Abstract
Background
Meta-analysis of case-control genome wide association studies (GWAS) for early onset and morbid obesity identified four variants in/near the PRL, PTER, MAF and NPC1 genes.
Objective
We aimed to validate association of these variants with obesity-related traits in population-based samples.
Design
Genotypes and anthropometric traits were available in up to 31 083 adults from the Fenland, EPIC-Norfolk, Whitehall II, Ely and Hertfordshire studies and in 2 042 children and adolescents from the European Youth Heart Study. In each study, we tested associations of rs4712652 (near-PRL), rs10508503 (near-PTER), rs1424233 (near-MAF) and rs1805081 (NPC1), or proxy variants (r2>0.8), with the odds of being overweight and obese, as well as with BMI, percentage body fat (%BF) and waist circumference (WC). Associations were adjusted for sex, age and age2 in adults and for sex, age, age-group, country and maturity in children and adolescents. Summary statistics were combined using fixed effects meta-analysis methods.
Results
We had 80% power to detect ORs of 1.046 to 1.092 for overweight and 1.067 to 1.136 for obesity. Variants near PRL, PTER and MAF were not associated with the odds of being overweight or obese, or with BMI, %BF or WC after meta-analysis (P > 0.15). The NPC1 variant rs1805081 showed some evidence of association with %BF (beta=0.013 SD/allele, P =0.040), but not with any of the remaining obesity-related traits (P >0.3).
Conclusion
Overall, these variants, which were identified in a GWAS for early onset and morbid obesity, do not seem to influence obesity-related traits in the general population.
Keywords: Obesity-susceptibility loci, genome-wide association, morbid, early-onset, anthropometric traits, children and adolescents, population-based
Introduction
Meta-analyses of genome-wide association studies (GWAS) for continuous obesity-related traits have so far identified 32 loci for BMI and 14 loci for waist-hip ratio1,2. Meyre et al3 previously hypothesized that children with early-onset obesity (before the age of six yrs) and adults with morbid obesity (BMI ≥ 40 kg/m2) may be enriched for genetic variants that contribute to obesity in the general population. After a two-stage meta-analysis of case-control GWAS, including 3 447 obese cases and 3 827 normal weight controls, four variants in or near previously unanticipated genetic regions showed association with the risk of early onset and morbid obesity. These variants were located near PRL (Chr 6), near PTER (Chr 10), near MAF (Chr 16) and in NPC1 (Chr 18). Subsequent follow-up of these hits in two population-based samples of 5 291 children and 4 417 adults showed limited evidence of association with BMI, with each additional obesity risk-allele increasing BMI by 0.001 to 0.091 kg/m2 in children and by 0.017 to 0.04 kg/m2 in adults3. P-values for association with BMI ranged from 3.2×10−6 to 0.98 in children and from 4.6×10−2 to 0.61 in adults3. Hence, in spite of associations with early onset and morbid obesity in case-control studies, the relevance of these variants for obesity and adiposity variation in the general population remains inconclusive.
In the present study, we examined whether the four loci identified in GWAS for early onset and morbid obesity also contribute to obesity-susceptibility and adiposity variation in the general population. To ensure sufficient power to find associations, we meta-analysed summary statistics in up to 31 083 adults from the Fenland, EPIC-Norfolk, Whitehall II, Ely and Hertfordshire studies and in 2 042 children and adolescents from the European Youth Heart Study (EYHS) (Tables 1-2; Supplementary Table 1).
Table 1.
Descriptive characteristics of children and adolescents of the EYHS stratified by sex
| Children | Adolescents | |||
|---|---|---|---|---|
| Boys | Girls | Boys | Girls | |
| n | 586 | 655 | 350 | 434 |
| Age (years) | 9.7 ± 0.4 | 9.6 ± 0.4 | 15.5 ± 0.5 | 15.5 ± 0.5 |
| BMI (kg/m2) | 17.1 ± 2.2 | 17.1 ± 2.6 | 20.5 ± 2.5 | 20.5 ± 2.7 |
| Weight (kg) | 33.3 ± 6.0 | 33.1 ± 6.8 | 62.5 ± 10.1 | 56.1 ± 8.2 |
| Height (m) | 139.1 ± 6.6 | 138.7 ± 6.7 | 174.4 ± 7.6 | 165.3 ± 6.0 |
| Waist circumference (cm) | 59.4 ± 5.5 | 58.4 ± 6.6 | 71.3 ± 5.9 | 66.8 ± 5.8 |
| Percentage body fat (%) | 23.4 ± 4.5 | 28.7 ± 5.5 | 17.7 ± 3.1 | 24.8 ± 3.5 |
| Normal weight (n / %) | 495 / 84.5 | 568 / 86.7 | 312 / 89.1 | 386 / 88.9 |
| Overweight, non-obese (n / %) | 75 / 12.8 | 73 / 11.1 | 35 / 10.0 | 45 / 10.4 |
| Obese (n / %) | 16 / 2.7 | 14 / 2.1 | 3 / 0.9 | 3 / 0.7 |
Data are presented as means ± SD for individuals with data on at least one SNP Obese: BMI ≥ 99th percentile; Overweight, non-obese: 90th percentile ≤ BMI < 99th percentile; Normal weight: BMI < 90th percentile
Table 2.
Descriptive characteristics of adults per cohort stratified by sex
| Fenland 1 | Fenland 2 | EPIC-Norfolk | Whitehall | Ely | Hertfordshire | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | Women | |
| n | 838 | 1 032 | 1 486 | 1 698 | 10 184 | 10 480 | 1 699 | 535 | 744 | 855 | 1 224 | 971 |
| Age (years) | 44.7 ± 7.3 | 45.3 ± 7.1 | 46.9 ± 7.2 | 46.9 ± 7.0 | 59.1 ± 9.3 | 58.6 ± 9.3 | 60.5 ± 5.8 | 60.6 ± 5.8 | 61.5 ± 9.1 | 60.8 ± 9.2 | 65.9 ± 2.9 | 66.6 ± 2.7 |
| BMI (kg/m2) | 27.8 ± 4.0 | 26.8 ± 5.6 | 27.0 ± 4.1 | 26.5 ± 5.5 | 26.5 ± 3.3 | 26.2 ± 4.2 | 26.6 ± 3.6 | 27.1 ± 5.3 | 27.4 ± 4.0 | 27.3 ± 5.4 | 27.1 ± 3.7 | 27.5 ± 5.0 |
| Weight (kg) | 87.3 ± 14.4 | 71.9 ± 15.7 | 85.4 ± 14.1 | 71.2 ± 15.5 | 80.3 ± 11.4 | 67.8 ± 11.7 | 81.9 ± 12.4 | 70.9 ± 14.5 | 83.1 ± 13.3 | 71.1 ± 14.3 | 82.4 ± 12.7 | 71.3 ± 13.7 |
| Height (m) | 177.2 ± 6.4 | 163.8 ± 6.4 | 1.78 ± 0.07 | 1.64 ± 0.06 | 174.0 ± 6.6 | 161.0 ± 6.2 | 175.2 ± 6.5 | 161.6 ± 6.4 | 174.2 ± 6.7 | 161.4 ± 6.1 | 174.2 ± 6.4 | 160.8 ± 5.9 |
| Waist circumference (cm) | 99.0 ± 11.5 | 87.1 ± 13.0 | 96.4 ± 11.2 | 85.2 ± 12.8 | 95.7 ± 9.7 | 82.1 ± 10.8 | 95.1 ± 10.3 | 86.0 ± 12.9 | 100.0 ± 10.6 | 87.6 ± 12.6 | 100.7 ± 10.5 | 92.2 ± 12.9 |
| Percentage body fat (%) | 24.3 ± 5.4 | 35.9 ± 7.1 | 23.7 ± 5.7 | 35.7 ± 7.2 | 23.5 ± 6.2* | 39.7 ± 9.1* | 24.3 ± 5.4 | 36.3 ± 6.8 | 26.6 ± 5.2 | 39.9 ± 6.5 | 28.7 ± 5.3 | 39.9 ± 5.0 |
| Normal weight (n / %) | 205 / 24.5 | 469 / 45.5 | 494 / 33.2 | 806 / 47.5 | 3 387 / 33.3 | 4 684 / 44.7 | 573 / 33.7 | 198 / 37.0 | 210 / 28.2 | 331 / 38.7 | 352 / 28.8 | 320 / 33.0 |
| Overweight, non-obese (n / %) | 438 / 52.3 | 323 / 31.3 | 695 / 46.8 | 542 / 31.9 | 5 466 / 53.7 | 4 125 / 39.4 | 850 / 50.0 | 210 / 39.3 | 372 / 50.0 | 322 / 37.7 | 624 / 51.0 | 386 / 39.8 |
| Obese (n / %) | 184 / 18.2 | 210 / 17.3 | 285 / 17.6 | 311 / 16.0 | 1 307 / 12.4 | 1 575 / 13.9 | 271 / 15.4 | 109 / 15.4 | 155 / 18.5 | 226 / 16.9 | 244 / 19.1 | 247 / 22.0 |
| Morbidly obese (n / %) | 11 / 5.1 | 30 / 6.0 | 12 / 2.4 | 39 / 4.6 | 24 / 0.7 | 96 / 2.0 | 5 / 0.9 | 18 / 8.3 | 7 / 3.2 | 178 / 16.9 | 4 / 1.1 | 18 / 5.3 |
Data are presented as means ± SD for individuals with data on at least one SNP; Percentage body fat for EPIC-Norfolk is from second health check (first follow-up, four yrs after baseline measurements; n = 6 265 and 6 206 for men and women, respectively)
Obese: BMI ≥ 30 kg/m2; Overweight, non-obese: 25 kg/m2 ≤ BMI < 30 kg/m2; Normal weight: BMI < 25 kg/m2 In comparison, the most recent National Health Survey for England (2009) indicates that 61.3% of adults had a BMI ≥ 25 kg/m2 and 23% had a BMI ≥ 30 kg/m2. In the latter health survey, average WC was 96.4 cm in adult men and 87.4 cm in adult women (http://www.dh.gov.uk/en/publichealth/obesity)
Materials and Methods
Study population and anthropometry
Genetic information and data on anthropometric traits were available in up to 31 083 adults from the Fenland, EPIC-Norfolk, Whitehall II, Ely and Hertfordshire studies, as well as in 1 252 children and 790 adolescents from the EYHS (Tables 1-2). Detailed information about study designs, participant recruitment and phenotype collection has been described elsewhere4-12.
Body mass and height were measured using standard procedures with participants dressed in light clothing and barefoot13. Overweight and obesity were defined as BMI ≥ 25 and ≥ 30 kg/m2 in adults and using age- and sex-specific thresholds of BMI in the EYHS (≥ 90th percentile for overweight; ≥ 99th percentile for obesity)14. Normal weight was defined as BMI < 25 kg/m2 in adults and a BMI < 90th percentile in the EYHS.
Waist circumference (WC) was measured midway between the lower rib margin and the iliac crest using a non-stretchable tape measure in all studies5, 6, 8, 9, 12, 15. We applied several techniques to determine percentage body fat (%BF). Dual energy X-ray absorptiometry (DEXA) (Lunar Prodigy Advanced fan beam scanner; GE Healthcare, Bedford, UK) was used in a subsample of the Fenland study (subsample 1, n = 1 870)9, 15, whereas we used bio-impedance in a second, independent subsample of the Fenland study (subsample 2, n = 3 184) (Tanita BD-418AM, Tanita Europe GmbH, Sindelfingen, Germany) 9, as well as in the Whitehall II (Tanita TBF-300; Tanita Europe GmbH, Sindelfingen, Germany), Ely (Bodystat 1500; Bodystat, Isle of Man, UK)6 and EPIC-Norfolk (Tanita 531; Tanita Europe GmbH, Sindelfingen, Germany) studies. In data from 2 535 Fenland participants, %BF as assessed using bio-impedance and DEXA were strongly correlated (r=0.92)16. In the Hertfordshire study, %BF was derived from the sum of four skinfolds (triceps brachial, biceps brachial, sub-scapular and supra-iliacal sites in mm) using the method of Durnin and Wormsley17. Maturity specific equations (pre-pubertal and pubertal)18 were used to derive %BF from the sum of two skinfolds in the EYHS (triceps brachial and sub-scapular sites in mm)19. All techniques used explain 80%-90% of the variance in body composition as assessed using the four-compartment model18, 20-22. Sexual maturity was assessed in the EYHS using the five-stage Tanner scale for breast development in girls and pubic hair in boys23.
Each study was approved by the local scientific committees and was performed in accordance with the declaration of Helsinki. All adults gave their written informed consent. In the EYHS, parents gave written informed consent for their child to participate and all children and adolescents gave verbal consent.
Genotyping
In subsample 1 of the Fenland study, as well as in the EYHS and Hertfordshire studies, we genotyped rs4712652 near PRL, rs10508503 near PTER, rs1424233 near MAF and rs1805081 in NPC1. The latter three were genotyped using a Sequenom iPLEX ® platform (Sequenom, San Diego, Ca, USA)24, whereas rs4712652 was genotyped using Custom TaqMan ® SNP Genotyping Assays, according to the manufacturer’s protocol (Applied Biosystems, Warrington, UK). Taqman assays were also used to genotype rs4712652, rs10508503, rs10514465 (a proxy for rs1424233 (r2 = 1.000; D′ = 1.000)) and rs1805081 in the EPIC-Norfolk study.
In the Ely and Whitehall II studies, as well as in subsample 2 of the Fenland study, genotype information was available from the metabochip; a custom iSELECT array containing ~195k SNPs that was designed to support large-scale follow-up of putative associations with metabolic and cardiovascular traits. The metabochip contains genetic information for rs6901423 (a proxy for rs4712652 (r2 = 0.803; D′ = 0.927)), rs8048408 (a proxy for rs1424233 (r2 = 0.967; D′ = 1.000) and rs1805081.
All markers passed quality control criteria; call rates > 95%, P-value for Hardy-Weinberg equilibrium > 0.0125 (Bonferroni correction for four independent tests at α = 0.05), as determined by a Chi2 test with one degree of freedom. An exception was rs4712652 in the EPIC-Norfolk, Whitehall II and Hertfordshire studies (P < 0.0125) (Supplementary Table 1). For association analyses of rs4712652, we excluded the latter samples from further analyses.
Statistical analysis
Before testing for associations, all traits were transformed to normal distributions with a mean of zero and SD of one in each study separately using inverse normal transformation. Hence, effect sizes can be interpreted as Z-scores, which allow comparison across traits and age-groups.
Logistic regression was used to test the association of each SNP with the odds of being overweight or obese compared with non-overweight individuals (BMI < 25 kg/m2; control group). A sensitivity analysis showed that using non-obese individuals (BMI < 30 kg/m2) as the control group did not change the results. We also tested the association of each SNP with BMI, %BF and WC using linear regression. For the near-PRL variant (rs4712652), associations with BMI (n = 4 879) and %BF (n = 3 068) were recently reported in the PPP-Botnia study25, whereas for the near-PTER (rs10508503), near-MAF (rs1424233) and NPC1 (rs1805081) variants, associations with BMI and WC were recently described in the Inter99 study (n = 6 514)26. Additional meta-analyses were performed in which the latter results were pooled with those of the current study. To the best of our knowledge, the PPP-Bothnia and Inter99 studies represent the only population-based samples with data from individuals of white European descent in which the association of these SNPs with adiposity-related traits have been described since they were discovered.
All associations were tested assuming an additive model of inheritance. However, based on the results of the discovery study3, we additionally examined associations under alternative models for the near-PRL (recessive model) and near-PTER (dominant model) variants. Risk-alleles were defined as the obesity-susceptibility increasing alleles in the discovery study3. All associations were adjusted for sex, age and age2 in adults, and for sex, age, age-group (children versus adolescents), country and maturity in the EYHS. In all studies, associations with WC were additionally adjusted for height.
In a secondary analysis, we examined interactions of SNPs with sex and age for case-control analyses and continuous traits by adding an interaction term to the model (SNP*sex, SNP*age). Interactions with sex and age were considered significant if P < 6.25×10−3 (Bonferroni correction for eight independent tests, i.e. four SNPs and two interactions per SNP, at α = 0.05).
Study-specific betas and SEs of the main effect associations and interaction terms were meta-analysed for continuous outcomes using the inverse variance weighted fixed effects method. We used the same method to perform meta-analyses of study specific odds ratios and 95% CIs for the case-control analyses. P-values for associations reported in the PPP-Botnia25 and Inter99 studies26 were pooled with those of the current studies using the weighted Z-score approach.
Power calculations were performed to estimate the power of our study to detect the odds ratios for obesity that were observed after meta-analysis of four case-control studies for early onset and morbid obesity in the replication stage of the discovery study3. In addition, we estimated the odds ratio for risk of overweight and obesity that we would be able to identify with 80% power at an α-level of 0.05 given our sample size. Given the relatively small sample of the EYHS, we had insufficient power to conclusively validate associations in children and adolescents. Nevertheless, we choose to provide and cautiously interpret the results as they are the first describing associations between these four loci and obesity-related traits in a population-based sample of children and adolescents. This way, the summary statistics of these associations will be valuable for others to use in future meta-analyses in young individuals. Furthermore, summary statistics from the EYHS were included in the full meta-analysis of our study, thereby further increasing the power to find associations.
We performed statistical analyses using SAS version 9.2 for windows (SAS Institute, Cary, NC, USA). We used STATA software for inverse variance weighted meta-analysis (metan) (STATA version 10.1, StataCorp, College Station, TX), whereas METAL was used for the weighted Z-score meta-analysis27. Power calculations were performed using Quanto (Quanto v1.1.1: http//hydra.usc.edu/gxe). Associations presented are for meta-analyses of all new data assuming an additive model unless stated otherwise. A two-sided P-value ≤ 0.05 was considered statistically significant.
Results
Power
For the near-PTER, near-MAF and NPC1 variants, we had >99% power to detect the ORs observed by Meyre et al for overweight and obesity (Supplementary Table 2). The power was generally lower for the near-PRL variant (88% for overweight; 61% for obesity) as the sample size was smaller. With the available sample size, we had 80% power to detect ORs of 1.046 to 1.092 for overweight and 1.067 to 1.136 for common obesity (Supplementary Table 2).
Meta-analysis
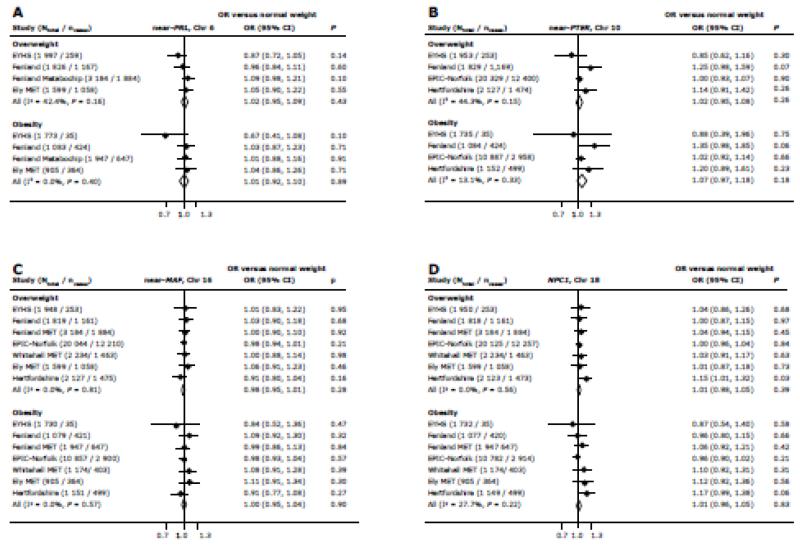
None of the risk alleles were associated with increased odds of being overweight or obese after meta-analysis of results from our population-based studies (P > 0.15) (Figure 1).
Figure 1.
Associations of variants near PRL (A), near PTER (B), near MAF (C) and in NPC1 (D) with the odds of being overweight and obese compared with non-overweight (control group), calculated using logistic regression in individual studies and after fixed effects meta-analysis of ORs and 95% CI. Associations were adjusted for age, age2 and sex in adults and for age, age-group, sex, country and maturity in children and adolescents. Odds ratios (OR) represent the change in outcome for each additional risk allele under an additive model, with risk alleles defined as the obesity-susceptibility increasing alleles in the discovery study.
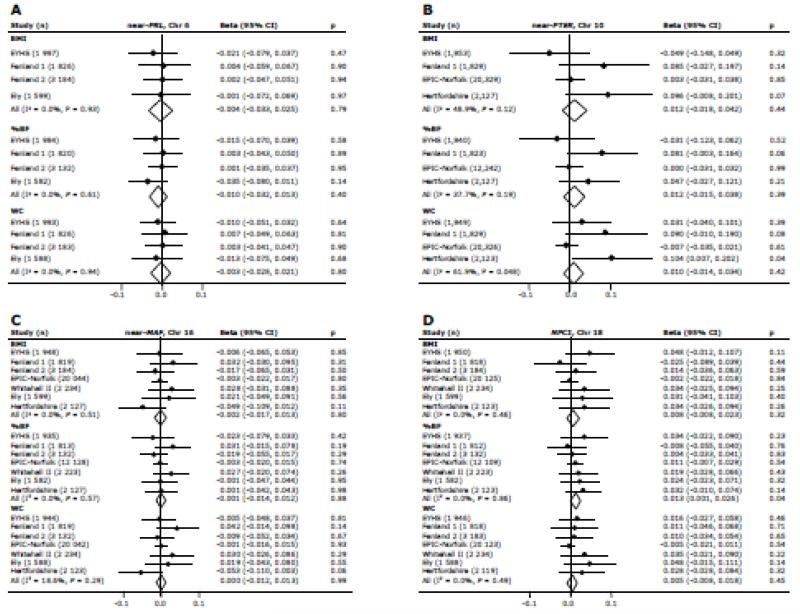
We found no evidence of association between any of the four variants and BMI after meta-analysis (P > 0.3) (Figure 2). We subsequently examined associations of the four variants with %BF and WC. Except for an association of the NPC1 variant (rs1805081) with %BF (beta = 0.013 SD/allele, P = 0.040), none of the variants showed association with %BF (P > 0.3) or WC (P > 0.4) (Figure 2). Heterogeneity in effect sizes was low to moderate for the near-PRL, near-PTER, near-MAF and NPC1 variants (I2 = 0.0% - 61.9%) (Figures 1-2).
Figure 2.
Associations of variants near PRL (A), near PTER (B), near MAF (C) and in NPC1 (D) with BMI, percentage body fat (%BF) and waist circumference (WC), calculated using linear regression in individual studies and after fixed effects meta-analysis of betas and standard errors. Associations were adjusted for age, age2 and sex in adults and for age, age-group, sex, country and maturity in children and adolescents. Associations with WC were additionally adjusted for height. As all traits were inverse normally transformed, betas represent the change in outcome for each additional risk allele under an additive model, expressed in SD units/ allele. Risk alleles are defined as the obesity-susceptibility increasing alleles in the discovery study.
Including recently reported associations of the near-PRL variant with BMI and %BF25 and of the near-PTER, near-MAF and NPC1 variants with BMI and WC26 in the meta-analysis did not change the results (Supplementary Table 3). Furthermore, results remained unchanged after assuming an alternative mode of inheritance for the near-PRL (recessive model) and near-PTER (dominant model) variants, based on the results of the discovery study (Supplementary Table 4)3. A sex-specific association of the near-PRL variant with BMI and %BF was reported in the PPP-Botnia study25. In a secondary analysis, none of the four variants showed evidence for an interaction with sex or age in either case-control analyses (Supplementary Table 5), or for BMI, %BF or WC (Supplementary Table 6) after considering the additional number of tests performed.
Discussion
Based on a meta-analysis of GWAS for early onset and morbid obesity, variants in/near PRL, PTER, MAF and NPC1 were previously considered relevant for common obesity and BMI variation3. So far, it remains unclear whether these variants are indeed associated with adiposity variation and obesity-susceptibility in the general population, as was confirmed for many of the loci identified in large-scale meta-analyses of GWAS for BMI2, waist-to-hip ratio adjusted for BMI1 and %BF16. While our study had sufficient power to identify small effects on the risk of overweight and obesity, none of the variants showed consistent and convincing evidence of such associations. These variants were not associated with the odds of being overweight or obese, nor were they associated with BMI, %BF and WC after meta-analysis of data from up to 33 045 population-based samples. Taken together, our findings suggest that the contribution of these variants to adiposity in the general population is likely limited. However, based on our findings, we cannot exclude the possibility that individuals with early onset and morbid obesity are enriched for rare, functional variants in high LD with the variants studied here. We had 62% power to detect the odds ratio for obesity (OR = 1.10) that was observed by Meyre et al for the near-PRL variant3. However, in our meta-analyses, ORs were 1.02 for overweight and 1.01 for obesity, suggesting that even with a larger sample and thus more power we were not able to confirm the associations observed in the case/control analyses for early onset and morbid obesity. Adding published results from the PPP-Botnia study25 to the meta-analysis for BMI and %BF did not change the results.
Recently, the near-PRL variant was shown to significantly interact with sex for BMI (P = 0.05) and %BF (P = 0.04) in up to 4 879 adults of the population-based PPP-Botnia study. Associations with BMI (beta = 0.36 kg/m2, P = 0.0047) and %BF (beta = 0.60%, P = 0.025) were restricted to male participants, with no associations observed in women (BMI: beta = 0.004 kg/m2, P = 0.98; %BF: beta = 0.21%, P = 0.45)25. However, in line with results from the discovery study3, our study does not show evidence for a sex-specific association of the near-PRL variant with BMI and %BF, with marginally larger effect sizes in women than in men (P > 0.4).
The observation that the near-PTER and near-MAF variants were not associated with any of the obesity-related traits in our study, either with or without inclusion of the recently published Inter99 data26, is consistent with a lack of association of these variants with BMI in adults of the population-based DESIR study (n = 4 417, P > 0.1)3, as well as in adults of the Inter99 cohort, in which effect sizes pointed in the opposite direction (n = 6 514, P > 0.05). In addition, no evidence for association with BMI was observed recently for the near-PTER and near-MAF variants after a large-scale meta-analysis of GWAS (n = 123 865) performed by the GIANT consortium (P = 0.64 for near-PTER; P = 0.25 for near-MAF)2. In another recent meta-analysis of GWAS, these variants did not show evidence of association with %BF either (n = 26 034, P = 0.31 for near-PTER; n = 36 309, P = 0.48 for near-MAF)16. Taken together, these results do not support an association of the near-PTER and near-MAF variants with obesity-related traits in the general population. Unfortunately, the aforementioned meta-analyses of GWAS for BMI and %BF did not provide results for associations with the near-PRL variant2, 16.
Of the four variants, only the near-MAF variant was associated with BMI in children of the population-based NFBC86 study (n = 5 291, P = 3.2×10−6)3. With BMI and genotype information available in 1 948 children and adolescents, we had only 16% power to validate the association of the near-MAF variant with BMI in the EYHS. Still, since the association with all obesity-related traits was either null or in the opposite direction in the EYHS, we cautiously conclude that our results do not support a role for the near-MAF variant in the aetiology of early onset obesity in the general population.
Only the association between rs1805081 in NPC1 and %BF, the most representative measure of overall adiposity that was available in the present study, reached nominal statistical significance, and was directionally consistent with results presented in the discovery study3. Associations with BMI and WC did not reach significance but were directionally consistent, with slightly smaller effect sizes compared with %BF. Each additional copy of the risk allele was associated with a 0.013 SD increase in %BF, corresponding with ~0.07% body fat, without any heterogeneity in effect size between studies. rs1805081, a non-synonymous (His215Arg) variant in exon 6 of NPC1, also showed limited evidence of association with BMI (P=2.5×10−3) and %BF (P=4.5×10−2) in recent GWAS meta-analyses2, 16. NPC1 is functionally important for the regulation of LDL cholesterol trafficking in mammalian cells28 and plays a central role in maintaining cellular, tissue and whole body lipid homeostasis29, 30. Recent human and animal studies highlighted NPC1 as a candidate gene for (diet-induced) obesity31, 32.
In conclusion, the results of the present study show that with the possible exception of rs1805081 in NPC1, variants identified in case-control data sets for early onset and morbid obesity do not seem to have an effect on obesity-related traits in the general population.
Supplementary Material
Acknowledgments
The authors would like to thank the study teams, who collected the data used in these analyses. We also acknowledge the volunteers, who gave their time to take part in the individual studies.
The Fenland study was supported by the Wellcome Trust; the Medical Research Council; the Support for Science Funding programme; and CamStrad. The EPIC Norfolk Study was supported by Cancer Research United Kingdom; and the Medical Research Council. The Whitehall II Study was supported by grants from the Medical Research Council (MRC); the British Heart Foundation; the United Kingdom Health and Safety Executive; the United Kingdom Department of Health; the US National Heart, Lung, and Blood Institute (grant HL36310); the US National Institute on Aging (grant AG13196); the US Agency for Health Care Policy and Research (grant HS06516); and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socio-economic Status and Health. The MRC Ely Study was supported by the Medical Research Council; and the Wellcome Trust. The Hertfordshire Study was supported by the Medical Research Council UK; and the University of Southampton UK. The EYHS was supported by grants from The Danish Heart Foundation; The Danish Medical Research Council Health Foundation; The Danish Council for Sports Research; The Foundation in Memory of Asta Florida Bolding Renée Andersen; The Faculty of Health Sciences, University of Southern Denmark; and The Estonian Science Foundation [grant 3277, 5209].
Footnotes
None of the authors declare a conflict of interest.
Supplementary information is available at The International Journal of Obesity’s website.
References
- 1.Heid IM, Jackson AU, Randall JC, Winkler TW, Qi L, Steinthorsdottir V, et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nature genetics. 2010;42(11):949–60. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nature genetics. 2010;42(11):937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nature genetics. 2009;41(2):157–9. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- 4.Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer. 1999;80(Suppl 1):95–103. [PubMed] [Google Scholar]
- 5.Dykes J, Brunner EJ, Martikainen PT, Wardle J. Socioeconomic gradient in body size and obesity among women: the role of dietary restraint, disinhibition and hunger in the Whitehall II study. Int J Obes Relat Metab Disord. 2004;28(2):262–8. doi: 10.1038/sj.ijo.0802523. [DOI] [PubMed] [Google Scholar]
- 6.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ. Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the Medical Research Council Ely Study. Diabetes Care. 2005;28(5):1195–200. doi: 10.2337/diacare.28.5.1195. [DOI] [PubMed] [Google Scholar]
- 7.Marmot M, Brunner E. Cohort Profile: the Whitehall II study. International journal of epidemiology. 2005;34(2):251–6. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 8.Riddoch C, Edwards D, Page A, Froberg K, Andersen A, Wedderkopp N, et al. The European Youth Heart Study-Cardiovascular disease risk factors in children: rationale, aims, study design, and validation of methods. Journal of physical activity and health. 2005;2:115–129. [Google Scholar]
- 9.Rolfe Ede L, Loos RJ, Druet C, Stolk RP, Ekelund U, Griffin SJ, et al. Association between birth weight and visceral fat in adults. The American journal of clinical nutrition. 2010;92(2):347–52. doi: 10.3945/ajcn.2010.29247. [DOI] [PubMed] [Google Scholar]
- 10.Syddall HE, Aihie Sayer A, Dennison EM, Martin HJ, Barker DJ, Cooper C. Cohort profile: the Hertfordshire cohort study. International journal of epidemiology. 2005;34(6):1234–42. doi: 10.1093/ije/dyi127. [DOI] [PubMed] [Google Scholar]
- 11.Syddall HE, Simmonds SJ, Martin HJ, Watson C, Dennison EM, Cooper C, et al. Cohort profile: The Hertfordshire Ageing Study (HAS) International journal of epidemiology. 2010;39(1):36–43. doi: 10.1093/ije/dyn275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vimaleswaran KS, Li S, Zhao JH, Luan J, Bingham SA, Khaw KT, et al. Physical activity attenuates the body mass index-increasing influence of genetic variation in the FTO gene. The American journal of clinical nutrition. 2009;90(2):425–8. doi: 10.3945/ajcn.2009.27652. [DOI] [PubMed] [Google Scholar]
- 13.Europe. Co . The Eurofit Test Battery. Council of Europe; Strasburg, France: 1988. [Google Scholar]
- 14.Cole T, Bellizzi M, Flegal K, Dietz W. Establishing a standard definition for child overweight and obesity worldwide: international survey. British Medical Journal. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finucane FM, Sharp SJ, Purslow LR, Horton K, Horton J, Savage DB, et al. The effects of aerobic exercise on metabolic risk, insulin sensitivity and intrahepatic lipid in healthy older people from the Hertfordshire Cohort Study: a randomised controlled trial. Diabetologia. 2010;53(4):624–31. doi: 10.1007/s00125-009-1641-z. [DOI] [PubMed] [Google Scholar]
- 16.Kilpelainen TO, Zillikens MC, Stancakova A, Finucane FM, Ried JS, Langenberg C, et al. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nature genetics. 2011;43(8):753–60. doi: 10.1038/ng.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32(1):77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 18.Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60(5):709–23. [PubMed] [Google Scholar]
- 19.Lohman T, Roche A, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books; Champaign: IL: 1991. [Google Scholar]
- 20.LaForgia J, Dollman J, Dale MJ, Withers RT, Hill AM. Validation of DXA body composition estimates in obese men and women. Obesity. 2009;17(4):821–6. doi: 10.1038/oby.2008.595. [DOI] [PubMed] [Google Scholar]
- 21.Sopher AB, Thornton JC, Wang J, Pierson RN, Jr., Heymsfield SB, Horlick M. Measurement of percentage of body fat in 411 children and adolescents: a comparison of dual-energy X-ray absorptiometry with a four-compartment model. Pediatrics. 2004;113(5):1285–90. doi: 10.1542/peds.113.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun SS, Chumlea WC, Heymsfield SB, Lukaski HC, Schoeller D, Friedl K, et al. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. The American journal of clinical nutrition. 2003;77(2):331–40. doi: 10.1093/ajcn/77.2.331. [DOI] [PubMed] [Google Scholar]
- 23.Tanner J. Growth at adolescence. 2nd edition edn Blackwell; Oxford: 1962. [Google Scholar]
- 24.Li S, Zhao JH, Luan J, Luben RN, Rodwell SA, Khaw KT, et al. Cumulative effects and predictive value of common obesity-susceptibility variants identified by genome-wide association studies. The American journal of clinical nutrition. 2010;91(1):184–90. doi: 10.3945/ajcn.2009.28403. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson L, Olsson AH, Isomaa B, Groop L, Billig H, Ling C. A common variant near the PRL gene is associated with increased adiposity in males. Mol Genet Metab. 2010;102(1):78–81. doi: 10.1016/j.ymgme.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Sandholt CH, Vestmar MA, Bille DS, Borglykke A, Almind K, Hansen L, et al. Studies of metabolic phenotypic correlates of 15 obesity associated gene variants. PLoS One. 2011;6(9):e23531. doi: 10.1371/journal.pone.0023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millard EE, Gale SE, Dudley N, Zhang J, Schaffer JE, Ory DS. The sterol-sensing domain of the Niemann-Pick C1 (NPC1) protein regulates trafficking of low density lipoprotein cholesterol. J Biol Chem. 2005;280(31):28581–90. doi: 10.1074/jbc.M414024200. [DOI] [PubMed] [Google Scholar]
- 29.Garver WS, Jelinek D, Francis GA, Murphy BD. The Niemann-Pick C1 gene is downregulated by feedback inhibition of the SREBP pathway in human fibroblasts. J Lipid Res. 2008;49(5):1090–102. doi: 10.1194/jlr.M700555-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gevry N, Schoonjans K, Guay F, Murphy BD. Cholesterol supply and SREBPs modulate transcription of the Niemann-Pick C-1 gene in steroidogenic tissues. J Lipid Res. 2008;49(5):1024–33. doi: 10.1194/jlr.M700554-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Jelinek D, Heidenreich RA, Erickson RP, Garver WS. Decreased Npc1 gene dosage in mice is associated with weight gain. Obesity (Silver Spring, Md. 2010;18(7):1457–9. doi: 10.1038/oby.2009.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uronen RL, Lundmark P, Orho-Melander M, Jauhiainen M, Larsson K, Siegbahn A, et al. Niemann-Pick C1 modulates hepatic triglyceride metabolism and its genetic variation contributes to serum triglyceride levels. Arterioscler Thromb Vasc Biol. 2010;30(8):1614–20. doi: 10.1161/ATVBAHA.110.207191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.