, and of good accuracy, repeatability and resolution, and hence can assist in the control quality of fried Fructus xanthii as well as its dispensing granule in an efficient manner.
Keywords: Dispensing granule, fried Fructus xanthii, phenolic acids, ultra-pressure liquid chromatography, Xanthium sibiricum Part
INTRODUCTION
Fructus xanthii, the dried mature fruit with involucre of Xanthium sibiricum Part, is embodied in current Pharmacopoeia of the People′s Republic of China (Year 2010 Edition, Volume I) as a frequently utilized Chinese herbal medicine to dispel cold wind, dehumidify, smooth the nose and resuscitate. It was popularly used to cure symptoms such as cold, headache, chronic sinus inflammation, rhinorrhea, rubella itching, arthritis with refractory pain caused by dampness, and muscular contracture, etc. in clinic practice.[1] In decades, it has been found that Fructus xanthii contains a large number of phenolic acids.[2] And, according to some reports,[3] phenolic acids isolated from anti-inflammatory ingredients of the herb include caffeic acid, chlorogenic acid, 1,5-dicaffeoylqunic acid, 4-o-caffeoylquinic acid [Figure 1], etc. Pharmacology studies indicate that caffeoylquinic acid compounds such as chlorogenic acid possess various bioactivities, e.g. antibiosis, antiviral property, and antioxidation. Synergy of multiple compounds present in this herb was deemed to be the crucial material foundation corresponding to its clinical efficacy.
Figure 1.
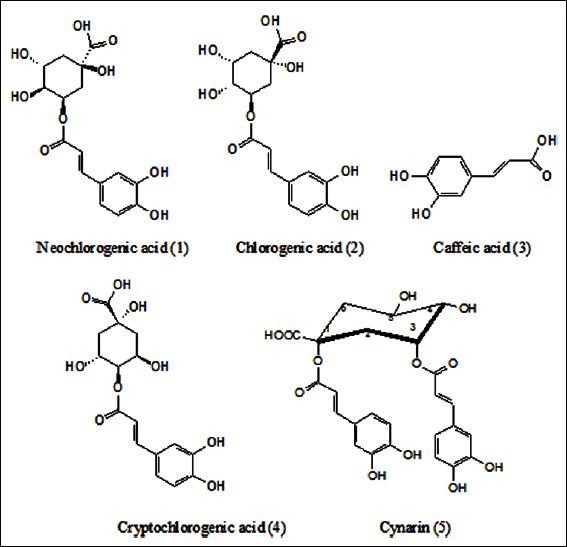
Chemical structures of five phenolic acids
Currently, Fructus xanthii in clinical prescription is in its processed form called fried Fructus xanthii as the raw material has toxicity to human′s lung, which is traditionally required to remove thorn and fry. Main purpose of this processing is to reduce toxicity and make it easier to clean the herb.[4] Fried Fructus xanthii′s dispensing granule was prepared by water extraction, concentration, drying, and granulation in sequence after frying the raw material in conformity with relevant regulations stipulated in the Pharmacopoeia. Due to their various bioactivities, phenolic acids in the herb, as the key material base corresponding to its efficacy, are considered to be the markers of fried Fructus xanthii as well as its dispensing granule. Ultra-pressure liquid chromatography (UPLC), effecting as a new-generation chromatographic technology of higher efficiency and accuracy than traditional high performance liquid chromatography,[5–9] was employed in this study with the aim to simultaneously determine the content of aforementioned 5 phenolic acids in the fried Fructus xanthii collected from 23 different production sites and 3 batches of its dispensing granule purchased in the market. And, it is also expected to provide a rapid and reliable assay protocol of those phenolic acids for the purpose of quality control of fried Fructus xanthii and its dispensing granule.
MATERIALS AND METHODS
Instruments
Acquity UPLC system (a quaternary pressure gradient pump, a vacuum degasser, an automatic sampler, a column oven, a DAD, and an Empower 2 chromatographic working station, US Waters Company); Mettler 0.0001 electronic balance, Millipore ultrafiltration instrument for making ultrapure water.
Chinese medicine materials and reagents
Fried Fructus xanthii was obtained through collecting its raw material from various production sites followed by frying them in Chinese Medicine processing facility. Three batches of fried Fructus xanthii dispensing granule were purchased from Jiangsu Jiangyin Tianjiang Pharmaceutical Co., Ltd. (B/N: 1010158, 1012058, 1001093, net weight of each package is 0.5 g, equivalence to 10 g of the herb). Chlorogenic acid (B/N: 110753-200413, purity: 98.7%) and caffeic acid (B/N: 110885-200102, purity: 99.1%) were the certified standard from National Institute for the Control of Pharmaceutical and Biological Products of China. And, the other three standards including neochlorogenic acid (B/N: N100620), cryptochlorogenic acid (B/N: C100616), and cynarin (B/N: D100429) were purchased from Chengdu Purify Science and Technology Development Co., Ltd. Their purities were all above 99.0%. Methanol is of chromatography grade, and other reagents are of analytical grade.
UPLC conditions
Chromatographic column: An Acquity BEH C18 column (100 mm × 2.1 mm, 1.7 μm); mobile phase: The mixture of 0.1% phosphoric acid aqueous solution (A) and methanol (B). Gradient elution time program: 0 min, B: 3%; 2 min, B: 7%; 10 min, B: 15%; flow rate: 0.4 mL/min; column temperature: 40°C; detection wavelength: 325 nm. A typical UPLC chromatogram was obtained as shown in Figure 1.
Preparation of standard solution
Chlorogenic acid (2.74 mg), caffeic acid (1.68 mg), neochlorogenic acid (2.74 mg), cryptochlorogenic acid (2.81 mg), and cynarin (2.35 mg) were accurately weighed and were transferred into volumetric flask of 5 mL, respectively. 3% methanol aqueous solution was added to dissolve the individual compound and then top up till the calibration mark in order to obtain standard solutions for each analyte.
Preparation of sample solution
2 g of fried Fructus xanthii powder from different production sites and 0.5 g of the dispensing granule were weighed and then transferred into a volumetric flask of 50 mL. 50% methanol was added to extract with the total weight taken, prior to ultrasonic extraction for 60 min. The suspension was cooled to room temperature, and then the lost weight was made up with 50% methanol. After shaking for homogenization, it was subject to suction filtration. The initial filtrate was discarded, and the subsequent filtrate was collected and centrifuged prior to injection into UPLC system.
Linearity
3.5 mL of neochlorogenic acid solution, 2.8 mL of chlorogenic acid solution, 0.30 mL of caffeic acid solution, 1.2 mL of cryptochlorogenic acid solution, and 1.0 mL of cynarin solution prepared before were pipetted into a brown volumetric flask, and 3% methanol was added in to prepare the mixture of standard solution. And then, a series dilution of 2, 5, 10, 20, 50, and 100 times was conducted to obtain working solutions for UPLC analysis. After centrifugation, the solutions were analyzed under the above chromatographic conditions.
Precision
Sample solutions of the dispensing granule (B/N: 1010158), fried Fructus xanthii (jiangsu), and standard solutions were prepared, and then 3 μL of the solution was injected continuously for 6 times to evaluate inter-day precision. The inter-day precision was investigated over 3 consecutive days.
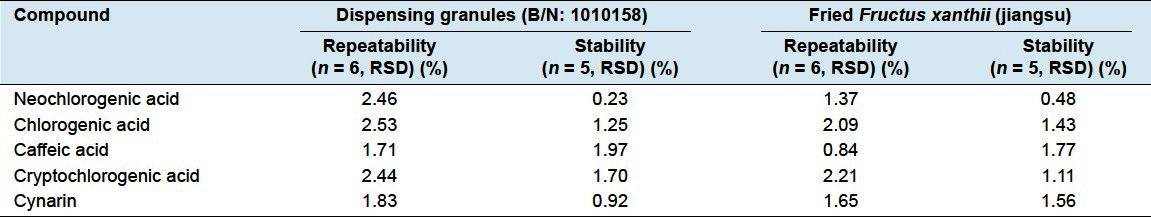
Repeatability
Six sample solutions of dispensing granule (B/N: 1010158) and fried Fructus xanthii (jiangsu) were prepared, and 4 μL of the solution was analyzed in accordance under the same chromatographic conditions.
Stability
Freshly prepared sample solutions of fried Fructus xanthii (jiangsu) and Dispensing Granule (B/N: 1010158) were injected immediately and then tested after storage at room temperature for 2, 4, 6, and 8 h.
Accuracy
Recovery test was conducted to evaluate the accuracy of the developed method. The standards were spiked into 6 Dispensing Granule (B/N: 1010158) and fried Fructus xanthii (jiangsu) samples of same batch. The sample solutions were prepared and tested using the developed method. The percentage recoveries were calculated based on the following formula: (Detected amount−Original amount)/Spiked amount × 100.
Determination of 6 analytes in the samples
The content of phenolic acids in the samples was determined in triplicate under the UPLC conditions as above.
RESULTS
Regression equation of peak area (Y) to concentration (X) was plotted, and correlation coefficient (r2), linearity range, LOD, and LOQ of the phenolic acids were calculated as well. The results are shown in Table 1, and the chromatogram of standard mixture is illustrated in Figure 2a.
Table 1.
Linear regression, LOD, and LOQ of the phenolic acids
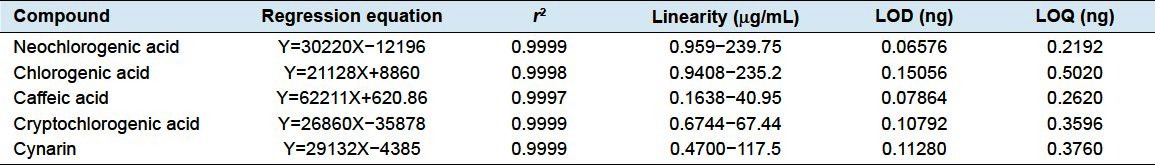
Figure 2.
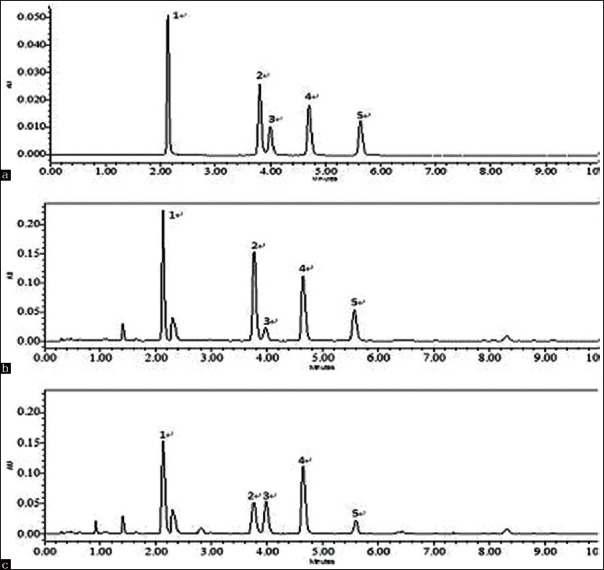
Typical UPLC chromatograms of standard and sample solutions (a) Reference; (b) Dispensing granules; (c) Fried Fructus xanthii)
The results for precisions, repeatability, and stability are summarized in Tables 2 and 3. It was revealed that the sample solution was stable over this period.
Table 2.
The results of precision
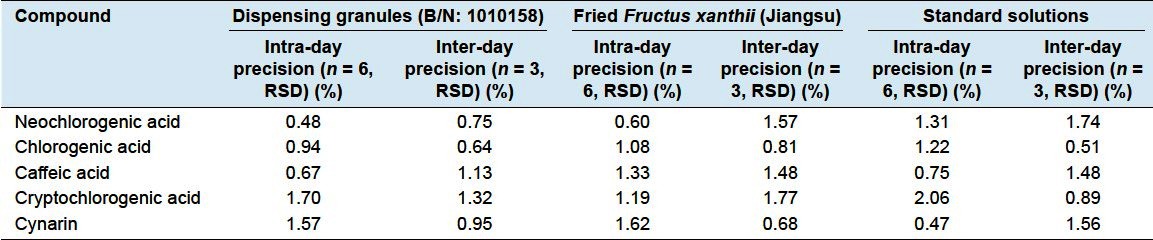
Table 3.
The results of repeatability and stability
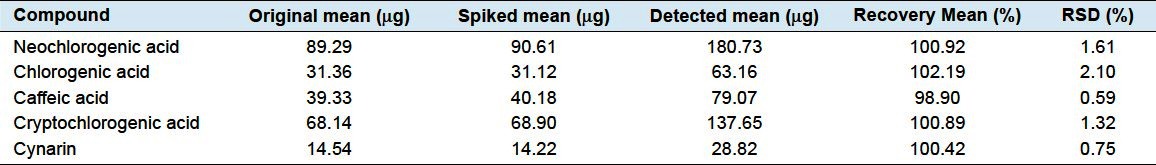
For accuracy, the results are presented in Tables 4 and 5. And, the recoveries of 5 phenolic acids were within the range of 98.90–102.19%, with the RSD values ranging from 0.59% to 2.10%, very well verifying the high accuracy of this developed method.
Table 4.
Dispensing granules (B/N: 1010158) determination result of recovery test (n = 6)
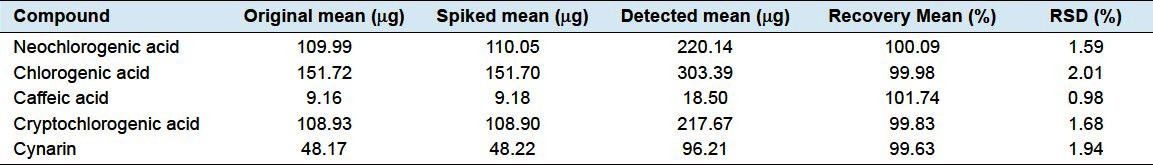
Table 5.
Fried Fructus xanthii (Jiangsu) determination result of recovery test (n = 6)
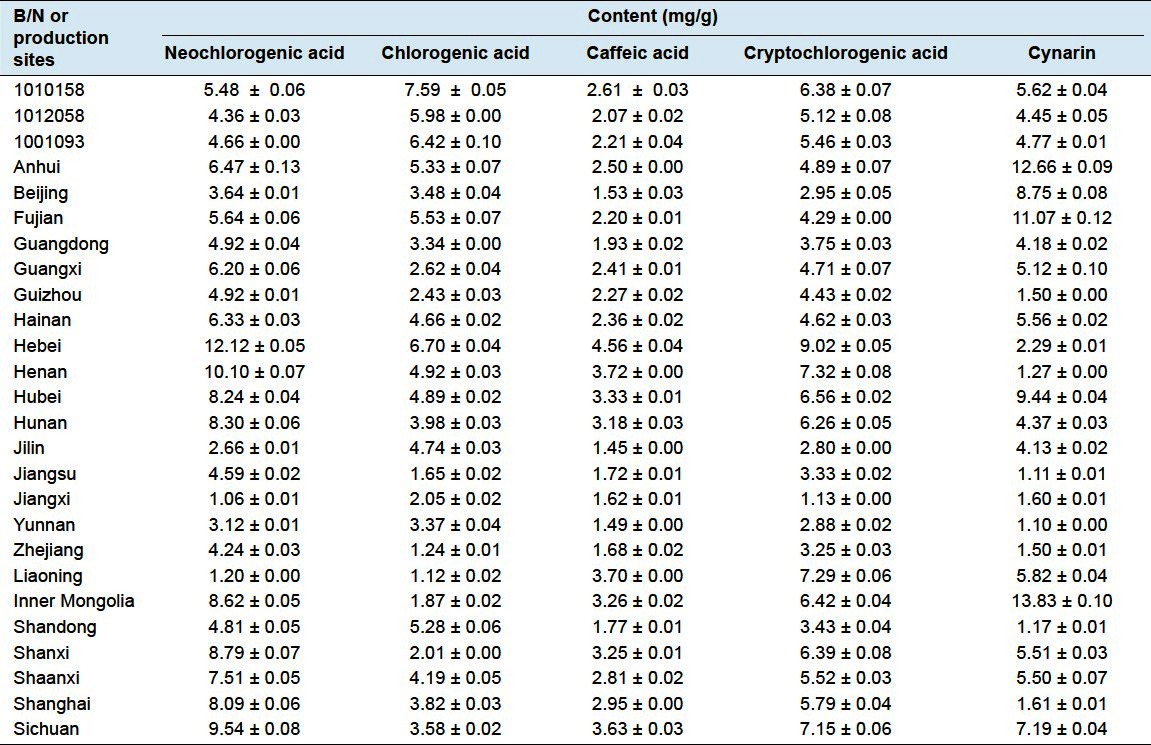
The chromatograms of fried Fructus xanthii and its dispensing granule are illustrated in Figure 2b and c. And, the results of the contents of 5 analytes are shown in Table 6.
Table 6.
The contents of 5 phenolic acids in sample (n = 3)
DISCUSSION
Currently, Fructus xanthii used in clinical practice is in their fried form. However, few reports on the quality control of this processed herb have been published. On the other hand, with the acceleration of people′s pace of life, clinical application of dispensing granule of a single herb is becoming more and more popular.[10,11] Chinese medicinal granules, a modern form of traditional Chinese medicines, can overcome some shortcomings such as big volume of service and difficulty in storage. It can be taken conveniently with reliable quality. In recent years, fried Fructus xanthii dispensing granule is undergoing an increasingly extensive application in clinical practice. Chinese medicines contain extremely complicated chemical compositions, and their effects are due to the synergy of various active ingredients intrinsic in the herb. The genuine feature of Chinese medicines hasn′t been comprehensively demonstrated by the traditional standard using single compound as the index in quality control. Therefore, specification for quality control established for Chinese medicines in their ready-in-use form and its dispensing granule should be higher than the prevailing one, and as many as compounds should be selected as markers so as to ensure their effectiveness.
In this study, fried Fructus xanthii and its dispensing granule from different production sites or batches were selected as an object. From preliminary study, 5 phenolic acids such as chlorogenic acid were selected as markers for the purpose of quality control. The results indicate that much difference is present in the fried Fructus xanthii from different production sites in term of the content of 5 phenolic acids. Accordingly, it is important to optimize the origin of raw Fructus xanthii. In addition, difference in content of 5 phenolic acids was also observed in the dispensing granule of fried Fructus xanthii among different batches of same manufacturer. If one of the acids is high, all the acids tested are higher than others, which might be relating to harvest time, origin, and process of Fructus xanthii. By taking the average of the 5 phenolic acids contained in the samples of dispensing granule of 3 different batches, it was noticed that their content just accounts for 10–15% of counterpart crude material,[12] which implies that a large number of components may have lost during the process, leading to the reduced transfer rate of active compositions. By analyzing existing problems of dispensing granule, our research group has raised a proposal about enhancing the quality, namely four standardizations and one further requisite,[13] and also pointed out that standardization of Chinese medicine formula granule was the foundation of their clinical efficacy. Therefore, it was suggested that manufacturing process shall be further perfected, and criterions for multiple components quality control needs to be established, with the expect to regulate the quality of fried Fructus xanthii dispensing granule in a more comprehensive manner so as to ensure reliability as well as consistency of its clinical efficacy.
CONCLUSION
It has been demonstrated that the newly proposed UPLC method in this study is a rapid and reliable method for the determination of 5 phenolic acids in the herbal products, namely fried Fructus xanthii and its dispensing granule. Furthermore, this validated method can also be applied for the purpose of quality control of this herb.
ACKNOWLEDGMENTS
This work was financially supported by National Natural Science Foundation of China (81102812), Science and Technology Research Fund from JSTCM (LZ09061), the Talents in TCM of Jiangsu Province (2006) and Changzhou Science and Technology Research Program (CE20125042).
Footnotes
Source of Support: National Natural Science Foundation of China (81102812), Science and Technology Research Fund from JSTCM (LZ09061), the Talents in TCM of Jiangsu Province (2006) and Changzhou Science and Technology Research Program (CE20125042)
Conflict of Interest: None declared.
REFERENCES
- 1.National Pharmacopoeia Committee. Chinese Pharmacopoeia ed. vol. 1. Beijing: Chemical Industry Press; 2010. p. 151. [Google Scholar]
- 2.Han T, Le HL, Hu Y. Content determination of phenolic acid compounds, various species and total phenolic acid in Fructus xanthii. J Chin Integrative Med. 2006;2:194–8. doi: 10.3736/jcim20060217. [DOI] [PubMed] [Google Scholar]
- 3.Han T. Biologically active components and quality evaluation of Fructus xanthii. Shanghai: Second Military Medical University; 2006. [Google Scholar]
- 4.Wu HE, Wei ZY, Wei JH. Content determination of chlorogenic acid and caffeic acid in fried Fructus xanthii dispensing granule with HPLC. Chin J Modern Appl Pharma. 2010;8:744–6. [Google Scholar]
- 5.Papageorgiou VP, Assimopoulou AN, Samanidou AF, Papadoyannis I. Analytical methods for the determination of alkannins and shikonins. Current Org Chem. 2006;5:583–622. [Google Scholar]
- 6.Assimopoulou AN, Karapanagiotis I, Vasiliou A, Kokkini S, Papageorgiou VP. Analysis of alkannin derivatives from Alkanna species by high-performance liquid chromatography/photodiode array/mass spectrometry. Biomed Chromatogr. 2006;20:1359–74. doi: 10.1002/bmc.705. [DOI] [PubMed] [Google Scholar]
- 7.Assimopoulou AN, Ganzera M, Stuppner H, Papageorgiou VP. Simultaneous determination of monomeric and oligomeric alkannins and shikonins by high-performance liquid chromatography-diode array detection-mass spectrometry. Biomed Chromatogr. 2008;22:173–90. doi: 10.1002/bmc.912. [DOI] [PubMed] [Google Scholar]
- 8.Assimopoulou AN, Sturm S, Stuppner H, Papageorgiou VP. Preparative isolation and purification of alkannin/shikonin derivatives from natural products by high-speed counter-current chromatography. Biomed Chromatogr. 2009;23:182–98. doi: 10.1002/bmc.1101. [DOI] [PubMed] [Google Scholar]
- 9.Noula E, Samanidou VF, Assimopoulou AN, Papageorgiou VP, Papadoyannis IN. Solid-phase extraction for purification of alkannin/shikonin samples and isolation of monomeric and dimeric fractions. Anal Bioanal Chem. 2010;397:2221–32. doi: 10.1007/s00216-010-3717-5. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Jia CB, Chen Y. Standardization research on schisandra chinensis dispensing granule process. Chin J Chin Mater Med. 2006;24:2078–9. [Google Scholar]
- 11.Chen Y, Cai Y, Jia XB. Content determination of 5 primary flavonoid fractions in herba epimedii dispensing granule with HPLC. Chin J Experimental Tradit Med Formulae. 2007;11:1–3. [Google Scholar]
- 12.Yang L, Su ZJ, Xu SJ. Content determination of 4 phenolic acids compositions in Fructus xanthii with UPLC. Pharma J. 2010;12:1537–40. [PubMed] [Google Scholar]
- 13.Jia XB, Huang LX, Chen Y. Standardization of Chinese medicine formula granule, foundation of clinical effects. Chin Arch Tradit Chin Med. 2008;6:1200–2. [Google Scholar]


