Abstract
Background:
Angelica sinensis is a famous traditional Chinese medicinalherb, which is predominantly used in the treatment of gynecological conditions. It is the first report for the simultaneous determination of six major active components in Chinese Angelica, which is important for quality control.
Objective:
A validated HPLC-PAD method was first developed to evaluate the quality of crude and processed Radix Angelica through simultaneous determination of six bioactive compounds, namely ferulic acid, senkyunolide I, senkyunolide H, coniferyl ferulate, Z/E-ligustilide and Z/E-butylidenephthalide.
Materials and Methods:
Samples were separated on a Xtimate™C18 column (250 × 4.6 mm, 5 μm) and detected by PAD. Mobile phase was composed of (A) aqueous phosphoric acid (0.02%, v/v) and (B) acetonitrile (MeCN) (including 10% tetrahydrofuran, v/v) using a gradient elution. Analytes were performed at 30°C with a flow rate of 1.0 mL/min.
Results:
All calibration curves showed good linear regression (r2 ≥ 0.9963) within the tested ranges, and the recovery of the method was in the range of 91.927–105.859%.
Conclusion:
The results demonstrate that the developed method is accurate and reproducible and could be readily utilized as a suitable quality control method for the quantification of Radix Angelica.
Keywords: Chinese Angelica, high performance liquid chromatography, photodiode-array detector, quality control, simultaneous determination
INTRODUCTION
Angelica sinensis (Apiaceae, Angelica, A. sinensis), called “Danggui” in Chinese, has been used in the treatment of gynecological conditions, namely dysmenorrhea, amenorrhea, menopausal syndromes[1] for thousands of years in traditional Chinese, Korean and Japanese medicines, which was first cited in Shenlong Bencao Jing (200–300 A.D., Han Dynasty).[2,3] Besides that, it has been widely applied to treat anemia, abdominal pain, migraine headaches, cardiovascular disease and hepatic fibrosis.[4]
Many kinds of compounds have been isolated and identified from Angelica sinensis, including essential oils (mainly including monomeric phthalides as well as phthalide dimmers), coumarins, organic acids and their esters, polysaccharides, amino acids, and others.[5,6] But it isquite distinct in different crude resources. Avula et al. have quantitative determined eight coumarin constituents from Angelica sinensis.[7] Instead of coumarins in Korean Angelica, phthalides are the principal components in Chinese Angelica, andeven coumarins have not been found in the latter.[8] Based on a large amount of pharmacological research, the constituents such as ferulic acid, senkyunolide I, senkyunolide H, coniferyl ferulate, Z/E-ligustilide, Z/E-butylidenephthalide were found to be responsible for the biological activities in Chinese Angelica. Their chemical structures are shown in Figure 1.
Figure 1.
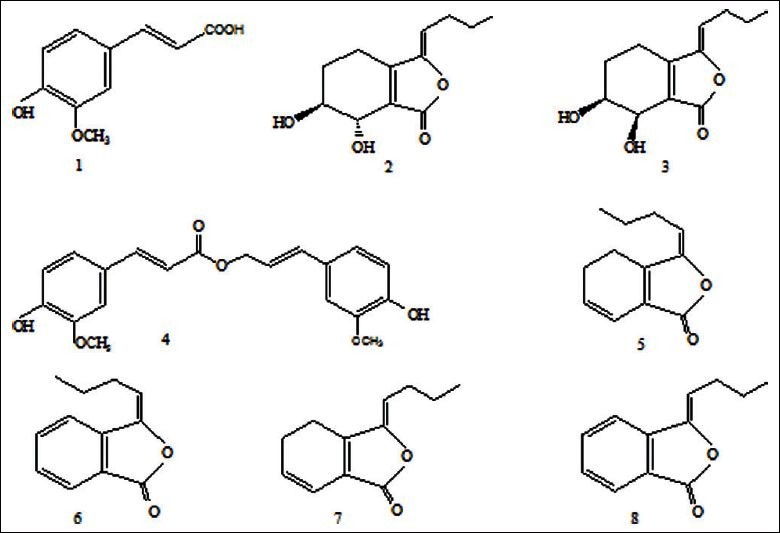
Chemical structures of the 6 active components contained in Chinese Angelica (1) ferulic acid, (2) Senkyunolide I, (3) Senkyunolide H, (4) Coniferyl ferulate, (5) E-Ligustilide, (6) E-Butylidenephthalide, (7) Z-Ligustilide, (8) Z-Butylidenephthalide
Since curative effect of TCMs is an integrative resultof a number of bioactive compounds, quantitative determination not only for the qualitycontrol of crude drugs but also for elucidating the therapeuticprinciple, which lead quality control of herbal medicines is necessary and important. Currently, only a few analytical studies have been reported to determine the active components in Chinese Angelica. Guang-Hua Lu et al. developed a high-performance liquid chromatographic fingerprints which could analyse Chinese Angelica a squalitative.[9] But they only focused on the qualitative analysis of chemical constituents in Chinese Angelica and lacked the information of quantitative determination for quality control. To date, the methods for the simultaneous separation and quantitative determination of multiple active components in a single running for Chinese Angelica are still not available. Therefore, an accurate and reliable method is needed for the quality control of this famous traditional Chinese medicinal.
The HPLC-PDA method applied for the simultaneous quantitative determination of 6 active components (including ferulic acid, senkyunolide I, senkyunolide H, coniferyl ferulate, Z/E-ligustilide and Z/E-butylidenephthalide) contained in Chinese Angelica is the first time reported in this work. The developed HPLC-PDA coupled method is very simple, accurate and reliable for the routine analysis and quality control of Chinese Angelica.
MATERIALS AND METHODS
Plant materials
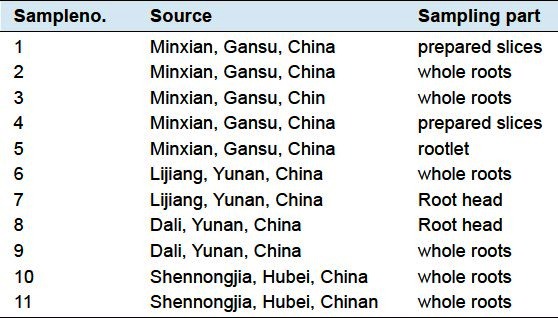
Elevencommercial products were purchased from different provinces′ herbal marketon July, 2011 in China, which werecrude plant material. The identity, sampling part and sample source of 11 tested samples are summarized in Table 1, and those samples have been authenticated by Dr. H. Zhao, from theInstitute of Materia Medica, The Fourth Military Medical University. The 11 samples were cut into smaller pieces, further ground into powder, and stored at desiccatorbefore use.
Table 1.
A summary of the tested samples
Chemicals and Reagents
All the solvents used in this experiment were HPLC-grade. Methanol (MeOH) was purchased from Burdick and Jackson (SK Chemical, UIsan, Korea), acetonitrile (MeCN) from Honeywell (Muskegon, MI, USA). The deionized water was prepared from Millipore water purification system (Milford, MA, USA) and filtered with a 0.22-μm membrane. Other reagents were all of analytical grade. A membrane filter (diameter-13 mm, poresize-0.22 μm, Advantec, CA, USA) was used to filter eachsample.
The standards of ferulic acid, senkyunolide I, senkyunolide H, coniferyl ferulate, Z/E-ligustilide and Z/E-butylidenephthalide were purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China), and the purity was shown to be greater than 98%.
Instrumentation and HPLC conditions
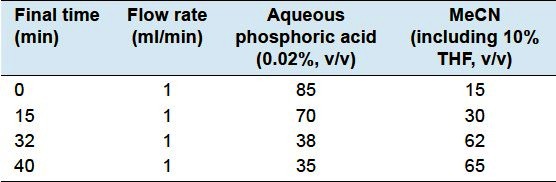
An Waters 2695 Alliance HPLC system (Waters Corp, Milford, MA, USA), equipped with Empower™ Software and comprised of a quaternary solvent delivery system, an on-line degasser, an autosampler, a thermostated compartment and a 2996 photodiode array detection, was used for the chromatographic analysis. All separations were performed on a Xtimate™ C18 column (250 × 4.6 mm, 5 μm), and the solvent gradient conditions areshown in Table 2. The temperature was maintained at 30°C, and the flow rate was 1.0 ml/min. The injection volume was 20 μL, and re-equilibration duration was 10 min between individual runs. Monitoring of the analytes and quantitation was performed at the wavelength of maximum absorbance for each analyte. Ferulic acid and Z/E-ligustilide were monitored at 322 nm, senkyunolide I and senkyunolide H at 277 nm, coniferyl ferulate at 216 nm, and Z/E-butylidenephthalide at 237nm. Peak identification was performed both by retention timesand by spectral information provided by the PAD. The components were quantified based on peak areas at the maximum wavelength in their UV spectrum.
Table 2.
Solvent gradient conditions for HPLC–PAD
Preparation of standard solutions and sample solutions
Stock solutions for standard compounds were prepared withHPLC-grade methanol as solvent and stored away from light at 4°C. Working calibration solutionscontaining the six compounds were prepared byappropriate serial dilution of the stocksolution with methanol and the final concentrations were 6.4, 60, 200, 20, 1200 and 300 μg/ml.
Accurately weighed about 1.0g dried and powdered samples of Chinese Angelica, added 20 mL ofmethanol, weighed the mixture andsonicate it for 30 min, made up the weight loss with methanol after cooling down to ambient temperature. The extract was then filtered with a 0.22-μm microporous membraneinto an amber glass HPLC vial prior to analysis.
RESULTS AND DISCUSSION
Optimization of chromatographic conditions
Regarding the choice of solvent for optimal extraction, methanol was thepreferred choice of extraction solvent in the present studyas a variety of compounds with different polarity can be coextracted effectively. Extraction efficiencyof methanol-water at ratios of 100:0, 90:10, 80:20, 70:30,60:40 and 50:50 were examined, by ultrasonic extraction 0.5h and 1h, respectively. It was observed that compounds included coniferyl ferulate, Z/E-ligustilide, and Z/E-butylidenephthalide were decreasing with ratios of methanol went down and those compounds of adenosine and ferulic acid were opposite, and the influence of time is ignorable. Considering the content and pesticide effect of water-solubility compounds is tiny, purely methanol was chosen inthis study. Besides, the interference from sugars in the raw herbs could also be minimized by extraction using methanol.
In general, a suitable chromatographic column, mobile phase, elution mode and detection wavelength are critically important for good separation. In the present study, different columns, such as Xtimate™ C18 column, Yilite Hypersil BDS C18 column, Yilite SinoChrom ODS-BP C18 column and Phenomenex Luna 5u C18 column were employed. Various mobile phases consisting of MeCN-water, methanol-water, methanol-MeCN-water, methanol-THF-water and MeCN-THF-water with different gradient elution modes were tested. In addition, the water modifiedby phosphoric acid, aceticacid and formic acid with different pH values were tested. The flow rate of 0.8 ml/minand 1.0 ml/min were also optimized. Since the structures of Z/E-ligustilide, and Z/E-butylidenephthalide are extremely similar, the separating degree of them in chromatograms presented were not so good. After add THF into the mobile phases, the separating degree had improved obviously.
The detection wavelength was selected according to the maximum adsorption wavelengths of 216, 237, 277, 322 nm, respectively, shown in UV spectra with three-dimension chromatograms of photodiode array detection. The desired components from Chinese Angelica were identified by comparing both the retention times and UV spectra with those of the authentic standard.
After many tests, Xtimate™ C18 column with the MeCN-THF-phosphoric acid solution system using gradient elution was found suitable for the simultaneous separation and determination. Excellent agreement between standard and sample spectra was found in all analyzed samples, indicating that under the proposed analytical conditions, the six marker constituents were sufficiently resolved and successfully separated. Typical chromatograms of the authentic standards and Chinese Angelica are shown in Figure 2.
Figure 2.
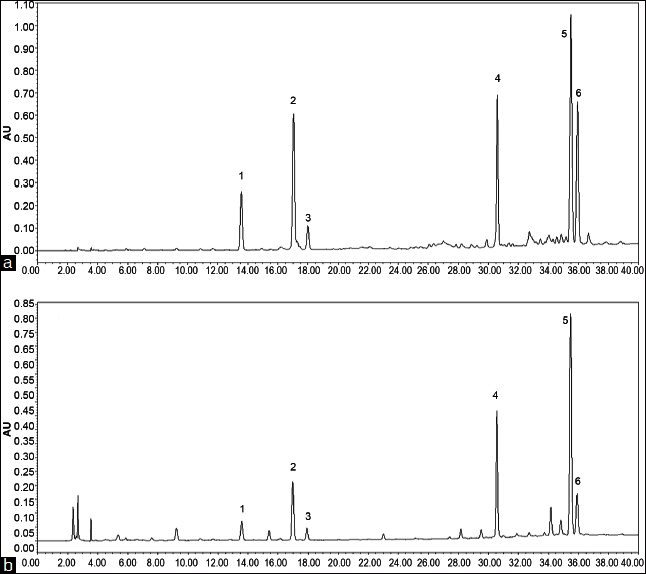
Chromatograms of the authentic standards and Chinese Angelica Typical chromatograms of the standard mixture (a) and Chinese Angelica (b) at 270 nm(1) ferulic acid, (2) senkyunolide I, (3) senkyunolide H, (4) coniferyl ferulate, (5) Z/E-ligustilide, (6) Z/E-Butylidenephthalide
METHOD VALIDATION
The HPLC method was validated by defining the linearity, limits of detection, identification andquantification of the precision, stability and recovery.
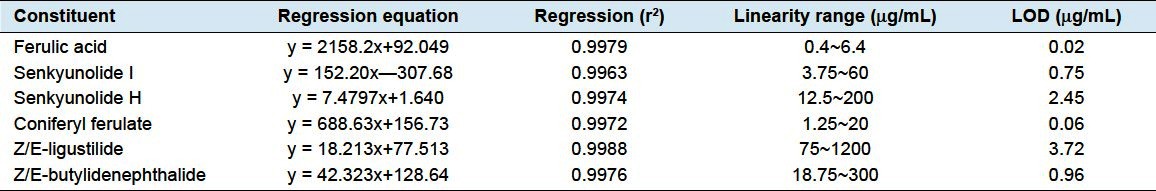
Calibrations working standard solutions were freshlyprepared in methanol by appropriate dilution of the stocksolutions. All calibration curves were constructedby analysis of a mixture containing sixstandard substances atvarious concentration levels and plotting peak area againstthe concentration of each reference standarda good correlation was found betweenthe peak area (y) and the concentrations (x) (r2 ≤ 0.9963) forall the compounds in the range of concentration tested attheir detected wavelengths.
The limits of detection (LOD) were determined according to International Conference onHarmonization (ICH) recommendations.[10] LODvalue was calculated by means of serial dilution based on a signal-to-noise (S/N)ratio of 3:1, which confirmed the applicabilityof the proposed method. The regression equations, correlation coefficients, and linear ranges and LODs for the analysis of the six marker constituents are shown in Table 3.
Table 3.
Regression equation, linear range and LODs of calibration curves
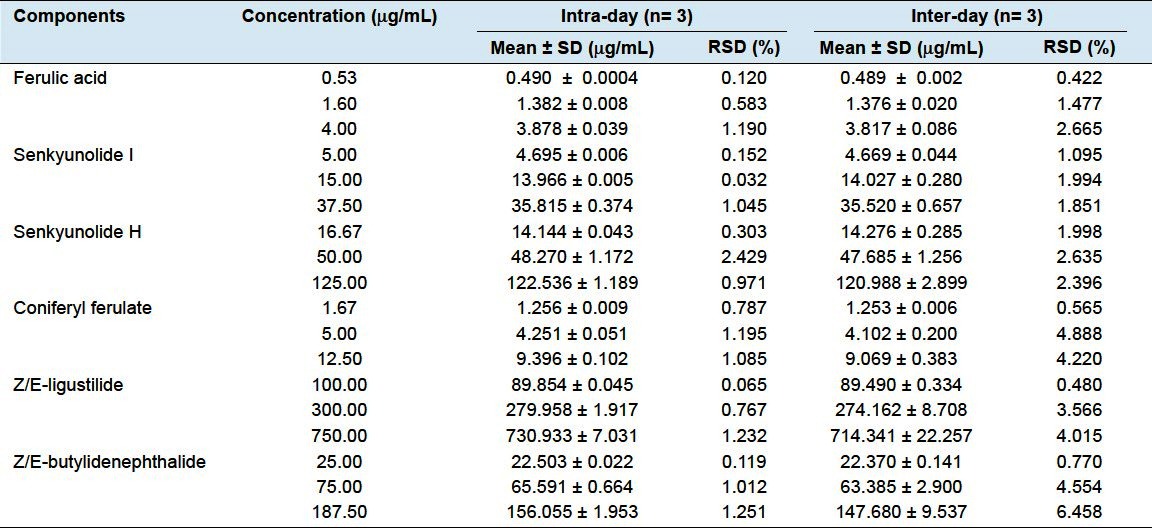
The intradayand interday precisions were determinedby assaying standard solutions at three concentrations during a single day and on three consecutive days, respectively. The resultsare shown in Table 4. From Table 4, it appears that the RSDs of intra day were not exceeding 2.429%, while the interdayprecisions were not allless than 5.0%. Since some constituents in Chinese Angelica were chemical instability mostly, which could decomposeat high temperature and direct sunlight. It was proposed that the concentration of compounds converted during the storage period of sample solution. Some studies have authenticated Z/E-ligustilide and Z/E-butylidenephthalide were volatile and unstable compounds, which can bechanged to other phthalides through oxidation, isomerization, dimerization, etc.[11]
Table 4.
Analytical results of intra and inter-day test
Recovery test was used toevaluate the accuracy of this method, showed in Table 5. Anappropriate amount of Chinese Angelica powder wasweighed and spiked with a known amount of each standardcompound. They were then extracted and analyzed as describedabove. The recovery percentage wascalculated by using the formula: Recovery(%) = (amountfound-original amount)/amount spiked × 100. Therecoveries were found in the range of 91.927–105.859%.
Table 5.
Recoveries of the analyte

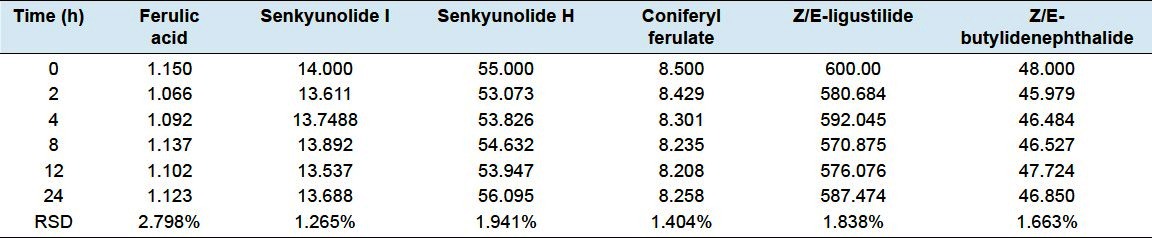
Stability was tested with Chinese Angelica at room temperature and analyzed at 0, 2, 4,8 12and24 h, respectively. The results are shown in Table 6. The RSDs were less than 2.798% for all analytes. The similarityof these results indicated that the sample remained stable within 24 h.
Table 6.
Stability of the analyte
Analysis of commercial products by HPLC-PAD
The newly established method has been applied to the determination of six marker constituents in 11 batches of Chinese Angelica samples, and the results are shown in Table 7. Altogether 11 Chinese Angelica samples including 6 whole roots, 2 root head, 1 rootlet and 2 prepared slices were analyzed which were collected from a varietyof sources and conditions. These included different cultivationareas, various cultivating environments, different processing methods and different parts of roots, etc.
Table 7.
Content of the 6 active components in 11 batches of Chinese Angelica
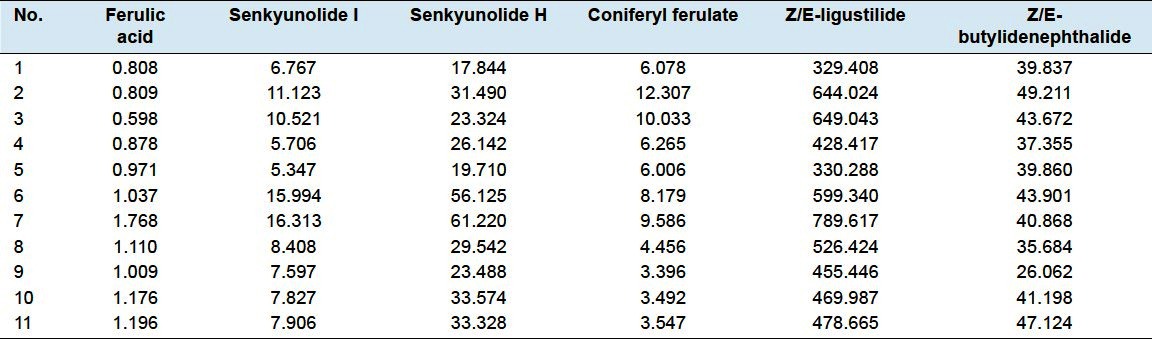
The results indicated that their chromatographic patterns were generally consistent although the absorption intensityof some peaks was different. As shown in Table 7, the contents of compounds in whole roots and root head were higher than rootlet and prepared slices, and some kind of compounds with low abundance cannot be found in prepared slices, which suggested to maintain the active components, the whole roots of Chinese Angelica should be a better choice than prepared slices for medicine trade, which was demonstrate by previous study.[12] In addition, Chinese Angelica which is cultivated in Minxian County, Gansu Province, China, is regarded as the authentic herb according to traditional experience; however, the contents of active components in substitute herbs cultivated in Yunan Province proved to be similar with the authentic herb in Gansu Province.
CONCLUSIONS
An accurate and reliable HPLC method to simultaneously determine multiple active components in Chinese Angelica was developed. This is the first report for the simultaneous determination of six major active components in Chinese Angelica by using reverse phase high performance liquid chromatography coupled with photodiode array detection. The results demonstrate that the developed method is accurate and reproducible and could bereadily utilized as a suitable quality control method for the quantification of Chinese Angelica. It also suggest that the analytes should be test as soon as possible since some componentscan bechanged to other phthalides, and the content of major active components in whole roots of Chinese Angelica would be higher than prepared slices, which demonstrates the whole roots of Chinese Angelica should be a better choice for medicine trade.
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China (30701081 and 81173513).
Footnotes
Source of Support: National Natural Science Foundation of China (30701081 and 81173513)
Conflict of Interest: None declared.
REFERENCES
- 1.Lee YY, Lee S, Jin JL, Yun-Choi HS. Platelet anti-aggregatory effects of coumarins from the roots of Angelicagenuflexa and A. gigas. Arch Pharm Res. 2003;26:723–6. doi: 10.1007/BF02976681. [DOI] [PubMed] [Google Scholar]
- 2.Chinese Pharmacopoeia Commission, Pharmacopoeia of the Peoples Republic of China. Vol. 1. Beijing: Chemical Industry Press; 2002. p. 425. [Google Scholar]
- 3.Chinese Pharmacopoeia Commission, Pharmacopoeia of the Peoples Republic of China. Vol. 1. Beijing: Chemical Industry Press; 2000. p. 101. [Google Scholar]
- 4.Yi LZ, Liang YZ, Wu H, Yuan DL. The analysis of Radix Angelicae Sinensis (Danggui) J Chromatogr A. 2009;1216:1991–2001. doi: 10.1016/j.chroma.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Huang WH, Song CQ. Research progresses in the chemistry and pharmacology of Angelica sinensis. Zhongguo Zhong Yao Za Zhi. 2001;26:147–51. [PubMed] [Google Scholar]
- 6.Chao WW, Lin BF. Bioactivities of major constituents isolated from Angelica sinensis (Danggui) Chin Med. 2011;19:6–29. doi: 10.1186/1749-8546-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avula B, Joshi VC, Reddy VL, Choi YW, Khan IA. Simultaneous determination of eight coumarins in Angelica gigas and in various other Angelica species by high performance liquid chromatography and comparative micro-morphology study of Angelica species. Planta Med. 2007;73:1509–16. doi: 10.1055/s-2007-990260. [DOI] [PubMed] [Google Scholar]
- 8.Ahn MJ, Lee MK, Kim YC, Sung SH. The simultaneous determination of coumarins in Angelica gigasroot by high performance liquid chromatography-diode arraydetector coupled with electrospray ionization/mass spectrometry. J Pharm Biomed Anal. 2008;46:258–66. doi: 10.1016/j.jpba.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Lu GH, Chan K, Liang YZ, Leung K, Chan CL, Jiang ZH, et al. Development of high-performance liquid chromatographic fingerprints for distinguishing Chinese Angelica from related umbelliferae herbs. J Chromatogr A. 2005;1073:383–92. doi: 10.1016/j.chroma.2004.11.080. [DOI] [PubMed] [Google Scholar]
- 10.European Agency for the Evaluation of Medical Products, Human medicines evaluation unit. Note for guidance onvalidation of analytical procedures: Methodology. London: 1996. ICH Topic Q 2B. CPMP/ICH/281/95. [Google Scholar]
- 11.Yi T, Zhang H, Xie J, Xue D. A new procedure for the preparative separation and isolation of Z-ligustilide from the roots of Angelica sinensis. J Sep Sci. 2007;30:1973–8. doi: 10.1002/jssc.200700001. [DOI] [PubMed] [Google Scholar]
- 12.Wu YY, Shang MY, Cai SQ. HPLC fingerprint of the components of Radix Angelicae Sinensis. Yao Xue Xue Bao. 2008;43:728–32. [PubMed] [Google Scholar]


