Abstract
Background:
Panax ginseng C. A. Meyer, a perennial herb from the Araliaceae family, is a commonly used medicinal plant. Many studies have been conducted on the biologically active constituents of whole parts of P. ginseng (i.e., roots, leaves, flower buds, and fruits). However, the seeds of P. ginseng have not been intensively investigated. A new sterol glucoside,3-O-b-d-glucopyranosyl-5,22,24-stigmastatrienol (1), and a known sterol, 5,22-stigmastadienol (2), were isolated from seeds of P. ginseng and were evaluated for their inhibitory activities on tumor necrosis factor (TNF)α-induced nuclear factor (NF)-κB and inducible nitric oxide synthase (iNOS) transcription in transfected HepG2 cells. The present work deals with the isolation, identification, and antiinflammatory activities of the two compounds.
Materials and Methods:
The compounds were isolated by a combination of silica gel and YMC R-18 column chromatography, and their structures were identified by analysis of spectroscopic data (1D, 2D-NMR, and MS). The antiinflammatory activities of the isolated compounds 1 and 2 were evaluated by luciferase reporter gene assays.
Results:
Two sterols have been isolated from the seeds of P. ginseng. Compound 1 is a previously unreported glucosidyl sterol. Compounds 1 and 2 both inhibited NFκB-luciferase activity, with IC50 values of 8.1 and 4.8΅M, respectively. They also inhibited iNOS-luciferase activity in TNFα-induced HepG2 cells, with IC50 values of 2.2 and 2.9΅M, respectively.
Conclusion:
The two isolatedsterols have inhibitory effects on inflammation-related factors in HepG2 cells, as determined by luciferase reporter gene assays. Thus, seeds of P. ginseng are worthy of consideration for the development and research of antiinflammatory agents.
Keywords: Antiinflammtiory activity, Panax ginseng, seeds, 3-O-β-d-glucopyranosyl-5, 22, 24-stigmastatrienol
INTRODUCTION
Nuclear factor κB (NFκB) comprises a group of transcription factors known to regulate inflammatory responses[1] and can be activated by the expression of inflammatory cytokines, such as tumor necrosis factor (TNF) or interleukin-1 (IL-1), and the presence of oxidant-free radicals.[2] It is required for inducible nitric oxide synthase (iNOS) induction and is implicated in the induction of the human iNOS gene.[3] Additionally, inhibitors of NF-κB activation minimally decrease iNOS expression.[4]
Ginseng (Panax ginseng C.A. Meyer), a traditional herbal medicine, has been used as a tonic for the treatment of various diseases.[5,6] The entire ginseng plant, including leaves, flower buds, and berries, has been comprehensively studied, and many dammarane-type triterpenes have been characterized as principal components.[7,8] However, the seeds of P. ginseng have not been investigated intensively. In our search for antiinflammatory components from the seeds of P. ginseng, a new sterol glucoside, 3-O-β-d-glucopyranosyl-5,22,24-stigmastatrienol (1), and a known sterol, 5,22-stigmastadienol (2), were isolated from seeds of P. ginseng using a combination of silica gel and YMC RP-18 column chromatography Figure 1. The structures were established on the basis of nuclear magnetic resonance (NMR), electrospray ionization mass spectrometry (ESI-MS), and a comparison with reports in the literature.[2,9] Compounds 1 and 2 were evaluated for their inhibitory activitieson TNF-α-induced NF-κB and iNOS transcription in transfected HepG2 cells.
Figure 1.
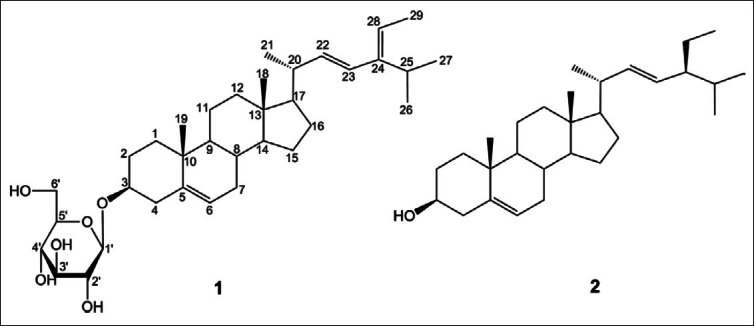
The structures of sterols (1 and 2) isolated from seeds of P. ginseng
MATERIALS AND METHODS
General
Optical rotations were measured with a DIP-360 digital polarimeter (Jasco, Easton, MD, USA). NMR spectra were obtained at room temperature on Bruker DRX 400 NMR spectrometers with tetramethylsilane (TMS) as internal standard. ESI-MS spectra were recorded on a Model 1100 LC-MSD Trap spectrometer (Agilent, Santa Clara, CA). Column chromatography was carried out silica gel (70–230 and 230–400 mesh, Merck, Darmstadt, Germany), YMC RP-18 resins (30–50 μm, Fuji Silysia Chemical Ltd., Aichi, Japan), and HP-20 Diaion (Mitshubishi Chemical, Tokyo, Japan). Analytical thin-layer chromatography (TLC) was performed on Kieselgel 60F254 or RP-18F254S (Merck, Darmstadt, Germany) glass plates and spots were visualized by spraying with 10% aqueous H2SO4 solution, followed by heating.
Plant material
The seeds of P. ginseng were collected in Geumsan province, which is well-known for ginseng cultivation in Korea, in August 2009, and were taxonomically identified by one of us (Young Ho Kim). Voucher specimens (CNU09105) have been deposited at the College of Pharmacy, Chungnam National University.
Extraction and Isolation
The powdered seeds of P. ginseng (4.0kg) were extracted in MeOH three times (5.0L × 3, 50°C) and the combined extracts were concentrated in vacuo to dryness. The MeOH residue (202.0g) was suspended in H2O (0.8L), then partitioned with ethyl acetate (EtOAc, 0.8L × 3), and the EtOAc-soluble fraction (83.7g) was subjected to a silica gel column eluted with a gradient of EtOAc in n-hexane (100% n-hexane, 10, 20, 25, 50, and 100% EtOAc; v/v) to give seven fractions (Fr. 1 ~ Fr. 7). Fr. 3 (5.1g) was purified by YMC RP-18 column with MeOH: acetone: H2O (15:1:0.5, v/v/v) to obtain compound 2 [11.5mg, 0.005% (w/w) of MeOH extract]. The water layer was subjected to a Diaion HP-20 column eluted with a gradient of MeOH in H2O (0, 25, 50, 75, and 100% MeOH; v/v) to give six fractions, F1~F6. 5 (6.6g) was purified on silica gel columns and eluted with CHCl3 :MeOH (7:0.1, v/v) to obtain 1 [37.0 mg, 0.018% (w/w)].
3-O-β-d-Glucopyranosyl-5,22,24-stigmastatrienol (1)
White amorphous powder; mp 280–300°C; ESI-MS: m/z573.4 [M + H]+; IR (KBr): 3481, 2933, 1463, 1379, 1164, 1075, 1024/cm; 1H-NMR (400MHz, C5D5 N): δ 0.67 (3H, s, CH3 -18), 0.93 (3H, s, CH3-19), 1.03 (3H, d, J = 6.6Hz, CH3-27), 1.04 (3H, d, J = 6.6Hz, CH3 -26), 1.10 (3H, d, J = 6.0Hz, CH3-21), 1.64 (3H, d, J = 6.6Hz, CH3 -29), 4.07 (1H, m, H-3), 5.09 (1H, d, J = 15.0Hz, H-23), 5.24 (1H, dd, J = 6.0, 15.0Hz, H-22), 5.26 (1H, q, J = 6.6Hz, H-28), 5.36 (1H, m, H-6), glucose unit: 5.07 (1H, d, J = 7.8Hz, H-1′), 3.97 (1H, m, H-2′), 4.31 (1H, m, H-3′), 4.30 (1H, m, H-4′), 3.94 (1H, m, H-5′), 4.58 (1H, d, J = 12.0Hz, H-6a′), 4.43 (1H, m, H-6b′); 13C-NMR (100MHz, C5D5N): δ 146.1 (C-24), 141.2 (C-5), 139.2 (C-22), 129.8 (C-23), 122.2 (C-6), 117.4 (C-28), 102.8 (C-1′), 78.9 (C-5′), 78.8 (C-3′), 78.3 (C-3), 75.6 (C-2′), 71.9 (C-4′), 63.0 (C-6′), 56.4 (C-14), 56.3 (C-17), 50.5 (C-9), 42.5 (C-13), 40.1 (C-20), 39.5 (C-4), 37.1 (C-1), 36.6 (C-10), 32.4 (C-8), 32.2 (C-7), 30.4 (C-12), 29.6 (C-2), 28.5 (C-25), 26.5 (C-16), 24.7 (C-15), 21.3 (C-11), 19.4 (C-19), 21.2 (C-26), 21.1 (C-27), 19.2 (C-21), 12.7 (C-29), 12.1 (C-18).
5,22-Stigmastadienol (2)
White amorphous powder; mp 164–165°C; ESI-MS: m/z 411.2 [M-H]-; This compound exhibited comparable spectroscopic data (1H- and 13C-NMR) to published values.[9]
Luciferase assay
NFκB-Luc and iNOS-Lus plasmids were kindly provided by Dr. Kyoon E. Kim (Chungnam National University, Daejeon, Korea). According to the manufacturer′s protocol, all cells were transfected with optimized amount of DNA plasmids (NFκB-Luc and iNOS-Luc plasmids) using Promega luciferase assay kits (Promega, Madison, WI). HepG2 cells were seeded at 1.5 × 105 cells/well in a 12-well plate and grown for 24h prior to transfection. The transfected Hep-G2 cells were pretreated for 1h with either vehicle (dimethyl sulfoxide [DMSO]) and a positive control, followed by 1h of treatment with 10 ng/mL TNFα. Unstimulated Hep-G2 cells were used as a negative control. Luciferase activity was assayed using an LB 953 Autolumat (EG&G Berthold) as described previously.[10,11] The data were presented as a mean SD of three independent experiments, performed in triplicate.
Cytotoxicity assay
An 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega Celltiter 96-Aqueous One Solution Assay) was used to analyze the effect of compounds on cell viability as described[12] by the manufacturer (Promega).
RESULTS AND DISCUSSION
Compound 1 was obtained as a white amorphous powder, and HRESI results yield a molecular formula of C35H57O6, based on the experimental [M+H]+peak at m/z 573.4183 (calculated 573.4149). The infrared (IR) spectrum showed the presence of a hydroxyl at 3481/cm. Based on1H- and 13C-NMR data, 1 was identified as a sterol glucoside, containing a β-d-glucopyranosyl unit. The large coupling constant (J = 7.8Hz) of the anomeric proton at δH 5.07 in the 1H-NMR spectrum of 1 indicates the β configuration. The deshielded oxymethine signal at δC 78.3 for C-3 indicates the presence of a 3-O-β-d-glucopyranosyl bond in 1.[2] The 1H-NMR spectrum showed six methyl groups at δH 0.67 (3H, s, CH3 -18), 0.93 (3H, s, CH3 -19), 1.03 (3H, d, J = 6.6Hz, CH3 -27), 1.04 (3H, d, J = 6.6Hz, CH3 -26), 1.10 (3H, d, J = 6.0Hz, CH3 -21), and 1.64 (3H, d, J = 6.6Hz, CH3 -29), as well as an oxygenated methine proton at δH 3.98 (1H, m, H-3), and three types of olefinic protons at δH 5.09 (1H, d, J = 15.0Hz, H-23), 5.26 (1H, dd, J = 6.0, 15.0Hz, H-22), 5.24 (1H, q, J = 6.6Hz, H-28), and at δH 5.36 (1H, m, H-6), which are characteristicfor a sterol with trans-double bond protonin the alkyl chain. As shown in Figure 2, key heteronuclear multiple-bond correlation spectroscopy (HMBC) correlations were observed between the following protonand carbon atoms: H-1′ and C-3; H-3 and C-2, and C-4; H-6 and C-4, and C-8; H-18 and C-13, and C-14; H-19 and C-5, and C-9; H-21 and C-20, and C-22; H-22 and C-17, C-20, and C-23; H-23 and C-20, and C-25; H-26 and C-24;H-27 and C-24; and H-29 and C-24, and C-28. Thus, compound 1 was determined to be 3-O-β-d-glucopyranosyl-5,22,24-stigmastatrienol, which has not been previously reported.
Figure 2.
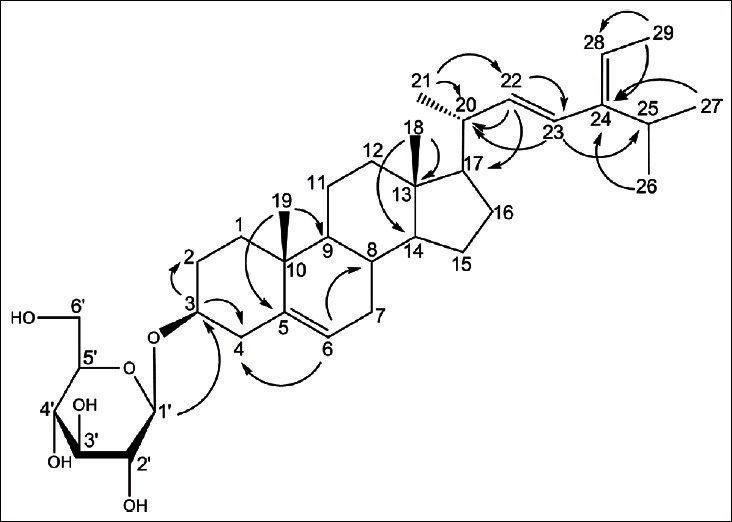
Selected HMBC (H→C) correlations of compound 1
Compound 2 was obtained as a white powder and showed a molecular ion peak at m/z 411.2 [M-H]- in the ESIMS spectrum. The 1H- and 13C-NMR spectra of 2 indicated a typical sterol structure containing 29 carbons. They showed six methyl groups (H-18, H-19, H-21, H-26, H-27, and H-29), two types of olefinic protons (H-22, H-23, and H-6), and an oxygenated methine proton (H-3). The configuration of the hydroxyl group at C-3 (δC = 71.8) was determined to be a by comparison with previously reported chemical shift: a with an upfield-shifted C-3 at δ 72.7and β with a downfield-shifted C-3 at δ 84.4.[2] From the above evidence and a comparison with the literature,[9] compound 2 was identified as 5,22-stigmastadienol.
To investigate the effect of the two sterols (compounds 1 and 2) from seeds of P. ginseng on TNFα-induced NFκB and iNOS, the nuclear transcription luciferase-reporter system was used. Compounds 1 and 2 inhibited NFκB activity with IC50 values of 8.1 and 4.8μM, respectively, compared with an IC50 value of 3.8μM for the positive control. Compounds 1 and 2 also inhibited iNOS activity with IC50 values of 2.2 and 2.9μM, respectively, compared with an IC50 value of 0.7μM for the positive control [Table 1]. To confirm the antiinflammatory activity of the tested compounds, cell viability was simultaneously determined using a colorimetric MTS assay (Promega). Neither compound 1 nor compound 2 exhibited a significant effect on cell viability at the concentration tested (data not shown). Thus, this study suggests that the compounds 1 and 2 from seeds of P. ginseng are consideration for the development and research of antiinflammatory agents.
Table 1.
Effects of compounds 1 and 2 on NF-κB and iNOS luciferase activities in TNFα-induced HepG2 cells
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Tak PP, Firestein GS. NF-кB: A key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JA, Yang SY, Koo JE, Koh YS, Kim YH. Lupane-type triterpenoids from the steamed leaves of Acanthopanax koreanum and their inhibitory effects on the LPS-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Bioorg Med Chem Lett. 2010;20:6703–7. doi: 10.1016/j.bmcl.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Salvemini D, Billiar TR, Vodovotz Y. Nitric oxide and inflammation (Progress in inflammation research) Switzerland: Birkhauser Verlag Basel; 2001. [Google Scholar]
- 4.Taylor SB, de Vera EM, Ganster WR, Wang Q, Shapiro AR, Morris MS, et al. Multiple NF-кB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem. 1998;273:15148–56. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- 5.Ernst E. Panax ginseng: An overview of the clinical evidence. J Ginseng Res. 2010;34:259–3. [Google Scholar]
- 6.Khorolragchaa A, Parvin S, Shim JS, Kim YJ, Lee OR, In JG, et al. Isolation of sesquiterpene synthase homolog from Panax ginseng C. A Meyer. J Ginseng Res. 2010;34:17–22. [Google Scholar]
- 7.Tung NH, Cho K, Kim JA, Song GY, Kim YH. Dammarane-type glycosides from the steamed flower-buds of Panax ginseng. Bull Korean Chem Soc. 2010;31:1381–4. [Google Scholar]
- 8.Tung NH, Song GY, Kim JA, Hyun JH, Kang HK, Kim YH. Dammarane-type saponins from the flower buds of Panax ginseng and their effects on human leukemia cells. Bioorg Med Chem Lett. 2010;20:309–14. doi: 10.1016/j.bmcl.2009.10.110. [DOI] [PubMed] [Google Scholar]
- 9.De-Eknamkul W, Potduang B. Biosynthesis of β-sitosterol and stigmasterol in Croton sublyratus proceeds via a mixed origin of isoprene units. Phytochemistry. 2003;62:389–98. doi: 10.1016/s0031-9422(02)00555-1. [DOI] [PubMed] [Google Scholar]
- 10.Kim KK, Park KS, Song SB, Kim KE. Upregulation of GW112 Gene by NF-κB promotes an antiapoptotic property in gastric cancer cells. MolCarcinog. 2010;49:259–70. doi: 10.1002/mc.20596. [DOI] [PubMed] [Google Scholar]
- 11.Kim JA, Yang SY, Song SB, Kim YH. Effects of impressic acid from Acanthopanax koreanum on NF-кB and PPA Rgactivities. Arch Pharm Res. 2011;34:1347–51. doi: 10.1007/s12272-011-0815-7. [DOI] [PubMed] [Google Scholar]
- 12.Kim JA, Song SB, Yang SY, Kim YH. Components from the steamed leaves of Acanthopanax koreanum and their effects of PPAR activity in HepG2 cells. Nat Prod Commun. 2011;6:1233–6. [PubMed] [Google Scholar]


