Abstract
Background:
Mushrooms are an important natural source represents a major and untapped potent pharmaceutical product. Ganoderma lucidum (G. lucidum) an important medicinal mushroom has been shown to contain high amount of antioxidant. However, in vivo studies on G. lucidum fruiting bodies are lacking.
Objectives:
To determine the effects of G. lucidum fruiting bodies ethanolic extract (GLEet) on expression of xenobiotic enzymes, oxidant-antioxidant and hormonal status on 7,12-dimethyl benz[a]antheracene (DMBA) induced experimental breast cancer was investigated in female Sprague dawley rats.
Materials and Methods:
Cancer bearing female Sprague dawley rats was orally treated with GLEet (500mg/kg body weight) for 16 weeks. Incidence and tumor volume in each groups, and biochemical parameters were carried out in plasma, liver, and mammary tissues of animals. Histopathological and immunohistochemical analysis were also determined.
Result:
Oral administration of GLEet on tumor bearing animals significantly diminished the levels of lipid peroxidation thereby enhancing the nonenzymatic antioxidants and also positively regulated the estrogen receptor hormones level to near normal when compared with DMBA treated rats. Moreover, it also positively modulates the xenobiotic metabolizing enzymes. Therefore, the dietary administration of G. lucidum may be efficiently used as a chemopreventive agent against mammary carcinogenesis.
Conclusion:
We concluded that G. lucidum is a potent chemopreventive agent, thereby it offers maximum protection against DMBA-induced mammary carcinogenesis.
Keywords: Antioxidant, 7, 12-dimethylbenz(a)anthracene, Ganoderma lucidum, mammary cancer, xenobiotic metabolizing enzymes
INTRODUCTION
Breast cancer is one of the most frequent cancer types and is responsible for the highest mortality rate among women.[1] For breast cancer diagnosis the traditional triple test are physical examination, mammography, and aspiration cytology. Unfortunately, all these methods are either not adequate or require an expert to identify breast cancer in earlystages.[2] The etiology of breast cancer is multifactorial. Significantly breast cancer risk factors include age, early age at menarche, late age of menopause, and late age at first pregnancy, obesity, oral contraception, hormone replacement therapy, diet, family history, lactation, and prior history of benign breast diseases.[3] As estimated in 2011, 230,480 new cases of female and 2140 male cases were diagnosed to have invasive breast cancer and 57,650 additional cases of noninvasive (in situ) breast cancer have also been diagnosed.[4] In India, the incidence of breast cancer is expected to increase by more than two-third over the next two decades to approximately 1.7 million new cases per year.[5]
Chemical carcinogen such as 7,12-dimethylbenz(a)anthracene (DMBA), is commonly employed to initiate and promote neoplastic transformation on experimental animals.[6] Oxidation is essential for many living organisms for the production of energy to fuel biological processes. However, oxidative stress resulting from increased free radical generation and decreased antioxidant status in the target cells and tissues play an important role in carcinogenesis.[7] Even though, all organisms possess antioxidant defense and repair systems that have evolved to protect them against oxidative damage, these system are insufficient to prevent the damage entirely.[8] Considerable, natural products with antioxidant activity may be used to help the human body to reduce oxidative damage. Many fruits, vegetables, herbs, cereals, sprouts, seeds, and edible mushrooms have been investigated for their antioxidant and anticancer activities.[9]
In recent years, mushroom consumption has been markedly increased throughout the world and involves a variety of edible species like Volvariella volvacea (Fam: Pluteaceae, Ver: Paddy straw mushroom), Agaricus bisporus (Fam: Agaricaceae, Ver: Button mushroom), and Calocybe indica (Fam: Tricholomataceae, Ver: Milky mushroom) are abundant in India.[10,11] For thousands of years, medicinal properties of mushrooms have been well known in Eastern Asian.[12] Of special interest is the implication of several mushrooms in breast cancer prevention including the popular mushrooms Ganoderma lucidum (Ling Zhi or Reishi) that has been widely used for the general promotion of health and longevity in Asia. Chemical investigations on the fruiting bodies, spores and mycelia of G. lucidum reveal that they contain various bioactive substances.[13] These bioactive compounds belong to the group of polysaccharides and antioxidants, which protect the body against free radicals that damage body cells to induce diseases and cancers.[14]
Xenobiotic metabolizing enzymes expressed in the breast may be “activating” (metabolizing substrates to more reactive species) or “detoxifying” (reducing the DNA reactivity of genotoxic species and increasing their excretion). Some enzymes may possess activating and detoxification roles, depending on the substrate.[15] However, G. lucidum has demonstrated to possess a great ability to produce ligninolytic enzymes, mainly laccases[16] and some studies have explored its capability to degrade xenobiotics, including dyes.[17] A crucial development in the evaluation of breast Carcinoma has been the realization that the presence of hormone (estrogen and progesterone) receptors in the tumor tissue correlates well with response to hormone therapy and chemotherapy.[18] The possible mechanism of action of phytoestrogens against breast cancer prevention and concluded that the mechanism of phytoestrogens action in breast cancer have yet to be elucidated due to the dual (chemopreventive and adverse effect) role of the phytoestrogen.[19] However, the previous study elucidated that G. lucidum contain biologically active compound with specificity against estrogen receptor (ER) and NF-κB signaling inhibits proliferation of human breast cancer cells.[20]
Thus the present study investigated the effect of G. lucidum fruiting bodies ethanolic extract of by monitoring the tumor incidence and volume as well as body mass index, biochemical status like (nonenzymatic antioxidants, phase I and phase II detoxification enzymes) during DMBA-induced mammary carcinogenesis. Furthermore, biological materials from them can be used for further histopathological and immunohistochemical studies, techniques that could possibly indentify the mechanisms and some indicators of malignancy and clinical prognosis.
MATERIALS AND METHODS
Chemicals
7,12-dimethylbenz(a)anthracene, Nitrobluetetrazolium (NBT), Phenazinemethosulphate (PMS), Nicotinamide adenine dinucleotide phosphate (NADPH), 5,5′-dithio-bis-nitrobenzoicacid (DTNB) were purchased from Sigma chemical Pvt. Ltd., Bangalore, India. All other chemical reagents used in this experiment were analytical grade.
Animals
Six weeks old, female Sprague dawley rats, weighing approximately 130–150g were obtained from National Institute of Nutrition, Hyderabad and maintained in the Central Animal House, Rajah Muthiah Medical College and Hospital, Annamalai University. All the rats were acclimatized for a week under standard husbandry conditions. The rats were housed in polypropylene cages (45 × 24 × 15cm), maintained at room temperature (27 ± 2°C), in 12 h light/12 h dark conditions. The animals were fed on a standard pellet diet (Amrut Laboratory Animal Feed, Mysore Feed Limited, Bangalore, India) and water ad libitum was available to the animals throughout the experimental period and replenished daily. The standard pellet diet comprised of 21% protein, 5% lipids, 4% crude fiber, 8% ash, 1% calcium, 0.6% phosphorous, 3.4% glucose, 2% vitamin, and 55% nitrogen free extract (carbohydrate) and it provides metabolisable energy of 3600 Kcal/kg.
Animal handling and experimental procedure were approved by the Institutional Animal Ethical Committee of Rajah Muthiah Medical College (Reg.No:160/1999, Proposal number: 775 CPCSEA), and the experiments were performed in accordance with the “Guide for the case and use of laboratory animal” (NIH, 1985) and committee for the purpose of control and supervision on experimental animals (CPCSEA).
Induction of mammary carcinogenesis
Mammary carcinogenesis was induced in Sprague Dawley rats using a single subcutaneous injection of 25 mg of DMBA in 1 mL emulsion of sunflower oil (0.5 mL) and physiological saline (0.5 mL) to each rat.[21]
Material
Ganoderma lucidum mushrooms were collected in and around areas of Meghamalai, Theni district, Tamil Nadu. The Plant was taxonomically identified and authenticated by Dr. V. Venkatesalu, Associate professor, Department of Botany, Annamalai University. A voucher specimen (No: 208) was deposited at the Herbarium of Botany, Department of Botany, Annamalai University.
Ethanolic extraction
The fresh fruiting bodies of G. lucidum were dried in shade conditions and the dried materials were pulverized in a blender to get coarse powder. For G. lucidum fruiting bodies ethanolic extraction, the coarse powder material of (500 g) was extracted by stirring with 1000 mL of 95% ethanol at 30°C for 24h at 150 rpm and filtering through Whatman No. 1 filter paper. The residue was then extracted with two additional 100 mL portions of ethanol as described above. The ethanolic supernatant was evaporated at 40°C under reduced pressure and controlled temperature (40–50°C). The ethanolic extracts were redissolved in ethanol for the antioxidant activity.[22] A dark semisolid material (yield 19g) obtained was stored at 0-4°C until use. A known amount of the residual extracts were suspended in distilled water and was orally administrated to the animals by gastric intubation.
Experimental design
In the experiment, rats were randomized and divided into four groups of six animals each, which are shown in Figure 1. The mode of administration for all groups was through gastric intubation. At the end of the experimental period (16 weeks), all the rats were kept overnight fast and anesthetized using ketamine chloride (24mg/kg body weight) by intramuscular injection and sacrificed by cervical decapitation between 8.00 am to 10.00 am. Blood was collected in clean dry test tubes with few drops of heparin and plasma obtained was used for various biochemical estimations. Tissues such as liver and mammary tissues were removed, cleared off blood, and immediately transferred to ice-cold containers containing 0.9% NaCl. The tissues were homogenized in an appropriated buffer and used for the estimation of various biochemical parameters.
Figure 1.
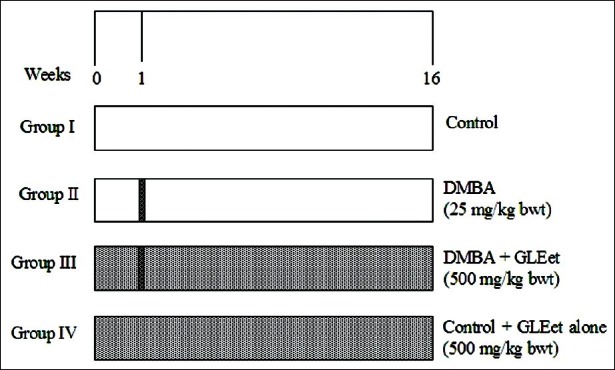
Schematic representation of experimental protocol
Histopathological examination
For histopathological study, three rats from each group were perfused with physiological saline, followed by formaline (10% formaldehyde). The mammary tissues were excised immediately and fixed in 10% formalin. The mammary tissues were sliced and embedded in paraffin wax, 3–5 μm thick sections were cut in a rotary microtome and were stained with hematoxylin and eosin. The specimens were evaluated with a light microscope. All histopathological changes were examined by the pathologist.
Immunohistochemical study
Paraffin embedded tissue section were dewaxed and rehydrated through graded ethanol to distilled water. Endogenous peroxidase was blocked by incubation with 3% H2O2 in methanol for 10min. The antigen retrieval was achieved by microwave in citrate buffer solution (2.1g citric acid/LD.H2O; 0.37g EDTA/LD. H2O; 0.2g Trypsin) (pH 6.0) for 10min, followed by washingwith Tris-buffered saline (TBS) (8g Nacl; 0.605g Tris) (pH 7.6). The tissues section was then incubated with power BlockTM reagent (Biogenex, San Ramon, CA, USA). Universal proteinaceous blocking reagent were incubated for 15min at room temperature to block nonspecific binding sites. The tissues sections were then incubated with the respective primary antibody (rabbit monoclonal antibodies) (Dako, Netherland) overnight at 4°C for ER. The bounded primary antibody was detected by incubated with (mouse polyclonal antibody) (BioGenex, San Ramon, CA, USA) the secondary antibody conjugated with horseradish peroxidase (BioGenex, San Ramon, CA, USA) for 30min each at room temperature. After rising with TBS, the antigen-antibody complex was detected using 3,3′-diamminobenzidine (Sigma, USA). When accepted color intensity was reached, the slides were washed, counter stained with hematoxylin, and covered with a mounting medium.
For negative control, the primary antibody was replaced with TBS. Positive control for each antibody was also processed simultaneously. The percentage positive tumor expressing these proteins was graded as follows: 3+= strong staining, more than 50% of cells were stained; 1+= week staining, between 5% and 25% of cells were stained; 0 = negative, less than 5% of cell staining.
Biochemical estimations
Lipid peroxidation was estimated as evidenced by the formation of thiobarbituric acid reactive substances (TBARS). TBARS in plasma liver and tissues was assayed by the method of Niehaus and Samuelson.[23] The activities of reduced glutathione (GSH), Vitamin C and Vitamin E in plasma, liver, and mammary tissues was determined by the methods of Ellman′s method,[24] Roe and Kuether et al.[25] and Baker et al.[26] respectively. The activity of DT-diophorase (DTD) was determined by the method of Ernster et al.[27] Cytochrome P450 was assayed by the method of Omura and Sato.[28]
Tumor volume
Tumor volume was measured as described by Escrich et al.[29] V=4/3π (d1/2) × (d2/2)2, where d1 and d2 are the two diameter of the tumor (d1>d2). At killing, the volume of each tumor calculated using its three diameters wasV=4/3π (d1/2)×(d2/2)×(d3/3);(d1>d2>d3)
Statistical analysis
Statistical analysis was performed using SPSS software package (South wacker drive, Chicago) version 11.5. The values were analyzed by one way analysis of variance (ANOVA) followed by Duncan′s Multiple Range Test (DMRT). All these results were expressed as mean ± SD for six rats in each group: P-values <0.05 were considered as significant.
RESULTS
Figure 2 shows the body weight changes during initial and final stage of experiment. The body weights were found to be significantly reduced in DMBA treated tumor bearing animals. Whereas, oral administration of GLEet (500 mg/kg body weight) showed significant (P < 0.05) increases in the body weight. No significant changes in body weight were observed in control and GLEet alone treated groups of rats.
Figure 2.
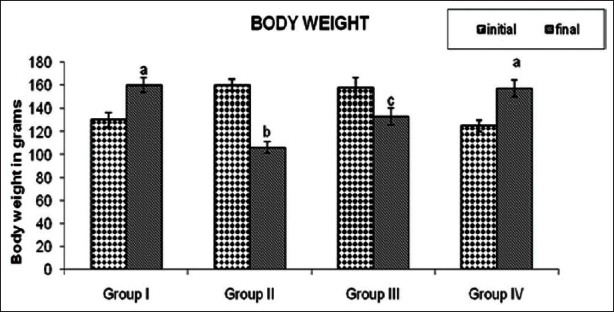
Effect of GLEet on the changes of body weight of control and experimental rats. Group I: Control, Group II: DMBA, Group III: DMBA + GLEet, Group IV: Control + GLEetalone. Values are expressed as mean ± SD for six animals in each group. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT)
The carcinogenic parameter like tumor incidence and tumor volume was observed and is shown in Table 1. The period of time from carcinogen administration to the palpable detection of tumor was recorded. The study demonstrated 100% tumor incidence in DMBA group. The tumor volume was increased in both the groups DMBA and DMBA + GLEet during the induction period. But oral administration of the GLEet in DMBA-induced tumor bearing rats, showed there was a significant (P < 0.05) decreases in the tumor incidence to 66% and decreased tumor volume when compared with DMBA group rats.
Table 1.
Incidence of mammary tumor in control and experimental rats in each group
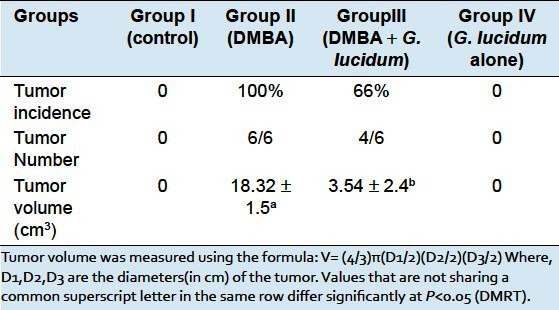
Figure 3a–c show the morphological appearance in DMBA and DMBA + GLEet treated rats. Figure 4 shows the histopathological features in control, DMBA, DMBA + GLEet, and GLEet alone. The immunohistochemical expression of hormone receptor status in mammary carcinoma of Sprague dawley rats were shown in Figure 5. In DMBA administration rats, the expression of ER was significantly higher compared with control animals. Whereas, oral supplementation of GLEet to tumor bearing rats showed down regulated expression of ER status. Administration of GLEet alone also showed mild expression of ER.
Figure 3.
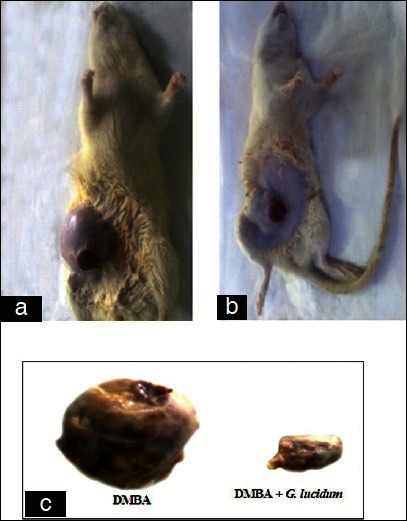
a, b and c Show the morphological appearance of DMBA and DMBA + GLEet treated rats. (a) The morphological appearance of the mammary adenocarcinoma in DMBA treated female Sprague dawley rats and (b) The morphological appearance of the mammary adenocarcinoma in DMBA + GLEet treated female Sprague dawley rats. (c) Tumor size of DMBA and DMBA +GLEet treated rats
Figure 4.
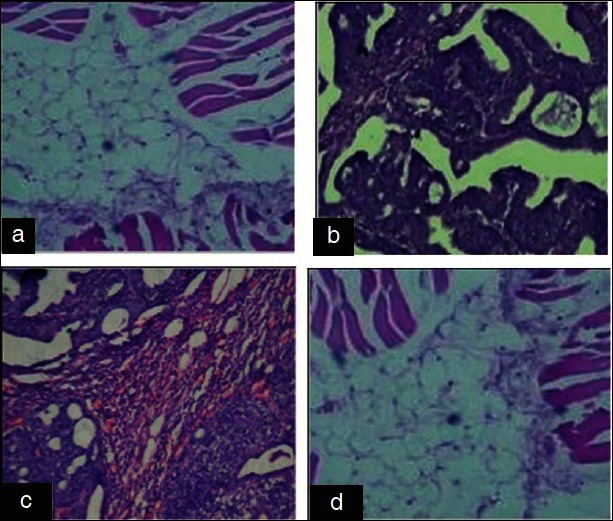
(a) Group I: Control showing normal structure. (b) Group II: DMBA showing pleomorphic with increased mitotic activity of adenocarcinoma. (c) Group III: DMBA + GLEet showing ductal hyperplasia. (d) Group IV: Control + GLEet alone showing normal structure (H and E, ×40)
Figure 5.
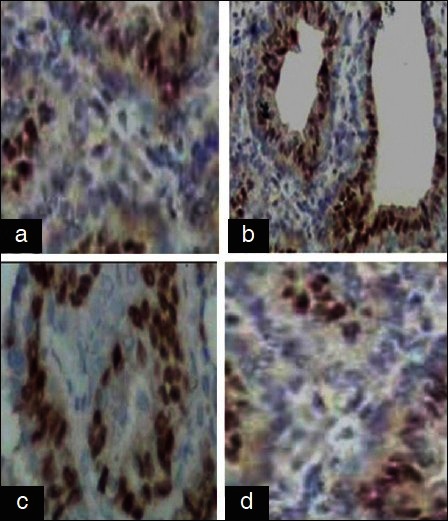
(a) Group I: Control showing mild expression. (b) Group II: DMBA showing overexpression.(c) Group III: DMBA + GLEet showing down regulation. (d) Group IV: Control + GLEet showing mild expression (H and E, ×40)
Figure 6 shows the level of TBARS in plasma, breast and liver tissues of various experimental groups. In plasma, breast and liver of DMBA group cancer bearing rats show a significant increase in TBARS levels when compared with control group rats. However, the level of TBARS was decreased significantly (P < 0.05) in DMBA +GLEet rats when compared with DMBA group rats. No significant changes were found in control and GLEet alone group rats.
Figure 6.
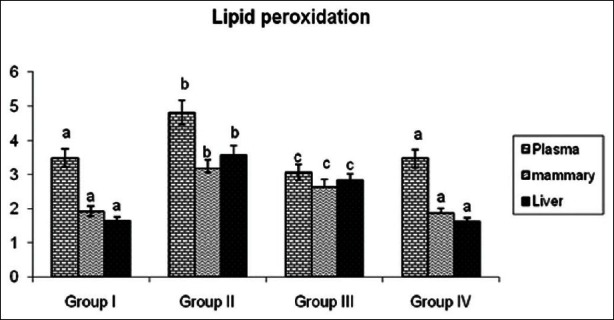
Effects of GLEet on lipid peroxidation in Plasma, mammary and liver tissues of control and experimental rats. Plasma (mM/dL), mammary and liver tissues (mM/100g wet tissue). Group I: Control, Group II: DMBA, Group III: DMBA + GLEet extract, Group IV: Control + GLEet alone. Values are expressed as mean ± SD for six animals in each group. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT)
The activities of nonenzymatic antioxidants like GSH, Vitamin C and Vitamin E in plasma, breast and liver tissues of control and experimental animals are depicted in Table 2. DMBA group cancer bearing rats showed a significant reduction in nonenzymatic antioxidant levels when compared with control group rats. Oral administration of GLEet in DMBA-induced tumor bearing rats shows significantly (P < 0.05) increased the antioxidant levels when compared with DMBA group rats. No significant changes were found in control and G. lucidum alone group of rats.
Table 2.
Effect of G. lucidum extract on non-enzymatic antioxidants in control and experimental animals
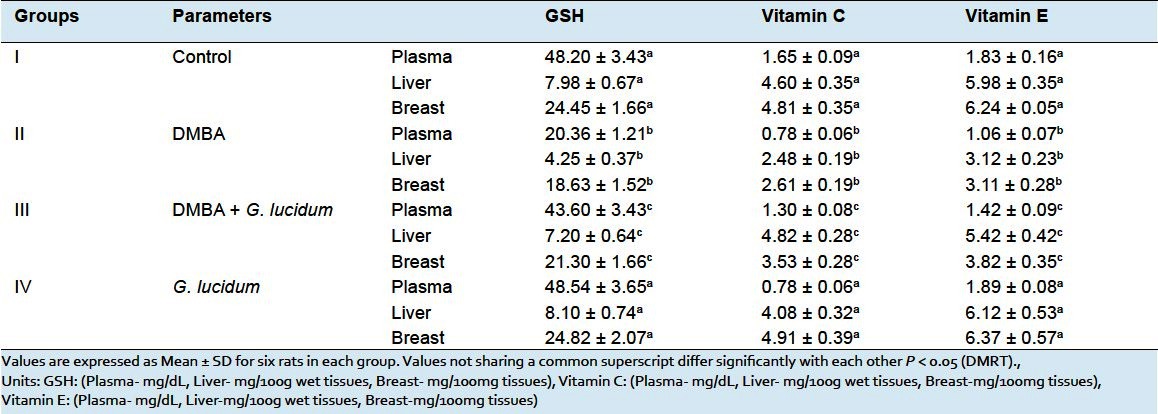
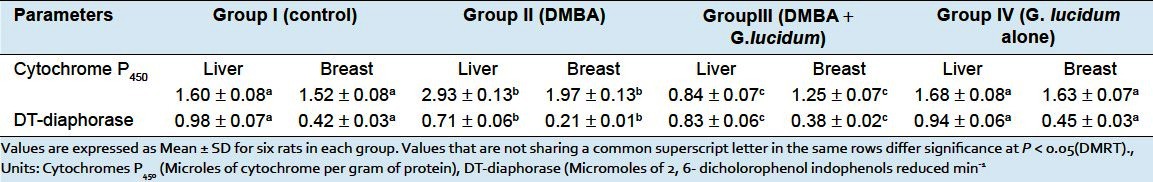
Table 3 shows the activities of phase II detoxification enzymes in liver tissues were significantly decreased whereas the activities of phase I enzymes were increased in DMBA group rats as compared with control rats. Oral administration of GLEet to DMBA-induced tumor bearing rats shows significantly (P < 0.05) restore the status of above said biochemical parameter to near normal. No significant changes were observed in control and GLEet alone group of rats. Whereas, the level of breast tissues activities of phase I enzymes were significantly increased and phase II detoxification enzymes were decreased in DMBA group rats as compared with control group of rats. Oral administration of GLEet to DMBA + GLEet treated rats shows significantly (P < 0.05) decreased the activities of phase I and increased the activities of phase II detoxification enzyme. No significant changes were observed in control and GLEet alone group of rats.
Table 3.
Effects of G. lucidum on Phase I and Phase II detoxification agents in Liver and Breast tissues of control and experimental rats in each group
DISCUSSION
Many recent studies explore the potential of naturally occurring antioxidants in cancer prevention and their possible use in intervention trials for the prevention of cancer in humans. In the present investigation, we studied the antioxidant and anticancer effect of GLEet on DMBA-induced rat mammary carcinogenesis. The body weight of the animals was recorded from the day of tumor induction until the completion of the experimental period. In tumor bearing rats, there were notable reductions in body weight, which might be due to cancer cachexia. The syndrome of cachexia is characterized by the features of anorexia and early statiety, weight loss and marked muscle weakness, anemia of nonspecific type, and altered host metabolism.[30] The weight loss is due to depletion of both adipose tissues and skeletal muscle mass; while the nonmuscle protein compartment is relatively preserved, thus distinguishing cachexia from simple starvation.[31] In addition, muscle loss occurs due to increased amount of alanine originating from muscle degradation and utilization for gluconeogenesis.[32] Whereas, oral administration of GLEet showed a gradual increased in body weight, which could be due to the presence of bioactive compounds like polysaccharides, triterpenoids, alkaloid, and proteins (include glycoprotein) which mediate these favorable changes due to their antioxidant, antiperoxidative, and antiinflammatory properties.[33]
Moreover, G. lucidum effectively decreased tumor volume, size, and burden which might be due to the inhibitory action of the drug on tumor growth in rats. G. lucidum also has shown its protective effect in tumor cell lines like human breast cancer MCF-7.[34] Other studies also reported that triterpene-enriched extracts from G. lucidum inhibit the growth of cancer cells through suppressing protein kinase C, activating mitogen-activated protein kinase and G2- phase cell cycle arrest which might be leads to apoptosis.[35]
Reactive oxygen species (ROS)-mediated biomembrane lipid peroxidation is regarded as one of the basic mechanisms of cell and tissue damage. The TBARS was measured by lipid peroxidation process. Lipids, especially polyunsaturated fatty acids (PUFA) are very susceptible to free radical attack, which can initiate lipid peroxidation.[36] High level of ROS has been reported to damage many biomolecules and exert diverse cellular and changes in gene expression that leads to initiation and promotion of carcinogenesis.[37] Plasma TBARS serve as an index to assess the extent of tissue damage. We observed that elevated TBARS levels in breast cancer rat can be related to overproduction and diffusion of free radicals from the damaged tumor tissues when compared with control rats. But, oral supplementation of GLEet to DMBA-induced rats decreased the status of TBARS to near normal range. This protective effect is probably based on the antioxidant activity of the extract which reduces the oxidative damage by blocking the production of free radicals and inhibits lipid peroxidation. In line with our findings, Joseph et al. reported that G. lucidum inhibits the progress of lipid peroxidation, which was induced by Fe2+ ascorbate in rat.[38]
Nonenzymatic antioxidants form the second line of defense mechanism to scavenge excessively generated ROS in the system. The evidence for increased action of oxidants in breast cancer is the increase in the level of oxidative damage and decreased in antioxidant status.[39] The antioxidants, such as GSH, vitamin C and vitamin E, play an excellent role in protecting the cells from oxidative damage.
GSH is an important nonprotein cellular thiol which in conjugation with GPx and GST, play a significant role in protecting cells against cytotoxic and carcinogeneicchemicals.[40] GSH serves as a marker for evaluation of oxidative stress and it act as an antioxidant at both intracellular and extracellular levels.[41] In our study, the concentration of GSH was significantly reduced in cancer-bearing animals, which was in constituentwith other reports.[42] The reduced levels may due to the changes in their intracellular GSH levels and consequently these could be reflected in their antioxidant machineries.[43] The reduced form of GSH therefore becomes readily oxidized to Glutathione disulfide (GSSG) on interacting with free radicals.[44]
Antioxidants other than GSH may also play a key role in preventing lipid peroxidation under experimental conditions. Vitamin C and vitamin E are naturally occurring free radical scavengers.[45] Vitamin C (ascorbic acid) is an important H2O soluble antioxidant in biological fluids and an essential micronutrient required for normal metabolic functioning of the body. It is shown to react directly with superoxide, hydroxyl radicals, and singlet oxygen.[45–48] Vitamin C undergoes synergistic interactions with tocopheroxyl radical in the regeneration of α-tocopherol.[49] Decreased levels of water-soluble antioxidant in cancer-bearing animals may be due to the utilization of antioxidants to scavenge free radicals. Vitamin E, the major lipid soluble antioxidant present in all cellular membranes, acts as a powerful terminator of lipid peroxidation.[50] Vitamin E is a potent oxygen radical scavenger that protects cell membranes from oxidative damage initiated by carcinogens.[51] The free radical clearing capacity of vitamin E is due to the localization of an unpaired electron on its conjugated double bond. Decreased levels of vitamin E were observed in plasma and tissues of DMBA rats, which may be due to reduced concentration of vitamin C and GSH levels which can result in reduced conversion of α-tocopheroxyl radical to α-tocopherol. Our results were corroborating with previous reports.[52]
Oral administration of GLEet with DMBA significantly increased the antioxidant status in plasma and tissues by quenching and detoxifying the free radicals. This potent antioxidant property of the extract is mainly due to the presence of high content of polysaccharides, polysaccharides peptide complexes triterpenoids, nucleosides, β-glucans, and lectins, which could have significantly attenuated the lipid peroxidation activity and resulted in increased antioxidant levels. A recent report has stated that G. lucidum acts as an effective antioxidant against degenerative diseases caused by oxidative stress,[53,38] this result strongly lends credence to our study.
The critical events in the initiation of carcinogenesis, is the conversion of DMBA into reactive oxygen species (epoxide) by an array of Xenobiotic metabolizing enzymes. Cytochrome P450 and cytochrome b5 are the key enzymes responsible for the metabolic activation and the carcinogenic potential of DMBA. DTD is an inducible enzyme that may protect the cell from oxidative damage by preventing oxyradical formation from one-electron reduction from quinines and subsequent formation of superoxide radicals via dismutation of the semiquinone.[54] GSH transferases usually associated with the phase II biotransformation of xenobiotics. They may products cellular proteins against oxidation through the glutathione redox cycle and also directly detoxify reactive oxygen species and neutralize reactive intermediates generated from exposure to xenobiotics including chemical carcinogens.[55] Mathivathani et al. reported that increased phase I and decreased phase II detoxification enzyme in the liver and mammary tissues of DMBA treated rats.[56] Similarly, our results were also in line with these finding. But oral administration of GLEet altered the changes of the tissue to near normal which might have either inhibited the metabolic activation of DMBA or stimulated the activation of detoxification against to excrete the active metabolites of DMBA, dihydodiolepoxide. In line with our findings, Gao et al reported that G. lucidum extract modulates the expression of hepatic phase I and phase II enzymes, which showed promising efficacy and well tolerated for the management of various hepatic injuries.[57]
Histopathological examination of mammary tissues in DMBA-induced mammary carcinoma rats showed the pleomorphic with increased mitotic activity of adenocarcinoma. Whereas, GLEet treatment in tumor bearing rat show regression in tumor with ductal glands were seen. Administration of GLEet alone did not show any changes when compared with control breast tissues. Immunohistochemical examination of DMBA-induced mammary carcinoma rats showed and higher level of ER expression. Oral administration of GLEet in tumor bearing rats showed down regulated expression of ER status. Similarly, Jianga et al. also reported that G. lucidum down-regulates the expression of ERα, inhibits estrogen-inducible ER transactivation and inhibits TNF-α stimulated activation of NF-κB in MCF-7 cells.[20]
CONCLUSION
On the basis of our results on biochemical, histopathological, and immunohistochemical expressions studies have demonstrated that G. lucidum extract showed potent chemopreventive effects against DMBA-induced mammary cancer. Owing to the presence of the phytochemicals such as polysaccharides and triterpenes are responsible for the preventive action of G. lucidum. Therefore, dietary consumption of G. lucidum might be used as a potent chemopreventive agent for mammary cancer prevention. Further pharmacological and biochemical investigations are underway to find out the active constituents that are responsible for the chemopreventive activity and to elucidate its mechanism of action against various cancers.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Joanitti GA, Azevedo RB, Freitas SM. Apoptosis and lymsosome membrane permeabilization induction on breast cancer cells by an anticarcinogenic Bowman-Birk protease inhibition from vignaunguiculata seeds. Cancer Lett. 2010;293:73–81. doi: 10.1016/j.canlet.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Mitka M. Researches seek mammography alternatives. JAMA. 2003;290:450–1. doi: 10.1001/jama.290.4.450. [DOI] [PubMed] [Google Scholar]
- 3.Gönenç A, Erten D, Aslan S, Akinci M, Simşek B, Torun M. Lipid peroxidation and antioxidant status in blood and tissues of malignant breast tumor and benign breast diseases. Cell BiolInt. 2006;30:376–80. doi: 10.1016/j.cellbi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 4.U. S Breast cancer statistics 19 oct 2011. [Last accessed on 2012 Jan 3]. Available from: http://www breast cancerorg/symptoms/understand-bc/statistics.jsp .
- 5.Indian stastistics/csis.org. [Last accessed on 2012 Jan 3]. Available from: http://medical xpress.com/news/2011-10-largest-cancer-india.html .
- 6.Cardeiro MC, Kaliwal BB. Antioxidant activities of Bark extract of Brideliaretusa Spreng on DMBA induced mammary carcinogenesis in female Sprague Dawley rats. J Pharmacol. 2011;2:14–20. [Google Scholar]
- 7.Huang YL, Sheer JY, Lin TH. Association between oxidative stress and changes of trace element in patients with breast cancer. Clin Biochem. 1999;32:131–6. doi: 10.1016/s0009-9120(98)00096-4. [DOI] [PubMed] [Google Scholar]
- 8.Simic MG. Mechanisms of inhibition of free radicals processed in mutagenesis and carcinogenesis. Mutat Res. 1988;202:377–86. doi: 10.1016/0027-5107(88)90199-6. [DOI] [PubMed] [Google Scholar]
- 9.Dimitrios B. Source of natural phenolic antioxidants. Trends Food Sci Technol. 2006;17:505–12. [Google Scholar]
- 10.Mirunalini S, Dhamodharan G, Deepalakshmi K. Antioxidant Potential and Current Cultivation Aspects of an Edible Milky Mushroom-CalocybeIndica. Int J Pharm Pharmsci. 2012;4:137–43. [Google Scholar]
- 11.Dhamodharan G, Mirunalini S. A novel medicinal characterization of Agaricus Bisporus (White Button Mushroom) Pharmacology online. 2010;2:456–63. [Google Scholar]
- 12.Mahajna J, Dotan N, Zaidman BZ, Petrova RD, Wasser SP. Pharmacological values of medicinal mushroom for prostate cancer theraphy: The case of Ganodermalucidum. Nutr Cancer. 2009;61:16–26. doi: 10.1080/01635580802379323. [DOI] [PubMed] [Google Scholar]
- 13.Lu QY, Jin YS, Zhang Q, Zhang Z, Heber D, Go VL, et al. Ganodermalucidum extracts inhibit growth and induce actin polymerization in bladder cancer cells in vitro. Cancer Lett. 2004;216:9–20. doi: 10.1016/j.canlet.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Deepalakshmi K, Mirunalini S. Therapeutic Properties and Current Medical Usage of Medicinal Mushroom: Ganoderma Lucidum. Int J Pharm Sci Res. 2011;2:1922–9. [Google Scholar]
- 15.Williams JA, Phillips DH. Mammary expression of xenobiotic metabolizing enzymes and their potential role in breast cancer. Cancer Res. 2000;60:4667–77. [PubMed] [Google Scholar]
- 16.Ko EM, Leem YE, Choi HT. Purification and characterization of laccaseisoenzymes from the white-rot basidimycete Ganodermalucidum. Appl Microbiol Biotechnol. 2001;57:98–102. doi: 10.1007/s002530100727. [DOI] [PubMed] [Google Scholar]
- 17.Murugesan K, NamI H, Kim YM, Chang YS. Decolorization of reactive dyes by a thermostablelaccase produced by Ganodermalucidum in solid state culture. Enz Microbial Technol. 2007;40:1662–72. [Google Scholar]
- 18.Barnes DM, Hanby AM. Oestrogen and Progesterone receptors in breast cancer: Past, Present and future. Histopathology. 2001;8:271–4. doi: 10.1046/j.1365-2559.2001.01060.x. [DOI] [PubMed] [Google Scholar]
- 19.Menes SM, Hei TK, Ganju RK, Bhat HK. Phytoestrogens and breast cancer prevention: Possible mechanisms of action. Environ Health Perspect. 2008;116:426–33. doi: 10.1289/ehp.10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang J, Slivova V, Sliva D. Ganodermalucidum inhibits proliferation of human breast cancer cells by down-regulation of estrogen receptor and NF-κB signaling. Int J Oncol. 2006;29:695–703. [PubMed] [Google Scholar]
- 21.Samy RP, Gopalakrishnakone P, Ignacimuthu S. Anti- tumor promoting potential of luteolin against 7, 12-dimethybenz(a)anthracene-induced mammary tumours in rats. ChemBiol Interact. 2006;164:1–14. doi: 10.1016/j.cbi.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Trease GE, Evans WC. A Textbook of Pharmacognosy. 7th. London: Oxford University Press; 1978. pp. 591–610. [Google Scholar]
- 23.Niehaus WG, Samuelson B. Formation of malondialdehyde from and glucose 6- phosphate dehydrogenase from fermenting yeast and phospholipids arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 24.Ellman GL Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 25.Roe JH, Kuether CA. The determination of ascorbic acid in whole blood and urine through the 2,4-dinitrophenylhydrazine derivative of dehydroascorbic acid. J Biol Chem. 1943;11:145–64. [Google Scholar]
- 26.Baker H, Frank O, DeAngelis B. Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr Res. 1980;21:531–6. [Google Scholar]
- 27.Ernster L. DT-diaphorase. Methods Enzymol. 1967;10:309–17. doi: 10.1016/0076-6879(90)86122-c. [DOI] [PubMed] [Google Scholar]
- 28.Omura T, Sato R. The carbon monoxide binding pigment of liver. J Biol Chem. 1964;239:2370–8. [PubMed] [Google Scholar]
- 29.Escrich E, Moral R, García G, Costa I, Sánchez JA, Solanas M. Identification of Noval differential expressed genes by the effect of a high-fat-n-6 diet in experimental breast cancer. Mol Carcinog. 2004;40:73–8. doi: 10.1002/mc.20028. [DOI] [PubMed] [Google Scholar]
- 30.Argiles JM, Aczon-Bieto J. The metabolic environment of cancer. Mol Cell Biochem. 1998;81:3–17. doi: 10.1007/BF00225648. [DOI] [PubMed] [Google Scholar]
- 31.Fearon KC. The mechanisms and treatment of weight loss in cancer. Proc Nutr Soc. 1992;51:251–65. doi: 10.1079/pns19920036. [DOI] [PubMed] [Google Scholar]
- 32.Leij-Halfwerk S, Dagnelie PC, van Den Berg JW, Wattimena JD, Hordijk-Luijk CH, Wilson JP. Weight loss and elevated gluconeogenesis from alanine in lung cancer patients. Am J Clin Nutr. 2000;71:583–9. doi: 10.1093/ajcn/71.2.583. [DOI] [PubMed] [Google Scholar]
- 33.Mau JL, Lin HC, Chen CC. Antioxidant properties of several medicinal mushrooms. J Agric Food Chem. 2002;50:6072–7. doi: 10.1021/jf0201273. [DOI] [PubMed] [Google Scholar]
- 34.Hu H, Ahn NS, Yang X, Lee YS, Kang KS. Ganodermalucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int J Caner. 2002;102:250–3. doi: 10.1002/ijc.10707. [DOI] [PubMed] [Google Scholar]
- 35.Lin SB, Li CH, Lee SS, Kan LS. Triterpene-enriched from Ganodermalucidum inhibit growth of hepatoma cell via suppressing protein kinase C activing mitogen- activated protein kinase and G2-phase cell cycle arrest. Life Sci. 2003;72:2381–90. doi: 10.1016/s0024-3205(03)00124-3. [DOI] [PubMed] [Google Scholar]
- 36.Halliwell B, Gutteridge JM. Free radicals in biology and medicine. 3rd. UK: Oxford Science Publications; 1999. p. 192. [Google Scholar]
- 37.Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans. 2003;31:1441–4. doi: 10.1042/bst0311441. [DOI] [PubMed] [Google Scholar]
- 38.Joseph S, Sabulal B, George V, Sinina TP, Janardnan KK. Antioxidative and Antinflammatory activities of the chloroform extract of Ganodermalucidum found in South India. Sci Pharm. 2009;77:111–21. [Google Scholar]
- 39.Tas F, Hansel H, Belce A, Ilvan S, Argon A, Camlica H. Oxidative stress in Breast cancer. Med Oncol. 2005;22:11–5. doi: 10.1385/MO:22:1:011. [DOI] [PubMed] [Google Scholar]
- 40.Obrador E, Nauarro J, Mompo J, Asensi M, Pellicer JA, Estrela JM. Gluathione and the rate of cellular proliferation determine tumor cell sensitivity to tumor necrosis factor in vivo. Biochem J. 1997;325:183–9. doi: 10.1042/bj3250183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Comporti M. Three models of free-radicals induced cell injury. Chem Biol Interact. 1989;72:1–56. doi: 10.1016/0009-2797(89)90016-1. [DOI] [PubMed] [Google Scholar]
- 42.Anbuselvam C, Vijayavel K, Balasubramanian MP. Protective effect of Operculinaturpethum against 7, 12-dimethylbenz(a)anthracene induced oxidative stress with reference to breast cancer in experimental rats. Chem Biol Interact. 2007;168:229–36. doi: 10.1016/j.cbi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Navarro F, Arroyo A, Martin SF, Bello RI, Cabo R, Burgess JR, et al. Protective role of ubiquinone in vitamin E and Selenium-deficient plasma membranes. Biofactors. 1999;9:163–70. doi: 10.1002/biof.5520090211. [DOI] [PubMed] [Google Scholar]
- 44.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–62. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 45.Hemila H, Roberts P, Wikstrom M. Activated polymorphonuclear leucocytes consume vitamin C. FEBS Lett. 1984;178:25–30. doi: 10.1016/0014-5793(84)81232-6. [DOI] [PubMed] [Google Scholar]
- 46.Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun. 1975;63:463–8. doi: 10.1016/0006-291x(75)90710-x. [DOI] [PubMed] [Google Scholar]
- 47.Bielski BH. Chemistry of Ascorbic acid radicals. Ascorbic acid: Chemistry, metabolism and uses. Adv Chem Ser. 1982;200:81–100. [Google Scholar]
- 48.Bodannes RS, Chan PC. Ascorbic acid as a scavenger of singlet oxygen. FEBS Lett. 1979;105:195–6. doi: 10.1016/0014-5793(79)80609-2. [DOI] [PubMed] [Google Scholar]
- 49.Packer L, Fuchs J. Vitamin C and Redox Cyclic Antioxidants in Vitamin C in health and Disease. New York: Marcel Dekker Inc.; 1997. p. 95. [Google Scholar]
- 50.Machlin LJ. Vitamin E: A comprehensive treatise. New York: Marcel Dekker Inc.; 1980. [Google Scholar]
- 51.Schindler R, Mentlein R. Flavanoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J Nutr. 2006;136:1477–82. doi: 10.1093/jn/136.6.1477. [DOI] [PubMed] [Google Scholar]
- 52.Padmavathi R, Senthilnathan P, Chodon D, Sakthisekaran D. Therapeutic effect of paclitaxel and propolis on lipid peroxidation and antioxidant system in 7,12 dimethylbenz(a)anthracene-induced breast cancer in female Sprague dawley rats. Life Sci. 2006;78:2820–5. doi: 10.1016/j.lfs.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Karaman MA, Mimica-Dukic Neda M, Matavulj M. Lignicolous fungi as potential natural sources of antioxidants. Arch Biol Sci. 2005;57:93–100. [Google Scholar]
- 54.Smart RC, Zinnoni VG. DT-diaphorase and peroxidase influence the covalent binding of the metabolites of phenol, the major metabolite of benzene. Mol Pharmacol. 1984;26:105–11. [PubMed] [Google Scholar]
- 55.Mishra P, Kale RK, Kar A. Chemoprevention of mammary tumor genesis and chemodulation of the antioxidantive enzymes and preoxidative damage in prepubertal Spraugedawley rats by Biochanin A. Mol Cell Biochem. 2008;312:1–9. doi: 10.1007/s11010-008-9714-8. [DOI] [PubMed] [Google Scholar]
- 56.Mathivadhani P, Shanthi P, Sachdanandam P. Effect of semecarpusanacardium Linn. Nut extract on mammary and hepatic expression of xenobiotic enzymes in DMBA-induced mammary carcinoma. Environ Toxicol Pharmacol. 2007;23:328–34. doi: 10.1016/j.etap.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Gao Y, Gao H, Chan E, Tango W, Li X, Liang J, et al. Protective effect of Ganoderma (a mushroom with Medicinal properties) against various Liver Injuries. Food Rev Int. 2007;21:27–52. [Google Scholar]


