Abstract
Background:
Schisandra chinensis, one of the well-known traditional Chinese herbal medicines, is derived from the dry ripe fruits of Schisandra chinensis (Turcz.) Baill. according to the 9th China Pharmacopeia. Lignans are the main components isolated from extracts of S. chinensis and their content varies depending on where S. chinensis was collected. We have established a qualitative and quantitative method based on the bioactive lignans for control of the quality of S. chinensis from different sources.
Materials and Methods:
To develop a high-performance liquid chromatography method, an Elite ODS C18 column (250 mm Χ 4.6 mm, 5μm) at a column temperature of 30°C and flow rate of 1.0ml/min using acetonitrile (A) and water (B) as the mobile phase with a linear gradient and the peaks were monitored at 217 nm.
Results:
All calibration curves showed good linearity (r ≥ 0.9995) within test ranges. This method showed good repeatability for the quantification of these eleven components in S. chinensis with intra- and inter-day relative standard deviations less than 0.43% and 1.21%, respectively. In the recovery test, results of accuracy ranged from 99.51% to 101.31% with RSD values less than 2.
Conclusion:
The validated method can be successfully applied to quantify the eleven investigated components in 22 samples of S. chinensis from different sources.
Keywords: Schisandra chinensis, lignans, quality control, high-performance liquid chromatography
INTRODUCTION
Schisandra chinensis (Wuweizi in China), the dry ripe fruits of Schisandra chinensis (Turcz.) Baill., officially listed as a sedative and tonic in the China Pharmacopoeia,[1] has been used as an important component in various prescriptions in traditional Chinese medicine (TCM) and more recently in western-based medicine for its antihepatotoxic and anti-HIV effects.[2] Its traditional effects are astringency of sweating, seminal emission, diarrhea and tranquillizing of the mind. Phytochemical investigations have resulted in the isolation and identification of many compounds such as lignans, polysaccharides, essential oils, organic acids, and vitamins,[3–8] among which, the lignans with a dibenzocyclooctadiene skeleton are the major bioactive compounds in S. chinensis. Pharmacological studies showed that they had good biological activities, including antihepatotoxic, anti-cancer,[9] antioxidant,[10] antitumor,[11] platelet activating factor antagonistic[12] and central nervous system protecting activities,[13] etc. So it is necessary to establish a qualitative and quantitative method based on the bioactive lignans for control of the quality of S. chinensis from different sources.
To our knowledge, there are previously reported analytical methods were developed to quantify lignans present in S. chinensis, but these methods only measure the contents of total lignans[14] or a mixture of some specific lignans.[15–18] To date, there has been no report of the quantitative determination of the eleven bioactive lignans in S. chinensis from different sources. In this paper, a simple and accurate HPLC-UV method was developed for the simultaneous quantification of the eleven major characteristic lignans in S. chinensis, namely schisandrin, gomisin J, schisandrol B, angeloylgomisin H, gomisin G, schisantherin A, schisantherin B, deoxyschisandrin, γ-schisandrin, schisandrin B, and schisandrin C [Figure 1].
Figure 1.
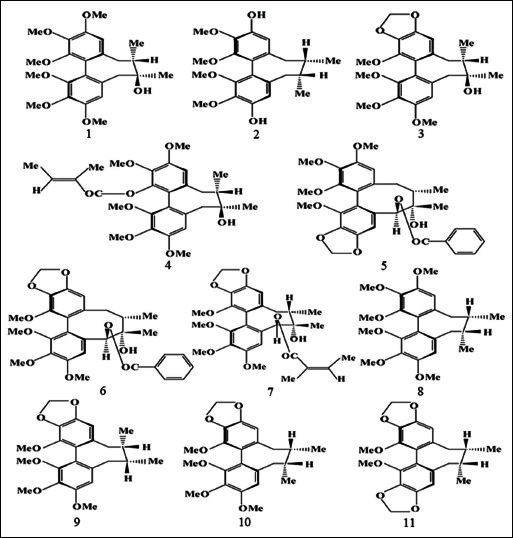
Structures of eleven lignans in Schisandra chinensis: Schisandrin (1), Gomisin J (2), Schisandrol B (3), Angeloylgomisin H (4), Gomisin G (5), Schisantherin A (6), Schisantherin B (7), Deoxyschisandrin (8), γ-schisandrin (9), Schisandrin B (10) and Schisandrin C (11).
The validated method is selective, precise, and accurate and thus can be used for the routine analysis of pharmaceutical preparations in quality control laboratories of the pharmaceutical industry. The method was successfully applied to simultaneous determination of these bioactive components in S. chinensis procured from different habitats in China.
MATERIALS AND METHODS
Materials and reagents
Schisandrin, schisandrol B, schisantherin A, deoxyschisandrin, and schisandrin B (> 99% purity) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Nanjing, China). Schisandrin C (> 98% purity) was provided by Shanghai Sunny Biotech Co. Ltd. Gomisin J, angeloylgomisin H, gomisin G, Schisantherin B and γ-schisandrin (> 98% purity) were provided by Shanghai Tauto Biotech Co. Ltd. Acetonitrile for HPLC was provided by Tedia Company (USA) and methanol for HPLC was purchased from Shandong Yuwang Industrial Co. Ltd. Water for the HPLC mobile phase was purified in a Milli-Q system (Millipore, Bedford, MA, USA).
Plant materials
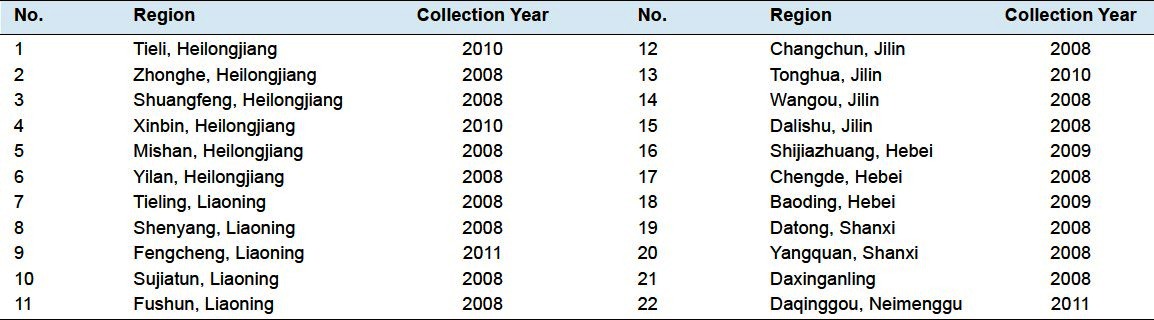
The 22 samples of S. chinensis from different sources were identified by Professor J.W. Chen, College of Pharmaceutical Science, Nanjing University of Chinese Medicine, Nanjing, PR China, and are shown in Table 1.
Table 1.
Details of the herbal materials collected
Preparation of sample
The sample was pulverized and the powder was screened through 60 mesh sieves. The fine powder of studied samples, was accurately weighed (0.3 g), and transferred into 25 ml volumetric flasks. They were extracted with 25 ml of methanol in an ultrasonic bath for 20 min at room temperature. Additional methanol was added to make up the lost. The supernatants were centrifuged for 10min at 14000 rpm prior to injection into the HPLC system.
Preparation of standard solutions
The standard stock solutions of schisandrin (250.2 μg/ml), gomisin J (52.00 μg/ml), schisandrol B (109.9 μg/ml), angeloylgomisin H (141.0 μg/ml), gomisin G (25.50 μg/ml), schisantherin A (37.90 μg/ml), schisantherin B (83.20 μg/ml), deoxyschisandrin (51.60 μg/ml), γ-schisandrin (91.00 μg/ml), schisandrin B (207.0 μg/ml) and schisandrin C (45.60 μg/ml) were prepared in methanol. Working standard solutions containing each of the eleven compounds were prepared by diluting the stock solution with methanol to the proper volumes within the ranges, respectively. The standard stock solutions and working solutions were stored at 4°C. The solutions were centrifuged for 10min at 14000 rpm prior to injection into the HPLC system.
HPLC analysis
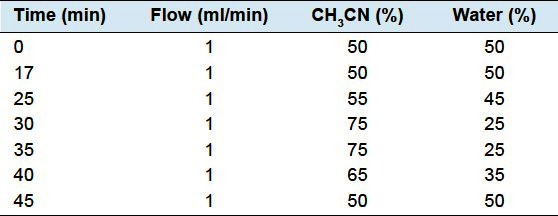
The high-performance liquid chromatography system consist of a Agilent 1100 HPLC (Agilent Technologies, Palo Alto, CA, USA), equipped with a quaternary pump, an auto-sampler, a vacuum degasser, an automatic thermostatic column compartment, and a UV detector. The chromatography was carried out on Elite ODS C18 column (250 mm × 4.6 mm, 5 μm) at a column temperature of 30°C and flow rate of 1.0ml/min using acetonitrile (A) and water (B) as mobile phase with a linear gradient Table 2. The injection volume was 10 μL and the detection wavelength was set at 217nm.
Table 2.
Time program of the gradient elution
RESULT AND DISCUSSION
Extraction procedure
Various extraction methods, solvents and times were evaluated in an effort to optimize the extraction procedures. The results revealed that ultrasonic extraction was better than refluxing, so the further experiments were carried out with ultrasonic extraction. Various solvents including 20% methanol, 50% methanol, 80% methanol and pure methanol were screened with ultrasonic extraction to evaluate the efficiency of the solvent extraction. The results showed that pure methanol was the most suitable extraction solvent, which allowed extraction of all the major constituents to be in high yields. The influence of the extraction time (10, 20, 30 and 60 min) on the efficiency of extraction was also investigated. We found that ultrasonic extraction for 20 min obtained optimal results, and there was no obvious difference between 20 and 30 min. Finally, we choose the extraction process was as follow: ultrasonic extraction of the samples in pure methanol for 20 min.
Optimization of separation conditions
Different compositions of mobile phase were tried to obtain chromatograms with good resolution of adjacent peaks. Various mixtures of water and methanol were used as mobile phase but separation was not satisfactory. However, when methanol was replaced by acetonitrile, the situation improved a lot and satisfactory resolution was obtained. Furthermore, other chromatographic variables were also optimized, including analytical columns (Elite ODS C18 and Kromasil C18), column temperatures (25 and 30°C) and flow rates (0.8 and 1.0ml/min). Eventually, the optimal separation was achieved on an Elite Hypersil ODS C18 column (250 mm × 4.6 mm, 5 μm) at a column temperature of 30°C with a flow rate of 1.0 ml/min. Under the optimal chromatographic conditions, the peaks corresponding to the different analytes were well-separated within 45 min. Representative chromatograms for the eleven standard analytes and herb samples were shown in Figure 2. The peaks of these lignans were identified by two methods: (i) by comparing the retention times of the peaks with those of the reference compounds eluted under the same conditions and (ii) by spiking the sample with stock standard solutions of analytes.
Figure 2.
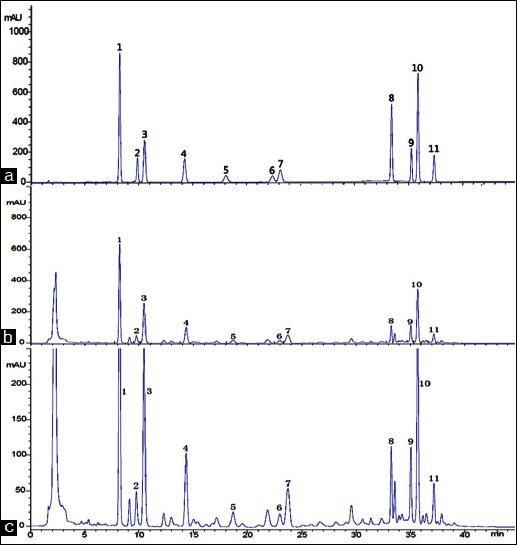
HPLC Chromatograms of (a) mixed standards: Schisandrin (125.10 ìg/ml), Gomisin J (26.00 ìg/ml), Schisandrol B(54.95 ìg/ml), Angeloylgomisin H(70.50 ìg/ml), Gomisin G (12.75 ìg/ml), Schisantherin A(18.95 ìg/ml), Schisantherin B(41.60 ìg/ml), Deoxyschisandrin (25.80ìg/ml), γ-Schisandrin(45.50 ìg/ml), Schisandrin B(103.50 ìg/ml) and Schisandrin C(22.80 ìg/ml); (b) Schisandra chinensis (produced from Tieling, Liaoning); (c) amplified (b), respectively
Calibration curves, limits of detection and quantification
Methanol stock solutions containing Schisandrin, Gomisin J, Schisandrol B, Angeloylgomisin H, Gomisin G, Schisantherin A, Schisantherin B, Deoxyschisandrin, γ-Schisandrin, Schisandrin B and Schisandrin C were prepared and diluted to appropriate concentrations for plotting the calibration curves. At least six concentrations of the eleven analytes solution were analyzed in triplicate, and then the calibration curve of each analyte was constructed by plotting the peak area versus the concentration. The calculated results are shown in Table 3. All calibration curves showed good linear regression (r ≥ 0.9995) in a relatively wide concentration range.
Table 3.
Linear regression data, LOD, LOQ, precision of eleven lignans in Schisandra chinensis
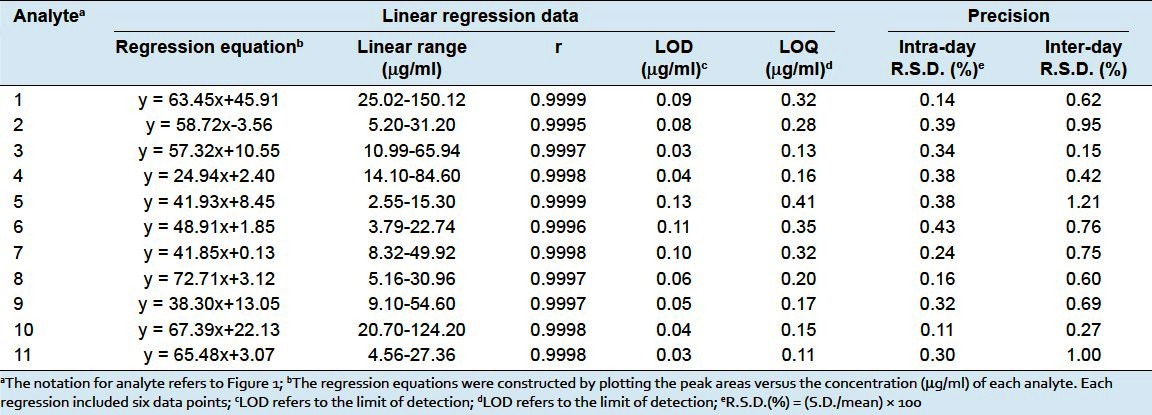
The working solutions of the analytes were further diluted with methanol to a series of appropriate concentrations. The limit of detection (LOD) and limit of quantification (LOQ) for each analyte were calculated with corresponding standard solution on the basis of a signal-to-noise ratio (S/N) of 3 and 10, respectively. The values can be seen in Table 3.
Precision, accuracy and stability
The intra- and inter-day precisions were determined by analyzing known concentrations of the eleven analytes in six replicates during a single day and by duplicating the experiments on five consecutive days. Variations were expressed by the relative standard deviations (R.S.D.) for intra- and inter-day. The results are shown in Table 3, where it can be seen that the intra- and inter-day R.S.D. values of the eleven compounds were all less than 1.21%, confirming the good reproducibility of the developed method.
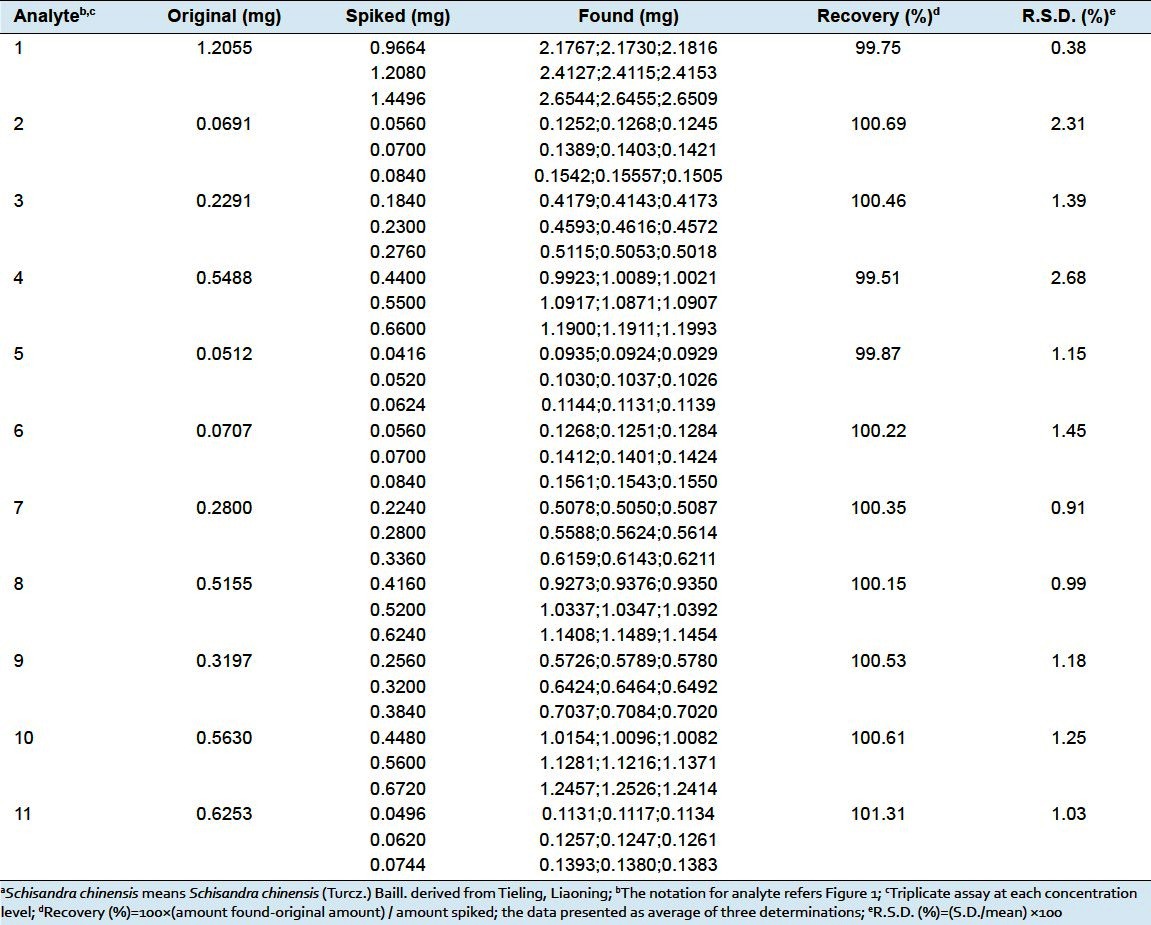
The recovery test was used to evaluate the accuracy of the method by adding three known amounts of individual standards to a certain amount (0.15 g) of S. chinensis (Tieling, Liaoning). Three replicates were performed for the test. The mixture was extracted and analyzed with the proposed method. The average percent recoveries were evaluated by calculating the ratio of the detected amount versus the added amount. The recovery of the method was in the range of 99.51–101.31% with R.S.D. values less than 2.68% as shown in Table 4. The results show that the method is accurate.
Table 4.
Recoveries for the assay of eleven lignans in Schisandra chinensisa
Stability of a sample solution was tested at room temperature when the sample solution was protected from sunlight. The sample solution was analyzed in triplicate every 12h over a period of 72h. The analytes were found to be very stable in methanol solution (R.S.D. ≤ 1.89%) over the test period.
Sample analysis
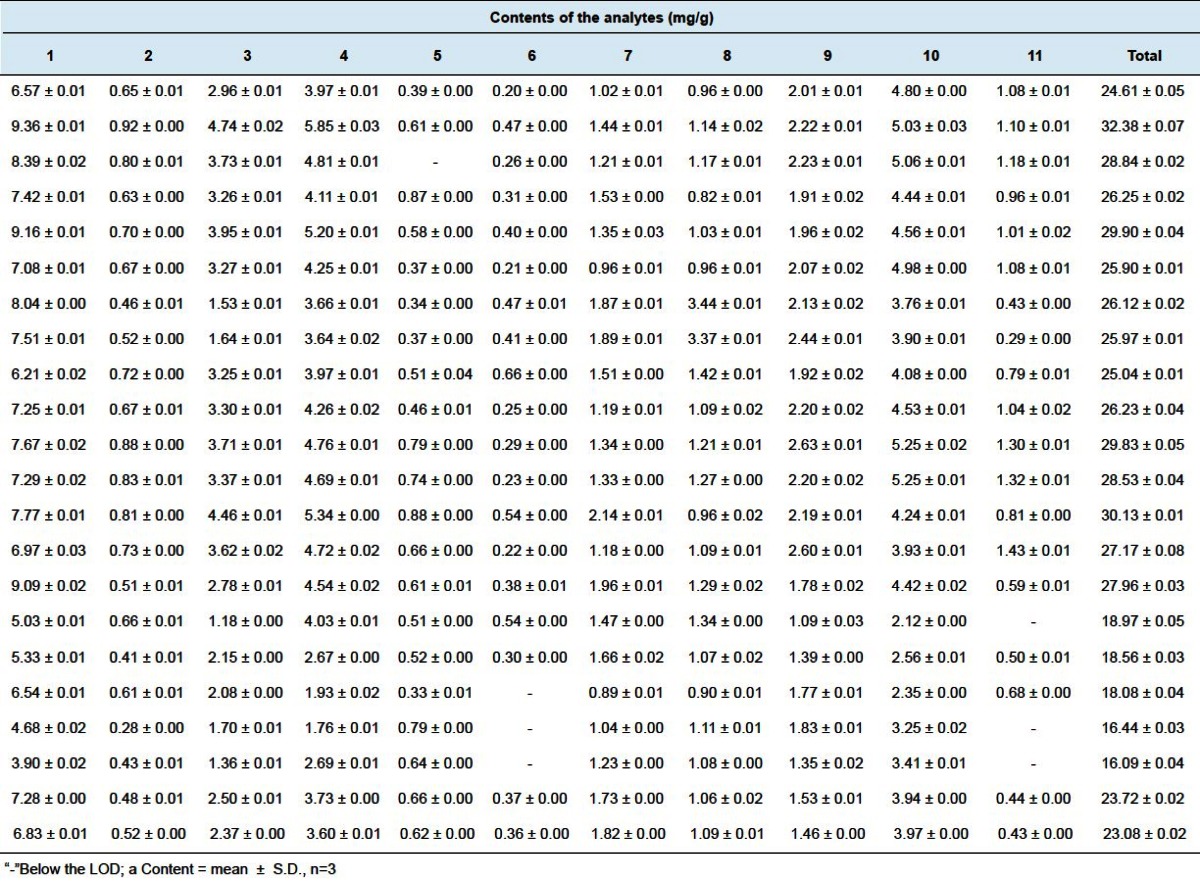
The method was applied to simultaneously determine eleven characteristic lignans in 22 samples of S. chinensis collected from different sources. The contents of the eleven compounds in the samples were quantified and the results of the mean values of three replicate injections are shown in Table 5. Among the analyzed compounds, the content of Schisandrin (1) was the highest in most of the samples. Significant variation in the total content of lignans was found in 22 samples of S. chinensis from different sources. The highest content obtained was 32.38 mg/g in the sample from Zhonghe, Heilongjiang Province, while very low in some regions, such as Yangquan, Shanxi Province.
Table 5.
Contents of eleven lignans in 22 samples of Schisandra chinensis
CONCLUSION
This study describes the development of an HPLC-UV method for simultaneous determination of the eleven characteristic lignans in 22 samples from different sources of S. chinensis. The qualitative and quantitative method for the quality control of bioactive lignans of S. chinensis from different sources was established for the first time. High linearity, repeatability, intra-day and inter-day assay precision, accuracy and reliability were presented in the method validation procedures. The proposed method is promising to improve the quality control of S. chinensis and its processed products. Moreover, based on this multi-components assay method, further studies of, phytochemical pharmacokinetic and statistics are expected to follow.
ACKNOWLEDGEMENTS
The research was supported by Major Program of State Commission of Science Technology of China (NO.2009ZX09308-004), Educational Commission of Jiangsu Province of China (NO.09KJA360001), and Key Laboratory of Acupuncture of Jiangsu Province of China (NO.KJA200906).
Footnotes
Source of Support: Major Program of State Commission of Science Technology of China (NO.2009ZX09308-004), Educational Commission of Jiangsu Province of China (NO.09KJA360001), and Key Laboratory of Acupuncture of Jiangsu Province of China (NO.KJA200906)
Conflict of Interest: None declared.
REFERENCES
- 1.China Pharmacopoeia Commission, China Pharmacopoeia. Vol. 1. Beijing: Chemical Industry Press; 2010. pp. 61–2. [Google Scholar]
- 2.Fujihashi T, Hara H, Sakata T, Mori K, Higuchi H, Tanaka A, et al. Anti-human immunodeficiency virus (HIV) activities of halogenated gomisin J derivatives, new nonnucleoside inhibitors of HIV type 1 reverse transcriptase. Antimicrob Agents Chemother. 1995;39:2000–7. doi: 10.1128/aac.39.9.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan YL, Cao P, Yu KB, Liao X. Studies on the chemical constituents from the fruit of Schisandra chinensis. Chinese Trad Herbal Drugs. 2006;37:185–7. [Google Scholar]
- 4.Willfor SM, Smeds AI, Holmbom BR. Chromatographic analysis of lignans. J Chromatogr A. 2006;1112:64–77. doi: 10.1016/j.chroma.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 5.Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail: an overview of Russian research and uses in medicine. J Ethnopharmacol. 2008;118:183–212. doi: 10.1016/j.jep.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Drasar P, Moravcova J. Recent advances in analysis of Chinese medical plants and traditional medicines. J Chromatogr B: Analyt Technol Biomed Life Sci. 2004;812:3–21. doi: 10.1016/j.jchromb.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 7.Ding AW. Chinese Materia Medical Preparation. Beijing: Higher Education Press; 2007. pp. 291–2. [Google Scholar]
- 8.Ye DJ, Yuan ST. Dictionary of Chinese Herbal Processing Science. Shanghai: Shanghai Science and Technology Press; 2005. p. 196. [Google Scholar]
- 9.Liu KT, Cresteil T, Columelli S, Lesca P. Pharmacological properties of dibenzo[a,c]cyclooctene derivatives isolated from Fructus Schizandrae chinensis. II. Induction of phenobarbital-like hepatic monooxygenases. Chem Biol Interact. 1982;39:315–30. doi: 10.1016/0009-2797(82)90048-5. [DOI] [PubMed] [Google Scholar]
- 10.Lu H, Liu GT. Anti-oxidant activity of dibenzocyclooctene lignans isolated from Schisandraceae. Planta Med. 1992;58:311–3. doi: 10.1055/s-2006-961473. [DOI] [PubMed] [Google Scholar]
- 11.Kim SR, Lee MK, Koo KA, Koo SH, Sung SH, Lee NG, et al. Dibenzocyclooctadiene lignans from Schisandra chinensis protect primary cultures of rat cortical cells from glutamate-induced toxicity. J Neurosci Res. 2004;76:397–405. doi: 10.1002/jnr.20089. [DOI] [PubMed] [Google Scholar]
- 12.Jiang SL, Zhang YY, Chen DF. Effects of heteroclitin D, schisanhenol and (+)-anwulignan on platelet aggregation. Fudan Univ J Med Sci. 2005;32:467–71. [Google Scholar]
- 13.Huang F, Xiong YT, Xu LH, Ma SP, Dou CG. Sedative and hypnotic activities of the ethanol fraction from Fructus Schisandrae in mice and rats. J Ethnopharmacol. 2007;110:471–5. doi: 10.1016/j.jep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Yin FZ, Lu TL, Cai BC. Influence of processing on total lignans in fruit of Schisandra chinensis. Chinese Trad Herbal Drugs. 2006;37:1341–4. [Google Scholar]
- 15.Halstead CW, Lee S, Khoo CS, Hennell JR, Bensoussan A. Validation of a method for the simultaneous determination of four schisandra lignans in the raw herb and commercial dried aqueous extracts of Schisandra chinensis (Wu Wei Zi) by RP-LC with DAD. J Pharm Biomed Anal. 2007;45:30. doi: 10.1016/j.jpba.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Avula B, Choi YW, Srinivas PV, Khan IA. Quantitative determination of lignan constituents from Schisandra chinensis by liquid chromatography. Chromatogra. 2005;61:515. [Google Scholar]
- 17.Hu JY, Lu TL, Mao CQ, Huang ZJ. HPLC simultaneous determination of six lignans in Fructus Schisandrae. Chin J Pharm Anal. 2011;31:27–30. [Google Scholar]
- 18.Chen AJ, Li CH, Gao WH, Hu ZD, Chen XG. Separation and determination of active components in Schisandra chinensis Baill.and its medicinal preparations by non-aqueous capillary electrophoresis. Biomed Chromatogr. 2005;19:481–7. doi: 10.1002/bmc.464. [DOI] [PubMed] [Google Scholar]


