Abstract
Background:
This research is among the few that has been conducted on the feasibility of subcritical water extraction (SWE) as a rapid and efficient extraction tool for polysaccharides.
Objective:
The aim of the study was to extractand optimize the parameter conditions of SWE of polysaccharides from Grifola frondosa using response surface methodology.
Materials and Methods:
In the study, SWEwas applied to extractbioactive compounds from G. frondosa. A preliminary analysis was made on the physical properties and content determination of extracts using SWE and hot water extraction (HWE). Analysis of the sample residues and antioxidant activities of the polysaccharides extracted by SWE and HWE were then evaluated.
Results:
The optimal extraction conditions include: extraction temperature of 210°C, extraction time of 43.65 min and the ratio of water to raw material of 26.15:1. Under these optimal conditions, the experimental yield of the polysaccharides (25.1 ± 0.3%) corresponded with the mean value predicted by the model and two times more than the mean value obtained by the traditional HWE. The antioxidant activities of polysaccharides extracted by SWE were generally higher than those extracted by HWE. From the study, the SWE technology could be a time-saving, high yield, and bioactive technique for production of polysaccharides.
Keywords: Antioxidant activity, Grifola frondosa, polysaccharide, response surface methodology, subcritical water extraction
INTRODUCTION
The subcritical water extraction (SWE) technique is one of the approaches used for the isolation of valuable components from plants. In applying SWE technique the critical temperature and pressure of water are 374°C and 22.1 MPa, respectively.[1] SWE technology uses water at temperatures between 100°C and 374°C with some amount of pressure (above 5 Mpa and below 22 Mpa) in order to maintain water in its liquid state.[1] Compared with supercritical fluid extraction, the required equipments for the SWE are relatively simpler and not much higher pressure is required. As the temperature increases to 250°C, the dielectric constant of water also reduces from 80 to 27, which is much similar to the dielectric constant of ethanol at ambient temperature.[2] The ion product of the subcritical water substantially increases with temperature, which can catalyze chemical reactions such as hydrolysis and degradation without any additional catalyst.[3] Under the SWE condition, the cellular structure of plant tissues can be broken and consequently result in releasing several chemical compounds to the extra-cellular medium that are dissolved in hot water.[4] SWE technology is considered to be time-saving, results in high yield, and has no toxic solvent residues for production of plant polysaccharides when compared with the traditional methods.
Grifola frondosa (edible mushroom), which belongs to the polyporaceae family, is a basidiomycete fungus. Polysaccharides from G. frondosa (PGF) has recently attracted considerable attention for its various physiological activities including antioxidant,[5,6] antitumor,[7] antiatherosclerotic,[8] antifatigue effects,[9,10] and immunostimulating activities.[11] Different methods are established for the extraction of polysaccharides from different plant, including traditional hot water extraction (HWE),[12–14] microwave-assisted extraction,[15] ultrasonic extraction,[16,17] andenzyme-assisted extraction.[5,18] In recent years, many papers have been published on the applicability of SWE for the extraction of bioactive compounds from plants. Examples of such studies include, essential oils from Bunium persicum Boiss,[19] extraction of phenolic compounds from potato peel,[2] and extraction of flavonol quercetin from onion skin.[20] Nevertheless, little research has been conducted on the feasibility of SWE as a rapid and efficient extraction tool for polysaccharides.
The traditional one-factor-at-a-time approach in which one factor varies at a time while all others are kept constant, has several drawbacks including its more laborious and time-consuming nature. Response surface methodology (RSM) is an effective statistical and mathematical technique used to optimize the conditions in food and pharmaceutical research. It can determine interaction between the variables and reduce number of experimental trials, which evaluate multiple parameters and their interactions.
The main objective of this study was to optimize the parameter conditions of the SWE of polysaccharides from G. frondosa and analyze the effects of extraction parameters on the yields of polysaccharides with RSM. A preliminary analysis was made on physical properties and content determination of extracts by SWE and HWE. Analysis of the sample residues and antioxidant activities of polysaccharides extracted by SWE and HWE were carried out.
MATERIALS AND METHODS
Materials and reagents
Fruit bodies of G. frondosa identified by Dr. Guanghua Mao were provided by FangGe Pharmaceutical Co., Ltd. (Zhejiang Province). The fruit bodies were oven-dried at 60°C for 24 h and then crushed into powdered form (200 μm) with a mill. All solvents and chemicals used were of analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Deionized water was used in this experiment as the extraction solvent.
Apparatus of subcritical water extraction
The experiment was carried out with a laboratory-built apparatus in accordance with recommended methoddescribed by He et al.[21] In the typical experimental instance, the sample was loaded on to a Polytetrafluoroethylene (PTFE) reactor (inner volume, 100 mL), with stainless steel jacket in an oven. On top of the reactor was a pressure sensor and a channel connected with nitrogen cylinder.
Extraction of polysaccharides
Subcritical water extraction
The oven for the experiment was heated to the designed temperature before the extraction was started. Nitrogen was flushed to remove the air in the reaction chamber until the reactor pressure exceeded 5 MPa. The reactor was carried out within a given time period and had to subsequently be changed according to the extraction temperature. Each of the extraction process with temperatures of 100°C, 120°C, and 130°C were allowed for 2 min of given time while temperatures of 150°C and 180°C extraction processes were allowed for 3 min each. The temperatures of 200°C, 210°C, and 230°C extraction processes were allowed for 4 min each. After the extraction, the reactor was cooled with tap water to temperature below 30°C. The sample was extracted with subcritical water two times. After filtering and centrifuging to remove the water-insoluble fractions, the supernatants were concentrated with a rotary evaporator at a reduced pressure, then precipitated by EtOH (5:1, v/v) at 4°C for 12 h and further centrifuged at 5000′g for 10 min. The precipitate was lyophilized to obtain polysaccharide extracts.
Traditional hot water extraction
The traditional HWE was carried out in a water bath using reported method[22] with some little modification. With this method, 10 g of dried powdered G. frondosa was extracted with 200 mL of boiling water for 3 h. After repeating the extraction process for three times, the extract was filtered. The process used in the SWE was then applied.
Experimental design and statistical analysis
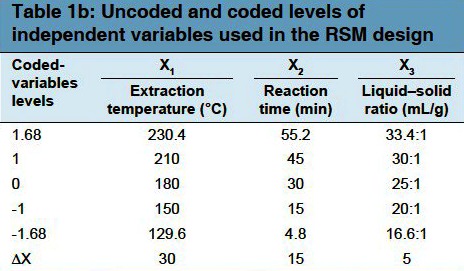
A five level and three variable central composite design (CCD) was applied in order to optimize the best combination of extraction variables for the response. Three independent variables (extraction temperature (X1), reaction time (X2), and ratio of water to raw material (X3), and a set of 20 experiments were employed [Table 1a]. The optimization was conducted for yields of polysaccharides as the responses Y. As shown in Table 1b, it exhibited the ranges of the independent variables and experimental design levels. Three factors (X1, X2, and X3) were categorized into three levels with codes +1, 0, and -1 for high, intermediate, and low values, respectively. The relationship between the coded values and actual values were determined by the equation below:
Table 1.
(a) Experimental design for optimization of subcritical water extraction of polysaccharide from Grifola frondosa (b) Uncoded and coded levels of independent variables used in the RSM design (c) Regression coefficients of the secondorder polynomial model for the response variables (d) Analysis on variance (ANOVA) of RSM for subcritical water extraction
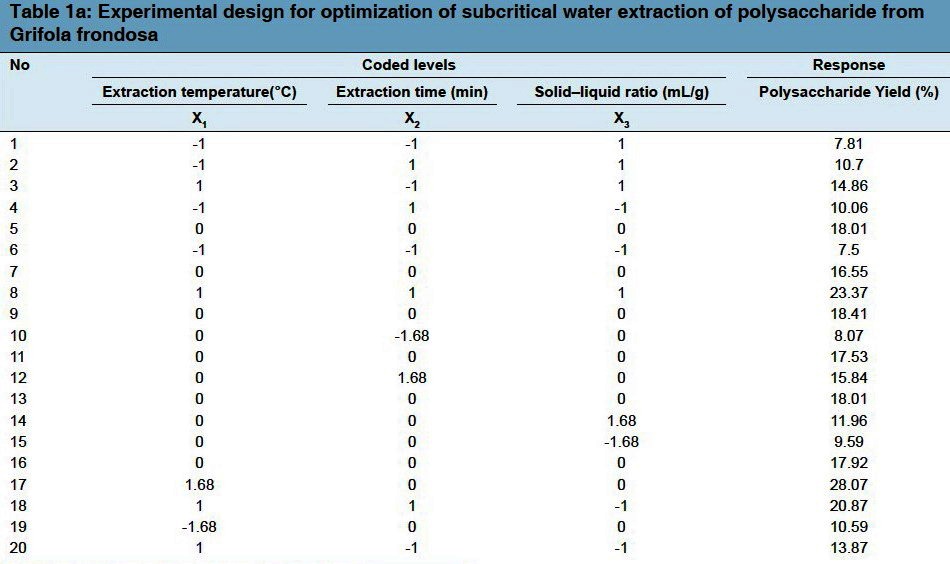
where xi is the coded value of the independent variable; Xi is the actual value of the variable; X0 is the actual value at the center point; and ΔX is the step change in the variable Xi. The quadratic equation for the variables is given below:
![]()
where Y is predicted response, A0 is constant, Ai is the coefficients of the linear, Aii is the squared coefficient, Aij is the interaction coefficient. Xi and Xj are the independent variables, respectively.
A software Design-Expert 7.1.3 Trial (State-Ease, Inc., Minneapolis MN, USA) was used to obtain the coefficients of the quadratic polynomial model. The significances of all the terms in the polynomial were determined statistically by computing the F-value at a probability (P). When the P-values are less than 0.05, it is considered statistically significant.
Total polysaccharide and total protein determination
The phenol-sulfuric acid method was employed for the measurement of total polysaccharide using glucose as the standard.[23] Protein concentration was determined using the Bradford assay method,[24] and bovine serum albumin (BSA) used as a standard to construct the calibration curve.
Viscosity determination
The viscosities of the polysaccharide extracts using SWE and HWE were measured with a rotational viscometer (DigitalNDJ-1B, Shanghai). For each sample, the runtime was recorded five times at a control temperature of 25°C.
FT-IR spectrometer
Fourier transform infrared (FT-IR) of polysaccharides extracted using SWE and HWE were carried out using potassium bromide (KBr) pellet method on a FTIR (Nicolet Avatar-370, USA), in the range of 400 - 4000/cm.
Analysis of the sample and residues by SEM and optical microscope
The shape and surface characters of the sample residues were recorded on the scanning electron microscope (JSM-7001F, JEOL, Ltd, Japan). The cellular structure of plant tissues was observed byoptical microscope NikonECLIPSE TS100/10, Japan).
Assay of antioxidant activity of PGF in vitro
Reducing power
Reducing power was determined using reported method[25] with some little modification. An aliquot of each sample was mixed with sodium phosphate buffer (0.2 M, pH 6.6) to 2.5 mL. Afterwards, 2.5 mL of 1% (w/v) K3Fe(CN)6 solution was added and the mixture incubated in a water bath at a temperature of 50°C for 20 min. After adding 2.5 mL of 10% trichloroacetic acid, the supernatant (2.5 mL) was then taken out and combined with 2.5 mL of H2O and 0.5 mL of 0.1% ferric chloride, followed by the absorbance analysis at 700 nm. The IC50 value is the concentration at the absorbance of 0.5 and ascorbic acid used for comparison.
DPPH radicals scavenging assay
1, 1-diphenyl-2- picrylhydrazyl (DPPH) radical-scavenging activity was determined using reported method[26] with little modification. An aliquot(2.0 mL) of each sample (with required dilution) was added to 2.0 mL of ethanolic DPPH solution. After 30 min of incubation at normal room temperature in the dark, the absorbance was read against a blank at 517 nm. Sample concentration with 50% inhibition (IC50) was determined from the graph plotted with inhibition percentage against extract concentration. Ascorbic acid was used for the comparison. The DPPH radical scavenging effect was calculated using the following equation:
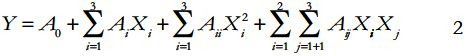
Statistical Analysis
Data were presented as mean ± SD (standard deviation) of triplicate determinations. Statistical calculations were carried out using SPSS version 16.0 (SPSS Inc., Chicago, USA). One-way analysis of variance (ANOVA) was used to determine the statistic differences. P < 0.05 was considered as significantly different.
RESULTS AND DISCUSSION
The effect of extraction temperature on polysaccharide yield
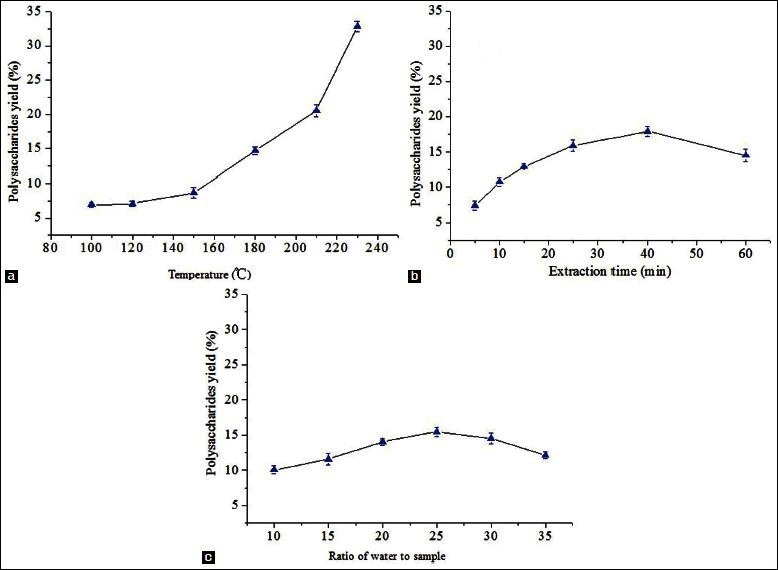
As shown in Figure 1a, the extraction temperature displayed a positive linear effect on the yield of the polysaccharides. The extraction parameters used in this case include extraction time of 30 min with the ratio of water to sample of 20:1. Owing to gelatinization of polysaccharides at a temperature of 230°C, the maximum temperature expected was 230°C. The extraction yield of PGF significantly increased from 6.9% to 32.8%, as the temperature increased from 100°C to 230°C. The increased yield of the polysaccharides was likely due to the rising temperature of the water and high pressure which could reduce its surface tension and increase the diffusion rate from the solid phase to the liquid phase.[27] SWE could also improve cell wall damage and decompose cell wall material.[28]
Figure 1.
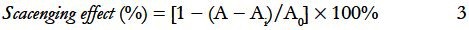
Effects of different (a) extraction temperatures (b) extraction time and (c) ratios of water to raw material on extraction yield of polysaccharides
The effect of extraction time on polysaccharide yield
Extraction time was another important factor that could influence the extraction yield of PGF. Research had shown that a long extraction time favored the production of polysaccharides.[29,30] Different extraction time was set in the range of 5–60 min in this study. Other extraction parameters included extraction temperature of 180°C and the ratio of water to sample of 20:1. Figure 1b indicates that the polysaccharides yield significantly increased from 7.4% to 17.9%, with the increasing of the extraction time from 5 to 40 min. However, with the continuous increase in the reaction time, the extraction yields of PGF decreased. This might be due to the partial nature in which the polysaccharides were hydrolyzed.[31] The results indicated that 40 min was considered to be the optimal parameter in this experiment.
The effect of ratio of water to sample on polysaccharide yield
The effect of the ratio of water to sample on the yield of polysaccharide was investigated [Figure 1c]. With the ratio of water to sample, which was increased from 10:1 to 35:1, other experimental conditions including extraction temperature of 180°C and extraction time of 30 min were held constant. When the ratio of water to sample was increased from 10:1 to 25:1, the extraction yield correspondingly increased from 10.1% to 15.5% and reached the maximum as a result of the increasing in the driving force of the increasing solvent molecules.[32,33] The yield of PGF indicated a descending tendency subsequently. Thus, the ratio of the water to the sample (25:1) was considered to be optimal in this experiment.
Statistical analysis and the model fitting
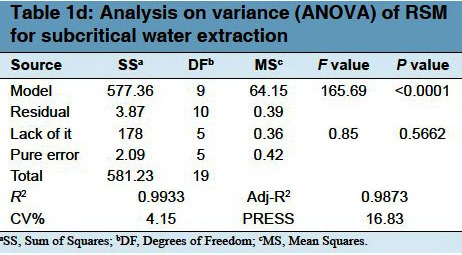
The design matrix and the corresponding results of RSM experiments to determine the effects of the three independent variables including extraction temperature (X1), extraction time (X2), and ratio of water to sample (X3) are shown in Table 1c. By employing multiple regression analysis on the experimental data, the predicted response Y for the yield of polysaccharides could be obtained using the following second-order polynomial equation:
The statistical significance of the regression equation was checked using P values in Table 1c. It could be seen from the equation that the linear coefficients (X1, X2, X3), the quadratic term coefficients (X12, X22, X32) and the cross product coefficient (X1 × X2) were significant, with small P values (P < 0.05). The results indicated that the extraction temperature, time and ratio of water to sample were all significantly correlated with the yield of PGF. The ANOVA for the response surface quadratic polynomial model was carried out by software Design-Expert and is presented in Table 1d. The model had been found to be significant with Prob >F-value less than 0.05. The value of the determination coefficient (R2) determined from the quadratic regression model was 0.9933, which indicated that 99.33% of the variability in the response could be explained by the model. The value of the adjusted determined coefficient (RAdj2 = 0.9873) indicated a high degree of correlation between the observed and predicted values. A very low value (4.15) coefficient of variation (CV) clearly indicated a high degree of precision and a good deal of reliability for the experimental values. Further, the results of the error analysis indicated that the lack of fitness was insignificant (P = 0.5662), indicating that the model equation was adequate to predict the extraction yield of the polysaccharides within the range of experimental variables. The P-value was used as a tool to check the significance of each coefficient. This in turn might indicate the pattern of the interaction between the variables. The smaller the value of P, the more significant is the corresponding coefficient. Values of “Prob >F” less than 0.05 indicate that the model terms are significant. In this case X1, X2, X3, X12, X11, X22, X33 were significant model terms. While the other coefficients were not significant (P > 0.05). To determine the optimal levels of the variables for the polysaccharides yield, the response surfaces and contour plots were determined using Eq. (4).
Optimization for extraction of polysaccharide yield
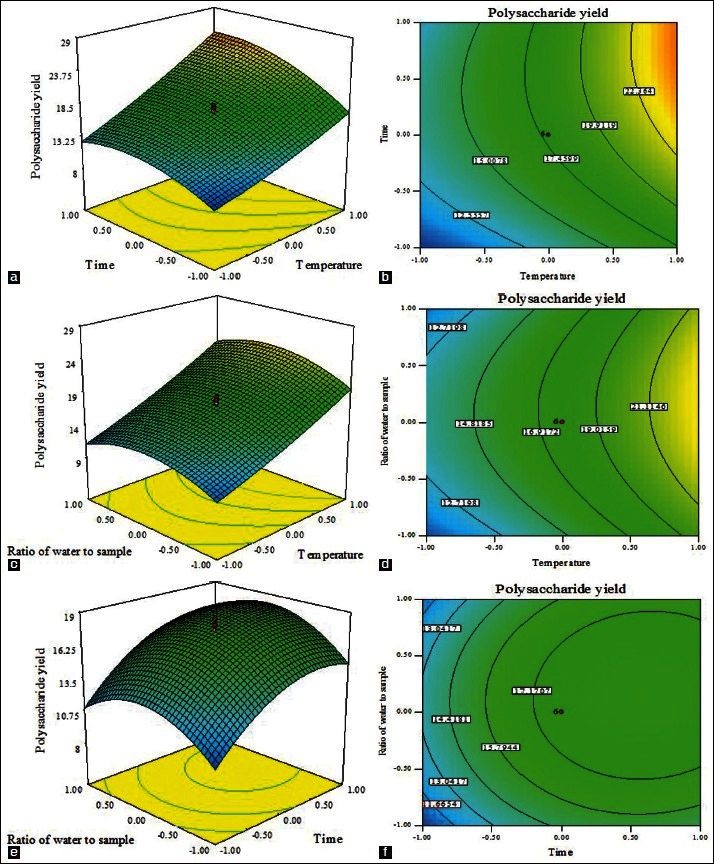
The fitted three-dimensional response surface plots and two-dimensional contour plots indicated in Figure 2 for the model were generated by the Stat-Ease Design-Expert (Cabit Information Technology Co., LTD) software, which was used to study the effects of parameters and their interactions on polysaccharide yield. These plots indicated effects of two factors on the response at a time, while the other factor was kept at zero level.
Figure 2.
Response surface (a, c, e) and contour plots (b, d, f) for the effect of extraction temperature (X1), extraction time(X2) and ratio of water to raw material (X3) on the polysaccharides yield
Figure 2a and b shows the 3-D plot and the contour plot at varying extraction temperature and time at constant ratio of water to raw material of 1:25. It can be seen from the figures that there was significant interaction between the extraction temperature and time. When the extraction time (X2) was kept constant, there occured a linear increase in the yield of the polysaccharide with increasing extraction temperature (X1). Figure 2c and d shows that the extraction temperature (X1) and the ratio of water to raw material (X3) as extraction time (X2) constantly kept at 30 min. When the extraction temperature (X1) was kept constant, the yield increased initially and then decreased with increasing ratio of water to sample (X3). Figure 2e and f indicates that the effects of extraction time and ratio of raw material to water on the polysaccharide yield at a constant catalyst temperature of 180°C. Temperature was the most influential factor for the mutual interactions that could induce cell wall materials solubilization through biodegradation. It can also enhance the opening up of the cell walls in order to facilitate the release of polysaccharide. However, the extraction time and the ratio of water to raw material were also significant. The optimal condition obtained by RSM at a three-variable and five-level CCD included extraction temperature of 210°C, extraction time of 43.65 min, and ratio of water to raw material of 26.15:1. The predicted yield of the polysaccharides was recorded as 25.0%. Additional experiment was carried out to validate the optimization result. The mean value of 25.1 ± 0.3% (n = 3) obtained from the real experiments demonstrated the validity of the RSM model. The correlation between the real and the predicted results, which was not significant (P > 0.05) confirmed that the response model was adequate to reflect the expected optimization.
Comparison of SWE with HWE
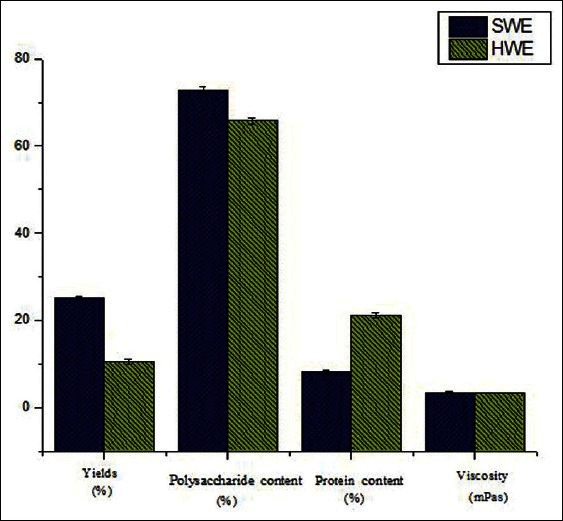
The physical properties and content determination of the polysaccharides extracted from G. frondosa extracted using SWE and HWE are shown in Figure 3. It could be seen that higher yield was given by the SWE technique (yield twice the value obtained by HWE). The content of polysaccharides by SWE was also higher than that by HWE. However, the content of the protein by SWE (8.13/100 g) was much lower than that by HWE (21.13/100 g). Cacace et al.[34] and Ho et al.[35] reported that there were possible continuous increases in protein and carbohydrate levels in extracts obtained by SWE as the extraction temperature increased over the range of 100–160°C. However, the proteincontent decreased during the SWE in this study. The reason might be that Maillard reaction (the reaction of reducing sugars with amino acids decomposed from protein) happened with the increase in temperature and time.[4,36] In addition, the time used for SCW extraction (43.8 min) was less than that of conventional extraction (9 h). The viscosities of PGF extracted by SWE and HWE, whichwere determined as 3.45 and 3.40 mPas (2 mg/mL), were not appreciable different. It was an indication that the molecular weights of polysaccharides were scarcely affected by SWE.
Figure 3.
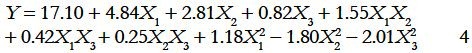
The physical properties and content determination of polysaccharide extracts by SWE and HWE
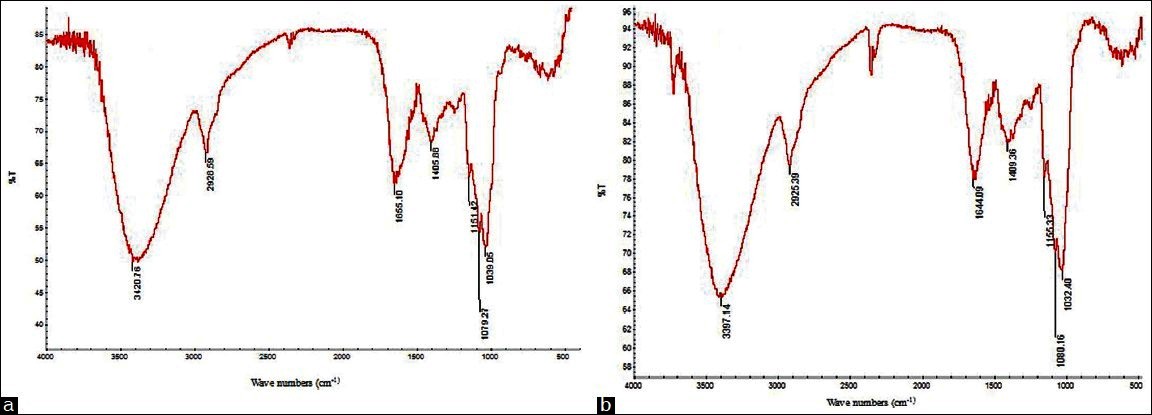
Figure 4 exhibit the typical signals of polysaccharides in the range of 4000–400/cmand indicated that the spectra′s of PGF by HWE Figure 4a and SWE Figure 4b were similar. The spectra were dominated by a broad band at about 3400/cm which might be due to the results of the stretching vibration modes of the OH groups. The bands in the region of 2925/cm were assigned to the CH stretching of the CH2 groups. The band of 1650/cmwas possibly the characteristic of the C = O stretching vibration of the carboxylic acid of the glucuronic acid in the starting polysaccharide. The three bandsfound in between 1200 and 1000/cm might be due to the characteristics absorption of the groups of saccharide ring.
Figure 4.
FTIR spectra of the polysaccharides of hot water extraction (a) subcritical water extraction (b)
Surface properties of residues by SEM
The scanning electron micrographs of the residues untreated and the residues after extraction with HWE and SWE are shown in Figure 4. The structure of treated material changed significantly when compared with the raw material, which showed more compact structure. As shown in Figure 5, the materials treated by SWE had the largest ′pores′ and a lot of debris in the surface. Meanwhile, it indicated that SWE changed the inner structure of the materials, in which various components contained were effectively removed and contributed to the higher extraction efficiency of polysaccharides. The reason might be that decomposition and conversion from cellulose to unknown structural substances seemed to be enhanced with the increase in temperature and high pressure.[37,38]
Figure 5.
Scanning electron microscopy images of Grifola frondosa powders. The residues untreated (a) the residues obtained by hot water extraction (b) and the residues obtained by subcritical water extraction (c)
Antioxidant activity
The reducing capacity is a significant reflection of the antioxidant activity in assessing potential antioxidants. Antioxidant compounds cause the reduction of ferric (Fe3+) form to the ferrous (Fe2+) form as a results of their reductive capabilities. Reduction can be determined by measuring the formation of Perl′s Prussian blue at 700 nm. As shown in Figure 6a, subcritical water and hot water extracts showed an increased ferric reducing power with the increasing concentration as standard antioxidants. The antioxidant activity of polysaccharides by SWE was higher than that by HWE, which might be as a result of the antioxidant components that were produced during SWE.[1,36] The IC50 was calculated and indicated in Table 2. From the table, IC50 values of the absorbance of PGF by SWE and HWE were 78.98 ± 24.36 μg/mL and 104.15 ± 27.58 μg/mL, respectively. The lower the IC50 value, the greater the free radical-scavenging activity.
Figure 6.
Antioxidant activities of polysaccharides by SWE and HWE in Reducing power (a) DPPH free radical-scavenging (b) assays. Ascorbic acid was used as a positive control. Data were presented as mean ± SD (n = 3)
Table 2.
IC50 values in antioxidant properties of polysaccharide extracts
The DPPH free radical, which is a stable radical with a maximum absorption at 517 nm, has been widely applied to evaluate the total antioxidative activity in natural compounds. As shown in Figure 6b, the scavenging abilities of the polysaccharides by SWE and HWE increased with increasing concentration of the extract samples. IC50 values of the DPPH radical-scavenging activity of PGF by SWE and HWE were 674.68 ± 36.83 μg/mL and 881.44 ± 43.47 μg/mL, respectively Table 2. According to our previous study,[5] the IC50 value of the DPPH radical-scavenging activity of polysaccharides obtained from G. frondosa by enzymolysis was 883.86 ± 26.39 μg/mL, which was similar to HWE, but higher than that of SWE in this study. As in the case of reducing power, the DPPH radical-scavenging activity of polysaccharides by SWE was stronger than that by HWE.
CONCLUSION
In this study, the response surface methodology was used and proved to be useful for the optimization of the polysaccharides extraction from G. frondosa. Compared with the HWE, SWE technique employed for the extraction of PGF was time-saving, energy-saving, and more high-yielding. The antioxidant activities of polysaccharides extracted by SWE were higher than those by HWE. It is therefore recommended that further purification, characterization, and the action of the active polysaccharide extracts in G. frondosa should be studied.
ACKNOWLEDGMENTS
This work was supported by the “Graduate innovative projects in Jiangsu Province” (NO.CXLX11-0601). The authors gratefully acknowledge the Fangge Pharmaceutical and Nutraceutical Co., Ltd. for providing Grifola frondosa. We are thankful to Dr. Mohammed Takase for language polishing.
Footnotes
Source of Support: Graduate innovative projects in Jiangsu Province” (NO.CXLX11-0601)
Conflict of Interest: None declared.
REFERENCES
- 1.Hassas-Roudsari M, Chang PR, Pegg RB, Tyler RT. Antioxidant capacity of bioactives extracted from canola meal by subcritical water, ethanolic and hot water extraction. Food Chem. 2009;114:717–26. [Google Scholar]
- 2.Singh PP, Saldaña MD. Subcritical water extraction of phenolic compounds from potato peel. Food Res Int. 2011;44:2452–8. [Google Scholar]
- 3.Öztürk İ, Irmak S, Hesenov A, Erbatur O. Hydrolysis of kenaf (Hibiscus cannabinus L.) stems by catalytical thermal treatment in subcritical water. Biomass Bioenergy. 2010;34:1578–85. [Google Scholar]
- 4.Plaza M, Amigo-Benavent M, del Castillo MD, Ibáñez E, Herrero M. Neoformation of antioxidants in glycation model systems treated under subcritical water extraction conditions. Food Res Int. 2010;43:1123–9. [Google Scholar]
- 5.Fan Y, Wu X, Zhang M, Zhao T, Zhou Y, Han L, et al. Physical characteristics and antioxidant effect of polysaccharides extracted by boiling water and enzymolysis from Grifola frondosa. Int J Biol Macromol. 2011;48:798–803. doi: 10.1016/j.ijbiomac.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Mau J-L, Chang C-N, Huang S-J, Chen C-C. Antioxidant properties of methanolic extracts from Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. Food Chem. 2004;87:111–8. [Google Scholar]
- 7.Cui FJ, Tao WY, Xu ZH, Guo WJ, Xu HY, Ao ZH, et al. Structural analysis of anti-tumor heteropolysaccharide GFPS1b from the cultured mycelia of Grifola frondosa GF9801. Bioresour Technol. 2007;98:395–401. doi: 10.1016/j.biortech.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Mori K, Kobayashi C, Tomita T, Inatomi S, Ikeda M. Antiatherosclerotic effect of the edible mushrooms Pleurotus eryngii (Eringi), Grifola frondosa (Maitake), and Hypsizygus marmoreus (Bunashimeji) in apolipoprotein E-deficient mice. Nutr Res. 2008;28:335–42. doi: 10.1016/j.nutres.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Chi A-P, Chen J-P, Wang Z-Z, Xiong Z-Y, Li Q-X. Morphological and structural characterization of a polysaccharide from Gynostemma pentaphyllum Makino and its anti-exercise fatigue activity. Carbohydr Polym. 2008;74:868–74. [Google Scholar]
- 10.Zhang HL, Li J, Li G, Wang DM, Zhu LP, Yang DP. Structural characterization and anti-fatigue activity of polysaccharides from the roots of Morinda officinalis. Int J Biol Macromol. 2009;44:257–61. doi: 10.1016/j.ijbiomac.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Švagelj M, Berovič M, Boh B, Menard A, Simčič S, Wraber B. Solid-state cultivation of Grifola frondosa (Dicks: Fr) S.F Gray biomass and immunostimulatory effects of fungal intra- and extracellular β-polysaccharides. N Biotechnol. 2008;25:150–6. doi: 10.1016/j.nbt.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Paradossi G, Cavalieri F, Pizzoferrato L, Liquori AM. A physico-chemical study on the polysaccharide ulvan from hot water extraction of the macroalga Ulva. Int J Biol Macromol. 1999;25:309–15. doi: 10.1016/s0141-8130(99)00049-5. [DOI] [PubMed] [Google Scholar]
- 13.Song T, Pranovich A, Holmbom B. Effects of pH control with phthalate buffers on hot-water extraction of hemicelluloses from spruce wood. Bioresour Technol. 2011;102:10518–23. doi: 10.1016/j.biortech.2011.08.093. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Zhang L, Cheung PCK, Ooi VE. Molecular weight and anti-tumor activity of the water-soluble polysaccharides isolated by hot water and ultrasonic treatment from the sclerotia and mycelia of Pleurotus tuber-regium. Carbohydr Polym. 2004;56:123–8. [Google Scholar]
- 15.Rodriguez-Jasso RM, Mussatto SI, Pastrana L, Aguilar CN, Teixeira JA. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr Polym. 2011;86:1137–44. [Google Scholar]
- 16.Yan Y-l, Yu C-h, Chen J, Li X-x, Wang W, Li S-q. Ultrasonic-assisted extraction optimized by response surface methodology, chemical composition and antioxidant activity of polysaccharides from Tremella mesenterica. Carbohydr Polym. 2011;83:217–24. [Google Scholar]
- 17.Tang X, Yan L, Gao J, Ge H, Yang H, Lin N. Optimization of extraction process and investigation of antioxidant effect of polysaccharides from the root of Limonium sinense Kuntze. Pharmacogn Mag. 2011;7:186–92. doi: 10.4103/0973-1296.84225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Jia S, Liu Y, Wu S, Ran J. Optimization of enzyme-assisted extraction of the Lycium barbarum polysaccharides using response surface methodology. Carbohydr Polym. 2011;86:1089–92. [Google Scholar]
- 19.Mortazavi SV, Eikani MH, Mirzaei H, Jafari M, Golmohammad F. Extraction of essential oils from Bunium persicum Boiss. using superheated water. Food Bioprod Process. 2010;88:222–6. [Google Scholar]
- 20.Ko M-J, Cheigh C-I, Cho S-W, Chung M-S. Subcritical water extraction of flavonol quercetin from onion skin. J Food Eng. 2011;102:327–33. [Google Scholar]
- 21.He L, Zhang X, Xu H, Xu C, Yuan F, Knez Ž, et al. Subcritical water extraction of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant activities with HPLC-ABTS+ assay. Food Bioprod Process [Google Scholar]
- 22.Chen J, Zhang T, Jiang B, Mu W, Miao M. Characterization and antioxidant activity of Ginkgo biloba exocarp polysaccharides. Carbohydr Polym. 2012;87:40–5. doi: 10.1016/j.carbpol.2011.06.083. [DOI] [PubMed] [Google Scholar]
- 23.Wei X, Chen M, Xiao J, Liu Y, Yu L, Zhang H, et al. Composition and bioactivity of tea flower polysaccharides obtained by different methods. Carbohydr Polym. 2010;79:418–22. [Google Scholar]
- 24.Carlsson N, Borde A, Wölfel S, Åkerman B, Larsson A. Quantification of protein concentration by the Bradford method in the presence of pharmaceutical polymers. Anal Biochem. 2011;411:116–21. doi: 10.1016/j.ab.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Chung JW, Kim JJ, Kim SJ. Antioxidative effects of cinnamomi cortex: A potential role of iNOS and COX-II. Pharmacogn Mag. 2011;7:314–9. doi: 10.4103/0973-1296.90412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wintola OA, Afolayan AJ. Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox Mill. Pharmacogn Mag. 2011;7:325–33. doi: 10.4103/0973-1296.90414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carr AG, Mammucari R, Foster NR. A review of subcritical water as a solvent and its utilisation for the processing of hydrophobic organic compounds. Chem Eng J. 2011;172:1–17. [Google Scholar]
- 28.Gehrke CW, Wixom RL, Bayer E. World Reference Laboratory. Chapter 5 Prominent chromatographers and their research seminal concepts in chromatography/separation sciences. In: Gehrke CW, Bayer E, editors. Journal of Chromatography Library. vol. 64. Elsevier; 2001. pp. 99–599. [Google Scholar]
- 29.Tian S, Zhou X, Gong H, Ma X, Zhang F. Orthogonal test design for optimization of the extraction of polysaccharide from Paeonia sinjiangensis K.Y. Pan. Pharmacogn Mag. 2011;7:4–8. doi: 10.4103/0973-1296.75874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Zhang J, Zhao B, Wang X, Wu Y, Yao J. A comparison study on microwave-assisted extraction of Potentilla anserina L. polysaccharides with conventional method: Molecule weight and antioxidant activities evaluation. Carbohydr Polym. 2010;80:84–93. doi: 10.1016/j.ijbiomac.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Shao Q, Deng Y, Shen H, Fang H, Zhao X. Optimization of polysaccharides extraction from Tetrastigma hemsleyanum Diels et Gilg using response surface methodology. Int J Biol Macromol. 2011;49:958–62. doi: 10.1016/j.ijbiomac.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Ye C-L, Jiang C-J. Optimization of extraction process of crude polysaccharides from Plantago asiatica L. by response surface methodology. Carbohydr Polym. 2011;84:495–502. [Google Scholar]
- 33.Liu J, Sun Y, Liu L, Yu C. The extraction process optimization and physicochemical properties of polysaccharides from the roots of Euphorbia fischeriana. Int J Biol Macromol. 2011;49:416–21. doi: 10.1016/j.ijbiomac.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Cacace JE, Mazza G. Pressurized low polarity water extraction of lignans from whole flaxseed. J Food Eng. 2006;77:1087–95. [Google Scholar]
- 35.Ho CH, Cacace JE, Mazza G. Extraction of lignans, proteins and carbohydrates from flaxseed meal with pressurized low polarity water. LWT - Food Sci Technol. 2007;40:1637–47. [Google Scholar]
- 36.Plaza M, Amigo-Benavent M, del Castillo MD, Ibáñez E, Herrero M. Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res Int. 2010;43:2341–8. [Google Scholar]
- 37.Kruse A, Dinjus E. Hot compressed water as reaction medium and reactant: Properties and synthesis reactions. J Supercrit Fluids. 2007;39:362–80. [Google Scholar]
- 38.Tanaka M, Takamizu A, Hoshino M, Sasaki M, Goto M. Extraction of dietary fiber from Citrus junos peel with subcritical water. Food Bioprod Process. 2012;2:180–6. [Google Scholar]


