Abstract
Background:
Vedic Guard is a polyherbal formulation used in the treatment of various ailments, however, is not scientifically assessed for its effect on doxorubicin-induced cardiotoxicity.
Objective:
To find out the preventive role of Vedic Guard against doxorubicin-induced myocardial toxicity in rats.
Materials and Methods:
Cardiotoxicity was produced by doxorubicin (15 mg/kg for 2 weeks). Vedic Guard (270 mg/kg, orally) was administered as pre-treatment for 2 weeks and then for 2 weeks alternated with doxorubicin (DXR). The general observations, mortality, histopathology, biomarker like lactate dehydrogenase (LDH), creatine phosphokinase (CPK), aspartate aminotransferase (AST), alanine transaminase (ALT), electrocardiographic (ECG) parameters, antioxidants such as glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) were monitored after 3 weeks of last dose.
Results:
The repeated administration of DXR causes cardiomyopathy associated with an antioxidant deficit. Pre-treatment with Vedic Guard decreases serum enzyme viz LDH, CPK, AST, and ALT levels to that of normal values. Vedic Guard significantly protected the myocardium from the toxic effect of DXR, by increasing the levels of antioxidants such as GSH, SOD, and CAT and decreased the elevated level of malondialdehyde. The study shows significant alteration of ECG pattern in DXR administered rats. The characteristic findings were elevation of ST segment, reduction in P waves, QRS complex, and R-R interval. Vedic Guard showed a protective effect against DXR-induced altered ECG pattern. It also reduced the severity of cellular damage of the myocardium confirmed by histopathology.
Conclusion:
The results of the present study indicated cardioprotective effect of Vedic Guard might be attributed to its antioxidant activity.
Keywords: Antioxidant, cardioprotective, cardiotoxicity, doxorubicin, vedic guard
INTRODUCTION
Doxorubicin (DXR), an anthracycline derivative, is one of the effective and useful anti-neoplastic agent commonly used for the treatment of variety of tumors including solid and malignant lymphoma. However, its clinical use is restricted due to its cardiotoxic effect.[1] Congestive heart failure, cardiomyopathy, and electrocardiographic changes were demonstrated after cumulative DXR administration.[2]
The mechanism of DXR-induced cardiomyopathy is not completely understood, but several hypothesis have been postulated, which include inhibition of nucleic acid,[3] protein synthesis,[4] release of vasoactive amines,[5] alterations in sarcolemmal Ca2+ transport,[6] alterations in membrane bound enzyme abnormalities in mitochondria,[7] lysosomal alterations,[8] and an imbalance of myocardial electrolytes.[9] However, recent studies have postulated the involvement of oxygen-free radicals in the development of cardiomyopathy due to DXR. The presence of semi-quinone in the tetracyclic aglycone molecule of DXR is reported to increase oxygen-free radical activity[10] as well as peroxidation of unsaturated lipids within the membranes.[11]
Polyherbal formulations/antioxidant compounds have shown protective effect in DXR-induced cardiotoxicity without reducing their therapeutic efficacy. Moreover, there is a growing interest in the usage of natural antioxidants as a protective strategy against the cardiovascular-related problems such as ischemia reperfusion[12] and DXR-induced cardiotoxicity.[13,14] Earlier studies have reported that Lipistat,[15] Cardipro[16] and Abana,[17] a polyherbal formulations, have shown protective effect in DXR-induced cardiotoxicity. Vedic Guard (VG) is a poly herbal formulation, containing extract of well-known plants in Ayurveda possessing antioxidant activity, which protect the cell from degenerative changes. The herbal composition of Vedic Guard is Terminalia arjuna, Withania somnifera, Piper longum, Emblica officinalis, Curcuma longa, Tinosporia cordifolia, Terminalia chebula, Glycyrrhiza glabra, Commiphora mukul, Bacopa monnieri, Sida cordifolia, Mesua ferrea, Eclipta alba, Tribulus terrestris, Puereria tuberose, and Asphaltum. Vedic Guard is used in the treatment of hypertension, diabetes, stress and strain condition, cardiovascular diseases and as co-therapy in the management of tuberculosis, cancer, and liver cirrhosis. Earlier studies have shown that Vedic Guard prevents hepatotoxicity induced by anti-tubercular drugs in rats due to its potent antioxidant property.[18] In view of this, DXR-induced cardiotoxicity is linked to oxidative stress; therefore, the present study is designed to investigate the protective effect of Vedic Guard against doxorubicin-induced cardiotoxicity in rats.
MATERIALS AND METHODS
Male Wistar rats weighing 150–200 g were used and were procured from Sri Venkateswara enterprises Bangalore. They were housed in a group of 6 under environmentally-controlled room with 12 h light/dark cycle and had free access to food and water. After 7 days of acclimatization period, they were randomly selected for different experimental groups.
All the experimental procedures were carried out in accordance with committee for the purpose of control and supervision of experiments on animal (CPCSEA) guidelines. All the experimental procedures were approved by the institutional animal ethical committee (IAEC).
Materials - Doxorubicin was a generous gift from Get Well Pharmaceuticals, India. Vedic Guard and other chemicals used were of analytical grade and procured locally. Analyzing kits were obtained from ERBA Diagnostics, Daman, India.
Dosage fixation - Doxorubicin (2.5 mg/kg body weight i.p.) in 6 equal injections every alternative day to make a total cumulative dose of 15 mg/kg body weight was used in the present study based on previous report.[19] VG suspension was prepared in 0.5% CMC by using distilled water. Dose of VG 270 mg/kg body weight was selected according to therapeutically-equivalent dose.
Experimental design - After 1 week of acclimatization, the animals were randomly divided into 4 groups of 6 animals in each. Group 1 served as normal control and received normal saline 5 ml/kg body weight (i.p.). Group 2 animals were treated with DXR (2.5 mg/kg body weight i.p.) in 6 equal injections every alternative day to make a total cumulative dose of 15 mg/kg body weight. Group 3 animals received VG daily (270 mg/kg body weight p.o.) for 2 weeks and then alternatively with vehicle for next 2 weeks. Group 4 animals received VG (270 mg/kg body weight p.o., for 2 weeks) as a pre-treatment, followed by doxorubicin administration as in DXR group.
Enzyme assays - After 36 h of the last treatment, orbital blood samples were obtained under light ether anesthesia using heparinized microcapillaries for the estimation of cardiac biomarkers CPK,[20] LDH,[21] ALP,[22] AST and ALT.[23] Both control and treated animals were observed for as long as 3 weeks after the last injection for the general appearance, behavior, and mortality. At the end of 3 weeks post-treatment period, animals were anesthetized with light anesthetic ether, and ECG parameters were recorded using computerized data acquisition system (Biopac MP 35). The animals were sacrificed under ether anesthesia, and a midline abdominal incision was performed, and heart tissue was quickly dissected out, washed in ice-cold saline, dried on filter paper, and weighed immediately. A portion of each heart was taken from all the groups, and a 30% w/v homogenate was prepared in 0.9% buffered KCl (pH 7.4) for the estimation of glutathione (GSH),[24] superoxide dismutase (SOD),[25] catalase (CAT),[26] and malondialdehyde (MDA).[27] The remaining portion of the heart tissue was used for histopathological studies.
Statistical analysis - The results were expressed as the mean ± SE. The results obtained were analyzed using one-way ANOVA followed by Dunnett′s multiple comparison tests. Data were computed for statistical analysis by using Graph Pad Prism Software.
RESULTS
Chronic administration of doxorubicin-induced cardiac toxicity and effect of VG was established by significant increase in cardiac biomarker enzymes, ECG pattern, and endogenous antioxidants and heart tissue histopathology.
General observations - The general appearance of all groups of animals was recorded throughout the study. In doxorubicin-treated group, the animal fur became scruffy and developed a pink tinge. These animals also had red exudates around the eyes and nose, soft watery feces. Necrosis was also observed at the site of DXR injection. These changes were less pronounced in case of VG pre-treated group. There was 50% mortality in DXR-treated group, whereas in pre-treated group, there was no mortality.
Heart weight, body weight, and ratio of heart weight to body weight - Effect of DXR on heart weight, body weight, liver weight, ratio of heart weight to body weight, and liver weight to body weight is shown in Table 1. The food and water intake in DXR-treated group was significantly decreased as compared to control group. The food and water intake in the VG+DXR group was significantly increased as compared to DXR group. The heart weight, body weight, liver weight, ratio of heart weight to body weight, and liver weight to body weight in DXR treated rats were significantly increased compared with normal rats. The heart weight, body weight, liver weight, ratio of heart weight to body weight, and liver weight to body weight in VG+DXR group was significantly decreased compared with DXR group.
Table 1.
Effect of Vedic Guard on heart weight, body weight, liver weight, and ratio of heart weight to body weight in doxorubicin-induced cardiotoxicity in rats
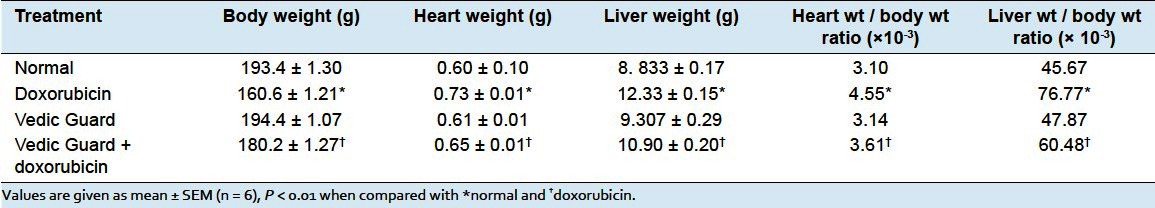
Cardiac damage enzyme markers - Animals treated with DXR produced significant increase in the levels of ALP, CPK, LDH ALT, and AST as compared to control group. VG+DXR group produced significant decrease in the level of ALP, CPK, LDH, ALT, and AST as compared to DXR group [Figure 1].
Figure 1.
Effect of Vedic Guard on different enzyme levels in doxorubicin-induced cardiotoxicity in rats. Values are expressed as mean ± SEM (n = 6), P < 0.01 when compared with FNx01normal and †doxorubicin of respective enzyme levels
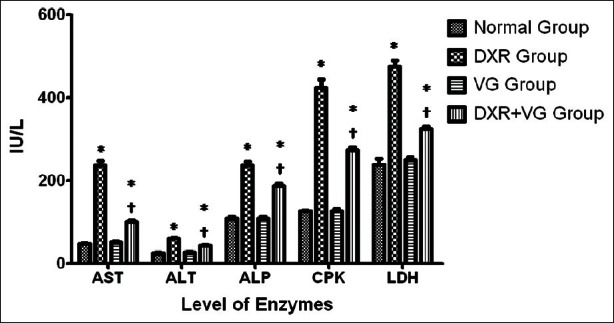
Electrocardiographic pattern - Electrocardiograph abnormalities are the main criteria generally used for the definite diagnosis of myocardial injury. The study show significant alteration of ECG patterns in DXR-administered rats as compared to normal control rats Figures [2 and 3]. The characteristic findings were elevation of ST segment, reduction in P waves, QRS complex, and R-R interval. In addition, there was a prolongation of QT interval. VG administration showed a protective effect against DXR-induced altered ECG parameter and eliminated the acute fatal complication by protecting the cell membrane damage.
Figure 2.
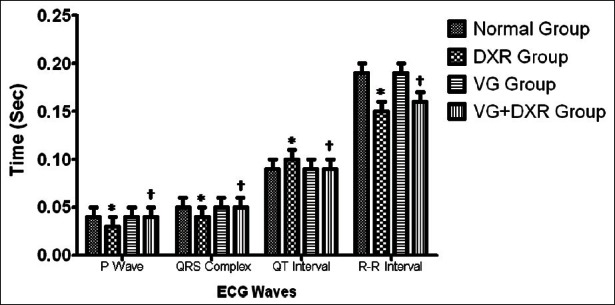
Effect of Vedic Guard on ECG parameters in doxorubicin-induced cardiotoxicity in rats. Values are expressed as mean ± SEM (n = 6), P < 0.01 when compared with FNx01normal and †doxorubicin of respective ECG parameter
Figure 3.
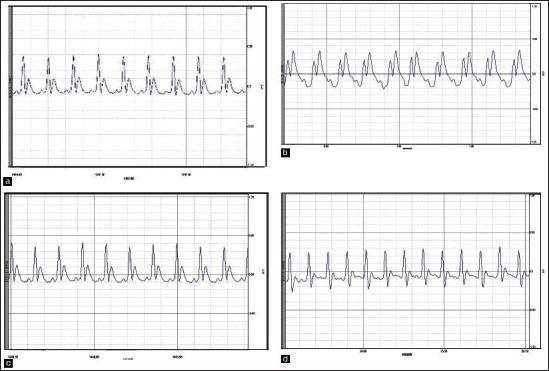
Effect of Vedic Guard on electrocardiographic pattern of (a): Normal; (b): Doxorubicin alone; (c): Vedic Guard; and (d): VG + DXR in rats.
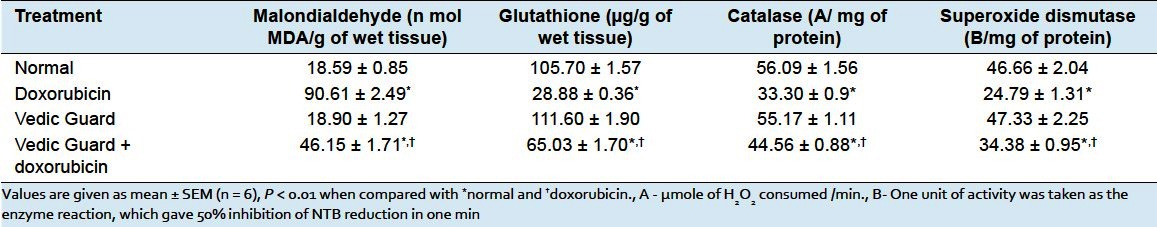
Antioxidant status - Effect of doxorubicin on tissue lipid peroxidation and antioxidant enzymes is shown in Table 2. The malondialdehyde level was increased; GSH, SOD, and CAT level were significantly decreased in doxorubicin-treated group as compared to normal animals. VG+DXR group produced significant decrease in the level of MDA and increase in the status of antioxidant and antioxidant enzymes.
Table 2.
Effect of Vedic Guard on malondialdehyde, glutathione, catalase, and superoxide dismutase in doxorubicin-induced cardiotoxicity in rats
Histopathological observation - The histology of the heart tissue from control and VG-treated animals showed normal morphological appearances, whereas in DXR group, disruptions of loss of myofibrils and vacuolization of the cytoplasm were observed. The histology of heart tissues from VG+DXR group showed less loss of myofibrils and vacuolization of the cytoplasm [Figure 4].
Figure 4.
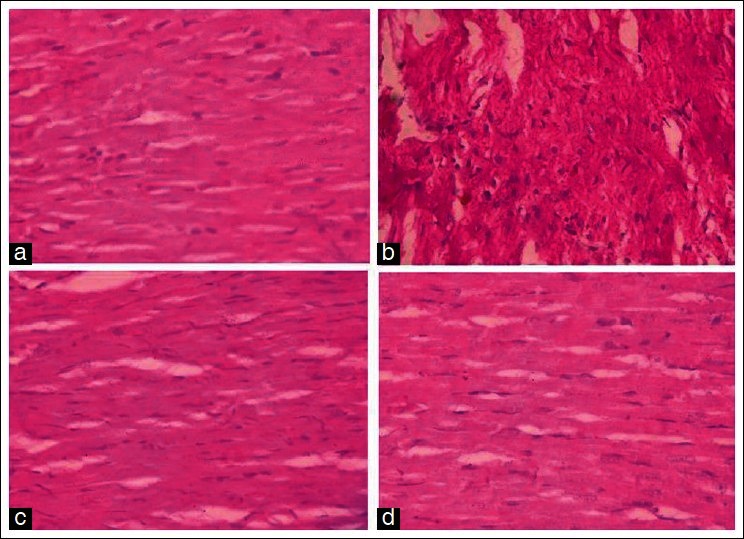
Histopathological studies of various treated groups (a): Normal; (b): Doxorubicin alone; (c): Vedic Guard and (d): VG + DXR in rats. The stain used to illustrate the histopathological changes was (H and E, x100)
DISCUSSION
Our results confirmed that a cumulative dose of DXR (15 mg/kg) induces cardiotoxicity in mice as evidenced by increase in mortality, decrease in heart weight, increased levels of biomarker enzymes, ECG changes, and loss of cardiomyocytes. Frequent administration of DXR has been shown to cause cardiomyopathic changes in patients and as well as in a variety of animal models. Administration of VG, a polyherbal preparation, reduced DXR-induced mortality in mice.
The experimental study reveals severe biochemical changes as well as oxidative damage in the cardiac tissue after the chronic treatment with DXR (cumulative dose of 15 mg/kg body weight). Doxorubicin is a well-known cardiotoxic agent; due to its ability, it will destruct myocardial cells. As a result of this, lactate dehydrogenase (LDH), transaminase (AST, ALT), and creatine kinase (CK) were released into blood stream and served as the diagnostic markers of myocardial tissue damage. The amount of these cellular enzymes present in the blood reflects the alteration in plasma membrane integrity and/or permeability.
In the present study, DXR-treated rats showed significant elevation in the levels of these diagnostic marker enzymes (AST, ALT, CK, LDH, and ALP). Moreover, elevated levels of these enzymes are an indicator of the severity of DXR-induced myocardial damage, which is in line with an earlier report.[28] The prior administration of VG showed significant reduction in DXR-induced elevated serum marker enzymes. This reduction in the enzyme level confirms that VG is responsible for maintenance of normal structural and architectural integrity of cardiac myocytes, thereby restricting the leakage of these enzymes, which can be accounted for membrane-stabilizing property of VG.
Electrocardiograph abnormalities are the main criteria generally used for the definite diagnosis of myocardial injury. The study show significant alteration of ECG patterns in DXR-administered rats as compared to normal control rats. The characteristic findings were elevation of ST segment, reduction in P waves, QRS complex, and R-R interval. In addition, there was a prolongation of QT interval. Moreover, ECG changes are an indicator of the severity of DXR-induced myocardial damage, which is in line with an earlier report.[29] The consecutive loss of cellular membrane damage due to oxidative stress might be characterized by ST elevation. VG administration showed a protective effect against DXR-induced altered ECG parameter and eliminated the acute fatal complication by protecting the cell membrane damage.
The mechanism of cardiotoxicity induced by a DXR is not clearly known from the present study, although large body of evidence supports that DXR administration is associated with a decrease in endogenous antioxidants and increase in oxygen-free radicals, resulting in increased oxidative stress, which is followed by development of a variety of sub-cellular changes in the myocardium, typical of doxorubicin-induced cardiac injury.[30] In rat treated with DXR, we found significant increase in heart tissue MDA levels, suggesting increased lipid per oxidation and decreased in levels of GSH, SOD, and CAT. Pre-treatment with VG efficiently counteracted the doxorubicin-induced cardiac tissue damage by significant decrease in MDA and increase in GSH, SOD, and CAT levels. Cardiac tissue damage may be due to increased oxidative stress and depletion of antioxidants as reported earlier.[31]
Histopathological report suggests that VG pre-treated group attenuates the doxorubicin-induced loss of myofibrils, vacuolization of the cytoplasm, and swelling of mitochondria. The histopathological changes observed in the doxorubicin-treated rats were similar to those previously reported.
Collectively, these electrocardiographic, biochemical, and histopathological results provide a possible and potential cardioprotection against DXR toxicity. Therefore, the antioxidant mechanism of VG may include its well-known ingredients in Ayurveda possessing antioxidant activity, which protects the cell from degenerative changes. Thus, in this work, VG effectively prevented tissue damage by decreasing the oxidative stress and restoring the antioxidant status.
Constituents of VG such as Terminalia arjuna,[32] Withania somnifera,[33] Piper longum,[34] Tinosporia cordifolia, [35] and Curcuma longa,[36] by virtue of their antioxidant property, prevent free radicals-mediated cardiotoxicity. In the present study, VG might serve as novel combination to limit free radical-mediated organ injury by restoring hemodynamic, biochemical, and histopathological alternations in rats via augmentation of endogenous antioxidants and maintenance of the myocardial antioxidant status.
Finally, we conclude that the cardiotoxicity induced by DXR is in relationship with oxidative stress. Our study suggests that Vedic Guard may be considered as a potentially useful candidate in combination with DXR to limit free radical-mediated myocardial injury.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Vergely C, Delemasure S, Cottin Y, Rochette L. Preventing the cardiotoxic effects of anthracyclines: From basic concepts to clinical data. Heart Metab. 2007;35:1–17. doi: 10.1016/j.ancard.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Bonadonna G, monfardini S, De Lena M, Fassati-Bellani F, Beretta G. Phase I and preliminary phase II evaluation of adriamyin. Cancer Res. 1970;30:2572–82. [PubMed] [Google Scholar]
- 3.Arena E, Bionda F, D′Alessndro N, Dusoncher L, Gebbia N, Gerbasi R. DNA, RNA protein synthesis in heart, liver and brain of mice treated with denaturation of adriamycin. Int Res Commun Syst Med Sci. 1984;2:10543–1061. [Google Scholar]
- 4.Buja LM, Ferrans VJ, Mayer RJ, Roberts WC, Hinderson ES. Cardiac ultrastructural changes induced by daunorubucin therapy. Cancer. 1973;32:771–88. doi: 10.1002/1097-0142(197310)32:4<771::aid-cncr2820320407>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR, Sagemen WS, Scott RH, Billingham ME, Bowden RE, Kernoff RS, et al. Acute and chronic cardiovascular effects of doxorubicin in the dog: the cardiovascular pharmacology of drug-induced histamine release. Cardiovasc Pharmacol. 1980;2:487–515. doi: 10.1097/00005344-198009000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Singal PK, Pierce GN. Adriamycin stimulates Ca 2+ -binding and lipid peroxidation but depress myocardial function. Am J Physiol. 1986;250:H419–24. doi: 10.1152/ajpheart.1986.250.3.H419. [DOI] [PubMed] [Google Scholar]
- 7.Singal PK, Panagia V. Direct effect of adriamycin on the rat heart sarcolemma. Res Commun Chem Pathol Pharmacol. 1984;43:67–77. [PubMed] [Google Scholar]
- 8.Singal PK, Segstro RU, Singh RP, Kutryk MJ. Changes in lysosomal morphology and enzyme activities during development of adriamycin induced cardiomyopathy. Can J Cardiol. 1985;1:139–47. [PubMed] [Google Scholar]
- 9.Oslan HM, Young DM, Prieur DJ, LeRoy AF, Reagan RL. Electrolyte and oxidation products of certain lipids. J Biol Chem. 1974;174:257–64. [Google Scholar]
- 10.Lee V, Randhawa AK, Singhal PK. Adriamycin induced myocardial dysfunction in vitro is mediated by free radicals. Am J Physiol. 1991;261:H989–95. doi: 10.1152/ajpheart.1991.261.4.H989. [DOI] [PubMed] [Google Scholar]
- 11.Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: The role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977:165–7. doi: 10.1126/science.877547. [DOI] [PubMed] [Google Scholar]
- 12.Gauthaman K, Mohamed Saleem TS, Ravi V, Patel SS, Devaraj SN. Alcoholic extract of Terminalia arjuna Protect the rabbit heart against Ischemic-reperfusion injury: Role of antioxidant enzymes and heart shock protein. Proc World Acad Sci Eng Technol. 2008;32:494–504. [Google Scholar]
- 13.Singh G, Singh AT, Abraham A, Bhat B, Mukherjee A, Verma R, et al. Protective effect of Terminalia arjuna against doxorubicin induced cardiotoxicity. J Ethanopharmacol. 2008;117:123–9. doi: 10.1016/j.jep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Hamza A, Amin A, Daoud S. The protective effect of a purified extract of Withania somnifera against doxorubicin induced cardiac toxicity in rats. Cell Biol Toxicol. 2008;24:63–73. doi: 10.1007/s10565-007-9016-z. [DOI] [PubMed] [Google Scholar]
- 15.Koti BC, Vishwanathswamy AH, Jyoti W, Thippeswamy AH. Cardioprotective effect of lipistat against doxorubicin induced myocardial toxicity in albino rats. Indian J Exp Biol. 2009;47:41–6. [PubMed] [Google Scholar]
- 16.Mohan IK, Kumar KV, Naidu MU, Khan M, Sundaram C. Protective effect of Cardipro against doxorubicin induced cardiotoxicity in mice. Phytomedicine. 2006;13:222–9. doi: 10.1016/j.phymed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Sasikumar SC, Shyamala Devi CS. Protective effect of Abana, a polyherbal formulation, on isoproterenol induced myocardial infarction in rats. Ind J Pharmacol. 2000;32:198–201. [Google Scholar]
- 18.Razdan R, Imranulla, Amar Dev MG. Preventive and curative effects of Vedic Guard against anti-tubercular drugs induced hepatic damage in rats. Phcog Mag. 2008;4:182–8. [Google Scholar]
- 19.Ayaz SA, Bhandari U, Pillai KK. Influence of DL α-lipoic acid and vitamin-E against doxorubicin-induced biochemical and histological changes in the cardiac tissue of rats. Indian J Pharmacol. 2005;37:294–9. [Google Scholar]
- 20.Rosalki SB. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967;69:696–705. [PubMed] [Google Scholar]
- 21.Henry RJ, Chiamori N, Goiub OJ, Berkman S. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase and lactic acid dehydrogenase. Am J Clin Path. 1960;34:381–98. doi: 10.1093/ajcp/34.4_ts.381. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson JH, Boutwell JH, Winsten S. Evaluation of a new system for the kinetic measurement of serum alkaline phosphatase. Clin Chem. 1969;15:487–95. [PubMed] [Google Scholar]
- 23.Reitman S, Frankel S. A colorimetric method for thedetermination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 25.Mishra HP, Fridovich I. The role of superoxide anion in the auto-oxidation of Epinephrine and a simple assay for superoxide dismuatse. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 26.Clairborne A. In: Catalase activity, in Handbook of methods for oxygen radical research. Greenwald R A, editor. CRC Press Boca Raton; 1985. p. 283. [Google Scholar]
- 27.Ohkawa H, Ohish N, Yogi K. Assay for lipid peroxidase in animal tissues by thiobarbituric acid. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 28.Wakade AS, Shah AS, Kulkarni MP, Juvekar AR. Protective effect of Piper longum L. on oxidative stress induced injury and cellular abnormality in adrimycin induced cardiotoxicity in rats. Indian J Exp Biol. 2008;46:528–33. [PubMed] [Google Scholar]
- 29.Villani F, Monti E, Piccinini F, Favalli L, Lanza E, Rozza Dionigi A, et al. Relationship between doxorubicin-induced ECG changes and myocardial alterations in rats. Tumori. 1986;72:323–9. doi: 10.1177/030089168607200315. [DOI] [PubMed] [Google Scholar]
- 30.Ayaz SA, Bhandari U, Pillai KK. Influence of DL α-lipoic acid and vitamin-E against doxorubicin-induced biochemical and histological changes in the cardiac tissue of rats. Indian J Pharmacol. 2005;37:294–9. [Google Scholar]
- 31.Thippeswamy AH, Akshay S, Koti BC, Jaffar AS, Praveen DM, Viswanatha Swamy AH, et al. Protective role of Phyllantus niruri extract in doxorubicin-induced myocardial toxicity in rats. Indian J Pharmacol. 2011;43:31–5. doi: 10.4103/0253-7613.75663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh G, Singh AT, Abraham A, Bhat B, Mukherjee A, Verma R, et al. Protective effect of Terminalia arjuna against doxorubicin induced cardiotoxicity. J Ethnopharmacol. 2008;117:123–9. doi: 10.1016/j.jep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Hamza A, Amin A, Daoud S. The protective effect of a purified extract of Withania somnifera against doxorubicin induced cardiac toxicity in rats. Cell Biol Toxicol. 2008;24:63–73. doi: 10.1007/s10565-007-9016-z. [DOI] [PubMed] [Google Scholar]
- 34.Wakade AS, Shah AS, Kulkarni MP, Juvekar AR. Protective effect of Piper longum L. on oxidative stress induced injury and cellular abnormality in adrimycin induced cardiotoxicity in rats. Ind J Exp Biol. 2008;46:528–33. [PubMed] [Google Scholar]
- 35.Rao PR, Kumar VK, Viswanath RK, Subbaraju GV. Cardioprotective activity of alcoholic extract of Tinospora cordifolia in ischemia-reperfusion induced myocardial infarction in rats. Biol Pharm Bull. 2005;28:2319–22. doi: 10.1248/bpb.28.2319. [DOI] [PubMed] [Google Scholar]
- 36.Mohanty IR, Arya DS, Gupta SK. Dietary Curcuma longa protects myocardium against isoproterenol induced hemodynamic, biochemical and histopathological alternations in rats. Int J Appl Res Nat Prod. 2008;1:19–28. [Google Scholar]


