Abstract
Strongyloides stercoralis is an intestinal nematode causing endemic infection, mostly in immunocompromised individuals, in tropical and subtropical regions. Herewith, we are reporting a rare case of this kind in immunocompetent patient. A 31-year-old male patient presented with chief complaints of chronic diarrhea and loss of weight since last 4 months. He reported passing watery and foul smelling stool. He also had loss of appetite since last 2 months and was diagnosed as diabetic since last 4 months but he was not given any treatment for this and his fasting blood sugar was 110 mg/dl. His HIV status was negative. Stool examination done on three occasions showed plenty of S. stercoralis larvae. Patient responded well to albendazole therapy. Strongyloidiasis is not always associated with compromise in immune status. It should be suspected in immunocompetent individuals with history of long-term diarrhea and weight loss.
KEYWORDS: Strongyloides stercoralis, immunocompetent, hyperinfection
INTRODUCTION
Strongyloides stercoralis is an intestinal nematode causing gastrointestinal (GI) infections that are often asymptomatic. It is endemic in tropical and subtropical countries.[1] Strongyloidiasis is generally benign and asymptomatic, eosinophilia and larvae in stool are the only indicators of infection.[2] In immunocompromised host, it can cause substantial intestinal disease and can disseminate widely to extra-intestinal sites such as lungs, kidney, or brain (hyperinfection syndrome); however, in immunocompetent hosts, these parasites cause a low-grade chronic infection, which has been seen even upto 40 years after exposure.[3] We report a case of 31-year-old immunocompetent male presented with features of malabsorption due to hyperinfection of S. stercoralis.
CASE REPORT
A 31-year-old male farmer was admitted to our hospital with chief complaints of chronic diarrhea since last 4 months. He had a history of passing watery and foul smelling stool. He complained of lower abdominal pain, fever, and loss of weight for 4 months as well as loss of appetite for last 2 months. The patient was diagnosed as pre-diabetic earlier and his fasting blood sugar was 110 mg/dl and post-prandial blood sugar was 145 mg/dl on admission. His weight was 53 kg and systemic examination findings including abdominal examination were normal. There was no lymphadenopathy, clubbing, and/or pedal edema. His laboratory investigations were as follows: Total leucocyte count, 11,200/cmm3; neutrophil, 64%; lymphocyte, 29%; eosinophil, 6%; monocyte, 0%; and basophil, 1%. Ultrasound of abdomen was normal; erythrocyte sedimentation rate, 5 mm in 1 h; Na+, 126 mEq/l; and K+, 2.6 mEq/l. Colonoscopy was normal. Serum urea and creatinine were 34 mg/dl and 0.9 mg/dl, respectively. Thyroid stimulating hormone (TSH) was 4.55 mIU/L and HIV - Human immunodeficiency virus, ELISA - Enzyme linked immunosorbent assay status (ELISA method) was negative. Urine examination (routine microscopy as well as culture) was normal. Stool examination done on three occasions showed larvae of S. stercoralis [Figures 1–3 and Video Clip]. He was treated with albendazole 10 mg/kg/day for 7 days. Stool examination was repeated after 1 week of therapy, which was negative for larvae. On follow-up, we found that diarrhea subsided and he had started gaining weight.
Figure 1.

Anterior end of the larva
Figure 3.
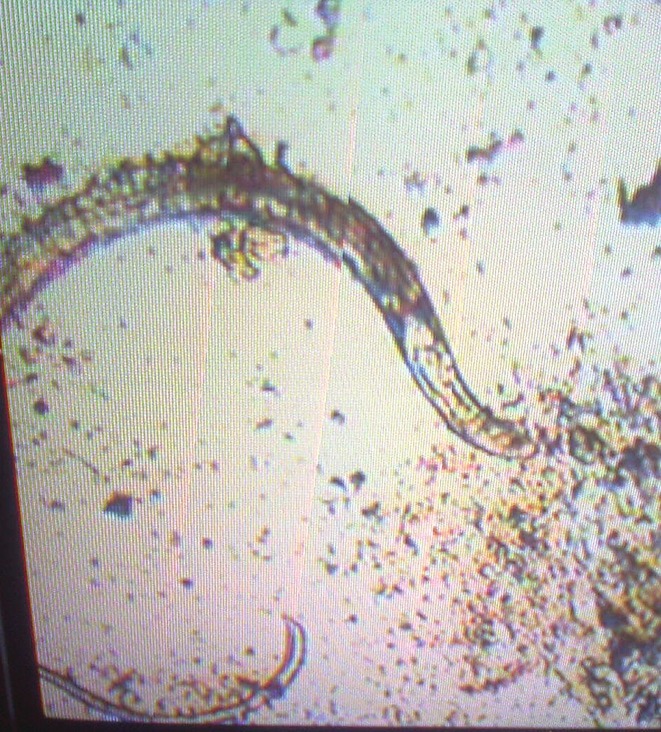
Complete larva
Figure 2.
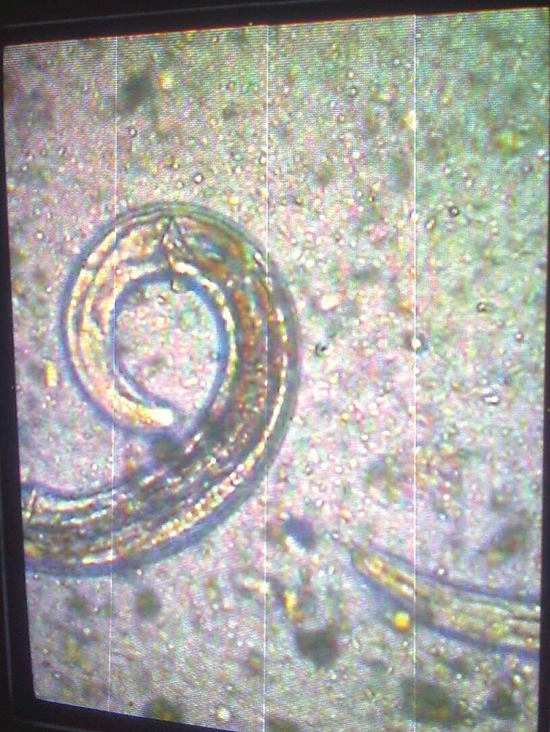
Posterior end of larva
DISCUSSION
S. stercoralis is an intestinal nematode of humans. It is endemic in tropical and subtropical areas. Most infected individuals are asymptomatic, but S. stercoralis infestation is capable of transforming into a fulminant fatal illness under certain conditions associated with a compromise of host immunity.[4] Low socioeconomic status, alcoholism, white race, and male gender have been associated with higher prevalence of Strongyloides stool positivity.[5] Initial invasion occurs when the patient's skin is exposed to contaminated soil for feces. The filariform (infective) larvae penetrate the dermis and migrate through the venous system to the lungs, ascend to the trachea, and are swallowed into the digestive tract and infect the small intestinal mucosa. Some larvae, however, re-enter the blood stream through the bowel wall and migrate through lungs bypassing the soil cycle. This ability for autoinfection implies that infestation can be life-long and extremely heavy.[6] Hyperinfection means accelerated autoinfection characterized by development or enhanced GI and pulmonary symptoms with increased numbers of larvae seen in stool or sputum, usually restricted to the organ of autoinfective cycle. The term dissemination implies migration of larvae to the organs other than GI and pulmonary organs. Involvement of brain, heart, gall bladder, and kidney has been reported.[7]
The clinical manifestation of hyperinfection syndrome varies widely and the onset may be acute or insidious. GI syndrome manifestations include watery diarrhea, weight loss, vomiting, and occasional bleeding. Usually symptoms of pulmonary strongyloidiasis include cough, dyspnea, wheezing, pulmonary hemorrhage, and pleural effusion; peripheral eosinophilia is common. Skin manifestations like pruritic linear streaks in lower trunk (larva currens) also frequently accompany hyperinfection. Eosinophilia is commonly seen in chronic infection but is less common in hyperinfection.[7] Diagnosis of Strongyloides is usually on the basis of detection of larvae in the stool or sputum, although the sensitivity of a single examination is only 50%.[8] Detection of larvae in the duodenal aspirates is more sensitive and hence should be considered when there is a clinical suspicion of hyperinfection.[9]
Strongyloides infection is treated with the aim to eradicate the infection. In chronic infection, ivermectin (200 mg/kg orally, once daily) for 1-2 days or albendazole (400 mg orally, twice daily) for 7 days is sufficient. Recent evidence suggests that ivermectin is the drug of choice[10] but disseminated or hyperinfection may need prolonged therapy. Strongyloidiasis is commonly reported in immunocompromised patients; however, our patient was immunocompetent as his HIV status was negative, the history of diabetes mellitus was of short duration and blood sugar was not highly raised. The patient responded well to albendazole therapy (400 mg twice daily for 7 days) and there were no Strongyloides larvae in the stool when examined after completion of therapy.
Video available at www.tropicalparasitology.org
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Genta RM. Global prevalence of strongyloidiasis: Critical review with epidemiologic insights into the prevention of disseminated disease. Rev Infect Dis. 1989;11:755–67. doi: 10.1093/clinids/11.5.755. [DOI] [PubMed] [Google Scholar]
- 2.Azira NM, Zeehaida M. Strongyloides stercoralis hyperinfection in a diabetic patient: Case report. Trop Biomed. 2010;27:115–9. [PubMed] [Google Scholar]
- 3.Gill GV, Bell DR. Strongyloides stercoralis infection in former far east prisoners of war. Br Med J. 1979;2:572–4. doi: 10.1136/bmj.2.6190.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampath PK, Partho PB, Erli AI, Renu G’Boy V, Prasanna CS, Ramji R, et al. An unusual presentation of Strongyloides stercoralis infestation. Infect Dis Clin Pract. 2008;16:271–2. [Google Scholar]
- 5.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–17. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vigg A, Mantri S, Reddy VA, Biyani V. Acute respiratory distress syndrome due to Strongyloides stercoralis in non-Hodgkin's lymphoma. Indian J Chest Dis Allied Sci. 2006;48:67–9. [PubMed] [Google Scholar]
- 7.Murali A, Rajendiran G, Ranganathan K, Shanthakumari S. Disseminated infection with Strongyloides stercoralis in a diabetic patient. Indian J Med Microbiol. 2010;28:407–8. doi: 10.4103/0255-0857.71854. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–7. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto K, Hokama A, Hirata T, Ihama Y, Nakamoto M, Kinjo N, et al. Endoscopic and histopathological study on the duodenum of Strongyloides stercoralis hyperinfection. World J Gastroenterol. 2008;14:1768–73. doi: 10.3748/wjg.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitisuttithum P, Supanaranond W, Chindanond D. A randomized comparative study of albendazole and thiabendazole in chronic Strongyloidiasis. Southeast Asian J Trop Med Public Health. 1995;26:735–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


